Abstract
Epithelial cells are the first line of mucosal defense. In the intestine, a single layer of epithelial cells must establish a selectively permeable barrier that supports nutrient absorption and waste secretion while preventing leakage of potentially harmful luminal materials. Key to this is the tight junction which seals the paracellular space and prevents unrestricted leakage. The tight junction is a protein complex established by interactions between members of the claudin, zonula occludens, and tight junction-associated MARVEL protein (TAMP) families. Claudins form the characteristic tight junction strands seen by freeze-fracture microscopy and create paracellular channels, but the functions of ZO-1 and occludin, founding members of the zonula occludens and TAMP families, respectively, are less well-defined. Recent studies have revealed that these proteins have essential non-canonical (non-barrier) functions that allow them to regulate epithelial apoptosis and proliferation, facilitate viral entry, and organize specialized epithelial structures. Surprisingly, neither is required for intestinal barrier function or overall health in the absence of exogenous stressors. Here, we provide a brief overview of ZO-1 and occludin canonical (barrier-related) functions, and a more detailed examination of their noncanonical functions.
Keywords: permeability, intestine, barrier, claudin, actin
Introduction
Epithelial surfaces form tissue barriers that are essential for complex life.1–5 In simplest terms, the plasma membranes of epithelial cells are the principal component of these barriers. The space between adjacent epithelial cells, referred to as the shunt pathway, must, however, also be sealed. The apical junctional complex, composed of tight junctions, adherens junctions, and desmosomes, accomplishes this while also conferring structural integrity to epithelial structures.6 Nevertheless, permeability of the seal formed by the apical junctional complex, primarily the tight junction, varies widely across tissues.7 At some sites, including the skin and urinary bladder, the barrier is nearly absolute. In contrast, significant paracellular flux occurs within the renal tubules and the gut. Defining characteristics of tight junction flux and paracellular permeability are that they are passive and symmetrical, respectively. Net paracellular transport is therefore determined by tight junction permeability and transepithelial concentration gradients. The latter are typically established by active transepithelial transport and a mechanism by which transmembrane transporters regulate paracellular flux.8–14
The large claudin protein family forms the substructure of tight junctions, and different claudins can create the paracellular barrier or form highly-selective paracellular channels barriers.15–18 These have been reviewed extensively.19–21 Here, we will review canonical (barrier-related) and focus on recently elucidated noncanonical (non-barrier) functions of two non-claudin proteins, ZO-1 and occludin.
Primary structures
ZO-1 was the first tight junction protein to be discovered.22 It is a cytosolic peripheral membrane protein with multiple domains specialized for protein interactions. Among these are three PDZ (PSD95/Dlg/ZO-1) domains, a Src homology-3 (SH3) domain, an actin binding region (ABR), and a ZU5 domain. These allow ZO-1 to bind to claudins, occludin, ZO-2, ZO-3, cingulin, afadin, JAM-A, α-catenin, shroom2, F-actin, angulin, and numerous other proteins.23–29 This has led to the hypothesis that ZO-1 forms a scaffold that organizes proteins at the tight junction, although only limited data suggest that this occurs and is significant in living cells.30–32 More recently, ZO-1 has been implicated in mechanotransduction, where it can take on elongated or globular forms and undergo phase separation.26, 27, 33–35
In contrast to ZO-1, occludin is a 522 amino acid protein that spans the membrane four times to form two extracellular loops and one intracellular loop. The short N-terminal and much longer C-terminal domains are intracellular.36 The 257 amino acid C-terminal domain comprises nearly half of occludin and is highly conserved throughout evolution. The distal 40% of the C-terminal tail forms three α-helices that arrange into the anti-parallel coiled-coils OCEL domain that homology to the C-terminus of ELL family of RNA polymerase II elongation factors. The OCEL domain is required for occludin trafficking to the tight junction and has been implicated in occludin interactions with a wide range of proteins.37–43
Contributions of occludin and ZO-1 to tight junction structure and barrier function
Occludin was initially postulated to be an essential tight junction component, but this idea was discarded due to the presence of tight junctions within occludin gene (Ocln) knockout embryoid bodies and the viability of Ocln knockout mice. Moreover, the absence of obvious spontaneous disease in Ocln knockout mice led many to conclude that occludin is not an important protein. Occludin gene knockout mice do, however, have significant defects, including chronic gastritis, hearing loss, brain clarifications, male infertility, and failure of females to adequately suckle pups 44, 45. These abnormalities can all be linked to epithelial and endothelial barrier dysfunction, consistent with the potential role of occludin as a regulator of barrier function. Occludin may, therefore, be a regulator, rather than requisite structural component, of tight junctions.
