Abstract
African histoplasmosis is a granulomatous mycosis caused by Histoplasma capsulatum var. duboisii. Treatment is usually extrapolated from guidelines for classical histoplasmosis, and includes 2–4 weeks of amphotericin B followed by a step-down maintenance therapy with itraconazole. Pediatric usage of posaconazole, an oral second-generation azole, remains off-label, but recent surveys show that it is safe and well tolerated in children. We report a case of disseminated African histoplasmosis in a 12-year-old boy from Guinea-Bissau. Therapy with amphotericin B and itraconazole led to a progressive clinical deterioration. A dramatic and lasting improvement was observed using posaconazole. He completed 12 months of therapy. No relapse was noted during or 3 months after treatment. We report that posaconazole may be a safe and efficacious drug in the salvage management of disseminated AH, either in patients with disease refractory to conventional anti-fungal therapy, or in patients whose serious adverse effects of first-line drugs preclude its use.
Keywords: Posaconazole, Histoplasmosis, Salvage therapy
Introduction
Histoplasmosis is a granulomatous disease caused by the dimorphic fungus Histoplasma capsulatum. There are two recognized subspecies of this fungus pathogenic to humans, namely Histoplasma capsulatum var. capsulatum (Hcc) and Histoplasma capsulatum var. duboisii (Hcd). Classical histoplasmosis is caused by the former, and is endemic in North and Latin America. Infection by Hcd is endemic in Central and West Africa and Madagascar,1 and is responsible for African histoplasmosis (AH). All reported cases of AH in Europe are imported from endemic areas.2
Inhaled H. capsulatum conidia result in subclinical infection in the majority of exposed individuals, but disseminated disease is not uncommon. The main clinical features of AH are involvement of the skin, subcutaneous tissues, lymph nodes and bones, and rarely the lungs and other internal organs.3
Treatment of AH is usually extrapolated from the guidelines of the Infectious Diseases Society of America established for classical histoplasmosis,4 and includes 2–4 weeks of liposomal amphotericin B followed by a step-down maintenance therapy with oral itraconazole for at least 12 months.
Posaconazole, an oral second-generation azole, was approved by FDA in 2006 for prophylaxis of invasive Candida and Aspergillus infections in adult immunocompromised hosts. Pediatric usage of posaconazole has been described in some small series, and its use remains off-label. Recent surveys in which posaconazole was used as salvage therapy show that it is safe and well tolerated in children 3–17 years old.5, 6
We report the first case of posaconazole usage as salvage therapy in African histoplasmosis.
Case presentation
A 12-year-old African boy from Guinea-Bissau was referred to our hospital with a presumptive diagnosis of lymphoma. Upon review, he had a 2-year history of multiple nodular formations in cervical, axillary, and inguinal regions, which were enlarging with time, and were associated with cutaneous fistulas. He had no fever, weight loss or respiratory symptoms. He had been treated in his home country with large-spectrum antibiotics for several weeks without any clinical improvement.
On physical examination he appeared pale, but was fully conscious, and had normal vital signs. Cervical, axillary, and inguinal nodular formations were discharging a yellowish pus. There were no abdominal palpable masses. The other physical examination was unremarkable.
Initial workup revealed anemia (hemoglobin = 7.2 g/dL), leucocytosis (15,860/μL) with neutrophilia, thrombocytosis (967,000/μL), and elevated C-reactive protein (98.3 mg/L). Liver and kidney function tests were normal. Evaluation for immunologic profile did not show any evidence of immunosuppression and human immunodeficiency virus testing was negative. Computerized tomography (CT) of the chest revealed a condensation in the left lower lobe with peripheral micronodules and hilar calcifications. Abdominal ultrasound was normal.
