Abstract
Background
Pneumococcal conjugate vaccines (PCVs) have significantly reduced pneumococcal disease, but disease from non-PCV serotypes remains. The safety, tolerability, and immunogenicity of a 20-valent PCV (PCV20) were evaluated.
Methods
This pivotal phase 3, randomized, double-blind study enrolled adults into 3 age groups (≥60, 50–59, and 18–49 years) at US and Swedish sites. Participants were randomized to receive 1 PCV20 or 13-valent PCV (PCV13) dose. After 1 month, participants aged ≥60 years also received 1 dose of saline or 23-valent polysaccharide vaccine (PPSV23). Safety assessments included local reactions, systemic events, adverse events, serious adverse events, and newly diagnosed chronic medical conditions. Opsonophagocytic activity geometric mean titers 1 month after PCV20 were compared with 13 matched serotypes after PCV13 and 7 additional serotypes after PPSV23 in participants aged ≥60 years; noninferiority was declared if the lower bound of the 2-sided 95% confidence interval for the opsonophagocytic activity geometric mean titer ratio (ratio of PCV20/saline to PCV13/PPSV23 group) was >0.5. PCV20-elicited immune responses in younger participants were also bridged to those in 60–64-year-olds.
Results
The severity and frequency of prompted local reactions and systemic events were similar after PCV20 or PCV13; no safety concerns were identified. Primary immunogenicity objectives were met, with immune responses after PCV20 noninferior to 13 matched serotypes after PCV13 and to 6 additional PPSV23 serotypes in participants aged ≥60 years; serotype 8 missed the statistical noninferiority criterion. PCV20 induced robust responses to all 20 vaccine serotypes across age groups.
Conclusions
PCV20 was safe and well tolerated, with immunogenicity comparable to that of PCV13 or PPSV23. PCV20 is anticipated to expand protection against pneumococcal disease in adults.
Clinical Trials Registration
Keywords: clinical trial, pneumococcal conjugate vaccine, Streptococcus pneumoniae
The 20-valent pneumococcal conjugate vaccine (PCV20) was safe and well tolerated, with immunogenicity comparable to that of the 13-valent pneumococcal conjugate vaccine or 23-valent polysaccharide vaccine. PCV20 is anticipated to expand protection against pneumococcal disease in adults.
Streptococcus pneumoniae is a significant public health concern worldwide, causing invasive pneumococcal disease (IPD; eg, bacteremia, bacterial meningitis, and bacteremic pneumonia) and noninvasive disease (eg, sinusitis and nonbacteremic pneumonia) [1–3]. In 2016, S. pneumoniae was the most common cause of lower respiratory tract infection across 195 countries, associated with approximately 1.2 million deaths [4]. In adults, pneumococci were among predominant bacterial pathogens of community-acquired pneumonia requiring hospitalization in 2010–2012 [5]; individuals with certain comorbid conditions and immunocompromising medical conditions are at increased risk of disease [4]. Older adults are particularly susceptible, with infection primarily manifesting as pneumonia (with or without associated bacteremia) and with approximately 693 000 deaths globally in 2015 among individuals aged ≥70 years [1–4].
The majority of pneumococcal disease is caused by a limited subset of the >95 identified serotypes [1, 6]; vaccines targeting the capsular polysaccharides of prevalent serotypes are used to prevent disease [1]. Unconjugated polysaccharide vaccines, such as the 23-valent polysaccharide vaccine (PPSV23), elicit T-cell–independent responses that do not induce long-lasting immunity [7]. Consensus regarding PPSV23 effectiveness against nonbacteremic pneumonia, particularly among the elderly, is lacking owing to conflicting findings [8]. In contrast, pneumococcal conjugate vaccines (PCVs) induce robust T-cell–dependent responses associated with long-lasting immunity and are effective against vaccine-type IPD and nonbacteremic pneumococcal pneumonia in older adults [7, 9–12].
Introduction of PCVs into immunization programs worldwide has significantly reduced pneumococcal disease burden [13]. In the United States, 7-valent PCV (PCV7) was introduced in 2000 and reduced IPD burden by 76% for young children (aged <5 years) and by 45% for all ages [14]. The 13-valent PCV (PCV13) expanded coverage by 6 serotypes, replacing PCV7 and further reducing pneumococcal disease burden among young children and adults [15]. However, certain non-PCV13 serotypes persistently cause a substantial proportion of global disease [16–19]. Thus, expanding PCV coverage to include these additional serotypes is essential toward further curtailing pneumococcal disease [13, 16, 19].
