Abstract
Introduction
The relationship between autoimmune pancreatitis (AIP) type 2 and inflammatory bowel disease (IBD) has been established and previously described within International Consensus Diagnostic Criteria. However, it is unknown if the presence of IBD changes the natural disease course of AIP type 2. Our aim was to investigate the association between AIP type 2 and IBD as well as to systematically summarize all the existing evidence in the literature.
Methods
Electronic medical record analysis was conducted in two centers (in Stockholm, Sweden, and Milan, Italy; records dated between January 2001 and June 2021). Additionally, we conducted a systematic review of the literature.
Results
A total of 35 patients (18 females, 51.4%) fulfilled the diagnostic criteria of AIP type 2 and were included in the study. A diagnosis of IBD was established in 29 patients (82.8%), ulcerative colitis in 17 (58.6%) and Crohn's disease in 11 (37.9%). Median follow‐up was 54 months. AIP patients with IBD commonly presented with abdominal pain and/or acute pancreatitis at diagnosis, the latter was prevailing in concomitant and later IBD onset. These patients more frequently used steroids, but there were no differences in relapse rates. Concomitant onset of IBD was associated with the development of diabetes mellitus. There were no cases of colon or pancreatic malignancy during follow‐up. In our systematic analysis, a total of 693 AIP type 2 patients were included from 24 single‐center retrospective studies and 8 multicenter retrospective studies. A diagnosis of IBD was reported in 330 (47.8%) patients. Relapse rate was 20.0%.
Conclusions
Clinical and radiological remission of AIP type 2 was high, while the cumulative incidence of relapse is around 20%. Our results show that concomitance of IBD imposes no obvious risk of a different disease course for AIP type 2.
Keywords: autoimmune, inflammatory bowel disease, pancreatitis, systematic review
Abbreviations
- AIP
autoimmune pancreatitis
- AP
acute pancreatitis
- AZA
zathioprine; CD, Crohn's disease
- DM
diabetes mellitus
- FE1
fecal elastase‐1
- GC
glucocorticoids
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICDC
International Consensus Diagnostic Criteria
- L
interleukin
- OOI
other organ involvement
- PEI
pancreatic exocrine insufficiency
- TNF
tumor necrosis factor
- UC
ulcerative colitis
INTRODUCTION
Autoimmune pancreatitis (AIP) is a unique form of pancreatic inflammation that often causes chronic pancreatitis with consequent pancreatic exocrine insufficiency (PEI) and endocrine insufficiency.1, 2 To date, histopathological observations have identified two AIP subtypes: lymphoplasmacytic sclerosing pancreatitis, also called AIP type 1, and idiopathic duct‐centric chronic pancreatitis, or AIP type 2. 3 The former belongs to the IgG4‐related disease spectrum, being its most prevalent manifestation in the digestive system, characterized by fibrotic lesions rich in IgG4 + plasma cells.2, 4 Hence, multiple other organ involvement (OOI), positive IgG4 serology in the majority of patients and a significant relapse rate after treatment are typical AIP type 1 features. 4 In contrast, AIP type 2 is less frequent, unrelated to IgG4 and associated with a lower risk of relapse after treatment.5, 6 Involvement of organs typically described in type I AIP is not observed in AIP type 2, where inflammatory bowel diseases (IBD) are more commonly described 5 and acknowledged within the International Consensus Diagnostic Criteria (ICDC). Indeed, the presence of IBD in patients with imaging evidence for AIP suggests a probable AIP type 2 diagnosis. 3 Definite AIP type 2 diagnosis still requires either histologically confirmed idiopathic duct‐centric pancreatitis or the presence of IBD and positive glucocorticoid (GC) trial. 3
Key summary.
Summarise the established knowledge on this subject
The relationship between autoimmune pancreatitis (AIP) type 2 and inflammatory bowel disease (IBD) has been established and previously described within International Consensus Diagnostic Criteria.
It is unknown if the presence of IBD changes the natural disease course of AIP type 2.
Our aim was to investigate the association between AIP type 2 and IBD as well as to systematically summarize all the existing evidence in the literature.
What are the significant and/or new findings of this study?
Clinical and radiological remission of AIP type 2 was high, while the cumulative incidence of relapse is around 20%.
Our results show that concomitance of IBD imposes no obvious risk of a different disease course for AIP type 2.
However, AIP should be considered as a differential diagnosis in patients with IBD presenting with gastrointestinal complaints unexplained by the underlying bowel disorder.
Overlooking a diagnosis of IBD in the setting of AIP‐type‐2‐associated pancreatic exocrine insufficiency may not be infrequent as both diseases can present with diarrhea and abdominal discomfort. 7 Moreover, there is a certain amount of similarity and overlap between both initial IBD and AIP treatment (GC, azathioprine),2, 8, 9 so the choice of AIP treatment might be influenced by the synchronous IBD, and vice versa. More importantly, IBD concurrent with AIP might hypothetically lead to a broadening of the available treatment options since a recent report described the successful use of tumor necrosis factor (TNF) inhibitors in AIP type 2 patients with and without concomitant IBD. 10
While the prevalence of IBD in different types of pancreatitis11, 12, 13 and the influence of AIP on the natural disease course of IBD 14 have been described before, whether the presence of IBD influences the natural history of AIP type 2 remains unknown. The present study aims to examine the relationship between AIP type 2 and IBD in patients from two European tertiary care centers and to summarize the existing data in the literature on this topic.
METHODS
We analyzed the medical records of all AIP patients at two large tertiary care centers, namely the Pancreas Outpatient Clinic at the Department of Upper Abdominal Disease at the Karolinska University Hospital in Stockholm, Sweden, and the Pancreas Center at the San Raffaele Hospital in Milan, Italy, dating between January 2001 and June 2021.
Inclusion and exclusion criteria
The patient selection process is outlined in Figure 1. All patients with a definite or probable diagnosis of AIP type 2 according to ICDC were included in the study. 3 Patients diagnosed before publication of the ICDC (2011) were retrospectively reviewed by two senior pancreatologists per center to ensure a correct diagnosis when the diagnosis was not based on histology. Patients with AIP type 1 and AIP not otherwise specified (NOS) were excluded. Follow‐up was defined as the time from the AIP diagnosis until the last contact with the patient. Patients followed for less than 6 months were excluded from the long‐term outcomes analysis.
FIGURE 1.
