Abstract
The zoonotic potential to cause human and/or animal infections among multidrug-resistant extraintestinal pathogenic Escherichia coli from avian origin was investigated. Twenty-seven extraintestinal pathogenic E. coli isolates containing the increased survival gene (iss) were obtained from the livers of healthy and diseased poultry carcasses at two slaughterhouses in Salvador, northeastern Brazil. The antimicrobial resistance-susceptibility profiles were conducted with antibiotics of avian and/or human use by the standardized disc-diffusion method. Antimicrobial resistance was higher for levofloxacin (51.8%), amoxicillin/clavulanic acid (70.4%), ampicillin (81.5%), cefalotin (88.8%), tetracycline (100%) and streptomycin (100%). The minimum inhibitory concentrations above the resistance breakpoints of doxycycline, neomycin, oxytetracycline and enrofloxacin reached, respectively, 88.0%, 100%, 75% and 91.7% of the isolates. Strains with high and low antimicrobial resistance were i.p. administered to Swiss mice, and histopathological examination was carried out seven days after infection. Resistance to goat and human serum complement was also evaluated. The results show that Swiss mice challenged with strain 2B (resistant to 11 antimicrobials) provoked a severe degeneration of hepatocytes besides lymphocytic infiltration in the liver, whereas the spleen showed areas of degeneration of the white and red pulp. Conversely, the spleen and liver of mice challenged with strain 4A (resistant to two antimicrobials) were morphologically preserved. In addition, complement resistance to goat and human serum was high for strain 2B and low for strain 4A. Our data show that multidrug resistance and pathogenesis can be correlated in extraintestinal pathogenic E. coli strains obtained from apparently healthy poultry carcasses, increasing the risk for human public healthy.
Keywords: ExPEC, MDR strains, Increased survival gene, Experimental infections
Introduction
The spread of multidrug resistance among avian Escherichia coli is usually attributed to the selective pressure exerted by the antimicrobials included in broiler feed for the past 60 years.1 Johnson et al.2 reinforced this suspicion since antibiotic-resistant and antibiotic-susceptible E. coli isolates from retail poultry products had similar phylogenetic background, and otherwise emerged from the same source population. Regarding antimicrobial exposure in poultry production, multidrug resistance among extraintestinal pathogenic E. coli (ExPEC) allied to community acquired infections is increasing in prevalence in many parts of the globe.3 The indiscriminate use of antimicrobials is high in developing countries, where human antibiotics are often accessible for non-therapeutic uses in healthy animals.4 Therefore, colonization of asymptomatic poultry by multidrug resistant ExPEC augments the probability of resistance gene acquisition by human strains through the food-chain.
Avian pathogenic E. coli (APEC) is commonly reported as an ExPEC pathotype, but its genotype is not clearly defined. According to Kwon et al.5 at least five virulence genes are present in APEC, many of them found in plasmid pTJ100.6 However, the increased survival gene (iss) has been identified as a virulence marker to distinguish between avian and human ExPEC.7 This gene exerts anti-complement resistance and has been found in conserved regions of ColV and ColBM plasmids,8, 9 which are often identified in APEc strains.6, 10 Recent findings also suggest that APEC and human neonatal meningitis E. coli (NMEC) are subpathotypes from ExPEC involved in pathogenesis of both avian and human infections.11 Therefore, hybrid plasmids (ColV- and ColBM-associated plasmids) harboring a number of distinct virulence genes and MDR-encoding islands can also be present in ExPEC isolates.11 Consequently, it is now clear that selected high-virulent and multidrug resistant ExPEC strains of avian origin represent subpathotypes of great danger to human public health.
Brazil has ranked first in the world in exports of poultry meat since 2004.12 Typical selections of poultry in Brazilian slaughterhouses take into account macroscopic alterations that can result in discarding carcasses.13 However, sub-clinical symptoms are not always perceived and often do not show a clue of the presence of pathogenic microorganisms. Thus, the risk of virulence and drug resistance gene transmission between avian E. coli obtained trough the food-chain and intestinal commensal E. coli is increasing. The goal of the present study was to investigate the pathogenesis to mammalian hosts of selected multidrug resistant ExPEC strains obtained from healthy poultry carcasses in Salvador, Brazil.
Material and methods
ExPEC strains
Extraintestinal pathogenic E. coli strains were obtained from the livers of poultry carcasses with (n = 9) or without (n = 18) macroscopic alterations at two slaughterhouses in Salvador, capital of the state of Bahia, northeast Brazil (Table 1). Carcasses without macroscopic alterations were considered healthy and approved for human consumption. The organs were collected under sterile conditions. All bacterial strains were identified through biochemical tests, and the presence of the iss gene was determined according to the method reported in a previous study.14 The isolates were maintained in tubes containing brain heart infusion agar at 8 °C until use.
Table 1.