The 20-valent PCV (PCV20) contains PCV13 components and polysaccharide conjugates for 7 additional serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F), selected based on their relative prevalence for causing pneumococcal disease, generalized geographic distribution, and associations with antibiotic resistance, invasive potential, or disease severity [16, 20–26]. Among US adults (aged 19–64 years) and older adults (aged ≥65 years), these 7 additional serotypes caused 32% and 28% of IPD cases, respectively, in 2017–2018 [19], and they create considerable economic burden [27].
In a phase 1 study in healthy adults (aged 18–49 years) [25] and a phase 2 study in older adults (aged 60–64 years) [26], PCV20 elicited robust immune responses to 20 vaccine serotypes with a safety profile consistent with other PCVs. Phase 2 study findings showing potential for PCV20 to provide substantial improvement over available vaccines and address an unmet medical need for serious diseases led to breakthrough therapy designation in ≥18-year-olds from the US Food and Drug Administration [28]. This trial and 2 additional phase 3 trials evaluated PCV20 safety and immunogenicity in adults supporting the US approval for ≥18-year-olds in June 2021 [29]. We present results from the pivotal phase 3 study evaluating PCV20 safety, tolerability, and immunogenicity in adults aged ≥18 years, including noninferiority assessments of PCV20 immune responses to specific corresponding serotypes within PCV13 and PPSV23.
METHODS
Study Design and Participants
This phase 3, randomized, active-controlled, double-blind, multicenter study was conducted between December 2018 and December 2019 in the United States and Sweden (NCT03760146; Figure 1). The study population comprised ≥18-year-olds in 3 cohorts based on age at enrollment (≥60, 50–59, and 18–49 years). Additional details are in the Supplementary Appendix and the study protocol, available at ClinicalTrials.gov. Participants were to be generally healthy or with a stable underlying disease. Key exclusion criteria included previous vaccination with any pneumococcal vaccine, diagnosis of a serious unstable chronic disorder or immunocompromising condition, or treatment with immunosuppressive therapies (detailed in Supplementary Appendix).
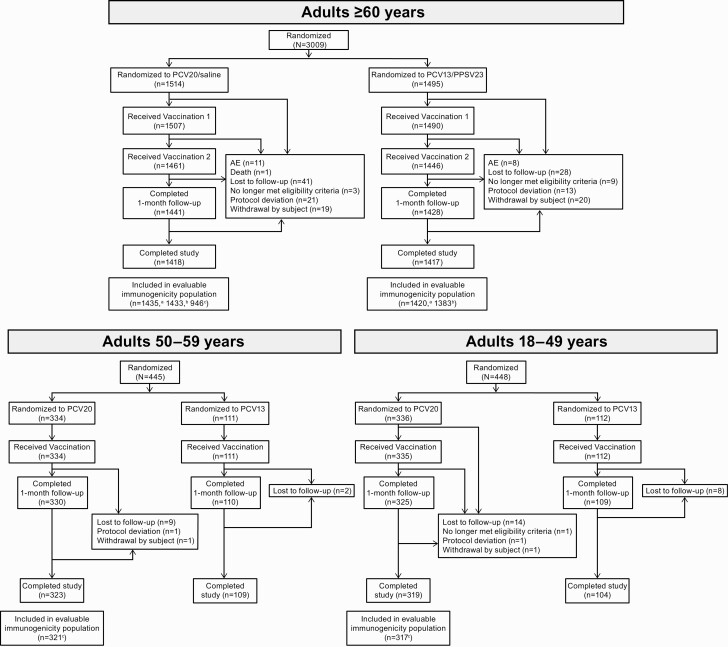
Figure 1.
Study design. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Participants aged ≥60 years were randomized 1:1 to receive a 0.5-mL dose of PCV20 or PCV13 at the first visit then a 0.5-mL dose of saline or PPSV23, respectively, 1 month later. The 1-month interval between control vaccines, while not a formally recommended schedule [30], provided a contemporaneous control for all 20 serotypes and was previously used in the phase 2 study [26]. Randomization was stratified by age subgroup; approximately one-third of participants aged ≥60 years were planned to be aged ≥65 years. Participants aged 18–49 or 50–59 years were randomized 3:1 to receive a 0.5-mL dose of PCV20 or PCV13 (Figure 1). The study was conducted in accordance with local ethical principles and guidelines (see Supplementary Appendix).
