Summary
Background
Simple, bedside prediction of infection-related mortality in low-resource settings is crucial for triage and resource-utilisation decisions. We aimed to evaluate mortality prediction by combining point-of-care venous lactate with the quick Sequential Organ Failure Assessment (qSOFA) score in adult patients admitted to hospital with suspected infection in southeast Asia.
Methods
We performed a cohort study by prospectively enrolling patients aged 18 years or older who had been admitted to hospital within the previous 24 h for suspected infection (with at least three documented systemic manifestations of infection according to the 2012 Surviving Sepsis Campaign) at Sunpasitthiprasong Hospital in Ubon Ratchathani, Thailand (derivation cohort). Venous lactate concentration was determined by a point-of-care device and multiple scores were developed. We then evaluated candidate 28-day mortality prediction models combining qSOFA and the lactate scores. A final model was compared with the qSOFA score, a lactate score, and a modified Sequential Organ Failure Assessment (SOFA) score for mortality discrimination using the area under the receiver operating characteristic curve (AUROC). Mortality discrimination of the qSOFA-lactate score was then verified in an external, prospectively enrolled, multinational cohort in southeast Asia.
Findings
Between March 1, 2013, and Jan 26, 2017, 5001 patients were enrolled in the derivation cohort; 4980 had point-of-care lactate data available and were eligible for analysis, and 816 died within 28 days of enrolment. The discrimination for 28-day mortality prediction of a qSOFA-lactate score combining the qSOFA score and a lactate score was superior to that of the qSOFA score alone (AUROC 0·78 [95% CI 0·76–0·80] vs 0·68 [0·67–0·70]; p<0·0001) and similar to a modified SOFA score (0·77 [0·75–0·78]; p=0·088). A lactate score alone had superior discrimination compared with the qSOFA score (AUROC 0·76 [95% CI 0.74–0.78]; p<0·0001). 815 patients were enrolled in the external validation cohort and 792 had point-of-care lactate data and were included in the analysis; the qSOFA-lactate score (AUROC 0·77 [95% CI 0·73–0·82]) showed significantly improved 28-day mortality discrimination compared with the qSOFA score alone (0·69 [0·63–0·74]; p<0·0001).
Interpretation
In southeast Asia, rapid, bedside assessments based on point-of-care lactate concentration combined with the qSOFA score can identify patients at risk of sepsis-related mortality with greater accuracy than the qSOFA score alone, and with similar accuracy to a modified SOFA score.
Funding
National Institutes of Health, Wellcome Trust.
Introduction
Sepsis—organ dysfunction from a dysregulated immune response to infection—is a major cause of death worldwide.1 Low-income and middle-income countries (LMICs) are particularly affected, as millions of sepsis-related deaths are likely to occur each year among LMICs.2 In southeast Asia, sepsis-associated mortality is particularly high, due to a variety of causes.3 Early and accurate assessment of the clinical trajectory during severe infection is imperative, particularly in settings with limited tertiary health care.2
Clinical scoring systems, such as the Sequential Organ Failure Assessment (SOFA), have been proposed for predicting sepsis-related outcomes, including mortality.4,5 Nonetheless, SOFA requires multiple laboratory and clinical data, which are potentially challenging to obtain in low-resource areas.6 Alternative scores developed for LMICs were derived in emergency room or intensive care settings, have not been widely validated, and have uncertain utility in populations with sepsis.7–9 The quick SOFA (qSOFA), requiring only three clinical examination components (systolic blood pressure, respiratory rate, and Glasgow Coma Scale), was initially developed to help clinicians identify patients at risk of sepsis, by assessing predictive validity using mortality as an outcome more likely to occur in patients with sepsis.10 The predictive validity of the qSOFA score for sepsis has been extended to low-resource settings.11 However, the discrimination of sepsis-related mortality by the qSOFA score is typically lower than more rigorous methodologies.12,13 Despite relatively low sensitivity for mortality prediction, the qSOFA score has been widely adopted as a screening tool for sepsis-related severity of illness, particularly in LMICs.14
The benefit of combining the qSOFA score with a concurrent lactate concentration for assessing sepsis risk or outcome prediction is unclear. When the qSOFA score was being developed, Seymour and colleagues10 examined the utility of adding 1 point to the qSOFA score for a lactate concentration of 2 mmol/L or greater, making a 0–4-point score. However, this expanded score did not improve the clinical utility of the qSOFA score to identify patients at risk of sepsis outside of the intensive care unit (ICU) and so was not part of the final recommended score.10 When this expanded score was further assessed in patients presenting to the emergency department in a large, multicentre, European cohort, no improvement in mortality discrimination was observed compared with the qSOFA score alone.15 Several more recent studies have reported an improved ability of the qSOFA score to predict sepsis-related mortality with the addition of a lactate component.14,16,17 However, these reports either used arterial samples or did not differentiate between arterial and venous lactate measurements, and did not account specifically for point-of-care testing, which limits their applicability to low-resource areas.
Diagnostic point-of-care testing has been proposed as a crucial technology for health-care triage and prognostication in low-resource settings.18 Point-of-care lactate assessment devices are widely available, and point-of-care lactate concentrations have been shown to predict sepsis-related mortality in communities with high HIV prevalence, although there are few data from other populations with sepsis in LMICs.19 Whether point-of-care venous lactate could augment the qSOFA score in predicting mortality in patients hospitalised with suspected infection in low-resource settings remains unknown. We aimed to evaluate mortality prediction by point-of-care venous lactate combined with the qSOFA score in adult patients admitted to hospital with suspected infection in southeast Asia.