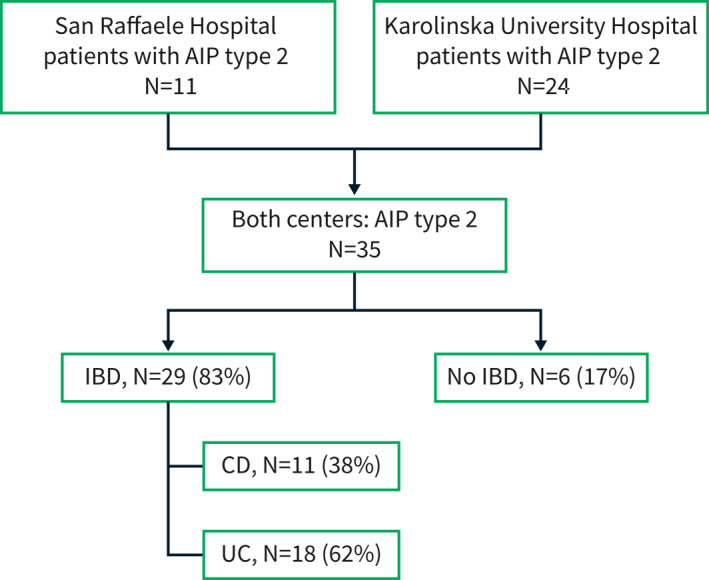
AIP‐autoimmune pancreatitis, IBD‐inflammatory bowel disease, CD‐Crohn's disease, UC‐ulcerative colitis
Definition of exposure and outcomes
Exposure was defined as the diagnosis of IBD according to the European Crohn's and Colitis Organization (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) joint collaborative guidelines. 15 Exposure was classified in a sub‐analysis according to IBD onset in relation to AIP. IBD in relation to the date of AIP diagnosis was subclassified as: before AIP diagnosis (IBDb), IBD concomitant (maximum 2 months diagnostic delay) with AIP (IBDc), and IBD after AIP diagnosis (IBDa). The outcome was treatment efficacy in terms of AIP remission and relapse. Relapse was defined as the recurrence of clinical and/or radiological features consistent with AIP type 2 after the first‐line treatment. Remission was defined as the normalization of radiological and/or clinical abnormalities. Clinical remission without maintenance treatment was defined as the absence of both IBD and AIP‐related systematic treatments (GC, azathioprine (AZA), infliximab, adalimumab, ustekinumab, golimumab, vedolizumab, budesonide, rituximab). Pancreatic exocrine function (using fecal elastase 1 = FE1) and endocrine function (presence of diabetes mellitus (DM)) at last follow‐up was also recorded.
At the baseline, we retrieved data regarding age at diagnosis of AIP, gender, symptoms at the time of AIP diagnosis (asymptomatic, new onset diabetes, acute pancreatitis, obstructive jaundice, weight loss, abdominal pain), OOI and pancreatic insufficiency in terms of endocrine dysfunction and exocrine dysfunction. Information related to AIP and IBD treatment was retrieved, as well as surgical procedures.
Data related to IBD was also collected, including the subtype (ulcerative colitis (UC), Crohn's disease (CD), indeterminate colitis), the onset of diagnosis in relation to AIP diagnosis (before, concomitant, after) and the treatment. The extent of colonic inflammation in UC, as well as the localization and behavior of the CD, were described according to Montreal classification. 16
Ethics
The study adhered to the latest version of the Declaration of Helsinki and was approved by both the Clinic Ethical Committee in Stockholm, Sweden (Dnr. 2016/1571‐31 and 2020–02209) and the Ethical Committee in Milan, Italy (22/INT/2018).
Statistical analysis
Data is expressed as median and interquartile range (IQR) for numerical data, or percentages for categorical data. The comparison of data was undertaken using appropriate non‐parametric statistical tests, for categorical data the chi‐square or Fisher's exact test, for numerical data the Mann‐Whitney U test or Kruskal‐Wallis test. Univariable and multivariable analyses were performed to identify possible predictors for relapse, based on a Cox‐proportional hazards regression model. Hazard ratios (HR) were expressed with 95% confidence intervals (CI 95%). The analysis was performed using IBM SPSS Statistics 28. A p‐value <0.05 (two‐sided) was considered statistically significant.
Systematic review of the literature
Search strategy and study selection
A literature search following the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines 17 was conducted to identify all relevant original articles referring to the relation between type 2 AIP and IBD. Cochrane, Embase, Google Scholar, PubMed and Web of Science databases were searched from 2003 until 15 August 2021 using the following search terms (‘autoimmune pancreatitis’ OR ‘autoimmune pancreatitis type 2’) AND (‘inflammatory bowel disease’ OR ‘ulcerative colitis’ OR ‘Crohn's Disease’). Prisma checklist is presented in Table S1 and search strategy in Table S2. Eligibility assessment was performed by screening the titles and, subsequently, the abstracts and full articles. This was undertaken by three independent reviewers (SN, ML, NP). All disagreements were resolved by MV and JML. Review articles, editorials, conference reports, comments on other studies, animal studies, non‐English‐language articles, book sections and theses, overlapping articles and articles that did not contain individual data on the prevalence of IBD in patients with AIP type 2 were excluded. When the results of a single study were reported in more than one publication, only the most recent or complete data was included in the analysis. Using a “snowball method”, additional articles were identified by hand‐searching the reference lists of all the articles retrieved to identify potentially relevant studies. The same above‐mentioned inclusion criteria were then applied. Studies in which a distinction between AIP type 1 and type 2 was not made were excluded. The proportions of patients having IBD subtypes, definite AIP diagnosis and certain treatment options were calculated by referring to the total number of patients in the respective study. The quality of the studies was assessed according to a checklist based on a modified version of the Newcastle–Ottawa quality assessment scale, with a score ranging 0–9. The selection process of articles for the review is summarized in Figure 2.
FIGURE 2.
