Abstract
Background
TB patients co-infected with HIV have worse treatment outcomes than non-coinfected patients. How clinical characteristics of TB and socioeconomic characteristics influence these outcomes is poorly understood. Here, we use polytomous regression analysis to identify clinical and epidemiological characteristics associated with unfavorable treatment outcomes among TB-HIV co-infected patients in Brazil.
Methods
TB-HIV cases reported in the Brazilian information system (SINAN) between January 1, 2001 and December 31, 2011 were identified and categorized by TB treatment outcome (cure, default, death, and development of MDR TB). We modeled treatment outcome as a function of clinical characteristics of TB and patient socioeconomic characteristics by polytomous regression analysis. For each treatment outcome, we used cure as the reference outcome.
Results
Between 2001 and 2011, 990,017 cases of TB were reported in SINAN, of which 93,147 (9.4%) were HIV co-infected. Patients aged 15–19 (OR = 2.86; 95% CI: 2.09–3.91) and 20–39 years old (OR = 2.30; 95% CI: 1.81–2.92) were more likely to default on TB treatment than those aged 0–14 years old. In contrast, patients aged ≥60 years were more likely to die from TB (OR = 2.22; 95% CI: 1.43–3.44) or other causes (OR = 2.86; 95% CI: 2.14–3.83). Black patients were more likely to default on TB treatment (OR = 1.33; 95% CI: 1.22–1.44) and die from TB (OR = 1.50; 95% CI: 1.29–1.74). Finally, alcoholism was associated with all unfavorable outcomes: default (OR = 1.94; 95% CI: 1.73–2.17), death due to TB (OR = 1.46; 95% CI: 1.25–1.71), death due to other causes (OR = 1.38; 95% CI: 1.21–1.57) and MDR-TB (OR = 2.29; 95% CI: 1.46–3.58).
Conclusions
Socio-economic vulnerability has a significant effect on treatment outcomes among TB-HIV co-infected patients in Brazil. Enhancing social support, incorporation of alcohol abuse screening and counseling into current TB surveillance programs and targeting interventions to specific age groups are interventions that could improve treatment outcomes.
Keywords: Tuberculosis, HIV, Coinfection, Logistic regression
Background
Disease caused by co-infection with Mycobacterium tuberculosis and Human Immunodeficiency Virus (TB-HIV) is a major global health problem.1 In Brazil, which is one of 22 high TB burden countries in the world, HIV prevalence among adults was estimated to be 0.6% between 2000 and 2006.2 A 2014 study based on the Brazilian National Surveillance System (SINAN) reported the prevalence of TB-HIV co-infection to be 19%.3 This study reported poor TB treatment outcomes in TB-HIV patients,3 similar to other studies.4, 5, 6, 7, 8, 9, 10, 11
Most prior studies of tuberculosis (TB) treatment outcomes in co-infected patients in Brazil examined dichotomous outcomes such as cure versus default or cure versus non-cure.7, 8, 9, 10, 11 None examined polytomous treatment outcomes, distinguishing between all possible outcomes present in the Notification System (cure, default, death from TB, MDR-TB, and death from other causes) of SINAN among TB-HIV patients.4, 5, 6, 7, 8, 9, 10, 11 This type of analysis provides a more detailed understanding of the influence of clinical and social characteristics on all TB treatment outcomes.12 Identification of TB-HIV patients who are more likely to have poor treatment outcomes is essential for the development of targeted interventions to improve treatment outcomes in this at-risk group. Building on the 2014 study cited above,3 which compared TB-HIV patients with a TB only group covering a 5-year period, we performed a polytomous regression analysis to identify clinical and epidemiological characteristics associated with unfavorable treatment outcomes among co-infected patients in Brazil.
Methods
Study design
A cross-sectional study design utilizing the Brazilian national TB reporting system (SINAN/TB) database was conducted. SINAN was developed in the early 1990s to collect and process data on disease notifications throughout the country. SINAN reports only confirmed cases and it is the primary information system from which data are extracted for epidemiological analyses using secondary data in Brazil.13
Study population
The study population was comprised of all TB cases with HIV infection reported in Brazil between January 1, 2001 and December 31, 2011. TB cases were defined as patients with a confirmed bacteriological diagnosis (smear or culture-positive) or by a combination of clinical and epidemiological data with results of diagnostic tests (smear and/or culture and/or histopathology.14
A patient was considered HIV infected when a screening test by enzyme-linked immunosorbent assay (ELISA) tested positive, followed by a confirmatory western blot test.15
Patients were classified into five (5) groups by treatment outcome: cure (completed treatment with at least two subsequent negative smear examinations), default (those that did not attend regular appointments for more than 30 days), death by TB, other cause of death (died during TB treatment of another cause), and MDR-TB (multidrug-resistant tuberculosis). Patients who initially were thought to have TB but were subsequently found to have a different diagnosis were excluded from the analysis.
Variables
The following socio-demographic covariates were extracted from SINAN: age group (<20 years, 20–39 years, 40–59 years, and ≥60 years), gender, skin color (white, black, brown, and other), education (illiterate, 1–3 years of schooling, 4–7 years, ≥8 years, and not applicable for children less than 6 years old), area of residence (urban, rural, or peri-urban) and whether the individual was institutionalized (prison/shelter/orphanage/mental hospital/others). The presence or absence of the following comorbidities was also determined: diabetes, alcoholism and AIDS.
Covariates related to the characteristics of tuberculosis and its treatment were also obtained. These included: type of entry in SINAN (new TB case: no prior TB diagnosis; relapse – completed a previous TB treatment; return after default: individuals that defaulted from a previous TB treatment regimen and returned to continue treatment), site of TB at presentation (pulmonary, extra-pulmonary, pulmonary and extra-pulmonary), tuberculin skin test result, existence of chest X-ray suspicious for TB, result of initial sputum smear test, result of initial culture examination and second month smear, result of initial histopathological examination, and whether they received directly observed therapy (DOT).
