Abstract
Background:
Though recent literature provides promising support for the analgesic properties of alcohol, potential differences in alcohol analgesia as a function of chronic pain status are not well understood. Thus, this study examined chronic pain status as a potential moderator of alcohol analgesia and distinguished between multiple aspects of pain experience and sensitivity: pain threshold, pain intensity, pain unpleasantness, and perceived relief.
Methods:
Social drinkers with (N=19) and without (N=29) chronic jaw pain completed two testing sessions in a counterbalanced order: alcohol (target BrAC=.08 g/dl) and placebo. In each, pressure algometry was performed at the insertion of the masseter. Alcohol analgesia was assessed by examining main and interactive effects of beverage condition, pressure level (4-, 5-, or 6-lbf.), and chronic jaw pain status (chronic pain vs. pain-free control) on quantitative sensory testing (QST) measures and pain relief ratings following noxious stimuli.
Results:
Analyses indicated significant increases in pain threshold and pain relief, as well as reductions in pain unpleasantness and pain intensity, under the alcohol condition. Chronic pain participants, compared to controls, demonstrated lower pain thresholds, and greater pain intensity and pain unpleasantness ratings.
Conclusions:
Findings provide experimental evidence of alcohol’s analgesic and pain-relieving effects and suggest these effects do not significantly differ by chronic pain status. Results suggest that individuals, regardless of chronic pain status, who self-medicate pain via alcohol consumption may be at high risk for engaging in hazardous drinking patterns and experiencing adverse alcohol-related consequences.
Keywords: alcohol, chronic pain, analgesia, pain relief
Introduction
Pain is a major public health issue, affecting more than 116 million U.S. adults and resulting in $635 billion annually in costs related to health care expenditure and lost productivity (Tsang et al., 2008; Gaskin & Richard, 2012). Similarly, hazardous alcohol use has a substantial public health impact, costing an estimated $249 billion annually and representing the third-leading preventable cause of death in the U.S. (Sacks et al., 2015; Mokdad et al., 2004; NIAAA, 2021).
Despite potentially critical associations between alcohol use and pain, much of the current literature regarding pain and substance use interactions focuses on opiate use/misuse. However, in recent years, a growing interest in the relationship between pain and alcohol use/misuse has prompted empirical investigations, resulting in emerging evidence that suggests a strong correlation between pain and alcohol use/misuse. For instance, individuals with AUD are more likely to report current moderate-severe pain compared to the general population (i.e. 43–75% vs. 18%; Brennan et al., 2005), and individuals in residential treatment with AUD report chronic pain at substantially higher rates than community-dwelling low-risk drinkers (54% vs. 32%; Boissoneault et al., 2019). Similarly, individuals with chronic pain are more likely to report heavy drinking compared to the general population (i.e. 25% vs. 4.5–7%; Zale et al., 2015; NIAAA, 2020) and self-management of pain via alcohol consumption is common (25–28%; Riley & King, 2009). Furthermore, experimentally induced pain has been shown to increase urge to consume alcohol (Moskal et al., 2018) and alcohol demand (Stennett et al., 2021) in healthy adults.
A growing body of literature points to shared neural mechanisms underlying chronic pain and alcohol/substance misuse, suggesting chronic pain status may moderate the analgesic effects of alcohol (Ditre et al., 2019; Egli et al., 2012). Studies of the functional neural correlates of alcohol-induced neurobehavioral compromise indicate disruption of functional activation and glucose utilization in brain structures including anterior cingulate gyrus, prefrontal cortex, medial frontal cortex, and the basal ganglia (Anderson et al., 2011; Marinkovic et al., 2012; Soderlund, et al., 2005, Volkow et al., 2006). Notably, these areas and the evaluative, limbic, and executive networks in which they are integrated are implicated as modulators of acute and chronic pain, indicating a common neural framework underlying both acute alcohol effects and the pain experience (Hashmi et al., 2013). Furthermore, recent research has provided evidence that acute oral alcohol intake produces elevations in perceived pain relief (Williams et al., 2021), pain threshold (Thompson et al., 2017; Williams et al., 2021), and conditioned pain modulation (Horn-Hofmann et al., 2019) as a result of beverage consumption. However, additional studies are needed to elucidate underlying mechanisms.
Thus, the overall aim of this study was to determine the analgesic effects of acute alcohol administration in regular drinkers with and without chronic jaw pain. This condition was chosen (vs. other potential chronic musculoskeletal pain conditions) for several reasons. Specifically, chronic jaw pain is common in the general population and use of alcohol to self-manage pain is widespread among affected individuals (Riley & King, 2009). In addition, pressure-based quantitative sensory testing (QST) involving application of multiple pressure levels to the insertion of the masseter muscle results in evoked pain qualitatively similar to that experienced day-to-day by jaw pain sufferers. Together, these considerations increased feasibility of participant recruitment and helped to ensure clinical relevance of the investigation. Finally, self-reported changes in pain intensity are typically assumed to also reflect perceived relief. However, this is likely a faulty assumption (Leknes et al., 2008). Thus, we also separately examined alcohol-related changes in pain relief, pain intensity, and pain unpleasantness.
Our study included two groups: individuals with (chronic pain) and without (control) chronic jaw pain. Based on previous research (Williams et al., 2021; Thompson et al., 2017), we hypothesized strong analgesic responses to alcohol among both participant groups, such that alcohol intake would be associated with reduced pain intensity/unpleasantness and greater perceived pain relief compared with placebo. We also predicted that chronic pain status would moderate the magnitude of alcohol effects on pain sensitivity. However, because literature on the directionality of this effect is lacking, we posed as an empirical question whether the effect of alcohol on pain sensitivity would be stronger or weaker in this group. Attenuated analgesic response in individuals with chronic jaw pain would be consistent with rat studies suggesting neuropathic pain alters opioid dose-response curves, increasing opioid self-administration (Egli et al., 2012). Conversely, an exaggerated analgesic response would suggest that alcohol use may be especially negatively reinforcing (Ditre et al., 2019).
