Abstract
Background:
Transitions of care (ToC) aim to provide continuity while preventing loss of information that may result in poor outcomes such as hospital readmission. Readmissions not only burden patients, they also increase costs. Given the high prevalence of coronary artery diseases (CAD) in the United States (US), patients with CAD often make up a significant portion of hospital readmissions.
Objective:
To conduct a systematic review evaluating the impact of pharmacist-driven ToC interventions on post-hospital outcomes for patients with CAD.
Methods:
MEDLINE, Scopus, and CINAHL were searched from database inception through 03/2020 using key words for CAD and pharmacists. Studies were included if they: (1) identified adults with CAD at US hospitals, (2) evaluated pharmacist-driven ToC interventions, and (3) assessed post-discharge outcomes. Outcomes were summarized qualitatively.
Results:
Of the 1,612 citations identified, 11 met criteria for inclusion. Pharmacist-driven ToC interventions were multifaceted and frequently included medication reconciliation, medication counseling, post-discharge follow–up and initiatives to improve medication adherence. Hospital readmission and emergency room visits were numerically lower among patients receiving versus not receiving pharmacist-driven interventions, with statistically significant differences observed in one study. Secondary prevention measures and adherence tended to be more favorable in the pharmacist-driven intervention groups.
Conclusion:
Eleven studies of multifaceted, ToC interventions led by pharmacists were identified. Readmissions were numerically lower and secondary prevention measures and adherence were more favorable among patients receiving pharmacist-driven interventions. However, sufficiently powered studies are still required to confirm these benefits.
Keywords: pharmaceutical care, acute coronary syndrome, myocardial Infarction, cardiology, medication therapy management, discharge planning
INTRODUCTION
Transitions of care (ToC) occur when patients move between healthcare systems, care settings or providers.1 An effective care transition promotes care coordination and continuity, while avoiding miscommunications or loss of information that may result in errors and poor outcomes such as hospital readmissions.2 Readmissions not only burden patients, they also increase costs. The Centers for Medicare and Medicaid Services (CMS) estimates that one in five Medicare patients discharged from a hospital is readmitted within 30 days, which costs over $26 billion annually.3 In October 2012, CMS started to reduce Medicare payments to hospitals with excess readmissions through the Hospital Readmission Reduction Program. The program targets hospital-wide all-cause admissions and readmissions for conditions such as acute myocardial infarction, chronic obstructive pulmonary disease, heart failure, pneumonia, coronary artery bypass graft surgery, and total hip and/or knee replacement.4 Given the high prevalence of coronary artery diseases (CAD) in the United States, patients with CAD often make up a significant portion of these readmissions.5
Pharmacist ToC interventions, such as medication review and reconciliation, medication education, outpatient clinic visits, and telephone follow-up, have been shown to reduce hospital readmissions while increasing medication adherence in patients with CAD.6–16 However, many evaluations of these interventions were conducted at only one institution, thus limiting applicability. As a result, a summary of the totality of the evidence is needed to better understand the role of pharmacist-led t ToC programs in the CAD population. Therefore, we conducted a systematic literature review to evaluate the impact of pharmacist-driven ToC interventions on post-hospital outcomes for patients with CAD.
METHODS
This report is prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.17 Prior to the start of the systematic review, a protocol was developed and submitted to the Prospective Register of Systematic Reviews (PROSPERO) online repository.
Search strategy and study selection
PubMed, Scopus, and CINAHL were systematically searched from database inception through March 27, 2020. The search strategy was developed by a medical librarian after discussion with the study team and utilized key words and Medical Subject Headings (MeSH) terms for CAD and pharmacist (Supplementary Table 1). Bibliographic database searches were supplemented with manual searches of the references sections of included articles and meeting abstracts from the American Society of Health-System Pharmacists (ASHP) and the American College of Clinical Pharmacy (ACCP) from 2015-2020.
Identified citations were independently reviewed by two investigators with disagreement resolved by a third investigator. Studies were included if they met the following criteria: (1) included adult patients with CAD (defined as those with acute coronary syndrome [ACS] events, coronary lesions and/or undergoing coronary revascularization procedures) being treated at United States hospitals, (2) evaluated pharmacist-driven interventions aimed at impacting ToC (defined as the discharge and/or follow-up process from an acute care center to an outpatient setting or outside facility), and (3) compared outcomes following hospital discharge in patients with CAD receiving the pharmacist-driven intervention to a control group. Citation screening was performed using an online systematic review management tool.18
Data abstraction and synthesis
All data were abstracted by one investigator, with a second investigator verifying data entries. The following data were abstracted from each study: first author’s last name, year of publication, sample size, study design, characteristics of the study population, key components of the intervention and duration of follow-up.
