Abstract
Obesity is an increasing health problem all over the world. In combination with the current COVID-19 pandemic, this has turned into a massive challenge as individuals with overweight and obesity at all ages show a significant increase in their risk of getting severe COVID-19. Around 20% of all patients that were hospitalized for COVID-19 suffered from obesity alone, whereas obesity in combination with other metabolic comorbidities, such as type 2 diabetes and hypertension, account for up to 60% of all hospitalizations in relation to COVID-19. Therefore, it is of immense importance to put the spotlight on the high incidence of obesity present already in childhood both by changing the individual minds and by encouraging politicians and the whole society to commence preventive interventions for achieving a better nutrition for all social classes all over the world. In the current review, we aim to explain the different pathways and mechanisms that are responsible for the increased risk of severe COVID-19 in people with overweight and obesity. Furthermore, we discuss how the pandemic has led to weight gains in many people during lockdown. At the end, we discuss the importance of preventing such an interface between a non-communicable disease like obesity and a communicable disease like COVID-19 in the future.
Key words: obesity, metabolic syndrome, COVID-19, long-COVID, inflammation
Introduction
Obesity increases the risk of severe COVID-19 by giving rise to a worse clinical outcome and increased mortality, when compared to the general population 1 . Obesity alone is responsible for 20% of COVID-19 hospitalizations, whereas obesity in combination with type 2 diabetes and hypertension accounts for up to 60% of all COVID-19 hospitalizations 2 . In addition, infected people with obesity (particularly those under 60 years of age) are more likely to require acute care, admission to the intensive care unit, intubation, and mechanical ventilation 3 . Even young patients are at higher risk for a nonfavorable COVID-19 prognosis if they suffer from metabolic dysfunctions 4 5 . Children usually develop an asymptomatic to moderate infection causing few hospitalizations; but a recent meta-analysis indicates that even childhood obesity is likely to increase the risk of severe COVID-19 6 . Albeit severe courses of COVID-19 in children are rare, a novel pediatric hyperinflammatory condition termed pediatric inflammatory multisystem syndrome, temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome (in children) (MIS(-C)), causes a severe to fatal disease. Even though underlying factors are unclear, it turns out that childhood obesity is a significant comorbidity 7 . This is a worldwide problem since, according to WHO reports, around 40% of the world population was estimated to be overweight or obese in 2016, and the numbers are still increasing, thus obesity has reached pandemic levels 8 ( Table 1 ).
Table 1 Key facts about obesity 7 .
|
Emerging data suggest that several mechanisms are responsible for this increased susceptibility of people with obesity for severe COVID-19 including, amongst others, an impaired immune system and changes in SARS-CoV-2 entry receptors in obese individuals 9 10 11 12 . In the current review, we discuss these mechanisms in order to understand why patients with obesity have a higher risk of developing severe COVID-19 symptoms not only in the acute phase of the disease but also in relation to long-COVID, vaccine breakthrough infections and re-infections. Furthermore, we discuss the effects of lockdown on obesity, and we comment on possibilities for avoiding this interface between metabolic and infectious diseases in potential future pandemics.
Adipose tissue
The adipose tissue is the largest endocrine organ in humans and, in addition to adipocytes, it consists of pre-adipocytes, endothelial cells, fibroblasts, leukocytes, and bone-marrow-derived macrophages 13 . Adipose tissue is classified into two main types, white adipose tissue and brown adipose tissue. White adipose tissue is the more predominant form in the human body, where it plays a major role in energy storage. The main function of brown adipose tissue is thermogenesis 14 15 16 . It is becoming increasingly clear that adipose depots serve distinct functions in males and females and have specific physiological roles. However, the mechanisms that regulate the size and function of specific adipose tissues in men and women remain poorly understood 17 .
In addition to energy storage via triacylglycerols stored in adipocytes, adipose tissue secretes “adipocytokines” or “adipokines”, including, for example, adiponectin, leptin, resistin, and visfatin 13 . Other important factors produced include the cytokines tumor necrosis factor (TNF), interleukin-6 (IL-6), interleukin-1 (IL-1), CC-chemokine ligand 2 (CCL2), plasminogen activator inhibitor type I (PAI-I), and a number of complement factors 18 19 . Most of these factors are known as pro-inflammatory mediators that induce immune cell infiltration (e. g., macrophages) and play a major role in infectious diseases.