Consistent with a regulatory function, occludin overexpression enhances epithelial barrier function in vitro.46, 47 This may correlate with the disruption of tight junction strand structure in MDCK II cells lacking both tricellulin and occludin.48 In vivo, TNF-α–induced, myosin light chain kinase-mediated barrier loss is associate with progressive increases in numbers of intracellular occludin vesicles (Fig. 1A) that were accompanied by near complete occludin depletion of tight junction segments (Fig. 1B),49 Transgenic occludin overexpression prevented occludin depletion (Fig. 1C) and attenuated barrier loss induced by TNF-α (Fig. 1D).49 Quantitative electron microscopy demonstrated that occludin internalization was accompanied by the appearance of a small population of vesicles that increased in size over time (Fig. 1E) and contained both occludin and caveolin-1.49 Consistent with an essential role of caveolar endocytosis in TNF-α–induced, occludin-dependent barrier loss, TNF-α was unable to trigger occludin internalization and barrier loss in Cav1−/− mice (Fig. 1F).49 Further study using in vitro models showed that TNF-α–induced occludin endocytosis requires occludin interactions with ZO-1.40
Figure 1.
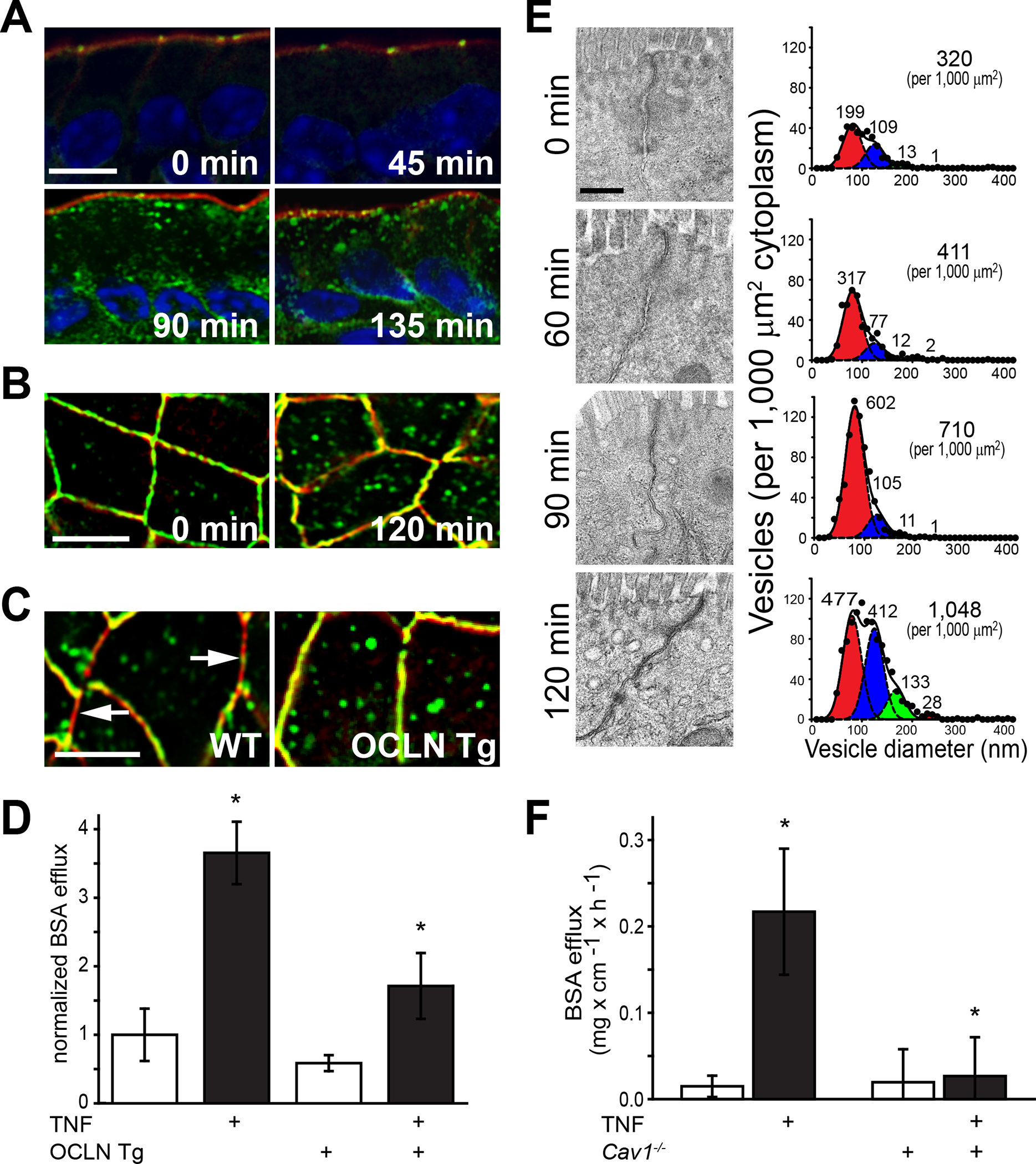
Occludin endocytosis mediates TNF-α–induced tight junction regulation in vivo. (A) Wild-type (WT) mice received TNF-α by i.p. injection and jejunal segments were harvested and stained for occludin (green), F-actin (red), and nuclei (blue) at designated times. Note the onset of occludin endocytosis at 90 min after TNF-α administration. Bar, 10 μm. (B) When viewed en face, it is clear that, focally, TNF-α induced complete depletion of tight junction-associated occludin. Bar, 10 μm. (C) Intestinal epithelial-specific occludin overexpression (OCLN Tg) prevents depletion of tight junction-associated occludin at 120 min after TNF-α administration. Compare to areas of perijunctional F-actin (red) that lack occludin (green) in WT mice (arrows). Bar, 10 μm. (D) In vivo perfusion assays show that TNF-α–induced paracellular leakage of BSA from the bloodstream to the intestinal lumen is markedly reduced by transgenic occludin overexpression. (E) Electron micrographs of jejunum, morphometric analyses, and Gaussian fits show a marked increase in numbers of 80 nm cytoplasmic vesicles 90 min after TNF-α injection. The decrease in 80 nm cytoplasmic vesicle numbers at 120 min coincides with increase numbers of larger vesicles, suggesting endosomal maturation. Bar, 500 nm. (F) In vivo perfusion data show that paracellular BSA leakage is not increased by TNF-α in caveolin-1 gene knockout (Cav1−/−) mice. Originally published in Marchiando et al.49