Materials and Methods
Participant Eligibility and Recruitment
Social drinkers with and without chronic jaw pain were recruited from the north central Florida area using flyers, print media, and word of mouth. Upon contacting the laboratory, interested individuals were informed of the basic inclusionary criteria, including (a) being between the ages of 21 and 45; (b) having no history of chronic pain, except for chronic jaw pain (i.e., at least 50% of the days over the past six months; chronic pain group only), major psychiatric disorder, neurological disease, or other serious medical illness that may affect pain perception; (c) having no history of drug or alcohol problems; (d) no current use of opioid analgesics or prescription medications which contraindicate alcohol use; (e) being a non-smoker; and (f) consuming, on average, one or more alcoholic beverages per month over the past half year. All procedures were approved by the University of Florida Institutional Review Board and participants provided written consent prior to participation in the study.
Screening Session
During an initial screening session, information regarding demographics, typical drinking behaviors (Alcohol Use Disorders Identification Test [AUDIT]; Saunders et al., 1993), and alcohol use histories (Alcohol Use Questionnaire [AUQ]; Cahalan et al., 1969) was collected. To avoid potential confounding effects of hazardous alcohol use, an AUDIT score ≥ 8 was exclusionary. Participants reporting chronic jaw pain were evaluated by a practicing orthodontist and orofacial pain expert (JKN) in accordance with published Research Diagnostic Criteria for TMD (Schiffman et al., 2014). They also completed the Oral Health Impact Profile-Temporomandibular Disorders (OHIP-TMDs; Yule et al., 2015), a jaw-pain specific inventory of pain severity and quality-of-life impacts. OHIP-TMDs scores range from 0–88, with higher scores representing more severe symptomatology, and previous studies show good test-retest reliability (ICC=.805; Yule et al., 2015). Eligible participants were scheduled for two testing sessions.
Testing Sessions
Upon arrival for each testing session, participants confirmed their adherence to pre-session criteria: abstaining from food for at least 4 hours, alcohol consumption for at least 24 hours, and use of medications that may affect responses to alcohol or pain testing for at least 12 hours. After confirming, participants consumed a meal replacement bar and completed drug, pregnancy (if applicable), and baseline breath alcohol concentration (BrAC) testing. One hour later, participants consumed the study beverage. Thirty minutes later, participants began the quantitative sensory testing (QST) procedures.
Beverage administration.
Participants completed two testing sessions: active alcohol (target BrAC: 0.08 g/dL) and placebo (target BrAC: 0.00 g/dL); the condition order was counterbalanced across participants. In the active alcohol condition, the participant’s alcohol dose was determined using a modified version of the Widmark formula (Watson et al., 1981), which calculates the amount of 95% ethanol required to achieve 0.08 g/dL BrAC, and mixed in a 1:3 ratio with sugar-free, lemon-lime soda. In the placebo condition, the beverage consisted of only soda. To enhance placebo efficacy, all serving glasses and trays were misted with alcohol and a small amount of alcohol was dropped on the rim and surface of the beverage. To maintain the double-blind, members of the research team who were not involved with QST procedures calculated, verified, prepared, and administered the alcohol and soda doses. Following beverage preparation, participants were given five minutes to consume the beverage. Participants received limited details about the conditions and beverages during the informed consent process (e.g., that they may or may not receive alcohol in either session) and were not provided suggestions regarding the potential pain-relieving effects of alcohol.
Breath Alcohol Concentration (BrAC).
Following beverage consumption, participants were asked to thoroughly swish their mouths with water to minimize inaccurate breath alcohol concentration (BrAC) sampling. Beginning immediately after beverage consumption, BrAC samples were collected at ten-minute intervals using a standard breathalyzer (CMI Inc., Owensboro, KY, USA). After QST concluded and BrAC was ≤ 0.02 g/dL, participants were transported home using a rideshare service (Brown et al. 2014).
Quantitative Sensory Testing (QST) Procedures.
QST was conducted in a private exam room and involved application of a Wagner Force One pressure algometer (Wagner Instruments, Greenwich, CT) to the insertion of the masseter muscle. To locate the masseter muscle, participants were instructed to clench their jaw and the area was palpated. To ensure a consistent stimulation site, the location of the masseter muscle was marked with ink. For chronic pain participants, the stimulation side (right or left) was the most affected side, as determined during the clinical examination. For healthy controls, the stimulation side was randomized. Stimulation side remained the same for all study sessions.
In each study session, participants completed a single bout of QST comprising four blocks of 3 stimuli each. In the first block, slowly-ramping stimuli were applied at a rate of .5 lbf/sec. Participants indicated the moment (i.e., pound-feet (lbf)) when the sensation transitioned from pressure to pain (threshold), at which point stimulation ceased. In the remaining three blocks, stimuli (4-, 5-, or 6-lbf, applied in pseudorandom order) were applied, resulting in 3 applications of each stimulus pressure. For these stimuli, pressure was increased over a 1s duration, maintained for 2s, and then removed. Research assistants conducting QST recorded the actual pressure achieved for each stimulus. Participant ratings from stimuli that were not within .5 lbf of the target pressure were excluded from analyses.
In all four blocks, immediately after stimulus removal, participants provided ratings of pain intensity and pain unpleasantness. For each rating, a 100mm visual analogue scale (VAS) anchored from “no pain at all/not at all unpleasant” to “most intense imaginable/most unpleasant imaginable” was used. Participants also rated their perceived relief as a result of beverage consumption from “no relief at all” to “most profound relief imaginable” on a 100 mm VAS. After completing the 3 ratings, a 60s break was taken before beginning the next stimulus. Pain intensity, unpleasantness, and relief ratings from threshold and each pressure level were averaged for analyses.
