Abstract
Objective
The aim of this study was to describe the most frequently found bacterial microorganisms in bloodstream isolates taken from patients in intensive care units in Colombia and their resistance profiles.
Methods
This was a multicentre descriptive observational study that was carried out between January 2001 and June 2008 with laboratory data from 33 participating hospitals in a surveillance network.
Results
The most frequently found microorganisms were coagulase-negative Staphylococci 39.6%, Staphylococcus aureus 12.3%, Klebsiella pneumoniae 8.2%, Escherichia coli 5.7%, Acinetobacter baumannii, 4.0% and Pseudomonas aeruginosa 3.8%. Coagulase-negative Staphylococci registered greater than 70% oxacillin resistance rate. S. aureus presented a change in its multiresistance profile during the years of follow-up. There was a trend towards a lower resistance rate among E. coli and K. pneumoniae isolates during the study period while A. baumannii carbapenem resistance rate exceeded 50%.
Discussion
There has been a change in the frequency of species being isolated with a higher frequency of enterobacteriaceae compared to Gram-positive microorganisms, in general with a high resistance rate.
Keywords: Bacteremia, Intensive care unit, Drug resistance, Anti-bacterial agents, Colombia/epidemiology, Epidemiologic surveillance
Introduction
Bacteremia acquired in the hospital is a frequent cause of morbidity and mortality in intensive care units (ICU)],1 having an important clinical impact on patients’ length of stay and costs associated with health care.1, 2 According to the World Health Organization (WHO), 8.7% of hospital acquired infections correspond to bacteremia2 and retrospective studies have shown that the incidence of nosocomial bacteremia is close to six episodes per 1000 hospital admissions. ICU ranks first amongst hospital services (51% of events) followed by adult internal medicine services (38%), surgical services (20%) and paediatric rooms (13.5%).1, 2 It has been reported that ICU patients present a greater tendency for developing episodes of nosocomial bacteremia compared to those admitted to different hospital wards.3 This is related to patients’ susceptibility due to immunosuppression and frequent use of invasive devices, such as intravascular and urinary catheters, and tubes for supporting mechanical ventilation.3
Knowing the most frequent pathogenic microorganisms that colonise or infect this type of patient allow for effective empirical antibiotic treatment taking into account microorganisms’ susceptibility and resistance patterns. A general increase in bacterial resistance rates has been reported around the world, giving rise to multiresistant strains within hospitals and thereby generating serious problems for health-care institutions.2, 3 There have been reports of resistance of several important pathogens in the hospital setting, such as Staphylococcus aureus, coagulase-negative Staphylococcus (CNS), Enterococcus spp., Escherichia coli, Klebsiella pneumoniae and non-fermenting enterobacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii.2
This study describes the frequency and resistance patterns of microorganisms associated with bacteremia presented in critically ill patients in ICUs of a third-level hospital network in Colombia.
Materials and methods
Design
This was a descriptive, observational study, based on multicentre surveillance of bacterial isolates from blood samples taken from patients admitted to ICU belonging to the Bogotá Bacterial Resistance Control Group's (GREBO – Grupo para el Control de la Resistencia Bacteriana de Bogotá) surveillance system from January 2001 to June 2008.
Surveillance network
This network consists of thirty-three institutional laboratories of third-level hospitals (hospital capacity ranging from 100 to 700 beds), located in five regions of Colombia: Bogotá (capital city of Colombia), Ibagué and Neiva (Southwest part of the country), Manizales (mountainous area adjacent to the coffee growing area) and Cúcuta (near the border with Venezuela). The surveillance network started with 12 institutions that have common procedures for detecting resistance, reporting it, and centralized quality control.4
All institutions have automated identification and bacterial susceptibility test systems (MicroScan, Dade Behring, USA or Vitek, Biomerieux, France) and are run in line with the 2007 Clinical and Laboratory Standard Institute (CLSI) guidelines.5 Criteria for blood sampling was decided individually upon the discretion of the assistant physician in all participating ICU. The number of samples and the blood volume taken were decided by each institutional protocol.
