Abstract
Background:
Irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) are chronic gastrointestinal (GI) disorders. GI symptom-specific anxiety (GSA) is the cognitive, affective, and behavioral response stemming from fear of GI symptoms. The Visceral Sensitivity Index (VSI) measures GSA and is validated in IBS and may be useful in IBD.
Methods:
We compared VSI scores in IBD participants to IBS participants and healthy controls (HCs). Using validated questionnaires, we assessed the VSI’s correlation with anxiety, health related quality of life (HRQOL), and IBD activity.
Key Results:
We recruited 222 age and sex-matched participants (74 IBD [23 Crohn’s disease; 51 ulcerative colitis], 74 IBS, and 74 HCs). IBD and IBS participants had higher VSI scores compared to HCs (IBD= 26.62±16.64, IBS= 38.83±15.06; HCs= 3.42±5.06; all p’s<0.001). VSI scores were lower in IBD vs IBS (p<0.001). In IBD, VSI modestly correlated with current anxiety (R=0.35, P=0.002) and the physical component of HRQOL (R=−0.45, P=0.0001) but less with the mental component of HRQOL (R=−0.23, P=0.05).
Conclusions & Inferences:
Our findings suggest the VSI is a useful measure in IBD. The VSI in IBD is related to general anxiety but is measuring a different construct and is not affected by the presence of trait anxiety. IBD patients have GSA that is associated with decreased HRQOL, which can negatively affect treatment compliance and other long-term disease outcomes. Future studies are needed to further validate the VSI in IBD and to assess its correlation with disease activity.
Keywords: Anxiety, Inflammatory Bowel Diseases, Irritable Bowel Syndrome, Quality of Life, Severity of Illness Index
Graphical Abstract
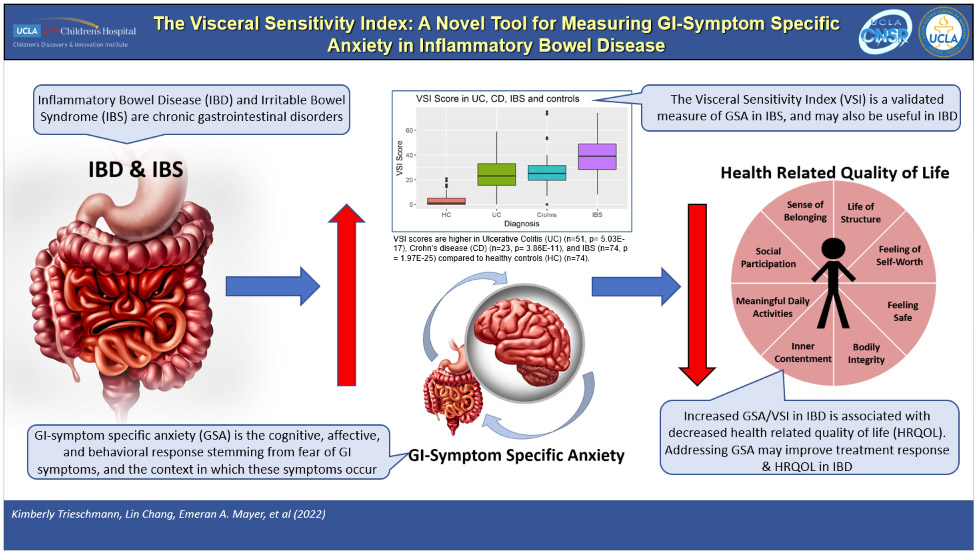
Inflammatory Bowel Disease (IBD) and Irritable Bowel Syndrome (IBS) are gastrointestinal (GI) disorders with increased GI-symptom specific anxiety (GSA) and decreased health related quality of life (HRQOL). The Visceral Sensitivity Index (VSI) measures GSA in IBS and may be useful in IBD to identify GSA and improve HRQOL.
INTRODUCTION
Irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), comprised of both Crohn’s disease (CD) and ulcerative colitis (UC), are gastrointestinal (GI) disorders associated with chronic or recurrent abdominal pain, alterations in bowel habits, and psychological distress1. While IBD results in objective mucosal inflammation, biochemical or structural abnormalities have not been identified for IBS.”
The clinical course of IBD is highly variable across individuals with disease activity that may fluctuate between remission and flares. The literature suggests that both GI symptom severity and psychological factors are related to health-related quality of life (HRQOL) in IBS and IBD2-4. This helps to explain why even during periods of minimally active inflammation, IBD patients continue to have impaired HRQOL5. Numerous studies have shown that a significant number of IBS and IBD patients have comorbid psychiatric disorders, such as affective disorders, somatization disorders, and anxiety, and these disorders may contribute to overall disease severity and impaired HRQOL5-8.
The importance of psychological symptoms in both IBD and IBS was supported by our previous study which measured the interrelationships between GI symptoms, psychological symptoms and HRQOL in adult IBD and IBS patients9. In both IBD and IBS, psychological distress was found to have a stronger direct effect on HRQOL than GI symptoms. However, the association of GI symptoms and psychological distress was significantly stronger in IBD than IBS, suggesting that psychological distress is more dependent on GI symptoms in IBD than IBS even though the degree that psychological distress impacts HRQOL is similar.
Recent studies in IBS demonstrate that GI symptom-specific anxiety (GSA) may be a uniquely important variable in determining symptom persistence10. GSA is defined as the cognitive, affective, and behavioral response stemming from fear of GI sensations, symptoms, and the context in which these visceral sensations and symptoms occur11. While anxiety, depression and other psychological domains were measured in our previous study of IBS and IBD patients,9 GSA was not. There remains a need to better understand how GSA may impact patients with IBD in order to guide multidisciplinary management of symptoms and HRQOL.
The Visceral Sensitivity Index (VSI) is a self-reported scale developed as a measure of GSA and is validated in adult IBS patients11, 12. VSI scores correlate with IBS symptom severity and HRQOL more than current anxiety symptoms11. We hypothesized that the VSI may also be a useful measure of GSA in IBD.
The aims of this study were: 1) to determine if VSI scores are significantly different between IBD patients, IBS patients and healthy controls (HCs), 2) to assess the construct internal consistency, reliability and validity of the VSI in adult patients with IBD, 3) to assess the convergent validity between VSI scores in IBD with general anxiety symptoms, trait anxiety, and HRQOL, and 4) to determine divergent validity between VSI scores and disease activity in adults with IBD.