Study outcomes were extracted and summarized qualitatively. Outcomes of interest were all-cause and cardiovascular-related hospital readmissions and emergency room (ER) visits. Mortality, CAD secondary prevention measures (e.g., low density lipoprotein [LDL] levels) and medication adherence were also assessed. Outcomes were only included if they were collected within one year of the index hospitalization and were compared among the study intervention and control groups.
The internal validity of each study was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool for cohort studies and the Cochrane Collaboration revised tool to assess risk of bias in randomized trials (RoB-2) for randomized controlled trials (RCTs).19–20 The following domains were assessed using the ROBINS-I tool: confounding, selection, intervention classification, deviation from intervention, missing data, measurement of outcome, and selection of reported results.20 Risk of bias could be deemed low, moderate, serious, or critical. For the Cochrane Collaboration RoB-2 tool, the following domains were assessed: randomization process, deviation from intended intervention, missing outcome data, measurement of the outcome, and selection of the reported results.19 Risk of bias could be deemed low, some concerns, or high.
RESULTS
Of the 1,612 unique citations identified through searching, 1,494 were excluded after title and abstract screening (Figure 1). A total of 107 articles were excluded after full text screening, with the reasons for exclusion displayed in Figure 1. This left 11 articles evaluating pharmacist-driven interventions aimed at impacting ToC among patients with CAD for inclusion in this systematic review (Table 1).6–16
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
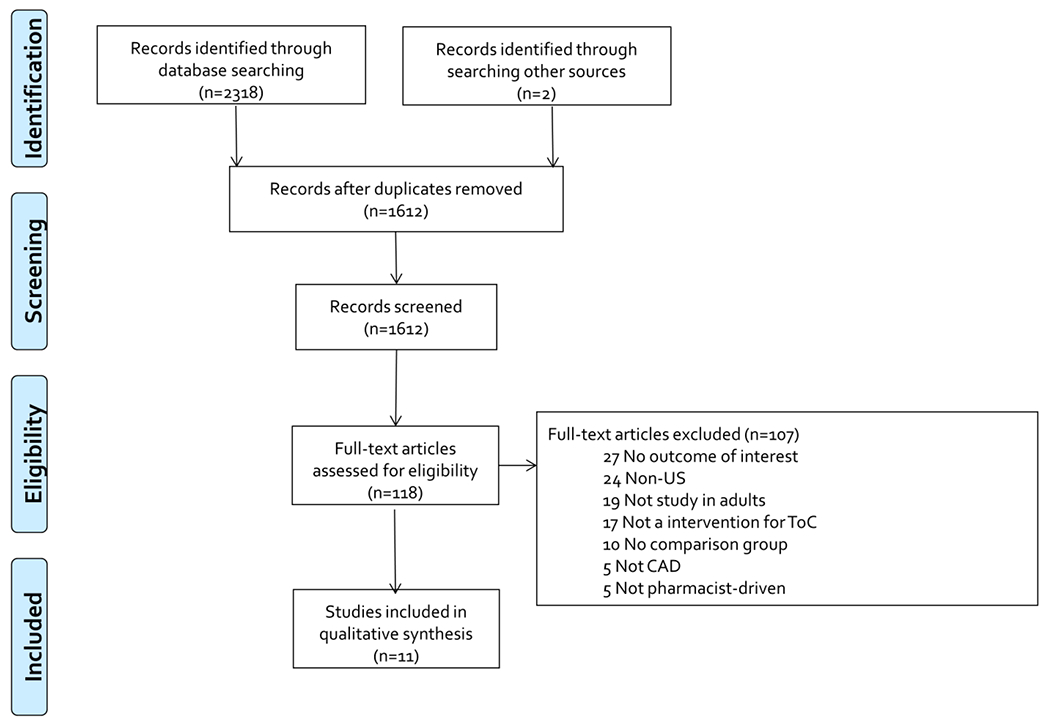
CAD=coronary artery disease; ToC= transitions of care
Table 1.
