Abstract
The stereoselective synthesis of molecules bearing stereogenic phosphorus(V) centers represents an enduring challenge in organic chemistry. Although stereospecific nucleophilic substitution at P(V) provides a general strategy for elaborating optically active P(V) compounds, existing methods for accessing the requisite chiral building blocks rely almost entirely on diastereocontrol using chiral auxiliaries. Catalytic, enantioselective methods for the synthesis of synthetically versatile stereogenic P(V) building blocks offer an alternative approach to stereogenic-at-P(V) targets without requiring stoichiometric quantities of chiral controlling elements. Herein, we report an enantioselective hydrogen-bond-donor-catalyzed synthesis of aryl chlorophosphonamidates, and the development of these products as versatile chiral P(V) building blocks. We demonstrate that the chlorophosphonamidates possess two leaving groups that can be displaced sequentially and stereospecifically to access a wide variety of stereogenic-at-P(V) compounds featuring diverse substitution patterns.
One Sentence Summary:
An enantioselective sulfinamidourea-catalyzed synthesis of chlorophosphonamidates underlies a general approach to chiral P(V) compounds
Introduction
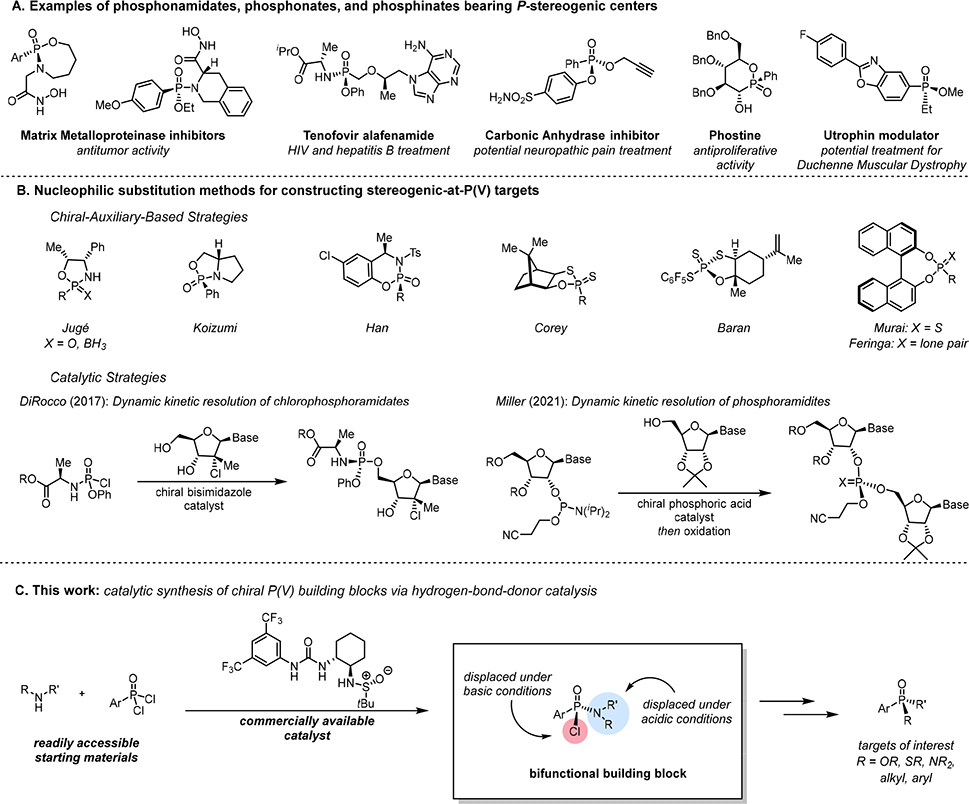
Phosphorus(V) stereocenters are present in a wide assortment of important molecules, including several recently developed pharmaceuticals (Figure 1A). The absolute stereochemistry at phosphorus is often directly associated with the biological activity of those molecules (1–7). Stereogenic-at-phosphorus compounds also serve as broadly useful ligands and catalysts in asymmetric organic synthesis (8, 9). Though a variety of natural products bearing P-stereogenic centers have been identified (10), these molecules are not practical synthetic building blocks due to their sparsity. Thus, whereas the synthesis of compounds bearing C-stereogenic centers has historically drawn heavily on nature’s chiral pool (11), access to P-stereogenic molecules relies entirely on de novo synthesis. Nucleophilic substitution at stereogenic P(V) centers can occur stereospecifically, thereby providing a powerful strategy for the synthesis of complex, optically active compounds from simple P(V) building blocks bearing one or more leaving groups attached to phosphorus (9, 11–13).
Fig. 1. Methods for accessing stereogenic P(V) targets.
(A) Representative bioactive compounds bearing P-stereogenic centers. (B) Synthetic approaches to stereogenic-at-P(V) targets using chiral auxiliaries (14–22) and stereoselective catalysis (23, 24). (C) A general approach to chiral P(V) building blocks via enantioselective catalysis/stereospecific substitution.
Effective methods for accessing stereogenic-at-phosphorus targets have relied primarily on the use of covalently attached chiral auxiliaries to achieve diastereocontrol, and a variety of chelating auxiliaries have been developed successfully for this purpose (Fig. 1B) (14–22). Their applicability depends on stereospecific displacement of the auxiliary to forge P(V) stereocenters with absolute stereocontrol. Among noteworthy recent advances using the chiral auxiliary approach, Baran and co-workers reported the development of highly reactive oxathiaphospholane-sulfide building blocks (19, 20). The propensity of the P–S bonds in these building blocks to undergo substitution by both alcohols and organometallic reagents was demonstrated and enables the synthesis of a variety of stereogenic-at-P(V) compounds, ranging from oligonucleotides to chiral phosphine oxides.