METHODS AND MATERIALS:
Study subjects:
Participants who were at least 18 years of age were obtained from a list of IBD (both CD and UC) and IBS participants and HCs who previously participated in physiologic research studies conducted by our Center from 6/2008 to 2/2018 and had completed the questionnaires detailed below. IBS and HC participants were age and sex matched to the IBD participants. The IBS and HC participants were recruited predominantly from community advertisements. Clinical characteristics including age, sex, and body mass index (BMI) were collected.
IBD patients:
All IBD participants were diagnosed by expert clinicians based on clinical presentation and pathologic confirmation. Participants with all levels of disease activity, from remission to severely active disease, were included. However, one of the main studies contributing IBD participants only included those in clinical remission. Exclusion criteria included known comorbid IBS, presence of other chronic GI disease (i.e. celiac disease, malignancy, cirrhosis), and comorbid psychiatric disease (i.e. major depression, anxiety disorder, schizophrenia, etc.).
IBS patients:
All IBS participants fulfilled the Rome III or IV diagnostic criteria13, 14 and the diagnosis of IBS was confirmed by a clinician with expertise in this area.
Healthy controls:
HCs did not have a history of IBS, other gastrointestinal (GI) disorders, or chronic pain disorder.
Exclusion criteria for both IBS and HC groups included presence of organic GI disease (i.e., celiac disease, IBD, malignancy, cirrhosis) and psychiatric disease (i.e. major depression, anxiety disorder, schizophrenia, etc.).
Questionnaires:
We administered the VSI11, 12 to all participants. Using validated questionnaires, we also measured current bowel symptoms (Bowel Symptom Questionnaire [BSQ])12, 15, 16, current anxiety symptoms (Hospital Anxiety and Depression Scale [HADs])17, trait anxiety (State Trait Anxiety Inventory [STAI])18, and HRQOL (Medical Outcomes Study [MOS] 12-item Short Form [SF-12])19. In IBD patients, IBD disease activity (Powell Tuck Index for UC [PTIUC]20, 21 and the CD Activity Index [CDAI]22) was measured.
Visceral Sensitivity Index (VSI) (Supplementary Figure 1)
The 15-item, Likert-scale, self-reported questionnaire is designed to measure those unique aspects of fear, anxiety, and hypervigilance that can accompany misappraisals of visceral sensations and discomfort. This scale has demonstrated reliability and validity in IBS patients11, 12. Items on the VSI are reverse scored (i.e. 1-6 becomes 5-0) and totaled to yield a range of possible scores from 0 (no GSA) to 75 (severe GSA).
Bowel Symptom Questionnaire (BSQ)
The BSQ is a self-report measure of GI symptoms. It includes multiple questions including the Rome diagnostic questions for IBS and bowel habit subtypes, overall GI symptom severity, and individual GI symptoms. IBD and IBS participants rated the current overall intensity of their GI symptoms over the past week on a 20-point ordinal scale from “none” to “the most intense imaginable”. Patients who reported having abdominal pain or discomfort also rated their usual symptom severity over time and was self-classified from a multiple-choice question as mild (can be ignored if I don’t think about it), moderate (cannot be ignored, but does not affect lifestyle), severe (affects lifestyle, or very severe (markedly affects lifestyle)12, 15, 16.
Hospital Anxiety and Depression Scale (HADs)
The HADs is a self-assessment mood scale specifically designed for use in non-psychiatric settings17. It has been extensively validated and is one of the most widely used brief inventories for assessment of symptoms of anxiety and depression. The HADs provides two 7-item subscales: anxiety and depression, with scores ranging from 0-21. To provide context for the scores for both subscales, scores range from 0-7 to represent ‘non-cases’, 8-10 ‘doubtful cases’, and 11-21 ‘cases’.
State-Trait Anxiety Inventory (STAI)
The STAI is a self-reported measure of the presence and severity of current symptoms of anxiety and a generalized propensity to be anxious. There are 2 subscales within this measure, each of which has 20 questions. First, the State Anxiety Scale (STAI-S) evaluates the current state of anxiety, asking how respondents feel “right now”. The second, Trait Anxiety Scale (STAI-T) evaluates relatively stable aspects of “anxiety proneness”, including general states of calmness, confidence, and security18, 23. For our study, we used the STAI-T subscale. STAI scores range from 0 to a maximum score of 80 on both the STAI-T and STAI-S subscales. STAI scores are commonly classified as “no or low anxiety” (20-37), “moderate anxiety” (38-44), and “high anxiety” (45-80).
Medical Outcomes Study 12-Item Short Form Health Survey (SF-12)
The SF-12 was developed for the Medical Outcomes Study (MOS), a multi-year study of patients with chronic conditions. It is a self-reported measure of HRQOL. It is divided into Physical and Mental Health Composite Scores (PCS & MCS) which are computed using the scores of twelve questions ranging from 0-100, where zero indicates the lowest level of health and 100 indicates the highest level of health19.
Crohn’s Disease Activity Index (CDAI)
The CDAI is a tool to assess disease activity and response to treatment in patients with CD. It consists of eight variables, two of which are subjective, and each weighted according to its ability to be predictive of disease activity. The total score ranges from 0 to over 600. No upper numerical limit is defined because one variable is based on hematocrit and another on body weight. Nevertheless, there are cutoff values: CDAI scores <150 represent clinical remission, 150-220 mild disease, 220-450 moderate disease, and >450 severe disease22.
Powell-Tuck Index for Ulcerative Colitis (PTIUC)
The PTIUC is a measure of disease activity and response to treatment in patients with UC. It includes 10 clinical variables that encompass general health and gastrointestinal symptoms. Scores range from 0-20: scores 0-1 represent clinical remission, 2-5 mild disease, 6-8 moderate disease, >9 severe disease20, 21.
Statistical Analysis:
Power Analysis. G*Power 3.124 was used to perform a formal power analysis based on our sample size, 74 per group and the primary analyses for testing group differences on the VSI scores. Specifically, based on a two-tailed Wilcoxon-Mann-Whitney U, and an alpha=0.0167 (controlling for 3 contrasts of interest, 0.05/3), 74 participants per group provided adequate power (80%) to detect an effect size difference as small as Cohen’s d=0.55, tcrit(139)=2.42, noncentrality parameter=3.27, if it existed. Additionally, 74 IBD participants provided the power to detect a correlation effect size, as small as ∣r∣=0.37 for convergent validity analyses tcrit(72)=2.56, noncentrality parameter=3.43.