The major adipokines in adipose tissue are leptin and adiponectin, where leptin is pro-inflammatory, and adiponectin is anti-inflammatory. In obesity, leptin is increased and adiponectin is decreased compared to normal weight individuals 20 . Oppositely, circulating adiponectin concentrations increase during caloric restriction 21 .
Leptin is almost exclusively expressed in differentiated adipocytes of the white adipose tissue with subcutaneous fat showing a higher expression than visceral adipose tissue 22 23 . Leptin released from adipocytes acts on neurons to reduce appetite and to increase energy expenditure 20 . Leptin is closely linked to the immune system where it stimulates the proliferation and activation of immune cells and cytokine production 20 .
Disease-specific subpopulations of adipose-resident immune cells can be found in adipose tissue. These immune cells can be further separated into populations specific for either visceral or subcutaneous adipose tissue 24 . An example of these immune cells are the macrophages, which are heterogenous and can generally be defined in two separate polarization states, M1 and M2 25 26 . M1 macrophages are induced by pro-inflammatory mediators, such as lipopolysaccharide (LPS) and interferon-γ (IFN-γ), produce pro-inflammatory cytokines (TNF-α, IL-6, IL-12) and generate reactive oxygen species, such as nitric oxide (NO) via activation of iNOS ( Nos2 ) 27 . M2 macrophages are induced, by among others, IL-4 and IL-13, and they produce high levels of the anti-inflammatory cytokines IL-10 and IL-1rα. Additionally, iNOS activity is blocked 27 . Overall, M2 macrophages are believed to participate in the inhibition of inflammatory responses and in the promotion of tissue repair and angiogenesis 25 . Both in mice and humans, it has been shown that distinct macrophage populations with unique characteristics direct inflammatory versus physiological changes in adipose tissue 28 .
Infection with SARS-CoV-2
Entry of SARS-CoV-2 into cells depends on binding of the viral spike glycoproteins to extracellular domains of cellular angiotensin-converting enzyme 2 (ACE2). ACE2 exists in two forms, a membrane-spanning cellular and an unbound soluble form 29 . Membrane-bound ACE2 (mACE2) constitutes the majority of ACE2; it contains a transmembrane domain anchoring the cleavable N-terminal domain. A membrane-bound protease (secretase) generates soluble ACE2 (sACE2) by enzymatic cleavage of mACE2. sACE2 appears in the circulation in very low concentrations. Both mACE2 and sACE2 are capable of binding the spike protein on the surface of SARS-CoV-2. After binding to mACE2, the spike proteins are proteolytically activated by host cell proteases 29 30 31 , resulting in fusion of the viral envelope with the plasma membrane or the endosome membrane of the host and viral entry into the cell.
ACE2 is part of the renin-angiotensin-aldosterone system (RAAS), where it mainly controls the generation of the vasodilating angiotensin 1–7 from angiotensin II. ACE2 also cleaves angiotensin I to angiotensin 1–9, which can be further converted to angiotensin 1–7 by ACE 29 . ACE2, Ang-(1–7), and its mitochondrial assembly (Mas) receptor constitute the vasoprotective arm of the RAAS leading to anti-inflammatory and anti-fibrotic responses 29 32 33 34 .
Diet and obesity have been shown to affect the expression of ACE2 in adipose tissue 35 . Recently, it was demonstrated that a decrease in sACE2 during weight loss was associated with improvements in metabolic health 36 . Another factor, neuropilin 1 (NRP-1), known to facilitate SARS-CoV-2 cell entry is highly abundant in subcutaneous adipose tissue, and both NRP-1 and ACE2 levels are decreased after weight loss 37 . However, it is still not clear whether a high or a low expression is beneficial in relation to health (reviewed in 32 ). Similarly, it is debated whether high levels of ACE2 in adipose tissue in relation to SARS-CoV-2 is an advantage or not. Thus, it seems that not only the abundance but also the functionality of the enzyme may be of importance.