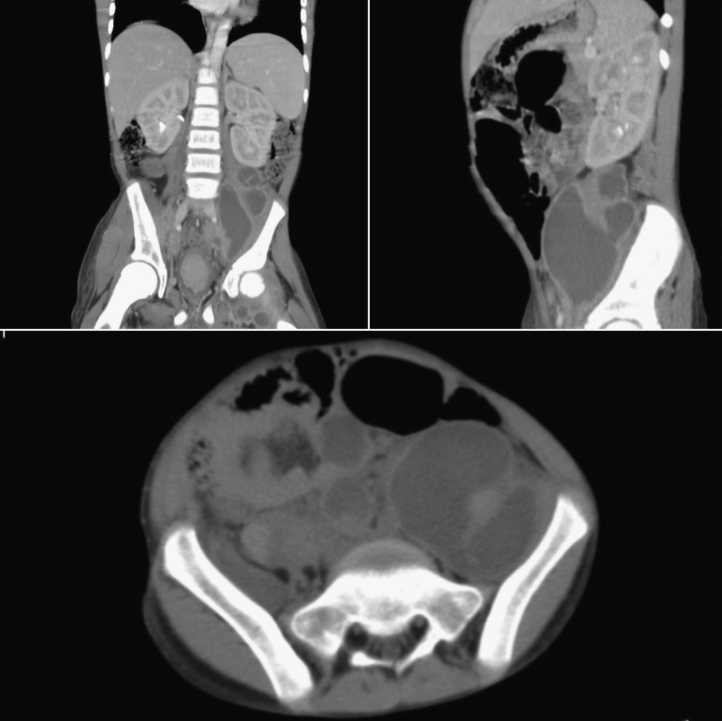
On day 3, he started a 6-month course of anti-tuberculous therapy due to a positive polymerase chain reaction (PCR) sample of Mycobacterium tuberculosis (Mt) in a gastric aspirate. This infection was later confirmed by a positive sputum culture. Meanwhile, a cervical nodular biopsy revealed necrotic areas along with others containing numerous histiocytes with multinucleated giant cells, polymorphic inflammatory infiltrate, and numerous uninucleate thick-walled large yeasts, budding from a narrow base (Fig. 1, arrow). Based on this morphology, a diagnosis of Hcd infection was made and he began therapy with liposomal amphotericin B (5 mg/kg/day). Histoplasmosis was later confirmed by multiple positive swab cultures of the exudates. After 4 weeks of amphotericin B therapy, he was switched to oral itraconazole (10 mg/kg/day). At this stage, a small clinical improvement was observed, with only minor reduction in the size of lymphadenopathies. In the following weeks, the patient clinical status was rapidly deteriorating, with enlargement of the previously noted adenopathies, along with appearance of new cutaneous fistulas. A painful supra-pubic abdominal mass appeared, and abdominal CT identified numerous new retroperitoneal lymphadenopathies and left hydronephrosis due to direct compression of the ureter (Fig. 2). Amphotericin B was restarted, with no apparent clinical benefit.
Fig. 1.
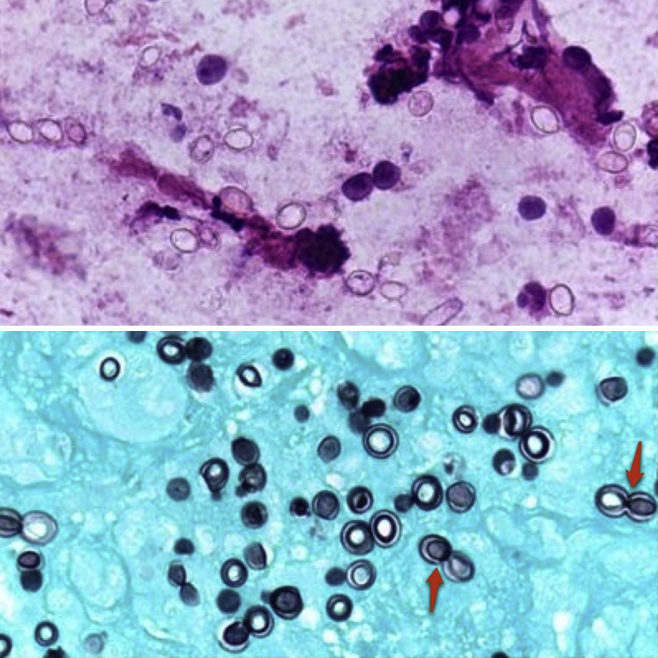
Histology from a cervical nodule – remark the presence of numerous uninucleate thick-walled large yeasts Histoplasma capsulatum var. duboisii (Hcd) along with numerous histiocytes (HE – 400×). Below a Grocott stain displays the thick wall of the yeasts as well as the budding (600×).
Fig. 2.
Abdominal computerized tomography showing multiple retroperitoneal lymphadenopathies.