Power Analysis
Power analyses for this project were based on effect sizes derived from an initial feasibility study (Hill et al., 2018) and were performed assuming two-tailed hypothesis tests with α=.05. Analyses indicated N=50 would provide excellent (98%) power to detect analgesic effects of alcohol administration on QST measures (Cohen’s d > .58). Critically, this sample size also provided adequate (>80%) power to detect small-to-medium sized effects of interest (d > .36; r > .18; R2 > .03) for which prior estimates were not available (e.g., the interactive effects of chronic pain status with beverage condition on alcohol analgesia and perceived pain relief).
Analysis Strategy
Data were analyzed in R (R Core Team, 2021) and figures were produced using the ggplot2 package (Wickham, 2016). A mixed, factorial ANOVA was used to examine main and interactive effects of beverage condition (alcohol vs. placebo), pressure level (4-, 5-, or 6-lbf), and chronic jaw pain status (chronic pain vs. healthy control) on QST measures: pain threshold (lbf) and ratings (0–100mm VAS) of pain intensity, pain unpleasantness, and perceived pain relief. An additional exploratory mixed ANOVA including beverage condition, chronic jaw pain status, and QST outcome (i.e., pain threshold vs. pain intensity vs. pain unpleasantness vs. pain relief) was conducted to determine whether the effect size of beverage condition differed between changes in pain threshold/pain percept and perceived relief. T-tests were used to examine differences between participant groups in demographic characteristics, typical drinking patterns, and alcohol disorder symptomatology. Descriptive statistics are reported as (M [SD]) and effect sizes are reported as ηp2.
Results
Sample demographics
Forty-eight community-dwelling adults with (N=19; 89.5% female) and without (N=29; 65.5% female) chronic jaw pain participated in this study. A diagram of participant flow is demonstrated in Figure 1.
Figure 1:
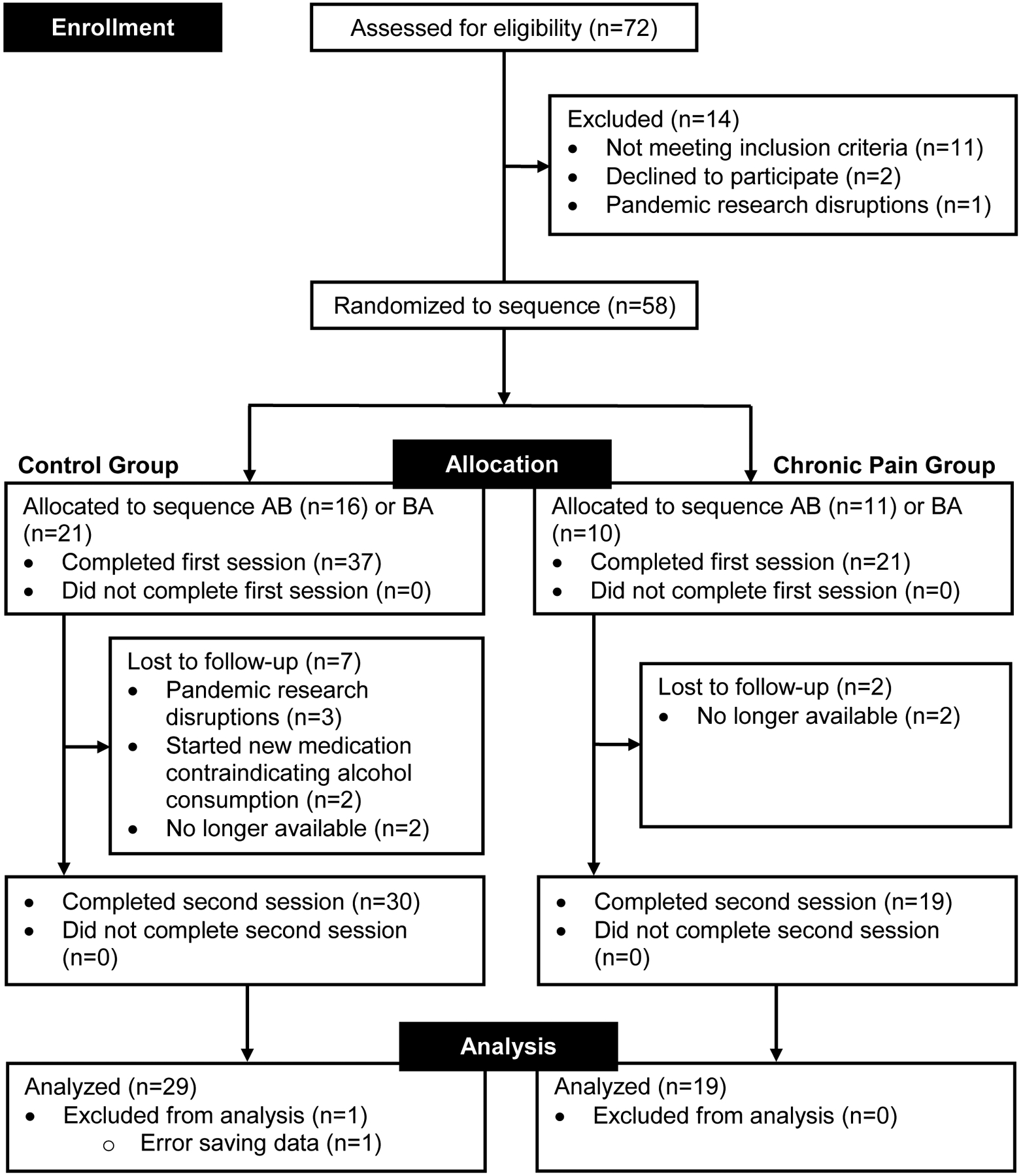
Flow diagram outlining participant enrollment, allocation to condition sequence, attrition between sessions, and analysis. Note: Condition A = Placebo. Condition B = Alcohol. Sequence order indicates order of conditions for study sessions.