Methods
Study design and participants
We prospectively enrolled patients in two cohorts. Patients aged 18 years or older admitted to Sunpasitthiprasong Hospital in Ubon Ratchathani, Thailand, with suspected infection were prospectively enrolled into the derivation cohort between 2013 through 2017 (Ubon-sepsis cohort).20 In brief, recruitment was performed by the study team by screening medical records of patients admitted to the emergency department, medical wards, and medical ICUs. Enrolment occurred if patients had been admitted within the previous 24 h with at least three documented systemic manifestations of infection, according to the 2012 Surviving Sepsis Campaign.10
Patients aged 18 years or older admitted to hospital with suspected infection at 13 referral hospitals in southeast Asia were prospectively enrolled into the external validation cohort between 2013 through 2015 (SEAICRN cohort); this cohort has been described previously.3 As in the derivation cohort, enrolment occurred if patients had been admitted to the study hospitals within the previous 24 h with at least three documented systemic manifestations of infection, according to the 2012 Surviving Sepsis Campaign.10
Written informed consent was obtained from all study participants or their representatives before enrolment. This study was approved by the appropriate local and national ethics committees.
Procedures
In both cohorts, venous whole blood lactate concentration was measured at enrolment using a point-of-care device (Lactate Pro 2; Arkray, Kyoto, Japan). Additional clinical and laboratory data were obtained from the patient’s medical records. After enrolment, patients were cared for by the hospital’s medical team and treated by the local standard of care.
A qSOFA score and modified SOFA score were calculated for all patients at the time of enrolment in the derivation and external validation cohorts. SOFA modifications were necessary due to the absence of some datapoints including inotrope and vasopressor doses and partial pressure of oxygen in arterial blood and are described in the appendix (p 15). The highest qSOFA and modified SOFA scores at or before enrolment were calculated. For consistency with previous approaches, where additional components of the qSOFA or modified SOFA scores were not available, they were assumed to be normal and 0 points were given during score calculations.10,11,21 The Charlson comorbidity index, a summary score of comorbidity, was calculated as described previously.22
Outcomes
The primary outcome was 28-day mortality, in both the derivation and external validation cohorts. 28-day mortality data were collected via telephone contact if patients were no longer hospitalised and had been discharged alive.
Statistical analysis
In the derivation cohort, using point-of-care lactate concentrations, we first developed binary (0 points for a lactate concentration <2·0 mmol/L or 1 point for a lactate concentration ≥2·0 mmol/L) and ternary (0 points for a lactate concentration <2·0 mmol/L, 1 point for a lactate concentration ≥2·0 to <4·0 mmol/L, and 2 points for a lactate concentration ≥4·0 mmol/L) lactate scores. We then evaluated models combining qSOFA score and the different lactate scores using the likelihood ratio test and integrated discrimination improvement analysis to assess added improvements in model performance.23,24 When the candidate model was chosen, association with 28-day mortality was evaluated by logistic regression, and calibration was assessed by generating plots of expected and actual observed events.25 Prediction of 28-day mortality was subsequently assessed by generating area under the receiver operating characteristic curve (AUROC), using qSOFA score, lactate score, and modified SOFA models as comparative standards.
Mortality discrimination was further verified by ten-fold internal cross-validation, dividing the cohort into ten subgroups for repeated model development and testing.26 To assess clinical utility, the net benefit of selected models across a range of risk thresholds was compared using decision curve analysis.27 In a secondary analysis assessing predictive validity for sepsis-associated mortality, a baseline risk model was developed by assessing variables selected a priori (age, sex, Charlson comorbidity index, and transfer status) in a multivariable regression model. Baseline risks were combined with score models and 28-day mortality discrimination was assessed compared with the baseline risk model. The candidate model developed in the derivation cohort was subsequently analysed in a similar way in the external validation cohort.
Data were summarised using proportions for discrete variables and medians with IQR for continuous variables. Differences between groups in proportions were assessed by the χ2 test and in medians were assessed by the Mann–Whitney U test. Correlation comparisons were based on Spearman’s correlation coefficient. Univariate associations with 28-day mortality were determined by logistic regression. Lactate was assessed as a discrete variable for model simplicity. AUROC curves were compared using the Stata command roccomp. All analyses were performed using Stata/SE (version 14.2).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
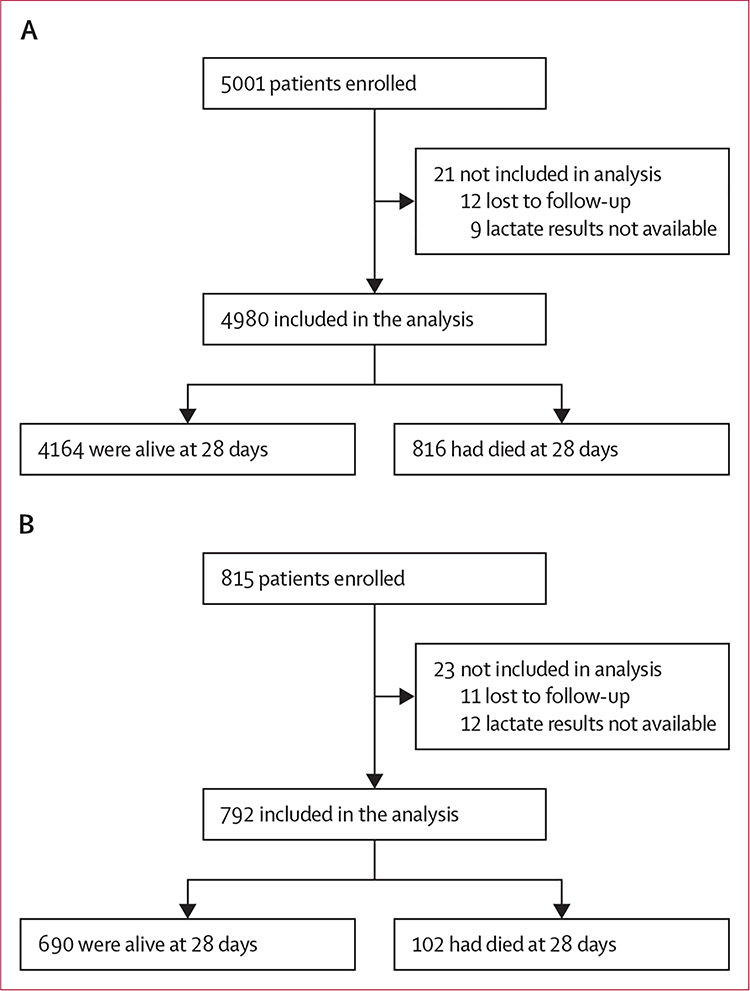
Between March 1, 2013, and Jan 26, 2017, 5001 patients were enrolled in the derivation cohort. 4874 patients had point-of-care lactate values available and another 106 had point-of-care lactate concentrations of less than the level of device detection and were considered to have a concentration at the lower limit (0·8 mmol/L). Therefore, 4980 patients had lactate data available and were followed up for 28 days and included in the final analysis (figure 1A). Of the patients included in the analysis, 816 (16%) died within 28 days of enrolment. The median age in the derivation cohort was 57 years (IQR 41–71), and most patients (3808 [77%]) had been transferred from another facility (table 1; appendix pp 2–3).