Despite important advances in the stereoselective synthesis of chiral P(V) compounds by the chiral auxiliary approach, there is both practical and fundamental motivation for developing asymmetric catalytic strategies toward these targets. In that vein, there have been several recent breakthroughs (Fig. 1B). Dirocco and coworkers developed a chiral bisimidazole catalyzed synthesis of phosphoramidate prodrugs through the diastereoselective addition of nucleosides to chlorophosphoramidates, proceeding via a cooperative mechanism of covalent activation of P(V) and general-base activation of the alcohol nucleophile (23). An alternative approach was demonstrated by Miller and co-workers in the catalytic, stereodivergent synthesis of P-stereogenic oligonucleotides from phosphoramidites via chiral phosphoric acid catalysis (24). Finally, in work that appeared as this study was being completed, Dixon and co-workers reported a catalytic, enantioselective desymmetrization of diaryl phosphonate esters by substitution with ortho-substituted phenols (25). Although high levels of stereoselectivity were achieved in these catalytic, nucleophilic substitution reactions, each is limited to a narrow class of nucleophiles that are not further displaced. We conceived that the catalytic, enantioselective installation of a nucleophile that could further serve as a leaving group for stereospecific substitution at P(V) could provide a generalizable strategy for the synthesis of chiral P(V) targets with the broad synthetic scope of state-of-the-art auxiliary approaches while avoiding the need for the stoichiometric use of chiral control elements.
We selected chlorophosphonamidates as potential targets of an enantioselective catalytic approach (Fig. 1C). The chloride and amino groups on P(V) possess orthogonal reactivity that might permit sequential and stereospecific displacement en route to chiral P(V) targets bearing a broad range of substitution patterns. Given that P–Cl bonds in particular are susceptible to substitution by a wide variety of nucleophiles (26–28), chlorophosphonamidates would be highly versatile precursors to a multitude of P(V) frameworks. We report here the development of an enantioselective method for the synthesis of chlorophosphonamidate intermediates using a commercially available hydrogen-bond-donor catalyst, and the application of these P(V) building blocks to the synthesis of P(V) compounds featuring diverse substitution patterns.
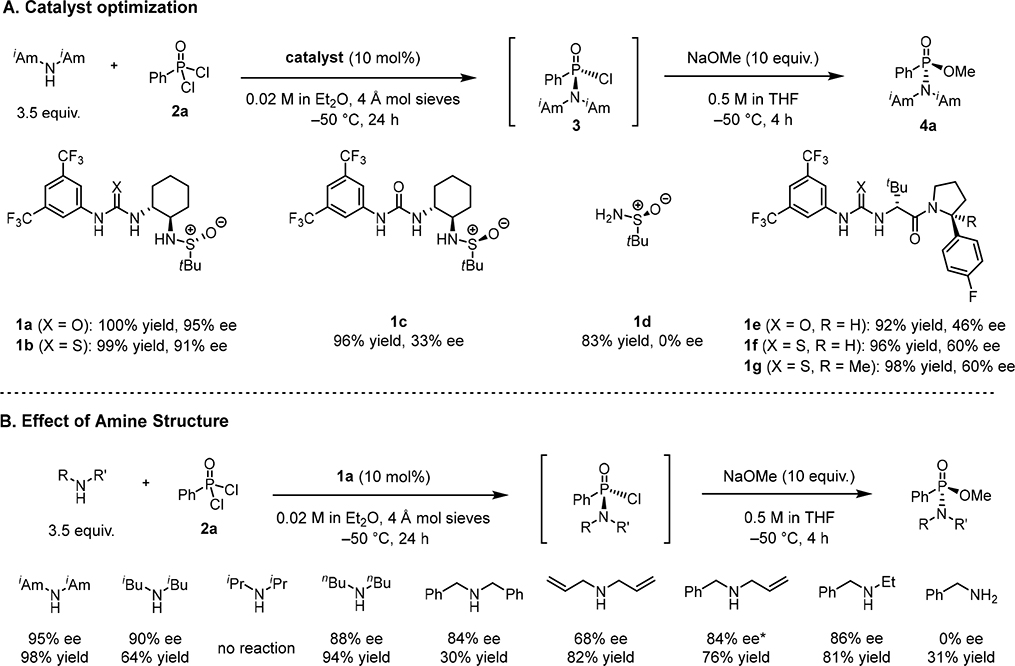
We recognized that a most concise enantioselective synthesis of chlorophosphonamidates would be realized via a catalytic desymmetrization reaction of phosphonic dichlorides with amines. Dual-hydrogen-bond-donor catalysts have been applied broadly and successfully to promote stereoselective nucleophilic substitution reactions via chloride-abstraction pathways (29–32), and we hypothesized that this reactivity principle could serve to activate one of the two enantiotopic chlorides of a phosphonic dichloride electrophile toward displacement by an amine. Phenyl phosphonic dichloride 2a was selected as a model substrate in reactions with various amine nucleophiles and potential chiral catalysts (Fig. 2). The chlorophosphonamidate products were found to be too reactive to isolate in pure form, but solutions of 3 were stable and could be separated from other reaction components by filtration through silica. Epimerization of chlorophosphonamidate 3 was not observed under the catalytic conditions, even in the presence of added tetrabutylammonium chloride. However, concentrated solutions of 3 underwent racemization slowly at room temperature over multiple hours (Table S10). For purposes of isolation and analysis, the