ExPEC strains obtained from the liver of poultry carcasses.
| E. coli | Macroscopic aspect | Presence of gene issa | Presence of gene stxb |
|---|---|---|---|
| 4A | No alteration | + | − |
| 5A | No alteration | + | − |
| 36A | No alteration | + | − |
| 41A | No alteration | + | − |
| 43A | No alteration | + | + |
| 44A | No alteration | + | − |
| 2B | No alteration | + | − |
| 5B | No alteration | + | − |
| 37B | No alteration | + | − |
| 38B | No alteration | + | + |
| 39B | No alteration | + | − |
| 42B | No alteration | + | − |
| 15C | No alteration | + | − |
| 21C | No alteration | + | − |
| 42C | No alteration | + | − |
| 32 | No alteration | + | − |
| 35 | No alteration | + | − |
| 46 | No alteration | + | + |
| 24A | Salmonella septicemia | + | − |
| 30C | Salmonella septicemia | + | − |
| 31C | Salmonella septicemia | + | − |
| 48A | Ascitis | + | − |
| 48B | Ascitis | + | − |
| 52B | Cachexy | + | − |
| 54B | Cachexy | + | − |
| 60A | Colibacillosis | + | − |
| 55B | Colibacillosis | + | − |
Increased serum survival gene.
Shiga-like toxin gene.
Antimicrobial resistance-susceptibility profile of ExPEC strains
The resistance-susceptibility profiles to antimicrobials of avian or human use were determined by the standard disc-diffusion method, following the recommendations of the Clinical and Laboratory Standards Institute (formally, National Committee for Clinical Laboratory Standards).15 Briefly, filter paper discs 5 mm in diameter impregnated with antibiotics (Cecon) were added to cultures in Petri dishes (0,5 on the McFarland scale, corresponding to 108 cells/mL) containing Mueller–Hinton agar (Oxoid). After 24 h incubation at 35 °C, the diameter of the inhibition zone was measured with a caliper. All tests were carried out in duplicate against 13 antimicrobials and the results were interpreted as sensitive, moderately sensitive or resistant. The breakpoints for resistance were those recommended by the CLSI. The overall resistance rate was calculated as the number of non-susceptible isolates divided by the total number of isolates. Multidrug resistance was determined when bacterial isolates were resistant to four antimicrobials of at least three different classes. The isolates obtained from a single sample with an identical antibiotic resistance/sensitivity profile were treated as a single strain. In this case, bacterial replicates were not conducted.
Minimum inhibitory concentration of typical antimicrobials used on Brazilian farms
The minimum inhibitory concentration (MIC) of antimicrobials commonly used on Brazilian farms (doxycycline, neomycin, oxytetracycline and enrofloxacin) was measured through the broth dilution method in concentrations ranging from 1,9 to 1000 μg/mL.16 The MIC was the lowest concentration that caused visible inhibition of growth, while the minimum bactericidal concentration (MBC) was the lowest concentration resulting in no growth after the incubation period of 24 h at 37 °C. All assays were performed in duplicate with 25 ExPEC strains resistant to 4–11 antimicrobials through the disc-diffusion method. A list of antimicrobials authorized by the Brazilian government to be included in broiler feed is shown in Table 2.
Table 2.
Antimicrobials authorized by Brazilian authorities used as growth promoters in broiler feed.
| Antimicrobial class | Antimicrobials | Dosage (g/ton) |
|---|---|---|
| Oligosaccharide | Avilamicin | 2,5–10 |
| Peptide | Bacitracin methylene disalicylate | 4–55 |
| Peptide | Zinc bacitracin | 4–55 |
| Benzene derivative | Chlorhexidine | 10–20 |
| Macrolide | Spiramycin | 5 |
| Peptide | Enramycin | 3–10 |
| Phosphoglycolipids | Flavomycin | 1–2 |
| Lincosamide | Lincomycin | 2,2–4,4 |
| Peptide | Colistin sulfate | 2–10 |
| Streptogramin | Virginiamycin | 5,5–16.5 |
| Quinolone | Clorohidroxiquinolin | 15–30 |
| Macrolide | Tylosin tartrate/phosphate | 4–55 |
Source: Brazilian Ministery of Agriculture.
Complement resistance assay
The complement resistance test was carried out with selected ExPEC strains obtained from healthy carcasses, and with distinct drug resistance profile, following the method adapted from Samuelsen et al.17 Samples of blood were obtained from goats and a healthy human volunteer under sterile conditions and allowed to coagulate. The blood was centrifuged (7000 rpm/5 min) and blood serum was separated into a new tube. Briefly, 190 μL of the serum plus 10 μL of E. coli strains (107 cells/mL) were cultured together in wells of sterile Elisa plates, and incubated at 37 °C for 180 min. Then, aliquots of 10 μL were sampled at times 0, 60, 120 and 180 min, and added to Petri dishes containing MacConkey agar for enumeration of the colony forming units. The absence of specific antibodies for the ExPEC strains used in this study was confirmed by in vitro agglutination tests.