Analysis
Each institution collected information monthly in databases exported from automated systems. Each database converted to a standard format using Baclink 2.0 software (WHO, Switzerland) and then analysed using Whonet 5.4 software (WHO, Switzerland).6 Results of blood cultures collected at services identified as ICU were extracted for analysis. Only the first positive blood culture of each patient was included in the analysis. Annual absolute and relative resistance frequencies were analysed. Comparative tables, tendency plots, and histograms were prepared in Excel 2007 software (Microsoft, USA). Resistant markers were chosen for the most commonly found microorganisms: oxacillin was chosen for staphylococci resistance, third generation cephalosporins for Gram-negative enteric bacilli, ceftazidime for P. aeruginosa, imipenen for A. baumannii, and vancomycin for Enterococcus faecalis.
Results
The hospital network reported 21,183 isolates from blood samples of patients admitted to their ICUs from January 2001 to June 2008. An annual increase in the number of isolates was observed, from 1175 in 2001 to 5069 in 2007. Such an increase was sustained in 2008, when 2437 isolates being recorded in just the first half of the year. This annual incremental trend was maintained after standardizing for the number of isolates per number of hospitals in each period (Table 1).
Table 1.
Frequency of isolated microorganisms standardized by year. 2001–first half of 2008.
| Year | Number of isolates (n) | Number of hospitals | Total months notified | Average months notified | Average of isolates per month per hospital |
|---|---|---|---|---|---|
| 2001 | 1176 | 12 | 127 | 10.6 | 9.3 |
| 2002 | 1942 | 13 | 142 | 10.9 | 13.7 |
| 2003 | 1841 | 16 | 188 | 11.8 | 9.8 |
| 2004 | 2788 | 19 | 216 | 11.4 | 12.9 |
| 2005 | 2959 | 22 | 262 | 11.9 | 11.3 |
| 2006 | 3420 | 22 | 258 | 11.7 | 13.3 |
| 2007 | 5070 | 28 | 326 | 11.6 | 15.6 |
| 2008 | 2438 | 33 | 197 | 6.0 | 12.4 |
Microorganism frequency
Table 2 describes the 10 microorganisms more frequently isolated in order of frequency in the network ICU.
Table 2.
Most frequently isolated microorganisms from bloodstream infections in patients in the ICU, 2001–first half of 2008.
| Microorganism | Number of isolates | (%) |
|---|---|---|
| Coagulase-negative Staphylococcus | 8384 | 39.6% |
| Staphylococcus aureus | 2615 | 12.3% |
| Klebsiella pneumoniae | 1727 | 8.2% |
| Escherichia coli | 1208 | 5.7% |
| Acinetobacter baumannii | 840 | 4.0% |
| Pseudomonas aeruginosa | 814 | 3.8% |
| Enterobacter cloacae | 718 | 3.4% |
| Enterococcus faecalis | 616 | 2.9% |
| Serratia marcescens | 542 | 2.6% |
| Klebsiella oxytoca | 309 | 1.5% |
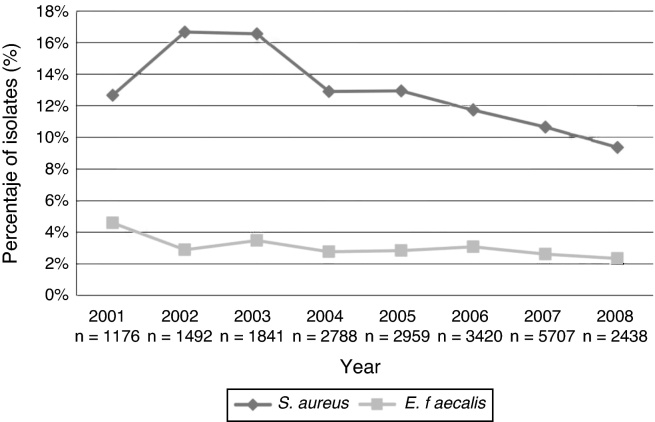
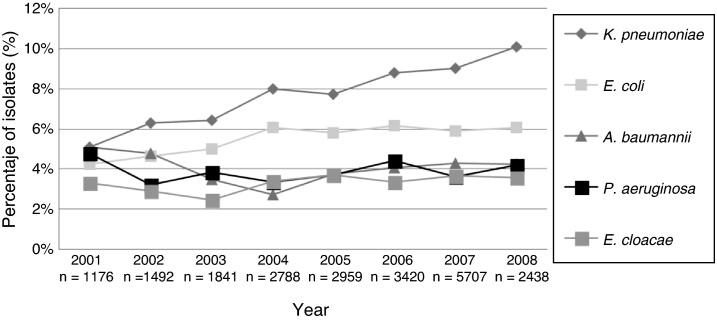
From 2001 to 2007, the rates of positive blood cultures for coagulase negative Staphylococci (CNS) were 41%, 39%, 40%, 41%, 39%, 39% and 36%, respectively; a rate of 40% was recorded for the first half of 2008. Fig. 1 describes the annual relative frequency pattern for other Gram-positive microorganisms isolated from blood in the ICUs (S. aureus and E. faecalis). Fig. 2 shows the pattern for some Gram-negative bacilli selected by frequency and their pathogenic potential.