Statistical analyses. Normality of distribution of all the variables was tested using Kolmogorov-Smirnov (K-S) test25 and non-parametric correlation tests were used when the two sample K-S test p value were <0.05. Based on this testing the VSI scores were found to be positively skewed violating assumptions for normality. To detect the group differences in the clinical characteristics, we applied general linear model and report the results for main effects of groups and post-hoc pairwise group comparisons (IBD vs HC, IBD vs IBS and IBS vs HC). Given that log transformation did not improve the normality of the distribution, we used the Wilcoxon-Mann-Whitney test to compare the pairwise group differences for VSI. To control for multiple comparisons, significance for the Wilcoxon-Mann-Whitney test was considered at p<.05/3=.0167. Cronbach’s α was calculated and factor analysis was applied to assess the internal consistency, reliability, and construct validity of the VSI. In IBD patients, Spearman correlations were used to determine the convergent validity of the VSI with general anxiety, trait anxiety, and HRQOL and divergent validity with IBD disease activity. Correlations were considered significant at p <.05/4=.0125. For interpretation, Cohen26 provided the following rules of thumb for interpreting effect sizes (r=.10, small, r=.30, medium, and r=.50 large). Internal consistency and construct validity were assessed using ‘psych’27 and ‘corrr’28 packages in R software (https://CRAN.R-project.org).
Ethical consideration:
This study was approved by the University of California Los Angeles Institutional Review Board. All study subjects signed a written informed consent prior to inclusion in these studies. Participants were compensated for completion of a history, physical examination and a variety of questionnaires.
RESULTS:
Clinical characteristics
Characteristics of the IBD, IBS, and HC groups can be seen in Table 1. Our study population was comprised of 74 IBD participants (51 UC, 23 CD; mean age 29 yrs, 40% female) and two age- and sex-matched comparison groups, 74 IBS participants, (mean age 29.3 yrs, 40% female), and 74 HCs. There were significant differences in the race of participants in each of the three groups, with the IBD group being predominantly Caucasian. There were no significant group differences in education level. HCs had significantly higher BMI compared to both IBD and IBS participants (P<0.001), however there was no significant difference in BMI between IBD and IBS participants (P=0.519). Both usual and current GI-symptom severity were significantly higher in the IBS group compared to the IBD group (P<0.001 for both), with IBS participants on average having moderate-severe usual symptom severity (none=1.3%, mild=12.2%, moderate=52.7%, severe=31.1%, very severe=2.7%, and not applicable=0%) while IBD participants on average had mild-moderate usual symptom severity (none=5.4%, mild=22.9%, moderate=37.8%, severe=5.4%, very severe=2.7%, and not applicable=25.6%). HCs had significantly higher HRQOL (i.e. higher level of health) for both the physical and mental components of HRQOL compared to both IBD and IBS participants (P<0.001). IBD participants had significantly higher HRQOL scores for the mental component compared to IBS participants (P=0.006). However, there was no difference for the physical component of HRQOL (P=0.338). IBS participants had significantly higher trait anxiety compared to both IBD participants and HCs (P<0.001). There was no difference in trait anxiety between IBD participants and HCs (P=0.097). Both IBD and IBS participants had significantly higher HAD Anxiety scores compared to HCs (P<0.001), however there was no difference HAD Anxiety scores between IBD and IBS participants (P=0.55). There was no difference in HAD Depression scores in IBD participants compared to HCs (P=0.138), however IBS participants had higher HAD Depression scores compared to both IBD participants and HCs (P=0.004, P<0.001 respectively).
Table 1:
Baseline Clinical Characteristics of Study Subjects
| Healthy Control (n=74) |
IBD (n=74) |
IBS (n=74) |
P-Value | ||||
|---|---|---|---|---|---|---|---|
| HC:IBD: IBS |
HC:IBD | HC:IBS | IBS:IBD | ||||
| Age [mean (st. dev.) | 29.14 (9.64) | 29.05 (9.51) | 29.36 (9.24) | 0.908 | |||
| Female [n (%)] | 30 (40%) | 30 (40%) | 30 (40%) | 1 | |||
| Race [n (%)] | 0.002 | 0.005 | 0.911 | <0.001 | |||
| Caucasian | 34 (49%) | 56 (79%) | 32 (44%) | ||||
| Asian | 20 (29%) | 8 (11%) | 21 (29%) | ||||
| African American | 5 (7%) | 3 (4%) | 7 (10%) | ||||
| Other/Mixed | 10 (14%) | 4 (6%) | 12 (17%) | ||||
| Education [n (%)] | 0.558 | ||||||
| High School Graduate or less | 5 (7%) | 2 (2%) | 7 (9%) | ||||
| Some College | 25 (36%) | 22 (31%) | 29 (39%) | ||||
| College Graduate | 21 (30%) | 26 (36%) | 20 (27%) | ||||
| Any post-graduate work | 19 (27%) | 22 (30%) | 18 (24%) | ||||
| BMI [mean (st. dev.)] | 27.1 (5.51) | 23.83 (3.99) | 24.51 (4.48) | <0.001 | <0.001 | 0.004 | 0.519 |
| Usual GI-Symptom Severity [mean (st. dev.)]* | 2.26 (1.05) | 3.2 (0.74) | <0.001 | ||||
| Current GI-Symptom Severity [mean (st. dev.)]** | 5.52 (3.92) | 9.84 (4.52) | <0.001 | ||||
| VSI Score [mean (st. dev.)]# | 3.42 (5.06) | 26.62 (16.64) | 38.83 (15.06) | NA## | <0.001 | <0.001 | <0.001 |
| HRQOL [mean (st. dev.)]*** | |||||||
| Physical composite score | 55.11 (3.3) | 52.2 (7.22) | 50.6 (7.88) | <0.001 | 0.014 | <0.001 | 0.338 |
| Mental composite score | 53.04 (7.13) | 48.27 (10.21) | 43.22 (11.58) | <0.001 | 0.009 | <0.001 | 0.006 |
| Trait Anxiety [mean (st. dev.)]+ | 46.04 (8.03) | 48.53 (8.56) | 56.01 (12.73) | <0.001 | 0.097 | <0.001 | 0.002 |
| HAD Anxiety [mean (st. dev.)]++ | 3.62 (2.7) | 6 (2.98) | 8.05 (4.96) | <0.001 | <0.001 | <0.001 | 0.055 |
| HAD Depression [mean (st. dev.)]++ | 1.68 (1.75) | 2.45 (2.57) | 3.73 (2.9) | <0.001 | 0.138 | <0.001 | 0.004 |
St. dev., standard deviation; BMI, Body Mass Index; VSI, Visceral Sensitivity Index; HRQOL, Health Related Quality of Life; HADs, Hospital Anxiety and Depression Scale; IBD, Inflammatory Bowel Disease; IBS, Irritable Bowel Syndrome; HC, Healthy Control.