Characteristics of included studies evaluating pharmacist-driven interventions
| Study N= | Study design | Timing of Study | Study population | Age (y) [mean (SD)] | Male | Key Components of Intervention |
|---|---|---|---|---|---|---|
|
Murphy 2019 N=251 |
Prospective, single-center, comparison to historical institution data | 8/2013-6/2015 | AMI | 64 (12) | 54% | Inpatient • High-touch patient education by pharmacy, dietician and providers • Discharge medication reconciliation • Outpatient cardiology visit scheduled in week 1 Outpatient • High-touch service including telephone and in-person follow up from provider, dietician and pharmacist (to assess understanding of and adherence to medications) in week 1 and 3 • Post-discharge pharmacist-led MTM appointment in week 1-2, with optional appointment in week 4 |
|
Dempsey 2018 N=4a |
Prospective, single-center, comparison to historical institution data | 9/2015-3/2016 | ACS, ≥65 years | 73 (10) | 64% | Inpatient and outpatient • Pharmacist provided education • Medication history, reconciliation and review • Medication bedside delivery • TOC coaching to encourage patients to take active role in process • Patient Activation Assessment assessing patient self-management • Distribution of adherence tools (e.g., pillboxes) • Review of insurance and cost-saving opportunities • Telephone follow-up 48-72 hours |
|
Gorman 2018 N=300 |
Retrospective, single-center, comparison to a matched, historical cohort | 7/2016-12/2016 | ACS | 63 (13) | 71% | Inpatient • Medication history and reconciliation • Assess insurance, obtain prior authorization, and individualize P2Y12 agent selection at discharge • Discharge counseling Outpatient • Telephone follow-up days 3-5 for counseling-related interventions including: ▪ Scheduling follow-up medical visits ▪ Medicine adherence assessment ▪ Side effect management ▪ OTC recommendations ▪ Smoking cessation counseling |
|
Budiman 2016 N=135 |
Prospective, single-center, comparison to historical institution data | 1/2015-4/2015 | STEMI with cardiac stents | 67 (13) | 78% | Inpatient • Reconciliation of prior-to-admission medication list • Assess medication adherence and literacy • Customized discharge medication list • Medication education • Facilitate delivery of discharge medication by resolving patient’s insurance issues before discharge • Discharge counseling on lifestyle management including diet, exercise, and smoking cessation Outpatient • Telephone follow-up 48-72 hours and 30 days |
|
Gasbarro 2015 N=548 |
Prospective, single-center, comparison to historical institution data | 11/2013 - 1/2014 | AMI | 62 (14) | 66% |
Inpatient • Medication education • Medication reconciliation • Medication cost optimization • Laboratory monitoring • Discharge counseling on exercise, moderation of alcohol intake, smoking cessation Outpatient • Telephone follow-up 48 hours |
|
Anderegg 2014 N = 71 |
Prospective, single-center, comparison to historical institution data | 10/2012 - 12/2012 | AMI | 54 (16) | 50% | Inpatient • Admission medication history and reconciliation • Discharge medication reconciliation with pended orders for provider review • Discharge education/counseling and take-home medication calendars |
|
Ho 2014 N=241 |
Multicenter RCT, allocated to intervention or usual care | 7/1/10-3/31/13 | Veterans with ACS | 64 (9) | 98% | Inpatient • Patient education on medications at hospital discharge Outpatient • Medication history, reconciliation, and education by a pharmacist 7-10 days after discharge either in person or over the phone • Medication history, reconciliation, and education by a pharmacist at one month after discharge • Distribution of adherence tools (e.g., pillboxes) • Pharmacist informed PCP or cardiologist about patient treatment to allow for communication • A voice messaging system contacted patients at regularly scheduled intervals about medication reminders and medication refills |
|
Calvert 2012 N=143 |
Multicenter RCT, allocated to intervention or usual care | 7/5/06-7/2/09 | ACS, coronary lesion ≥50%, prior angioplasty, stent or CABG discharged on a statin, beta-blocker and antiplatelet agent |
Median (IQR) 63 (54-71) | 66% | Inpatient • Pharmacist counseled on the importance of medication adherence and the purpose of each medication and addressed barriers to adherence. • The following were provided: a pocket medication card, a list of tips for remembering to take medications, and a pillbox • Before discharge, pharmacist called community pharmacy that patient fills at to discuss identified medication adherence barriers Outpatient • At discharge, patient’s discharge medication regimen, barriers to medication adherence, and contact information of the PCP were faxed to the patient’s pharmacy • Letter faxed to patient’s PCP about enrollment into medication adherence intervention • Pharmacist called patients 1-2 weeks after discharge to confirm discharge medications were filled • Community pharmacists were reminded to verify the intervention patient’s adherence to triple therapy immediately after discharge and at 6, 12, 18, and 24 weeks post discharge and to contact physician about adherence as necessary • Patients were contacted by a pharmacist at 6 months after hospital discharge to assess current medications, pharmacy(s) used, and patient satisfaction |