Objectives and End Points
The primary safety objective was to describe PCV20 safety in adults aged ≥18 years. End points included percentage of participants with prompted local reactions and systemic events within 10 and 7 days after PCV20, respectively, adverse events (AEs) within 1 month after PCV20, and serious AEs (SAEs) and newly diagnosed chronic medical conditions within 6 months after PCV20 (see Supplementary Appendix).
Primary immunogenicity objectives were to show noninferiority of PCV20-elicited immune responses to the corresponding 13 matched serotypes after PCV13 and 7 additional serotypes after PPSV23, based on serotype-specific opsonophagocytic (OPA) geometric mean titers (GMTs) 1 month after vaccination in ≥60-year-old participants. Demonstration of comparable immune responses served as an immunologic bridge, with further support based on the known efficacy and mechanism of action of PCV13 against IPD and pneumonia to infer PCV20 effectiveness [31].
Secondary objectives included noninferiority evaluations of OPA GMTs 1 month after vaccination with PCV20 in participants aged 18–49 or 50–59 years, compared with GMTs in those aged 60–64 years. Additional secondary objectives were to describe serotype-specific immune responses to PCV20 using geometric mean fold rises (GMFRs) in OPA titers from before to 1 month after vaccination, the percentage of participants with a ≥4-fold rise in OPA titers from before to 1 month after vaccination, and the percentage with OPA titers greater than or equal to the lower limit of quantitation (LLOQ) 1 month after vaccination.
Blood samples were collected from all participants before (baseline) and 1 month after vaccination with PCV20 or PCV13. For participants aged ≥60 years, blood samples were also collected 1 month after saline or PPSV23 administration.
Statistical Analysis
Population Size
Based on assumptions supported by prior studies [26, 31], 2700 evaluable participants aged ≥60 years (1350 per vaccine group) were planned, to provide 96% power to demonstrate noninferiority for OPA GMTs for ≥19 of 20 serotypes and 72% power for all 20 serotypes for the primary immunogenicity objectives. For secondary bridging objectives (ie, bridging to responses in 60–64-year-olds), the planned sample size of approximately 300 evaluable participants in the PCV20 group of each younger cohort provided >90% probability of showing noninferiority for all 20 serotypes.
Safety End Points
Safety end points were analyzed in the safety population for each cohort, which included all participants in that age group who received ≥1 vaccine dose with safety follow-up. PCV13 was the main control for safety assessments in all cohorts, although control participants aged ≥60 years also received PPSV23 1 month after PCV13.
Immunogenicity
The primary immunogenicity analysis used an evaluable immunogenicity population, which included participants without major protocol deviations enrolled into the appropriate age group, received vaccination(s) as randomized, and had ≥1 valid OPA titer from a blood sample collected within a specified window 1 month after vaccination (see Supplementary Appendix). OPA titers to 20 vaccine serotypes were measured in serum samples collected at baseline and 1 month after PCV20 or PCV13; OPA titers to the 7 additional serotypes were measured only in serum samples collected 1 month after saline or PPSV23 in participants aged ≥60 years. For participants aged ≥60 years, PCV13 served as control for immunogenicity to the 13 matched serotypes and PPSV23 as control for immunogenicity to the 7 additional serotypes. For participants aged 18–49 or 50–59 years who received PCV20, the control for immunogenicity was participants aged 60–64 years in the PCV20 group.
In participants aged ≥60 years, hypothesis testing was performed to assess the primary immunogenicity end point. Noninferiority for each serotype was evaluated by a confidence interval (CI) for the ratio of OPA GMTs (PCV20/saline group to PCV13/PPSV23 group) 1 month after vaccination, and noninferiority was declared if the lower bound of the 2-sided 95% CI was >0.5. The OPA titer geometric mean ratio (GMR) and CI for each serotype were calculated using a linear regression model that included terms for age, sex, smoking status, corresponding baseline OPA titer, and vaccine group (see Supplementary Appendix).
Hypothesis testing for the secondary end point was performed similarly to that for the primary end point. Noninferiority for each vaccine serotype was declared if the lower bound of the model-based 2-sided 95% CI for the OPA GMR (PCV20 recipients aged 18–49 or 50–59 years to PCV20 recipients aged 60–64 years) was >0.5 ( Supplementary Appendix).
OPA GMTs at baseline and 1 month after vaccination, OPA GMFRs and percentages of participants with ≥4-fold rises in OPA titer from before to 1 month after vaccination, and percentages of participants with OPA titers at or above the LLOQ were descriptively summarized for each vaccine group in the ≥60-year age group and PCV20 recipients in younger groups, with associated 2-sided 95% CIs for each vaccine serotype.