Figure 1: Study profiles.
Flow diagrams for the analysis of the Ubon-sepsis derivation cohort (A) and the SEAICRN external validation cohort (B).
Table 1:
Patient characteristics
| Derivation cohort (n=4980) | External validation cohort (n=792) | |
|---|---|---|
|
| ||
| Age | 57 (41–71) | 51 (33–65) |
| Sex | ||
| Female | 2325(47%) | 343 (43%) |
| Male | 2655 (53%) | 449 (57%) |
| Pre-existing conditions | ||
| Charlson comorbidity index | 2 (0–4) | 1 (0–3) |
| Diabetes | 1006 (20%) | 122 (15%) |
| Chronic liver disease | 132 (3%) | 14 (2%) |
| Chronic kidney disease | 545 (11%) | 49 (6%) |
| Chronic cardiovascular disease | 282 (6%) | 43 (5%) |
| Chronic lung disease | 392 (8%) | 35 (4%) |
| Cancer | 82 (2%) | 9 (1%) |
| HIV | 62 (1%) | 2 (<1%) |
| Transferred from another facility | 3808 (77%) | 501 (63%) |
| Admission characteristics | ||
| Duration of symptoms, days | 3 (1–4) | 3(2–8) |
| Received mechanical ventilation | 801 (16%) | 68 (9%) |
| Received vasoactive medications | 1275 (26%) | 163 (21%) |
| 28-day mortality | 816 (16%) | 102 (13%) |
Data are median (IQR) or n (%).
Median modified SOFA and qSOFA scores in patients who died were significantly higher than in patients who were alive at 28 days after enrolment (p<0·0001 for both; appendix p 4). Additionally, patients who died had higher median point-of-care lactate concentration than those who were alive at 28 days (3·4 mmol/L [IQR 2·1–7·1] vs 1·7 mmol/L [1·2–2·3]; p<0·0001).
2519 (51%) of 4980 patients presented with a qSOFA score of 2, with a corresponding 28-day mortality of 17% (95% CI 16–19; figure 2A). 434 (9%) patients had a qSOFA score of 3 and had a 28-day mortality of 51% (95% CI 46–56). 2854 (57%) of 4980 patients presented with a lactate concentration of less than 2·0 mmol/L and 732 (15%) had a lactate concentration of 4·0 mmol/L or greater (figure 2B). Ordinal qSOFA score and lactate concentration, as a continuous variable, were weakly correlated (Spearman’s r=0·31; p<0·0001). Mortality progressively increased with higher qSOFA score and lactate concentration. For example, patients with a qSOFA score of 1 and a lactate concentration from 2·0 mmol/L to less than 4·0 mmol/L had a significantly higher mortality than those with a qSOFA score of 1 and a lactate concentration of less than 2·0 mmol/L (14% vs 5%; p<0·0001; appendix p 5).
Figure 2: Distribution of patients and 28-day mortality by qSOFA score, lactate concentration, and qSOFA-lactate score in the derivation cohort.