Fig. 1.
Frequency of selected Gram-positive cocci associated with bloodstream infections in patients in intensive care units, excluding coagulase negative Staphylococci. 2001–first half of 2008.
Fig. 2.
Frequency of selected Gram-negative bacilli associated with bloodstream infections in patients admitted to ICU. 2001–first half of 2008.
Antimicrobial resistance
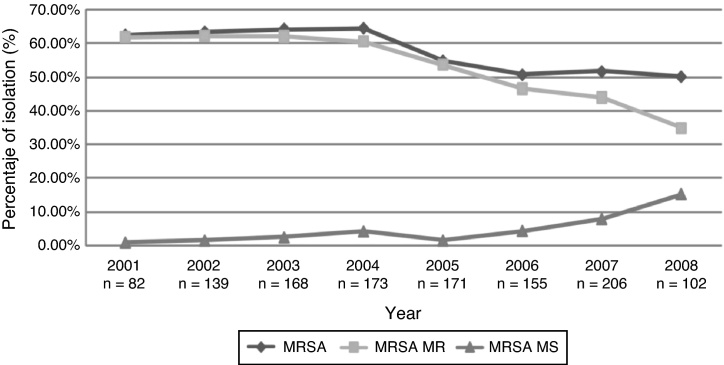
Table 3 summarizes the frequencies of resistance for selected markers of resistance among microorganisms isolated from blood at the participating ICUs per year. Reduced rates of oxacillin resistance of S. aureus and a change in multiresistance profile were seen (Fig. 3), as well as recovery of K. pneumoniae susceptibility to third-generation cephalosporins. A. baumannii presented a fivefold increase in its rate of initial resistance to carbapenems, rising from 10.5% in 2001 to 49.5% in the first half of 2008.
Table 3.
Resistance markers for most frequently isolated microorganisms from bloodstream infections in patients in ICU. 2001–first half of 2008.