Data analysis and statistics
Pearson's chi-square test was used to compare proportions between the treatment outcome groups. Variables associated with the outcome of interest (p ≤ 0.05) were included in the hierarchical multinomial logistic regression model.
We performed polytomous regression analysis with outcome category as the dependent variable.12 For each possible outcome (default, death from TB, development of MDR-TB, and death from other case) the reference outcome was cure.
The present model was based on a conceptual framework for social determinants of TB in which independent variables are grouped into five levels: socio-demographic (Level 1), environmental (Level 2), previous comorbidities (Level 3), clinical features of TB (Level 4), and type of entry in SINAN and DOT (Level 5).16 At each level of analysis, those covariates associated with the outcome (p ≤ 0.05) were retained in the model. All our analyses were conducted with Stata, version 13.0 (Stata Corp, College Station, TX, USA).
Ethics statement
The databases were obtained under the rules for release of the Secretariat of Health Surveillance and Health Care Department of the Ministry of Health, ensuring the confidentiality and nondisclosure of individual identifiers. The Federal University of Espirito Santo (UFES) Institutional Review Board approved the study design by registration number 242,831.
Results
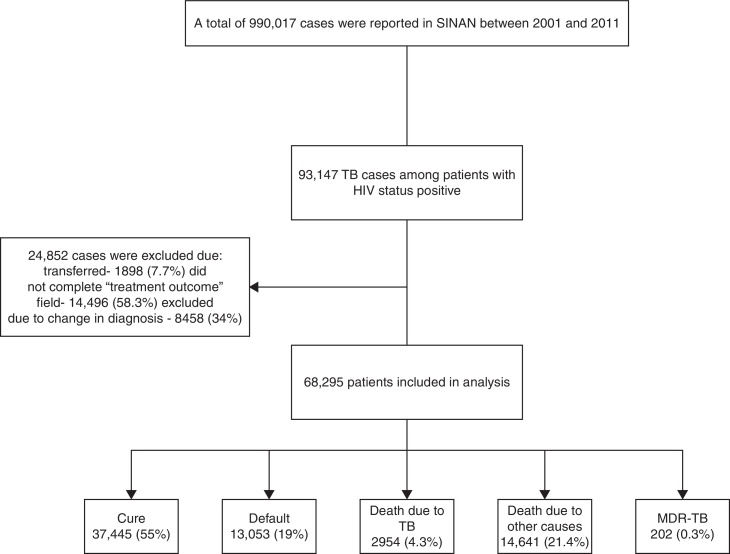
Between 2001 and 2011, 990,017 cases of TB were reported in SINAN, of which 93,147 (9.4%) were HIV co-infected (Fig. 1). Because of missing information on TB treatment outcome, 24,852 were excluded. Therefore, 68,295 patients were included in our analysis.
Fig. 1.
Study flow diagram for selection of patients included in our study.
The results of univariate analyses of socio-demographic and clinical characteristics by treatment outcome are shown in Table 1, Table 2. The proportions of all socio-demographic and clinical characteristics examined varied significantly between treatment outcome groups. The hierarchical polytomous regression model (Table 3) showed that default was more likely in individuals aged 15–19 years old (OR = 2.9, 95% CI 2.12–3.96), blacks (OR = 1.33, 95% CI 1.22–1.44), institutionalized persons (OR = 2.92, 95% CI 1.11–7.69), and persons with alcohol dependence (OR = 1.91, 95% CI 1.70–2.13). In addition, default was more likely to occur in persons diagnosed with AIDS (OR = 1.53, 95% CI 1.31–1.80), those diagnosed with pulmonary and extra-pulmonary TB simultaneously (OR = 1.21, 95% CI 1.01–1.46), individuals with positive cultures (OR = 1.44, 95% CI 1.11–1.86), notification in SINAN as a relapse (OR = 1.30, 95% CI 1.08–1.57) or as a return after default (OR = 5.13, 95% CI 4.36–6.02).
Table 1.
Distribution of socio-demographic characteristics and clinical history among TB-HIV cases by treatment outcome. Brazil, 2001–2011.
| Characteristics | Outcome |
||||
|---|---|---|---|---|---|
| Cure n (%) | Default n (%) | Death due to TB n (%) | Death due to other causes n (%) | MDR-TBan (%) | |
| Gender (n = 82,776)b | |||||
| Female | 10,745 (28.70) | 3909 (29.95) | 907 (30.70) | 4310 (29.44) | 63 (31.19) |
| Male | 26,692 (71.30) | 9141 (70.05) | 2047 (69.30) | 10,329 (70.56) | 139 (69.08) |
| Age, years (n = 82,789)b | |||||
| 0–14 | 897 (2.40) | 155 (1.19) | 39 (1.32) | 157 (1.07) | 1 (0.50) |
| 15–19 | 506 (1.35) | 247 (1.89) | 39 (1.32) | 123 (0.84) | 4 (1.98) |
| 20–39 | 22,313 (59.59) | 9116 (69.84) | 1551 (52.51) | 8167 (55.78) | 119 (58.91) |
| 40–59 | 12,785 (34.14) | 3398 (26.03) | 1179 (39.91) | 5613 (38.34) | 73 (36.14) |
| ≥60 | 944 (2.52) | 137 (1.05) | 146 (4.94) | 581 (3.97) | 5 (2.48) |
| Skin color (n = 57,577)b | |||||
| White | 13,372 (52.79) | 4231 (48.50) | 969 (36.59) | 5068 (53.57) | 100 (53.76) |