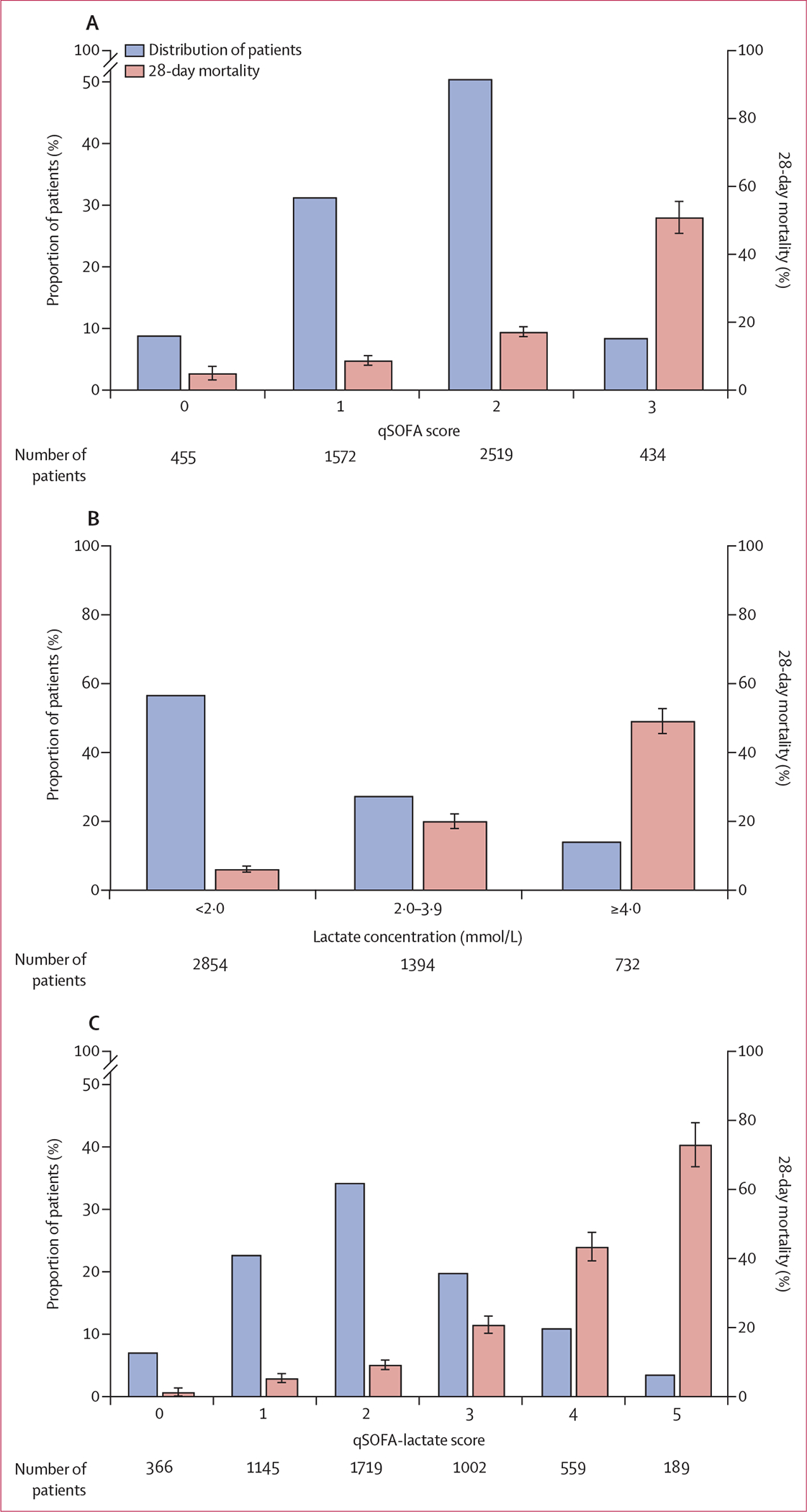
Bars show distribution of patients (blue bars) and 28-day mortality (red bars) by qSOFA score (A), lactate concentration (B), or qSOFA-lactate score (C). Error bars show 95% CIs for 28-day mortality. qSOFA=quick Sequential Organ Failure Assessment.
The addition of either binary or ternary lactate score significantly improved the 28-day mortality discrimination of the qSOFA score model (likelihood ratio p<0·0001 after addition of either the binary or ternary lactate scores; appendix p 6). Discrimination analysis showed a higher integrated discrimination improvement when the ternary lactate score was added to the qSOFA score than with the addition of the binary lactate score (0·11 [plus or minus 0·006] for the ternary lactate score vs 0·07 [plus or minus 0·004] for the binary lactate score, both p<0·0001). A final model combining the qSOFA score (0–3 points) and the ternary lactate score (0–2 points) was chosen for further analysis as a qSOFA-lactate score (0–5 points).
Before the comparative analysis, we next characterised the selected 0–5-point qSOFA-lactate score. The distribution of patients across the score range was more normalised than with either the qSOFA score or lactate score alone (figure 2). The proportion of patients who died within 28-days progressively increased as the qSOFA-lactate score increased (figure 2C).
Risk prediction models for the qSOFA-lactate score and ternary lactate score were developed and compared with the qSOFA score and a modified SOFA score for discrimination of 28-day mortality. First, a calibration plot of the qSOFA-lactate score model was generated, which showed strong concordance between actual and expected probabilities (appendix p 9). Therefore, the model was carried forward for evaluation of mortality discrimination, assessed by calculating the AUROC for each model. Compared with the qSOFA score model, the qSOFA-lactate score model significantly improved 28-day mortality discrimination (AUROC 0·78 [95% CI 0·76–0·80] vs 0·68 [0·67–0·70]; p<0·0001; table 2, figure 3A). Furthermore, the qSOFA-lactate score model performed better than a ternary lactate score model (AUROC 0·76 [95% CI 0·74–0·78]; p=0·0001) and similarly to a modified SOFA score model (0·77 [0·75–0·78]; p=0·088). The mortality discrimination of the ternary lactate score model was also superior to the qSOFA score model (p<0·0001) and similar to the modified SOFA score model (p=0·34). Discrimination of all models was validated through ten-fold internal cross-validation (table 2). When stratifed by transfer status, the qSOFA-lactate score model maintained a significant improvement over the qSOFA score model (appendix p 7).