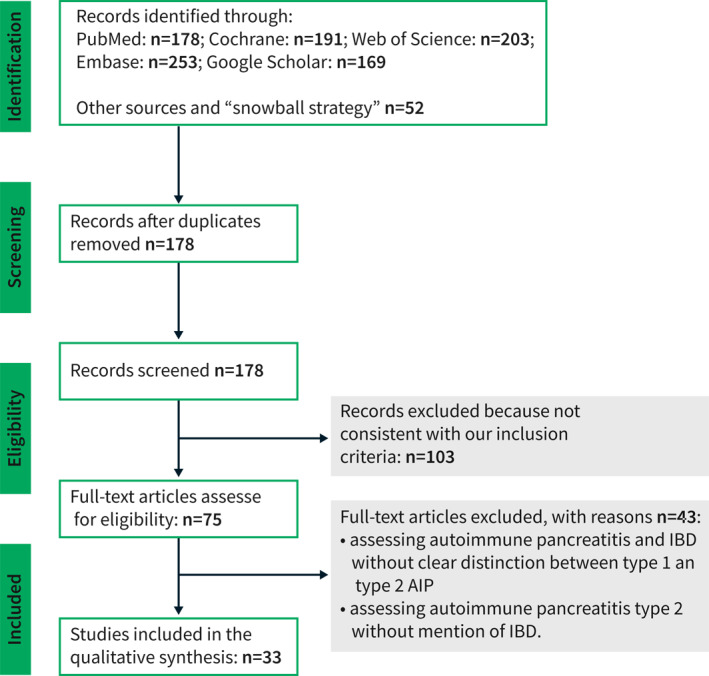
AIP‐autoimmune pancreatitis, IBD‐inflammatory bowel disease
RESULTS
Baseline characteristics of patients with AIP
In total, 35 patients (18 females, 51.4%) fulfilled the diagnostic criteria of AIP type 2 and were included in the study. Baseline demographic and clinical features are reported in Table 1. Twenty‐two patients (62.9%) received a definite AIP type 2 diagnosis, while 13 (37.1%) had probable AIP type 2 according to ICDC. 3 The median age at diagnosis was 41 years (IQR 26). The clinical presentation included abdominal pain (30 patients, 85.7%), acute pancreatitis (16 patients, 45.7%), weight loss (7 patients, 20.0%), jaundice (4 patients, 11.4%) and new‐onset diabetes (2 patients, 5.6%). Of note, in 2 of the 5 (14.3%) asymptomatic patients, the diagnosis of type 2 AIP had been considered incidental as it was unveiled during imaging workup after IBD diagnosis. Median follow‐up time for AIP was 54 months (IQR 46.5 months). Two patients were excluded from the long‐term analysis because of a short follow‐up time (<6 months).
TABLE 1.
Baseline characteristics of autoimmune pancreatitis (AIP) type 2 patients in relation to inflammatory bowel diseases (IBD)
| Patients | Total | IBD | |
|---|---|---|---|
| n = 35 | YES (n = 29, 83%) | NO (n = 6, 17%) | |
| Female, n (%) | 18 (51.4) | 14 (48.3) | 4 (66.7%) |
| Age at AIP diagnosis (median, IQR) | 41, 26 | 38.0, 24.0 | 60.0, 35.0 |
| Follow‐up (months)* (median, IQR) | 54, 46.5 | 54.0, 47.0 | 61.5, 77.0 |
| Definite AIP 2, n (%) | 22 (62.9) | 16 (55.2) | 6 (100.0) |
| Alcohol consumption >5 U | 1 (2.9) | 1 (3.6) | 0 |
| Smoker | |||
| Never | 18 (51.4) | 17 (58.6) | 1 (16.7) |
| Former | 14 (40.0) | 11 (37.9) | 3 (50.0) |
| Active | 3 (8.6) | 1 (3.4) | 2 (33.3) |
| Diagnosis by histology, n (%) | 8 (22.9) | 3 (10.3) | 5 (83.3) |
| AIP symptoms at diagnosis, n (%) | |||
| Abdominal pain | 30 (85.7) | 27 (93.1) | 3 (50.0) |
| Weight loss | 7 (20.0) | 6 (20.7) | 1 (16.7) |
| Acute pancreatitis | 16 (45.7) | 15 (51.7) | 1 (16.7) |
| Jaundice | 4 (11.4) | 2 (6.9) | 2 (33.3) |
| New onset diabetes | 2 (5.7) | 1 (3.4) | 1 (16.7) |
| Incidental finding | 5 (14.3) | 3 (10.3) | 2 (33.3) |
| PEI, n (%) | |||
| At diagnosis | 11 (31.4) | 10 (34.5) | 1 (16.7) |
| FE‐1 (μg/g, median, IQR) | 225.3 | 220.2 | 500.3 |
| At follow‐up* | 10 (30.3) | 8 (29.6) | 2 (33.3) |
| FE‐1 (μg/g, median, IQR)* | 260.3 | 231.3 | 292.2 |
| Diabetes mellitus, n (%) | |||
| At diagnosis | 4 (11.4) | 2 (6.9) | 2 (33.3) |
| At follow‐up* | 7 (21.2) | 5 (18.5) | 2 (33.3) |
| AIP treatment, n (%) | 31 (88.6) | 25 (86.2) | 6 (100.0) |
| Surgery | 7 (20.0) | 2 (6.9) | 5 (83.3) |
| Steroids | 26 (74.3) | 25 (86.2) | 1 (16.7) |
| Azathioprine | 8 (22.9) | 8 (27.6) | 0 |
| AIP relapse, n (%)* | 8 (24.2) | 7 (25.9) | 1 (17.7) |
| AIP maintenance treatment | 4 (12.1) | 4 (14.8) | 0 |
| Clinical remission at last contact* | 33 (100.0) | 27 (100.0) | 6 (100.0) |
| Clinical remission without systemic therapy for both IBD and AIP | 22 (66.7) | 16 (59.3) | 6 (100.0) |
| Radiological remission at last contact* | 31 (93.9) | 25 (92.6) | 6 (100%) |
Note: (normal >200 μg/g; measured up to 800 μg/g). For variables with *, n = 33.
Abbreviations: AIP, autoimmune pancreatitis; CD, Crohn's disease; DM, diabetes mellitus; FE‐1, fecal elastase‐1; IBD‐inflammatory bowel disease; IQR‐interquartile range; PEI‐pancreatic exocrine insufficiency; UC‐ulcerative colitis.
Characteristics of AIP patients in relation to IBD
A diagnosis of IBD was established in 29 patients (82.8%). UC and CD were diagnosed in 17 (58.6%) and 11 (37.9%) patients, respectively. In one patient (3.5%), the subtype could not be established. The Montreal classification of the 29 IBD patients is shown in the (Table S3). Most of the UC patients had left side colitis. All CD patients had a non‐stricturing, non‐penetrating disease. IBD was diagnosed after AIP in 10 (34.5%) patients (IBDa patients), while 13 (44.8%) patients received an IBD diagnosis before AIP onset (IBDb patients). In 6 (20.7%) patients, IBD and AIP were diagnosed concomitantly (IBDc patients). AIP patients with IBD showed a trend toward lower median age compared to AIP without IBD (38.0 vs. 60.0 years, p = 0.06). AIP patients with IBD presented significantly more often with abdominal pain (27/29 (93.1%) versus 3/6 (50.0%), p = 0.02). The rate of abdominal pain in the three IBD subgroups (IBDa, IBDb, IBDc) was not statistically different (Table 2). On the other hand, acute pancreatitis as initial presentation of AIP type 2 was more prevalent in patients with IBDc and IBDa compared to IBDb: 5 (83.3%), 7(70.0%), and 3 (23.1%), respectively (p = 0.02).