Chronic pain participants averaged 26.74 years of age (SD=5.00) and 17.82 years of education (SD=2.19); pain-free control participants averaged 25.72 years of age (SD=4.12) and 16.20 years of education (SD=2.13). Similar to prior studies (Yule et al., 2015), OHIP-TMDs total scores averaged 31.79 (SD=14.74) for chronic pain participants and 10.14 (SD=11.26) for controls. A total of 63.2% of chronic pain participants identified as White, 10.5% as Asian, and 26.3% as another race or multiple races. A total of 58.6% of healthy control participants identified as White, 10.3% as Black or African American, 13.8% as Asian, 17.2% as another race or multiple races. In all, 21.1% of chronic pain participants and 31.0% of healthy control participants identified as Hispanic/Latino/a/x. Detailed self-reported demographic information is shown in Table 1.
TABLE 1.
Participant demographic and alcohol-related characteristics
| Chronic Pain (N=19) | Control (N=29) | |
|---|---|---|
| Variable | M (SD) or % | M (SD) or % |
| Age, in years | 26.74 (5.00) | 25.72 (4.12) |
| Education, in years | 17.82 (2.19) | 16.20 (2.13) |
| Sex | ||
| Male | 10.5% | 34.5% |
| Female | 89.5% | 65.5% |
| Race | ||
| White | 63.2% | 58.6% |
| Black or African American | 0.0% | 10.3% |
| Asian | 10.5% | 13.8% |
| Other or Multiple Races | 26.3% | 17.2% |
| Ethnicity | ||
| Hispanic/Latinx | 21.1% | 31.0% |
| Not Hispanic/Latinx | 78.9% | 69.0% |
| AUDIT, total | 4.37 (1.77) | 4.31 (1.67) |
| QFI, oz. abs. EtOH/day | 1.12 (2.68) | 0.43 (0.34) |
| Max Q, oz. abs. | 4.29 (2.01) | 3.74 (1.41) |
| OHIP-TMDs, total | 31.79 (14.74) | 10.14 (11.26) |
Note: QFI = quantity–frequency index; oz. = ounces; abs. = absolute; EtOH = ethanol; MAX Q = maximum quantity consumed in a single 24-hr period in the past 6 months; AUDIT = Alcohol Use Disorders Identification Test; OHIP-TMDs = Oral Health Impact Profile-Temporomandibular Disorders.
T- and X2-tests revealed non-significant differences between participant groups for all demographic variables, except for education and OHIP-TMDs total score: sex (t46=−1.91, p=.063), age (t46=−0.76, p=.448; d=.22), race (X2=2.56, p=.47), ethnicity (X2=.579, p=.45), education (t45=−2.53, p=.015; d=.75), and OHIP-TMDs (t31.45=−5.446, p<.0001; d=1.70).
Typical drinking habits and alcohol disorder symptomatology
Chronic pain participants reported an average daily consumption of 1.12 oz. (SD=2.68) of absolute ethanol (quantity-frequency index [QFI]; ~1.9 standard drinks); pain-free controls reported an average daily consumption of 0.43 oz. (SD=0.34) (~0.7 standard drinks). Chronic pain participants reported a maximum consumption of 4.29 oz. (SD=2.01) of absolute ethanol (maximum quantity [MaxQ]; ~7.2 standard drinks), compared to 3.74 oz. (SD=1.41) (~6.2 standard drinks) for healthy controls, within a single 24-hour period in the past 6 months. The average AUDIT score for chronic pain participants was 4.37 (SD=1.77); for controls, the average score was 4.31 (SD=1.67).
Two-sided independent sample t-tests revealed non-significant differences between participant groups for total QFI (t46=−1.39, p=.172; d=.36), MaxQ (t46=−1.13, p=.266; d=.32), and AUDIT score (t46=−0.12, p=.909; d=.04).
Breath alcohol concentration (BrAC) measures
No significant differences in BrAC immediately prior to QST were detected between control (M=.069, SD=.015) and chronic pain participants (M=.064, SD=.018, p=.356, d=0.27).
Quantitative sensory testing (QST)