Table2:
Mortality discrimination by model
| Cohort AUROC | p value* | Cross-validation AUROC† | |
|---|---|---|---|
|
| |||
| Derivation cohort | |||
| qSOFA | 0·68 (0·67–0·70) | ·· | 0·67 (0·65–0·69) |
| Lactate scored‡ | 0·76 (0·74–0·78) | <0.0001 | 0·74 (0·73–0·76) |
| qSOFA-lactate score§ | 0·78 (0·76–0·80) | <0·0001 | 0–77 (0·75–0·79) |
| Modified SOFA | 0·77 (0·75–0·78) | <0·0001 | 0·76 (0·75–0·78) |
| External validation cohort | |||
| qSOFA | 0·69 (0·63–0·74) | ·· | 0·69 (0·57–0·69) |
| Lactate scored‡ | 0·74 (0·69–0·80) | 0·13 | 0·75 (0·62–0·76) |
| qSOFA-lactate score§ | 0·77 (0·73–0·82) | <0·0001 | 0·78 (0·69–0·80) |
| Modified SOFA | 0·78 (0·74–0·83) | 0.0010 | 0·78 (0·72–0·82) |
Data are AUROC (95% CI) or p values. In the derivation cohort, the p value for qSOFA-lactate score model versus the lactate score model was 0·0001; the p value for qSOFA-lactate score model versus the modified SOFA model was 0·088. In the external validation cohort, the p value for qSOFA-lactate score model versus the lactate score model was 0·10; the p value for qSOFA-lactate score model versus the modified SOFA model was 0·82. AUROC=area under the receiver operating characteristic curve. qSOFA=quick Sequential Organ Failure Assessment. SOFA=Sequential Organ Failure Assessment.
p value for comparison of model versus qSOFA model.
Ten-fold internal cross-validation.
Lactate score was a 0–2-point score composed of 0 points for a lactate concentration of less than 2·0 mmol/L, 1 point for a lactate concentration from 2·0 to less than 4·0 mmol/L, and 2 points for a lactate concentration of 4·0 mmol/L or greater.
qSOFA-lactate score was a 0–5-point score composed of the 0–3-point qSOFA score plus the 0–2-point lactate score.
Figure 3: Receiver operating curves for mortality discrimination.
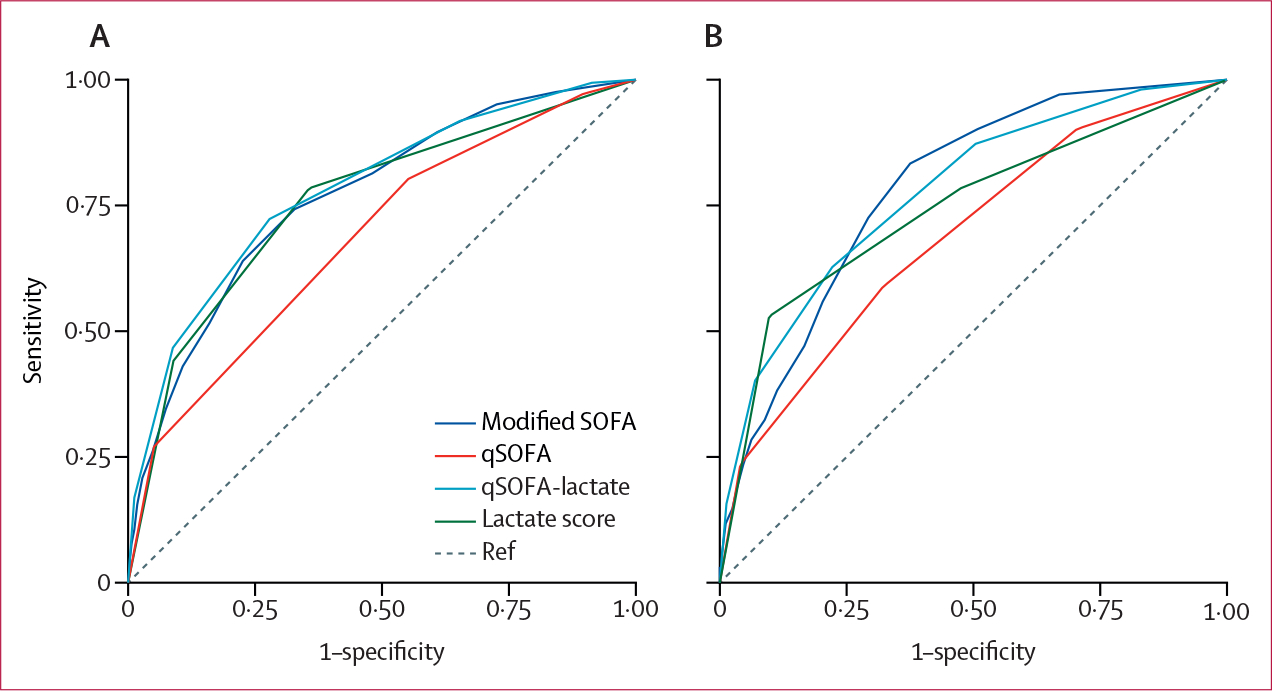
Area under the receiver operating curves (AUROC) for the modified SOFA, qSOFA, qSOFA-lactate, and ternary lactate score models for 28-day mortality discrimination in the derivation cohort (A) and external validation cohort (B). SOFA=Sequential Organ Failure Assessment. qSOFA=quick Sequential Organ Failure Assessment.
As the qSOFA-lactate score had stronger mortality discrimination than the qSOFA score or ternary lactate score alone and similar mortality discrimination to a modified SOFA score, we next assessed the clinical utility of these four models by decision curve analysis. The qSOFA-lactate model showed a higher net benefit compared with the qSOFA model for a risk threshold of death between 10% and 65%. The qSOFA-lactate model also showed a higher net benefit compared with the modified SOFA model and the ternary lactate model when the risk threshold of death was between 15% and 65% (appendix pp 10–11). The models showed similar clinical utility outside these risk thresholds.