Viruses including coronaviruses are primarily dependent on the host metabolism in several stages of their life cycle. For example, an association of dyslipidemia with the pathological development of COVID-19 was reported 38 . This raises the possibility that exploitation of the host lipid metabolism, by using potential inhibitors, can exhibit therapeutic benefits against COVID-19 39 . Additionally, specific lipid supplementation can represent another strategy to error-prone the formation of viral particles. Furthermore, switching the lipid metabolism through the implementation of ketogenic diet might be an approach to limit the effects of viral infection 40 . An experimental study associated with computational analysis identified the potential inhibitory effect of flavonoids against SARS-CoV-2 as they bind to essential viral targets required in virus entry and/or replication 41 . Flavonoids also showed excellent immunomodulatory and anti-inflammatory activities including the inhibition of various inflammatory cytokines. Further, flavonoids showed a significant ability to reduce the exacerbation of COVID-19 in the case of obesity via promoting lipid metabolism 41 .
Mechanisms responsible for an increased risk of severe COVID-19 in obesity
Obesity, in particular visceral obesity, is a risk factor for the development of metabolic syndrome, cardiovascular disease 42 43 , blood hypercoagulability 44 , and vitamin D deficiency 45 , which are furthermore all risk factors for COVID-19 severity 46 .
A number of mechanisms are responsible for the increased risk of severe COVID-19 and mortality in people with adiposity 47 48 49 . One explanation may be the physical stress on ventilation by obstructing diaphragm excursion. Furthermore, obesity is associated with an increased risk of pulmonary fibrosis, chronic obstructive pulmonary disorder, and reduced respiratory function 50 .
Another reason is an impairment of the immune system in people with adiposity. Obesity is characterized by hyperplasia and hypertrophy of adipocytes and accumulation of macrophages in the adipose tissue, resulting in the development of crown-like structures of necrotic adipocytes encircled by macrophages 42 ( Fig. 1 ). In obesity, a switch from an anti-inflammatory M2 type to the pro-inflammatory M1 form of macrophages is observed 14 . Adiponectin can also affect macrophages by stimulating the production of anti-inflammatory cytokines 51 . Similarly, adiponectin-deficient mice display an increased expression of pro-inflammatory M1 type markers and decreased anti-inflammatory M2 type markers 52 . Thereby, obesity may lead to a baseline state of chronic inflammation. In adipose tissue of people with obesity the expression of pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, is upregulated.
Fig. 1.
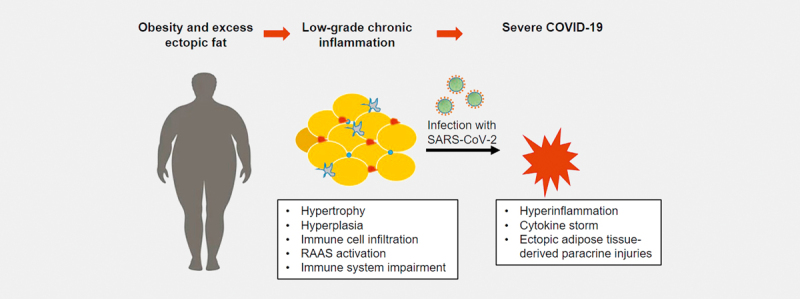
Chronic inflammation in adipose tissue of obese individuals: There are several reasons why obesity can lead to a severe course of COVID-19. One possible cause is the chronic inflammatory reaction in the adipose tissue. In adipose tissue with hypertrophic adipocytes, there is a mass production of pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α. In addition, more and more immune cells invade the adipose tissue. These cells produce inflammatory substances themselves. Being overweight thereby leads to a low-grade chronic inflammation. If an infection with SARS-CoV-2 then occurs, there is a high risk of an overreaction of the immune system leading to hyperinflammation and cytokine storm. This represents a potentially life-threatening derailment of the immune system, which can further lead to paracrine injuries.
In patients who died from COVID-19, a higher prevalence of CD68-positive macrophages in visceral adipose tissue was observed compared to control patients without COVID-19. As expected, these were accompanied by crown-like structures, signs of adipocyte stress and death 46 .