Usual GI-Symptom Severity was assessed by the Bowel Symptom Questionnaire (BSQ) and reflects a patient’s usual symptom severity over time. It is scored 1-5 (1=none, 2=mild, 3=moderate, 4=severe, 5=very severe).
Current GI-Symptom Severity was assessed by the BSQ and reflects a patient’s current symptom severity over the past week. It is scored 0-20 (“none” to “most intense imaginable”).
HRQOL was assessed by the MOS SF-12 which is divided into the physical- and mental-composite score. Each are scored 0-100 with 0 being poor health and 100 being highest level of health.
Trait anxiety was assessed by the State-Trait Anxiety Inventory (STAI), scores range 20-80 points.
HADs Anxiety and HADs Depression scores range 0-21 points.
We applied general linear model and report the results for main effects of groups and post-hoc pairwise group comparisons.
Given that log transformation did not improve the normality of the distribution, we used the Wilcoxon-Mann-Whitney U test to compare the pairwise group differences for VSI, and therefore the omnibus test is NA.
Clinical characteristics of the 74 IBD participants, including IBD severity (as measured by CDAI and PTIUC), history of IBD related surgery, current presence of an ostomy, and current IBD related medications, can be seen in Table 2. Approximately 90% of participants had no history of an IBD-related surgery. Only 25 (33%) IBD participants (CD N=12, UC N=13) completed disease activity indices. The overall disease activity in these participants was low with CDAI and PTIUC scores in the remission to mild disease range. In regards to current IBD medications, 18 (24%) were on a biologic, 26 (35%) were on an immunomodulator, and 41 (55%) were on a 5-aminosalicylic acid medication.
Table 2:
Clinical Characteristics and Disease Severity of the 74 IBD Participants*
| History of IBD Related Surgery [n (%)] | 8 (10%) |
| Ostomy present [n (%)] | 1 (1%) |
| CDAI Score [mean (st. dev.)]** | 46.83 (50.19) |
| PTIUC Score [mean (st. dev.)]*** | 3.85 (2.4) |
| Current IBD Medication [n (%)] | |
| Biologics | 18 (24%) |
| Infliximab | 8 (11%) |
| Adalimumab | 8 (11%) |
| Other Anti-TNF | 1 (1%) |
| Ustekinumab | 0 (0) |
| Vedolizumab | 1 (1%) |
| Tofacitinib | 0 (0) |
| Immunomodulator | 26 (35%) |
| Methotrexate | 4 (5%) |
| Mercaptopurine | 17 (23%) |
| Azathioprine | 5 (7%) |
| 5-Aminosalicylic Acid | 41 (55%) |
| Sulfasalazine | 4 (5%) |
| Mesalamine | 37 (50%) |
| Systemic Steroid | 2 (3%) |
| Enteral Steroid | 0 (0) |
| Rectal Medications | 6 (8%) |
| Mesalamine | 6 (8%) |
| Steroid | 0 (0) |
IBD, Inflammatory Bowel Disease; CDAI, Crohn’s Disease Activity Index; PTIUC, Powell-Tuck Index for Ulcerative Colitis.
25 (33%) IBD patients (Crohn’s Disease N=12, Ulcerative Colitis N=13) completed either the CDAI or PTIUC.
CDAI scores <150 represent clinical remission, 150-220 mild disease, 220-450 moderate disease, and >450 severe disease.
PTIUC scores 0-1 represent clinical remission, 2-5 mild disease, 6-8 moderate disease, and >9 severe disease.
VSI scores
Both IBD and IBS participants had significantly higher mean VSI scores compared to HCs (IBD = 26.62 ± 16.64, IBS = 38.83 ± 15.06; HC = 3.42 ± 5.06; all P’s<0.001, Table 1, Figure 1). However, VSI scores were significantly lower in IBD participants compared to IBS participants (P<0.001). VSI scores did not differ between CD and UC patients (P=0.59, Figure 1).
Figure 1:
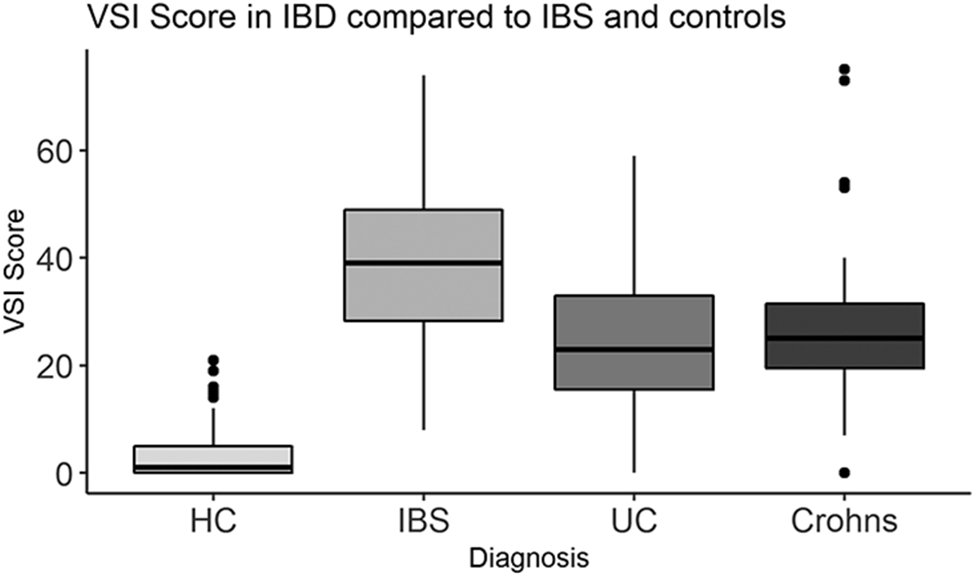
VSI scores were higher in in ulcerative colitis (UC, n=51, p<0.001), Crohn’s disease (CD, n=23, p<0.001), and Irritable Bowel Syndrome (IBS, n=74, p<0.001) compared to healthy controls (HC), n=74). VSI scores were similar between CD and UC (p=0.59). VSI scores were higher in IBS compared to IBD (p<0.001).