| Marker | 2001 |
2002 |
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Oxacillin-resistant CNS | 253 | 90.5 | 245 | 84.1 | 236 | 76.4 | 346 | 80.6 | 594 | 85.2 | 710 | 84.5 | 1113 | 84.8 | 517 | 78.5 |
| Oxacillin-resistant S. aureus | 146 | 62.3 | 244 | 63.5 | 296 | 64.9 | 341 | 58.1 | 369 | 47.2 | 391 | 43 | 533 | 43.3 | 216 | 33.4 |
| Third-generation Cephalosporin-resistant K. pneumoniae | 53 | 58.5 | 89 | 58.4 | 99 | 35.4 | 190 | 35.3 | 207 | 28 | 287 | 26.8 | 397 | 26.2 | 240 | 17.9 |
| Third-generation cephalosporin-resistant E. coli | 46 | 6.5 | 59 | 15.3 | 82 | 8.5 | 128 | 5.5 | 151 | 6.6 | 181 | 7.2 | 262 | 8.8 | 145 | 4.8 |
| Imipenem-resistant A. baumannii | 57 | 10.5 | 68 | 27.9 | 56 | 37.5 | 72 | 41.7 | 105 | 55.2 | 139 | 42.4 | 211 | 54.5 | 99 | 49.5 |
| Cephthazidim-resistant P. aeruginosa | 56 | 28.6 | 47 | 40.4 | 64 | 15.6 | 80 | 20 | 99 | 30.3 | 142 | 28.2 | 177 | 39 | 99 | 29.3 |
| Third-generation Cephalosporin-resistant E. cloacae | 39 | 33.3 | 42 | 40.5 | 41 | 36.6 | 73 | 17.8 | 93 | 25.8 | 102 | 29.4 | 154 | 26 | 77 | 16.9 |
| Vancomycin-resistant E. faecalis | 54 | 1.9 | 42 | 2.4 | 64 | 0 | 77 | 3.9 | 81 | 0 | 103 | 1.9 | 118 | 0 | 55 | 0 |
| Imipenem-resistant K. pneumoniae | 54 | 0 | 88 | 1.1 | 102 | 1 | 206 | 0.5 | 215 | 0.9 | 298 | 1.3 | 430 | 0.9 | 244 | 1.2 |
Fig. 3.
Tendency of oxacillin-resistant S. aureus, according to resistant phenotype tendency. 2001–first half of 2008. GREBO.
E. faecalis vancomycin resistance rates did not exceed 4% and were recorded in some institutions of the network. Such increase was not sustained in the following years. A marked reduction in proven high-level gentamicin resistance was simultaneously recorded for this microorganism, falling from 25% in 2001 and 2002 to less than 13% resistance rate from 2005 to 2007. Close to 3% carbapenem resistance was reported in K. pneumoniae isolates during the last two years of observation. The rate of ciprofloxacin-resistant E. coli ranged from 20% to 30% for isolates taken from blood during the observation period.
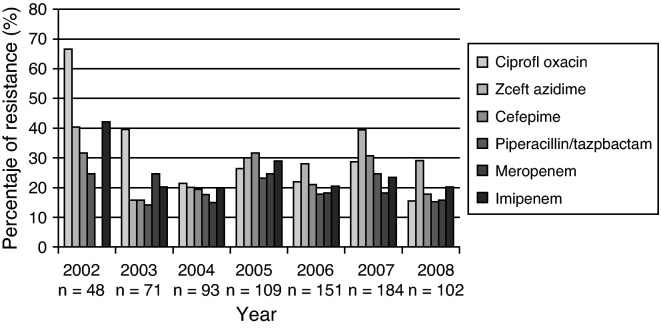
P. aeruginosa presented resistance to several antimicrobial drugs, varying from 15% to 30% during the observation period for isolates taken from blood (Fig. 4).
Fig. 4.
Resistance profile of P. aeruginosa causing bloodstream infections in patients admitted to ICU. 2001–first half of 2008. GREBO. Each bar represents the rate of resistance to the selected antibiotic. Number of isolates tested is below each year.
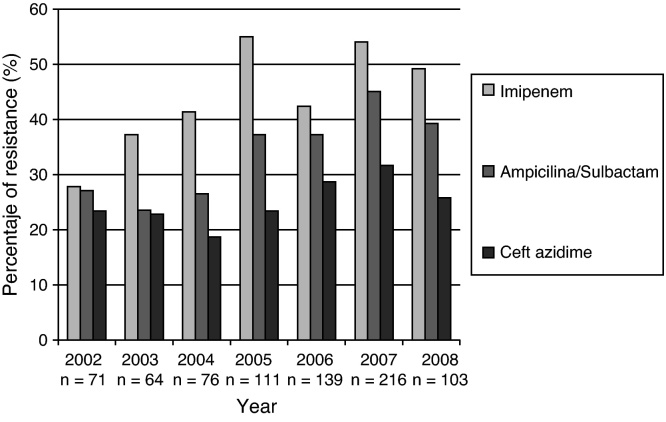
A. baumannii showed greater resistance to multiple antibiotics, ranging from 35% to 60% in the follow-up period. An overall increase of resistance to carbapenems of this microorganism was recorded, reaching rates higher than 50% (Fig. 5).