Previously, obesity was shown to increase the duration of type A influenza virus shedding in adults, whereas this was not the case for type B influenza 53 . Adipocytes and other adipose tissue-resident cells, such as adipo-stromal cells, and macrophages have also been shown to be targets for adenovirus subtype 36, but not subtype 2 54 . Therefore, it has been suggested that adipose tissue may act as a reservoir for the SARS-CoV-2 virus, whereby it would facilitate the spread of the virus and stimulate the immune response 54 . Indeed, a recent study showed that SARS-CoV-2 RNA could be found in adipose tissue of both men and women that had died due to COVID-19. In male individuals who were obese with a body mass index (BMI) >30, SARS-CoV-2 could also be detected in the liver. In women, there was no correlation between BMI and viral load in the adipose tissue 55 . In another study, the presence of SARS-CoV-2 in adipose tissue was confirmed in more than 60% of COVID-19 autopsy cases. In 25 out of the 29 COVID-19 cases in this study, comorbidities were present with 34% patients being overweight or with obesity 56 .
It was demonstrated that SARS-CoV-2 is able to infect mature differentiated and lipid-laden adipocytes but not preadipocytes or immature precursors 55 . Whether this is due to different ACE2 concentrations, or another mechanism is not known yet. An alteration in carbon metabolism with increased circulating levels of glucose and free fatty acids were observed in COVID-19 patients 57 . High levels of such free fatty acids may increase the levels of adipokines, myokines and cytokines, which further promote inflammatory processes. Furthermore, cytokines are able to damage the vascular endothelium and activate the RAAS, which may lead to increased blood pressure, atherosclerosis, and thrombosis 58 .
This chronic inflammation and imbalance between pro-inflammatory and anti-inflammatory factors in obesity is a risk factor for additional pathogenic infections, such as SARS-CoV-2, which may lead to an abnormal immune response reaching pathogenic levels 1 .
The upregulation of TNF-α, IL-6 and IL-1β in people with obesity inhibits insulin signaling 59 , and consecutively this cytokine upregulation leads to an increase in leptin and plasminogen activator inhibitor-1 and a reduced release of adiponectin 60 . An inverse correlation to glucose intolerance and type 2 diabetes has been observed 61 . Adiponectin modulates a number of metabolic processes, including glucose regulation and fatty acid oxidation 62 . Low adiponectin blood levels thereby cause an inappropriate increase in the immune response in COVID-19.
Overall, these impairments of the immune system may contribute to a chronic state of low-grade inflammation in the ectopic visceral adipose tissue in people with obesity ( Fig. 1 ). In combination with an infection like SARS-CoV-2, this may lead to an overreaction of the immune system, a so-called hyperinflammation resulting in a cytokine storm that can lead to paracrine injuries in other organs with progression to acute respiratory syndrome 63 .
Post-COVID and long-term consequences in relation to obesity
During the COVID-19 pandemic, social isolation and (semi)-lockdown were imposed upon populations in the interest of infection control. All over the world, obesity increased during the pandemic due to dramatic changes in the daily routines, such as a reduction in physical activity and negative changes in the eating habits 64 . In the US, the COVID-19 pandemic promoted weight gains in adults with those already being obese being more susceptible 65 . However, in particular children with obesity have been shown to be at a higher risk of negative lifestyle changes and weight gain during lockdown 66 . As such, several studies have shown that not just adults gain weight, but that also obesity in adolescents and children has increased due to COVID-19 lockdowns 67 . For example, in China, a study performed on 12 889 Chinese college students aged 17–27 years showed that their weight significantly increased during a 4-month lockdown in early 2020. This weight gain was associated with increased sedentary time and an increase in COVID-19-related stress and depression 68 . Another study from South Korea showed that in 226 children between 4 and 14 years old, school closure was significantly associated with an increased BMI 69 .
Different studies have shown that an unhealthy, high-fat diet might increase the susceptibility to various infectious diseases 70 . For example, experimental animals on a high-fat diet had exhibited a two-fold increase in mortality, an enhancement in respiratory lesions and an increased production of cytokines when infected with H1N1 influenza 71 . The individual nutrition pattern is also known to be able to change the gut microbiota, which might cause metabolic changes that might affect the susceptibility for getting infected with SARS-COV-2 in a positive or negative direction 70 .