In vitro occludin depletion by shRNA knockdown increased leak pathway flux of macromolecules with diameters up to ~125 Å across tight junctions in a manner similar to TNF-α–induced occludin removal from tight junctions.40, 50 Consistent with this, we have detected modest leak pathway permeability increases in intestinal epithelial-specific occludin knockout mice using a multiprobe gavage assay.51 Although similar changes were not detected in Ussing chamber studies of isolated intestine from occludin knockout mice,44, 52 this may reflect the small magnitude of the changes and variability in macromolecular flux across mouse intestine mounted in Ussing chambers.
The possibility that posttranslational modifications of occludin itself contribute to TNF-induced endocytosis has not been explored. However, VEGF-induced internalization of endothelial occludin requires phosphorylation at S490 and consequent ubiquitination. Some data suggest that interactions between ubiquitinated sites and Epsin-1 or Eps15 drive occludin endocytosis.53
Many other occludin phosphorylation sites are concentrated in a phosphorylation hotspot between Y398–S408 that is targeted by PKCη, PKCζ, Src, and CK2.54–56 These residues are located in an unstructured region that may interact with and modify tertiary structure of the α-helices41, 57–60 and occludin behaviors. For example, PKCη-mediated phosphorylation of T403 and T404 appears to promote occludin recruitment to tight junctions.61
Src-mediated phosphorylation of occludin Y398 and Y402 or CK2-mediated phosphorylation of occludin S408 have both been reported to reduce ZO-1 binding.30, 62 Despite this similarity, the impact and functional outcomes of these phosphorylation events are unique. Oxidative stress-induced Src-mediated Y398 and Y402 phosphorylation destabilizes tight junctions; expression of phosphomimetic occludinY398D/Y402D both delayed tight junction assembly and sensitized monolayers to oxidant-induced barrier loss.63, 64 The observation that occludinY398D/Y402D or nonphosphorylatable occludinY398A/Y402A expression similarly reduced the occludin mobile fraction and limited tight junction disruption by Ca2+ depletion suggests that dynamic phosphorylation and dephosphorylation of these residues may be critical to occludin anchoring.61
In contrast to Y398 and Y402, occludin S408 appears to be constitutively phosphorylated by CK2 at steady-state.30 S408 dephosphorylation enhanced occludin binding to ZO-1 and, in turn, ZO-1 binding to claudin-2.30 This increased TER by disrupting claudin-2 channels and reducing paracellular Na+ flux in vitro.30 In vivo studies have shown that CK2 inhibition also blocks claudin-2 channel activity in vivo and that this approach to claudin-2 channel inactivation attenuates progression of experimental inflammatory bowel disease.65 Occludin–ZO-1 interactions that are modulated by occludin phosphorylation are, therefore, significant regulators of barrier function and potential therapeutic targets.
