Abstract
Background
Community-acquired pneumonia (CAP) is a common cause for hospitalization and antibiotic overuse. We aimed to improve antibiotic duration for CAP across 41 hospitals participating in the Michigan Hospital Medicine Safety Consortium (HMS).
Methods
This prospective collaborative quality initiative included patients hospitalized with uncomplicated CAP who qualified for a 5-day antibiotic duration. Between 23 February 2017 and 5 February 2020, HMS targeted appropriate 5-day antibiotic treatment through benchmarking, sharing best practices, and pay-for-performance incentives. Changes in outcomes, including appropriate receipt of 5 ± 1–day antibiotic treatment and 30-day postdischarge composite adverse events (ie, deaths, readmissions, urgent visits, and antibiotic-associated adverse events), were assessed over time (per 3-month quarter), using logistic regression and controlling for hospital clustering.
Results
A total of 41 hospitals and 6553 patients were included. The percentage of patients treated with an appropriate 5 ± 1–day duration increased from 22.1% (predicted probability, 20.9% [95% confidence interval: 17.2%–25.0%]) to 45.9% (predicted probability, 43.9% [36.8%–51.2%]; adjusted odds ratio [aOR] per quarter, 1.10 [1.07–1.14]). Thirty-day composite adverse events occurred in 18.5% of patients (1166 of 6319) and decreased over time (aOR per quarter, 0.98 [95% confidence interval: .96–.99]) owing to a decrease in antibiotic-associated adverse events (aOR per quarter, 0.91 [.87–.95]).
Conclusions
Across diverse hospitals, HMS participation was associated with more appropriate use of short-course therapy and fewer adverse events in hospitalized patients with uncomplicated CAP. Establishment of national or regional collaborative quality initiatives with data collection and benchmarking, sharing of best practices, and pay-for-performance incentives may improve antibiotic use and outcomes for patients hospitalized with uncomplicated CAP.
Keywords: antibiotic duration, antibiotic stewardship, pneumonia, quality of care
In a 3-year prospective collaborative quality initiative including 41 Michigan hospitals and 6553 patients hospitalized with uncomplicated community-acquired pneumonia, the predicted probability of treatment with an appropriate 5-day antibiotic duration increased from 20.9% to 43.9%, while adverse events decreased.
Optimal antibiotic use is vital for effectively treating infections and protecting patients from antibiotic-associated adverse events, Clostridioides difficile infection (CDI), and multidrug-resistant organisms [1–4]. Pneumonia is the most common indication for inpatient antibiotic therapy and a major contributor to antibiotic overuse [5, 6]. Despite guidelines recommending a shorter antibiotic course [7–9], the majority of hospitalized patients with community-acquired pneumonia (CAP) receive an excessive duration of therapy [2, 10, 11].
Several studies have demonstrated that antibiotic stewardship interventions can decrease excessive-duration antibiotic therapy for CAP, but data from large multihospital collaboratives are lacking [12–14]. In 2017, the Michigan Hospital Medicine Safety Consortium (HMS), a statewide collaborative quality initiative (CQI), began collecting data, sharing best practices, and benchmarking performance related to antibiotic duration for uncomplicated CAP. The resulting antibiotic use and patient outcomes from the first 3 years are described here.
METHODS
Overview of the Program
HMS is a statewide CQI sponsored by Blue Cross Blue Shield of Michigan (BCBSM) and Blue Care Network with the goal of improving care for hospitalized medical patients. Hospitals joining HMS collect and share data to improve outcomes related to several focused initiatives. Of the 92 noncritical access, nongovernmental hospitals in Michigan, 47 (51%) have participated in HMS. For the current analysis, we included 41 hospitals (87%) that participated for at least half the study period (23 February 2017 through 5 February 2020).
In 2017, HMS began collecting data on patients treated for CAP as part of its antimicrobial use initiative, focusing on 3 quality improvement pillars (see Supplementary Figure 1 for timeline). First, HMS collects patient-level data from each hospital through medical record review by nurse abstractors employed by individual hospitals but trained by HMS. Patient data include signs and symptoms of infection, diagnostic testing, antibiotic prescriptions, and outcomes. These data are used to create hospital-level quality metrics, which are shared with participating hospitals to benchmark performance and identify areas for improvement. Data reports can be accessed in real time and are shared during triannual meetings (example in Supplementary Material).
Second, during triannual meetings, HMS disseminates national guidelines, key literature, and best practices related to pneumonia care and antibiotic stewardship. These meetings include presentations on successful strategies and brainstorming sessions to engage lower-performing hospitals. In March 2018, an antibiotic toolkit was disseminated via webinar and included curated literature, customizable guidelines/pocket cards, sample communications to clinicians, and examples of successful interventions (details in Supplementary Material). How hospitals use HMS resources is left to hospital discretion.