chlorophosphonamidates were quenched with sodium methoxide at low temperature to produce the corresponding phosphonamidates (e.g. 4a). After systematic evaluation of a series of chiral dual H-bond-donor catalysts and amine nucleophiles, the sulfinamido urea 1a (33, 34) was found to promote the nucleophilic substitution by diisoamylamine in 95% enantiomeric excess (ee) and quantitative yield (Fig. 2A, see supplementary materials for optimization studies). Multiple equivalents of amine were required to attain full conversion of 2a, as the amine functions both as a nucleophile and as a stoichiometric Brønsted base to trap the HCl byproduct produced in the reaction. Examination of the role of catalyst structure revealed the importance of both the H-bond donor and the sulfinamide group in promoting high enantioselectivity. Whereas sulfinamido urea 1a and its thiourea analog 1b proved similarly effective as catalysts, the sulfinamide 1d lacking the H-bond-donor motif induced little acceleration above the uncatalyzed rate (83% vs. 64% yield after 24 h) and afforded only racemic product. The sulfinamido urea 1c epimeric to 1a also induced severely diminished enantioselectivity, a stereochemical “mismatch” effect that has also been observed in other applications of this catalyst (33, 34) and one that is strongly suggestive of cooperative participation of the H-bond donor and the sulfinamide in the enantiodetermining step. Arylpyrrolidino (thio)ureas such as 1e–g, which have proven useful in a wide range of asymmetric anion-binding pathways (35) but lack the sulfinamide moiety, were catalytically active but generally poorly effective with respect to enantiocontrol. The enantioselectivity of the substitution was also closely tied to the identity of the amine, with diisoamylamine undergoing reaction with distinctly superior results relative to any of the other nucleophiles examined (Fig. 2B). Beyond a beneficial effect of distal alkyl branching, it is difficult to discern any straightforward correlation between the steric or electronic properties of the amine and enantioselectivity in the substitution reaction. Control studies suggest that the properties of the dialkylammonium chloride byproducts likely play a critical and complex role in influencing the observed enantioselectivity, either as inhibitors of the anion-binding H-bond-donor catalyst or by promoting a racemic pathway between 2a and the dialkylamine (Tables S8–S9).
Figure 2. Optimization studies.
Yield values reflect product quantification by 31P NMR relative to an internal standard. Reactions were carried out using a one-pot procedure without purification of 3. Concentration values correspond to the initial concentration of the limiting stoichiometric reagent. (A) Catalyst optimization for enantioselective reaction of diisoamylamine with phenyl phosphonic dichloride. Reactions were carried out on a 0.06 mmol scale. (B) Optimization of amine structure for enantioselective substitution reaction with phenyl phosphonic dichloride. Reactions were carried out on a 0.06 mmol scale. *Reaction performed at −40 °C for 48 h.
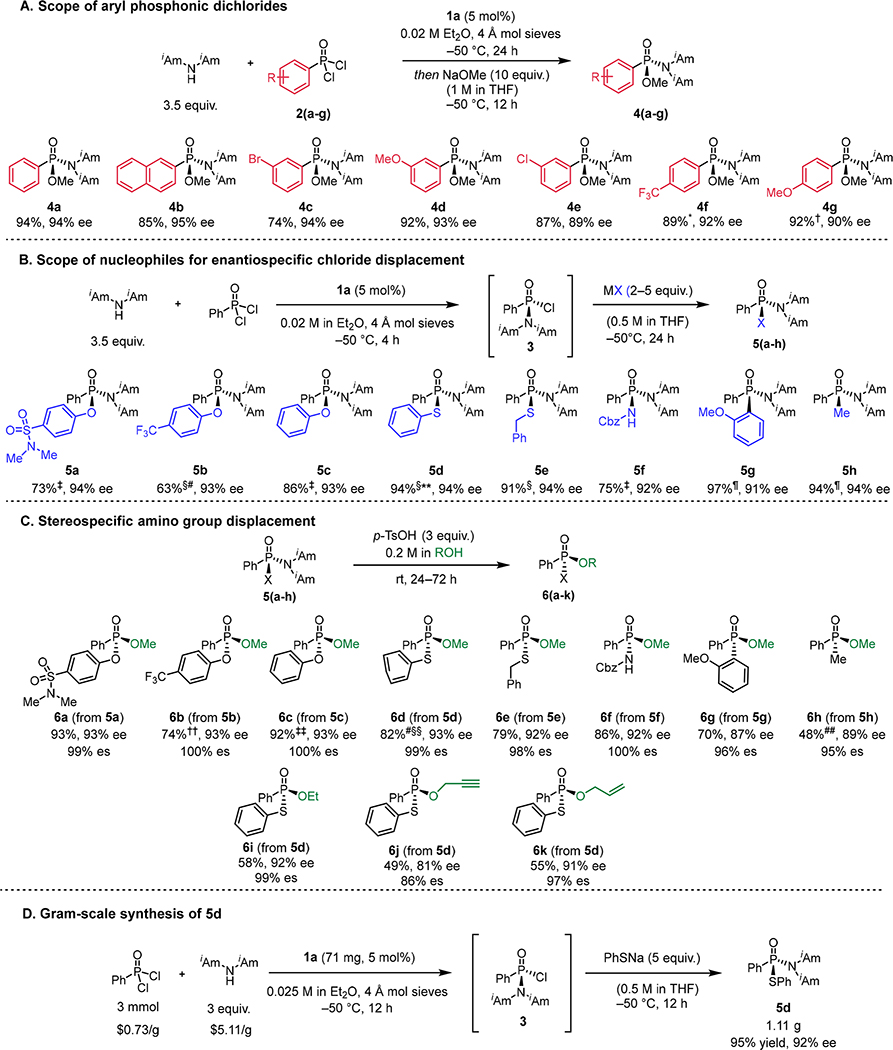
High levels of enantioselectivity were achieved in the reaction of a variety of aryl phosphonic dichlorides with diisoamylamine (Fig 3A). Substrates bearing arenes with either electron-withdrawing or electron-donating substituents underwent substitution with consistently high levels of enantioselectivity (4b–g). In contrast, alkyl phosphonic dichlorides are ineffective substrates for the enantioselective reaction. For example, hexylphosphonic dichloride was converted to the corresponding phosphonamidate in only 26% ee and 50% yield under the catalytic conditions.