TABLE 2.
Sub‐analysis of autoimmune pancreatitis (AIP) type 2 patient characteristics with inflammatory bowel diseases (IBD)
| Total (29 patients) | IBDb (N = 13, 44.8%) | IBDc (N = 6, 20.7%) | IBDa (N = 10, 34.5%) |
|---|---|---|---|
| Female, n (%) | 7 (53.8) | 4 (66.7) | 3 (30.0) |
| CD, n (%) | 4 (30.8) | 2 (33.3) | 5 (50.0) |
| UC, n (%) | 9 (69.2) | 3 (50.0) | 5 (50.0) |
| Age at AIP diagnosis (median, IQR) | 43, 23.0 | 36.0, 27.0 | 31.5, 28.0 |
| Follow‐up AIP (months)* (median, IQR) | 52, 47 | 54, 55 | 49, 35 |
| Abdominal pain | 12 (92.3) | 5 (83.3) | 10 (100.0) |
| Acute pancreatitis | 3 (23.1) | 5 (83.3) | 7 (70.0) |
| Weight loss | 1 (7.7) | 0 | 5 (50.0) |
| PEI, n (%) | |||
| at diagnosis | 4 (30.8) | 5 (83.3) | 1 (10.0) |
| at last contact* | 4 (33.3) | 2 (40.0) | 2 (20.0) |
| Diabetes n (%) | |||
| at diagnosis | 1 (7.7) | 1 (16.7) | 0 |
| at last contact* | 1 (8.3) | 4 (80.0) | 0 |
| Treatment of AIP, n (%) | |||
| Steroid | 9 (69.2) | 6 (100.0) | 10 (100.0) |
| Azathioprine | 5 (38.5) | 2 (33.3) | 1 (10.0) |
| Relapses of AIP n (%)* | 4 (33.3) | 1 (20.0) | 2 (20.0) |
| Clinical remission of AIP without systemic treatment for both AIP and IBD | 6 (50.0) | 2 (40.0) | 8 (80.0) |
Note: For variables with *, N = 33.
Abbreviations: AIP, autoimmune pancreatitis; CD, Crohn's disease; DM, diabetes mellitus; IBD‐inflammatory bowel disease; IQR‐interquartile range; PEI‐pancreatic exocrine insufficiency; UC‐ulcerative colitis.
Treatment
In our cohort, 26 (74.3%) patients with AIP type 2 received treatment with GC. GC were used more frequently in AIP patients with IBD (25 patients, 86.2%) compared to those without IBD (1 patient, 16.7%, p < 0.002). A representative example of the clinical effect of steroid treatment is shown in Figure 3. AZA was used to treat AIP only in patients with IBD (8 patients, 22.9%). Seven patients (20.0%) underwent a surgical procedure because a pancreatic malignancy could not be excluded based on clinical and radiological findings. The diagnosis of AIP in these 7 patients was established subsequently by histology according to the ICDC criteria. 3 It is of note that the diagnosis of IBD was made in only one patient at the time of surgery. One patient developed CD 2 years after surgery, while the other 5 patients had no IBD. A watchful waiting approach was chosen in 2 (5.2%) patients due to spontaneous regression of AIP.
FIGURE 3.
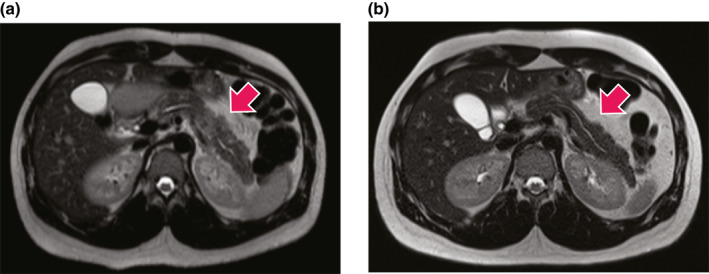
Note the focal inflammation (white arrow) with swelling, higher signal intensity (whiter) and compression of the main pancreatic duct present before (a) but not after (b) steroid treatment
Remission and relapse
At the last contact, all AIP patients (33/33, 100%) were in clinical remission and 31/33 (93.9%) had achieved radiological remission as well. AIP‐related maintenance therapy was indicated in 4/33 patients (12.1%). Relapse of AIP occurred in 8/33 (24.2%) patients. The only parameter associated with AIP relapse at the univariate analysis was age at diagnosis. In particular, higher the age, the lower appeared the risk of relapse, yet accounting for a very low HR (HR − 0.01 95%CI − 0.02 to −0.001, p = 0.04). Sub‐analysis according to IBD onset in AIP patients showed no differences in the above‐mentioned outcomes.
Long‐term consequences
At diagnosis, 11 (31.4%) patients displayed PEI and 4 (11.4%) had DM. At last contact, 10/33 (30.3%) had PEI and 7/33 (21.2%) patients had DM. Interestingly, IBDc patients presented with a higher prevalence of PEI at diagnosis (Table 2). However, no differences in PEI prevalence were recorded at follow‐up analysis. On the other hand, in the subgroup of IBDc patients, DM prevalence was significantly higher compared to the IBDb and IBDa groups – 4/5 (80%) versus 1/12 (8.3%) and 0/10 respectively, p = 0.001.
One patient without IBD developed malignant melanoma, while no pancreatic or colorectal cancer were detected during the follow‐up.
Systematic review
Study selection
Our primary search identified 260 titles. After the removal of duplicate articles, 178 studies remained. We excluded 103 articles because they were not consistent with our inclusion criteria. Finally, 75 studies were included in a qualitative synthesis and the full text of each one was reviewed to establish eligibility for quantitative analysis. After reviewing these articles, 43 more were excluded due to insufficient data related to IBD or AIP subtype. Thirty‐two studies fulfilled the inclusion criteria and were selected for the systematic review (Figure 2). The characteristics and key findings of the included studies are reported in the (Table S4).
Results of the individual studies
Our analysis included 24 single‐center retrospective studies and 8 multicenter retrospective studies, with a total of 693 AIP type 2 patients. Among the single‐center studies, 14 were performed in Europe, 4 in the USA, 9 in Asian countries and one in Australia. All relevant data that was possible to extract from systematic review studies are presented in the (Table S5).