Internal consistency and construct validity of the VSI in IBD participants
Cronbach’s α = 0.86 in IBD participants. Factor analysis revealed that one main factor accounted for 48% of the variance and the second factor accounted for 11% variance. Four items loaded uniquely on factor 1, and three items loaded uniquely on factor 2 (Table 3). A third factor accounted for an additional 7% variance. Seven items loaded on this factor, however, only items 2 and 9 loaded uniquely (Table 3). The average inter-item correlation was between 0.15 and 0.50 for all VSI questions with the exception of question (VQ) 12, which had an inter-item correlation of 0.55 (Supplementary Table 1).
Table 3:
Factor loading analysis of the VSI in 74 IBD participants.
| Item | Symptoms | Factor Loadings | ||
|---|---|---|---|---|
| Factor1* | Factor2** | Factor3*** | ||
| VQ01 | Believe eating causes bloating and distension in the belly | 0.864 | ||
| VQ02 | Experience anxiety going to a new restaurant | 0.805 | ||
| VQ03 | Frequent worry about abdominal problems | 0.683 | ||
| VQ04 | Focus on abdominal discomfort interferes with enjoyment | 0.711 | 0.449 | |
| VQ05 | Fear of lacking the ability to have a normal bowel movement | 0.685 | ||
| VQ06 | Avoid new foods due to fear of developing abdominal symptoms | 0.445 | 0.578 | |
| VQ07 | Believe eating causes discomfort | 0.807 | 0.359 | |
| VQ08 | Abdominal symptoms lead to worry and anxiety | 0.797 | ||
| VQ09 | Searching for a bathroom is a priority in new environment | 0.683 | ||
| VQ10 | Vigilant to feelings in belly | 0.537 | 0.411 | |
| VQ11 | Believe pain or discomfort in abdomen indicates serious illness | 0.677 | ||
| VQ12 | Upon awakening, worries about possible abdominal discomfort during the day | 0.525 | 0.544 | 0.403 |
| VQ13 | Experiences abdominal discomfort as frightening | 0.899 | ||
| VQ14 | Believe symptoms are experienced during stress | 0.628 | ||
| VQ15 | Vigilance towards bowels | 0.469 | 0.544 | |
Factor 1 accounts for 48% of the overall variance. VQ1, 3, 5, and 14 loaded uniquely onto Factor 1.
Factor 2 accounts for 11% of the overall variance. VQ8, 11, and 13 loaded uniquely onto Factor 2.
Factor 3 accounts for 7% of the overall variance. Only VQ2 and 9 loaded uniquely onto Factor 3
Construct validity: Correlation of VSI with GI and psychological symptoms and HRQOL in IBD participants
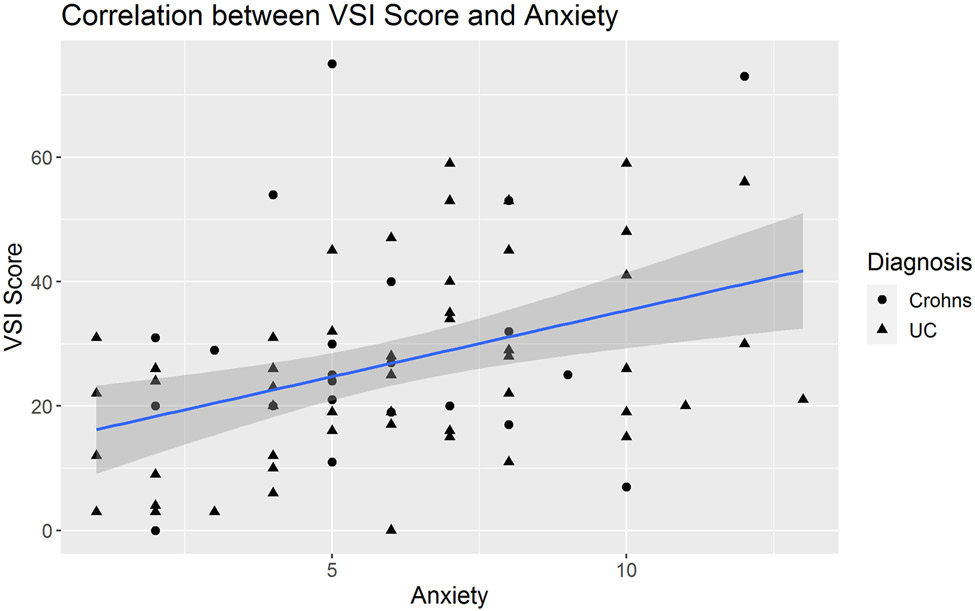
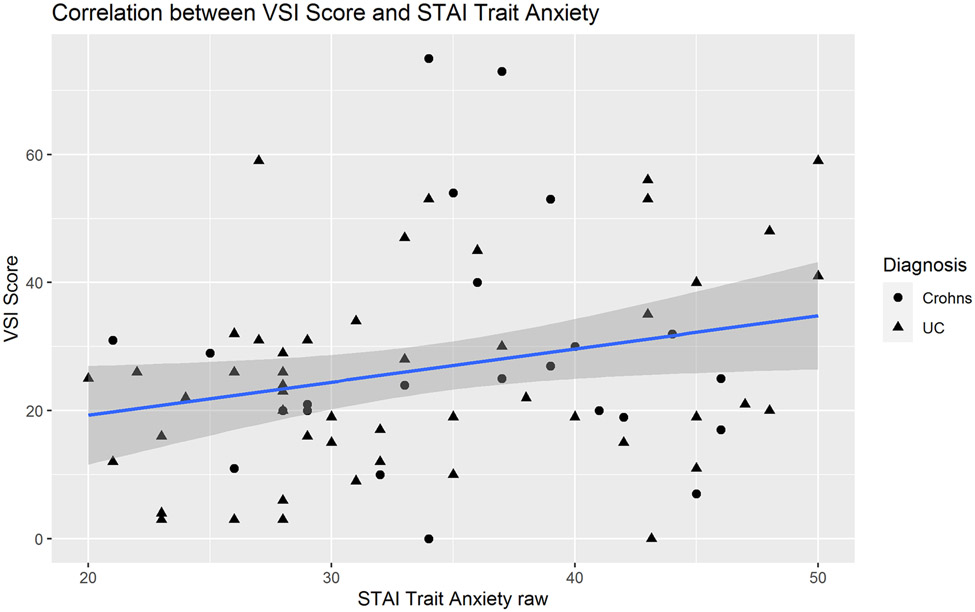
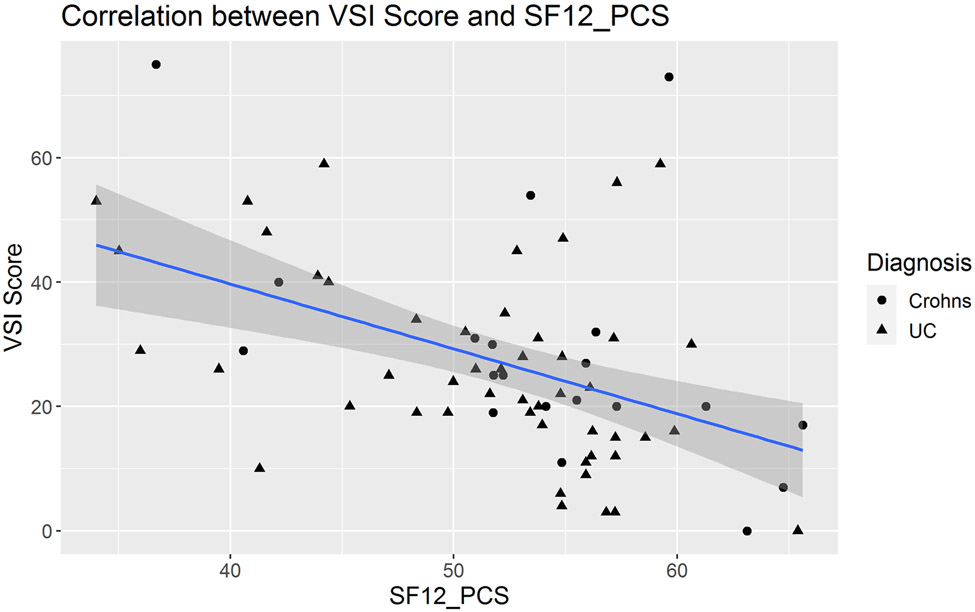