Numerous factors contribute to childhood and adolescent obesity, including amongst others gender, biology, geographical and socio-economical aspects 72 73 74 . Non-communicable diseases, such as overweight and obesity are largely preventable. At the individual level, people can choose to limit energy intake by eating healthier food consisting of, for example, fruit, vegetables and whole grains. Furthermore, regular physical activity spread throughout the week is important. However, for individuals to follow these recommendations, supportive environments and communities are fundamental in shaping people’s mind, by making the choice of healthier foods and regular physical activity the easiest choice. This means that the healthiest alternative should be accessible, available and affordable 8 .
Evidence from the SARS-CoV-1 outbreak in 2002–2003 suggests that there is a likelihood of long-term metabolic sequelae from COVID-19. In survivors of SARS-CoV-1, long-term metabolic abnormalities including dyslipidemia and cardiovascular disease as well as signs of abnormal glucose metabolism with insulin resistance and hyperglycemia, and diabetes have been observed for up to 12 years 75 76 . More and more studies are emerging showing similar tendencies after infections with SARS-CoV-2, where up to 40% of people that were infected with SARS-CoV-2 suffer from symptoms of long-COVID 77 78 79 , such as difficulties in concentration, cognitive dysfunction, amnesia, depression, fatigue, and anxiety 80 81 82 . Therefore, people post discharge following COVID-19 will need close monitoring for risk factor control 83 .
To avoid severe COVID-19, vaccination was proven to be highly effective 84 . However, currently a high number of SARS-CoV-2 vaccine breakthrough infections and reinfections occur when people are exposed to the Omicron SARS-CoV-2 variants. The relationship between obesity and vaccine efficacy remains unclear, but as T-cell responses in obese individuals are impaired, it might imply that COVID-19 vaccines are less effective in obese individuals 85 . This was supported in latest findings indicating that obesity and other metabolic dysfunctions might promote vaccine breakthrough SARS-CoV-2 infections 84 86 87 . Furthermore, for reinfections, it was recently shown that at least one of the comorbidities obesity, diabetes, asthma, heart disease, lung disease, and high blood pressure was present in 50% of all cases 88 .
Conclusion
The number of people with overweight and obesity is increasing all over the world making these people more susceptible to infectious diseases, such as COVID-19, which in the current corona pandemic turned out to be devastating. Therefore, the importance of preventing obesity already from childhood on has been further put into the spotlight 89 . The COVID-19 pandemic has taught us that nutrition education interventions, access to healthy food, as well as family nutrition counselling should be covered by pediatric services to prevent obesity, which worsens disease outcomes related to SARS-CoV-2 infection and to potential other new epidemics in the future 66 . Individually-targeted evidence-based health promotion, weight management, behavioral change and psycho-social support services need vigorous support from physicians and other health personnel 90 .
Acknowledgement
We thank Martina Talke, Nitzan Bornstein, Gregor Müller and Aline Günther for their contribution. The study was supported by GWT, the Deutsche Forschungsgemeinschaft (DFG, German Research foundation) project no. 314061271, TRR 205/2: „The Adrenal: Central Relay in Health and Disease“ and project no. 288034826, IRTG 2251: „Immunological and Cellular Strategies in Metabolic Disease“.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Steenblock C, Schwarz PE H, Ludwig B et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Hearn M, Liu J, Cudhea F et al. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10:e019259. doi: 10.1161/JAHA.120.019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goossens G H, Dicker D, Farpour-Lambert N J et al. Obesity and COVID-19: a perspective from the European association for the study of obesity on immunological perturbations, therapeutic challenges, and opportunities in obesity. Obes Facts. 2020;13:439–452. doi: 10.1159/000510719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Discacciati M G, Siani S, Campa A et al. Why should obese youth be prioritized in COVID-19 vaccination programs? A nationwide retrospective study. Lancet Reg Health Am. 2022;7:100167. doi: 10.1016/j.lana.2021.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Palmieri L, Vanacore N et al. Nonrespiratory complications and obesity in patients dying with COVID-19 in Italy. Obesity (Silver Spring) 2021;29:20–23. doi: 10.1002/oby.23007. [DOI] [PubMed] [Google Scholar]