| Black | 3732 (14.73) | 1610 (18.46) | 419 (15.82) | 1487 (15.72) | 34 (18.28) |
| Brown | 7937 (31.33) | 2797 (32.06) | 1228 (46.37) | 2810 (29.70) | 51 (27.42) |
| Other | 289 (1.14) | 85 (0.97) | 32 (1.21) | 95 (1.00) | 1 (0.54) |
| School level, years (n = 52,366)b | |||||
| Illiterate | 4788 (18.95) | 1916 (22.59) | 464 (25.88) | 1722 (21.45) | 34 (19.21) |
| 1–3 | 1015 (4.02) | 383 (4.52) | 209 (11.66) | 326 (4.06) | 14 (7.91) |
| 4–7 | 10,386 (41.10) | 3806 (44.88) | 513 (28.61) | 3555 (44.28) | 87 (49.15) |
| ≥8 | 8043 (31.83) | 2159 (25.46) | 430 (23.98) | 2186 (27.23) | 34 (19.21) |
| Not applicablec | 1039 (4.11) | 216 (2.55) | 177 (9.87) | 239 (2.98) | 8 (4.52) |
| Area of residence (n = 54,463)b | |||||
| Urban | 22,422 (95.70) | 8035 (97.28) | 2465 (95.91) | 8402 (96.52) | 186 (97.38) |
| Rural | 885 (3.78) | 198 (2.40) | 89 (3.46) | 264 (3.03) | 4 (2.09) |
| Periurban | 123 (0.52) | 27 (0.33) | 16 (0.62) | 39 (0.45) | 1 (0.52) |
| Institutionalized (n = 37,220)b | |||||
| No | 12,686 (88.33) | 4714 (88.69) | 2079 (90.39) | 4729 (89.67) | 123 (87.86) |
| Prison | 1204 (8.38) | 322 (6.06) | 105 (4.57) | 343 (6.50) | 11 (7.86) |
| Shelter | 16 (0.11) | 12 (0.23) | 5 (0.22) | 11 (0.21) | 1 (0.71) |
| Orphanage | 34 (0.24) | 10 (0.19) | 6 (0.26) | 9 (0.17) | 0 |
| Mental hospital | 22 (0.15) | 15 (0.28) | 4 (0.17) | 5 (0.09) | 1 (0.71) |
| Other | 400 (2.79) | 242 (4.55) | 101 (4.39) | 177 (3.36) | 4 (2.86) |
| Alcoholism (n = 40,838)b | |||||
| No | 14,173 (84.99) | 4667 (76.70) | 1831 (79.54) | 5089 (82.05) | 94 (68.61) |
| Yes | 2504 (15.01) | 1418 (23.30) | 471 (20.46) | 1113 (17.95) | 43 (31.39) |
| Diabetes (n = 40,240)b | |||||
| No | 15,826 (96.99) | 5817 (98.29) | 2261 (97.37) | 5972 (97.26) | 127 (96.95) |
| Yes | 491 (3.01) | 101 (1.71) | 61 (2.63) | 168 (2.74) | 4 (3.05) |
| AIDS (n = 74,396)b | |||||
| No | 1633 (4.99) | 397 (3.41) | 119 (4.18) | 285 (2.11) | 14 (7.91) |
| Yes | 31,123 (95.01) | 11,261 (96.59) | 2731 (95.82) | 13,240 (97.89) | 163 (92.09) |
MDR-TB, multidrug-resistant tuberculosis.
P < 0.001.
Patients less than 6 years old.
Table 2.
Distribution of clinical characteristics among TB-HIV cases by treatment outcome. Brazil, 2001–2011.
| Characteristics | Outcome |
||||
|---|---|---|---|---|---|
| Cure n (%) | Default n (%) | Death due to TB n (%) | Death due to other causes n (%) | MDR-TB n (%) | |
| Type of entry (n = 82,791)b | |||||
| New case | 29,462 (78.68) | 8080 (61.90) | 2153 (72.88) | 11,390 (77.80) | 56 (27.72) |
| Relapse | 3418 (9.13) | 1231 (9.43) | 221 (7.48) | 1239 (8.46) | 55 (27.23) |
| Return after default | 2500 (6.68) | 3132 (23.99) | 298 (10.09) | 1319 (9.01) | 50 (24.75) |
| Unknown | 190 (0.51) | 88 (0.67) | 58 (1.96) | 187 (1.28) | 1 (0.50) |
| Transferred | 1875 (5.01) | 522 (4.00) | 224 (7.58) | 506 (3.46) | 40 (19.80) |
| Tuberculin skin test (n = 55,302)b | |||||
| No reaction | 3022 (12.73) | 763 (9.06) | 302 (11.75) | 968 (10.91) | 9 (4.52) |
| Weak reaction | 600 (2.53) | 144 (1.71) | 30 (1.17) | 127 (1.43) | 0 |
| Strong reaction | 3230 (13.61) | 840 (9.98) | 74 (2.88) | 304 (3.42) | 10 (5.03) |
| Not performed | 16,887 (71.14) | 6671 (79.25) | 2165 (84.21) | 7477 (84.24) | 180 (90.45) |
| TB form (n = 82,786)b | |||||
| Pulmonary | 23,494 (62.74) | 8734 (66.91) | 1931 (65.37) | 8437 (57.65) | 169 (83.66) |
| Extra-pulmonary | 10,248 (27.37) | 2812 (21.54) | 596 (20.18) | 3929 (26.84) | 14 (6.93) |
| Pulmonary + extra-pulmonary | 3703 (9.89) | 1507 (11.55) | 427 (14.45) | 2270 (15.51) | 19 (9.41) |
| Initial sputum smear (n = 82,791)b | |||||
| Negative | 12,479 (33.33) | 3974 (30.45) | 928 (31.42) | 4702 (32.12) | 35 (17.33) |
| Positive | 13,799 (36.85) | 5264 (40.33) | 1004 (33.99) | 4574 (31.24) | 145 (71.78) |
