Abstract
Background:
Axicabtagene ciloleucel (axi-cel) is a standard-of-care for relapsed or refractory (r/r) large B-cell lymphoma (LBCL) with two or more lines of prior therapy. Patients receiving axi-cel in the real-world could have broader demographics, disease, and treatment profile compared to the cohort of the pivotal ZUMA-1 trial.
Objective:
To evaluate the outcomes of axi-cel in the real-world setting.
Study Design:
A total of 1297 patients receiving commercial axi-cel between 2017 and 2020 were selected from the Center for International Blood and Marrow Transplant Research (CIBMTR) data registry, among which 739 (57%) would have been ineligible for inclusion to the cohort of ZUMA-1. Efficacy and safety outcomes were described for the entire cohort and by ZUMA-1 eligibility. Their associations with age, ECOG performance score and comorbidities were evaluated using multivariable logistic and Cox regressions.
Results:
At a median follow-up of 12.9 months, overall response rate (ORR) was 73% with a 56% complete response (CR) rate. Median overall and progression-free survival (OS and PFS) were 21.8 months [95% confidence interval (CI), 17.4-28.8] and 8.6 months (95% CI, 6.5-12.1), respectively. Duration of response (DOR) for the ZUMA-1 ineligible patients (62% by 1 year, 95% CI, 57-66) was comparable to the ZUMA-1 eligible group (67% by 1 year, 95% CI, 62-72). Patients with age ≥ 65 years had favorable ORR [odds ratio (OR), 1.39; 95% CI, 1.05-1.83] despite having higher risk of cytokine release syndrome (CRS) (OR, 1.41; 95% CI, 1.02-1.94) and immune effector cell-associated neurotoxicity syndrome (ICANS) (OR, 1.77; 95% CI, 1.39-2.26). ECOG performance score ≥ 2 was associated with inferior efficacy outcomes [OR for ORR, 0.32; 95% CI, 0.18-0.56; hazard ratio (HR) for OS, 3.27; 95% CI, 2.37-4.52] and higher incidence of ICANS (OR, 2.63; 95% CI, 1.40-4.93).
Conclusions:
Patients ineligible to ZUMA-1 still had durable response with axi-cel. Elderly patients had favorable efficacy outcomes despite higher rates of CRS and ICANS. Patient selection for standard-of-care axi-cel should consider comorbidities and risk-to-benefit ratio rather than be strictly based on ZUMA-1 eligibility.
Keywords: Axicabtagene ciloleucel, CAR-T, large B-cell lymphoma, real-world evidence
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive blood cancer and the most common subtype of non-Hodgkin lymphoma (NHL) accounting for approximately 30% of newly diagnosed NHL cases. Thirty to forty percent of DLBCL patients relapse following successful treatment or present with a refractory disease 1,2. Patients with relapsed or refractory (r/r) DLBCL have poor outcomes with a median overall survival (OS) of 6.3 months 3.
Recent advances in immunotherapy have resulted in the development of chimeric antigen receptor-modified T-cell (CAR-T) therapy. Axicabtagene ciloleucel (axi-cel), a CD19-directed autologous CAR-T therapy, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of adult patients with r/r DLBCL, primary mediastinal large B-cell lymphoma (PMBCL), high grade B-cell lymphoma and transformed follicular lymphoma (TFL), collectively referred to as large B-cell lymphoma (LBCL), after two or more lines of systemic therapies. The approval was based on the primary analysis of the ZUMA-1 clinical trial4; in a subsequent 2-year follow-up analysis, 83% of patients treated with axi-cel had an overall response, 58% had a complete response (CR), and the median OS was not reached 5.
Since clinical trials often have stringent eligibility criteria, the efficacy and safety outcomes of trials may not be observed in real-world medical practice where treated patients could have more proliferative disease or comorbidities that would have excluded them from the trials. In a study with standard-of-care setting use of axi-cel across seventeen centers, including patients with comorbidities that would have made them ineligible for the ZUMA-1 trial, efficacy and safety outcomes were comparable to ZUMA-1 results 6. In a small multi-center real-world study, axi-cel use was shown to retain overall response and toxicity profile even though ZUMA-1 eligible patients had more favorable CR rates, DOR and OS7. In order to better understand the efficacy and safety outcomes of commercial axi-cel, we conducted this long-term follow-up study utilizing the infrastructure created by the Center for International Blood and Marrow Transplant Research (CIBMTR).