Fig. 5.
Resistance profile for A. baumannii causing bloodstream infections in patients admitted to ICU. 2001–first half of 2008. GREBO. Each bar represents the rate of resistance to the selected antibiotic. Number of isolates tested is below each year.
Discussion
The widespread distribution of resistant Gram-positive and Gram-negative microorganisms to currently available antimicrobial drugs has gained importance in Europe, North America and Latin-America.7 Reports in our setting have shown the widespread participation of multiresistant microorganisms in bacteremia of patients admitted to ICUs.8, 9
A rapid frequency increase in multiple-antibiotic-resistant pathogenic bacteria has been observed during the last few years7 with a change in the expected distribution of different markers. In spite of the high frequency of isolates for CNS and S. aureus, the frequency and participation of other Gram-negative bacteria (i.e. K. pneumoniae and E. coli) have increased in ICU-related bacteraemia.10, 11
The percentage of pathogens isolated from blood in our study was similar to the results reported in 2008 by Hidron et al., in a hospital network reporting to CDC.12 Different authors have reported these microorganisms as being the most frequently occurring agents causing ICU service-acquired infections.7, 13 The clinical importance of CNS as causal agent in our setting is beyond the scope of this report; however, its participation should be stressed in some populations at risk, especially in those with infections related to the use of external devices.14 High levels of oxacillin-resistant CNS were observed, a frequent finding in our region, since it has been described that around 80% of CNS isolates in Latin-America are oxacillin resistant.15 One of the greatest problem observed in laboratory system-based surveillance is to discriminate CNS pathogenic strains from contaminant strains (normal skin flora); this represents a challenge since CNS has been reported as being the microorganism most commonly causing contamination in microbiological cultures.14 Such high rate of contamination could be related to deficient cleaning practice amongst medical personnel, sterilizing instruments and surgical areas, thereby increasing the risk of pathogen dissemination and acquiring post-operation infection.16
The second microorganism in importance was S. aureus, for which a reduction in the frequency in ICUs was observed, simultaneously with a change in multiresistance profile. Some authors have previously reported such change8 and it has been proposed to be influenced by hospital clones being displaced by an increase in circulating community-derived clones having a multisensitivity pattern.11, 17 These clones’ participation has been identified in Colombia, North America and some Latin-American countries. A molecular study carried out in 2008 by Arias et al. described similarities in the pattern of strains circulating in Colombia and the USA; this reinforced the need for establishing public health action in health care centres due to the risk of crossed dissemination in hospital services of such resistant strain coming from the community.18
A change in the frequency of predominating Gram-negative was observed during the study period. Up to 100% increases were recorded when compared to the Gram-positive pattern; K. pneumoniae, E. coli and S. marcescens had similar increases to those reported in other studies.10 Enterobacteriaceae participation in the ICU setting and their increase have important repercussions, given its general association with the use of mechanical ventilation and other invasive devices such as urinary catheters.19
An important reduction in resistance to third-generation cephalosporins was recorded for K. pneumoniae, E. coli and Enterobacter cloacae, as has been observed in other studies of ICU patients but not among patients admitted to general wards in Colombia.20 This could have been related to improvement in infection control policies and better antimicrobial use, as some broad spectrum antibiotics have been the target for control action given their potential for inducing extended spectrum beta-lactamase (ESBL) enzymes.21