Fig. 3. Scope of enantioselective addition of diisoamylamine to aryl phosphonic dichlorides and stereospecific elaborations.
All yield values correspond to chromatographically purified, isolated products. Concentration values correspond to the initial concentration of the limiting stoichiometric reagent. (A) Substrate scope of addition of diisoamylamine to aryl phosphonic dichlorides catalyzed by 1a. Reactions were carried out on 0.2 mmol scale. The absolute stereochemistry of the products was assigned based on the x-ray crystal structure of 10 and the known optical rotation of 8a (Fig. 4, see supplementary materials). (B) Scope of nucleophiles for enantiospecific substitution with 3. (C) Enantiospecific displacement of the diisoamylamino group with alcohols. See supplementary materials for reaction conditions. (D) Gram-scale synthesis of 5d. Prices from Thermo Fisher Scientific (February, 2022). *Reaction was carried out at −78 °C with 20 mol% catalyst loading. †Reaction was carried out at −40 °C with 4.5 equivalents of diisoamylamine. ‡ Reaction was carried out in a two-pot procedure involving generation of 3 in solution and purification by filtration through silica and subsequent reaction with 2 equivalents of nucleophile. § Reaction was carried out using one-pot procedure without purification of 3 with 5 equivalents of nucleophile. ¶ Reaction was carried out in a two-pot procedure involving generation of 3 in solution and purification by filtration through silica and subsequent reaction with 5 equivalents of nucleophile. #Reaction was carried out on 0.9 mmol scale. **Reaction was carried out on 1.0 mmol scale. ††Reaction was carried out on 0.57 mmol scale. ‡‡Reaction was carried out on 0.24 mmol scale. §§ Reaction was run at 0.3 M concentration instead of 0.2 M. ##H3PO3 was used instead of para-tolylsulfonic acid.
The products of the enantioselective reactions feature two chemically distinct leaving groups on phosphorus that could be selectively and stereospecifically displaced to afford access to multiple classes of chiral P(V) compounds. We first explored the scope of nucleophiles capable of enantiospecific displacement of the remaining chloride (Fig. 3B). Reaction of 3 with alkoxides, phenoxides, thiolates, carbamides, and Grignard reagents afforded the desired products with high levels of enantiospecificity (es) in all cases (5a–h). The substitution reactions could be performed after the enantioselective catalytic step with or without purification of 3 in solution (see supplementary materials for details). We found that the reactions could be scaled up without loss of enantioselectivity or yield; thus, the synthesis of 5d was performed by the one-pot procedure on 3 mmol scale with 5 mol% catalyst, affording 1.11 grams of product in 95% yield and 92% ee (Fig. 3D).
The products of the chloride-displacement reactions could be further elaborated to afford alkoxy-substituted P(V) compounds via an acid-mediated stereoinvertive displacement of the diisoamylamino group (Fig. 3C). Substitution of 5a–h with methanol yielded a variety of enantioenriched phosphonates, phosphinates, and phosphonamidates (6a–h) with nearly complete enantiospecificity observed in every case. The slightly diminished stereospecificity observed with 5g and 5h is consistent with prior observations (14, 16). Substitution with other primary alcohols proceeded with varied but generally high levels of enantiospecificity (6i–k).