|
Ma 2010 N=689 |
Single-center RCT, allocated to intervention or usual care | 9/2000-8/2005 | Coronary lesion ≥50% | 60 (11) | 60% | Inpatient • Initial contact with pharmacist to establish relationship and provide discharge counseling and medication card • Pharmacist provided an educational packet, a dietary goal booklet, and a pillbox Outpatient • Computer-based tracking system to facilitate follow-up • Pharmacist-delivered telephone counseling to improve adherence at 2 weeks and 1, 3, 6, and 9 months • Pharmacist helped facilitate scheduling of lab work and provided prompts to patient and providers for LDL-C management |
|
Hilleman 2001 N=612 |
Prospective, single-center, comparison to historical institution data | 1/1999-3/1999 | AMI, CABG/ PCI or coronary lesion >70% without a history of hypercholesterolemia | 67 (10) | 66% | Inpatient • Pharmacist tracked patients Outpatient • Pharmacist sent letters and made phone calls to PCP treating patients who were recently discharged from the hospital • Letter or phone call made 2 weeks after discharge for 2 years • Review of lipid profile and recommendations of statin therapy if appropriate |
|
Faulkner 2000 N=30 |
Single-center RCT, allocated to intervention or usual care | NR | CABG and/or PTCA | 64 (12) | 53% | Outpatient • Pharmacist called patient weekly for 12 weeks to reinforce importance of medication and assess refills, side effects, overall wellbeing, and reasons for nonadherence • Pill and packet counts at 6 and 12 weeks and phone calls to pharmacies to assess adherence |
N represents patients initially admitted for ACS in the intervention group only.
AMI= Acute myocardial infarction; CAD=coronary artery disease; CABG= coronary artery bypass graft; ED= emergency department; IQR=interquartile range; MTM= medication therapy management; PCI=percutaneous coronary intervention; PTCA=percutaneous transluminal coronary angioplasty; RCT=randomized controlled trial; STEMI= ST-segment elevation myocardial infarction; TOC= transitions of care
A total of 3,024 patients were included in the 11 studies, with the average age of patients ranging from 54 to 73 years and most were male (range 50-98%; Table 1). Seven studies restricted the population to those with ACS events, while the remaining studies also identified hospitalized patients for inclusion based on the presence of coronary lesions and/or coronary revascularization procedures. Six studies were prospective single-center evaluations comparing data on pharmacist-driven interventions to historical data; while four studies were RCTs and one study utilized a retrospective design.
Key components of the pharmacist-driven ToC interventions can be found in Table 1. Common components of the interventions included pharmacist-provided medication education and counseling (n=10), discharge medication reconciliation (n=8) and post-discharge follow-up phone calls or visits (n=10). Ten interventions specifically assessed and/or addressed barriers to medication adherence through initiatives such as education, assessing medication cost, or providing adherence tools such as pillboxes. The average amount of time spent on the intervention was reported in 3 studies and ranged from 12 to 22 minutes.
Upon validity assessment, studies met the evaluated criteria or were deemed to have low risk of bias across most domains (Supplementary Tables 2 and 3). For the seven cohort studies assessed using the ROBINS-I tool, risk of bias was deemed to be low in all studies in the following domains: intervention classification, deviation from intervention, measurement of outcome, and selection of reported results (Supplementary Table 2). Risk of bias for all studies was rated as high in the domain assessing selection because the studies selected historical rather than concurrent controls. Only one study was deemed to have low risk of bias when confounding was assessed, as this was the only study that attempted to match those receiving the intervention to historical controls based on patient characteristics. As many studies were conducted at single-centers and did not have access to data outside of their institution, most were rated as high risk of bias for the domain assessing missing data. All RCTs evaluated using the Cochrane Collaboration RoB2 tool were deemed to have low risk of bias for the randomization process and selection of the reported results (Supplementary Table 2). The domain assessing deviation from the intended intervention was rated as high risk of bias for all studies, because of concerns that arose due to lack of participant and personnel blinding. Similarly, two of the four studies did not blind outcome assessment, which resulted in concerns that prompted high risk of bias ratings in the domain assessing measurements of outcomes. Lastly, two studies were deemed to have high risk of bias due to missing outcome data.