Ratings for pain intensity, pain unpleasantness, and pain relief, as well as pressure levels for pain threshold, are shown in Table 2. The main and interactive effects of beverage condition, chronic pain status, and pressure level on QST measures are reported below and illustrated in Figure 2a–d.
TABLE 2.
Quantitative sensory testing (QST) measures.
| Chronic Pain | Control | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | Placebo | Alcohol | Placebo | ||||||||||||
| Variable | M (SD) | M (SD) | M (SD) | M (SD) | |||||||||||
| Pain threshold | 4.56 (1.08) | 3.81 (0.84) | 5.27 (1.37) | 4.51 (1.21) | |||||||||||
| 4lbf | 5lbf | 6lbf | 4lbf | 5lbf | 6lbf | 4lbf | 5lbf | 6lbf | 4lbf | 5lbf | 6lbf | ||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||||
| Pain intensity | 23.96 (20.95) | 35.43 (19.67) | 37.19 (23.90) | 32.39 (19.95) | 41.81 (20.28) | 46.10 (22.97) | 12.76 (13.44) | 20.92 (17.02) | 26.95 (23.06) | 22.05 (17.72) | 29.98 (23.89) | 34.03 (22.94) | |||
| Pain unpleasantness | 21.61 (21.02) | 33.13 (21.07) | 36.08 (24.13) | 30.55 (21.59) | 40.83 (22.53) | 46.23 (24.96) | 13.33 (15.46) | 21.00 (19.57) | 24.76 (21.33) | 22.58 (19.01) | 31.55 (25.15) | 35.30 (25.10) | |||
| Pain relief | 40.58 (31.31) | 41.00 (28.27) | 39.72 (30.05) | 7.91 (13.71) | 7.20 (13.01) | 7.20 (13.41) | 39.79 (30.58) | 42.75 (30.14) | 43.65 (29.61) | 5.51 (11.36) | 5.57 (11.13) | 5.89 (13.11) | |||
Pain threshold was measured in lbf. Pain intensity, pain unpleasantness, and pain relief were measured on a 0–100 visual analogue scale (VAS). lbf=pound-feet
Figure 2:
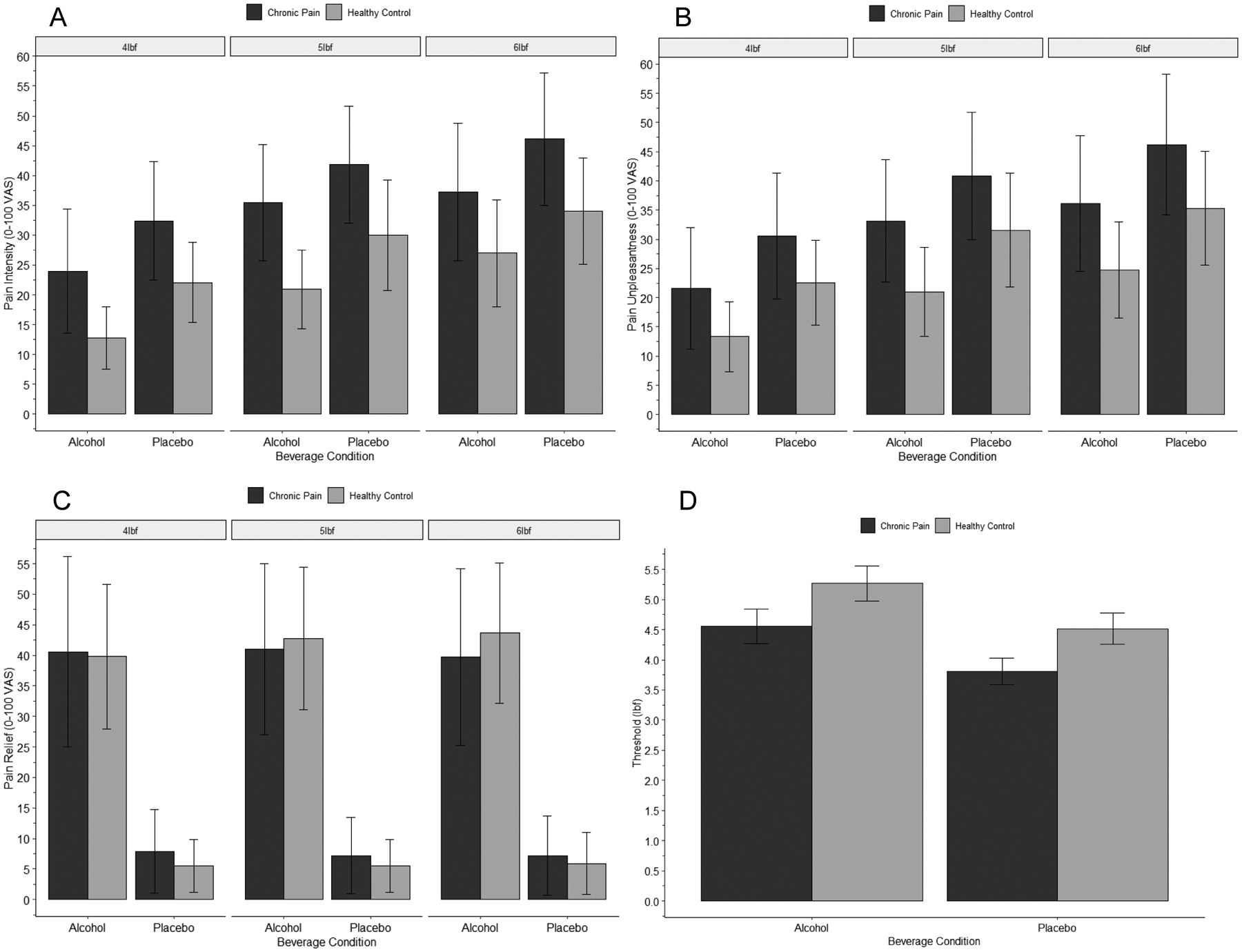
Effects of beverage condition, chronic pain status, and pressure level on pain intensity (A), pain unpleasantness (B), pain relief (C), and pain threshold (D). Note: Vertical bars represent standard deviation. Detailed statistics on reported effects are provided in the text. (A) Pain intensity was significantly greater in the placebo condition, compared to the alcohol condition; among chronic pain participants, compared to pain-free control participants; and for 6 lbf stimuli, compared to 5 lbf and 4 lbf stimuli. No interactive effects were apparent. (B) Pain unpleasantness was significantly greater in the placebo condition, compared to the alcohol condition; among chronic pain participants, compared to control participants; and for 6 lbf stimuli, compared to 5 lbf and 4 lbf stimuli. No interactive effects were observed. (C) Pain relief was significantly greater in the alcohol condition, compared to the placebo condition. No main effects of chronic pain status or pressure level, or any interactive effects, on pain relief were observed. (D) Pain threshold was significantly greater in the alcohol condition, compared to the placebo condition; and among control participants, compared to chronic pain participants. No interactive effects were apparent.
Pain threshold.
A significant main effect of beverage condition on pain threshold was observed, F(1, 46)=12.55, p<.0001, ηp2=.21, such that greater pain threshold was reported in the alcohol (M=4.99, SD=1.31) than placebo (M=4.24, SD=1.13) condition. Chronic pain status had a significant main effect on pain threshold, F(1, 46)=6.32, p=.015, ηp2=.12, with chronic pain participants reporting a lower pain threshold (M=4.18, SD=1.03) compared to control participants (M=4.89, SD=1.35). A Condition × Chronic Pain Status interaction for pain intensity was not apparent, F(1, 46)=.001, p=.99, ηp2=.000.
Pain intensity.