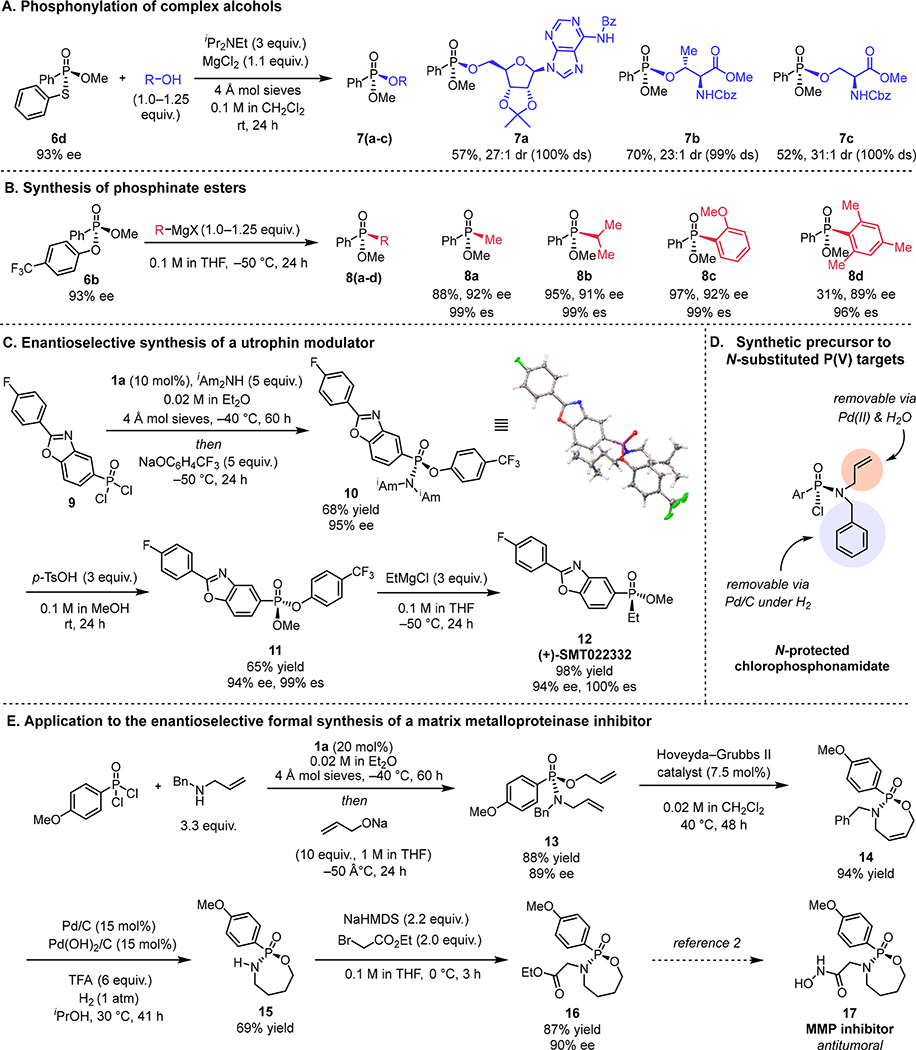
The phosphonate ester and thioester products 6b and 6d possess further readily displaceable substituents that render them useful synthetic building blocks for further elaboration to chiral P(V) compounds. For example, phosphonate thioester 6d underwent reaction with functionally complex alcohols to furnish the corresponding phosphonylated biomolecules with high levels of stereospecificity (7a–c, Fig. 4A). These substitutions are performed under Brønsted-acid–free conditions using little-or-no excess of the alcohol reagent, highlighting the utility of 6d for the phosphonylation of precious or acid-sensitive alcohols. Phosphonate 6b underwent efficient substitution with Grignard reagents with displacement of the electron-deficient aryloxide to yield highly enantioenriched phosphinate esters, known precursors to chiral phosphine oxides (Fig. 4B) (20). This three-step route to phosphinate esters was applied to the synthesis of (+)-SMT022332, a utrophin modulator developed as a potential treatment for Duchenne Muscular Dystrophy (36–38). An analogue of (+)-SMT022332 was previously accessed in 83% ee and 5% overall yield using a chiral auxiliary-based approach (4). Subjection of phosphonic dichloride 9 to the optimized conditions for the enantioselective substitution yielded phosphonamidate 10, which was characterized crystallographically (Fig. 4C). Subsequent methanolysis and phenol displacement furnished (+)-SMT022332 (12) in 94% ee and 43% overall yield over 3 steps.
Fig. 4. Application to the synthesis of chiral P(V) targets.
All yield values refer to chromatographically purified, isolated products. Concentration values correspond to the initial concentration of the limiting stoichiometric reagent. (A) Stereospecific phosphonylation of precious alcohols with 6d. Reactions were carried out on 0.1 mmol scale. (B) Stereospecific addition of Grignard reagents to 6b for the synthesis of enantioenriched phosphinate esters. Absolute stereochemistry of 8a was determined by comparison of optical rotation to literature value; others assigned by analogy. Reactions run on 0.05–0.1 mmol scale. *Prepared from 6b that was in 92% ee. (C) Application of method to the enantioselective synthesis of (+)-SMT022332. Yield values refer to isolated yields. Absolute stereochemistry of 10 assigned by the depicted X-ray crystal structure, and of 12 by comparison of the optical rotation to the literature value. (D) Orthogonally N-protected chlorophosphonamidate. (E) Formal synthesis of a matrix metalloproteinase inhibitor. TFA = trifluoroacetic acid.
In addition to serving as versatile synthetic building blocks, phosphonamidates are often synthetic targets themselves (2, 3, 5, 6, 39–43), and general access to these compounds by the catalytic procedure would be desirable. However, the structural requirements on the amine for achieving high enantioselectivity in catalytic reaction impose restrictions to the N-substituents that can be introduced directly (Fig. 2B). We therefore sought to identify amine derivatives that participate successfully in the enantioselective reaction while bearing orthogonally cleavable N-protecting groups that might provide centralized access to a variety of substituted phosphonamidates (Fig. 4D). High enantioselectivity was obtained using N-allyl benzylamine in the substitution reaction under modified conditions. The benzyl group and the allyl group on the chlorophosphonamidate products can each be cleaved successively, enabling their sequential replacement (see supplementary materials) (44–48). This strategy was exploited in the synthesis of phosphonamidate 17, a matrix metalloproteinase (MMP) inhibitor with demonstrated anticancer activity (Fig. 4E) (2). Phosphonic dichloride 2h effectively underwent the catalytic reaction with N-allylbenzylamine to produce, after quenching with allyl alkoxide, phosphonamidate 13 in 89% ee and 88% yield. Phosphonamidate 13 was elaborated over three steps to afford cyclic phosphonamidate 16 in 90% ee, completing the enantioselective formal synthesis of MMP inhibitor 17. We anticipate that N-allyl benzylamine’s versatility as a masked “–NH2” equivalent may enable access to a wide variety of phosphonamidate targets.