Third, HMS selects certain quality metrics to become pay-for-performance metrics that, if met, provide additional funding for hospitals from BCBSM. The amount of funding varies depending on hospital size (number of beds) and the number of CQIs in which the hospitals participate. Each year HMS has up to 3 pay-for-performance metrics for the antimicrobial use initiative determined by representatives from across the collaborative. BCBSM generally agrees to suggested metric targets suggested by HMS provided that they represent “reach targets” for the collaborative; each year, the target that must be reached for full funding increases to help raise the bar (eg, in 2018, >20% of those eligible for 5-day therapy must receive 5 ± 1 days of therapy; in 2019, >45%; and in 2020, >50%) (see Supplementary Material for target details).
Outcomes and Data Collection
The primary study outcome was a dichotomous variable representing whether a patient hospitalized with uncomplicated CAP who was eligible for a short, 5-day antibiotic course, appropriately received 5 (±1) days of effective antibiotic therapy (inpatient plus discharge duration). Patients potentially eligible for inclusion were identified based on presence of a discharge diagnostic code of pneumonia and receipt of antibiotics on day 1 or 2 of hospitalization. Patients not eligible for inclusion were those with any of the following: concomitant infection (eg, cellulitis), initial admission to the intensive care unit (ICU), pregnancy, severe immune compromise (eg, any transplant, neutropenia, human immunodeficiency virus with a CD4 cell count <200/µL), or fungal infection. Patients were included only if they received ≥3 days of antibiotic therapy and met diagnostic criteria for CAP, including chest imaging findings and ≥2 signs or symptoms consistent with pneumonia (Supplementary Material) [2].
Patients eligible for a 5-day antibiotic duration were identified using previously published methods based on national guidelines [2, 9, 11, 15]. Briefly, patients expected to have a 5-day antibiotic duration were those who reached clinical stability (afebrile for ≥48 hours and having ≤1 vital sign abnormality) by hospital day 5 [16] or were discharged by day 3 of hospitalization. Because we were targeting patients whose eligibility for a 5-day duration was clear, we excluded patients infected by an organism that may require longer treatment (ie, bacteremia, Legionella, Staphylococcus, or Pseudomonas species, or other nonfermenting gram-negative organisms) or a condition that may require longer treatment (ie, moderate immune compromise [ie, human immunodeficiency virus with CD4 cell count >200/µL, recent chemotherapy, treatment with biologic agents, congenital or acquired immunodeficiency], structural lung disease, moderate or severe chronic obstructive pulmonary disease) [9, 16]. Because HMS began before the removal of healthcare-associated pneumonia (HCAP) terminology from national guidelines [9], CAP was defined based on the 2007 CAP guidelines, and patients who met criteria for HCAP (eg, hospitalization within 90 days) were excluded [16]. Patients who died, were transferred to the ICU during hospitalization, or who were missing critical data to calculate antibiotic duration were also excluded (see Supplementary Figure 2 for definitions and flow diagram).
The goal for each hospital was to include 8 patients every 16 days, including weekends. To identify patients for inclusion, HMS abstractors at each hospital consecutively screened discharge lists in order of discharge time for eligibility. Once 1 case was included (or if no eligible cases were identified for that discharge day), the abstractor would begin screening the list for the subsequent discharge day until 8 patients were included. Abstractors undergo in-person and online case-based training on abstraction and eligibility and undergo random yearly audits for quality assurance (see Supplementary Material for details) [2, 15].
The secondary outcome of composite adverse events (ie, death, hospital readmission, urgent visit [any urgent visit not resulting in hospitalization, including emergency department or urgent care visit or observation stay], and antibiotic-associated adverse events) was collected 30 days after discharge, using a combination of record review (for all patients) and by a scripted patient phone call conducted by the trained nurse abstractors (for those eligible, see below). Antibiotic-associated adverse events included CDI, clinician-documented adverse events (eg, QT prolongation), and (for patients prescribed an antibiotic at discharge) patient-reported adverse events obtained via the 30-day postdischarge telephone call (“Have you had any side effects from your prescribed antibiotic?”). Patients confirmed by medical record to have died, to be hospitalized, or to be residing in an inpatient hospice, extended care facility, or prison at 30 days after discharge were ineligible for a follow-up phone call. Secondary outcomes were also assessed at the hospital level on a quarterly basis.
Hospital characteristics were collected from self-report or publicly available databases (see Supplementary Material for details). Because disparities due to patient demographics may exist, we report data on sex, race, and ethnicity, obtained from the medical record and categorized as noted in the Supplementary Material.