Given the mortality discrimination and clinical utility of the qSOFA-lactate score, we next determined the performance measures of the score at different cutoffs. The combination of sensitivity and specificity for 28-day mortality were maximised at a qSOFA-lactate score of 3 or greater (both 72%; table 3).
Table 3:
Clinical performance of the qSOFA-lactate score to predict 28-day mortality
| Score ≥2 | Score ≥3 | Score ≥4 | |
|---|---|---|---|
|
| |||
| Derivation cohort * | |||
| Sensitivity | 92% (90–94) | 72% (69–75) | 47% (43–50) |
| Specificity | 35% (33–36) | 72% (71–74) | 91% (90–92) |
| Positive predictive value | 22% (20–23) | 34% (32–36) | 51% (47–55) |
| Negative predictive value | 96% (94–97) | 93% (92–94) | 90% (89–91) |
| Positive likelihood ratio | 1·4 (1·4–1·5) | 2·6 (2·4–2·8) | 5·3 (4·7–6·0) |
| Negative likelihood ratio | 0·2 (0·2–0·3) | 0·4 (0·3–0·4) | 0·6 (0·6–0·6) |
| External validation cohort † | |||
| Sensitivity | 87% (79–93) | 62% (53–72) | 40% (31–50) |
| Specificity | 49% (46–53) | 78% (75–81) | 93% (91–95) |
| Positive predictive value | 20% (17–25) | 30% (24–36) | 46% (35–57) |
| Negative predictive value | 96% (94–98) | 93% (91–95) | 91% (89–93) |
| Positive likelihood ratio | 1·7 (1·6–1·9) | 2·8 (2·3–3·5) | 5·8 (4·0–8·3) |
| Negative likelihood ratio | 0·3 (0·2–0·4) | 0·5 (0·4–0·6) | 0·6 (0·5–0·8) |
Data are point estimate (95% CI).
Assuming a 28-day mortality of 16%.
Assuming a 28-day mortality of 13%.
We next assessed the predictive validity of the qSOFA-lactate score for excess mortality above baseline risk. The model combining the qSOFA-lactate score and baseline risk factors (AUROC 0·81 [95% CI 0·80–0·83]) significantly improved mortality discrimination compared with baseline risk factors alone (0·69 [0·67–0·71]; p<0·0001) or compared with a model combining the qSOFA score and baseline risk factors (0·75 [0·73–0·76]; p<0·0001; appendix pp 8, 12). The qSOFA-lactate plus baseline risk factor model had similar mortality prediction to a model combining a modified SOFA score plus baseline risk factors (AUROC 0·80 [95% CI 0·78–0·82]; p=0·089).
As the qSOFA-lactate score showed superior 28-day mortality discrimination compared with the qSOFA score in a single-centre cohort, we sought to validate our findings in the multicentre, multinational, external validation cohort.3 Of 815 adult patients enrolled in the SEAICRN cohort, 792 (97%) had point-of-care lactate data available and were followed up for 28 days and included in the analysis (figure 1B, table 1; appendix pp 2–4). qSOFA score was again weakly correlated with lactate concentration (Spearman’s r=0·31; p<0·0001), and patients with a qSOFA score of 1 and a lactate concentration of 2·0 mmol/L to less than 4·0 mmol/L had higher mortality than those with a qSOFA score of 1 and a lactate concentration of less than 2·0 mmol/L (11% vs 5%; p=0·051; appendix p 5). The calibration plot of the qSOFA-lactate model also showed good concordance between actual and expected probabilities in the external validation cohort (appendix p 9).
In the external validation cohort, the qSOFA-lactate score showed significantly improved mortality discrimination compared with the qSOFA score alone (AUROC 0·77 [95% CI 0·73–0·82] vs 0·69 [0·63–0·74]; p<0·0001), including when stratified by transfer status (figure 3B, table 2; appendix p 7). The qSOFA-lactate score had similar mortality discrimination to modified SOFA score (AUROC 0·78 [95% CI 0·74–0·83]; p=0·82) and ternary lactate score (0·74 [0·69–0·80]; p=0·10; figure 3B, table 2). The qSOFA-lactate model also showed a higher net benefit compared with the qSOFA model for a risk threshold of death between 2% and 59%, as well as a higher net benefit compared with the modified SOFA model for a risk threshold of death between 13% and 59% (appendix p 13). The sensitivity of the qSOFA-lactate score was 87% with scores of 2 or greater (table 3). In terms of predictive validity, the model combining the qSOFA-lactate score and baseline risk factors had significantly improved mortality discrimination compared with the model combining the qSOFA score and baseline risk factors (AUROC 0·83 [95% CI 0·80–0·87] vs 0·77 [0·72–0·81]; p<0·0001; appendix pp 8, 12).
Discussion
In this study, we evaluated whether point-of-care venous lactate testing could enhance the mortality prediction of qSOFA for patients admitted to hospital with suspected infection. We developed and tested a 5-point qSOFA-lactate score for mortality prediction in a large, prospectively enrolled cohort in Thailand and validated the model in a multicentre, multinational, prospectively enrolled cohort in southeast Asia. To our knowledge, this study is the largest prospective and externally validated analysis of point-of-care venous lactate concentrations and qSOFA for mortality prediction in a low-resource setting.