We expect the versatile enantioenriched chlorophosphonamidate intermediates accessed via synthetic strategies outlined herein to enable the facile synthesis of both known and new stereogenic-at-P(V) compounds of interest.
Supplementary Material
Acknowledgments
We thank Dr. S.-L. Zheng (Harvard University) for determination of the x-ray crystal structure, and R. Algera, J. Essman, and H. Sharma for helpful discussions.
Funding:
National Institutes of Health grant GM043214 (ENJ).
Footnotes
Data and materials availability:
Crystallographic data for compound 10 are available free of charge from the Cambridge Crystallographic Data Centre under reference CCDC 2155524. All other data are available in the main text or the supplementary materials.
REFERENCES
- 1.Pradere U, Garnier-Amblard EC, Coats SJ, Amblard F, Schinazi RF, Synthesis of Nucleoside Phosphate and Phosphonate Prodrugs. Chem. Rev 114, 9154–9218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sørensen MD, Blæhr LKA, Christensen MK, Høyer T, Latini S, Hjarnaa P-JV, Björkling F, Cyclic phosphinamides and phosphonamides, novel series of potent matrix metalloproteinase inhibitors with antitumour activity. Bioorg. Med. Chem 11, 5461–5484 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Sawa M, Kiyoi T, Kurokawa K, Kumihara H, Yamamoto M, Miyasaka T, Ito Y, Hirayama R, Inoue T, Kirii Y, Nishiwaki E, Ohmoto H, Maeda Y, Ishibushi E, Inoue Y, Yoshino K, Kondo H, New Type of Metalloproteinase Inhibitor: Design and Synthesis of New Phosphonamide-Based Hydroxamic Acids. J. Med. Chem 45, 919–929 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Babbs A, Berg A, Chatzopoulou M, Davies KE, Davies SG, Edwards B, Elsey DJ, Emer E, Figuccia ALA, Fletcher AM, Guiraud S, Harriman S, Moir L, Robinson N, Rowley JA, Russell AJ, Squire SE, Thomson JE, Tinsley JM, Wilson FX, Wynne GM, Synthesis of SMT022357 enantiomers and in vivo evaluation in a Duchenne muscular dystrophy mouse model. Tetrahedron. 76, 130819 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nocentini A, Alterio V, Bua S, Micheli L, Esposito D, Buonanno M, Bartolucci G, Osman SM, ALOthman ZA, Cirilli R, Pierini M, Monti SM, Di Cesare Mannelli L, Gratteri P, Ghelardini C, De Simone G, Supuran CT, Phenyl(thio)phosphon(amid)ate Benzenesulfonamides as Potent and Selective Inhibitors of Human Carbonic Anhydrases II and VII Counteract Allodynia in a Mouse Model of Oxaliplatin-Induced Neuropathy. J. Med. Chem 63, 5185–5200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nocentini A, Gratteri P, Supuran CT, Phosphorus versus Sulfur: Discovery of Benzenephosphonamidates as Versatile Sulfonamide-Mimic Chemotypes Acting as Carbonic Anhydrase Inhibitors. Chem. – Eur. J 25, 1188–1192 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Lee WA, He G-X, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, Cundy KC, Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother 49, 1898–1906 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imamoto T, Synthesis and applications of high-performance P-chiral phosphine ligands. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 97, 520–542 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutartre M, Bayardon J, Jugé S, Applications and stereoselective syntheses of P-chirogenic phosphorus compounds. Chem. Soc. Rev 45, 5771–5794 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Kolodiazhnyi OI, Phosphorus Compounds of Natural Origin: Prebiotic, Stereochemistry, Application. Symmetry. 13, 889 (2021). [Google Scholar]
- 11.Kolodiazhnyi OI, Kolodiazhna A, Nucleophilic substitution at phosphorus: stereochemistry and mechanisms. Tetrahedron Asymmetry. 28, 1651–1674 (2017). [Google Scholar]
- 12.Kolodiazhnyi OI, Recent developments in the asymmetric synthesis of Р-chiral phosphorus compounds. Tetrahedron Asymmetry. 23, 1–46 (2012). [Google Scholar]
- 13.Ye X, Peng L, Bao X, Tan C-H, Wang H, Recent developments in highly efficient construction of P-stereogenic centers. Green Synth. Catal 2, 6–18 (2021). [Google Scholar]
- 14.Jugé S, Genet JP, Asymmetric synthesis of phosphinates, phosphine oxides and phosphines by Michaelis Arbuzov rearrangement of chiral oxazaphospholidine. Tetrahedron Lett 30, 2783–2786 (1989). [Google Scholar]
- 15.Jugé S, Stephan M, Laffitte JA, Genet JP, Efficient asymmetric synthesis of optically pure tertiary mono and diphosphine ligands. Tetrahedron Lett 31, 6357–6360 (1990). [Google Scholar]
- 16.Koizumi T, Yanada(nee Ishizaka) R, Takagi H, Hirai H, Yoshii E, Grignard reaction of 2-phenyl-tetrahydropyrrolo-1,5,2-oxazaphospholes, observation of the stereospecific inversion of configuration. Tetrahedron Lett 22, 571–572 (1981). [Google Scholar]