Statistical Analysis
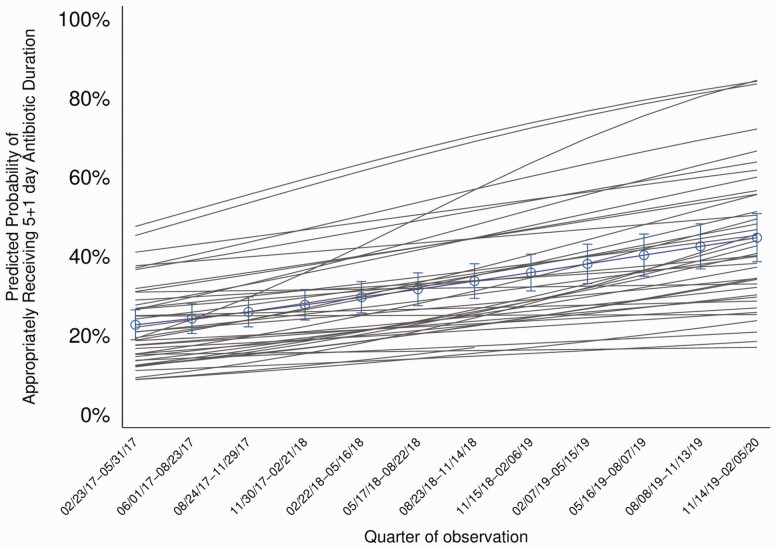
First, we describe how the percentage of patients with uncomplicated CAP treated appropriately with a 5-day duration changed during the study. Using a logistic regression model, adjusted for hospital clustering, and allowing for random intercepts and slopes, we obtained adjusted odds ratios (aORs) assessing the change by quarter in appropriate 5-day therapy for CAP. Because patient characteristics were used to define appropriateness, no additional patient-level adjustments were included. The random intercepts describe the baseline differences in treatment between hospitals before the intervention. The random slopes allow us to estimate how the rate of change in treatment over time varied across hospitals. Using these models, we were able to graphically present (Figure 1), the predicted probability of appropriate 5-day therapy averaged over all hospitals, as well as the smoothed individual adoption curves for each hospital.
Figure 1.
The predicted probability of a patient hospitalized with CAP who was eligible for a 5-day antibiotic duration actually receiving 5 (± 1) days of antibiotic treatment over time by quarter. The predicted probability of appropriate 5-day therapy is shown averaged over all hospitals (blue line) as well as smoothed individual adoption curves (gray lines) for each hospital. Predicted probabilities were obtained from a logistic regression model, adjusted for hospital clustering, and allowing for random intercepts and slopes. The aOR for appropriate 5-day treatment per quarter is 1.10, 95% CI: 1.07–1.14 per quarter; per year is 1.49 (1.33–1.66). Note: Dates on x-axis are given in month/date/year format.
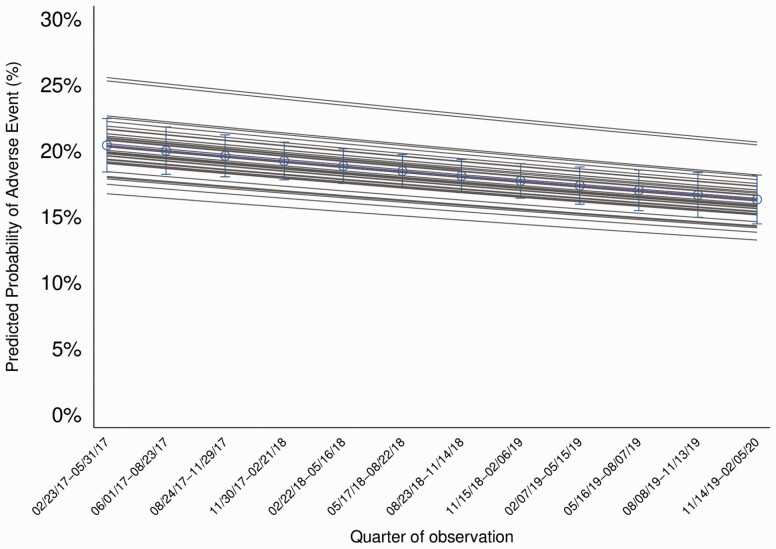
To describe how the secondary outcomes (ie, 30-day patient outcomes) changed during the study period, we used logistic regression models, adjusting for hospital clustering, to obtain aORs for change by quarter. For each model, we adjusted for patient factors associated with the outcome (details in Supplementary Material) [2]. As above, we constructed logistic regression models that allowed for random intercepts and slopes adjusting for patient risks by averaging over the covariate distribution in the population to produce risk-standardized outcome predictions. In this model, the variation across hospitals in the rate of change in secondary outcomes over time was small and not significant, so the final model includes only a random intercept allowing for different baseline secondary outcome rates. We again graphically present predicted overall rates of change (averaging across hospitals and the population patient covariate distribution) as well as individual smoothed hospital curves averaging over patient covariates (Figure 2). As a secondary analysis, we imputed missing data through a 10-fold multiple imputation procedure and combined using standard rules [17].
Figure 2.
The predicted probability of a patient hospitalized with CAP having an adverse event within 30-days of hospital discharge is shown over time by quarter (aOR of adverse event per quarter: .98, 95% CI: .96–.99). The average over all hospitals is shown in blue and smoothed individual hospital curves are shown in gray. Predicted probabilities were obtained from logistic regression models, adjusting for hospital clustering, allowing for random intercepts, and adjusting for patient risks to obtain aORs for change by quarter. Composite adverse events, measured at 30-days after discharge, include mortality, hospital readmission, urgent visit (includes any urgent visit not resulting in hospitalization including emergency department visit, urgent care visit, or observation stay), and antibiotic-associated adverse events obtained from a combination of chart review and patient phone calls at 30-days. Note: Dates on x-axis are given in month/date/year format.