Patients and Methods
Approval of CAR T-cells by the FDA and other National Health Authorities required implementation of a mechanism to follow patients for assessment of safety and efficacy outcomes for 15 years. The approach for axi-cel was the development of a prospective, non-interventional cohort study. This postauthorization safety study (PASS) was reviewed and approved by the FDA and by the National Marrow Donor Program (NMDP) central institutional review board (IRB), and administered by the Cellular Immunotherapy Data Resource (CIDR) infrastructure managed by the CIBMTR. Data collection utilizes a secondary database mechanism whereby recipients of axi-cel signed an informed consent for data sharing with the CIBMTR for research purposes. The accrual goal for the PASS was 1500 patients with r/r DLBCL, PMBCL and high-grade B-cell lymphoma and started in October 2017 along with the commercial approval of axi-cel and completed in August 2020 with 79 centers participating. Patients who received noncommercial axi-cel (e.g. enrolled in expanded access programs or clinical trials) were not eligible for the PASS study. Among the 1500 patients, 7 patients were excluded /for the following reasons: rescinded consent (n = 1), reporting error in query (n = 1), confirmed to be enrolled in a clinical trial after data query (n = 5). The eligibility for this analysis further excluded patients with a prior history of CAR T cell infusion (n = 31), last contacted less than 180 days post-infusion (n = 123) and unknown comorbidity (n = 42) (Supplementary Figure 1). A total of 1297 eligible patients from 78 centers with at least 6 months of follow-up were included in the analysis. Patients with pre-existing end-organ impairments were not excluded from the study and comorbidities were collected by categories according to the Hematopoietic Cell Transplant Comorbidity Index (HCT-CI) elements 8. The HCT CI elements as originally described were analyzed individually. All study data were obtained from clinical, laboratory, and diagnostic assessments conducted during routine medical practice. Participating sites were responsible for completing a data collection form at the time of axi-cel administration, at 3, 6, and 12 months after the infusion, and then annually for up to 15 years.
Endpoints and Assessments
Analyses of efficacy endpoints included: overall response rate (ORR), complete and partial response (CR and PR) rate, duration of response (DOR), progression-free survival (PFS), non-relapse mortality (NRM) and overall survival (OS). Disease responses were assessed per International Working Group (IWG) revised response criteria for malignant lymphoma 9 and Lugano Classification 10. Relapse and progression were determined by radiological and/or clinical assessment. PFS was defined as time from the first axi-cel infusion to the earliest documented relapse or progression, or death due to any causes, whichever happened first. NRM was defined as death without previous experience of relapse or progression.
Analyses of safety endpoints included cytokine release syndrome (CRS), immune-effector cell associated neurotoxicity syndrome (ICANS), hematologic recovery, serious infections, and subsequent neoplasms. The type and severity 11,12 of CRS and ICANS, as well as individual signs and symptoms, treatment, date to onset, and date of resolution were captured. Events of failed recovery of normal neutrophil and platelet counts by day 30 were recoded. The type and timing of viral, bacterial, or fungal infections were captured.
Statistical Analysis
Descriptive statistics, including mean, standard deviation, median, and range for continuous variables, and percentages for categorical variables, are provided. Event rates for dichotomous outcomes including ORR, CRS and ICANS were calculated with 95% Fisher’s exact confidence intervals (CIs). Event-free probabilities and 95% CIs for time-to-event (TTE) efficacy outcomes including DOR, PFS and OS were summarized using the Kaplan-Meier (KM) estimator. Cumulative incidences and 95% CIs for NRM and resolutions of CRS and ICANS were estimated based on the cumulative incidence function (CIF). Median follow-up time was estimated using the reverse KM method.
Multivariable analysis was conducted to explore potential risk factors for both efficacy and safety outcomes. The process of variable selection involved a combination of biologically, clinically and empirically data driven approaches. Certain key variables were forced into the model, including age at infusion (≥ 65 vs. < 65 years), sex (male vs. female), Eastern Cooperative Oncology Group (ECOG) performance score (PS) at infusion (< 2 vs. ≥ 2, with higher scores indicating greater disability; translated from the Karnofsky performance score)13, bridging therapy (yes or no) and comorbidities of moderate to severe pulmonary disease, cardiac, cerebrovascular or heart valve disease, obesity (body mass index > 35 kg/m2), moderate to severe renal disease, moderate to severe hepatic disease, and any prior malignancy other than non-melanoma skin cancer. A stepwise regression procedure was implemented for selection of other baseline variables. The proportionality assumption for the Cox model was tested via Schoenfeld residuals 14. Multiple comparison adjustments were not performed, and nominal p-values were reported. A p-value cutoff of 0.05 was used to report “significant” variables. All analyses were performed in SAS 9.4.
Results
Patient Characteristics
Baseline characteristics of the 1297 patients were summarized in Table 1, including 1029 (79%) with DLBCL, 39 (3%) with PMBCL, and 210 (16%) with high grade B-cell lymphoma. For the last group, 192 (15%) were high grade B-cell lymphoma (HGBCL) with C-MYC and either BCL-2 and/or BCL-6 translocations. Transformed large B-cell lymphoma was present in 361 (28%) of cases (Supplemental Table 1) Seven hundred and thirty-nine (57%) patients were considered ZUMA-1 ineligible based on the following criteria (Supplementary Table 2): One patient was diagnosed with primary central nervous system (CNS) lymphoma while 18 had secondary involvement of the CNS. Histologic transformation from CLL occurred in 22 patients. At infusion, five percent of the patients had an ECOG PS ≥ 2. Of the reported comorbidities, 28% of the patients had moderate to severe pulmonary disease, 13% had cardiac, cerebrovascular, or heart valve disease, 5% had inflammatory bowel or rheumatologic disease, 2% had moderate to severe renal disease, 2% had moderate to severe hepatic disease, 4% had infection requiring ongoing antimicrobial treatment, and 13% had prior malignancy other than non-melanoma skin cancer. Prior history of allogeneic HCT was reported for 18 patients while 35 previously received check-point inhibitor therapy.