Close to 3% carbapenem resistance has been observed during the last two years in enterobacteria; this has been compatible with the beginning of carbapenemase circulation in Colombia.22 The appearance of these resistant isolates might have to do with a change in the use of antimicrobials, increased use of carbapenems or other antibiotics, as well as clonal dissemination.23
There was high multiresistance rates in non-fermenting Gram-negative bacteria. P. aeruginosa showed a clear increase of its multiresistant phenotypes. Our results were similar to other studies which have recorded 12.8–20.8% resistance rates in North America, and 16.8–30% in Europe, leading to the common use of empirical combined antimicrobial therapy against P. aeruginosa despite the recognition of the inefficacy of such approach in different scenarios.24
A. baumannii expressed a greater multiresistance pattern, ranging from 35% to 60% in the study period. This multiresistance rates have been described in other countries, recognizing that the rates of susceptibility pattern of isolates in Latin-America are much lower than those recorded in North America and Europe.25 A fivefold increase in resistance was observed in Colombia between 2001 and 2008. Tognim et al. carried out a five-year follow-up (1997–2001) in Latin-American countries, finding a reduction in carbapenem susceptibility; the highest resistance rates were recorded in Argentina (20%), followed by Colombia (14%), Brazil (8.5%) and Chile (0.7%).26
This phenomenon has a multifactorial origin; widespread use of carbapenems, especially in ICU services, is considered to be the greatest risk factor for selecting high carbapenem-resistance and could be playing an important role,27 but also the easy dissemination of this microorganism and its ability to acquire multiple resistance mechanisms further complicate the scenario.13
The importance of our results in the identification of highly resistant patterns among microorganisms causing bloodstream infection, thereby leading to inadequate initial empirical therapy24; unexpected adverse results are thus obtained, increasing the risk of mortality, length of hospital stay and hospital costs.26 These surveillance data are the starting point for generating prevention and control measures to tackle this alarming problem. The resistance patterns circulating in given setting must be identified for determining the most suitable therapy; one must bear in mind that the resistance patterns vary between countries and between different hospital services.4
Using data based on a laboratory network's surveillance systems is of great utility to define isolate frequency and to decide empirical therapy. Some limitations, such as estimating prevalence of infection-causing resistant microorganisms and uniformity in processing samples must be considered when interpreting the information.28 However, establishing centralized quality control and following-up standards could help improving the quality of information.
Results of this study of bacteremia in critically ill patients highlight an incremental trend in Gram-negative bacilli whilst the relative frequency of Gram-positive cocci (S. aureus, CNS, E. faecalis) has decreased. These microorganism resistance rates were high, even when compared to those identified in other studies in Latin America, Europe and North America. This study has provided relevant information for guiding empirical therapy for ICU patients and sounding an alert concerning the need for generating strategies for controlling resistance in our settings.
Conflict of interest
All authors declare to have no conflict of interest.
Acknowledgements