Reencounter and mortality outcomes among those receiving pharmacist-driven interventions versus control groups are displayed in Table 2. Six studies evaluated all-cause hospital readmissions or ER visits and these events were numerically lower among patients receiving pharmacist-driven interventions in all but one study. Cardiovascular-related encounters were evaluated in three studies and were numerically lower among patients receiving pharmacist interventions in all studies. Mortality was numerically lower among those receiving the pharmacist intervention in two studies but slightly higher in a third study. Only one study reported statistically significant differences between the groups for the aforementioned outcomes, with significant decreases in 90-day hospital readmission and 30- and 90-day cardiovascular-related readmission observed in those receiving the pharmacist interventions.
Table 2.
Reencounter and mortality outcomes across included studies evaluating pharmacist-driven interventions
| Study | Outcome | Timing of Outcome Measurement | Results (% Intervention vs % Control) |
|---|---|---|---|
| All-cause encounters | |||
| Murphy 2019 | Hospital Readmission | 30 d | 17.2% vs 11.4%, p=0.25 |
| Murphy 2019 | ER Visit | 72 hr | 4.3% vs 4.3%, p>0.99 |
| Dempsey 2018 | Hospital Readmission | 30 d | 0% vs 9%, p=NR |
| Gorman 2018 | Hospital Readmission | 30 d | 9.3% vs 14.7%, p=0.16 |
| Gorman 2018 | Hospital Readmission | 90 d | 13.3% vs 24.7%, p=0.01 |
| Budiman 2016 | Hospital Readmission | 30 d | 5% vs 13%, p=0.18 |
| Gasbarro 2015 | Hospital Readmission | 30 d | 6% vs 11.6%, p=NR |
| Anderegg 2014 | Hospital Readmission or ER Visit | 30 d | 7.3% vs 23.3%, p=0.12 |
| CV-related encounters | |||
| Gorman 2018 | Any CV-related Hospital Readmission | 30 d | 4.7% vs 11.3%, p=0.03 |
| Gorman 2018 | Any CV-related Hospital Readmission | 90 d | 8% vs 16.7%, p=0.02 |
| Budiman 2016 | Readmission with PCI | 30 d | 0% vs 3.2%, p=NR |
| Budiman 2016 | Readmission due to In-stent thrombosis | 30 d | 0% vs 3.2%, p=NR |
| Budiman 2016 | Readmission due to Restenosis | 30 d | 0% vs 2.1%, p=NR |
| Ho 2014 | Revascularization | 1 yr | 11.5% vs 17.6%, p=0.24 |
| Ho 2014 | Readmission due to MI | 1 yr | 6.6% vs 4.2%, p=0.60 |
| Mortality | |||
| Murphy 2019 | All-cause | 30 d | 1.1% vs 2.5%, p=0.66 |
| Budiman 2016 | All-causea | 30 d | 0% vs 8%, p=NR |
| Ho 2014 | All-cause | 1 yr | 9% vs 7.6%, p=0.86 |
Only captured during hospital reencounters
CV=cardiovascular; ER=emergency room; MI=myocardial infarction; PCI=percutaneous coronary intervention; NR=not reported
Four studies compared secondary prevention measures (i.e., the proportion of patients with an LDL<100mg/dL or meeting blood pressure goals) in patients receiving pharmacist-driven interventions versus control groups (Table 3). The measures tended to be more favorable in those receiving pharmacist-driven interventions, with significant differences observed in two studies.
Table 3.
Secondary prevention measures across included studies evaluating pharmacist-driven interventions
| Study | Timing of Measurement | Results (% Intervention versus % Control) |
|---|---|---|
| LDL-C <100 mg/dl | ||
| Ho 2014 | 1 yr | 72% vs 83%, p=0.14 |
| Ma 2010 | 1 yr | 65% vs 60%, p=0.29 |
| Hilleman 2001 | 6 wk | 39% vs. 17%, p<0.05 |
| Hilleman 2001 | 12 wk | 51% vs 17%, p<0.05 |
| Hilleman 2001 | 24 wk | 52% vs 18%, p<0.05 |
| Hilleman 2001 | 1 yr | 54% vs 18%, p<0.05 |
| Faulkner 2000 | 12 wk | 67% vs. 67%, p=NR |
| Faulkner 2000 | 1 yr | 53% vs. 13%, p<0.05 |
| Blood Pressure Goal a | ||
| Ho 2014 | 1 yr | 59% vs 49%, p=0.23 |
<140/90mmHg or <130/80 mmHg for patients with diabetes/chronic kidney disease
Medication adherence was compared in four studies (Table 4). Adherence tended to be higher in patients receiving pharmacist-driven interventions when compared to control groups and differences were significant in 8 of the 19 comparisons made.