Secondary Analyses
In the models assessing change in appropriate 5-day treatment over time, we added potential hospital-level explanatory variables (eg, hospital size [number of beds]) to describe how rates of change in appropriate 5-day treatment varied by these factors. Because not all patients had data on patient-reported antibiotic-associated adverse events, we conducted a sensitivity analysis to determine whether composite adverse events changed over time if patient-reported antibiotic-associated adverse events were not included.
All statistical tests were performed at an α level of .05. Two-tailed estimates of effect (odds ratios) and 95% confidence intervals (CIs) are reported for all regression coefficients. Statistical analyses were performed with SAS (version 9.4; SAS Institute) and Stata (version 16; StataCorp) software. We followed SQUIRE 2.0 reporting guidelines (checklist in Supplementary Material). This study received a “not regulated” status from our institutional review board.
RESULTS
While 47 hospitals contributed data to HMS, 6 were excluded for participating in less than half of the study period (23 February 2017 through 5 February 2020), leaving 41 hospitals for our analysis (87%). Hospitals that dropped out were similar to those that participated (Supplementary Table 1). There were 6553 patients with uncomplicated CAP who were eligible for a 5-day duration and included in this study (see Supplementary Table 2 for patient characteristics). The most common empiric antibiotics were ceftriaxone, azithromycin, and levofloxacin. The most common discharge antibiotics were azithromycin, oral cephalosporins, and levofloxacin. Over the study period both empiric and discharge fluoroquinolone use decreased, replaced largely by cephalosporin and β-lactam use (Supplementary Figure 3A and 3B).
Over the study period, 34.0% of patients eligible for a 5-day duration (2228 of 6553) received an appropriate 5 ± 1–day duration. Proportions of patients with uncomplicated CAP treated with an appropriate 5-day duration ranged from 10.0% to 69.0% across hospitals; hospitals that were academic, nonprofit, larger, or part of a statewide healthcare system had higher rates of appropriate 5-day treatment (Table 1).
Table 1.
Hospital Characteristics Associated with Higher Rates of Appropriate 5-Day Treatment for Community-Acquired Pneumonia Treatment (n = 41 Hospitals)
| Hospital Characteristica | Patients, No. (%)b | P Valuec | |
|---|---|---|---|
| Appropriate Treatment Duration (n = 2228) | Excessive Treatment Duration (n = 4325) | ||
| Academic hospitald | 1856 (83.3) | 3313 (76.6) | <.001 |
| Profit typee | <.001 | ||
| Nonprofit | 2058 (92.4) | 3846 (88.9) | |
| For profit | 170 (7.6) | 479 (11.1) | |
| Hospital sizef | |||
| No. of beds, mean (SD) | 377 (256) | 352 (238) | <.001 |
| 51–100 beds | 164 (7.4) | 541 (12.5) | <.001 |
| 101–200 beds | 481 (21.6) | 815 (18.8) | |
| >200 beds | 1583 (71.1) | 2969 (68.6) | |
| No. of hospitalists, mean (SD)g | 22 (24) | 19 (20) | <.001 |
| Healthcare system)h | |||
| None | 214 (9.6) | 493 (11.4) | <.001 |
| State | 1700 (76.3) | 2834 (65.5) | |
| National | 314 (14.1) | 998 (23.1) | |
Abbreviation: SD, standard deviation.
Hospital characteristics associated with appropriate 5-day treatment are shown.
Data represent no. (%) of patients unless otherwise specified.
P values were calculated from χ2 tests, with differences considered significant at P < .05.
Academic hospital status was obtained from the American Hospital Association’s data hub.
Profit status was obtained from the American Medical Association’s data hub.
Hospital size (number of beds) was obtained from the 2019 Michigan Certificate of Need Annual Survey.
For participating hospitals, data on hospitalists are self-reported from the November 2019 hospital survey. For nonparticipants, data were collected from hospital Web sites.
Membership in a large healthcare system; data were obtained from the Michigan Health and Hospital Association (https://www.mha.org/About/Our-Hospitals/Michigan-Hospitals-By-Health-System).
Changes in Appropriate Antibiotic Duration Over Time
Over the study period, the proportion of patients treated with a 5-day antibiotic duration increased from 22.1% (predicted probability adjusted for hospital clustering, 20.9% [95% CI, 17.2%–25.0%]) to 45.9% (43.9% [36.8%–51.2%]; P < .001) (Figure 1). Each quarter was associated with higher odds of receiving an appropriate 5-day antibiotic duration (aOR per quarter, 1.10 [95% CI, 1.07–1.14]), for an annual aOR of 1.49 (1.33–1.66). Improvement in appropriate antibiotic duration over time was driven by a decrease in antibiotic duration at discharge (median [interquartile range] discharge duration, 5 [3–7] days in 2017 and 3 [2–5] days in 2020; P < .001).