In contrast to occludin, ZO-1 gene (Tjp1) knockout results in an embryonic lethal phenotype.66, 67 This has prevented in vivo analyses of ZO-1–deficient epithelia. To overcome this, we recently generated mice with intestinal epithelial-specific ZO-1 gene knockout.68 Surprisingly, the mice were healthy and born in expected Mendelian ratios. Similar to in vitro studies of ZO-1–deficient epithelial cells,31, 69, 70 there was a mild increase in macromolecular (leak pathway) permeability. Although not studied in the intestinal epithelial-specific ZO-1 gene knockout mice, in vitro data suggest that the role of ZO-1 in creating a barrier to macromolecular flux requires dynamic interactions with F-actin that can be regulated by myosin light chain kinase and depend, at least in part, on the ZO-1 ABR.31, 69, 70 This contrasts with the roles of ZO-1 PDZ1 and GuK domains, which mediate interactions with claudin and occludin, respectively.30
Noncanonical occludin contributions to intercellular interactions
Shortly after its discovery, occludin was found to enhance intercellular adhesion.71 This was initially taken as evidence of a role for occludin in tight junction assembly and paracellular barrier function. However, homotypic interactions may allow occludin to direct aPKC–PAR3 and PATJ assembly at the leading edge to orient epithelial migration in vitro.72 Conversely, some data indicate that occludin suppresses migration and invasion of tumor cells.73 Unfortunately, no subsequent studies have reconciled these potentially contradictory results, and conclusions based on these data must, therefore, be considered speculative.
Intraepithelial lymphocyte migration represents an additional process that occludin-mediated intercellular adhesion may facilitate. Although primarily expressed in epithelial and endothelial cells, small quantities of occludin are also present in γδ T cells. A series of in vitro and in vivo analyses have shown that occludin promotes intraepithelial lymphocyte migration.74 Occludin expression on both epithelial and γδ T cells contributes to this process, suggesting that homotypic occludin interactions promote direct interactions between these cell types.74, 75 Consistent with this, a dense ring of occludin accumulates around intraepithelial γδ T lymphocytes in vivo.74
In contrast to γδ T cells, whose migration is limited to the intestinal epithelium and lamina propria,74 neutrophils are able to traverse paracellular junctions, thereby crossing the epithelium and accumulating within the lumen to form crypt abscesses. This process requires dynamic interactions between neutrophils and tight junction proteins.76 Interestingly, disruption of the free N-terminal cytoplasmic domain of occludin enhanced transepithelial neutrophil migration, but deletion of the long C-terminal tail was without effect.77 This could suggest that tight junction-associated occludin restricts neutrophil transmigration. That would, however, not explain how deletion of 13 residues within the first extracellular loop of occludin increases TER and reduces neutrophil transmigration.77 It is also difficult to integrate these observations with reports that a peptide derived from the second extracellular loop enhanced neutrophil transmigration across endothelial monolayers.78 Further elucidation of these processes might benefit from comparisons between migration of neutrophils, which do not express occludin, and γδ T cells. For example, do the occludin mutants from the second extracellular loop peptide that modulates neutrophil transmigration have the same effects on intraepithelial migration of γδ T cells? It would also be of interest to know if epithelial occludin downregulation and increased neutrophil transmigration in inflammatory bowel disease are related functionally.79–81
Occludin as a cofactor in viral entry
Extracellular domains of tight junction proteins, including occludin, are typically sequestered within the protected paracellular space and are inaccessible to apical materials, including viruses. However, tight junction proteins are redistributed along lateral membranes and exposed to the intestinal lumen during epithelial extrusion.82, 83 The resulting transient unmasking of these proteins may explain, evolutionarily, why several tight junction proteins have been identified as viral receptors.84 Among these, the immunoglobulin domain-containing proteins JAM-A and CAR stand out as receptors for reovirus and retrovirus85, 86 or coxsackievirus and adenovirus,87–89 respectively. Similarly, claudin-1 serves as a viral receptor for HCV and dengue virus.90, 91 Occludin contributes to viral entry as an essential co-receptor for HCV and coxsackievirus entry.89, 92 This may reflect the ability of occludin to direct internalization via caveolar endocytosis in response to cytoskeletal and immune stimuli.49, 88, 89, 93
Occludin as an organizer of neoplastic epithelial architecture
One characteristic of neoplastic gastrointestinal epithelium is loss of the normally well-organized monolayer. This can be modeled in vitro by expressing oncogenic proteins, such as constitutively active c-RAF, in nonneoplastic epithelial cell lines.94–96 For example, c-RAF-mediated transformation of rat parotid epithelial cells activated MEK-ERK signaling, enabled anchorage-independent growth, and converted the growth pattern on semi-permeable supports from high TER monolayers to low resistance multilayered epithelia.94 These changes were accompanied by reduced expression of occludin and claudin-1 and redistribution of ZO-1 and E-cadherin.94 Remarkably, forced expression of occludin triggered claudin-1 re-expression, normalized distributions of ZO-1 and E-cadherin, suppressed anchorage-independent growth, restored monolayer growth morphology, and partially corrected TER.94 Further study showed that the second extracellular loop of occludin as well as the N- and C-terminal cytoplasmic tails were required for this reversal of c-RAF–mediated transformation.97 Activation of the epithelial to mesenchymal transformation-promoting transcription factor SLUG was responsible for suppression of the occludin promoter, and siRNA-mediated SLUG inhibition was sufficient to prevent occludin downregulation in c-RAF transformed cells.98 Although It has not been studied, this suggests that either SLUG knockdown or forced occludin expression might also inhibit the MEK–ERK signaling cascade that directs c-RAF–mediated transformation. Thus, despite many remaining questions, these data demonstrate the complex interaction between tight junction proteins and signal transduction pathways that are not typically considered to be related to barrier function.