RESULTS
Participants
A total of 3009 participants aged ≥60 years were randomized; 2835 (94.2%) completed the study (Figure 2). Of 2997 participants receiving the first vaccination, 66.2% were aged 60–64, 31.4% aged 65–79, and 2.3% aged ≥80 years (Supplementary Table 1). Approximately 17% of participants aged ≥65 years were enrolled in Sweden; the remaining participants were from US sites. Of 445 and 448 participants aged 50–59 or 18–49 years, respectively, randomized to receive PCV20 or PCV13, 432 (97.1%) and 423 (94.4%) completed the study. Demographic characteristics in each age-specific cohort were relatively diverse and generally similar between vaccine groups (Supplementary Table 1).
Figure 2.
Participant disposition. aEvaluable immunogenicity population for 13 matched serotypes. bEvaluable immunogenicity population for 7 additional serotypes. cEvaluable immunogenicity population for all 20 serotypes. See Supplementary Appendix for descriptions of evaluable populations. Abbreviations: AE, adverse event; PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Immunogenicity
PCV20 Noninferiority in Participants Aged ≥60 Years
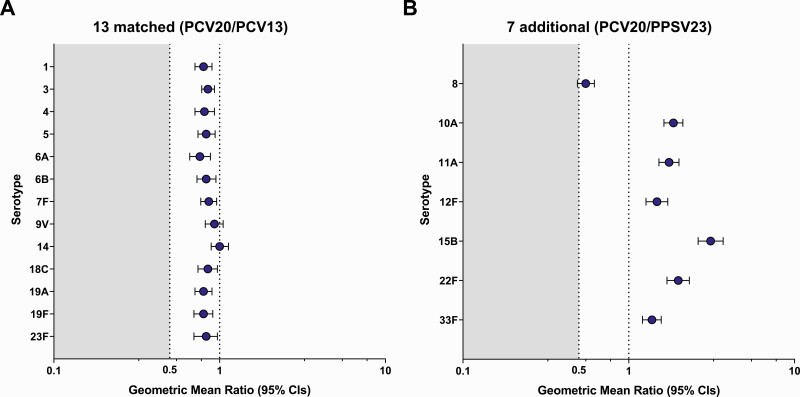
Noninferiority criteria were met for all 13 matched serotypes between PCV13 and PCV20 (Figure 3). Although OPA GMTs 1 month after vaccination were slightly lower in PCV20 than in PCV13 recipients for 13 matched serotypes, substantial increases in OPA GMTs from baseline to 1 month after vaccination were observed in both groups (Figure 4). Similar percentages of participants in PCV20 and PCV13 groups had ≥4-fold rises in OPA titers from before to 1 month after vaccination and OPA titers at or above the LLOQ 1 month after vaccination (Supplementary Figures 1 and 2).
Figure 3.
Model-based opsonophagocytic activity (OPA) geometric mean ratios (GMRs) in participants aged ≥60 years for the 13 matched serotypes (20-valent pneumococcal conjugate vaccine [PCV20]/13-valent pneumococcal conjugate vaccine [PCV13]) (A) and 7 additional serotypes (PCV20/23-valent pneumococcal polysaccharide vaccine [PPSV23]) (B) 1 month after vaccination. Noninferiority was declared if the lower bound of the 2-sided 95% confidence interval (CI) for the GMR was >0.5 (2-fold criterion). Noninferiority criteria were met for all 13 matched serotypes between PCV13 and PCV20. Assay results below the lower limit of quantitation (LLOQ) were set to 0.5 × LLOQ in the analysis. GMRs (PCV20/control) and 2-sided CIs were calculated by exponentiating the difference in least-squares means and the corresponding CIs based on analysis of log-transformed OPA titers using a regression model including terms of vaccine group, age at vaccination in years (continuous), sex, smoking status, and baseline log-transformed OPA titers. The numbers of subjects with valid and determinate OPA titers for the specified serotype varied by serotype and were 1399–1430 for PCV20 and 1390–1419 for PCV13 for the 13 matched serotypes, and 1157–1374 for PCV20 and 1201–1319 for PPSV23 for the 7 additional serotypes.
Figure 4.