In IBD patients, medium effect size correlations were observed between the VSI and HAD Anxiety scores (R=0.35, P=0.002, Figure 2). There was a non-significant trend toward a positive correlation between VSI and trait anxiety as measured by STAI-T (R=0.22, P=0.06, Figure 3). In IBD participants, VSI scores had a moderate correlation with the physical component of HRQOL (R=−0.45, P=0.0001, Figure 4), but less so with the mental component of HRQOL (R=−0.23, P=0.05). VSI scores in IBD participants also showed a trend for a positive association between the VSI and current overall severity (as measured by a subjective numeric rating scale), but this did not reach statistical significance (R=0.25, P=0.08). However, the VSI scores in IBD participants with none/mild usual severity (28.3% of patients) were significantly lower compared to those with moderate/severe usual severity (86.5% of patients) (P=0.008, Figure 5). Only 25 (33%) IBD patients (CD N=12, UC N=13) completed disease activity indices and their overall IBD severity was in the remission-mild range (CDAI mean= 46.83 ± 50.19 and PTIUC mean = 3.85 ± 2.4, Table 1). As the majority of patients had remission to mild clinical activity, there were no significant correlations noted between VSI and CDAI (R=0.04, P=0.9) or PTIUC (R=0.18, P=0.57).
Figure 2:
In adult participants with inflammatory bowel disease, visceral sensitivity index (VSI) scores modestly correlated with general anxiety as measured by the Hospital Anxiety and Depression Scale (HAD) – Anxiety subscale (R=0.35, P=0.002).
Figure 3:
In adult participants with inflammatory bowel disease, there was a non-significant trend toward positive correlation between visceral sensitivity index (VSI) scores and trait anxiety as measured by the State Trait Anxiety Inventory (STAI) (R=0.22, P=0.06).
Figure 4:
In adult participants with inflammatory bowel disease, visceral sensitivity index (VSI) scores had a moderate correlation with the physical component of health-related quality of life (HRQOL) (R=−0.45, P=0.0001). The physical component of HRQOL was assessed by the medical outcomes study (MOS) 12-item short form (SF-12) physical composite score (PCS) which is scored 0-100 with 0 being poor health and 100 being highest level of health.
Figure 5:
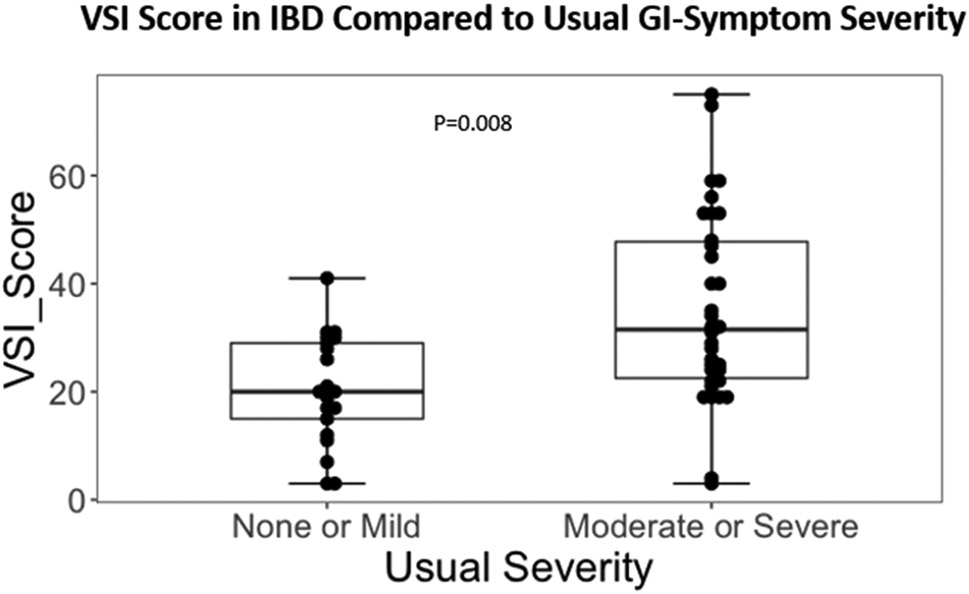
In adult participants with inflammatory bowel disease (IBD), visceral sensitivity index (VSI) scores were significantly higher in those with moderate/severe usual GI symptom severity (86.8% of IBD participants) compared those with none/mild usual GI symptom severity (28.3% of IBD participants) as measured by the Bowel Symptom Questionnaire (BSQ) (P=0.008).