Every year, the collaborative average was higher than the “pay-for-performance” target set by the CQI, though not all hospitals met the metric: 85% (35 of 41) met the metric in 2018 and 44% (18 of 41) in 2019. There was evidence of variation in adoption; hospitals with low baseline rates had, on average, larger increases in rates of appropriate treatment (Figure 1). The estimate of the variance of the random slopes across hospitals was 0.005 on the log odds scale (95% CI: .001–.008), which implies a wide variation in rates of change with 95% of hospitals falling into a range of 0.96–1.27 for OR per quarter. However, the hospital characteristics we evaluated did not explain the variation in slope of improvement across hospitals (Supplementary Table 3).
Changes in Outcomes Over Time
Composite adverse events (ie, death, readmission, urgent visit, or antibiotic-associated adverse event) occurred in 18.5% of patients (1166 of 6319) during the study. Raw composite and individual adverse events are shown in Table 2. The most common antibiotic-associated adverse events documented by providers were rash, diarrhea, itching, and gastrointestinal distress (Supplementary Table 4). Of the 5134 patients eligible for a follow-up phone call to ascertain patient-reported antibiotic-associated adverse events, 2967 (57.8%) responded; of those, 95 (3.2%) reported an adverse event. The most common antibiotic-associated adverse events reported by patients were diarrhea, gastrointestinal distress, and mucosal candidiasis (Supplementary Table 4).
Table 2.
Adverse Events in Hospitalized Patients with Uncomplicated Community-Acquired Pneumonia, by Quarter
| Patients, No. (%)a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | |||||||||||
| Outcome 30 d After Discharge | Q2(n = 669) | Q3(n = 468) | Q4(n = 500) | Q1 (n = 633) | Q2(n = 523) | Q3(n = 533) | Q4(n = 409) | Q1 (n = 471) | Q2(n = 596) | Q3(n = 501) | Q4(n = 483) | Q1(n = 533) | aOR per Q (95% CI)b | P Valueb |
| Composite adverse outcomec | 127 (19.0) | 99 (21.2) | 100 (20.0) | 114 (18.0) | 107 (20.5) | 116 (21.8) | 82 (20.0) | 82 (17.4) | 98 (16.4) | 76 (15.2) | 79 (16.4) | 86 (16.1) | 0.98 (.96–.99) | .008d |
| Deathc | 6 (0.9) | 11 (2.4) | 3 (0.6) | 8 (1.3) | 11 (2.1) | 6 (1.1) | 6 (1.5) | 1 (0.2) | 8 (1.3) | 5 (1.0) | 5 (1.0) | 4 (0.8) | 0.97 (.91–1.04) | .38 |
| Readmissionc | 49 (7.3) | 46 (9.8) | 38 (7.6) | 39 (6.2) | 43 (8.2) | 53 (9.9) | 43 (10.5) | 38 (8.1) | 42 (7.0) | 30 (6.0) | 45 (9.3) | 41 (7.7) | 1.00 (0.98–1.03) | .78 |
| Urgent visitc,d | 55 (8.2) | 42 (9.0) | 61 (12.2) | 69 (10.9) | 52 (9.9) | 59 (11.1) | 40 (9.8) | 44 (9.3) | 55 (9.2) | 45 (9.0) | 28 (5.8) | 45 (8.4) | 0.98 (.95–1.00) | .07 |
| Antibiotic-associated ADEc | 33 (4.9) | 21 (4.5) | 15 (3.0) | 26 (4.1) | 19 (3.6) | 15 (2.8) | 8 (2.0) | 6 (1.3) | 11 (1.8) | 7 (1.4) | 16 (3.3) | 8 (1.5) | 0.91 (.87–.95) | <.001d |
| CDIe | 2 (0.3) | 1 (0.2) | 1 (0.2) | 3 (0.5) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0 (0) | 3 (0.6) | 1 (0.2) | 1.00 (.86–1.17) | .97 |
| Physician reportedf | 12 (1.8) | 11 (2.4) | 7 (1.4) | 15 (2.4) | 8 (1.5) | 9 (1.7) | 2 (0.5) | 3 (0.6) | 5 (0.8) | 5 (1.0) | 9 (1.9) | 4 (0.8) | 0.93 (.87–.99) | .02d |
| Patient reportedf | 21 (6.2) | 10 (4.4) | 8 (3.3) | 12 (4.0) | 11 (4.5) | 8 (3.2) | 5 (2.9) | 2 (0.9) | 6 (2.1) | 2 (0.9) | 6 (2.8) | 4 (1.6) | 0.89 (.84–.95) | <.001d |
Abbreviations: ADE, adverse drug event; aOR, adjusted odds ratio; CDI, Clostridioides difficile infection; CI, confidence interval; Q, quarter.
Raw proportions of patients hospitalized with uncomplicated community-acquired pneumonia, by quarter, who had an adverse event within 30 days of hospital discharge.
aORs and the associated P values are shown for the change over time (by quarter) in adverse outcomes. aORs were obtained from logistic regression models, adjusting for hospital clustering and predictors known to be associated with each outcome.