The benefit of adding a lactate variable to a qSOFA score to improve mortality prediction observed in our study builds on the evidence from several previous studies. A large, multicentre study in Brazil reported strong predictive accuracy for mortality using a qSOFA score of 1 or greater or a serum lactate concentration of 2 mmol/L or greater; although the lactate concentrations might have been from arterial samples and measured by hospital laboratories.14 A single-centre study in Indonesia reported that a score combining qSOFA and point-of-care lactate in the emergency department had similar prediction of in-hospital mortality up to 28 days compared with a SOFA score.28 However, the generalisability of this study was limited due to its single-centre design and 35% in-hospital mortality. Furthermore, in-hospital mortality might underestimate sepsis-related deaths in LMICs, where patients might prefer to die at home. In our large, prospective, cohort study with 28-day mortality ranging from 13% to 16%, we found that a 5-point score combining qSOFA score and point-of-care venous lactate had significantly higher 28-day mortality predictive value than a qSOFA score alone.
The qSOFA-lactate score showed similar mortality discrimination to the more complex modified SOFA score, but might have superior clinical utility over a range of risk thresholds. In many low-resource settings, critically ill patients are treated outside an ICU or without advanced support measures, such as mechanical ventilation or vasoactive agents.11 In health-care centres without laboratory facilities and critical care resources, SOFA is difficult or impossible to calculate, making Sepsis-3 guideline adherence challenging. Our results offer an alternative and strongly support the utility of qSOFA in combination with a point-of-care venous lactate for predicting mortality in patients with suspected infection in southeast Asia.
In this study, a point-of-care venous lactate score alone had strong mortality prediction in both the derivation and external validation cohorts. This finding supports data from other patient populations and suggests that a point-of-care lactate assessment might have utility in suspected infection outcome prediction when there are few clinical data.19 Future studies could explore the utility of qSOFA and point-of-care lactate in triage protocols for patients with infection in low-resource settings.
This study has several strengths. To our knowledge, this is the largest study in hospitalised patients in LMICs to assess the utility of qSOFA and point-of-care lactate for infection-related morality prediction, and the only study to be externally validated. Both our derivation and external validation cohorts had minimal loss to follow-up and used 28-day mortality (not in-hospital mortality) as an outcome. Our external validation cohort was enrolled across multiple centres in various countries in southeast Asia, reducing the risk of local practice variation affecting our results.
This study also has several potential limitations. Reflecting the resource constraints of the study sites, arterial blood gases and dosage data of vasoactive medications were not available in both cohorts and many patients in the external validation cohort did not have a Glasgow Coma Scale included in their medical records. These factors prevented the calculation of a full SOFA score, and our analysis instead relied on a modified SOFA score. Similarly, some clinical data, particularly bilirubin concentrations, were not available for many patients in both cohorts, which might have affected score accuracy. Additionally, clinical scores were assessed at study enrolment and not followed up sequentially, potentially impacting mortality prediction. Lactate concentrations were measured on a point-of-care device at a single timepoint and lactate clearance was not assessed. Additionally, we did not assess the cost-effectiveness of point-of-care device implementation. Patients who were admitted to non-medical wards, such as a surgical ward, might not have been identified in our recruitment. Furthermore, all included study sites were tertiary care centres in southeast Asia, where care might differ from elsewhere in the world, and most patients in both cohorts were transferred from other health-care facilities. Therefore, the generalisability of our findings to other settings might be limited.
In conclusion, a simple score combining point-of-care venous lactate and the qSOFA score improved mortality prediction compared with the qSOFA score alone, and had similar mortality prediction to a modified SOFA score, in patients admitted to hospital with suspected infection. Our findings, derived from a large, prospective cohort in Thailand and validated in a multicentre prospective cohort in southeast Asia, indicate that combining bedside laboratory and clinical data could have substantial utility in identifying patients with increased risk of sepsis-related mortality and add to the data regarding Surviving Sepsis Guidelines in low-resource settings.29 Future studies will need to determine whether these results are applicable to other LMICs and whether the early identification of patients who are at high risk of death might alter management and improve patient outcomes.
Supplementary Material
Research in context.
Evidence before this study
When the quick Sequential Organ Failure Assessment (qSOFA) score was originally developed to identify patients at risk of sepsis using hospital databases in the USA and Germany, the addition of lactate did not meaningfully improve the predictive validity for in-hospital mortality. We searched PubMed on May 11, 2021, with no date or language restrictions, for published articles using the terms “lactate” AND “qSOFA”, and identified 75 studies. Several studies assessed the combination of lactate and qSOFA scores for sepsis outcome prediction using a variety of methods; however, the additive value of lactate to a qSOFA score remains unclear. Additionally, no identified studies used point-of-care venous lactate assessments with a 28-day mortality outcome in a low-income or middle-income setting.
Added value of this study
Most previous studies that assessed the predictive value of lactate and qSOFA scores were either performed in high-income settings or did not use bedside point-of-care lactate measurements. To our knowledge, this is the largest study in a low-income or middle-income setting to develop a mortality prediction score combining point-of-care venous lactate and qSOFA score in patients with suspected infection and the only study to assess 28-day mortality. Using two prospective cohorts of patients hospitalised with suspected infection in southeast Asia, we found that a simple score combining qSOFA and point-of-care venous lactate improves 28-day mortality prediction compared with qSOFA score alone and has similar efficacy to the more extensive modified Sequential Organ Failure Assessment (SOFA) score. The strengths of this study include its large derivation cohort and multicentre external validation cohort from multiple countries in southeast Asia. Furthermore, as in-hospital mortality assessments might underestimate mortality outcomes in low-income and middle-income settings, where critically ill patients might prefer to die at home, we followed up all patients to 28 days after admission, including after discharge from hospital.
Implications of all the available evidence
Our findings show that adding a bedside point-of-care lactate assessment could improve the prognostic accuracy of the qSOFA score and potentially replace a modified SOFA score in patients with suspected infection. In locations with limited health-care providers, clinical facilities, and laboratory capabilities, this simple rapid score could be applied during early sepsis assessments to identify patients at highest risk of death.