Pneumococcal opsonophagocytic activity (OPA) geometric mean titers (GMTs) and geometric mean fold rises (GMFRs) in participants aged ≥60 years for the 13 matched (A) and 7 additional (B) serotypes before and 1 month after vaccination. Substantial increases in OPA GMTs from baseline to 1 month after vaccination were observed in both groups. Assay results below the lower limit of quantitation (LLOQ) were set to 0.5 × LLOQ. The numbers of subjects with valid and determinate assay results for the specified serotypes varied by serotype and were 1360–1425 for 20-valent pneumococcal conjugate vaccine (PCV20) and 1294–1418 for 13-valent pneumococcal conjugate vaccine (PCV13) for the 13 matched serotypes and 973–1353 for PCV20 and 993–1293 for 23-valent pneumococcal polysaccharide vaccine (PPSV23) for the 7 additional serotypes.
Statistical noninferiority criteria were met for 6 of 7 additional serotypes between PCV20 and PPSV23 (Figure 3). Serotype 8 did not meet noninferiority, with an OPA GMR of 0.55 (95% CI, .49–.62). OPA GMTs for 6 of 7 additional serotypes were higher in the PCV20 group 1 month after vaccination, with robust increases in OPA GMTs for all 7 additional serotypes observed from baseline to 1 month after PCV20 (Figure 4).
There is no established minimum threshold of protection for OPA; nonetheless, OPA is used as a measure of response. In addition, OPA assays are uniquely optimized for individual serotypes, and it is therefore not appropriate to directly compare across vaccine-induced OPA GMTs. However, elements of relative response (eg, from baseline) to vaccine serotypes elicited by PCV13 can provide useful context. Additional assessments of immune response to serotype 8 after PCV20 showed robust responses within the range of those observed to 13 matched serotypes after PCV13, including OPA GMFR from before to 1 month after PCV20 of 22.1 (PCV13 range, 5.7–47.3) (Figure 4), with 77.8% of participants having ≥4-fold rises in OPA titers from baseline to 1 month after PCV20 (PCV13 range, 54.0%–84.0%; Supplementary Figure 1) and 92.9% having OPA titers at or above the LLOQ 1 month after PCV20 (PCV13 range, 76.0%–96.6%; Supplementary Figure 2).
PCV20 Noninferiority Comparison Between 18–59- and 60–64-Year-Old Participants
PCV20 also met the pivotal secondary immunogenicity objective, with OPA responses in participants aged 50–59 or 18–49 years showing noninferiority compared with those aged 60–64 years for all 20 serotypes 1 month after PCV20 (Supplementary Figure 3). The observed OPA GMTs for most serotypes were higher in younger participants, particularly those aged 18–49 years compared with those aged 60–64 years.
Safety
Local Reactions and Systemic Events
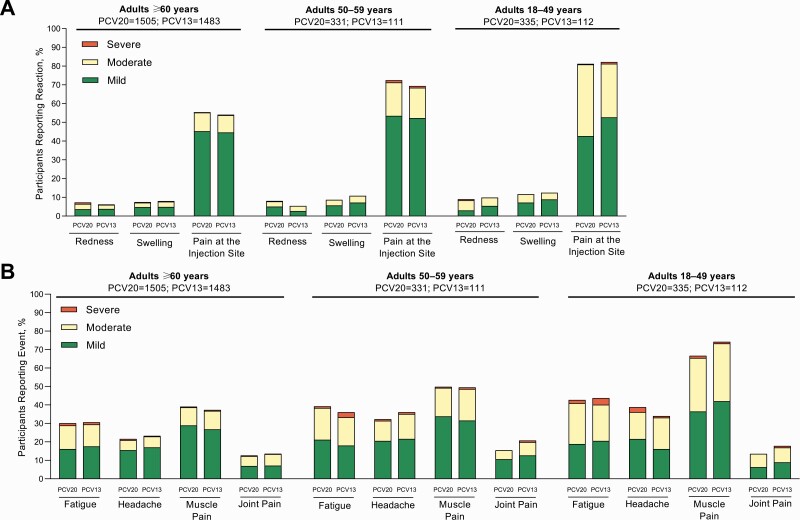
The frequency and severity of local reactions within 10 days after PCV20 or PCV13 were similar within each age group (Figure 5). Most local reactions were mild to moderate in intensity, and pain at the injection site was most frequent.
Figure 5.
Prompted reactogenicity events in participants aged ≥18 years, including local reactions within 10 days of vaccination (A) and systemic events within 7 days of vaccination (B). The frequency and severity of local reactions within 10 days after 20-valent or 13-valent pneumococcal conjugate vaccine (PCV20 or PCV13) were similar within each cohort.