Data on composite outcome, deaths, readmissions, and urgent visits were adjusted for age, Charlson comorbidity index, sex, pneumonia severity index, length of stay, Medicaid insurance status, and concurrent disease exacerbations (eg, chronic obstructive pulmonary disease, and heart failure).
Any urgent visit not resulting in hospitalization, including emergency department visits, urgent care visits, and observation stays.
CDI data were adjusted for age, Charlson comorbidity index, inflammatory bowel disease, immunosuppression medications, tube feeds, proton pump inhibitor, length of stay, antibiotic use in the prior 90 days, and number of antibiotics in the prior 90 days.
Data on physician- and patient-reported adverse events were adjusted for age, Charlson comorbidity index, and sex. Patient-reported ADEs were obtained via 30-day follow-up phone call. Of the 6669 patients contacted to ascertain patient-reported adverse events, 3888 (58.3%) responded.
After adjustments, composite adverse events decreased over time (aOR 0.98 [95% CI: .96–.99]) (Figure 2 and Table 2). This decrease appeared to be driven by a decrease in antibiotic-associated adverse events over time (physician-reported aOR per quarter, 0.93 [95% CI: .87–.99]; patient-reported aOR per quarter 0.89 [.84–.95]). The occurrence of deaths, readmissions, urgent visits, and CDI did not change over time (Table 2).
Additional Analyses
Missing data were uncommon and existed for 3.6% of patients (201 missing payer information, 32 missing inflammatory bowel disease, and 2 missing pneumonia severity index). Estimates for change over time in composite adverse events were similar when missing data were imputed (aOR, 0.98 [95% CI: .96–.99]). Patient-reported antibiotic-associated adverse events accounted for the bulk of the change in outcomes; when these were excluded from the secondary outcome, composite adverse events no longer significantly decreased over time (aOR, 0.99 [95% CI: .97–1.00]; P = .11).
DISCUSSION
Over the study period, appropriate antibiotic use for hospitalized patients with uncomplicated CAP increased substantially, and adverse events decreased. The HMS CQI model of a multipronged intervention including (1) data collection and benchmarking, (2) sharing of best practices, and (3) pay-for-performance incentives may be a successful model for improving antibiotic use across diverse hospitals.
Consistent with findings of previous studies, we found that the majority of patients with CAP received an excessive duration of antibiotics despite randomized trials, systematic reviews, and guidelines supporting the safety and efficacy of 5-day (or even 3-day) [18] treatment courses [2, 9–11, 19, 20]. Prior single-center studies have demonstrated that stewardship programs can reduce excessive-duration antibiotic treatment (though not adverse events) for CAP [12–14]. Our study builds on previous studies by demonstrating that a CQI model is associated with higher rates of appropriate antibiotic use and fewer adverse events across a diverse set of hospitals. Notably, appropriate antibiotic use increased over time even in small (<200-bed) hospitals, which historically have a harder time implementing stewardship [21]. One reason may be that the data infrastructure provided by HMS directly addresses the common barrier for small hospitals of obtaining metrics on antibiotic prescribing to inform improvement [21]. This strategy could also be helpful for other infectious disease states. HMS has already been targeting antibiotic stewardship in urinary tract infection and has reduced inappropriate treatment of asymptomatic bacteriuria over time through similar mechanisms [22].
Despite improved duration of therapy, we note that more than half of patients received antibiotics for an excessive duration even during the later quarters of CQI participation. Those numbers may be higher in the future as cases formerly categorized as HCAP become eligible for 5-day therapy under the new CAP guidelines [9]. Potential continued barriers to improvement are myriad. One is that >95% of excessive-duration antibiotic treatment occurs after hospital discharge [2]. Because discharge antibiotics are not part of national antibiotic use measures, they are often not a focus of hospital-based antibiotic stewardship programs [7, 23, 24]. Hospitals doing well on inpatient prescribing may miss opportunities for discharge stewardship [25]. In HMS, though we provided education and resources related to discharge prescribing (see Supplementary Material), we did not standardize an approach to discharge stewardship across hospitals. Antibiotic stewardship interventions at discharge, including pharmacist audit and feedback or order sets with 5-day stop dates, may be a way to speed improvement at the local hospital level [26–29].
Based on conversations during site visits to participating hospitals, many clinicians still hesitate to prescribe short-course therapy to patients with multiple comorbid conditions, even if they defervesce and stabilize quickly. Previously, our group found that patients with community-onset pneumonia (including CAP and HCAP) who had respiratory testing, longer stays, or receipt of antibiotics before hospitalization were more likely to receive excessive therapy [2]. Data-driven education with benchmarking has appeared to improve clinician comfort with short-course therapy, but changing prescribing habits takes time.