We would like to thank Andrés Fernando Meneses, Sandra Yamile Saavedra and María Victoria Ovalle for their scientific and technical support in maintaining both the network and the reference laboratory and María Isabel Ovalle (GREBO group secretary) for her logistical support. The following members belong to the Bogota Bacterial Resistance Control Group (GREBO): Martha Lucia Salinas, Norma Montoya (Clínica de Occidente); Mauricio Luna Ortiz, Martha Martínez (Clínica del Niño); Álvaro Ignacio Arango, Jaime Alberto Patiño, Martha Isabel Álvarez, Zenaida Montañez, Shirley Acosta (Fundación Cardio Infantil); Yaniz Hernández, Claudia Calderón, Jaime Saravia (Fundación San Carlos); Guillermo Prada, Clara Luz Rico, Blanca Stella Vanegas (Fundación Santa Fe de Bogotá); Gerson Arias, Nubia Escobar (Hospital Occidente de Kennedy); Elkin Lemos, Narda Olarte, Alberto Valderrama, Julia Stella Quijano, Martha Isabel Garzón (Hospital el Tunal); Carlos Gómez, Maria Nilse González (Hospital Militar Central); Carlos Arturo Álvarez, Claudia Linares, José Roberto Tamara, Beatriz Ariza, Sandra Valderrama (Hospital Universitario San Ignacio); Tailandia María Rodríguez Gutiérrez, Felipe Zamora, Constanza Correa (Hospital Simón Bolívar); Carlos Alquichire, Martha Ruiz (Clínica San Pedro Claver); Adriana Jiménez, Henry Mendoza, Claudia Fajardo Uribe (Hospital de San José); Luz Mila López, Gloria Inés Gallo (Hospital Santa Clara); Carlos Humberto Saavedra Trujillo, Clemencia Ávila, Claudia Clavijo (Hospital Universitario Clínica San Rafael); Myriam Lucia Galeano, Johana Montes (Hospital Universitario la Misericordia); Carlos Pérez, Beatriz Cuevas, Lucy Guzmán Cruz (Hospital Universitario la Samaritana); Sonia Isabel Cuervo, Claudia Patricia Arroyo (Instituto Nacional de Cancerología); Catherine Rojas, Nidia Stella Moya, Margarita Cardona (Centro Policlínico la Olaya); Giovanni Rodríguez Leguizamón, Claudia Cifuentes López (Clínica Infantil Colsubsidio); Johana Ávila, Claudia Cifuentes (Clínica Videlmédica); Tailandia Rodríguez, Luz Stella Gómez (Clínica Colsubsidio Orquídeas); Henry Mendoza, Martha Meléndez (Hospital Central de la Policía Nacional); Amparo Ovalle Garzón, Claudia Echeverri, (Hospital Federico Lleras Acosta); Ana Maria Pérez Fernández, Melba Sofía, (Clínica Central del Quindío); Sandra Gualtero, Luís Fernando Duran, Dagoberto Santofimio Sierra (Hospital Universitario Hernando Moncaleno Perdomo); Claudia Pilar Botero, Inés Elena Montoya (Clínica la Presentación de Manizales); Carlos Alquichire, Giovanni Peña (Clínica Jorge Piñeros Corpas-SaludCoop).
References
- 1.Lizaso D., Aguilera C.K., Correa M., et al. Epidemiología y factores de riesgo de mortalidad de las bacteriemias intrahospitalarias por bacilos gramnegativos. Rev Chilena Infectol. 2008;25:368–373. [PubMed] [Google Scholar]
- 2.WHO. Prevention of hospital-acquired infections: a practical guide; 2002 (updated 2002; cited). Available from: http://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_EPH_2002_12/en/ [Revised: march 7, 2011].
- 3.Ribas R.M., Freitas C., Gontijo Filho P.P. Nosocomial bloodstream infections: organisms, risk factors and resistant phenotypes in the Brazilian University Hospital. Braz J Infect Dis. 2007;11:351–354. doi: 10.1590/s1413-86702007000300010. [DOI] [PubMed] [Google Scholar]
- 4.Leal A.L., Eslava-Schmalbach J., Alvarez C., Buitrago G., Mendez M. Canales endémicos y marcadores de resistencia bacteriana, en instituciones de tercer nivel de Bogotá, Colombia. Rev Salud Publica (Bogota) 2006;8:59–70. doi: 10.1590/s0124-00642006000400006. [DOI] [PubMed] [Google Scholar]
- 5.Institute CaLS . CLSI; Wayne, PA: 2008. M100-S18, performance standards for antimicrobial susceptibility testing. Eighteen informational supplement. [Google Scholar]
- 6.O’Brien T.F., Stelling J.M. WHONET: removing obstacles to the full use of information about antimicrobial resistance. Diagn Microbiol Infect Dis. 1996;25:162–168. [PubMed] [Google Scholar]
- 7.Sader H.S., Jones R.N., Andrade-Baiocchi S., Biedenbach D.J. Four-year evaluation of frequency of occurrence and antimicrobial susceptibility patterns of bacteria from bloodstream infections in Latin American medical centers. Diagn Microbiol Infect Dis. 2002;44:273–280. doi: 10.1016/s0732-8893(02)00469-8. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez C., Cortes J., Arango A., Correa C., Leal A. Resistencia antimicrobiana en Unidades de Cuidado Intensivo de Bogotá, Colombia, 2001–2003. Rev Salud Publica (Bogota) 2006;8:86–101. doi: 10.1590/s0124-00642006000400008. [DOI] [PubMed] [Google Scholar]