Animals
Adult male Swiss mice weighing approximately 35 g, obtained from Keizo-Azami Immunopathology Laboratory (LIKA/UFPE), were used. The animals were kept in an animal house with free access to water and commercial sterile diet (Purina, Paulínia, SP, Brazil). The mice were handled according to established experimental procedures.
Experimental infection with selected ExPEC strains
The mice were separated into three groups (n = 5) and challenged by the intraperitoneal (i.p.) route with 0,2 mL of bacterial suspensions containing 106 CFU/mL. Experimental infections were carried out with strains 4A, 41A and 2B. Clinical symptoms such as prostration, weight loss and mortality were observed daily for seven days post-infection. Survivors were sacrificed under anesthesia with Halothane (Halocarbon Laboratories, USA). Sections of the liver were submitted to enumeration of colony forming units (CFU/g) and histological examination.
Enumeration of colony forming units
The spleens and livers were dissected, weighed and macerated in PBS (1:10 or 1:100, w/v) under sterile conditions. Serial decimal dilutions were made and 0,1 mL aliquots were plated onto MacConkey agar (Oxoid). The colonies were counted after incubation at 37 °C for 24 h and the results expressed as CFU/g of organ.
Histological examination
Tissues were fixed in 10% formaldehyde and processed for paraffin embedding. The sections (5 μm) were stained with hematoxylin–eosin and the slides were coded and examined by a single pathologist, who was unaware of the experimental conditions of each group.
Statistical analyses
The statistical significance of data was assessed by analysis of variance (ANOVA), followed by Student's t-test. The level of significance was determined as p < 0,05.
Results
The antimicrobial resistance-susceptibility profiles of 27 extraintestinal pathogenic E. coli strains from carcasses of healthy and diseased poultry are shown in Table 3. The majority of ExPEC were resistant to at least four antibiotics from different classes. The most prevalent phenotypes were resistant to levofloxacin (51.8%), amoxicillin/clavulanic acid (70.4%), ampicillin (81.5%), cefalotin (88.8%), tetracycline (100%) and streptomycin (100%). The overall multidrug resistance varied from 4 to 11 antimicrobials and reached 92.6% of E. coli strains. In addition, 40.7% were simultaneously resistant to streptomycin, levofloxacin, ciprofloxacin and tetracycline. The proportion of highly multidrug resistant strains (8–11 antimicrobials) reached 22.2%. Conversely, the aminoglycoside amikacin of avian and human use were very effective against 89.9% of ExPEC. The MIC and MBC of typical antimicrobials used in Brazilian farms were determined for those ExPEC strains resistant to at least 4 antimicrobials. The level of resistance against doxycycline, neomycin, enrofloxacin and oxytetracycline reached, respectively, 88.0%, 100%, 75% and 91.7% of the strains (Table 4).
Table 3.
Resistance-susceptibility profiles of ExPEC strains to antimicrobials of avian or human use in Brazil.