| Not performed | 11,167 (29.82) | 3815 (29.23) | 1022 (34.60) | 5365 (36.64) | 22 (10.89) |
| Sputum smear 2nd month (n = 30,199)b | |||||
| Negative | 3358 (28.97) | 1036 (23.83) | 554 (23.65) | 985 (25.14) | 26 (18.71) |
| Positive | 3152 (27.19) | 1208 (27.78) | 469 (20.03) | 683 (17.43) | 69 (49.64) |
| Not performed | 5081 (43.84) | 2104 (48.39) | 1319 (56.32) | 2250 (57.43) | 44 (31.65) |
| Sputum culture (n = 82,790)b | |||||
| Negative | 3447 (9.21) | 969 (7.42) | 143 (4.84) | 995 (6.80) | 10 (4.95) |
| Positive | 4696 (12.54) | 1796 (13.76) | 233 (7.89) | 1290 (8.81) | 97 (48.02) |
| Result not available | 2707 (7.23) | 1133 (8.68) | 260 (8.80) | 1133 (7.74) | 23 (11.39) |
| Not performed | 26,594 (71.02) | 9155 (70.14) | 2318 (78.47) | 11,223 (76.65) | 72 (35.64) |
| X-ray suggestive of TB (n = 77,258)b | |||||
| Negative | 4586 (13.04) | 1262 (10.37) | 240 (8.50) | 1563 (11.77) | 8 (4.02) |
| Suspicious of TB | 26,557 (75.52) | 9480 (77.92) | 2321 (82.19) | 10,349 (77.96) | 169 (84.92) |
| Not performed | 4022 (11.44) | 1425 (11.71) | 263 (9.31) | 1363 (10.27) | 22 (11.06) |
| Histopathological (n = 74,040)b | |||||
| AFB-positive | 2971 (8.90) | 887 (7.72) | 200 (7.11) | 907 (7.15) | 10 (5.03) |
| Suggestive of TB | 2995 (8.97) | 715 (6.22) | 133 (4.73) | 643 (5.07) | 7 (3.52) |
| Not suggestive of TB | 440 (1.32) | 140 (1.22) | 23 (0.82) | 186 (1.47) | 1 (0.50) |
| Result not available | 852 (2.55) | 284 (2.47) | 117 (4.16) | 333 (2.62) | 3 (1.51) |
| Not performed | 26,132 (78.26) | 9461 (82.36) | 2338 (83.17) | 10,623 (83.70) | 178 (89.45) |
| DOTd(n = 70,034)b | |||||
| No | 23,921 (72.71) | 8601 (77.04) | 1765 (69.03) | 8813 (74.18) | 114 (57.58) |
| Yes | 8979 (27.29) | 2564 (22.96) | 792 (30.97) | 3067 (25.82) | 84 (42.42) |
aMDR-TB, multidrug-resistant tuberculosis.
P < 0.001.
cAFB, acid-fast bacilli.
DOT, directly observed therapy.
Table 3.
Multinomial logistic regression with a hierarchical model of socio-demographic and clinical covariates associated with tuberculosis treatment outcomes in patients with HIV status positive. Brazil, 2001–2011.
| Characteristics | Default | Death due to TB | Death due to other causes | MDR-TBa |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Level 1 | ||||
| Gender | ||||
| Female | Ref | Ref | Ref | Ref |
| Male | 0.94 (0.89–1.01) | 0.92 (0.83–1.03) | 1.02 (0.96–1.09) | 0.82 (0.60–1.13) |
| Age, years | ||||
| 0–14 | Ref | Ref | Ref | Ref |
| 15–19 | 2.86 (2.09–3.91) | 1.67 (0.99–2.82) | 1.20 (0.82–1.77) | 5.22 (0.58–47.19) |
| 20–39 | 2.30 (1.81–2.92) | 1.29 (0.88–1.87) | 1.86 (1.45–2.39) | 3.49 (0.48–25.24) |
| 40–59 | 1.42 (1.11–1.81) | 1.67 (1.15–2.44) | 2.09 (1.62–2.69) | 4.09 (0.56–29.76) |
| ≥60 | 0.71 (0.50–1.00) | 2.22 (1.43–3.44) | 2.86 (2.14–3.83) | 3.13 (0.34–28.26) |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.33 (1.22–1.44) | 1.50 (1.29–1.74) | 1.05 (0.96–1.14) | 0.99 (0.65–1.50) |
| Browns | 1.08 (1.01–1.15) | 2.19 (1.96–2.45) | 0.94 (0.88–1.01) | 0.83 (0.58–1.19) |
| Other | 0.90 (0.67–1.20) | 1.72 (1.12–2.63) | 0.89 (0.67–1.19) | 0.46 (0.06–3.33) |
| School level, years | ||||
| Illiterate | Ref | Ref | Ref | Ref |
| 1–3 | 0.91 (0.79–1.03) | 2.00 (1.68–2.40) | 0.89 (0.77–1.02) | 1.66 (0.87–3.17) |
| 4–7 | 0.89 (0.82–0.95) | 0.86 (0.75–0.98) | 1.01 (0.93–1.09) | 1.75 (1.16–2.64) |
| ≥8 | 0.68 (0.63–0.74) | 0.82 (0.71–0.94) | 0.78 (0.71–0.84) | 0.78 (0.47–1.28) |
| Not applicable | 0.51 (0.43–0.59) | 1.69 (1.40–2.05) | 0.65 (0.56–0.76) | 1.02 (0.47–2.23) |
| Level 2 | ||||
| Area of residence | ||||
| Urban | Ref | Ref | Ref | Ref |
| Rural | 0.53 (0.41–0.68) | 0.88 (0.67–1.15) | 0.53 (0.40–0.69) | 0.53 (0.16–1.69) |
| Periurban | 0.68 (0.36–1.29) | 0.90 (0.43–1.93) | 0.95 (0.52–1.73) | 1.38 (0.18–10.16)) |