Table 4.
Medication adherence across included studies evaluating pharmacist-driven interventions
| Study | Adherence Measurement | Timing of Measurement | Results (Intervention versus Control) |
|---|---|---|---|
| Ho 2014 | Percent of patients with a PDC >80% based on prescription claims data | 1 yr | Statin: 93.2% vs 71.3%, p<0.001 ACEI/ARB: 93.1% vs 81.7%, p =0.03 Clopidogrel: 86.8% vs 70.7%, p=0.03 Beta-blocker: 88.1% vs 84.8%, p= 0.59 Overall (Cardiac Medications): 89.3% vs 73.9%, p=0.003 |
| Calvert 2012 | Percent of patients with a PDC >75% based on prescription claims data | 6 mo | Beta-blocker: 71% vs 49%, p=0.03 Statin: 58% vs 49%, p=0.34 Overall (Statin/Beta-blocker): 53% vs 38%, p=0.11 |
| Calvert 2012 | Percent of patients reporting taking medications as prescribed | 6 mo | Aspirin: 100% vs 100%, p=NR Beta-blocker: 90% vs 96%, p=0.50 Statin: 98% vs 98%, p=0.99 Overall (Aspirin/Statin/Beta-blocker): 91% vs 94%, p=0.50 |
| Calvert 2012 | Median score on a 4-item Morisky Adherence Scale using a 5-item Likert scale | 6 mo | 19 vs 19, P = 0.40 |
| Ma 2010 | Mean (SD) Continuous multiple-intervala | 1 yr | Statin: 0.88 (0.3) vs 0.9 (0.3), p=0.51 Beta-blocker: 0.97 (1.1) vs 0.91 (0.4), p=0.56 ACEI: 1.02 (0.8) vs 0.93 (0.3), p=0.25 |
| Faulkner 2000 | Average proportion of doses taken based on pill count | 6 wk | Statin: 92% vs 89%, p>0.05 |
| Faulkner 2000 | Average proportion of doses taken based on pill count | 12 wk | Statin: 88% vs 86%, p>0.05 |
| Faulkner 2000 | Percent of patients filling 80% or more of their prescriptions based on prescription refill records | 1 yr | Statin: 67% vs 33%, p <0.05 |
Ratio of days’ supply obtained to total days between refill records
ACEI= Angiotensin-converting-enzyme inhibitors; PDC=proportion of days covered; SD=standard deviation
DISCUSSION
This systematic review included 11 studies evaluating the impact of pharmacist-driven ToC interventions among adults with CAD. All interventions were multifaceted, with most including pharmacist-provided medication education and counseling, discharge medication reconciliation, and post-discharge follow-up phone calls or visits. Re-encounter, mortality, CAD secondary prevention, and medication adherence outcomes tended to be more favorable in those receiving the pharmacist-driven interventions.
Several important gaps exist in the available literature on pharmacist-driven ToC interventions in the CAD population. None of the included studies in this systematic review reported the impact of their interventions on medication errors. A meta-analysis of pharmacist ToC interventions demonstrated that pharmacist-driven interventions reduce medication errors by at least 37%21; however, this meta-analysis included studies performed in various clinical settings and patient populations. Individual studies that included patients with CAD or ACS and other diagnoses, such as chronic obstructive pulmonary disease and heart failure, exhibited inconsistent impacts of pharmacist ToC interventions on medication errors.22–24 Additionally, most of the studies included in this systematic review were underpowered to detect significant differences in important outcomes like mortality and readmissions or did not report these outcomes. Sufficiently powered studies with rigorous designs are needed to evaluate the impact of pharmacist-driven ToC interventions on medication errors, hospital readmissions, and mortality in the CAD population.