| Antimicrobial class | Antimicrobials | Disc content (μg) | Use: avian (A) human (H) |
Resistance breakpoint (mm) | Healthy carcasse |
Diseased carcasse |
Overall Resistance %b | Resistant strains | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S %a | I % | R % | S % | I % | R % | |||||||
| Penicillin | Ampicillin | 10 | A/H | ≤13 | 11.2 | 5,5 | 83.3 | 11.3 | 11 | 77.7 | 81.5 | 44A, 15C, 48B, 36A, 43A, 5B, 21C, 42C, 35, 31C, 52B, 55B, 5A, 24A, 54B, 6 60A, 38B, 37B, 3 39B, 46, 41A, 2 2B |
| Aminoglycoside | Amikacin | 30 | A/H | ≤14 | 72.4 | 11 | 16.6 | 100 | 0 | 0 | 1,1 | 36A, 42B, 2B |
| Gentamicin | 10 | A/H | ≤12 | 61.3 | 11 | 27.7 | 56.6 | 0 | 44.4 | 33.3 | 32, 48B, 52B, 55B, 42B, 54B, 37B, 41A, 2B |
|
| Streptomycin | 10 | H | ≤11 | 0 | 0 | 100 | 0 | 0 | 100 | 100 | 4A, 48A, 32, 44A, 15C, 30C, 48B, 36A, 43A, 5B, 21C, 42C, 35, 31C, 52B, 55B, 5A, 42B, 24A, 54B, 60A, 38B, 37B, 39B, 46, 41A, 2B |
|
| Fluoroquinolone | Ciprofloxacin | 5 | A/H | ≤15 | 22.3 | 27.7 | 50 | 67.7 | 0 | 33.3 | 44.4 | 30C, 43A, 35, 42B, 24A, 60A, 38B, 37B, 39B, 46, 41A, 2B |
| Levofloxacin | 5 | H | ≤13 | 27.9 | 11 | 61.1 | 55.6 | 11.1 | 33.3 | 51.8 | 15C, 30C, 43A, 42C, 35, 5A, 42B, 24A, 60A, 38B, 37B, 46, 41A, 2B |
|
| Phenicol | Chloramphenicol | 30 | A/H | ≤12 | 66.8 | 5,5 | 27.7 | 88.9 | 11.1 | 0 | 18.5 | 37B, 39B, 46, 41A, 2B |
| Cephalosporin | Ceftazidim | 30 | H | ≤14 | 56.7 | 27.7 | 16.6 | 66.7 | 22.2 | 11.1 | 14.8 | 54B, 39B, 46, 41A |
| Cefalotin | 30 | H | ≤14 | 5,7 | 5,5 | 88.8 | 0 | 11.1 | 88.9 | 88.8 | 32, 44A, 15C, 30C, 48B, 36A, 43A, 5B, 21C, 42C, 31C, 52B, 55B, 5A, 42B, 24A, 54B, 60A, 38B, 37B, 39B, 46, 41A, 2B |
|
| Carbapenem | Imipenem | 10 | H | ≤13 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | - |
| Tetracycline | Tetracycline | 30 | A/H | ≤14 | 0 | 0 | 100 | 0 | 0 | 100 | 100 | 4A, 48A, 32, 44A, 15C, 30C, 48B, 36A, 43A, 5B, 21C, 42C, 35, 31C, 52B, 55B, 5A, 42B, 24A, 54B, 60A, 38B, 37B, 39B, 46, 41A, 2B |
| Monobactam | Aztreonam | 30 | H | ≤15 | 33.8 | 22.2 | 44 | 44.5 | 44.4 | 11.1 | 33.3 | 5B, 21C, 31C, 5A, 54B, 38B, 9B, 46, 41A, 2B |
| Beta-lactam/beta-lactamase inhibitor | Amoxycillin/clavulanic acid | 20/10 | H | ≤13 | 22.3 | 5,5 | 72.2 | 33.4 | 0 | 66.6 | 70.4 | 44A, 36A, 5B, 21C, 42C, 35, 31C, 52B, 55B, 5A, 24A, 54B, 60A, 38B, 37B, 39B, 46, 41A, 2B |
Percentage of E. coli strains sensitive (S), intermediary sensitive (I) or resistant (R) out of the total of isolates.
The overall resistance rate is given by the number of non-susceptible isolates divided by the total number of isolates submitted to antibiogram tests.
Table 4.
Resistance of multidrug resistant ExPEC strains from poultry carcasses to antimicrobials commonly used in Brazilian farms.
| Strain | Antimicrobials (μg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Doxycycline |
Neomycin |
Enrofloxacin |
Oxytetracycline |
|||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 4A | 125 | – | >1000 | – | 1,9 | 31.2 | >1000 | – |