| Institutionalization | ||||
| No | Ref | Ref | Ref | Ref |
| Prison | 0.80 (0.67–0.96) | 0.65 (0.48–0.86) | 0.92 (0.76–1.12) | 1.51 (0.79–2.87) |
| Shelter | 2.88 (1.10–7.52) | 1.28 (0.27–5.98) | 1.84 (0.61–5.52) | – |
| Orphanage | 1.09 (0.48–2.49) | 1.05 (0.36–3.09) | 1.29 (0.54–3.04) | – |
| Mental hospital | 1.67 (0.71–3.92) | 1.24 (0.35–4.34) | 0.51 (0.11–2.26) | 5.24 (0.70–42.47) |
| Other | 1.53 (1.23–1.89) | 1.34 (1.00–1.78) | 1.37 (1.08–1.75) | 1.28 (0.46–3.52) |
| Level 3 | ||||
| Diabetes | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.63 (0.45–0.88) | 0.68 (0.46–1.01) | 0.80 (0.59–1.08) | 0.89 (0.27–2.88) |
| Alcoholism | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.94 (1.73–2.17) | 1.46 (1.25–1.71) | 1.38 (1.21–1.57) | 2.29 (1.46–3.58) |
| AIDS | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.44 (1.21–1.70) | 2.04 (1.57–2.65) | 2.72 (2.17–3.42) | 1.04 (0.55–1.96) |
| Level 4 | ||||
| TB form | ||||
| Pulmonary | Ref | Ref | Ref | Ref |
| Extra-pulmonary | 0.75 (0.64–0.87) | 0.75 (0.61–0.93) | 0.92 (0.78–1.07) | 0.75 (0.27–2.03) |
| Pulmonary + extra-pulmonary | 1.10 (0.94–1.30) | 1.58 (1.30–1.92) | 1.77 (1.51–2.08) | 1.34 (0.72–2.50) |
| Tuberculin skin test | ||||
| No reaction | Ref | Ref | Ref | Ref |
| Weak reaction | 0.96 (0.64–1.42) | 0.48 (0.26–0.89) | 0.88 (0.60–1.29) | – |
| Strong reaction | 1.09 (0.89–1.35) | 0.24 (0.17–0.35) | 0.32 (0.25–0.43) | 0.86 (0.27–2.74) |
| Not performed | 1.65 (1.40–1.95) | 1.37 (1.13–1.65) | 1.29 (1.11–1.51) | 2.05 (0.87–4.82) |
| Initial sputum smear | ||||
| Negative | Ref | Ref | Ref | Ref |
| Positive | 0.97 (0.82–1.15) | 0.77 (0.62–0.96) | 0.86 (0.72–1.04) | 1.66 (0.80–3.46) |
| Not performed | 0.84 (0.70–1.01) | 0.79 (0.62–1.01) | 0.93 (0.76–1.13) | 0.83 (0.30–2.31) |
| X-ray | ||||
| Negative | Ref | Ref | Ref | Ref |
| Suspicious of TB | 0.93 (0.78–1.13) | 1.09 (0.84–1.41) | 0.93 (0.76–1.11) | 0.82 (0.30–2.22) |
| Not performed | 1.02 (0.82–1.27) | 0.69 (0.49–0.96) | 0.86 (0.68–1.09) | 0.88 (0.28–2.76) |
| Histopathological examination | ||||
| AFB-positiveb | Ref | Ref | Ref | Ref |
| Suggestive of TB | 0.89 (0.70–1.14) | 0.59 (0.40–0.86) | 0.59 (0.45–0.77) | 0.45 (0.05–3.87) |
| Not suggestive of TB | 0.99 (0.63–1.57) | 0.49 (0.22–1.10) | 0.94 (0.59–1.51) | 1.51 (0.17–13.17) |
| Result not available | 1.25 (0.92–1.70) | 1.31 (0.88–1.95) | 0.91 (0.65–1.28) | 2.08 (0.40–10.73) |
| Not performed | 1.06 (0.89–1.25) | 1.18 (0.93–1.50) | 1.08 (0.91–1.29) | 2.15 (0.91–5.08) |
| Sputum culture | ||||
| Negative | Ref | Ref | Ref | Ref |
| Positive | 1.50 (1.18–1.91) | 1.19 (0.83–1.69) | 1.02 (0.77–1.36) | 6.0 (2.35–15.49) |
| Result not available | 1.55 (1.21–1.98) | 1.52 (1.08–2.14) | 1.27 (0.96–1.68) | 1.52 (0.52–4.49) |
| Not performed | 1.18 (0.96–1.44) | 1.44 (1.08–1.90) | 1.55 (1.24–1.94) | 0.41 (0.15–1.09) |
| Sputum smear 2nd month | ||||
| Negative | Ref | Ref | Ref | Ref |
| Positive | 1.09 (0.91–1.32) | 0.95 (0.74–1.23) | 0.76 (0.62–0.95) | 1.20 (0.56–2.57) |
| Not performed | 1.37 (1.15–1.63) | 1.47 (1.17–1.84) | 1.23 (1.02–1.48) | 1.36 (0.63–2.94) |
| Level 5 | ||||
| Type of entry | ||||
| New case | Ref | Ref | Ref | Ref |
| Relapse | 1.27 (1.07–1.50) | 0.78 (0.60–1.00) | 1.08 (0.88–1.33) | 6.47 (3.74–11.18) |
| Return after default | 4.78 (4.13–5.55) | 1.69 (1.34–2.12) | 1.90 (1.54–2.36) | 8.13 (4.59–14.43) |
| Unknown | 1.45 (–--) | 3.67 (1.05–12.78) | 2.21 (0.55–8.91) | – |
| Transferred | 1.06 (0.89–1.27) | 0.99 (0.79–1.25) | 0.80 (0.63–1.00) | 3.41 (1.71–6.80) |
| DOTc | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.74 (0.67–0.83) | 0.81 (0.70–0.94) | 0.81 (0.71–0.93) | 1.71 (1.10–2.64) |
The values in bold were statistically significant.
MDR-TB, multidrug-resistant tuberculosis.