- 17.Han ZS, Goyal N, Herbage MA, Sieber JD, Qu B, Xu Y, Li Z, Reeves JT, Desrosiers J-N, Ma S, Grinberg N, Lee H, Mangunuru HPR, Zhang Y, Krishnamurthy D, Lu BZ, Song JJ, Wang G, Senanayake CH, Efficient Asymmetric Synthesis of P-Chiral Phosphine Oxides via Properly Designed and Activated Benzoxazaphosphinine-2-oxide Agents. J. Am. Chem. Soc 135, 2474–2477 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Corey EJ, Chen Z, Tanoury GJ, A new and highly enantioselective synthetic route to P-chiral phosphines and diphosphines. J. Am. Chem. Soc 115, 11000–11001 (1993). [Google Scholar]
- 19.Knouse KW, deGruyter JN, Schmidt MA, Zheng B, Vantourout JC, Kingston C, Mercer SE, Mcdonald IM, Olson RE, Zhu Y, Hang C, Zhu J, Yuan C, Wang Q, Park P, Eastgate MD, Baran PS, Unlocking P(V): Reagents for chiral phosphorothioate synthesis. Science. 361, 1234–1238 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Rivas-Bascón N, Padial NM, Knouse KW, Zheng B, Vantourout JC, Schmidt MA, Eastgate MD, Baran PS, Enantiodivergent Formation of C–P Bonds: Synthesis of P-Chiral Phosphines and Methylphosphonate Oligonucleotides. J. Am. Chem. Soc 142, 5785–5792 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Kuwabara K, Maekawa Y, Minoura M, Maruyama T, Murai T, Chemoselective and Stereoselective Alcoholysis of Binaphthyl Phosphonothioates: Straightforward Access to Both Stereoisomers of Biologically Relevant P -Stereogenic Phosphonothioates. J. Org. Chem 85, 14446–14455 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Mondal A, Thiel NO, Dorel R, Feringa BL, P-chirogenic phosphorus compounds by stereoselective Pd-catalysed arylation of phosphoramidites. Nat. Catal 5, 10–19 (2022). [Google Scholar]
- 23.DiRocco DA, Ji Y, Sherer EC, Klapars A, Reibarkh M, Dropinski J, Mathew R, Maligres P, Hyde AM, Limanto J, Brunskill A, Ruck RT, Campeau L-C, Davies IW, A multifunctional catalyst that stereoselectively assembles prodrugs. Science. 356, 426–430 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Featherston AL, Kwon Y, Pompeo MM, Engl OD, Leahy DK, Miller SJ, Catalytic asymmetric and stereodivergent oligonucleotide synthesis. Science. 371, 702–707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Formica M, Rogova T, Shi H, Sahara N, Farley AJM, Christensen KE, Duarte F, Dixon DJ, Catalytic Enantioselective Nucleophilic Desymmetrisation of Phosphonate Esters (2021), doi: 10.26434/chemrxiv-2021-5714s-v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauduin C, Moulin D, Kaloun EB, Darcel C, Jugé S, Highly Enantiomerically Enriched Chlorophosphine Boranes: Synthesis and Applications as P-Chirogenic Electrophilic Blocks. J. Org. Chem 68, 4293–4301 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Kimura T, Murai T, Enantiomerically pure P-chiral phosphinoselenoic chlorides: inversion of configuration at the P-chirogenic center in the synthesis and reaction of these substances. Chem. Commun, 4077–4079 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Kimura T, Murai T, P-Chiral Phosphinoselenoic Chlorides and Optically Active P-Chiral Phosphinoselenoic Amides: Synthesis and Stereospecific Interconversion with Extrusion and Addition Reactions of the Selenium Atom. Chem. Lett 33, 878–879 (2004). [Google Scholar]
- 29.Doyle AG, Jacobsen EN, Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev 107, 5713–5743 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Kutateladze DA, Strassfeld DA, Jacobsen EN, Enantioselective Tail-to-Head Cyclizations Catalyzed by Dual-Hydrogen-Bond Donors. J. Am. Chem. Soc 142, 6951–6956 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bendelsmith AJ, Kim SC, Wasa M, Roche SP, Jacobsen EN, Enantioselective Synthesis of α-Allyl Amino Esters via Hydrogen-Bond-Donor Catalysis. J. Am. Chem. Soc 141, 11414–11419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford DD, Lehnherr D, Kennedy CR, Jacobsen EN, Anion-Abstraction Catalysis: The Cooperative Mechanism of α-Chloroether Activation by Dual Hydrogen-Bond Donors. ACS Catal 6, 4616–4620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan KL, Jacobsen EN, Indium-Mediated Asymmetric Allylation of Acylhydrazones Using a Chiral Urea Catalyst. Angew. Chem. Int. Ed 46, 1315–1317 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Zuend SJ, Woll MG, Tao Y, Jacobsen EN, Asymmetric Cooperative Catalysis of Strong Brønsted Acid–Promoted Reactions Using Chiral Ureas. Science. 327, 986–990 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strassfeld DA, Jacobsen EN “The Aryl-Pyrrolidine-tert-Leucine Motif as a New Privileged Chiral Scaffold: The Role of Noncovalent Stabilizing Interactions” in Supramolecular Catalysis: New Directions and Developments, van Leeuwen PWNM, Raynal M, Eds. (Wiley, 2022), chap. 25, pp. 361–385. [Google Scholar]