| 48A | 31.2 | – | >1000– | – | 1,9 | 250 | >1000 | – |
| 32 | >1000 | – | 500 | 500 | 62.5 | 1000 | 250 | 250 |
| 44A | 62.5 | 500 | >1000 | – | 15.6 | 250 | >1000 | – |
| 15C | 62.5 | 1000 | >1000 | – | 62.5 | – | 500 | – |
| 30C | 1,9 | 250 | 500 | 500 | 1,9 | 1,9 | 1,9 | 500 |
| 48B | 125 | 125 | 125 | 125 | 1,9 | 62.5 | >1000 | – |
| 36A | 1,9 | 500 | 250 | 500 | 1,9 | 500 | >1000 | – |
| 43A | 62.5 | 500 | 62.5 | 250 | 31.2 | 250 | 250 | – |
| 5B | 62.5 | 500 | >1000 | – | 1,9 | 1000 | 250 | – |
| 21C | 125 | 500 | 500 | 1000 | 15.6 | – | 500 | 500 |
| 42C | 31.2 | – | 125 | 250 | 3,9 | 500 | 1000 | – |
| 35 | 125 | 250 | 500 | 500 | 31.2 | – | 62.5 | 500 |
| 31C | 125 | 500 | 250 | 250 | 15.6 | 1000 | >1000 | – |
| 52B | 31.2 | – | 500 | – | 7,8 | 500 | 500 | – |
| 55B | 62.5 | 1000 | >1000 | – | 31.2 | 1000 | 250 | – |
| 24A | 62.5 | 250 | >1000 | – | 125 | – | >1000 | – |
| 54B | 62.5 | 250 | >1000 | – | >1000 | – | 7,8 | 7,8 |
| 60A | 62.5 | 500 | >1000 | – | 15.6 | 1000 | 125 | – |
| 38B | 125 | 125 | >1000 | – | 62.5 | 125 | 125 | 125 |
| 37B | 62.5 | 125 | >1000 | – | 31.2 | 62.5 | 62.5 | – |
| 39B | 15.6 | 62.5 | 500 | 500 | 7,8 | 7,8 | >1000 | – |
| 46 | 62.5 | 125 | 500 | 500 | 31.2 | 250 | >1000 | – |
| 41A | 62.5 | 62.5 | >1000 | – | NT | NT | NT | NT |
| 2B | 125 | 125 | 125 | 125 | 31.2 | 31.2 | 1000 | – |
| Resistance breakpoint | ≥16 | ≥32 | ≥2 | ≥16 | ||||
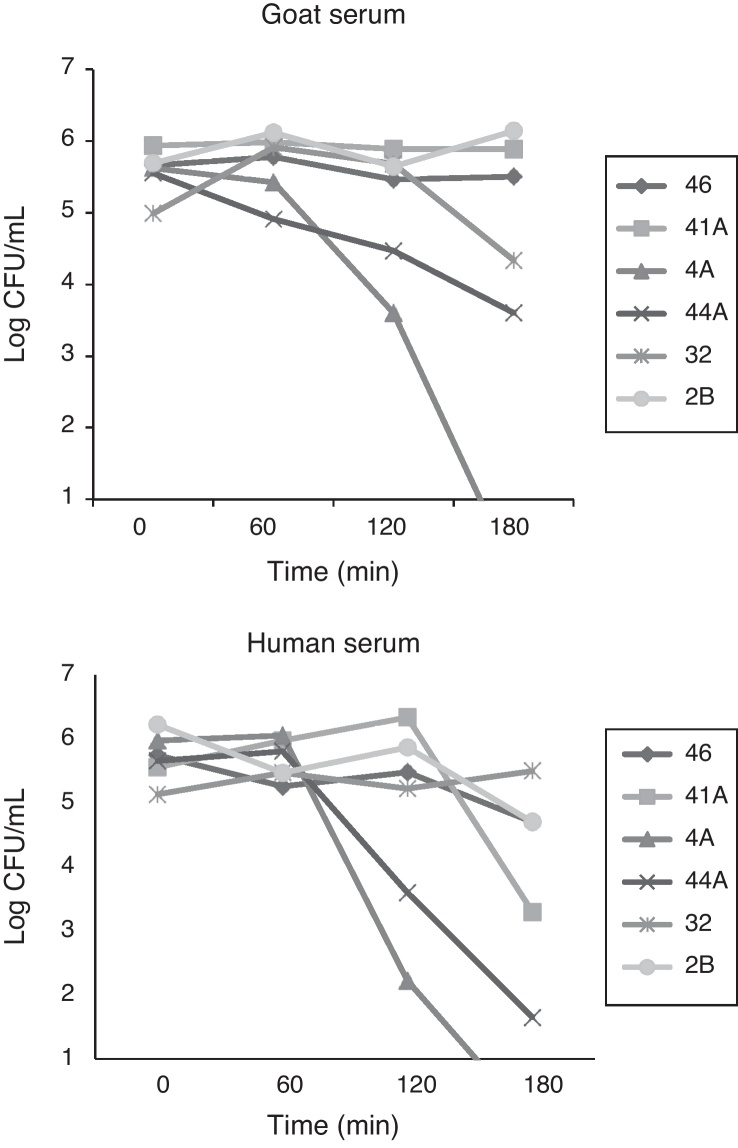
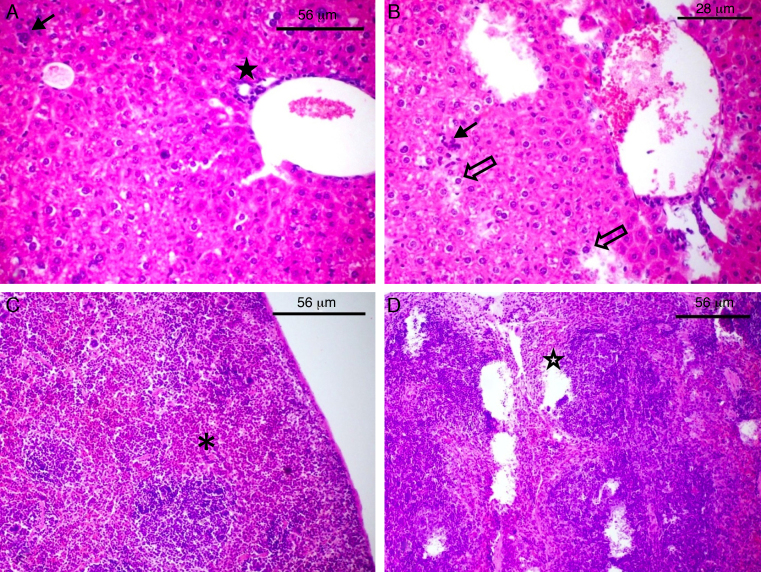
The complement resistance to goat serum was high for strains 2B and 46 and low for strains 4A and 41A, whereas resistance to human serum was high for strains 32, 46 and 2B and low for strains 44 and 4A (Fig. 1). Experiments carried out with laboratory animals have shown that Swiss mice challenged with strains 4A, 41A and 2B can survive seven days after infection. After this time, the bacterial load in the spleen and the liver reached respectively: 3,74 × 103 and 8,54 × 103 CFU/g for strain 4A; 4,8 × 102 and 1,56 × 103 CFU/g for strain 41A, and 4,0 × 101 and 1,2 × 104 CFU/g for strain 2B. However, strain 2B provoked severe degeneration of hepatocytes besides lymphocytic and lipid infiltration in the liver, whereas spleen showed areas of degeneration of the white and red pulp (Fig. 2B and D). Likewise, the liver of mice challenged with strain 41A showed marked vacuolization of hepatocytes and lymphocytic infiltration, whereas the spleen showed leukocyte infiltration and white pulp without boundaries (data not shown). Conversely, strain 4A showed hepatocytes, portal space and lobular veins preserved with mild lymphocytic infiltration in the liver. Also, the spleen was morphologically preserved with capsule, and white pulp with central arteriole besides red pulp (Fig. 2A and C). These histological findings were generally perceptible in all mice from each animal group independently of the bacterial load in the organs.
Fig. 1.
Resistance to serum complement of multidrug resistant avian ExPEC strains harboring the iss gene.