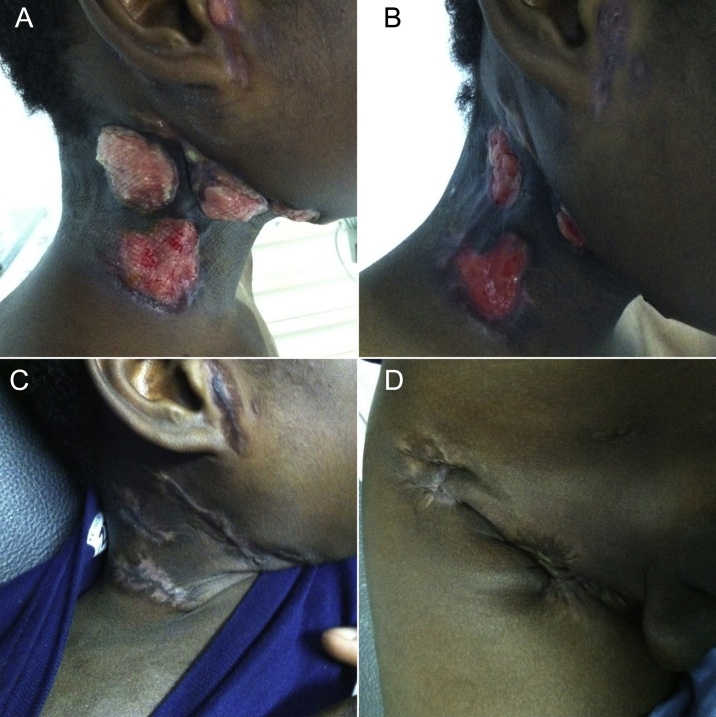
After approval by our hospital's ethics committee, posaconazole therapy was started, at a dosage of 400 mg twice daily. A dramatic and lasting improvement, both clinical (Fig. 3A-D) and biochemical, was observed. Imagiologic control 2 months after introduction of posaconazole demonstrated resolution of hydronephrosis and only minor (1 cm) lymphadenopathies. At day 127 of posaconazole therapy, complete cicatrization of all skin lesions was achieved (Fig. 3). Our patient completed 12 months of therapy with posaconazole. No relapse was noted during or 3 months after treatment.
Fig. 3.
Cervical nodular formations at days 4 (A) and 22 (B) of posaconazole therapy, along with cervical (C) and inguinal (D) regions after complete cicatrization.
Discussion
This case illustrates the many challenges that clinicians face when treating conditions more common in tropical areas. Despite its rarity, AH should be kept in mind as a possible diagnosis in Africa-born patients who have compatible signs or symptoms, particularly involving the skin and lymph nodes.
The pathogenesis of AH remains unclear. The main route of acquisition could be airborne contamination from the soil and, rarely, direct inoculation.7 In this case, we could not identify the source of contamination. AH, as observed in our patient, is usually not associated with immunosuppression.7
Fungal culture remains the gold standard for diagnosis of histoplasmosis, but is hampered by the slow growth of this microorganism. In our patient, histological analysis revealing granulomas and fungi within giant cells was the key for a rapid diagnosis. We should note that the observed giant cell granulomatosis could also suggest tuberculosis, but the histological presence of yeasts, the negativity of PCR for Mycobacterium in the histology sample, and the absence of clinical improvement with anti-tuberculous therapy suggest that tuberculosis was not responsible for the cutaneous and abdominal masses. Tuberculosis is nowadays a global emergency, responsible for 1.7 million deaths/year.8 In Guinea-Bissau it is an endemic infection, with a prevalence of 134/100,000, as shown in a recent cross-sectional survey.9 In our patient, tuberculosis might have contributed to AH infection, but we cannot establish any causal relationship with these two entities.
In the previously described cases of AH, the most common therapeutic regimens included amphotericin B and ketoconazole, fluconazole or itraconazole. In some of these reports, the disease proved fatal, either due to its natural course or due to side effects, mainly associated with amphotericin B.10 Posaconazole, though already successfully used as salvage therapy in classical histoplasmosis,11 has never been used in refractory AH.
Although firm recommendations regarding use of the newer azoles in histoplasmosis cannot be made, posaconazole appears to be a potential valuable treatment option, even in comparison with established effective agents such as itraconazole. In murine models infected with Histoplasma spp., although the minimal inhibitory concentration for itraconazole and posaconazole was similar, posaconazole was at least 10 times more effective than itraconazole in reducing fungal burden.12 Moreover, itraconazole, being a substrate for cytochrome P450 3A4 (CYP3A4), has a greater propensity for drug interactions.13 This also might have contributed to itraconazole failure in our patient, since he was being treated with the antituberculosis drug rifampicin, an inducer of CYP3A4.14
Pharmacokinetic and efficacy data for posaconazole usage in pediatric patients are still very limited,15 but even using high doses (400 mg twice daily), it provided a very fast, stable, and safe therapy in our patient. However, more studies are needed to optimize posaconazole dosage and length of therapy in pediatric patients.