Acknowledgments
We thank the patients and staff at all the study hospitals and Mahidol-Oxford Tropical Medicine Research Unit. This work was supported by the US National Institutes of Health (grant numbers T32GM086270, 5K12HD047349, K08HL157562, K23GM141463, R01HL113382, and R01AI137111). This research was also funded, in part, by the Wellcome Trust (090219/Z/09/Z and 101103/Z/13/Z).
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Shelton W Wright, Division of Pediatric Critical Care Medicine, Department of Pediatrics University of Washington, Seattle, WA, USA.
Viriya Hantrakun, Mahidol-Oxford Tropical Medicine Research Unit Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Kristina E Rudd, Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Chuen-Yen Lau, Collaborative Clinical Research Branch, Division of Clinical Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Khie Chen Lie, Department of Internal Medicine, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia.
Nguyen Van Vinh Chau, Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam; Department of Internal Medicine, Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam.
Prapit Teparrukkul, Department of Internal Medicine, Sunpasitthiprasong Hospital, Ubon Ratchathani, Thailand.
T Eoin West, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine University of Washington, Seattle, WA, USA; Department of Global Health University of Washington, Seattle, WA, USA; Department of Microbiology and Immunology Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Direk Limmathurotsakul, Mahidol-Oxford Tropical Medicine Research Unit Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; Department of Tropical Hygiene Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Data sharing
Deidentified data and a data dictionary are available at https://doi.org/10.6084/m9.figshare.14938068.
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 2020; 395: 200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Kissoon N, Limmathurotsakul D, et al. The global burden of sepsis: barriers and potential solutions. Crit Care 2018; 22: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudarmono P, Aman AT, Arif M, et al. Causes and outcomes of sepsis in southeast Asia: a multinational multicentre cross-sectional study. Lancet Glob Health 2017; 5: e157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–10. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286: 1754–58. [DOI] [PubMed] [Google Scholar]
- 6.Lie KC, Lau CY, Van Vinh Chau N, West TE, Limmathurotsakul D. Utility of SOFA score, management and outcomes of sepsis in southeast Asia: a multinational multicenter prospective observational study. J Intensive Care 2018; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore CC, Hazard R, Saulters KJ, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. BMJ Glob Health 2017; 2: e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riviello ED, Kiviri W, Fowler RA, et al. Predicting mortality in low-income country ICUs: the Rwanda mortality probability model (R-MPM). PLoS One 2016; 11: e0155858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haniffa R, Mukaka M, Munasinghe SB, et al. Simplified prognostic model for critically ill patients in resource limited settings in south Asia. Crit Care 2017; 21: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudd KE, Seymour CW, Aluisio AR, et al. Association of the quick sequential (sepsis-related) organ failure assessment (qSOFA) score with excess hospital mortality in adults with suspected infection in low- and middle-income countries. JAMA 2018; 319: 2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmedding M, Adegbite BR, Gould S, et al. A prospective comparison of quick sequential organ failure assessment, systemic inflammatory response syndrome criteria, universal vital assessment, and modified early warning score to predict mortality in patients with suspected infection in Gabon. Am J Trop Med Hyg 2019; 100: 202–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranzani OT, Prina E, Menéndez R, et al. New sepsis definition (Sepsis-3) and community-acquired pneumonia mortality: a validation and clinical decision-making study. Am J Respir Crit Care Med 2017; 196: 1287–97. [DOI] [PubMed] [Google Scholar]
- 14.Machado FR, Cavalcanti AB, Monteiro MB, et al. Predictive accuracy of the quick sepsis-related organ failure assessment score in Brazil: a prospective multicenter study. Am J Respir Crit Care Med 2020; 201: 789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of Sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017; 317: 301–08. [DOI] [PubMed] [Google Scholar]
- 16.Ho KM, Lan NSH. Combining quick Sequential Organ Failure Assessment with plasma lactate concentration is comparable to standard Sequential Organ Failure Assessment score in predicting mortality of patients with and without suspected infection. J Crit Care 2017; 38: 1–5. [DOI] [PubMed] [Google Scholar]
- 17.Shetty A, MacDonald SPJ, Williams JM, et al. Lactate ≥2 mmol/L plus qSOFA improves utility over qSOFA alone in emergency department patients presenting with suspected sepsis. Emerg Med Australas 2017; 29: 626–34. [DOI] [PubMed] [Google Scholar]
- 18.Drain PK, Hyle EP, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 2014; 14: 239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore CC, Jacob ST, Pinkerton R, et al. Point-of-care lactate testing predicts mortality of severe sepsis in a predominantly HIV type 1-infected patient population in Uganda. Clin Infect Dis 2008; 46: 215–22. [DOI] [PubMed] [Google Scholar]
- 20.Hantrakun V, Somayaji R, Teparrukkul P, et al. Clinical epidemiology and outcomes of community acquired infection and sepsis among hospitalized patients in a resource limited setting in northeast Thailand: a prospective observational study (Ubon-sepsis). PLoS One 2018; 13: e0204509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 2017; 317: 290–300. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–72. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010; 21: 128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. BMJ 2015; 351: h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015; 162: W1–73. [DOI] [PubMed] [Google Scholar]
- 27.Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 2008; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinto R, Suwarto S, Lie KC, Harimurti K, Widodo D, Pohan HT. Prognostic accuracy of the quick Sequential Organ Failure Assessment (qSOFA)-lactate criteria for mortality in adults with suspected bacterial infection in the emergency department of a hospital with limited resources. Emerg Med J 2020; 37: 363–69. [DOI] [PubMed] [Google Scholar]
- 29.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47: 1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data and a data dictionary are available at https://doi.org/10.6084/m9.figshare.14938068.