- 6.Tsankov B K, Allaire J M, Irvine M A et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180:2019–2034. doi: 10.1007/s00431-021-03993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation WHO . 2022. Obesity and overweight. In: [Google Scholar]
- 9.Sattar N, McInnes I B, McMurray JJ V. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 10.Sudhakar M, Winfred S B, Meiyazhagan G et al. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol Cell Biochem. 2022;477:1155–1193. doi: 10.1007/s11010-022-04356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Zhang X, Ye S et al. Obesity and COVID-19: mechanistic insights from adipose tissue. J Clin Endocrinol Metab. 2022;107:1799–1811. doi: 10.1210/clinem/dgac137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudhakar M, Winfred S B, Meiyazhagan G et al. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol Cell Biochem. 2022;477:1155–1193. doi: 10.1007/s11010-022-04356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen A R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 14.Hornung F, Rogal J, Loskill P et al. The inflammatory profile of obesity and the role on pulmonary bacterial and viral infections. Int J Mol Sci. 2021;22:3456. doi: 10.3390/ijms22073456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch C A, Sharda P, Patel J et al. Climate change and obesity. Horm Metab Res. 2021;53:575–587. doi: 10.1055/a-1533-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner J B, Kumar A, Koch C A. The effects of indoor and outdoor temperature on metabolic rate and adipose tissue – the Mississippi perspective on the obesity epidemic. Rev Endocr Metab Disord. 2016;17:61–71. doi: 10.1007/s11154-016-9358-z. [DOI] [PubMed] [Google Scholar]
- 17.Karastergiou K, Fried SK. Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. Adv Exp Med Biol. 2017;1043:29–51. doi: 10.1007/978-3-319-70178-3_3. [DOI] [PubMed] [Google Scholar]
- 18.Calle E E, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 19.Wellen K E, Hotamisligil G S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VdO Leal, Mafra D. Adipokines in obesity. Clinica Chimica Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Cawthorn W P, Scheller E L, Learman B S et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajzová M, Kováciková M, Vítková M et al. Retinol-binding protein 4 expression in visceral and subcutaneous fat in human obesity. Physiol Res/Acad Sci Bohem. 2008;57:927–934. doi: 10.33549/physiolres.931379. [DOI] [PubMed] [Google Scholar]
- 23.Zha J M, Di W J, Zhu T et al. Comparison of gene transcription between subcutaneous and visceral adipose tissue in Chinese adults. Endocr J. 2009;56:935–944. doi: 10.1507/endocrj.k09e-091. [DOI] [PubMed] [Google Scholar]
- 24.Vijay J, Gauthier M F, Biswell R L et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon S, Taylor P R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Sica A, Sozzani S et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Lumeng C N, Bodzin J L, Saltiel A R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill D A, Lim H W, Kim Y H et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oz M, Lorke D E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed Pharmacother. 2021;136:111193. doi: 10.1016/j.biopha.2020.111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls A C, Park Y J, Tortorici M A et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–2.92E288. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oz M, Lorke D E, Kabbani N. A comprehensive guide to the pharmacologic regulation of angiotensin converting enzyme 2 (ACE2), the SARS-CoV-2 entry receptor. Pharmacol Ther. 2021;221:107750. doi: 10.1016/j.pharmthera.2020.107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoes e Silva A C, Silveira K D, Ferreira A J et al. ACE2, angiotensin-(1-7) and mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swamy S, Koch C A, Hannah-Shmouni F et al. Hypertension and COVID-19: updates from the era of vaccines and variants. J Clin Transl Endocrinol. 2022;27:100285. doi: 10.1016/j.jcte.2021.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Zorita S, Milton-Laskibar I, Garcia-Arellano L et al. An overview of adipose tissue ACE2 modulation by diet and obesity. Potential implications in COVID-19 infection and severity. Int J Mol Sci. 2021;22:7975. doi: 10.3390/ijms22157975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cauwenberghs N, Prunicki M, Sabovcik F et al. Temporal changes in soluble angiotensin-converting enzyme 2 associated with metabolic health, body composition, and proteome dynamics during a weight loss diet intervention: a randomized trial with implications for the COVID-19 pandemic. Am J Clin Nutr. 2021;114:1655–1665. doi: 10.1093/ajcn/nqab243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soll D, Beer F, Spranger L et al. Effects of weight loss on adipose and muscular neuropilin 1 mRNA expression in obesity: potential implication in SARS-CoV-2 infections? Obes Facts. 