Within 7 days after PCV20 or PCV13, fever was reported by 0.9%–1.5% of PCV20 and 0.8%–1.8% of PCV13 recipients. The frequency and severity of systemic events after PCV20 and PCV13 were similar within each age group (Figure 5). Most systemic events were mild to moderate in intensity; muscle pain was most frequently reported.
AEs and SAEs
Frequencies of AEs within 1 month after PCV20 or PCV13 were similar for each age group (Supplementary Table 2). Less than 1.5% of reported AEs were considered by the investigator to be related to study vaccine. Immediate AEs were reported by <1.0% of participants. Severe AEs within 1 month after PCV20 or PCV13 were infrequent. One month after saline or PPSV23, 7.4% and 12.2% of participants reported AEs, respectively.
The SAEs collected through 6-month follow-up were infrequent and similar between vaccine groups in each age group; none were vaccine related (Supplementary Table 2). In all 3 age groups, ≤1% of participants reported a newly diagnosed chronic medical condition within 1 month after PCV20 or PCV13; through 6-month safety follow-up, newly diagnosed chronic medical conditions were reported by ≤ 2.3% of participants and were consistent with medical events that may be observed in the population. None of these conditions were considered vaccine related.
Eleven participants in the PCV20/saline group and 8 in the PCV13/PPSV23 group, all aged ≥60 years, discontinued the study owing to AEs. These were considered vaccine related by the investigator in 5 PCV20 recipients (redness or swelling with or without pruritis at the injection site in 3, palpitations and anxiety in 1, and feeling abnormal in 1) and 4 PCV13 recipients (injection site pain with muscle pain, injection site reaction, headache, and bronchial hypersensitivity 6 days after vaccination in 1 participant each). One death in the PCV20/saline group was due to a traumatic injury, unrelated to study vaccination.
DISCUSSION
Addressing the global pneumococcal disease burden remains a public health imperative. Introduction of PCVs, such as PCV13, into immunization programs worldwide led to striking decreases in pneumococcal disease [13, 14, 21]. However, certain serotypes not covered by available PCVs persistently cause disease and threaten at-risk populations, including adults aged ≥65 years [16–18]. The 7 additional serotypes targeted by PCV20 but not PCV13 annually cause an additional 82 722 cases of pneumonia and other serious disease among US adults at risk because of age or other factors [27]. Although PPSV23 also contains these serotypes [32], it induces T-cell–independent immune responses lacking immunological memory, which can significantly limit effectiveness compared with PCVs [1, 7, 33], and, unlike PCVs [11], may not prevent nonbacteremic pneumococcal pneumonia [8, 31, 34]. Thus, expanding coverage through conjugated vaccines without these limitations is necessary to further reduce the burden of this serious and potentially fatal disease [13, 16, 19, 20, 23, 24].
Here, we present findings from the pivotal phase 3 study in adults aged ≥18 years evaluating safety and immunogenicity of investigational PCV20. The study design was modeled from clinical studies supporting PCV13 licensure in adults, which involved first showing comparable or higher OPA responses to PPSV23 in adults aged ≥60 years, with bridging to adults aged 50–59 and 18–49 years [35, 36]. PCV13 efficacy against vaccine-type IPD and pneumococcal pneumonia was subsequently shown in the CAPiTA trial (Community-Acquired Pneumonia Immunization Trial in Adults) in adults aged ≥65 years [31, 37].
PCV20 elicited robust, functional immune responses as measured by OPA to all 20 vaccine serotypes 1 month after vaccination in participants aged ≥18 years. In those aged ≥60 years, PCV20 induced immune responses for 13 matched serotypes that were noninferior to those elicited by PCV13. Noninferiority was also shown for 6 of the 7 additional serotypes in both PCV20 and PPSV23. Although serotype 8 missed the statistical noninferiority criterion, additional immunogenicity analyses showed that PCV20 elicited robust responses against serotype 8 within the range of those observed for 13 matched serotypes after PCV13. Given the established efficacy and immunologic properties of PCVs, including PCV13 [1, 7, 31], PCV20 will likely provide similar protection against this and other vaccine serotypes. As adults aged <60 years may also be candidates for PCV20, this study also bridged PCV20-elicited immune responses in younger participants to those in adults aged 60–64 years.