Beverage condition had a significant main effect on ratings of pain intensity, F(1, 41)=4.53, p=.04, ηp2=.10, with participants reporting greater pain intensity in the placebo (M=33.27, SD=22.45) than alcohol condition (M=27.08, SD=21.02). A significant main effect of chronic pain status was revealed, F(1, 41)=6.24, p=.017, ηp2=.13, such that chronic pain participants reported greater pain intensity (M=36.88, SD=18.57) than pain-free control participants (M=23.47, SD=17.66). A significant main effect of pressure level on pain intensity was observed, F(2, 82)=48.41, p<.0001, ηp2=.54, such that participants reported greater pain intensity for stimuli of 6 lbf (M=35.88, SD=23.79) compared to 5 lbf (M=31.66, SD=21.53) and 4 lbf (M=23.00, SD=18.73). No Condition × Chronic Pain Status interaction for pain intensity was revealed, F(1, 41)=.85, p=.36, ηp2=.02. Neither a Pressure Level × Chronic Pain Status, F(2, 82)=.20, p=.76, ηp2=.007, nor Pressure Level × Condition, F(2, 82)=.66, p=.52, ηp2=.02, interaction was observed for pain intensity. No significant Beverage Condition × Pressure-Level × Chronic Pain Status interaction for pain intensity was revealed, F(2, 82)=.54, p=.59, ηp2=.01.
Pain unpleasantness.
Alcohol had a significant main effect on ratings of pain unpleasantness, F(1, 41)=5.38, p=.025, ηp2=.12, with participants reporting greater unpleasantness in the placebo (M=33.33, SD=23.96) than alcohol condition (M=25.96, SD=21.27). Chronic pain status had a significant main effect on ratings of pain unpleasantness, F(1, 41)=4.11, p=.049, ηp2=.09, with chronic pain participants reporting greater pain unpleasantness (M=35.47, SD=4.55) compared to control participants (M=23.83, SD=3.50). A significant main effect of pressure level on pain unpleasantness was observed, F(2, 82)=49.00, p<.0001, ηp2=.54, such that participants reported greater pain unpleasantness for stimuli of 6 lbf (M=35.45, SD=24.61) compared to 5 lbf (M=31.17, SD=23.04) and 4 lbf (M=22.33, SD=19.61). No Condition × Chronic Pain Status interaction for pain unpleasantness was revealed, F(1, 41)=.49, p=.49, ηp2=.012. Neither a Pressure Level × Chronic Pain Status, F(2, 82)=.32, p=.73, ηp2=.008, nor Pressure Level × Condition, F(2, 82)=.21, p=.81, ηp2=.005, interaction for pain unpleasantness was observed. No significant Beverage Condition × Pressure-Level × Chronic Pain Status interaction for pain unpleasantness was revealed, F(2, 82)=1.29, p=.28, ηp2=.03.
Perceived relief.
Beverage condition had a significant main effect on ratings of perceived relief, F(1, 41)=55.02, p<.0001, ηp2=.57, with participants reporting greater relief in the alcohol (M=43.16, SD=29.52) than placebo condition (M=6.88, SD=12.30). A significant main effect of chronic pain status on perceived relief was not observed, F(1, 41)=.14, p=.71, ηp2=.003. Pressure level did not significantly affect perceived relief ratings, F(2, 82)=.34, p=.71, ηp2=.008. No Condition × Chronic Pain Status interaction for perceived relief was revealed, F(1, 41)=.001, p=.98, ηp2=.000. Neither a Pressure Level × Chronic Pain Status, F(2, 82)=3.06, p=.053, ηp2=.07, nor Pressure Level × Condition, F(2, 82)=1.58, p=.21, ηp2=.04, interaction for perceived relief was observed. Analyses did not find a significant Beverage Condition X Pressure-Level X Chronic Pain Status interaction for perceived relief, F(2, 82)=.69, p=.50, ηp2=.017.
Effect of QST measure.
A significant QST measure X beverage condition interaction was detected, F(1,45),=51.81, p<.0001, ηp2=.54, indicating that the effect of beverage condition on relief ratings (ηp2=.57) was significantly greater than on pain threshold (ηp2=.21), pain intensity (ηp2=.10) or unpleasantness (ηp2=.12). The three-way interaction of QST measure X Condition X Chronic Pain Status was non-significant, F(1,45)=0.30, p=.86, ηp2=.001.
Discussion
Overview
Consistent with previous findings (Thompson et al., 2017; Williams et al., 2021), pain threshold was significantly greater and pain intensity and unpleasantness were significantly lower in the alcohol condition compared to the placebo condition, supporting our hypothesis that alcohol intake would have acute analgesic effects. Additionally, as expected, participants reported significantly greater pain relief from their beverage under alcohol than placebo, suggesting alcohol’s pain-relieving effects may be an important component of the overall negative reinforcing effects of alcohol. Interestingly, the effect of alcohol on subjective pain relief was substantially greater than on pain intensity, pain unpleasantness, or pain threshold. This suggests that the negative reinforcing effects of alcohol may not be fully captured by simply measuring changes in pain intensity or unpleasantness, or pain threshold.
Taken together, our findings are in line with recent literature supporting the analgesic properties of alcohol in producing clinically-relevant reductions in ratings of pain intensity and increases in pain threshold (Thompson et al., 2017; Williams et al., 2021; Capito et al., 2020). Of note, there is limited prior research on the effects of acute alcohol intake on pain unpleasantness, with a single study demonstrating a statistically-significant increase in pain unpleasantness under a lower alcohol dose (0.06%) and no significant changes under a moderate alcohol dose (0.08%) in response to heat pain stimuli (Capito et al., 2020). Given our use of pressure-based pain stimuli in the present study, additional research is needed to determine whether mixed findings regarding pain unpleasantness depend on stimulus modality or other methodological differences (e.g., administration of both low and moderate alcohol doses).
As hypothesized, our analysis demonstrated a significant effect of chronic pain status on pain threshold, pain intensity, and pain unpleasantness, such that chronic pain participants, compared to pain-free controls, reported significantly greater pain sensitivity. Contrary to expectations, however, our analysis did not reveal any significant two-way or three-way interactive effects of beverage condition, chronic pain status, and/or pressure level on pain sensitivity, as measured via pain threshold, pain intensity, pain unpleasantness, and perceived pain relief. Indeed, effect sizes for the non-significant chronic pain status X beverage condition interactions were quite small and would require very large sample sizes to provide adequate power for reliable detection, suggesting low clinical relevance.