Ours is not the first study to show the benefit of CQIs for improving quality of care related to infections. Using a strategy similar to that of HMS, a statewide antibiotic stewardship CQI in Colorado was associated with improved antibiotic use for urinary tract and skin and soft-tissue infections [30]. CQIs have also demonstrated a reduction in catheter-associated urinary tract infections in non-ICU patients and catheter-related bloodstream infections in the ICU setting [31, 32]. Each of these CQIs developed an evidence-based quality metric, disseminated guidelines and best practices, and measured performance over time. Unique to the HMS CQI is the pay-for-performance incentive and the ability of hospitals to benchmark their prescribing compared with other hospitals. Taken together, the CQI model provides a cohesive path for widespread improvement in patient care across infections.
Our study has limitations. First, hospitals were not randomized to participate in the CQI, and thus we cannot provide causal evidence that participation led to improved outcomes. Second, although hospitals that dropped out were similar to participants, participants may have been more motivated to improve antibiotic use. Third, there was no control group. Although other studies suggest that antibiotic duration has improved less than seen in the current study [10–12, 29, 33–35], we cannot exclude secular trends as the reason for improvement. Fourth, the decline in adverse events was driven by patient-reported adverse events, which were not available for all patients. Although an important patient-centered outcome, adverse events are difficult to collect and subject to recall and ascertainment bias. Finally, the CQI was made possible through support by BCBSM and may not be generalizable. Our study has strengths. Our rigid definition of CAP, including a clear definition of “uncomplicated CAP” with record-level review, eliminated the need for severity adjustment often required in quality improvement studies. Additional strengths include participation of a large number of hospitals, ability to benchmark performance, and detailed collection of outcomes, including antibiotic-associated adverse events.
Our study has important implications. Participation in a stewardship CQI may improve not only antibiotic use but also patient outcomes. The observation that all hospitals, including small hospitals, benefited from CQI participation suggests that the CQI model could be a way to advance antibiotic stewardship, even in small, rural hospitals with fewer resources. Other states and healthcare systems should consider adopting this 3-pronged method for improvement, including data collection and benchmarking, sharing of best practices, and a pay-for-performance incentive.
In conclusion, over a 3-year period, HMS participation was associated with more appropriate use of short-course therapy and fewer adverse events in hospitalized patients with uncomplicated CAP across diverse hospitals. Establishment of national or regional CQIs with data collection and benchmarking, sharing of best practices, and pay-for-performance incentives may improve antibiotic use and outcomes for patients hospitalized with uncomplicated CAP.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. V. M. V., T. N. G., and D. R. had full access to all the data in the study and final responsibility for the decision to submit for publication. Substantial contributions to the conception or design of the work: V. M. V., T. N. G., T. P. H., L. A. P., A. N. M., S. A. F., D.R. Substantial contributions to acquisition, analysis, or interpretation of data for the work: T. P. H., D. R., J. K. H., E. S. M., T. C. Drafting the work: V. M. V., T. N. G., T. P. H., J. H. Revising it critically for important intellectual content- all authors. Final approval of the version to be published- all authors.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality (AHRQ) or the Department of Veterans Affairs. Blue Cross Blue Shield of Michigan (BCBSM) supported data collection at each participating site and funded the data coordinating center. No funder had a role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Financial support. This work was supported by BCBSM—through their Value Partnerships program, through funding to the Michigan Hospital Medicine Safety Consortium (HMS) collaborative (including data analytic time), and through payments to institutions as part of the HMS Consortium—and by the AHRQ (career development award K08HS026530 to VMV).
Contributor Information
Valerie M Vaughn, Division of General Internal Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA; Division of Health System Innovation & Research, Department of Population Health Science, University of Utah School of Medicine, Salt Lake City, Utah, USA; Division of Hospital Medicine, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Tejal N Gandhi, Division of Infectious Diseases, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Timothy P Hofer, Center for Clinical Management Research, VA Ann Arbor Health System, Ann Arbor, Michigan, USA; Division of General Internal Medicine, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Lindsay A Petty, Division of Infectious Diseases, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Anurag N Malani, Division of Infectious Diseases, Department of Internal Medicine, St Joseph Mercy Health System, Ann Arbor, Michigan, USA; Department of Infection Prevention and Control, St Joseph Mercy Health System, Ann Arbor, Michigan, USA.
Danielle Osterholzer, Hurley Medical Center, Flint, Michigan, USA; Michigan State University, College of Human Medicine, East Lansing, Michigan, USAand.
Lisa E Dumkow, Department of Clinical Pharmacy Services, Mercy Health Saint Mary’s, Grand Rapids, Michigan, USA.
David Ratz, Center for Clinical Management Research, VA Ann Arbor Health System, Ann Arbor, Michigan, USA.