A diagnosis of IBD was reported in 330 (47.8%) patients, being UC, CD or not assessed in 183 (27.3%), 53 (7.9%), and 94 (14.0%) cases, respectively. In 6 studies, the IBD subtype was not reported. The type of AIP diagnosis was reported in 6 studies, including a total of 147 patients.13, 18, 19, 20, 21, 22 Among these, a definite AIP type 2 diagnosis was established in 56 (38.0%) patients, a probable type 2 AIP diagnosis in 81 (55.1%) and in 10 (6.8%) the type was not known because the diagnosis of probable AIP type 2 was not established until 2011. Twelve studies (249 patients) reported the timing of both AIP and IBD diagnosis.5, 10, 13, 14, 18, 19, 20, 22, 23, 24, 25, 26 AIP type 2 was diagnosed before IBD in 35 (14.0%) cases. AIP clinical presentation was mentioned in 12 reports (227 patients).5, 10, 13, 18, 19, 21, 22, 24, 25, 27, 28, 29 Acute pancreatitis was the initial manifestation in 105 patients (46.2%) and jaundice in 19 (8.4%). AIP treatment was reported in 16 studies (316 patients).13, 14, 18, 19, 20, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33 Surgery was performed in 91 patients (28.8%), while systemic steroids were used in 94 patients (29.7%). A watchful waiting strategy was used in 49 patients (15.5%). A total of 40 (20.2%) AIP relapses were recorded in 9 studies (198 patients).14, 18, 20, 21, 22, 25, 27, 30, 31 IBD treatment was reported in 8 articles (180 patients).13, 14, 18, 22, 23, 24, 25, 33 Topical treatment, GC, standard therapy (azathioprine, methotrexate), and TNF‐inhibitors were used in 70 patients (38.8%), 15 patients (8.3%), 59 patients (32.8%) and 48 (26.7%) patients, respectively. Unfortunately, due to insufficient data, it was not possible to compare characteristics or outcomes between type 2 AIP patients with and without IBD.
DISCUSSION
AIP type 2 is a specific entity within the pancreatic diseases spectrum. 34 In this work, we aimed to examine the salient features in AIP type 2 patients from a large dual‐center cohort, with a special focus on the relationship with IBD occurrence. The existing evidence in the literature on this subject was also systematically evaluated.
The prevalence of IBD in our study was 82.8%, whereas the systematic review analysis reveals a prevalence of 47.8%. This discrepancy can be explained by significant heterogeneity among the selected studies, including different sample sizes (2–89 patients) and a wide range of reported IBD prevalence (10.4%–100%).10, 11, 13, 14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 31, 32, 33, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Interestingly, AIP patients with IBD in our cohort were more likely to have a definite diagnosis compared with studies included in the systematic review, 55% versus 38.0% respectively.13, 20, 21, 22, 42 In line with the observations of the systematic review, the ratio of UC to CD in our study was 3:1.10, 11, 13, 14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 31, 32, 33, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Moreover, the location and behavior of the CD according to the Montreal classification 16 were similar to the results reported by Lorenzo et al., 22 with predominantly non‐stricturing, non‐penetrating disease, located mainly in the colon. The location of UC was predominantly the left colon (Table S1).
AIP patients with IBD were more likely to have acute pancreatitis (not statistically significant) and abdominal pain at presentation. The prevalence of acute pancreatitis in our cohort was similar to that identified from the systematic review of the literature, 45.2% versus 45.7%.10, 21, 22, 24, 25, 27, 28, 29, 48 Hart et al. 24 observed a higher prevalence of acute pancreatitis in patients with concurrent IBD, which was also the case in our cohort (Table 1), but the difference was not statistically significant in our study. Moreover, AIP patients with either IBDc or IBDa were more likely to present with acute pancreatitis (Table 2). Interestingly, IBDa patients complained about weight loss significantly more often than those in the IBDc or IBDb groups. Thus, it may be important to consider the concomitant presence of IBD and AIP in patients with non‐typical clinical course.
In contrast to the findings of the systematic review, in our study GC treatment was the most prevalent therapy choice (74.3% vs. 29.2%), in comparison to surgery (20% vs. 28.8%) and watchful waiting (11.4% vs. 15.5%).1, 13, 14, 18, 19, 20, 22, 23, 25, 27, 28, 30, 32, 33, 40, 42 This observation is probably due to the generally higher proportion of IBD in our cohort. Consequently, surgery was significantly more prevalent as a treatment method in AIP type 2 patients without IBD, while steroids were used in AIP type 2 patients with IBD. We noted a trend towards a significant association between the onset of IBD and GC treatment for AIP in the IBDc and IBDa groups. The use of AZA is commonly considered in the setting of both AIP and IBD‐related maintenance therapy. Yet, the risk of AZA‐induced acute pancreatitis is significant, occurring in up to 7% of AZA‐exposed patients. 49
Biologic drugs, including TNF inhibitors, form a central part of the IBD armamentarium. Recently, Lorenzo et al. 10 have reported the successful use of adalimumab in the induction of remission in a patient with AIP type 2 in the absence of concomitant IBD. In addition, the authors treated three patients with active IBD and relapsing AIP with TNF inhibitors, achieving remission of the pancreatic disease. Indeed, granulocyte epithelial lesions are central in AIP type 2 diagnosis19, 50 and reflect an extensive neutrophils infiltration within the pancreatic parenchyma. As a result of neutrophilic chemotactic power, the overexpression of interleukin (IL) 8 was associated with both AIP type 2 (in ducts) and UC (in crypt epithelium). 27 This evidence suggests a putative common pathogenetic pathway. Given these data, it is reasonable to speculate that a common treatment strategy might be effective for both diseases. From this perspective, recent findings on the use of colchicine to target neutrophils in AIP type 2 are of interest and deserve further investigation in patients with concomitant IBD. 51
In our study, a high proportion of AIP type 2 patients achieved clinical and radiological remission without AIP‐related maintenance treatment (2/3 of the patients had neither AIP nor IBD‐related systemic treatment). The cumulative incidence of relapse during median follow‐up of 54 months was in line with the average 20% relapse in the analyzed literature where information on relapse was available.14, 21, 22, 25, 27, 28 Moreover, a low relapse rate in AIP type 2 in comparison to AIP type 1 has been noticed before. 52 In contrast to what is known about other organ involvement as a predictor of relapse in AIP type 1,53, 54 according to our results, AIP type 2 patients with IBD are not at higher risk of relapse in comparison to AIP type 2 patients without IBD. In fact, the onset of IBD did not seem to influence the remission or relapse rates. Nevertheless, more prospective studies should be performed to validate this assumption.