Table 1:
Baseline demographics and disease characteristics by ZUMA-1 eligibility
| Characteristic (%) | Ineligible for ZUMA-1 N = 739 |
Eligible for ZUMA- 1 or Unknown N = 558 |
Overall N = 1297 |
|---|---|---|---|
| Disease | |||
| DLBCL | 587 (80) | 442 (79) | 1029 (79) |
| PMBCL | 20 (3) | 19 (3) | 39 (3) |
| High grade B-cell lymphoma | 112 (15) | 98 (18) | 210 (16) |
| Other B-cell lymphoma | 19 (3) | 0 (0) | 19 (1) |
| Disease histology at diagnosis | |||
| DLBCL | 587 (80) | 442 (79) | 1029 (79) |
| Diffuse, large B-cell lymphoma - NOS | 569 (77) | 430 (77) | 999 (77) |
| T-cell/histiocytic rich large B-cell lymphoma | 10 (1) | 6 (1) | 16 (1) |
| Intravascular large B-cell lymphoma | 1 (< 1) | 0 (0) | 1 (< 1) |
| Primary cutaneous DLBCL, leg type | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| EBV+ DLBCL, NOS | 4 (< 1) | 4 (< 1) | 8 (< 1) |
| DLBCL associated with chronic inflammation | 1 (< 1) | 0 (0) | 1 (< 1) |
| Large B-cell lymphoma with IRF4 rearrangement | 1 (< 1) | 0 (0) | 1 (< 1) |
| ALK+ large B-cell lymphoma | 0 (0) | 1 (< 1) | 1 (< 1) |
| Primary mediastinal large B-cell lymphoma | 20 (3) | 19 (3) | 39 (3) |
| Primary mediastinal (thymic) large B-cell lymphoma | 20 (3) | 19 (3) | 39 (3) |
| High grade B-cell lymphoma | 112 (15) | 98 (18) | 210 (16) |
| High-grade B-cell lymphoma, NOS | 5 (< 1) | 13 (2) | 18 (1) |
| High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 rearrangements | 107 (14) | 85 (15) | 192 (15) |
| Other B-cell lymphoma | 19 (3) | 0 (0) | 19 (1) |
| Primary diffuse, large B-cell lymphoma of the CNS | 1 (< 1) | 0 (0) | 1 (< 1) |
| Nodal marginal zone B-cell lymphoma | 1 (< 1) | 0 (0) | 1 (< 1) |
| Other B-cell lymphoma | 4 (< 1) | 0 (0) | 4 (< 1) |
| B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and cHL | 5 (< 1) | 0 (0) | 5 (< 1) |
| Follicular, predominantly large cell (grade IIIB) | 6 (< 1) | 0 (0) | 6 (< 1) |
| Follicular, predominantly large cell (grade IIIA vs IIIB unspecified) | 2 (< 1) | 0 (0) | 2 (< 1) |
| Median (range), years | 63.1 (19.6-90.8) | 60.1 (21.5-80.6) | 62.1 (19.6-90.8) |
| ≥ 65 years | 310 (42) | 174 (31) | 484 (37) |
| Male sex | 469 (63) | 372 (67) | 841 (65) |
| Race | |||
| White | 609 (82) | 447 (80) | 1056 (81) |
| African American | 44 (6) | 23 (4) | 67 (5) |
| Asian | 35 (5) | 37 (7) | 72 (6) |
| Other | 51 (7) | 51 (9) | 102 (8) |
| Hispanic or Latino ethnicity | 68 (9) | 78 (14) | 146 (11) |
| ECOG PS ≥ 2 prior to infusion | 59 (8) | 0 (0) | 59 (5) |
| Comorbidities | |||
| Hepatic (moderate/severe) | 28 (4) | 0 (0) | 28 (2) |
| Cardiac/cerebrovascular/heart valve disease | 167 (23) | 0 (0) | 167 (13) |
| Renal (moderate/severe) | 30 (4) | 0 (0) | 30 (2) |
| Pulmonary (moderate/severe) | 367 (50) | 0 (0) | 367 (28) |
| Inflammatory bowel/rheumatologic disease | 59 (8) | 0 (0) | 59 (5) |
| Infection requiring ongoing antimicrobial treatment | 54 (7) | 0 (0) | 54 (4) |
| Prior malignancy (other than non-melanoma skin cancer) | 173 (23) | 0 (0) | 173 (13) |
| Obesity | 71 (10) | 46 (8) | 117 (9) |
| Chemo-resistant prior to infusion | 500 (68) | 354 (63) | 854 (66) |