Occludin regulates epithelial survival
Occludin knockdown in MDCK cells did not significantly affect steady-state TER but did cause modest increases in paracellular permeability of organic cations.50 The observations that cholesterol depletion neither activated rho nor reduced TER in occludin knockdown monolayers suggest that occludin may play an important role in regulating the composition of tight junction membrane microdomains. Curiously, occludin knockdown also limited extrusion of apoptotic cells from the monolayer.50 Subsequent studies suggested that occludin enhances mitochondrial-mediated, i.e., intrinsic pathway, apoptosis, possibly by promoting Ca2+ release from the endoplasmic reticulum, in hepatocellular carcinoma cell lines.73 Separate in vitro studies showed that a short sequence within the occludin second extracellular loop promoted extrinsic pathway of apoptosis.99, 100 Finally, ex vivo analyses show that mammary epithelial cells from occludin knockout mice are resistant to apoptosis induced by disruption of claudin-mediated interactions.99 Thus, scattered in vitro data indicate that occludin may regulate epithelial apoptosis and, conversely, survival.
The conclusion that occludin regulates epithelial survival seems inconsistent with the absence of spontaneous disease in universal occludin knockout mice. Moreover, intestinal epithelial-specific Ocln knockout mice lack nearly all of the abnormalities detected in universal Ocln knockout mice.101 This does not, however, exclude an important role for intestinal epithelial occludin as, for example, an essential component of responses to external challenges. To assess the potential contributions of intestinal epithelial occludin to exogenous stressors, occludin knockout mice were challenged with DSS, a form of chemical colitis that induces massive epithelial damage. Surprisingly, both universal and intestinal epithelial-specific occludin knockout mice were protected from DSS-induced mucosal damage, colitis, and systemic features of disease, including weight loss (Fig. 2A) and epithelial apoptosis (Fig. 2B).101 The observation that transgenic occludin expression within intestinal epithelium fully restored disease in universal occludin knockout mice indicates that DSS-resistance was entirely due to occludin loss in intestinal epithelial cells rather than other cell types, such as endothelial cells or intraepithelial γδ T cells (Fig. 2A). Moreover, this protection was not limited to DSS-induced colitis, as intestinal epithelial Ocln knockout mice were also resistant to TNBS-induced chemical colitis.101
Figure 2.