DISCUSSION:
Our main findings are: 1) The VSI shows good reliability and factor structure in IBD participants and is similar to its performance seen previously in IBS participants; 2) GSA in IBD participants, as measured by the VSI, is related to general anxiety but is a different construct which is not affected by the presence of trait anxiety; 3) IBD participants had significantly higher VSI scores compared to HCs but also had significantly lower VSI scores than IBS participants; 4) VSI scores did not differ between CD and UC participants, suggesting the VSI performs similarly between the two IBD subgroups; 5) In IBD participants, higher VSI scores were significantly associated with lower HRQOL, especially the physical component of HRQOL; 6) In IBD participants, higher VSI scores were significantly associated with greater usual GI symptom severity and had a non-significant trend towards a positive correlation with current overall GI symptom severity as measured by the BSQ; and 7) The limited data availability on IBD disease activity in our participants made it difficult to make any meaningful conclusions on the association of the VSI with IBD disease severity. Our analysis revealed no significant correlation between IBD disease severity and VSI scores, however it did show that the VSI remained elevated even in IBD participants with disease in remission or mild severity.
The VSI shows good reliability and internal consistency in IBD participants and its performance in IBD participants (Cronbach’s α = 0.86) is similar to that in IBS participants (Cronbach’s α = 0.93)11. Cronbach’s α is commonly used in questionnaire/scale development and is a measure of internal consistency and reliability. It describes the extent to which the items in a scale consistently measure the intended concept or construct29 (i.e. Cronbach’s α provides an assessment of how well the VSI measures GSA). In general, a score of at least 0.7 is considered good. Factor analysis is also commonly used in questionnaire/scale development and is particularly useful evaluating item/question interrelationships, assessing associations of items/questions with possible latent variables, and in determining scale dimensionality30. In our study, factor analysis revealed the presence of a prominent first factor and a much smaller second and third factors which was similar to that found for IBS. The items most associated with the first and second factors and the weightings of the items differed between IBS and IBD suggesting there are differences in how these two groups respond to the VSI questions. However, a unidimensional scale is still supported by this factor analysis. Additionally, average inter-item correlations were between 0.15 and 0.5 for all VSI questions except for VQ12 which had an inter-item correlation of 0.55. This suggests that all the VSI items are related to GSA and there is minimal collinearity between items. For reference, the ideal range of average inter-item correlation is 0.15-0.50; a value less than 0.15 suggests the items are not well correlated and do not measure the same construct; more than 0.50 suggests that the items are collinear31. In this current analysis, the aim was to see how well the current VSI could be applied to IBD. It was beyond the scope of this analysis to re-evaluate the impact of removing specific items on the reliability and validity of the test. However, future studies should explore whether items can be removed from the VSI without affecting the reliability and validity of the test.
IBD participants had increased GSA, as measured by the VSI, compared to HCs even after controlling for current anxiety symptoms as measured by HAD. In IBD participants, VSI scores modestly correlated with general anxiety (HAD-anxiety scores), i.e. higher GSA as measured by the VSI was associated with higher current anxiety symptoms. This suggests that even though the VSI is related to current anxiety symptoms, it is measuring a different construct. We also found that VSI scores are not significantly affected by the presence of trait anxiety in IBD. Previous studies have shown that IBD participants experience both anxiety and depression at significantly higher rates than the general population. A 2016 systematic review analyzed 171 studies for a combined total of 158,371 IBD participants and found that the prevalence of depressive symptoms was 21.6% and the prevalence of anxiety symptoms was 35.1% in IBD participants5. Given that our current study, as well as previous studies in IBS11, 12, show that GSA is a different construct than general anxiety, it is likely that an even greater number of IBD patients have GSA than general anxiety. This is important because GSA could be contributing to perceived GI-symptom severity and persistence, and to disease progression in IBD patients as has already been shown to be the case for IBS patients. In IBS patients, the VSI score was found to be the single strongest predictor of GI symptom severity and symptom persistence 10. We have previously shown that GI symptom severity positively correlates with psychological symptoms9. However, there is also evidence that inflammation plays a critical role in subgroups of patients with anxiety and depression, and that mood disorders alone may be associated with increased inflammatory cytokines32-35. Increased plasma levels of inflammatory cytokines associated with persistent mucosal inflammation in IBD may explain why symptoms of anxiety and depression are independently associated with clinical recurrence of IBD36. Thus, it is conceivable that increased mucosal inflammation is associated with increased GI symptom-related anxiety independent of general anxiety, particularly in patients without a mood disorder. However, further cross-sectional and longitudinal VSI studies in IBD patients with a range of disease activity are needed.
In the current study, VSI scores in IBD participants negatively correlated with the physical component of generic HRQOL, i.e. higher GSA was associated with lower physical HRQOL, but not with mental HRQOL. This suggests that VSI is significantly associated with overall physical health but less so with mental health. This finding supports our previous study on the importance of psychological symptoms in both IBD and IBS9. In that study, we found that in both IBD and IBS participants, psychological distress had a stronger direct effect on HRQOL than GI symptoms. However, the association of GI symptoms and psychological distress was significantly stronger in IBD than IBS9, highlighting the importance of recognizing the overall impact of psychological symptoms, including GI-symptom related anxiety, in IBD patients.
The current findings suggest that GSA may persist even during states of IBD remission. This is evidenced by our finding of elevated VSI scores in IBD participants who mainly had IBD in remission or with mild activity. This persistence of GSA during times of disease remission may help to explain the continued decreased HRQOL seen in IBD patients and may have broader implications such as increased work absenteeism and poor sleep quality. Future studies should address whether targeting VSI and GSA as an outcome measure in treatment trials will significantly improve the HRQOL of patients with IBD.
Another hypothesis as to why VSI scores remain elevated in IBD patients when disease is in remission is comorbid IBS. The IBD patients included in this study were participants of previous functional brain imaging studies comparing IBS patients, healthy controls, and IBD patients who were predominantly in remission or had mild disease. IBD patients with a known concomitant IBS diagnosis were excluded. Patients with IBD were not required to have a colonoscopy or inflammatory markers at or around the time of the VSI surveys, and therefore no additional assessment was done at the time of enrollment to screen for overlapping IBS. However, we can confirm 25% of IBD participants did not meet Rome criteria based on their BSQ responses. Additionally, IBD subjects are routinely asked if they also have a diagnosis of IBS. It is therefore unlikely there was a large proportion of IBD-IBS overlap in the IBD participants. However, we are currently conducting a larger study evaluating GSA as measured by VSI in patients with IBD and are specifically assessing for potential IBD-IBS overlap.