As previously noted, evidence suggests substantial overlap in the neural underpinnings of the acute neurobehavioral effects of alcohol and chronic pain. Chronic pain is associated with perturbation in structure and function within prefrontal and limbic regions of the brain (for review, see Apkarian et al., 2013), which are also affected by both acute and chronic alcohol intake (e.g., Marinkovic et al. 2012). Connectivity between mesocorticolimbic structures represent another area of overlap. Baliki and colleagues (2012) identified increased functional connectivity between medial prefrontal cortex and the nucleus accumbens in individuals with persistent back pain one year after acute or subacute presentation, compared to those who recovered. In healthy adults, a pain modulation training protocol that reduced experimental pain sensitivity also reduced resting connectivity between these structures (Sevel et al., 2020). At the same time, alterations in connectivity have been detected between these areas at rest following acute alcohol administration (Boissoneault et al., 2020; but see Han et al., 2021) and in abstinent heavy drinkers during a monetary reward task (Forbes et al., 2014).
These findings, among others reviewed in the Introduction, helped to form the basis for our expectation that the analgesic effects of alcohol would differ between pain-free controls and individuals with chronic jaw pain, even in the absence of strong evidence whether effects would be exaggerated or attenuated. The lack of any significant interaction effects would seem to suggest that although individuals with chronic orofacial pain may use alcohol to help self-manage their pain (e.g., Riley & King, 2009), and are at increased risk for hazardous drinking and alcohol-related consequences, they may not be differentially responsive to alcohol’s analgesic or negative reinforcing effects. Furthermore, the shared neural substrates underlying alcohol effects and chronic pain may have minimal implications for modulating the pain relieving effects of alcohol, per se. Our null results appear to challenge the notion that this vulnerability is due to a differential level of response to alcohol in the context of pain among individuals with chronic jaw pain (but see Limitations below). Rather, these individuals may simply experience pain more frequently and intensely than people without chronic pain, resulting in more opportunities for pain to act as a proximal antecedent for alcohol use (Ferguson et al., 2021).
Limitations and future directions
Although our data suggest that chronic pain status and intensity of noxious stimuli do not significantly affect the analgesic properties of alcohol in a population of social drinkers, additional studies are needed to characterize potential biopsychosocial moderators of alcohol analgesia and determine whether alcohol has differential effects in populations with alcohol use disorders, multiple or other chronic pain conditions, and/or poorer self-reported mental or physical health. In addition, although participants with chronic jaw pain were required to report pain on the majority of days over the past six months, we did not collect information on the overall duration of their pain. Given evidence from the preclinical literature that the analgesic effects of alcohol may change as duration of chronic pain increases (McGinn et al., 2020), it is also important that future work determine whether pain duration and age (especially given the relatively young age of our sample) might be critical determinants of the relevance of chronic pain as a moderator of alcohol analgesia.
Several additional limitations should be noted. First, the study sample was relatively homogenous, particularly in the chronic pain group. Although TMD is more prevalent in women than men (~2:1; Campbell et al., 2017), the chronic pain sample was nearly 90% women. Thus, additional studies are needed to examine potential sex differences in the analgesic effects of alcohol among individuals with chronic pain. In addition, a single oral alcohol dose consistent with a binge-like episode was used in this study. It is possible that chronic pain status may be a more relevant determinant of alcohol analgesia at lower BrACs than the 0.08 g/dL target used in this study. Thus, future work would benefit from inclusion of multiple doses, including those associated with more moderate consumption (e.g., 0.04 g/dL target BrAC). Finally, due to recruitment challenges related to the COVID-19 pandemic, we were not able to meet our original enrollment goal of 50 participants, resulting in a total N=48 and n=19 with chronic jaw pain. Although the slightly smaller and uneven sample reduces power to some degree compared with initial power analyses (see Methods), we note that we retained sufficient power to detect main effects of beverage condition and chronic pain status, and non-significant interactive effects were quite small (ηp2<.03).
Conclusion and implications
These findings may have important clinical implications. Significant reductions in pain sensitivity under the alcohol condition indicate individuals, regardless of chronic pain status, who self-medicate pain with alcohol may be vulnerable to engaging in hazardous drinking patterns (i.e., consuming relatively large quantities of alcohol over a limited period of time) to reduce pain sensitivity and maximize pain relief. In addition, results suggest that alcohol-related changes in pain sensitivity do not completely capture its negative reinforcing effects. Further study is needed to characterize individual factors underlying perception of pain relief subsequent to acute alcohol intake.
Acknowledgements
Support for this work was provided by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R21AA026805 (JB, PI). The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors thank Casey Alexander, BS for technical assistance and extend their sincerest gratitude to the individuals who participated in this study.
Footnotes
Disclosures of Interest
The authors report no relevant disclosures.
References
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, & Pearlson GD (2011). Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res, 35, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, & Regunathan S (2013). Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, & Apkarian AV (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nature Neuroscience, 15(8), 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J, Lewis B, & Nixon SJ (2019). Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol, 75, 47–54. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH (2005) Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction, 100(6), 777–786. [DOI] [PubMed] [Google Scholar]
- Brown FF, Robinson ME, Riley JL, Gremillion HA, McSolay J, Myers G (2000) Better Palpation of Pain: Reliability and Validity of a New Pressure Pain Protocol in TMD. Cranio, 18(1), 58–65. [DOI] [PubMed] [Google Scholar]
- Brown SA, de Wit H, O’Connor S, O’Malley SS, Ota-Wang V, Palmer LI, Chezem L, Peterson KP, Warren KR, Sher KJ, Swann AC, & Taylor RE (2014) Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation [Web site]. Available at: https://www.niaaa.nih.gov/research/guidelines-and-resources/administering-alcohol-human-studies [Accessed March 15, 2022].