We observed a significantly higher prevalence of PEI at diagnosis in IBDc patients. 55 At last contact with patients, that difference was no longer significant due to PEI recovery in 3 patients. This might be explained by the dilution effect of diarrhea on fecal elastase‐1 (FE1) concentration that is, falsely low FE1 values. 56 Interestingly, the opposite was observed in DM prevalence—it was significantly higher at the last contact. We believe that it is crucial to regard IBD as a reason for malabsorption syndrome and diarrhea in AIP patients, and especially vice versa. The risk of cancer seems to be low, as we detected no pancreatic or colon cancer during follow‐up.
Our work does not come without limitations. Besides the obvious—small sample size due to AIP type 2 being an orphan disease—the retrospective nature of the study might have affected data retrieval. Also, AIP type 2 awareness has changed over time, thus artificially modifying the temporal relationship with IBD, as previous AIP might have been overlooked and the diagnosis delayed. On the other hand, nowadays, AIP type 2 patients without IBD might be at risk of being underdiagnosed, potentially introducing a certain selection bias. Findings found from the comparison of AIP type 2 patients with and without IBD should be interpreted with caution due to the low number of patients without IBD. Since both our centers are tertiary, some referral bias might be present. Moreover, IBD treatment strategies might have reflected different local policies. For example, treatment with biologics was more common in Stockholm compared to Milan. Systematic review was not registered in the PROSPERO database which is a limitation of the study.
Despite these limitations, our work is based on the cohorts of two centers highly skilled in the management of pancreatic diseases. According to the systematic review we performed, this is the first paper exploring the influence of IBD on AIP‐related outcomes.
In conclusion, there was no obvious association between IBD and relapse or remission in AIP type 2. The prevalence of both clinical and radiological remission was very high, while the cumulative incidence of relapse was around 20%. Remarkably, IBD represents a common bystander that should be actively investigated and managed in a multidisciplinary approach. However, IBD does not seem to be a risk factor for a more aggressive AIP type 2 natural course. Potentially common pathogenetic pathways and effective treatment strategies for both diseases should be explored in further studies.
CONFLICT OF INTEREST
Miroslav Vujasinovic: Abbott (lecture fee), Mylan (lecture fee); Sara Nikolic: Ferring (lecture fee), Mylan (lecture fee), Krka (lecture fee); J.‐Matthias Löhr: Abbott (lecture fee), Mylan (lecture fee).
AUTHOR CONTRIBUTIONS
Study conception and design: Sara Nikolic, Marco Lanzillotta, Miroslav Vujasinovic. Acquisition of data: Sara Nikolic, Marco Lanzillotta, Miroslav Vujasinovic. Statistical analysis: Sara Nikolic, Marco Lanzillotta; Interpretation of data and drafting of the manuscript: all authors.
Supporting information
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Tabel S1
Tabel S2
Tabel S3
Tabel S4
Tabel S5
ACKNOWLEDGEMENTS
We thank our colleague Charlotte Hedin, MD, PhD Karolinska Institutet, Department of Medicine Solna, Stockholm, Sweden, for her valuable comments and help in the preparation of the manuscript.
We also thank the Swedish Society for Development of Pancreatology (SveSuP) for continuous support, promotion and creating awareness of pancreas diseases in Swedish society.
This article is part of Sara Nikolic's PhD thesis.
Miroslav Vujasinovic is supported by the Swedish Magtarmfondens stipendium for research and Ruth and Richard Julin Foundation for research (not related to the present study). Nikola Panic was supported by a mobility grant from the European Pancreatic Club. Sara Nikolic is supported by Pancreas 2000—an educational program for future pancreatologists.
Nikolic S, Lanzillotta M, Panic N, Brismar TB, Moro CF, Capurso G, et al. Unraveling the relationship between autoimmune pancreatitis type 2 and inflammatory bowel disease: results from two centers and systematic review of the literature. United European Gastroenterol J. 2022;10(5):496–506. 10.1002/ueg2.12237
Sara Nikolic and Marco Lanzillotta share first authorship.
J.‐Matthias Löhr and Miroslav Vujasinovic share senior authorship.
All authors approved the final version of the article, including the authorship list.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Hart PA, Krishna SG, Okazaki K. Diagnosis and management of autoimmune pancreatitis. Curr Treat Options Gastroenterol. 2017;15(4):538–47. 10.1007/s11938-017-0147-x [DOI] [PubMed] [Google Scholar]
- 2. Löhr JM, Beuers U, Vujasinovic M, Alvaro D, Frøkjær JB, Buttgereit F, et al. European Guideline on IgG4‐related digestive disease: UEG and SGF evidence‐based recommendations. United Eur Gastroenterol. J. 2020;8(6):637–66. 10.1177/2050640620934911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino‐Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352–8. 10.1097/mpa.0b013e3182142fd2 [DOI] [PubMed] [Google Scholar]
- 4. Löhr JM, Vujasinovic M, Rosendahl J, Stone JH, Beuers U. IgG4‐related diseases of the digestive tract. Nat Rev Gastroenterol Hepatol. 2021;19(3):185–97. 10.1038/s41575-021-00529-y [DOI] [PubMed] [Google Scholar]
- 5. de Pretis N, Frulloni L. Autoimmune pancreatitis type 2. Curr Opin Gastroenterol. 2020;36(5):417–20. 10.1097/mog.0000000000000655 [DOI] [PubMed] [Google Scholar]
- 6. Zen Y Type 2 autoimmune pancreatitis: consensus and controversies. Gut and Liver. 2021. 10.5009/gnl210241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aziz I, Simrén M. The overlap between irritable bowel syndrome and organic gastrointestinal diseases. Lancet Gastroenterol Hepatol. 2021;6(2):139–48. 10.1016/s2468-1253(20)30212-0 [DOI] [PubMed] [Google Scholar]
- 8. Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn's Colitis. 2021;16(1):2–17. 10.1093/ecco-jcc/jjab178 [DOI] [PubMed] [Google Scholar]
- 9. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn's Disease: medical treatment. J Crohn's Colitis. 2020;14(1):4–22. 10.1093/ecco-jcc/jjz180 [DOI] [PubMed] [Google Scholar]