- 36.Wilkinson IVL, Perkins KJ, Dugdale H, Moir L, Vuorinen A, Chatzopoulou M, Squire SE, Monecke S, Lomow A, Geese M, Charles PD, Burch P, Tinsley JM, Wynne GM, Davies SG, Wilson FX, Rastinejad F, Mohammed S, Davies KE, Russell AJ, Chemical Proteomics and Phenotypic Profiling Identifies the Aryl Hydrocarbon Receptor as a Molecular Target of the Utrophin Modulator Ezutromid. Angew. Chem. Int. Ed 59, 2420–2428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babbs A, Berg A, Chatzopoulou M, Davies KE, Davies SG, Edwards B, Elsey DJ, Emer E, Guiraud S, Harriman S, Lecci C, Moir L, Peters D, Robinson N, Rowley JA, Russell AJ, Squire SE, Tinsley JM, Wilson FX, Wynne GM, 2-Arylbenzo[d]oxazole Phosphinate Esters as Second-Generation Modulators of Utrophin for the Treatment of Duchenne Muscular Dystrophy. J. Med. Chem 63, 7880–7891 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Chatzopoulou M, Emer E, Lecci C, Rowley JA, Casagrande A-S, Moir L, Squire SE, Davies SG, Harriman S, Wynne GM, Wilson FX, Davies KE, Russell AJ, Decreasing HepG2 Cytotoxicity by Lowering the Lipophilicity of Benzo[d]oxazolephosphinate Ester Utrophin Modulators. ACS Med. Chem. Lett 11, 2421–2427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overtveldt MV, Heugebaert TSA, Verstraeten I, Geelen D, Stevens CV, Phosphonamide pyrabactin analogues as abscisic acid agonists. Org. Biomol. Chem 13, 5260–5264 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Buti M, Riveiro-Barciela M, Esteban R, Tenofovir Alafenamide Fumarate: A New Tenofovir Prodrug for the Treatment of Chronic Hepatitis B Infection. J. Infect. Dis 216, S792–S796 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Slusarczyk M, Serpi M, Pertusati F, Phosphoramidates and phosphonamidates (ProTides) with antiviral activity. Antivir. Chem. Chemother. 26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasper M-A, Glanz M, Stengl A, Penkert M, Klenk S, Sauer T, Schumacher D, Helma J, Krause E, Cardoso MC, Leonhardt H, Hackenberger CPR, Cysteine-Selective Phosphonamidate Electrophiles for Modular Protein Bioconjugations. Angew. Chem. Int. Ed 58, 11625–11630 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Lentini NA, Foust BJ, Hsiao C-HC, Wiemer AJ, Wiemer DF, Phosphonamidate prodrugs of a butyrophilin ligand display plasma stability and potent Vγ9Vδ2 T cell stimulation. J. Med. Chem 61, 8658–8669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Z, Zhu Q, Che S, Luo Z, Lian Y, Palladium/nickel-mediated cross coupling reaction between phosphorylamides and alkenes toward enephosphorylamides. Synth. Commun 50, 2338–2346 (2020). [Google Scholar]
- 45.Xiao J, Li P, Zhang Y, Xie D, Peng Z, An D, Dong W, Cobalt-catalyzed oxidative arylmethylation of phosphorylamides. Tetrahedron. 74, 4558–4568 (2018). [Google Scholar]
- 46.Xu Y, Su Q, Dong W, Peng Z, An D, The Chan-Evans-Lam N-arylation of phosphonic/phosphinic amides. Tetrahedron. 73, 4602–4609 (2017). [Google Scholar]
- 47.Zhong L, Su Q, Xiao J, Peng Z, Dong W, Zhang Y, An D, Ligand-Free Copper-Catalyzed Arylation of Phosphonamides and Phosphinamides with Aryl Siloxanes. Asian J. Org. Chem 6, 1072–1079 (2017). [Google Scholar]
- 48.Zhu Q, Che S, Luo Z, Zhao Z, Ligand-free copper-catalyzed denitrogenative arylation of phosphorylamides with arylhydrazines. Synth. Commun 50, 947–957 (2020). [Google Scholar]
- 49.Schull TL, Brandow SL, Dressick WJ, Synthesis of symmetrical triarylphosphines from aryl fluorides and red phosphorus: scope and limitations. Tetrahedron Lett 42, 5373–5376 (2001). [Google Scholar]
- 50.Ohmura N, Nakamura A, Hamasaki A, Tokunaga M, Hydrolytic Deallylation of N-Allyl Amides Catalyzed by PdII Complexes. Eur. J. Org. Chem 2008, 5042–5045 (2008). [Google Scholar]
- 51.Bruker AXS APEX3 (2015).
- 52.Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D, Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr 48, 3–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheldrick GM, SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheldrick GM, Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard J. a. K., Puschmann H, OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr 42, 339–341 (2009). [Google Scholar]
- 56.Accelrys Software Inc., Accelrys DS Visualizer v2.0.1 (2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystallographic data for compound 10 are available free of charge from the Cambridge Crystallographic Data Centre under reference CCDC 2155524. All other data are available in the main text or the supplementary materials.