| Disease characteristics at diagnosis | |||
| Histologic transformation | 219 (30) | 142 (25) | 361 (28) |
| HGBL with cMYC with either BCL2 either/or BCL6 translocations | 107 (14) | 85 (15) | 192 (15) |
| Ann Arbor stage III or IV | 440 (60) | 300 (54) | 740 (57) |
| Elevated LDH | 217 (29) | 151 (27) | 368 (28) |
| Two or more extranodal involvement sites | 194 (26) | 120 (22) | 314 (24) |
| No. of lines of prior therapies | |||
| Median (range) | 3 (2-18) | 3 (1-11) | 3 (1-18) |
| 1 to 2 lines | 177 (24) | 179 (32) | 356 (27) |
| 3 lines | 231 (31) | 176 (32) | 407 (31) |
| 4 lines | 122 (17) | 111 (20) | 233 (18) |
| 5 or more lines | 169 (23) | 70 (13) | 239 (18) |
| Prior history of HCT(s) | 217 (29) | 152 (27) | 369 (28) |
| Bridging therapy | 167 (23) | 114 (20) | 281 (22) |
| Days since leukapheresis | |||
| Median (range) | 28 (6-223) | 27 (5-145) | 27 (5-223) |
| ≥ 28 days | 383 (52) | 260 (47) | 643 (50) |
| Months since diagnosis | |||
| Median (range) | 15.2 (1.6-282.5) | 13.2 (1.2-405.8) | 14.2 (1.2-405.8) |
| ≥ 12 months | 450 (61) | 300 (54) | 750 (58) |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HGBCL, high grade B-cell lymphoma; LDH, lactate dehydrogenase; HCT, hematopoietic stem cell transplant; PMBCL, primary mediastinal large B-cell lymphoma; PS, performance score
Efficacy
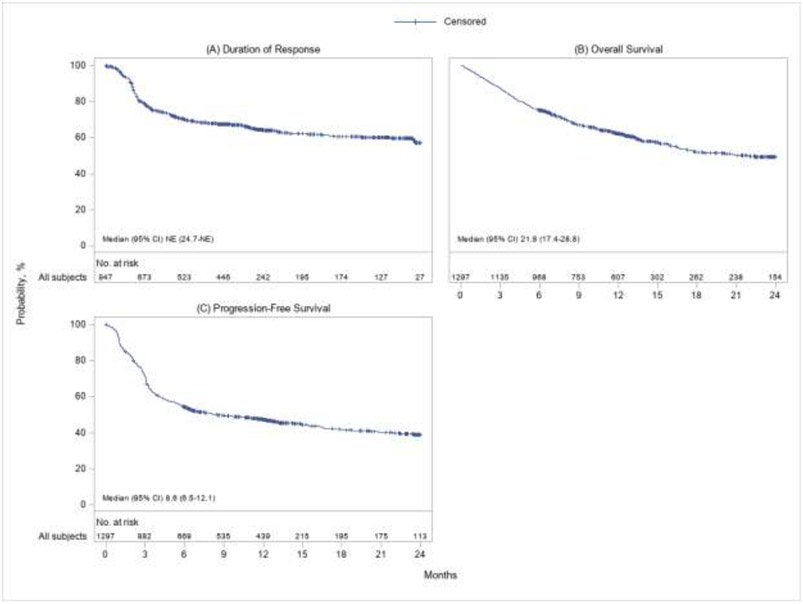
At a median follow-up of 12.9 months, ORR as best response among the 1297 patients was 73% (95% CI, 71 to 75) including a 56% (95% CI, 53 to 58) CR rate and a 18% (95% CI, 16 to 20) PR rate. The median DOR was not reached while 64% (95% CI, 61 to 67) and 57% (95% CI, 52 to 62) patients stayed relapse and progression-free by 12 months and 24 months since the initial response. The KM estimate for DOR is shown in Figure 1A. Median OS was 21.8 months (95% CI, 17.4 to 28.8), and 62% (95% CI, 60 to 65) and 50% (95% CI, 46 to 53) of the patients were expected to survive beyond 12 months and 24 months post-infusion. Median PFS was 8.6 months (95% CI, 6.5 to 12.1), and 47% (95% CI, 44 to 50) and 39% (95% CI, 36 to 43) of the patients were expected to have no relapse, disease progression or death at 12 months and 24 months respectively (Table 2). The KM estimates for OS and PFS were provided in Figure 1B and 1C. A total of 544 deaths (42%) were reported at the data cutoff. Disease relapse or progression was the mostly identified cause of death (74% among those who died), followed by infection (11%) and organ failure (5%) (Supplementary Table 3). NRM occurred in 1% (95% CI, < 1 to 2) of the population by 1 month and in 3% (95% CI, 2 to 4) by 3 months (Table 2). Efficacy outcomes by ZUMA-1 eligibility were shown in Table 2.
Figure 1: Kaplan-Meier plots for (A) DOR, (B) OS and (C) PFS.
Abbreviations: CI, confidence interval; CR, complete response; DOR, duration of response; NE, not estimable; OS, overall survival; PFS, progression-free survival; PR, partial response.
PFS and DOR were both censored at subsequent transplants or cellular therapies. DOR was estimated among patients who achieved CR/PR as best response.
Table 2.