Jennifer K Horowitz, Division of Hospital Medicine, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Elizabeth S McLaughlin, Division of Hospital Medicine, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Tawny Czilok, Division of Hospital Medicine, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Scott A Flanders, Division of Hospital Medicine, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
References
- 1. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE.. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 3. Kazakova SV, Baggs J, McDonald LC, et al. Association between antibiotic use and hospital-onset Clostridioides difficile infection in US acute care hospitals, 2006-2012: an ecologic analysis. Clin Infect Dis 2020; 70:11–8. [DOI] [PubMed] [Google Scholar]
- 4. CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 5. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 6. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. Available at https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed 1 June 2021. [Google Scholar]
- 8. Joint Commission on Hospital Accreditation. Approved: new antimicrobial stewardship standard. Jt Comm Perspect 2016; 36:1–3. [PubMed] [Google Scholar]
- 9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2018; 66:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magill SS, O’Leary E, Ray SM, et al. Assessment of the appropriateness of antimicrobial use in US hospitals. JAMA Network Open 2021; 4:e212007–e212007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–7. [DOI] [PubMed] [Google Scholar]
- 13. Haas MK, Dalton K, Knepper BC, et al. Effects of a syndrome-specific antibiotic stewardship intervention for inpatient community-acquired pneumonia. Open Forum Infect Dis 2016; 3:ofw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foolad F, Huang AM, Nguyen CT, et al. A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J Antimicrob Chemother 2018; 73:1402–7. [DOI] [PubMed] [Google Scholar]
- 15. Vaughn VM, Hersh AL, Spivak ES.. Antibiotic overuse and stewardship at hospital discharge: the reducing overuse of antibiotics at discharge (ROAD) home framework. Clin Infect Dis 2022; 74:1696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubin DB. Multiple imputations in sample surveys-a phenomenological Bayesian approach to nonresponse. In: Proceedings of the survey research methods section of the American Statistical Association. Vol. 1. American Statistical Association, 1978:20–34. [Google Scholar]
- 18. Dinh A, Ropers J, Duran C, et al. Discontinuing β-lactam treatment after 3 days for patients with community-acquired pneumonia in non-critical care wards (PTC): a double-blind, randomised, placebo-controlled, non-inferiority trial. Lancet 2021; 397:1195–203. [DOI] [PubMed] [Google Scholar]
- 19. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016; 176:1257–65. [DOI] [PubMed] [Google Scholar]
- 20. Li JZ, Winston LG, Moore DH, Bent S.. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med 2007; 120:783–90. [DOI] [PubMed] [Google Scholar]
- 21. Stenehjem E, Hyun DY, Septimus E, et al. Antibiotic stewardship in small hospitals: barriers and potential solutions. Clin Infect Dis 2017; 65:691–6. [DOI] [PubMed] [Google Scholar]
- 22. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med 2019; 179:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaughn VM, Petty LA, Flanders SA, et al. A deeper dive into antibiotic stewardship needs: a multihospital survey. Open Forum Infect Dis 2020; 7:ofaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baker DW, Hyun D, Neuhauser MM, Bhatt J, Srinivasan A.. Leading practices in antimicrobial stewardship: conference summary. Jt Comm J Qual Patient Saf 2019; 45:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaughn VM, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA.. The association of antibiotic stewardship with fluoroquinolone prescribing in Michigan hospitals: a multi-hospital cohort study. Clin Infect Dis 2019; 69:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yogo N, Shihadeh K, Young H, et al. Intervention to reduce broad-spectrum antibiotics and treatment durations prescribed at the time of hospital discharge: a novel stewardship approach. Infect Control Hospital Epidemiol 2017; 38:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leja N, Collins CD, Duker J.. Antimicrobial stewardship by transitions of care pharmacists at hospital discharge. Hospital Pharm 2020; 0:0018578720951170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daniels LM, Weber DJ.. Interventions to improve antibiotic prescribing at hospital discharge: a systematic review. Infect Control Hospital Epidemiol 2021; 42:96–9. [DOI] [PubMed] [Google Scholar]
- 29. Ciarkowski CE, Timbrook TT, Kukhareva PV, et al. A pathway for community-acquired pneumonia with rapid conversion to oral therapy improves health care value. Open Forum Infect Dis 2020; 7:ofaa497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jenkins TC, Hulett T, Knepper BC, et al. A statewide antibiotic stewardship collaborative to improve the diagnosis and treatment of urinary tract and skin and soft tissue infections. Clin Infect Dis 2018; 67:1550–8. [DOI] [PubMed] [Google Scholar]
- 31. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355:2725–32. [DOI] [PubMed] [Google Scholar]
- 32. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med 2016; 374:2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li DX, Ferrada MA, Avdic E, Tamma PD, Cosgrove SE.. Sustained impact of an antibiotic stewardship intervention for community-acquired pneumonia. Infect Control Hospital Epidemiol 2016; 37:1243–6. [DOI] [PubMed] [Google Scholar]
- 34. Vaughn VM, Seelye SM, Wang XQ, Wiitala WL, Rubin MA, Prescott HC.. Inpatient and discharge fluoroquinolone prescribing in Veterans Affairs hospitals between 2014 and 2017. Open Forum Infect Dis 2020; 7:ofaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madaras-Kelly KJ, Burk M, Caplinger C, et al. Total duration of antimicrobial therapy in veterans hospitalized with uncomplicated pneumonia: results of a national medication utilization evaluation. J Hospital Med 2016; 11:832–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.