AFB, acid-fast bacilli.
DOT, directly observed therapy.
TB-HIV patients who died due to TB were more likely to be over 60 years old (OR = 2.18, 95% CI 1.41–3.39), black (OR = 1.48, 95% CI 1.27–1.71) or brown (OR = 2.17, 95% CI 1.94–2.43), and report alcoholism (OR = 1.48, 95% CI 1.26–1.72). In addition, those who died due to TB were more likely to be diagnosed with AIDS (OR = 2.05, 95% CI 1.62–2.61), have pulmonary and extra-pulmonary TB simultaneously (OR = 1.68, 95% CI 1.30–2.22), and return after default (OR = 1.81, 95% CI 1.33–2.48). Death due to TB was less likely in patients with ≥8 years of schooling (OR = 0.68, 95% CI 0.60–0.78) and patients receiving DOT (OR = 0.75, 95% CI 0.61–0.91).
Regarding death due to other causes, the odds were higher for persons aged 40–59 (OR = 2.08, 95% CI 1.61–2.67), persons aged ≥60 (OR = 2.85, 95% CI 2.13–3.81), persons with alcohol dependence (OR = 1.49, 95% CI 1.23–1.58) and those diagnosed with AIDS (OR = 2.47, 95% CI 2.03–3.00). Diagnosis with of MDR-TB in TB-HIV patients was more likely when alcoholism was reported (OR = 2.23, 95% CI 1.44–3.45), the initial sputum smear was positive (OR = 2.52, 95% CI 1.29–4.92), and also with positive smear test at the second month of treatment (OR = 2.28, 95% CI 1.28–4.06). In addition, those who relapsed (OR = 7.76, 95% CI 4.18–14.43) or returned after default (OR= 10.25, 95% CI 5.44–19.34) had increased risk of developing MDR-TB.
Discussion
Tuberculosis remains a major public health concern worldwide and in Brazil, driven in part by TB-HIV co-infection.3 We found that the socio-demographic characteristics most associated with poor TB treatment outcomes were age (15–19 years old and ≥60 years old), alcohol use, homelessness, less education, and non-white race. The clinical factors associated with poor TB treatment outcomes were resuming treatment after failing a previous regimen (either relapse or return after dropout), AIDS and site of TB infection (pulmonary and extra-pulmonary TB simultaneously).
The rising rate of TB-HIV incidence found in our study may be explained by higher rates of HIV testing among TB cases observed from 2001 to 2011 in Brazil.17 The effect of age on treatment outcomes that we observed may be more complicated to clarify. Older age is a risk factor for mortality among TB patients with and without HIV.18, 19 It is also an independent risk factor for infectious diseases, in part due to age-related decreases in immunity.20 Age-related decreases in immunity could thus explain both the increase in TB mortality as well as increases in mortality from other causes.
The effect we observed of alcohol on treatment outcomes appears to be linked to age. In Brazil, younger TB-HIV patients have higher rates of high-risk behaviors, including alcohol use, smoking, and use of illicit drugs.21 In our study population, the majority of patients with alcohol use were of younger age (20–39 years old). Therefore, the higher rates of treatment default we observed may be attributable to these behaviors.
The effects of housing status, education and race are likely to have a common explanation: low socioeconomic status (SES). In the face of more pressing matters (e.g. financial and food insecurity) and reduced access to services, persons with low SES are less likely to prioritize TB care/treatment. It is thus unsurprising that housing status, education, and race, all of which are markers of SES, were associated with worse TB treatment outcomes.22 A good example of the difficulties faced by this group is that in some Brazilian cities, patients are able to receive HAART (Highly Active Antiretroviral Therapy) but not food vouchers or transportation subsidies. The absence of the latter two services makes successful treatment virtually impossible.3 The importance of access to services is reinforced by our results in persons with diabetes mellitus and in prisoners. Both of these groups had lower rates of treatment default, possibly because of better, more consistent contact with the healthcare system.
Unsuccessful prior TB treatment had a significant impact on TB treatment outcomes, with patients who relapsed and patients who returned to treatment after default doing poorly. These poor treatment outcomes were largely due to mycobacterial resistance that was a direct consequence of having been exposed to incomplete treatment, which could have happened multiple times. These patients had much higher rates of MDR-TB and, as a result, poorer outcomes.
Finally, the associations we observed between AIDS and site of TB infection with poorer treatment outcomes are almost certainly linked. Higher levels of immunosuppression increase the likelihood of extra-pulmonary or simultaneous extra-pulmonary and pulmonary TB, all of which are more difficult to treat.23, 24, 25, 26, 27 Although we did not have access to CD4 counts and could not estimate the mean level of immunosuppression in patients with AIDS, this possibility seems to be the likely explanation for poor outcomes in these groups.
Although we believe the results we report here to be important and clinically relevant, it is important to acknowledge a primary limitation of our analysis: there was a large amount of missing data regarding treatment outcome. These missing data could have biased our risk estimates, resulting in under or over estimation. To examine this possibility, we repeated our analysis including these data and observed only a negligible effect on our risk estimates (data not shown). Bias is always a concern with secondary data sources, but the SINAN database has already served as a source for several other robust population-based studies in Brazil.3, 28, 29 A second limitation of our results is their generalizability. Whether the social factors that are relevant in Brazil will also be relevant to other countries is unknown. However, each of the factors we identified is tied to poverty and while the specific factors may vary from country to country, we believe that the link between poverty and poorer TB-HIV treatment outcomes may remain consistent.