Fig. 2.
Histological damage to laboratory animals after challenge with avian ExPEC. The slides of the liver (A and B) and spleen (C and D) of Swiss mice challenged with strain 4A (A and C) and 2B (B and D) are shown. The full arrow (A and B) indicates leukocyte infiltration whereas empty arrow shows degeneration of hepatocytes (B). The full star shows portal space (A) and empty star shows degeneration of red besides white pulp (D). The asterisk shows the white pulp of the spleen in normal morphology (C).
Discussion
Poultry-associated diseases caused by ExPEC cause massive economic losses in the food industry.18, 19, 20 However, the broad use of antimicrobials by poultry farmers in recent decades has led to the emergence of multidrug resistant strains in many parts of the globe.21, 22, 23, 24 Additionally, among multidrug resistant E. coli from chickens and piglets in China, 97% harbored the iss gene, suggesting this gene could also be used as a multidrug resistance marker.22 In Brazil, a previous study showed that the rates of multidrug resistance among avian E. coli reached 77.5%.25 Regarding ExPEC strains harboring the iss gene, we confirmed that the majority were resistant to several antimicrobials beyond those authorized for inclusion in broiler feed. Although the number of strains investigated was small, the levels of antimicrobial resistance to ampicillin, gentamicin, streptomycin, ciprofloxacin, chlorramphenicol and tetracycline were very high in comparison to the levels found in a recent study carried out with 101 E. coli strains from broilers and layer hens with colibacillosis infections in Bangladesh.26
Additionally, we have shown that a great number of avian ExPEC are probably β-lactamase producing strains, since resistance to amoxycillin/clavulanic acid was present in 70.4% of the isolates. Oteo et al.27 reported that resistance to amoxicillin-clavulanic acid is increasing among E. coli from human origin in Spanish hospitals, affecting 5,1% of 9090 blood isolates. Considering the food-chain, it is worrying that the only inhibitory drug against all avian ExPEC is carbapenem imipinem, restricted to human use. Also of particular concern are the resistance of avian ExPEC to fluoroquinoles such as enrofloxacin, which are similar to antibiotics being used in human medicine.28 For instance, the increasing resistance of E. coli strains to ciprofloxacin has been detected in several international studies.29, 30 Enrofloxacin has been withdrawal from non-therapeutic use in the poultry industry in the United States.31 In Europe, the drug cannot be used in the animals from which eggs are produced for human consumption.32 However, in this study the level of antimicrobial resistance to doxycycline, neomycin, enrofloxacin and oxytetracycline reached alarming levels. Although we cannot confirm that Brazilian farmers restrict their use of such antimicrobials to treatment regimens, it was clear that these drugs should no longer be used on Brazilian poultry farms.