Studies have demonstrated the impact of pharmacist-driven interventions on outcomes following ToC in other medical conditions. The most studied cardiovascular condition is heart failure, where improvement in important clinical outcomes, such as readmission25–29 and all-cause mortality,25,28,29 have been demonstrated. Additionally, improved medication adherence28,29 and use of guideline concordant treatment with beta-blockers and angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARB) have also been established with these pharmacist-driven interventions in patients with heart failure.25,26 Like patients with heart failure, patients with CAD are at high-risk of poor outcomes following hospitalization if coordination is suboptimal as they transition between care settings. Coordination related to medication therapy is especially important, as improper discontinuation of medication therapy in these individuals increases mortality.30 For instance, in a study of 1,521 US patients discharged on beta-blockers, statins and aspirin following a myocardial infarction, discontinuation of these medications at one month was associated with over a three-fold increase in one-year mortality (hazard ratio=3.81; 95% confidence interval=1.88-7.72).30 It is therefore likely that individuals with CAD benefit from coordination of and education about medication therapy performed by pharmacists and other members of the healthcare team following hospital discharge.
The main limitation of our systematic review was the inability to perform a meta-analysis and thus quantitatively combine the results of included studies due to a high amount of heterogeneity across studies. Important differences existed in the design of included studies. Both cohort studies utilizing historic controls and RCTs that randomly allocated individuals to intervention or control groups were identified for inclusion. Even among the cohort studies, notable differences in study design existed. For example, one study was retrospective and utilized matching, while the others had a prospective component and did not attempt to control for differences between groups. Additionally, studies differed in their timing of outcome assessment. For instance, LDL cholesterol was measured anywhere from 6 weeks to 12 months following the index hospital encounter. Lastly, differences existed in how outcomes were defined and captured. For instance, some studies used proportion of days covered from prescription claims data to measure medication adherence, while others relied on patient reported adherence data. Moreover, cardiovascular-related readmissions were defined as any cardiovascular-related readmission in some studies, while others only captured readmissions where revascularization was utilized.
CONCLUSIONS
We identified 11 multifaceted, pharmacist-driven interventions aimed at improving outcomes in ToC for patients with CAD. Outcomes assessing re-encounters, mortality, CAD secondary prevention measures, and medication adherence tended to be more favorable in those receiving the pharmacist-driven interventions. However, most studies were underpowered to detect significant differences in outcomes. Sufficiently powered studies with more rigorous designs are needed to adequately evaluate the impact of pharmacist-driven ToC interventions on medication errors, hospital readmissions, and mortality in the CAD population.
Supplementary Material
Acknowledgements
The authors would like to thank Emily Brennan, MLIS for developing the search strategy with the study team and entering citations into the online systematic review management tool utilized in this study.
Funding:
This systematic review was performed related to a quality improvement project funded by the VA National Center for Patient Safety. Dr. Weeda received funding from the VA VISN-7 Research and Development Award.
Footnotes
Conflicts of interest: The authors report no potential conflicts of interest relevant to this article. This article represents the views of the authors and not those of the Medical University of South Carolina or Veterans Health Administration.
REFERENCES
- 1.Transitions of Care: The Need for a More Effective Approach to Continuing Patient Care. The Joint Commission, 2012, www.jointcommission.org/-/media/deprecated-unorganized/imported-assets/tjc/system-folders/topics-library/hot_topics_transitions_of_carepdf.pdf?db=web&hash=CEFB254D5EC36E4FFE30ABB20A5550E0. Accessed 10 Aug. 2020. [Google Scholar]
- 2.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving Health Reform. Health Affairs 2011;30:746–754. [DOI] [PubMed] [Google Scholar]
- 3.Community-Based Care Transitions Program. Centers for Medicare and Medicaid Services, 2012, https://innovation.cms.gov/innovation-models/cctp. Accessed 10 Aug. 2020. [Google Scholar]
- 4.Readmission Measures Overview. Centers for Medicare and Medicaid Services, https://www.qualitynet.org/inpatient/measures/readmission. Accessed 10 Aug. 2020. [Google Scholar]