We show here the significant influence of socio-demographic and clinical factors on treatment outcomes in TB-HIV co-infected patients in Brazil. We identify insufficient institutional support for the poor as a primary barrier to achieving better TB-HIV treatment outcomes. New strategies must be developed and implemented to mitigate these effects. One potential strategy is to have better coordination between TB and HIV control programs, through collaborative TB-HIV activities. These activities include: (1) HIV testing and counseling for patients with presumptive and diagnosed TB; (2) TB prevention through isoniazid and early antiretroviral therapy. Another potential strategy to is to take advantage of the Brazilian healthcare system's existing structure of community healthcare workers to develop community-based TB treatment programs.30, 31, 32, 33, 34 Both of the strategies we propose focus on the healthcare system, but for them to be most effective they must be coupled with efforts to improve patients’ social conditions. Providing food vouchers and transportation subsidies could help improve adherence to both TB and antiretroviral therapy. Similarly, given the strong influence of alcohol, incorporation of screening instruments such as the Alcohol Use Disorder Identification Test (AUDIT) and Screening and Brief Interventions into current TB surveillance activities could improve treatment adherence.35, 36 These and other initiatives aimed at addressing patients’ social conditions could have a significant impact on TB-HIV treatment outcomes.
Conclusions
The key factors driving unfavorable tuberculosis treatment outcomes in TB-HIV co-infected patients in Brazil were age (15–19 years old and ≥60 years old), alcohol use, homelessness, less education, non-white race, and the form of TB. Many of these factors are markers of socio-economic vulnerability. These findings have broad policy implications: providing better socio-economic support to patients is likely the key to improving outcomes. There is a pressing need to improve coordination between TB and HIV control programs. Specific initiatives could include the incorporation of alcohol abuse screening and counseling into current TB control programs, which could result in better treatment outcomes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by CNPq/Brazil edital Doenças negligenciadas 2012 and Universal 2010, the U.S. National Institutes of Health, under contract ICOHRTA 5 U2R TW006883-02 and FAPES (Fundação de Amparo à Pesquisa do Espírito Santo).
References
- 1.World Health Organization . World Health Organization; Geneva: 2010. Global tuberculosis control: 2010. [Google Scholar]
- 2.Brazilian Ministry of Health . 2011. Monitoraids – AIDS monitoring online system. http://sistemas.aids.gov.br/monitoraids/ [accessed 15.06.14] [Google Scholar]
- 3.Prado T.N., Miranda A.E., de Souza F.M., et al. Factors associated with tuberculosis by HIV status in the Brazilian national surveillance system: a cross sectional study. BMC Infect Dis. 2014;14:1–8. doi: 10.1186/1471-2334-14-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruza M., Ximenes R.A.A., Lacerda H.R. Desfecho do tratamento e confirmação do diagnóstico de tuberculose em pacientes com HIV/AIDS no Recife, Pernambuco, Brasil. J Bras Pneumol. 2008;34:394–403. doi: 10.1590/s1806-37132008000600010. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez M., Bartholomay P., Arakaki-Sanchez D., et al. Outcomes of TB treatment by HIV status in national recording systems in Brazil, 2003–2008. PLoS ONE. 2012;7:e33129. doi: 10.1371/journal.pone.0033129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prado T.N., Caus A.L., Marques M., Maciel E.L., Golub J.E., Miranda A.E. Epidemiological profile of adult patients with tuberculosis and AIDS in the state of Espírito Santo, Brazil: cross-referencing tuberculosis and AIDS databases. J Bras Pneumol. 2011;37:93–99. doi: 10.1590/s1806-37132011000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruza M., Albuquerque M.F., Coimbra I., et al. Risk factors for default from tuberculosis treatment in HIV-infected individuals in the state of Pernambuco, Brazil: a prospective cohort study. BMC Infect Dis. 2011;16:351. doi: 10.1186/1471-2334-11-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabahi M.F., Rodrigues A.B., Mello F.Q., Almeida Netto J.C., Kritski A.L. Concompliance with tuberculosis treatment by patients at a tuberculosis and AIDS reference hospital in midwestern Brazil. Braz J Infect Dis. 2002;6:63–73. doi: 10.1590/s1413-86702002000200002. [DOI] [PubMed] [Google Scholar]
- 9.Ngamvithayapong J., Uthaivoravit W., Yanai H., Akarasewi P., Sawanpanyalert P. Adherence to tuberculosis preventive therapy among HIV-infected persons in Chiang Rai, Thailand. AIDS. 1997;11:107–112. doi: 10.1097/00002030-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Tangüis H.G., Caylà J.A., García de Olalla P.G., Jansà J.M., Brugal M.T. Factors predicting non-completion of tuberculosis treatment among HIV-infected patients in Barcelona (1987–1996) Int J Tuberc Lung Dis. 2000;4:55–60. [PubMed] [Google Scholar]
- 11.Kittikraisak W., Burapat C., Kaewsa-ard S., et al. Factors associated with tuberculosis treatment default among HIV-infected tuberculosis patients in Thailand. Trans R Soc Trop Med Hyg. 2009;103:59–66. doi: 10.1016/j.trstmh.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Biesheuvel C.J., Vergouwe Y., Steyerberg E.W., Grobbee D.E., Moons K.G. Polytomous logistic regression analysis could be applied more often in diagnostic research. J Clin Epidemiol. 2008;61:125–134. doi: 10.1016/j.jclinepi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Cerqueira A.C.B., Sales C.M.M., Lima R., et al. Completude do sistema de informação de agravos de notificação compulsória de gestante HIV positivo entre 2001 e 2006, no Espírito Santo, Brasil. UFES Rev Odont. 2008;10:33–37. [Google Scholar]