Effectiveness Outcomes by ZUMA-1 Eligibility
| Measure (95% CI) | Ineligible for ZUMA-1 N = 739 |
Eligible for ZUMA-1 or Unknown N = 558 |
Overall N = 1297 |
|---|---|---|---|
| ORRa | 70.9 (67.5-74.2) | 75.8 (72.0-79.3) | 73.0 (70.5-75.4) |
| CRa | 52.4 (48.7-56.0) | 59.7 (55.5-63.8) | 55.5 (52.8-58.2) |
| PRa | 18.5 (15.8-21.5) | 16.1 (13.2-19.4) | 17.5 (15.5-19.7) |
| DORb | |||
| Median, months | 25.2 (23.6-NE) | NE (24.7-NE) | NE (24.7-NE) |
| At 1 year | 61.7 (56.8-66.2) | 67.0 (61.7-71.6) | 64.1 (60.6-67.4) |
| At 2 years | 53.7 (45.7-61.1) | 60.4 (52.3-67.6) | 57.0 (51.5-62.1) |
| OS | |||
| Median, months | 16.5 (15.1-21.8) | 28.0 (22.4-NE) | 21.8 (17.4-28.8) |
| At 1 year | 58.1 (54.3-61.7) | 67.8 (63.6-71.7) | 62.3 (59.5-64.9) |
| At 2 years | 45.2 (40.6-49.8) | 55.1 (49.7-60.2) | 49.5 (46.0-52.9) |
| PFS | |||
| Median, months | 6.4 (5.5-9.0) | 13.0 (8.3-21.8) | 8.6 (6.5-12.1) |
| At 1 year | 43.7 (39.9-47.4) | 52.0 (47.6-56.1) | 47.3 (44.4-50.1) |
| At 2 years | 35.8 (31.5-40.1) | 43.6 (38.4-48.5) | 39.2 (35.9-42.5) |
| NRM | |||
| At 1 month | 1.4 (0.7-2.5) | 0.9 (0.4-2.0) | 1.2 (0.7-1.9) |
| At 3 months | 3.8 (2.6-5.4) | 1.8 (0.9-3.3) | 3.0 (2.1-4.0) |
| Median follow-up, months | 12.9 (12.6-13.2) | 13.1 (12.7-13.5) | 12.9 (12.8-13.2) |
Abbreviations: CI, confidence interval; CR, complete response; DOR, duration of response; NE, not estimable; NRM, non-relapse mortality; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response
As best response.
Among patients who achieved CR/PR as best response (n = 947).
Based on the multivariable analysis (Supplementary Table 4), having an elevated ECOG PS ≥ 2 or being chemo-resistant prior to infusion were associated with inferior ORR (OR, 0.32; 95% CI, 0.18 to 0.56; and 0.54; 95% CI, 0.38 to 0.76), DOR (HR, 3.29; 95% CI, 2.00 to 5.40; and 1.39; 95% CI, 1.05 to 1.83), OS (HR, 3.27; 95% CI, 2.37 to 4.52; and 1.44; 95% CI, 1.15 to 1.81) and PFS (HR, 2.61; 95% CI, 1.90 to 3.60; and 1.48; 95% CI, 1.21 to 1.79). Moderate to severe pulmonary disease was associated with inferior ORR (OR, 0.75; 95% CI, 0.57 to 1.00) while moderate to severe hepatic disease was associated with worse DOR (HR, 2.63; 95% CI, 1.30 to 5.32), OS (HR, 2.69; 95% CI, 1.72 to 4.20) and PFS (HR, 2.38; 95% CI, 1.50 to 3.79). In addition, patients with age ≥ 65 years had favorable ORR (OR, 1.39; 95% CI, 1.05-1.83).
Safety
As shown in Table 3, among the 1297 patients, 1073 (83%) developed CRS of any grade, while 107 (8%) developed grade 3 or higher CRS 11. Seven hundred and fourteen patients (55%) developed ICANS of any grade, of which 313 (24%) were grade 3 or higher 12. Median onset time from axi-cel infusion was 4 days (range, 1 to 28) for CRS and 7 days (range, 1 to 36) for ICANS. Among all 1297 patients, 219 (17%) received tocilizumab without corticosteroids, 96 (7%) had corticosteroids but not tocilizumab, while 532 (68%) were treated with both. Tocilizumab was given to 79% of the patients experiencing both CRS and ICANS and 53% among those with only CRS. In addition, 81% of the patients having both CRS and ICANS received corticosteroids as treatment. Resolution rates by Week 3 since onset were 92% (95% CI, 90 to 94) for CRS and 77% (95% CI, 73 to 80) for ICANS. As of the data cutoff, 295 patients (24% among those who survived 30 days post-infusion) had prolonged cytopenia, of which 82 (7%) were neutropenia and 271 (22%) were thrombocytopenia. Five hundred and eighty patients (45%) experienced clinically significant infections. A total of 50 patients (4%) developed subsequent neoplasms, among which myelodysplasia syndrome (n = 15), squamous cell skin malignancy (n = 11), and myelodysplasia/myeloproliferative neoplasm (n = 4) were the most common. Two patients had multiple incidences of subsequent neoplasms.