2022;15:90–98. doi: 10.1159/000520419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shakartalla S B, Alhumaidi R B, Shammout OD A et al. Dyslipidemia in breast cancer patients increases the risk of SAR-CoV-2 infection. Infect Genet Evol. 2021;92:104883. doi: 10.1016/j.meegid.2021.104883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alketbi E H, Hamdy R, El-Kabalawy A et al. Lipid-based therapies against SARS-CoV-2. infection. 2021;31:e2214. doi: 10.1002/rmv.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soliman S, Faris M E, Ratemi Z et al. Switching host metabolism as an approach to dampen SARS-CoV-2 infection. Ann Nutr Metab. 2020;76:297–303. doi: 10.1159/000510508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alzaabi M M, Hamdy R, Ashmawy N S et al. Flavonoids are promising safe therapy against COVID-19. Phytochem Rev. 2022;21:291–312. doi: 10.1007/s11101-021-09759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo L, Lumeng C N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valencak T G, Osterrieder A, Schulz T J. Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017;12:806–813. doi: 10.1016/j.redox.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kott K A, Morel-Kopp M C, Vernon S T et al. Association of global coagulation profiles with cardiovascular risk factors and atherosclerosis: a sex disaggregated analysis from the BioHEART-CT study. J Am Heart Assoc. 2021;10:e020604. doi: 10.1161/JAHA.120.020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golzarand M, Hollis B W, Mirmiran P et al. Vitamin D supplementation and body fat mass: a systematic review and meta-analysis. Eur J Clin Nutr. 2018;72:1345–1357. doi: 10.1038/s41430-018-0132-z. [DOI] [PubMed] [Google Scholar]
- 46.Colleluori G, Graciotti L, Pesaresi M et al. Visceral fat inflammation and fat embolism are associated with lung’s lipidic hyaline membranes in subjects with COVID-19. Int J Obes (Lond) 2022;46:1009–1017. doi: 10.1038/s41366-022-01071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoong CW S, Hussain I, Aravamudan V M et al. Obesity is associated with poor Covid-19 outcomes: a systematic review and meta-analysis. Horm Metab Res. 2021;53:85–93. doi: 10.1055/a-1326-2125. [DOI] [PubMed] [Google Scholar]
- 48.Seidu S, Gillies C, Zaccardi F et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: a systematic review and meta-analysis. Endocrinol Diabetes Metab. 2020;4:e00176. doi: 10.1002/edm2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shekhar S, Wurth R, Kamilaris CD C et al. Endocrine conditions and COVID-19. Horm Metab Res. 2020;52:471–484. doi: 10.1055/a-1172-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayres J S. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2:572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumada M, Kihara S, Ouchi N et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 52.Ohashi K, Parker J L, Ouchi N et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maier H E, Lopez R, Sanchez N et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan P M, Caplice N M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in Coronavirus disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zickler M, Stanelle-Bertram S, Ehret S et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Cell Metab. 2022;34:1–2. doi: 10.1016/j.cmet.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poma A M, Bonuccelli D, Giannini R et al. COVID-19 autopsy cases: detection of virus in endocrine tissues. J Endocrinol Invest. 2022;45:209–214. doi: 10.1007/s40618-021-01628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas T, Stefanoni D, Reisz J A et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5:e140327. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasheghani M, Hessami Z, Rekabi M et al. Evaluating possible mechanisms linking obesity to COVID-19: a narrative review. Obes Surg. 2022;32:1689–1700. doi: 10.1007/s11695-022-05933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boucher J, Kleinridders A, Kahn C R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amin M N, Hussain M S, Sarwar M S et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab Syndr. 2019;13:1213–1224. doi: 10.1016/j.dsx.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 61.Turer A T, Khera A, Ayers C R et al. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diez J J, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 63.Lasbleiz A, Gaborit B, Soghomonian A et al. COVID-19 and obesity: role of ectopic visceral and epicardial adipose tissues in myocardial injury. Front Endocrinol (Lausanne) 2021;12:726967. doi: 10.3389/fendo.2021.726967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manca R, Bombillar F, Glomski C et al. Obesity and immune system impairment: A global problem during the COVID-19 pandemic. Int J Risk Saf Med. 2022;33:193–208. doi: 10.3233/JRS-227007. [DOI] [PubMed] [Google Scholar]