- 14.Brasil Ministério da Saúde . Secretaria de Vigilância em Saúde, MS; Brasília, Brazil: 2011. Manual de recomendações para o controle da tuberculose no Brasil. [Google Scholar]
- 15.Brazilian Ministry of Health . 2009. Decree number 59. Available: http://dtr2001.saude.gov.br/sas/PORTARIAS/Port2003/GM/GM-59.htm [accessed 12.01.12] [Google Scholar]
- 16.Maciel E.L. Saberes; Campinas: 2012. A Promoção da Saúde e os Determinantes Socais da Tuberculose: Elementos para a ação. Promoção da Saúde na diversidade humana e na pluralidade de itinerários terapeuticos; pp. 429–448. [Google Scholar]
- 17.Oliveira G.P., Torrens A.W., Bartholomay P., Barreira D. Tuberculosis in Brazil: last tem years analysis – 2001–2010. Braz J Infect Dis. 2013;17:218–233. doi: 10.1016/j.bjid.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan-Yeung M., Noertjojo K., Tan J., Chan S.L., Tam C.M. Tuberculosis in the elderly in Hong Kong. Int J Tuberc Lung Dis. 2002;6:771–779. [PubMed] [Google Scholar]
- 19.Lee J.H., Han D.H., Song J.W., Chung H.S. Diagnostic and therapeutic problems of pulmonary tuberculosis in elderly patients. J Korean Med Sci. 2005;20:784–789. doi: 10.3346/jkms.2005.20.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberger B., Herndler-Brandstetter D., Schwanninger A., Weiskopf D., Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- 21.Batista Jd., Militão de Albuquerque Mde F., Ximenes R.A., et al. Prevalence and socioeconomic factors associated with smoking in people living with HIV by sex, in Recife, Brazil. Rev Bras Epidemiol. 2013;16:432–443. doi: 10.1590/S1415-790X2013000200018. [DOI] [PubMed] [Google Scholar]
- 22.Nadjane Batista Lacerda S., Cristina de Abreu Temoteo R., Maria Ribeiro Monteiro de Figueiredo T., et al. Individual and social vulnerabilities upon acquiring tuberculosis: a literature systematic review. Int Arch Med. 2014;7:35. doi: 10.1186/1755-7682-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muñoz-Sellart M., Cuevas L.E., Tumato M., Merid Y., Yassin M.A. Factors associated with poor tuberculosis treatment outcome in the Southern Region of Ethiopia. Int J Tuberc Lung Dis. 2010;14:973–979. [PubMed] [Google Scholar]
- 24.Ronaidi N.N., Mohd N.S., Wan Mohammad Z., Sharina N.R., NikRosmawati N.H. Factors associated with unsuccessful treatment outcome of pulmonary tuberculosis in Kota Bharu, Kelantan. Malays J Public Health Med. 2011;11:6–15. [Google Scholar]
- 25.Braun M.M., Coté T.R., Rabkin C.S. Trends in death with tuberculosis during the AIDS era. JAMA. 1993;269:2865–2868. [PubMed] [Google Scholar]
- 26.Franco J., Blanquer R. Mortality from tuberculosis in Spain from 1970 to 1993: changes in epidemiological trends during the acquired immune-deficiency syndrome epidemic. Int J Tuberc Lung Dis. 1998;2:663–669. [PubMed] [Google Scholar]
- 27.Klautau G.B., Kuschnaroff T.M. Clinical forms and outcome of tuberculosis in HIV-infected patients in a tertiary hospital in São Paulo – Brazil. Braz J Infect Dis. 2005;9:464–478. doi: 10.1590/s1413-86702005000600004. [DOI] [PubMed] [Google Scholar]
- 28.Gomes T., Vinhas S.A., Reis-Santos B., et al. Extrapulmonary tuberculosis: Mycobacterium tuberculosis strains and host risk factors in a large urban setting in Brazil. PLOS ONE. 2013;8:e74517. doi: 10.1371/journal.pone.0074517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis-Santos B., Locatelli R., Horta B.L., et al. Socio-demographic and clinical differences in subjects with tuberculosis with and without diabetes mellitus in Brazil – a multivariate analysis. PLOS ONE. 2013;8:e62604. doi: 10.1371/journal.pone.0062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villa T.C.S., Assis E.G. de, Oliveira M.F. de, Arcêncio R.A., Cardozo Gonzales R.I., Palha P.F. Cobertura do tratamento diretamente observado (DOTS) no Estado de São Paulo (1998 a 2004) Rev Esc Enferm USP. 2008;42:98–104. doi: 10.1590/s0080-62342008000100013. [DOI] [PubMed] [Google Scholar]
- 31.Cardozo gonzales R.I., Monroe A.A., Arcêncio R.A., Oliveira M.F. de, Ruffino Netto A., Villa T.C.S. Performance indicators of DOT at home for tuberculosis control in a large city, SP, Brazil. Rev Latinoam Enferm (Ribeirão Preto) 2008;16:95–100. doi: 10.1590/s0104-11692008000100015. [DOI] [PubMed] [Google Scholar]
- 32.Maciel E.L., Silva A.P., Meireles W., Fiorotti K., Hadad D.J., Dietze R. Directly observed therapy using home-based supervisors for treating tuberculosis in Vitória, Brazil. J Bras Pneumol. 2008;34:506–513. doi: 10.1590/s1806-37132008000700011. [DOI] [PubMed] [Google Scholar]
- 33.Prado T.N., Wada N., Guidoni L.M., Golub J.E., Dietze R., Maciel E.L. Cost-effectiveness of community health worker versus home-based guardians for directly observed treatment of tuberculosis in Vitoria, Espirito Santo State, Brazil. Cad Saude Publica. 2011;27:944–952. doi: 10.1590/s0102-311x2011000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arshad A., Salam R.A., Lassi Z.S., Das J.K., Naqvi I., Bhutta Z.A. Community based interventions for the prevention and control of tuberculosis. Infect Dis Poverty. 2014;1:3–27. doi: 10.1186/2049-9957-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoeman J.H., Parry C.D., Lombard C.J., Klopper H.J. Assessment of alcohol screening instruments in tuberculosis patients. Tuberc Lung Dis. 1994;5:371–376. doi: 10.1016/0962-8479(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 36.Peltzer K., Naidoo P., Louw J., et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: results from a cluster randomized controlled trial. BMC Public Health. 2013;13:699. doi: 10.1186/1471-2458-13-699. [DOI] [PMC free article] [PubMed] [Google Scholar]