- 5.Khera R, Wang Y, Nasir K, Lin Z, Krumholz HM. Evaluation of 30-Day Hospital Readmission and Mortality Rates Using Regression-Discontinuity Framework. J Am Coll Cardiol. 2019;74:219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderegg SV, Wilkinson ST, Couldry RJ, Grauer DW, Howser E. Effects of a hospitalwide pharmacy practice model change on readmission and return to emergency department rates. Am J Health Syst Pharm. 2014;71:1469–79. [DOI] [PubMed] [Google Scholar]
- 7.Budiman T, Snodgrass K, Komatsu chang A. Evaluation of Pharmacist Medication Education and Post-discharge Follow-up in Reducing Readmissions in Patients With ST-Segment Elevation Myocardial Infarction (STEMI). Ann Pharmacother. 2016;50:118–24. [DOI] [PubMed] [Google Scholar]
- 8.Calvert SB, Kramer JM, Anstrom KJ, Kaltenbach LA, Stafford JA, Allen lapointe NM. Patient-focused intervention to improve long-term adherence to evidence-based medications: a randomized trial. Am Heart J. 2012;163:657–65.e1. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey J, Gillis C, Sibicky S, et al. Evaluation of a transitional care pharmacist intervention in a high-risk cardiovascular patient population. Am J Health Syst Pharm. 2018;75:S63–S71. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner MA, Wadibia EC, Lucas BD, Hilleman DE. Impact of pharmacy counseling on compliance and effectiveness of combination lipid-lowering therapy in patients undergoing coronary artery revascularization: a randomized, controlled trial. Pharmacotherapy. 2000;20:410–6. [DOI] [PubMed] [Google Scholar]
- 11.Gasbarro NM, Eginger KH, Street C. Impact of clinical pharmacist interventions on 30-day readmission rate in hospitalized patients with acute myocardial infarction. J Pharm Tech 2015:31;64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorman EM, Brown GW, Costello JN, Woodruff AE. Impact of a pharmacist‐driven transition of care program for patients with acute coronary syndromes. Journal of the American College of Clinical Pharmacy. 2018;1:74–80. [Google Scholar]
- 13.Hilleman DE, Monaghan MS, Ashby CL, Mashni JE, Woolley K, Amato CM. Physician-prompting statin therapy intervention improves outcomes in patients with coronary heart disease. Pharmacotherapy. 2001;21:1415–21. [DOI] [PubMed] [Google Scholar]
- 14.Ho PM, Lambert-kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2014;174:186–93. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Ockene IS, Rosal MC, Merriam PA, Ockene JK, Gandhi PJ. Randomized trial of a pharmacist-delivered intervention for improving lipid-lowering medication adherence among patients with coronary heart disease. Cholesterol. 2010;383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy JA, Schroeder MN, Rarus RE, Yakubu I, Mckee SOP, Martin SJ. Implementation of a Cardiac Transitions of Care Pilot Program: A Prospective Study of Inpatient and Outpatient Clinical Pharmacy Services for Patients With Heart Failure Exacerbation or Acute Myocardial Infarction. J Pharm Pract. 2019;32:68–76. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org [Google Scholar]
- 19.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Oliveira GS, Castro-Alves LJ, Kendall MC, McCarthy R. Effectiveness of pharmacist intervention to reduce medication errors and health-care resources utilization after transitions of care: a meta-analysis of randomized controlled trials. J Patient Saf 2017;00:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Hawes EM, Maxwell WD, White SF, Mangun J, Lin FC. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J Prim Care Community Health 2014;5:14–18. [DOI] [PubMed] [Google Scholar]
- 23.Farris KB, Carter BL, Xu Y, et al. Effect of a care transition intervention by pharmacists: an RCT. BMC Health Serv Res 2014;14:406. doi: 10.1186/1472-6963-14-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012;157:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart Failure Transitions of Care: A Pharmacist-Led Post-Discharge Pilot Experience. Prog Cardiovasc Dis. 2017;60:249–258. [DOI] [PubMed] [Google Scholar]
- 26.Neu R, Leonard MA, Dehoorne ML, Scalia SJ, Kale-Pradhan PB, Giuliano CA. Impact of Pharmacist Involvement in Heart Failure Transition of Care. Ann Pharmacother. 2020;54:239–246. [DOI] [PubMed] [Google Scholar]
- 27.McKay C, Park C, Chang J, et al. Systematic Review and Meta-analysis of Pharmacist-Led Transitions of Care Services on the 30-Day All-Cause Readmission Rate of Patients with Congestive Heart Failure. Clin Drug Investig. 2019;39:703–712. [DOI] [PubMed] [Google Scholar]
- 28.Jackevicius CA, de Leon NK, Lu L, Chang DS, Warner AL, Mody FV. Impact of a multidisciplinary heart failure post-hospitalization program on heart failure readmission rates. Ann Pharmacother. 2015;49:1189–1196. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Jackevicius CA, de Leon NK, Warner AL, Chang DS, Mody FV. Impact of a multidisciplinary heart failure postdischarge management clinic on medication adherence. Clin Ther. 2017;39:1200–1209. [DOI] [PubMed] [Google Scholar]
- 30.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