Avian ExPEC strains have been genetically associated with human E. coli causing urinary infections.6 Additionally, E. coli strains of the EcoR group B2 related to human and animal extraintestinal infections have been detected among APEC strains obtained from diseased and healthy chickens.33 In spite of the implications of avian ExPEC in carcasses of healthy poultry is unclear, a previous study in the UK showed that samples of imported chicken breasts were often positive for E. coli with CTX-M-2 genotype related to human infections in South America.34 Our data shows that resistance of avian E. coli to serum complement lyses in mammalian hosts was not directly related to the presence of the iss gene. This finding suggests that other genetic determinants among E. coli of avian origin must be related to human serum resistance. Additionally, multidrug-resistant ExPEC strains 41A and 2B were often more resistant to goat and human serum complement, and also more virulent to the experimentally infected mice than strain 4A, which is sensitive to the majority of antimicrobials.
Previous studies have shown the correlation between serum resistance and virulence of E. coli causing diseases in turkeys and chickens.35 Moreover, the characterization a transferable hybrid plasmid pAPEC-O103-ColBM encoding multidrug resistance and pathogenicity was recently described.11 Even though virulence and multidrug resistance genes are not uniformly distributed among conjugative plasmids, the diversity of avian ExPEC with zoonotic potential to cause human diseases can be more frequent than perceived. For example, in the present study the risk for human health was particularly observed for strain 2B, obtained from poultry carcasses approved for human consumption. Thus, an early diagnosis procedure should be followed on poultry farms and at slaughterhouses to identify hazardous microorganisms in asymptomatic poultry, and therefore eliminate carcasses that are not proper for human consumption. The data reinforce the general concern about the spread of multidrug-resistance and virulence genes between avian and human E. coli through the food-chain.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors thank the Brazilian Council of Research (CNPq) for research funding. Prof. Lima-Filho was supported by a Scholarship funding from Programa de Educação Tutorial (PET-MEC/SESu). The authors also thank Maria Helena (LIKA/UFPE) for providing the laboratory animals used in this study.
References
- 1.Jones F.T., Ricke S.C. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult Sci. 2003;82:613–617. doi: 10.1093/ps/82.4.613. [DOI] [PubMed] [Google Scholar]
- 2.Johnson J.R., McCabe J.S., White D.G., et al. Molecular analysis of Escherichia coli from retail meats (2002–2004) from the United States national antimicrobial resistance monitoring system. Clin Infect Dis. 2009;49:195–201. doi: 10.1086/599830. [DOI] [PubMed] [Google Scholar]
- 3.Collignon P. Resistant Escherichia coli—we are what we eat. Clin Infect Dis. 2009;49:202–204. doi: 10.1086/599831. [DOI] [PubMed] [Google Scholar]
- 4.Collignon P., Henrik C.W., Peter B., et al. The routine use of antibiotics to promote animal growth does little to benefit protein undernutrition in the developing world. Clin Infect Dis. 2005;41:1007–1013. doi: 10.1086/433191. [DOI] [PubMed] [Google Scholar]
- 5.Kwon S.G., Cha S.Y., Choi E.J., et al. Epidemiological prevalence of avian pathogenic Escherichia coli differentiated by multiplex PCR from commercial chickens and hatchery in Korea. J Bacteriol Virol. 2008;38:179–188. [Google Scholar]
- 6.Rodriguez-Siek K.E., Giddings C.W., Doetkott C., et al. Characterizing the APEC pathotype. Vet Res. 2005;36:241–256. doi: 10.1051/vetres:2004057. [DOI] [PubMed] [Google Scholar]
- 7.Johnson T.J., Wannemuehler Y., Johnson S.J., et al. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl Environ Microbiol. 2008;74:7043–7050. doi: 10.1128/AEM.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tivendale K.A., Allen J.L., Ginns C.A., et al. Association of iss and iucA, but not tsh, with plasmid-mediated virulence of avian pathogenic Escherichia coli. Infect Immun. 2004;72:6554–6560. doi: 10.1128/IAI.72.11.6554-6560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson T.J., Johnson S.J., Nolan L.K. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J Bacteriol. 2006;188:5975–5983. doi: 10.1128/JB.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson T.J., Giddings C.W., Horne S.M., et al. Location of increased serum survival gene and selected virulence traits on a conjugative R plasmid in an avian Escherichia coli isolate. Avian Dis. 2002;46:342–352. doi: 10.1637/0005-2086(2002)046[0342:LOISSG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Johnson T.J., Dianna J., Subhashinie K., et al. Sequence analysis and characterization of a transferable hybrid plasmid encoding multidrug resistance and enabling zoonotic potential for extraintestinal Escherichia coli. Infect Immun. 2010;78:1931–1942. doi: 10.1128/IAI.01174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazilian Chicken Producers and Exporters Association; 2012. http://www.abef.com.br/English/default.php [accessed 28.06.12].
- 13.Brasil. Portaria 2010; 1998. http://extranet.agricultura.gov.br/sislegis-consulta/servlet/VisualizarAnexo?id=3162 [accessed 28.06.12].