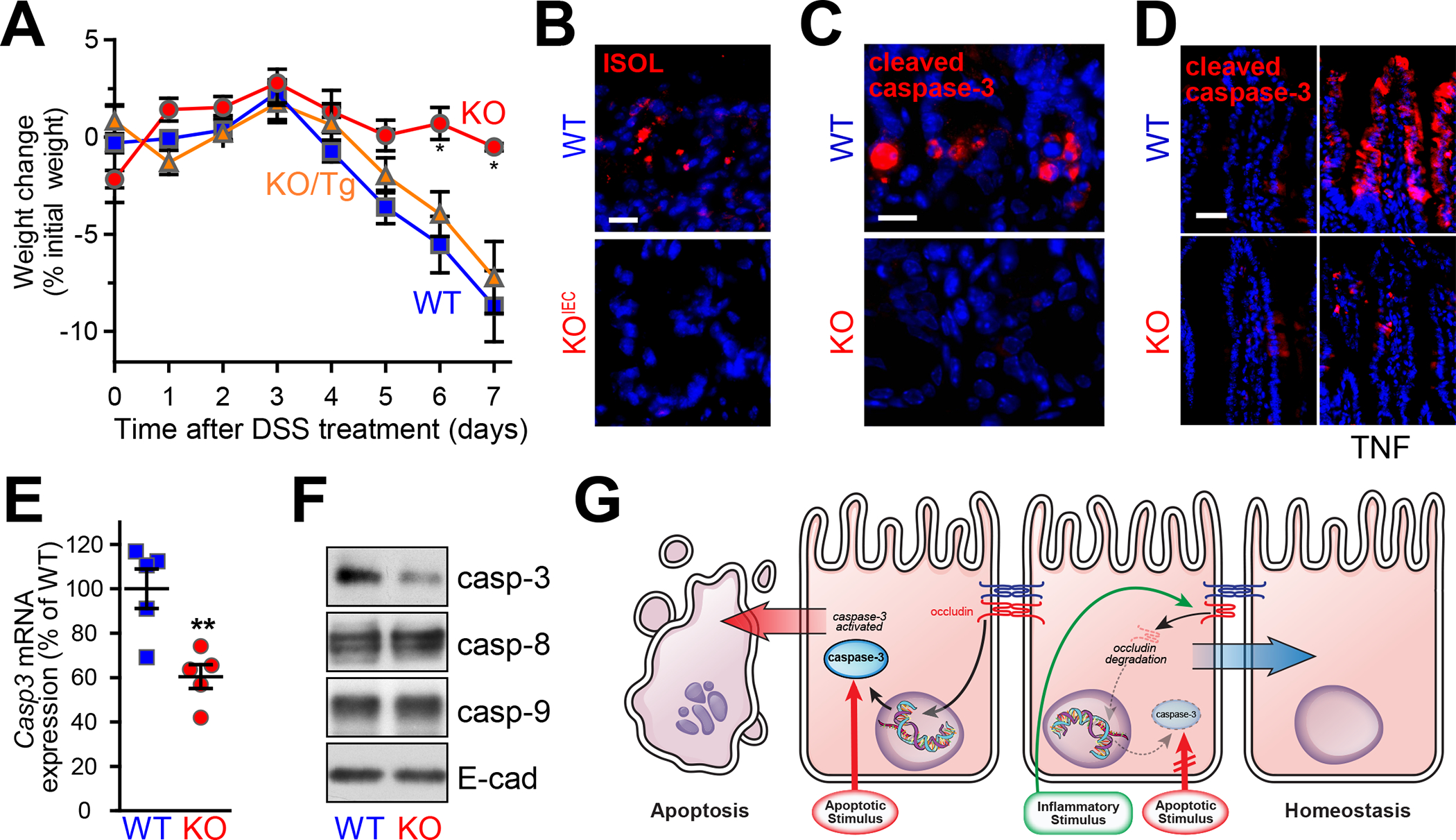
Occludin expression confers colitis sensitivity by enhancing caspase-3 expression. (A) Occludin gene knockout (KO) attenuates DSS-induced weight loss. Sensitivity of Ocln knockout mice is restored to that of wild-type mice (WT) by transgenic intestinal epithelial occludin expression (KO/Tg). (B) Apoptotic ISOL-positive cells (red) numbers are increased after DSS challenge in WT, but not intestinal epithelial–specific Ocln knockout (KOIEC) mice. Nuclei are shown in blue for reference. Bar = 20 μm. (C) 5-Fluoruracil induces epithelial apoptosis as detected by cleaved caspase-3 staining (red) in WT, but not occludin KO, mice. Bar = 20 μm. (D) TNF-α induces caspase-3 cleavage and apoptosis in WT, but not Ocln KO, mice. Bar = 50 μm. (E) Quantitative qRT-PCR demonstrates reduced Casp3 mRNA in jejunal epithelial cells from occludin KO, relative to WT, mice. (F) Caspase-3 protein expression is reduced in jejunal epithelial cells isolated from Ocln KO mice. Caspase-8, caspase-9, and E-cadherin expression are not affected by occludin deletion. (G) Occludin promotes apoptosis by enhancing caspase-3 transcription, suggesting that occludin downregulation in inflammatory conditions may confer apoptotic resistance and promote mucosal homeostasis. Originally published in Kuo et al.101
Although DSS and TNBS administration are useful colitis models, the means by which damage occurs are neither well-established nor relevant to human disease. Nevertheless, analysis of these models showed reduced numbers of apoptotic, cleaved caspase-3 positive intestinal epithelial cells in the absence of occludin expression. To better define how apoptotic resistance was induced by occludin deletion, mice were challenged with 5-fluorouracil (Fig. 2C), a chemotherapeutic nucleoside that disrupts DNA replication and triggers intrinsic pathway apoptosis or TNF-α (Fig. 2D), a classic activator of extrinsic pathway apoptosis. Intestinal epithelial occludin knockout conferred resistance to both intrinsic and extrinsic pathway apoptosis, suggesting that occludin deletion occurred downstream of mitochondrial apoptotic activation. Consistent with this, intestinal epithelial occludin deletion reduced caspase-3 transcription (Fig. 2E) and protein expression (Fig. 2F) in vivo and in vitro.101
Intestinal epithelial occludin deletion only reduced caspase-3 expression by ~50%, raising concerns that some other process might also contribute to the apoptotic resistance of occludin-deficient epithelial cells. However, this idea was refuted by analyses of mice in which one allele of Casp3 was deleted. Similar to intestinal epithelial occludin knockout, caspase-3 protein expression was reduced ~50% in Casp3+/− mice, and this was sufficient to prevent both DSS and TNF-α–induced intestinal epithelial apoptosis in vivo.101 Therefore, the protection from apoptosis afforded by occludin deletion can be fully explained by the reduction in caspase-3 expression.