- 10. Lorenzo D, Vullierme MP, Rebours V. Antitumor necrosis factor therapy Is effective for autoimmune pancreatitis type 2. Am J Gastroenterol. 2020;115(7):1133–4. 10.14309/ajg.0000000000000668 [DOI] [PubMed] [Google Scholar]
- 11. Schneider A, Hirth M, Weiss C, Weidner P, Antoni C, Thomann A, et al. Prevalence of inflammatory bowel disease in alcoholic, non‐alcoholic and autoimmune pancreatitis. Z Gastroenterologie. 2018;56(5):469–78. [DOI] [PubMed] [Google Scholar]
- 12. Almeida P, Almeida C, Gompertz M, Berger Z. Association between autoimmune pancreatitis and ulcerative colitis: a report of 12 patients. Rev Esp Enferm Dig. 2020;112(9):682–7. 10.17235/reed.2020.6677/2019 [DOI] [PubMed] [Google Scholar]
- 13. Kawa S, Okazaki K, Notohara K, Watanabe M, Shimosegawa T. Autoimmune pancreatitis complicated with inflammatory bowel disease and comparative study of type 1 and type 2 autoimmune pancreatitis. J Gastroenterol. 2015;50(7):805–15. 10.1007/s00535-014-1012-5 [DOI] [PubMed] [Google Scholar]
- 14. Kim JM, Hwang SW, Park SH, Song TJ, Kim MH, Lee HS, et al. Clinical course of ulcerative colitis patients who develop acute pancreatitis. World J Gastroenterol. 2017;23(19):3505–12. 10.3748/wjg.v23.i19.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO‐ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohn's Colitis. 2018;13(2):144–64K. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 16. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–53. 10.1136/gut.2005.082909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. 2009;339(4):b2700. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27(8):1119–27. 10.1097/00000478-200308000-00009 [DOI] [PubMed] [Google Scholar]
- 19. Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch: An Int J Pathol. 2004;445(6):552–63. 10.1007/s00428-004-1140-z [DOI] [PubMed] [Google Scholar]
- 20. Park SH, Kim D, Ye BD, Yang SK, Kim JH, Yang DH, et al. The characteristics of ulcerative colitis associated with autoimmune pancreatitis. J Clin Gastroenterol. 2013;47(6):520–5. 10.1097/mcg.0b013e31827fd4a2 [DOI] [PubMed] [Google Scholar]
- 21. Lopez‐Serrano A, Crespo J, Pascual I, Salord S, Bolado FD , Del‐Pozo‐Garcia AJ, et al. Diagnosis, treatment and long‐term outcomes of autoimmune pancreatitis in Spain based on the International Consensus Diagnostic Criteria: a multi‐centre study. Pancreatology: Off J Int Assoc Pancreatol. 2016;16(3):382–90. 10.1016/j.pan.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 22. Lorenzo D, Maire F, Stefanescu C, Gornet JM, Seksik P, Serrero M, et al. Features of autoimmune pancreatitis associated with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(1):59–67. 10.1016/j.cgh.2017.07.033 [DOI] [PubMed] [Google Scholar]
- 23. Ueki T, Kawamoto K, Otsuka Y, Minoda R, Maruo T, Matsumura K, et al. Prevalence and clinicopathological features of autoimmune pancreatitis in Japanese patients with inflammatory bowel disease. Pancreas. 2015;44(3):434–40. 10.1097/mpa.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 24. Hart PA, Levy MJ, Smyrk TC, Takahashi N, Abu Dayyeh BK, Clain JE, et al. Clinical profiles and outcomes in idiopathic duct‐centric chronic pancreatitis (type 2 autoimmune pancreatitis): the Mayo Clinic experience. Gut. 2016;65(10):1702–9. 10.1136/gutjnl-2015-309275 [DOI] [PubMed] [Google Scholar]
- 25. Roque Ramos L, DiMaio CJ, Sachar DB, Atreja A, Colombel JF, Torres J. Autoimmune pancreatitis and inflammatory bowel disease: case series and review of the literature. Dig Liver Dis. 2016;48(8):893–8. 10.1016/j.dld.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 26. Oh D, Song TJ, Moon SH, Kim DJH, Lee NJ, Hong SM, et al. Type 2 Autoimmune pancreatitis (idiopathic duct‐centric pancreatitis) highlighting patients presenting as clinical acute pancreatitis: a single‐center experience. Gut and Liver. 2019;13(4):461–70. 10.5009/gnl18429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ku Y, Hong SM, Fujikura K, Kim SJ, Akita M, Abe‐Suzuki S, et al. IL‐8 expression in granulocytic epithelial lesions of idiopathic duct‐centric pancreatitis (type 2 autoimmune pancreatitis). Am J Surg Pathol. 2017;41(8):1129–38. 10.1097/pas.0000000000000891 [DOI] [PubMed] [Google Scholar]
- 28. Rana SS, Gupta R, Nada R, Gupta P, Basher R, Mittal BR, et al. Clinical profile and treatment outcomes in autoimmune pancreatitis: a report from North India. Ann Gastroenterol. 2018;31(4):506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pattabathula K, Waters PS, Hwang J, Bettington M, Singh M, Bryant RD, et al. Diagnostic and therapeutic considerations in biopsy‐proven type 2 autoimmune pancreatitis: comparative analysis with biopsy‐proven type 1 autoimmune pancreatitis. ANZ J Surg. 2021;91(5):907–14. 10.1111/ans.16445 [DOI] [PubMed] [Google Scholar]
- 30. Detlefsen S, Zamboni G, Frulloni L, Feyerabend B, Braun F, Gerke O, et al. Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: a study of 114 surgically treated European patients. Pancreatology: Off J Int Assoc Pancreatol. 2012;12(3):276–83. 10.1016/j.pan.2012.03.055 [DOI] [PubMed] [Google Scholar]
- 31. Song TJ, Kim JH, Kim MH, Jang JW, Park DH, Lee SS, et al. Comparison of clinical findings between histologically confirmed type 1 and type 2 autoimmune pancreatitis. J Gastroenterol Hepatol. 2012;27(4):700–8. 10.1111/j.1440-1746.2011.06934.x [DOI] [PubMed] [Google Scholar]
- 32. Ikeura T, Detlefsen S, Zamboni G, Manfredi R, Negrelli R, Amodio A, et al. Retrospective comparison between preoperative diagnosis by International Consensus Diagnostic Criteria and histological diagnosis in patients with focal autoimmune pancreatitis who underwent surgery with suspicion of cancer. Pancreas. 2014;43(5):698–703. 10.1097/mpa.0000000000000114 [DOI] [PubMed] [Google Scholar]
- 33. Detlefsen S, Mortensen MB, Pless TK, Cribe AS, de Muckadell OB. Laparoscopic and percutaneous core needle biopsy plays a central role for the diagnosis of autoimmune pancreatitis in a single‐center study from Denmark. Pancreas. 2015;44(6):845–58. 10.1097/mpa.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 34. Buijs J, Cahen DL, van Heerde MJ, Rauws EA, de Buy Wenniger LJ, Hansen BE, et al. The long‐term impact of autoimmune pancreatitis on pancreatic function, quality of life, and life expectancy. Pancreas. 2015;44(7):1065–71. [DOI] [PubMed] [Google Scholar]