Table 3:
Safety outcomes by ZUMA-1 eligibility
| Measure (%) | Ineligible for ZUMA-1 N = 739 |
Eligible for ZUMA-1 or Unknown N = 558 |
Overall N = 1297 |
|---|---|---|---|
| CRS | |||
| Any grade | 612 (83) | 461 (83) | 1073 (83) |
| ≥ grade 3a | 73 (10) | 34 (6) | 107 (8) |
| Grade 4a | 38 (5) | 15 (3) | 53 (4) |
| Grade 5a | 14 (2) | 8 (1) | 22 (2) |
| Median time to onset (range), daysb | 4 (1-25) | 4 (1-28) | 4 (1-28) |
| Median duration (range), daysb | 7 (1-121) | 6 (1-81) | 6 (1-121) |
| Resolution rate by Day 21 since onset (95% CI)b | 91.1 (88.5-93.1) | 93.7 (91.0-95.6) | 92.2 (90.4-93.6) |
| ICANS | |||
| Any grade | 425 (58) | 289 (52) | 714 (55) |
| ≥ grade 3a | 193 (26) | 120 (22) | 313 (24) |
| Grade 4a | 73 (10) | 41 (7) | 114 (9) |
| Grade 5a | 8 (1) | 5 (< 1) | 13 (1) |
| Median time to onset (range), daysc | 7 (1-36) | 7 (1-25) | 7 (1-36) |
| Median duration (range), daysc | 7 (1-115) | 7 (1-112) | 7 (1-115) |
| Resolution rate by Day 21 since onset (95% CI)c | 75.4 (70.9-79.3) | 78.6 (73.5-82.9) | 76.7 (73.4-79.7) |
| Treatment for CRS or ICANS | |||
| Tocilizumab without corticosteroids | 122 (17) | 97 (17) | 219 (17) |
| Corticosteroids without tocilizumab | 56 (8) | 40 (7) | 96 (7) |
| Both tocilizumab and corticosteroids | 297 (40) | 235 (42) | 532 (41) |
| Prolonged cytopeniad | 195 (28) | 100 (18) | 295 (24) |
| Neutropenia | 45 (6) | 37 (7) | 82 (7) |
| Thrombocytopenia | 182 (26) | 89 (16) | 271 (22) |
| Clinically significant infection | 374 (51) | 206 (37) | 580 (45) |
| Subsequent neoplasms | 36 (5) | 14 (3) | 50 (4) |
Abbreviations: ASTCT, American Society of Transplant and Cellular Therapy; CI, confidence interval; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome
Lee criteria for CRS grade; ASTCT consensus criteria for ICANS grade
Among patients with CRS (n = 1073)
Among patients with ICANS (n = 714)
Among patients alive at Day 30 post-infusion (n = 1238)
Based on the multivariable analysis (Supplementary Table 5), age 65 years or older was associated with increased risk of CRS (OR, 1.41; 95% CI, 1.02 to 1.94) and ICANS (OR, 1.77; 95% CI, 1.39 to 2.26). Moderate to severe hepatic disease was associated with higher odds of grade 3 or higher CRS (OR, 3.70; 95% CI, 1.43 to 9.56) while ECOG PS ≥ 2 was associated with higher incidence of ICANS (OR, 2.63; 95% CI, 1.40 to 4.93) and ICANS of grade 3 or higher (OR, 3.23; 95% CI, 1.81 to 5.74).
Discussion
Based on the data of 1297 patients from 78 centers, this is the largest real-world prospective study thus far to report on patients treated with CAR-T cell therapy. Our findings demonstrated that the efficacy of real-world axi-cel use for r/r LBCL was comparable to response outcomes reported in clinical trials. We observed an ORR of 73% and a CR rate of 56% which align with the findings of the pivotal portion of the ZUMA-1 trial 4. Our data also showed that DOR for patients who would have been ineligible to ZUMA-1 (62% by 1 year; 95% CI, 57-66) was comparable to the ZUMA-1 eligible group (67% by 1 year; 95% CI, 62-72). Furthermore, certain comorbidities such as prior malignancy, which would have excluded patients from being eligible for ZUMA-1, were not associated with inferior efficacy outcomes. Similar observations have been reported in a much smaller number of patients 6. Notably, odds of achieving overall response is 39% higher among patients aged 65 years or older compared to those of younger age even after multivariable adjustment. NRM after axi-cel infusion also remained at a very low level.
The CRS rate in this study was similar to what was reported in ZUMA-1 but CRS of grade 3 or higher trended lower at 8% versus 11% in ZUMA-1 4,5. The median time to onset of CRS was 4 days (range, 1 to 28), and the median time until resolution was 6 days, compared to 2 days (range, 1 to 12) and 8 days in ZUMA-1 respectively. In the same vein, ICANS of grade 3 or higher in this study was 24% versus 28% in ZUMA-1. The median time to onset of ICANS was 7 days (range, 1 to 36) versus 5 days (range, 1 to 17) in ZUMA-1. Other than the association between moderate to severe hepatic disease and grade 3 or higher CRS, none of the comorbidities assessed was associated with CRS or ICANS. Low prevalence of thrombocytopenia, neutropenia and infections were observed relative to the pivotal clinical trial of the axi-cel and reported by single centers 15,16. The improved safety profile for grade 3 or higher CRS and ICANS in the real-world setting versus early clinical trials was mostly likely accounted for by an increased use of tocilizumab, corticosteroids, siltuximab, and other drugs as part of the emerging pattern of toxicity management of CAR-T therapy 17, while lower pretreatment levels of inflammation and disease burden due to improved bridging options may account for the longer median to onset for CRS and ICANS 18.