To our knowledge, this is the first study to use the VSI to assess GSA in IBD participants. Strengths of this study include comparison of the VSI in IBD participants to age and sex matched HCs and IBS participants. Additionally, the study had robust analysis of the association of the VSI with both general anxiety and trait anxiety as well as HRQOL. This study highlights a novel tool that may be helpful to the clinician in evaluating IBD patients’ GI symptoms in relation to GSA and how they may be interacting to contribute to their overall HRQOL. For example, a clinician may find that their patient reports severe symptoms but has biochemical evidence of remission-mild disease and the VSI reveals high GSA. This may help guide the physician to recommend brain targeted therapies such as cognitive behavioral therapy or central neuromodulators, to further reduce symptoms rather than continuing to focus on managing inflammation. Additionally, clinicians could use this tool to help educate their patients and help them to understand the intricacies of the brain-gut connection and how organic disease can lead to learned fear responses to stimuli that can persist even when the inflammation is under control.
There are several limitations in this study. Disease activity data for IBD participants were available in only a small subset of IBD participants with relatively milder disease activity compared to the moderate-severe disease activity in the IBS group. This difference in disease activity could explain why VSI scores were significantly lower in IBD participants compared to IBS participants. We would expect that if our IBD participants had disease activity in the moderate-severe range, they would have similar VSI scores as the IBS participants with moderate-severe disease. Further studies with a larger sample size and wider distribution of IBD disease activity are required in order to make any meaningful conclusions on the association of the VSI with IBD disease activity. However, this limitation does not negate our primary finding that the VSI is a useful tool for the assessment of GSA and the overall health status of IBD patients and may be used to improve HRQOL. Another limitation to this study was the inability to fully control for comorbid IBS in the IBD participants. Future studies should assess the effect of comorbid IBS and IBD on the VSI’s performance.
In summary, the VSI overall appears to be a useful measure of GSA in IBD patients and is a novel tool that clinicians can use to help guide care and allocate limited resources in these complex patients that require a comprehensive biopsychosocial approach. However, future larger studies are needed to further validate the VSI in IBD and to assess its correlation with disease activity in both the adult and pediatric IBD population. Finally, long term longitudinal studies would be useful to evaluate the VSI and its relationship to GI symptoms, IBD disease activity, and HRQOL in IBD patients over time and also if treating GSA, with interventions such as pharmacotherapy and behavioral therapy, impacts disease activity and HRQOL.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to acknowledge the invaluable assistance of Cathy Liu and the research staff of the G. Oppenheimer Center for Neurobiology of Stress and Resilience, University of California Los Angeles.
FUNDING
This work was supported by the National Institute of Health [P50 DK064539, P30 DK041301, R01 DK048351, K23 DK106528, and R01 AT007137 to EAM] and the UCLA Children’s Discovery and Innovation Institute (CDI) Harry Winston Fellowship Award [CDI-HWF-07012018 to K.T.].
Footnotes
CONFLICT OF INTEREST/DISCLOSURES STATEMENT: To the best of our knowledge, the named authors have no conflict of interest, financial or otherwise.
COMPETING INTERESTS: the authors have no competing interests.
Data Availability Statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES:
- 1.Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. 2010;35(5):653–662. [DOI] [PubMed] [Google Scholar]
- 2.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119(3):654–660. [DOI] [PubMed] [Google Scholar]
- 3.Simrén M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97(2):389–396. [DOI] [PubMed] [Google Scholar]
- 4.Pizzi LT, Weston CM, Goldfarb NI, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(1):47–52. [DOI] [PubMed] [Google Scholar]
- 5.Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res. 2016;87:70–80. [DOI] [PubMed] [Google Scholar]
- 6.Lydiard RB, Fossey MD, Marsh W, Ballenger JC. Prevalence of psychiatric disorders in patients with irritable bowel syndrome. Psychosomatics. 1993;34(3):229–234. [DOI] [PubMed] [Google Scholar]
- 7.Tkalcić M, Hauser G, Stimac D. Differences in the health-related quality of life, affective status, and personality between irritable bowel syndrome and inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2010;22(7):862–867. [DOI] [PubMed] [Google Scholar]
- 8.Drossman D, Corazziari E, Talley N, Thompson W, Whitehead W. Rome II. The Functional Gastrointestinal Disorders, Diagnosis, Pathophysiology and Treatment: A Multinational Consensus, 2nd edn. VA, 2000 [Google Scholar]
- 9.Naliboff BD, Kim SE, Bolus R, Bernstein CN, Mayer EA, Chang L. Gastrointestinal and psychological mediators of health-related quality of life in IBS and IBD: a structural equation modeling analysis. Am J Gastroenterol. 2012;107(3):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerndal P, Ringström G, Agerforz P, et al. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22(6):646–e179. [DOI] [PubMed] [Google Scholar]
- 11.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69(1):89–98. [DOI] [PubMed] [Google Scholar]
- 12.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20(1):89–97. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. [DOI] [PubMed] [Google Scholar]
- 14.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology. 2016. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Boyce PM, Owen BK, Newman P, Paterson KJ. Initial validation of a bowel symptom questionnaire and measurement of chronic gastrointestinal symptoms in Australians. Aust N Z J Med. 1995;25(4):302–308. [DOI] [PubMed] [Google Scholar]
- 16.Schmulson M, Lee OY, Chang L, Naliboff B, Mayer EA. Symptom differences in moderate to severe IBS patients based on predominant bowel habit. Am J Gastroenterol. 1999;94(10):2929–2935. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 18.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31 (Pt 3):301–306. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 20.Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13(7):833–837. [DOI] [PubMed] [Google Scholar]
- 21.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 23.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 25.Conover WJ. Practical Nonparametric Statistics. New York: John Wiley & Sons:295–301 (one-sample Kolmogorov test), 309-314 (two-sample Smirnov test) [Google Scholar]
- 26.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 27.Revelle W. psych: Procedures for Personality and Psychological Research. Evanston, Illinois, USA: Northwestern University, 2019 [Google Scholar]
- 28.Kuhn M, Jackson S, Cimentada J. corrr: Correlations in R. R package version 0.4.2, 2020 [Google Scholar]
- 29.Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annu Rev Psychol. 2000;51:201–226. [DOI] [PubMed] [Google Scholar]
- 31.Clark LA, Watson D. Constructing Validity: Basic Science Issues in Objective Scale Development. Vol. 7: Psychological Assessment, 1995:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajadinejad MS, Asgari K, Molavi H, Kalantari M, Adibi P. Psychological issues in inflammatory bowel disease: an overview. Gastroenterol Res Pract. 2012;2012:106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48–54. [DOI] [PubMed] [Google Scholar]
- 34.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. [DOI] [PubMed] [Google Scholar]
- 36.Mikocka-Walus A, Pittet V, Rossel JB, von Känel R, Group SICS. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2016;14(6):829–835.e821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.