- Cahalan D, Cissin L, & Crossley H (1969) American Drinking Practices: A National Study of Drinking Behaviors and Attitudes (Monograph No. 6). New Brunswick, NJ: Rutgers Center of Alcohol Studies. [Google Scholar]
- Campbell BK, Fillingim RB, Lee S, Brao R, Price DD, & Neubert JK (2017) Effects of High-Dose Capsaicin on TMD Subjects: A Randomized Clinical Study. JDR Clin Trans Res, 2(1), 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capito ES, Lautenbacher S, Wolstein J, Horn-Hofmann C (2020) Effects of oral alcohol administration on heat pain threshold and ratings of supra-threshold stimuli. Scand J Pain, 20(3), 623–634. [DOI] [PubMed] [Google Scholar]
- Chopra K, & Tiwari V (2012) Alcoholic neuropathy: possible mechanisms and future treatment possibilities. British journal of clinical pharmacology, 73(3), 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1992) A power primer. Psychological Bulletin, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Plawecki MH, Corbin W, King A, McCarthy DM, Ramchandani VA, Weafer J, O’Connor SJ (2020) To Infuse or Ingest in Human Laboratory Alcohol Research. Alcohol Clin Exp Res, 44(4), 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, LaRowe LR (2019) A Reciprocal Model of Pain and Substance Use: Transdiagnostic Considerations, Clinical Implications, and Future Directions. Annual Review of Clinical Psychology, 15(1), 503–528. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neuroscience & Behavioral Reviews, 26(10), 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, & Narendran R (2014). Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PloS one, 9(5), e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ & Richard P (2012) The economic costs of pain in the United States. J Pain, 13(8), 715–724. [DOI] [PubMed] [Google Scholar]
- Han J, Keedy S, Murray CH, Foxley S, & de Wit H (2021). Acute effects of alcohol on resting-state functional connectivity in healthy young men. Addictive behaviors, 115, 106786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, & Apkarian AV (2013). Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain, 136, 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Wesolowicz D, Robinson M, & Boissoneault J (2018). Sex Differences in the Acute Analgesic Effects of a Sub-Intoxicating Dose of Alcohol in Healthy Social Drinkers. Presented at the 41st Annual Meeting of the Research Society on Alcoholism (RSA), San Diego, CA (June 16–20, 2018). Alcoholism: Clinical and Experimental Research 41(s1): 188a. [Google Scholar]
- Horn-Hofmann C, Capito ES, Wolstein J, & Lautenbacher S (2019) Acute alcohol effects on conditioned pain modulation, but not temporal summation of pain. Pain, 160(9), 2063–2071. [DOI] [PubMed] [Google Scholar]
- LaRowe LR, Maisto SA, & Ditre JW (2021) A measure of expectancies for alcohol analgesia: Preliminary factor analysis, reliability, and validity. Addictive Behaviors, 116, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Brooks JCW, Wiech K, & Tracey I (2008) Pain relief as an opponent process: a psychophysical investigation. Eur J Neurosci, 28(4), 794–801. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, & Artsy E (2012). Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp, 33, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MA, Edwards KN, & Edwards S (2020). Chronic inflammatory pain alters alcohol-regulated frontocortical signaling and associations between alcohol drinking and thermal sensitivity. Neurobiology of Pain, 8, 100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004) Actual causes of death in the United States, 2000. JAMA, 291(10), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Moskal D, Maisto SA, De Vita M, & Ditre JW (2018). Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Experimental and clinical psychopharmacology, 26(1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). (2021) Alcohol Facts and Statistics. Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics [Accessed March 15, 2022].
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). (2020) Alcohol Facts and Statistics. Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/using-alcohol-to-relieve-your-pain [Accessed March 15, 2022].
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). (2021) Alcohol Facts and Statistics. Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics [Accessed March 15, 2022].
- R Core Team. (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Riley III JL, & King C (2009). Self-report of alcohol use for pain in a multi-ethnic community sample. The Journal of Pain, 10(9), 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, & Brewer RD (2015) National and State Costs of Excessive Alcohol Consumption. Am J Prev Med, 49(5), 73–79. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, … John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF; International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain (2014) Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. Journal of Oral & Facial Pain and Headache, 28(1), 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevel L, Boissoneault J, Alappattu M, Bishop M, & Robinson M (2020). Training endogenous pain modulation: a preliminary investigation of neural adaptation following repeated exposure to clinically-relevant pain. Brain imaging and behavior, 14(3), 881–896. [DOI] [PubMed] [Google Scholar]
- Slade GD (1997) Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol, 25(4), 284–290. [DOI] [PubMed] [Google Scholar]
- Soderlund H, Parker ES, Schwartz BL, & Tulving E (2005). Memory encoding and retrieval on the ascending and descending limbs of the blood alcohol concentration curve. Psychopharmacology (Berl), 182, 305–317. [DOI] [PubMed] [Google Scholar]
- Stennett B, Anderson MB, Vitus D, Ferguson E, Dallery J, Alappattu M, … & Boissoneault J (2021). Sex moderates the effects of experimentally induced musculoskeletal pain on alcohol demand in healthy drinkers. Drug and Alcohol Dependence, 219, 108475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Oram C, Correll CU, Tsermentseli S, & Stubbs B (2017) Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. Journal of Pain, 18(5), 499–510. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley-Browne MA, Posada-Villa J, Seedat S, Watanabe M (2008) Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain, 9(10), 883–891. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, & Kai Li T (2006). Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage, 29, 295–301. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, & Batt RD (1981) Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. J Stud Alcohol, 42(7), 547–556. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Williams MK, Vitus D, Ferguson E, Stennett B, Robinson M, & Boissoneault J (2021) Acute Tolerance to the Analgesic Effects of Alcohol. JSAD, 82(3), 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule PL, Durham J, Playford H, Moufti MA, Steele J, Steen N, Wassell RW & Ohrbach R. (2015). OHIP‐TMD s: a patient‐reported outcome measure for temporomandibular disorders. Community dentistry and oral epidemiology, 43(5), 461–470. [DOI] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, & Ditre JW (2015) Interrelations between pain and alcohol: An integrative review. Clinical Psychology Review, 37, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z & Yuan KH (2018) Practical Statistical Power Analysis Using Webpower and R. Granger, IN: ISDSA Press. [Google Scholar]