This is the first study of its size to have long follow-up of patients treated with axi-cel in the real-world with a high incidence of medical comorbidities. It is also the first PASS for a CAR-T cell product to have completed its targeted accrual to fulfill health authority requirement for the approval of these agents. These observations highlight the feasibility of using an existing secondary outcomes database to fulfill post-market requirements and evaluate the important outcomes of novel cellular therapies in large cohorts in the real-world setting.
There are some limitations to this study. Being an observational study, there was no way to prespecify an intervention for the patients, patient selection, or the type and timing of evaluations of outcome response. All response assessments were per the primary oncologists and a central review of response assessments was lacking. Certain key disease features known to be associated with response to axi-cel including tumor burden, systemic and tumor inflammation, and CAR-T cell expansion in the blood also could not be evaluated 18,19. Cell dose was from axi-cel manufacturing results was also not available to assess its impact with outcomes. Importantly, due to the nature of the data registry, characteristics of patients who never had a chance to receive axi-cel were not reported and the grouping of ZUMA-1 eligibility was not based on intention-to-treat. The higher efficacy of axi-cel in patients greater than 65 raises the question of whether this is due to selection bias based upon disease aggressiveness or comorbidities, or if there are biological differences in immunity in older patients impacting outcomes with CAR-T. Further studies may be necessary to uncover the biology behind the observed association.
In conclusion, our findings suggest that patients not meeting eligibility criteria for the pivotal ZUMA-1 trial still had durable response with axi-cel. Elderly patients had favorable efficacy outcomes with axi-cel despite higher rates of CRS and ICANS. Patient selection for standard-of-care axi-cel should consider comorbidities and the risk-to-benefit ratio, rather than be strictly based upon ZUMA-1 eligibility. These results significantly add to the growing body of evidence of axi-cel use in the real-world setting and expand the definition of the potential patient population that may benefit from it.
Supplementary Material
Supplementary Table 1: Detailed histologies of transformed lymphomas
Supplementary Table 2: Reason(s) for ZUMA-1 ineligibility
Supplementary Table 3: Primary cause of death
Supplementary Table 4: Multivariable analysis for efficacy outcomes
Supplementary Table 5: Multivariable analysis for safety outcomes
Supplementary Figure 1. Patient disposition diagram
HIghlights.
Patients ineligible to ZUMA-1 still had durable response with axi-cel
Elderly patients had favorable efficacy outcomes despite higher CRS and ICANS rates
Patient selection for axi-cel should consider comorbidity and risk-to-benefit ratio
Acknowledgements
Authors’ Disclosures of Potential Conflict of Interest
CAJ: honoraria from Kite, a Gilead Company, Celgene, Novartis, Humanigen, Pfizer, Precision BioSciences, Nkarta, Lonza, and AbbVie; consultancy or advisory role for Kite, a Gilead Company, Celgene, Novartis, Pfizer, Humanigen, Precision BioSciences, Nkarta, Lonza, Pfizer and AbbVie; speakers' bureau participation for Axis and Clinical Care Options; research funding from Pfizer; and travel support from Kite, a Gilead Company, Celgene, Novartis, Precision Biosciences, Lonza, Pfizer, and Humanigen. FLL: consulting or advisory role with ecoR1, Emerging Therapy Solutions Gerson Lehman Group, Allogene, Amgen, Bluebird Bio, BMS/Celegene, Calibr, Iovance, Kite, a Gilead Company, Janssen, Legend Biotech, Novartis, Umoja, Cowen, Cellular Biomedicine Group, GammaDelta Therapeutics, Wugen; research funding from Kite, a Gilead Company, Allogene and Novartis; and patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy. LM: employment with Kite, a Gilead Company. JA: employment with Kite, a Gilead Company. ZH: employment with Kite, a Gilead Company; and research funding from Kite, a Gilead Company, Novartis, and Bristol Myers Squibb. TS: consultancy or advisory role for AstraZeneca, PCYC, Celgene, Juno, Kite, a Gilead Company, and BeiGene; speakers' bureau participation for PCYC, Janssen, AstraZeneca, and Seattle Genetics; and research funding from PCYC, Juno, Kite, a Gilead Company, AstraZeneca, BeiGene, Oncternal, TG Therapeutics, and Celgene. SA: research funding from Seagen, Tessa Therapeutics, Merck, and Xencor. AG: consultancy or advisory role for Kite, a Gilead Company, Amgen, Atara, Wugen Inc., and Celgene; research funding from Kite, a Gilead Company, and Amgen; and honoraria from Kite, a Gilead Company. DBM: consultancy or advisory role for Kite, a Gilead Company, Novartis, Juno-Celgene-BMS, Allogene, Precision Bioscience, Adicet, Pharmacyclics, Janssen, Takeda, Adaptive Biotechnologies and Miltenyi Biotechnologies; research funding from Kite, a Gilead Company, Novartis, Juno-Celgene-BMS, Allogene, Precision Biosciences, Adicet, Adaptive Biotechnologies; and patents, royalties, or other intellectual property from