PCV20 was well tolerated, with a safety profile comparable to that of PCV13. Rates of prompted local reactions and systemic events were similar between vaccines; most were mild or moderate. No safety concerns were identified, and SAEs within 6 months after PCV20 or PCV13 were similarly infrequent.
Potential study limitations include that participants aged <65 years were enrolled from US sites, while those aged ≥65 years were also enrolled from Sweden to expand the population of pneumococcal vaccine-naive participants. While this could limit generalizability to global populations, based on efficacy and postmarketing effectiveness experience with PCV13 in adults [12, 31], PCV20 is likely to have similar effectiveness across regions. In addition, for most serotypes, immune responses to PCV20 relative to PCV13 or PPSV23 in participants aged ≥65 years had a trend generally similar to responses in those aged ≥60 years.
As in previous pivotal immunogenicity studies, immunocompromised or immunosuppressed individuals were excluded to support stringent noninferiority comparisons of PCV20 to licensed vaccines. Data from PCV studies, including PCV13, showed similar safety profiles in immunocompromised, immunosuppressed, and general populations and immune responses that may confer benefit against pneumococcal disease in high-risk populations [12, 38, 39]. Therefore, as PCV20 showed safety and immunogenicity comparable to PCV13 in this large study, PCV20 is anticipated to perform similarly to PCV13 in high-risk populations. In addition, only pneumococcal vaccine–naive adults participated, potentially limiting generalizability to populations with prior pneumococcal vaccination. Previous PCV13 studies showed that noninferiority to PPSV23 was met for both pneumococcal vaccine–naive and previously vaccinated populations, indicating expected applicability of these results regardless of prior vaccination [40].
In conclusion, in adults aged ≥18 years, PCV20 had a tolerability and safety profile similar to PCV13. PCV20 elicited functional immune responses in older adults that were noninferior to PCV13 responses for all 13 matched serotypes and to PPSV23 responses for 6 of 7 additional serotypes, with serotype 8 missing the statistical noninferiority criterion. The totality of immunogenicity data show that PCV20 elicits a robust response to serotype 8. Immune responses to PCV20 in adults aged 18–59 years were noninferior to those in adults aged 60–64 years. These data support potential PCV20 effectiveness against pneumococcal disease caused by these 20 serotypes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Editorial/medical writing support was provided by Emily Stackpole, PhD, of ICON (North Wales, Pennsylvania), and was funded by Pfizer Inc.
Financial support. This work was supported by Pfizer Inc.
Data availability. On request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or the European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Presented in part: IDWeek 2020, Oct 21–25, 2020.
Contributor Information
Brandon Essink, Meridian Clinical Research, Omaha, Nebraska, USA.
Charu Sabharwal, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Kevin Cannon, PMG Research of Wilmington, Wilmington, North Carolina, USA.
Robert Frenck, Cincinnati Children’s Hospital, Cincinnati, Ohio, USA.
Himal Lal, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Xia Xu, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Vani Sundaraiyer, Syneos Health, Somerset, New Jersey, USA.
Yahong Peng, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Lisa Moyer, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Michael W Pride, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Ingrid L Scully, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Kathrin U Jansen, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
William C Gruber, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Daniel A Scott, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Wendy Watson, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
References
- 1. World Health Organization. Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec 2012; 87:129–44. [PubMed] [Google Scholar]
- 2. Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect 2012; 18:7–14. [DOI] [PubMed] [Google Scholar]
- 3. Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20:45–51. [DOI] [PubMed] [Google Scholar]
- 4. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geno KA, Gilbert GL, Song JY, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 2015; 28:871–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clutterbuck EA, Lazarus R, Yu LM, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012; 205:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Vaccine 2015; 33:D60–5. [DOI] [PubMed] [Google Scholar]
- 9. Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066–73. [DOI] [PubMed] [Google Scholar]
- 10. Vadlamudi NK, Parhar K, Altre Malana KL, Kang A, Marra F. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to 23-valent pneumococcal polysaccharide in immunocompetent adults: a systematic review and meta-analysis. Vaccine 2019; 37:1021–9. [DOI] [PubMed] [Google Scholar]