- 14.Silva I.M.M., Evêncio-Neto J., Silva R.M., et al. Caracterização genotípica dos isolados de Escherichia coli provenientes de frangos de corte. Arq Bras Med Vet Zootec. 2011;63:333–339. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards . Clinical and Laboratory Standards Institute, NCCLS; Villanova, PA, USA: 2002. Padronização dos Testes de Sensibilidade a Antimicrobianos por Disco difusão: Norma Aprovada M2-A8, Oitava Edição-Tradução ANVISA. [Google Scholar]
- 16.Koneman E.W., Allen S.D., Janda W.M., et al. MEDSI; São Paulo, Brasil: 2001. Diagnóstico Microbiológico – Texto e Atlas Colorido. 2002(5) [Google Scholar]
- 17.Samuelsen Ø., Haukland H.H., Ulvatne H., et al. Anti-complement effects of lactoferrin-derived peptides. FEMS Immunol Med Microbiol. 2004;41:141–148. doi: 10.1016/j.femsim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Dho-Moulin M., Fairbrother J.M. Avian pathogenic Escherichia coli (APEC) Vet Res. 1999;30:299–316. [PubMed] [Google Scholar]
- 19.Ewers C.T., Janssen, Wieler L.H. Avian pathogenic Escherichia coli (APEC) Berl Munch Tierarztl Wochenschr. 2003;116:381–395. [PubMed] [Google Scholar]
- 20.Kabir S.M.L. Avian Colibacillosis and Salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronzwaer S.L., Cars O., Buchholz U., et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;8:278–282. doi: 10.3201/eid0803.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H., Sheng C., David G., et al. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J Clin Microbiol. 2004;42:3483–3489. doi: 10.1128/JCM.42.8.3483-3489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi T.Z., Bonab S.F. Antibiotics susceptibility pattern of Escherichia coli strains isolated from chickens with colisepticemia in Tabriz Province, Iran. Int J Poultry Sci. 2006;5:677–684. [Google Scholar]
- 24.Harada K., Tetsuo A. Role of antimicrobial selective pressure and secondary factors on antimicrobial resistance prevalence in Escherichia coli from food-producing animals in Japan. J Biomed Biotechnol. 2012 doi: 10.1155/2010/180682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanatta G.F., Kanashiro A.M.I., Castro A.G.M., et al. Suscetibilidade de amostras de Escherichia coli de origem aviária a antimicrobianos. Arq Inst Biol. 2004;71:283–286. [Google Scholar]
- 26.Hasan B., Faruque R., Drobni M., et al. High prevalence of antibiotic resistance in pathogenic Escherichia coli from large- and small-scale poultry farms in Bangladesh. Avian Dis. 2011;55:689–692. doi: 10.1637/9686-021411-Reg.1. [DOI] [PubMed] [Google Scholar]
- 27.Oteo J., Campos J., Lázaro E., et al. Increased amoxicillin–clavulanic acid resistance in Escherichia coli blood isolates. Spain Emerg Infect Dis. 2008;14:1259–1262. doi: 10.3201/eid1408.071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox L.A., Jr., Popken D.A. Quantifying potential human health impacts of animal antibiotic use: enrofloxacin and macrolides in chickens. Risk Anal. 2006;26:135–146. doi: 10.1111/j.1539-6924.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 29.Vellinga A., Tansey S., Hanahoe B., et al. Trimethoprim and ciprofloxacin resistance and prescribing in urinary tract infection associated with Escherichia coli: a multilevel model. J Antimicrob Chemother. 2012;67(10):2523–2530. doi: 10.1093/jac/dks222. [DOI] [PubMed] [Google Scholar]
- 30.Morfin-Otero R., Tinoco-Favila J., Sader H., et al. Resistance trends in gram-negative bacteria: surveillance results from two Mexican hospitals, 2005–2010. BMC Res Notes. 2012;5:277. doi: 10.1186/1756-0500-5-277. [PMID: 22676813] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Drug Administration; 2005. http://www.fda.gov/AnimalVeterinary/SafetyHealth/RecallsWithdrawals/ucm042004.htm [accessed 28.06.12].
- 32.The European Agency for the Evaluation of Medicinal Products, 1998. http://www.emea.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014142.pdf [accessed 28.06.12].
- 33.Ewers C., Esther-Maria A., Diehl I., et al. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia. Appl Environ Microbiol. 2009;75:184–192. doi: 10.1128/AEM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren R.E., Ensor V.M., O’Neill P., et al. Imported chicken meat as a potential source of quinolone-resistant Escherichia coli producing extended. J Antimicrob Chemother. 2008;61:504–508. doi: 10.1093/jac/dkm517. [DOI] [PubMed] [Google Scholar]
- 35.Allan B.J., Jvan den Hurk J.V., Potter A.A. Characterization of Escherichia coli isolated from cases of avian colibacillosis. Can J Vet Res. 1993;57:146–151. [PMC free article] [PubMed] [Google Scholar]