In vitro studies have shown that occludin expression is reduced by TNF-α. This raises the possibility that occludin downregulation in response to low-grade inflammatory stimuli may protect epithelial cells from more severe stressors. To test this hypothesis, wild-type and occluding-knockdown Caco-2 cells were treated with very low concentrations of TNF-α (0.5 ng/ml). This did not induce apoptosis but did reduce expression of both occludin and caspase-3 by ~50% in wild-type Caco-2.101 Low-dose TNF-α treatment did not affect viability or caspase-3 expression of occluding-knockdown Caco-2 cells. Vehicle and low-dose TNF-α–treated cells were subsequently challenged with either staurosporine, an activator of intrinsic pathway apoptosis, or high-dose TNF-α and cycloheximide. The low-dose TNF-α pretreatment reduced apoptosis of wild-type cells in response to either stimulus to levels comparable to occludin knockdown cells. In contrast, low-dose TNF-α pretreatment did not affect apoptotic sensitivity of occludin knockdown cells. Thus, occludin downregulation may be an adaptive mechanism that limits apoptosis under inflammatory conditions (Fig. 2G).
To test the idea that occludin downregulation might lead to reduced caspase-3 expression in human disease, quantitative immunostains were used to assess expression of both proteins in small intestinal epithelia of Crohn’s disease patients and healthy subjects (Fig. 3A) as well as colonic epithelia of ulcerative colitis patients and healthy controls. As expected, occludin expression was reduced in both sets of patients relative to the healthy control groups. Intestinal epithelial caspase-3 expression was also reduced in epithelia within patient biopsies (Fig. 3B). Moreover, the degree of caspase-3 downregulation correlated directly with that of occludin (Fig. 3C).101 Occludin is therefore a critical regulator of epithelial survival whose downregulation in inflammatory disease may represent an adaptive response to limit tissue injury.
Figure 3.
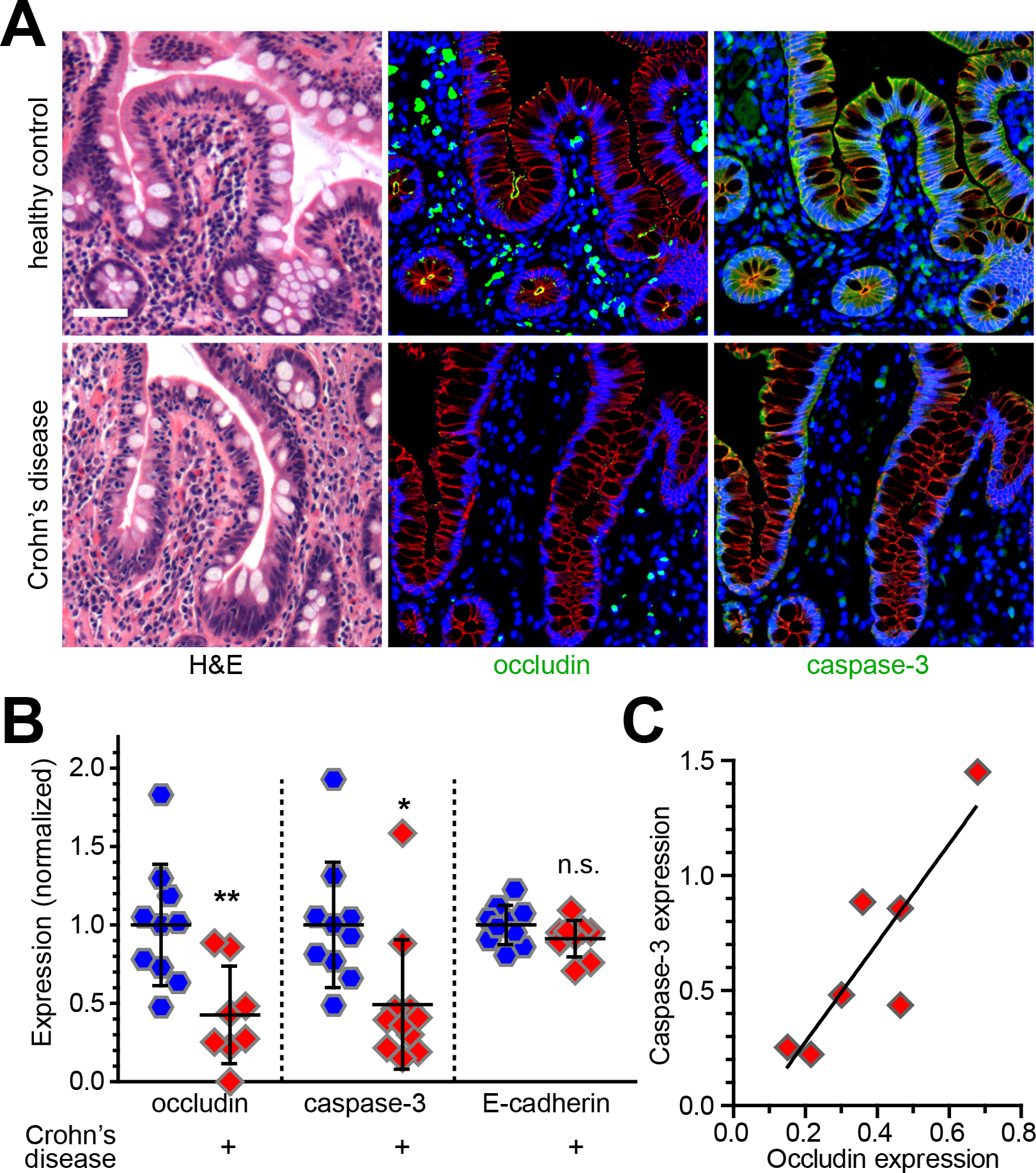
Occludin downregulation in inflammatory bowel disease is accompanied by reduced caspase-3 expression. (A) Hematoxylin-eosin (H&E) and immunofluorescent staining of occludin (green) and caspase-3 (green) in ileal biopsies from healthy control subjects and Crohn’s disease patients. E-cadherin (red) and nuclei (blue) are shown for reference. Bar = 50 μm. (B) Quantitative morphometry of occludin, caspase-3, and E-cadherin staining intensity within the intestinal epithelium. (C) Caspase-3 expression correlates with occludin expression (r2 = 0.76). Originally published in Kuo et al.101