- 35. Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149(1):39–51. 10.1053/j.gastro.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 36. Sah RP, Chari ST, Pannala R, Sugumar R, Clain JE, Levy MJ, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139(1):140–8. ; quiz e12‐3. [DOI] [PubMed] [Google Scholar]
- 37. Ikeura T, Manfredi R, Zamboni G, Negrelli R, Capelli P, Amodio A, et al. Application of international consensus diagnostic criteria to an Italian series of autoimmune pancreatitis. United Eur Gastroenterol. J. 2013;1(4):276–84. 10.1177/2050640613495196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Notohara K, Nishimori I, Mizuno N, Okazaki K, Ito T, Kawa S, et al. Clinicopathological features of type 2 autoimmune pancreatitis in Japan: results of a multicenter survey. Pancreas. 2015;44(7):1072–7. 10.1097/mpa.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 39. Rasch S, Phillip V, Schmid RM, Algül H. Epidemiology, clinical presentation, diagnosis and treatment of autoimmune pancreatitis: a retrospective analysis of 53 patients. Pancreatology: Off J Int Assoc Pancreatol. 2016;16(1):73–7. 10.1016/j.pan.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 40. Detlefsen S, de Vos JD, Tanassi JT, Heegaard NHH, Fristrup C, Schaffalitzky de Muckadell OB. Value of anti‐plasminogen binding peptide, anti‐carbonic anhydrase II, immunoglobulin G4, and other serological markers for the differentiation of autoimmune pancreatitis and pancreatic cancer. Medicine (Baltim). 2018;97(31):e11641. 10.1097/md.0000000000011641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupta R, Neyaz A, Chougule A, Akita M, Zen Y, Forcione D, et al. Autoimmune pancreatitis type 2: diagnostic utility of PD‐L1 immunohistochemistry. Am J Surg Pathol. 2019;43(7):898–906. 10.1097/pas.0000000000001282 [DOI] [PubMed] [Google Scholar]
- 42. Lee HM, Deheragoda M, Harrison P, Devlin J, Sellars M, Hadzic N, et al. Autoimmune pancreatitis in children: a single centre experience in diagnosis, management and long term follow up. Pancreatology: Off J Int Assoc Pancreatol. 2019;19(1):169–76. 10.1016/j.pan.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 43. Soliman H, Vullierme MP, Maire F, Hentic O, Ruszniewski P, Levy P, et al. Risk factors and treatment of relapses in autoimmune pancreatitis: rituximab is safe and effective. United Eur Gastroenterol. J. 2019;7(8):1073–83. 10.1177/2050640619862459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barresi L, Tacelli M, Crino SF, Attili F, Petrone MC, De Nucci G, et al. Multicentric Italian survey on daily practice for autoimmune pancreatitis: clinical data, diagnosis, treatment, and evolution toward pancreatic insufficiency. United Eur Gastroenterol. J. 2020;8(6):705–15. 10.1016/j.pan.2020.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Czakó L, Gyökeres T, Topa L, Sahin P, Takács T, Vincze A, et al. Autoimmune pancreatitis in Hungary: a multicenter nationwide study. Pancreatology: Off J Int Assoc Pancreatol. 2011;11(2):261–7. 10.1159/000327092 [DOI] [PubMed] [Google Scholar]
- 46. Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40(6):809–14. 10.1097/mpa.0b013e3182258a15 [DOI] [PubMed] [Google Scholar]
- 47. Balasubramanian G, Sugumar A, Smyrk TC, Takahashi N, Clain JE, Gleeson FC, et al. Demystifying seronegative autoimmune pancreatitis. Pancreatology: Off J Int Assoc Pancreatol. 2012;12(4):289–94. 10.1016/j.pan.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 48. de Pretis N, Vieceli F, Brandolese A, Brozzi L, Amodio A, Frulloni L. Autoimmune pancreatitis not otherwise specified (NOS): clinical features and outcomes of the forgotten type. Hepatobiliary Pancreat Dis Int: HBPD INT. 2019;18(6):576–9. 10.1016/j.hbpd.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 49. Wilson A, Wang Q, Choi YH, Ponich T, Gregor JC, Chande N, et al. Pretreatment HLADQA1‐HLADRB1 testing for the prevention of azathioprine‐induced pancreatitis in inflammatory bowel disease: a prospective cohort study. Clin Transl Gastroenterol. 2021;12(4):e00332. 10.14309/ctg.0000000000000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40(8):1172–9. 10.1097/mpa.0b013e318233bec5 [DOI] [PubMed] [Google Scholar]
- 51. Chiabrando F, Lanzillotta M, Palumbo D, Pedica F, Caruso M, Capurso G, et al. Treating type 2 autoimmune pancreatitis with colchicine: a case series. Ann Intern Med. 2021;174(12):1775–1776. 10.7326/l21-0281 [DOI] [PubMed] [Google Scholar]
- 52. Matsubayashi H, Ishiwatari H, Imai K, Kishida Y, Ito S, Hotta K, et al. Steroid therapy and steroid response in autoimmune pancreatitis. Int J Mol Sci. 2019;21(1):257. 10.3390/ijms21010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakamura A, Ozawa M, Watanabe T, Ito T, Muraki T, Hamano H, et al. Predictive factors for autoimmune pancreatitis relapse after 3 years of maintenance therapy. Pancreas. 2018;47(10):1337–43. 10.1097/mpa.0000000000001173 [DOI] [PubMed] [Google Scholar]
- 54. Miki M, Fujimori N, Oono T, Kawabe K, Ohno A, Matsumoto K, et al. Relapse patterns and predictors of IgG4‐related diseases involved with autoimmune pancreatitis: a single‐center retrospective study of 115 patients. J Dig Dis. 2019;20(3):152–8. 10.1111/1751-2980.12708 [DOI] [PubMed] [Google Scholar]
- 55. Vujasinovic M, Valente R, Maier P, von Beckerath V, Haas SL, Arnelo U, et al. Diagnosis, treatment and long‐term outcome of autoimmune pancreatitis in Sweden. Pancreatology: Off J Int Assoc Pancreatol. 2018;18(8):900–4. 10.1016/j.pan.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 56. Salvatore S, Finazzi S, Barassi A, Verzelletti M, Tosi A, Melzi d'Eril GV, et al. Low fecal elastase: potentially related to transient small bowel damage resulting from enteric pathogens. J Pediatr Gastroenterol Nutr. 2003;36(3):392–6. 10.1097/00005176-200303000-00018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Tabel S1
Tabel S2
Tabel S3
Tabel S4
Tabel S5
Data Availability Statement
Research data are not shared.