- 65.Seal A, Schaffner A, Phelan S et al. COVID-19 pandemic and stay-at-home mandates promote weight gain in US adults. Obesity (Silver Spring) 2022;30:240–248. doi: 10.1002/oby.23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cena H, Fiechtner L, Vincenti A et al. COVID-19 pandemic as risk factors for excessive weight gain in pediatrics: the role of changes in nutrition behavior. A narrative review. Nutrients. 2021;13:4255. doi: 10.3390/nu13124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiess W, Kirstein A S, Stein R et al. Obesity after the Covid-19 pandemic and beyond. J Pediatr Endocrinol Metab. 2022;35:135–138. doi: 10.1515/jpem-2022-2135. [DOI] [PubMed] [Google Scholar]
- 68.Dun Y, Ripley-Gonzalez J W, Zhou N et al. Weight gain in Chinese youth during a 4-month COVID-19 lockdown: a retrospective observational study. BMJ Open. 2021;11:e052451. doi: 10.1136/bmjopen-2021-052451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang H M, Jeong D C, Suh B K et al. The impact of the Coronavirus disease-2019 pandemic on childhood obesity and vitamin D status. J Korean Med Sci. 2021;36:e21. doi: 10.3346/jkms.2021.36.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller L, Berber E, Sumbria D et al. Controlling the burden of COVID-19 by manipulating host metabolism. Viral Immunol. 2022;35:24–32. doi: 10.1089/vim.2021.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milner J J, Rebeles J, Dhungana S et al. Obesity increases mortality and modulates the lung metabolome during pandemic H1N1 influenza virus infection in mice. J Immunol. 2015;194:4846–4859. doi: 10.4049/jimmunol.1402295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinson M L, Chang Y L, Han W J et al. Child overweight and obesity in Shanghai, China: contextualizing Chinese socioeconomic and gender differences. Int J Behav Med. 2018;25:141–149. doi: 10.1007/s12529-017-9688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nogueira H, Costeira EPM M, Costa D et al. The environment contribution to gender differences in childhood obesity and organized sports engagement. Am J Hum Biol. 2020;32:e23322. doi: 10.1002/ajhb.23322. [DOI] [PubMed] [Google Scholar]
- 74.Shah B, Tombeau Cost K, Fuller A et al. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr Prev Health. 2020;3:387–390. doi: 10.1136/bmjnph-2020-000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q, Zhou L, Sun X et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7:9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J K, Lin S S, Ji X J et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blomberg B, Mohn K G, Brokstad K A et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavli A, Theodoridou M, Maltezou H C. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575–581. doi: 10.1016/j.arcmed.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tabacof L, Tosto-Mancuso J, Wood J et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehabil. 2022;101:48–52. doi: 10.1097/PHM.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Y, Bitna H, Kim S W et al. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. 2022;22:93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nasserie T, Hittle M, Goodman S N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4:e2111417. doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rogers J P, Chesney E, Oliver D et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet. Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khunti K, Davies M J, Kosiborod M N et al. Long COVID – metabolic risk factors and novel therapeutic management. Nat Rev Endocrinol. 2021;17:379–380. doi: 10.1038/s41574-021-00495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dagan N, Barda N, Kepten E et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanckova M, Betakova T. Pandemics of the 21st century: the risk factor for obese people. Viruses. 2021;14:25. doi: 10.3390/v14010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Juthani P V, Gupta A, Borges K A et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21:1485–1486. doi: 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stefan N. Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat Rev Endocrinol. 2022;18:75–76. doi: 10.1038/s41574-021-00608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahman S, Rahman M M, Miah M et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci Rep. 2022;12:1438. doi: 10.1038/s41598-022-05325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saliba K, Cuschieri S. Amidst the COVID-19 pandemic childhood obesity is still an epidemic-spotlight on obesity’s multifactorial determinants. Health Sci Rev (Oxf) 2021;1:100006. doi: 10.1016/j.hsr.2021.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petrie J R, Boyle J G, Ali K et al. A post COVID-19 ‘Marshall Plan’ for type 2 diabetes. Diabet Med. 2021;38:e14439. doi: 10.1111/dme.14439. [DOI] [PMC free article] [PubMed] [Google Scholar]