In conclusion, we report that posaconazole may be safe and efficacious in the salvage management of disseminated AH, either in patients with disease refractory to conventional anti-fungal therapy, or in patients whose serious adverse effects or drug interactions of first-line drugs preclude its use.
Conflict of interest
All authors declare to have no conflict of interest.
Acknowledgements
The authors are grateful to Dra. Helena Barroca from our Hospital's Department of Pathology for providing the histology images for this article.
Contributor Information
Daniel Gonçalves, Email: danieldiasgoncalves@gmail.com.
Catarina Ferraz, Email: ferraz.catarina@gmail.com.
Luisa Vaz, Email: luisagvaz@netcabo.pt.
References
- 1.Wheat L.J. Histoplasmosis: a review from clinicians from non-endemic areas. Mycoses. 2006;49:274–282. doi: 10.1111/j.1439-0507.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi R., Mazzoni A., Nanetti A., Chiodo F. Histoplasmosis capsulate and duboisii in Europe: the impact of the HIV pandemic, travel and immigration. Eur J Epidemiol. 1994;10:675–681. doi: 10.1007/BF01719280. [DOI] [PubMed] [Google Scholar]
- 3.Gugnani H.C., Muotoe-Okafor F. African histoplasmosis: a review. Rev Iberoam Micol. 1997;14:155–159. [PubMed] [Google Scholar]
- 4.Wheat L.J., Freifeld A.G., Kleiman M.B., et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 5.Krishna G., Sansone-Parsons A., Martinho M., et al. Posaconazole plasma concentrations in juvenile patients with invasive fungal infection. Antimicrob Agents Chemother. 2007;51:812–818. doi: 10.1128/AAC.00454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehrnbecher T., Attarbaschi A., Duerken M., et al. Posaconazole salvage treatment in paediatric patients: a multicentre survey. Eur J Clin Microbiol Infect Dis. 2010;29:1043–1045. doi: 10.1007/s10096-010-0957-4. [DOI] [PubMed] [Google Scholar]
- 7.Loulergue P., Bastides F., Baudouin V., et al. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis. 2007;13:1647–1652. doi: 10.3201/eid1311.070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Organization; 2008. Global tuberculosis control – surveillance, planning, financing. WHO/HTM/TB/2008.393. [Google Scholar]
- 9.Bjerregaard-Andersen M., da Silva Z.J., Ravn P., et al. Tuberculosis burden in an urban population: a cross sectional tuberculosis survey from Guinea Bissau. BMC Infect Dis. 2010;10:96. doi: 10.1186/1471-2334-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakotoarivelo R.A., Razafimahefa S.H., Andrianiaina H.D., Randria M.J.D. Une histoplasmose africaine chez un patient malgache immunocompétent. Bull Soc Pathol Exot. 2010;103:19–21. doi: 10.1007/s13149-009-0030-7. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo A., Tobón A., Clark B., et al. Salvage treatment of histoplasmosis with posaconazole. J Infect. 2007;54:319–327. doi: 10.1016/j.jinf.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Connolly P., Wheat J., Schnizlein-Bick C., et al. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob Agents Chemother. 1999;43:322–328. doi: 10.1128/aac.43.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontoyiannis D.P. Invasive mycoses: strategies for effective management. Am J Med. 2012;125(1 Suppl.):S25–S38. doi: 10.1016/j.amjmed.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Nakamoto T., Hase I., Imaoka S., et al. Quantitative RT-PCR for CYP3A4 mRNA in human peripheral lymphocytes: induction of CYP3A4 in lymphocytes and in liver by rifampicin. Pharmacogenetics. 2000;10:571–575. doi: 10.1097/00008571-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Cesaro S., Milano G.M., Aversa F. Retrospective survey on the off-label use of posaconazole in pediatric hematology patients. Eur J Clin Microbiol Infect Dis. 2011;30:595–596. doi: 10.1007/s10096-010-1123-8. [DOI] [PubMed] [Google Scholar]