Pharmacyclics. YL: consultancy or advisory role for Kite, a Gilead Company, Janssen, Novartis, Celgene, Bluebird Bio, Juno, Legend, Sorrento, Gamida Cell, and Vineti; research funding from Kite, a Gilead Company, Janssen, Celgene, Bluebird Bio, Merck, and Takeda. MP: employment with Memorial Sloan Kettering Cancer Center; honoraria from AbbVie, Astellas, Bellicum, Celgene, Bristol Myers Squibb, Incyte, Karyopharm, Kite, a Gilead Company, Miltenyi Biotech, MorphoSys, Nektar Therapeutics, Novartis, Takeda, VectivBio AG and Vor Biopharma; consultancy or advisory role for Merck, Omeros, OrcaBio; research funding from Incyte, Kite, a Gilead Company, and Miltenyi; and other relationships with DSMB, Cidara Therapeutics, Medigene, Sellas Life Sciences and Servier. MAL: consultancy or advisory role for Kite, a Gilead Company, Celgene, Verastem, Janssen, Myeloid Therapeutics, AstraZeneca, Acrotech, ADC Therapeutics, Legend, Spectrum, Beigene, Daiichi-Sankyo, Morphosys, TG Therapeutics, Novartis, Kyowa Kirin, Karyopharm, and Abbvie. MH: no relevant financial relationships to disclose. BTH: honoraria from Kite, a Gilead Company; consultancy or advisory role for Kite, a Gilead Company; research funding from Kite, a Gilead Company; and travel support from Kite, a Gilead Company. SG: no relevant financial relationships to disclose. HD: employment with Kite, a Gilead Company SN: consultancy or advisory role for Kite, a Gilead Company, and Novartis. MH: employment with Kite, a Gilead Company, stock or other ownership in Gilead Sciences. JK: employment with Kite, a Gilead Company and Sierra Oncology, stock or other ownership in Gilead Sciences. HX: employment with Kite, a Gilead Company. MCP: honoraria from Celgene; consultancy or advisory role for Medigene, Pfizer, and Amgen; and research funding from Kite, a Gilead Company, Novartis, and Bristol Myers Squibb.
Funding, Support and Role of Sponsor
The study was supported in part by the National Cancer Institute CIDR (U24 CA233032) and Kite, a Gilead Company (study sponsor), and was conducted in a collaboration between the authors and the sponsor. Medical writing support was provided by the sponsor.
Other Acknowledgements
The authors thank the patients who participated in this trial and their families, caregivers, and friends; the trial investigators, coordinators, and health care staff at each clinical study site.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the American Society of Clinical Oncology annual meeting online, June 4-8 2021.
Data Access and Responsibility
MCP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. Jan 2014;3(1):66–70. doi: 10.4103/2278-330X.126531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. Jan 1 2015;125(1):22–32. doi: 10.1182/blood-2014-05-577189 [DOI] [PubMed] [Google Scholar]
- 3.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. Oct 19 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. The New England journal of medicine. Dec 28 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. Jan 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. Sep 20 2020;38(27):3119–3128. doi: 10.1200/JCO.19.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. Sep 20 2020;38(27):3095–3106. doi: 10.1200/JCO.19.02103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. Oct 15 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. Feb 10 2007;25(5):579–86. doi: 10.1200/jco.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. Sep 20 2014;32(27):3059–68. doi: 10.1200/jco.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. Jul 10 2014;124(2):188–95. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. Apr 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 13.National Marrow Donor Program, The Medical College of Wisconsin. Forms Instruction Manual Appendix L: Karnofsky/Lansky performance status. https://www.cibmtr.org/manuals/fim/1/en/topic/appendix-l-karnofsky-lansky-performance-status
- 14.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 15.Lin RJ, Lobaugh SM, Pennisi M, et al. Impact and safety of chimeric antigen receptor T-cell therapy in older, vulnerable patients with relapsed/refractory large B-cell lymphoma. Haematologica. Jan 1 2021;106(1):255–258. doi: 10.3324/haematol.2019.243246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. Apr 1 2021;106(4):978–986. doi: 10.3324/haematol.2019.238634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacoboni G, Villacampa G, Martinez-Cibrian N, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. May 2021;10(10):3214–3223. doi: 10.1002/cam4.3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. Oct 13 2020;4(19):4898–4911. doi: 10.1182/bloodadvances.2020002394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain MD, Zhao H, Wang X, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. May 13 2021;137(19):2621–2633. doi: 10.1182/blood.2020007445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Detailed histologies of transformed lymphomas
Supplementary Table 2: Reason(s) for ZUMA-1 ineligibility
Supplementary Table 3: Primary cause of death
Supplementary Table 4: Multivariable analysis for efficacy outcomes
Supplementary Table 5: Multivariable analysis for safety outcomes
Supplementary Figure 1. Patient disposition diagram