- 9.Tuon F.F., Gortz L.W., Rocha J.L. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. Braz J Infect Dis. 2012;16:351–356. doi: 10.1016/j.bjid.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Munoz P., Cruz A.F., Rodriguez-Creixems M., Bouza E. Gram-negative bloodstream infections. Int J Antimicrob Agents. 2008;32:S10–S14. doi: 10.1016/j.ijantimicag.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Tenorio M.T., Porfirio Z., Lopes A.C., Cendon S. Clinical and microbiological characteristics of bloodstream infections in a tertiary hospital in Maceio, Alagoas, Brazil. Braz J Infect Dis. 2010;14:175–179. doi: 10.1590/s1413-86702010000200011. [DOI] [PubMed] [Google Scholar]
- 12.Hidron A.I., Edwards J.R., Patel J., et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 13.Patwardhan R.B., Dhakephalkar P.K., Niphadkar K.B., Chopade B.A. A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J Med Res. 2008;128:178–187. [PubMed] [Google Scholar]
- 14.Olaechea P.M., Alvarez-Lerma F., Palomar M., et al. Impact of primary and intravascular catheter-related bacteremia due to coagulase-negative staphylococci in critically ill patients. Med Intensiva. 2011;35:217–225. doi: 10.1016/j.medin.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Secchi C., Antunes A.L., Perez L.R., Cantarelli V.V., d’Azevedo P.A. Identification and detection of methicillin resistance in non-epidermidis coagulase-negative staphylococci. Braz J Infect Dis. 2008;12:316–320. doi: 10.1590/s1413-86702008000400012. [DOI] [PubMed] [Google Scholar]
- 16.von Eiff C., Peters G., Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677–685. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez C.A., Yomayusa N., Leal A.L., et al. Nosocomial infections caused by community-associated methicillin-resistant Staphylococcus aureus in Colombia. Am J Infect Control. 2010;38:315–318. doi: 10.1016/j.ajic.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Arias C.A., Rincon S., Chowdhury S., et al. MRSA USA300 clone and VREF—a U.S–Colombian connection? N Engl J Med. 2008;359:2177–2179. doi: 10.1056/NEJMc0804021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marschall J., Agniel D., Fraser V.J., Doherty J., Warren D.K. Gram-negative bacteraemia in non-ICU patients: factors associated with inadequate antibiotic therapy and impact on outcomes. J Antimicrob Chemother. 2008;61:1376–1383. doi: 10.1093/jac/dkn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briceno D.F., Correa A., Valencia C., et al. Antimicrobial resistance of Gram negative bacilli isolated from tertiary-care hospitals in Colombia. Biomedica. 2010;30:371–381. [PubMed] [Google Scholar]
- 21.Hernandez J.R., Pascual A., Canton R., Martinez-Martinez L. Escherichia coli y Klebsiella pneumoniae productores de betalactamasas de espectro extendido en hospitales españoles (Proyecto GEIH-BLEE 2000) Enferm Infecc Microbiol Clin. 2003;21:77–82. [PubMed] [Google Scholar]
- 22.Saavedra S.Y., Nunez J.C., Pulido I.Y., et al. Characterisation of carbapenem-resistant Acinetobacter calcoaceticus–A. baumannii complex isolates in a third-level hospital in Bogota, Colombia. Int J Antimicrob Agents. 2008;31:389–391. doi: 10.1016/j.ijantimicag.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Nordmann P., Cuzon G., Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 24.van Delden C. Pseudomonas aeruginosa bloodstream infections: how should we treat them? Int J Antimicrob Agents. 2007;30:S71–S75. doi: 10.1016/j.ijantimicag.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Morales I.R. Terapia de bacterias productoras de b-lactamasas de espectro extendido. Rev Chil Infectol. 2003;20(Suppl. 1):S7–S24. [Google Scholar]
- 26.Tognim M.C., Andrade S.S., Silbert S., Gales A.C., Jones R.N., Sader H.S. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int J Infect Dis. 2004;8:284–291. doi: 10.1016/j.ijid.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Zavascki A.P., Cruz R.P., Goldani L.Z. Risk factors for imipenem-resistant Pseudomonas aeruginosa: a comparative analysis of two case-control studies in hospitalized patients. J Hosp Infect. 2005;59:96–101. doi: 10.1016/j.jhin.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Bax R., Bywater R., Cornaglia G., et al. Surveillance of antimicrobial resistance—what, how and whither? Clin Microbiol Infect. 2001;7:316–325. doi: 10.1046/j.1198-743x.2001.00239.x. [DOI] [PubMed] [Google Scholar]