- 11. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis 2018; 67:1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vadlamudi NK, Chen A, Marra F. Impact of 13-valent pneumococcal conjugate vaccine among adults: a systematic review and meta-analysis. Clin Infect Dis 2018; 69:34–49. [DOI] [PubMed] [Google Scholar]
- 14. Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 15. Wiese AD, Griffin MR, Grijalva CG. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert Rev Vaccines 2019; 18:327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hausdorff WP, Hanage WP. Interim results of an ecological experiment—conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother 2016; 12:358–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isturiz RE, Ramirez J, Self WH, et al. Pneumococcal epidemiology among US adults hospitalized for community-acquired pneumonia. Vaccine 2019; 37:3352–61. [DOI] [PubMed] [Google Scholar]
- 18. Van der Linden M, Falkenhorst G, Perniciaro S, Imohl M. Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PloS One 2015; 10:e0131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pilishvili T, Gierke R, Farley M, et al. Epidemiology of invasive pneumococcal disease (IPD) following 18 years of pneumococcal conjugate vaccine (PCV) use in the United States. Presented at: IDWeek; Oct 21–25, 2020; virtual meeting. [Google Scholar]
- 20. Metcalf BJ, Gertz RE Jr, Gladstone RA, et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect 2016; 22:e9–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PloS Med 2009; 6:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wantuch PL, Avci FY. Invasive pneumococcal disease in relation to vaccine type serotypes. Hum Vaccin Immunother 2019; 15:874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomczyk S, Lynfield R, Schaffner W, et al. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis 2016; 62:1119–25. [DOI] [PubMed] [Google Scholar]
- 25. Thompson A, Lamberth E, Severs J, et al. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2019; 37:6201–7. [DOI] [PubMed] [Google Scholar]
- 26. Hurley D, Griffin C, Young M, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis 2021; 73:e1489–e1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perdrizet J, Wasserman M, Farkouh RA, Chilson E. Estimated pneumococcal disease and economic burden for current and future vaccine serotypes in United States in children under five years of age. Presented at: International Symposium on Pneumococci and Pneumococcal Diseases. Digital Library, 2020. Available at https://isppd.kenes.com/digital-library/. Accessed 14 December 2021. [Google Scholar]
- 28. Pfizer. Pfizer granted FDA breakthrough therapy designation for 20-valent pneumococcal conjugate vaccine for the prevention of invasive disease and pneumonia in adults aged 18 years and older. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_granted_fda_breakthrough_therapy_designation_for_20_valent_pneumococcal_conjugate_vaccine_for_the_prevention_of_invasive_disease_and_pneumonia_in_adults_aged_18_years_and_older. Accessed 15 May 2019.
- 29. Food and Drug Administration. BLA approval and BLA accelerated approval letter Available at: https://www.fda.gov/media/150021/download. Accessed 18 June 2021.
- 30. Centers for Disease Control and Prevention. Pneumococcal vaccine timing for adults. US Department of Health and Human Services. Available at: https://www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 5 October 2021.
- 31. Bonten MJ, Huijts SM, Bolkenbaas M; CAPITA coauthors. Vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 373:93. [DOI] [PubMed] [Google Scholar]
- 32. Pneumovax® 23 (pneumococcal vaccine polyvalent). Full prescribing information. Whitehouse Station, NJ: Merck & Co, 2019. [Google Scholar]
- 33. Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2019; 68:1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gierke R, Wodi AP, Kobayashi M. Pneumococcal Disease. In: Epidemiology and Prevention of Vaccine-Preventable Diseases. Hall E, Wodi AP, Hamborsky J, et al., eds. 14th ed. Washington, DC: Public Health Foundation, 2021: 279–96. [Google Scholar]
- 35. Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–93. [DOI] [PubMed] [Google Scholar]
- 36. Bryant KA, Frenck R, Gurtman A, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 18–49 years of age, naive to 23-valent pneumococcal polysaccharide vaccine. Vaccine 2015; 33:5854–60. [DOI] [PubMed] [Google Scholar]
- 37. Lockhart SP, Scott DA, Jansen KU, Anderson AS, Gruber WC. Glycoconjugate vaccines: the clinical journey. In: Prasad KA, ed. Carbohydrate-based vaccines: from concept to clinic. Vol 1290. Washington, DC: American Chemical Society; 2018: 7–59. [Google Scholar]
- 38. Chilson E, Scott DA, Schmoele-Thoma B, Watson W, Moran MM, Isturiz R. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in patients with immunocompromising conditions: a review of available evidence. Hum Vaccin Immunother 2020; 16:2758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fletcher MA, Balmer P, Bonnet E, Dartois N. PCVs in individuals at increased risk of pneumococcal disease: a literature review. Expert Rev Vaccines 2015; 14:975–1030. [DOI] [PubMed] [Google Scholar]
- 40. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60–64 years of age. Vaccine 2014; 32:2364–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.