ZO-1 regulates apical epithelial structure
ZO-1 has numerous binding partners,24, 102, 103 the diversity of which likely explains the embryonic lethality of Tjp1 knockout mice as well as the disruption of junction-associated F-actin in C. elegans embryos lacking the ZO-1 orthologue.66, 104 In cultured epithelial cell lines, loss of both ZO-1 and its cousin ZO-2 prevents recruitment of claudin proteins to the tight junction27 and markedly impairs barrier function.105, 106 In these ZO-1/ZO-2–deficient cell lines, barrier function can be rescued by expression of full-length ZO-1, and truncated ZO-1 consisting only of the N-terminal half of the protein is sufficient to restore claudin recruitment.106 ZO-1 and ZO-2 deletion also disturbs apical cytoskeletal organization, and this also appears to be rescued by an N-terminal truncation mutant of ZO-1.106, 107 Thus, although the studies were performed in cell lines lacking both ZO-1 and ZO-2, the phenotypes observed may be primarily linked to ZO-1 loss.108
Consistent with a dominant role, ZO-1 loss with retained ZO-2 expression resulted in disruption of microvillous and apical membrane structures in vitro and in vivo.109 This was associated with hypercontraction of cortical actin that led to formation of a dome-like distensions of the apical membrane in vivo (Fig. 4A,B) and in vitro (Fig. 4C).109 Rescue experiments using ZO-1 deletion mutants showed that the U5 GuK region, specifically the U5 domain, is essential for ZO-1–mediated maintenance of apical membrane and microvillous structure. This is, perhaps, surprising, as no protein interactions with U5 domain alone have been described. However, the U5 domain does seem to be the primary region required for tight junction localization of ZO-1. Further study in cells lacking both ZO-1 and ZO-2 showed similar results except that ZO-1 lacking the PDZ1 domain was sufficient to rescue structure in ZO-1 knockdown, but not double knockdown cells. This suggests that interactions with claudins, or other proteins that bind to PDZ1, are essential for ZO-1 effects on apical structure and that, in ZO-2-sufficient cells, ZO-1ΔPDZ1 heterodimerization with ZO-2 allows interactions to occur via the ZO-2 PDZ1 domain.109
Figure 4.
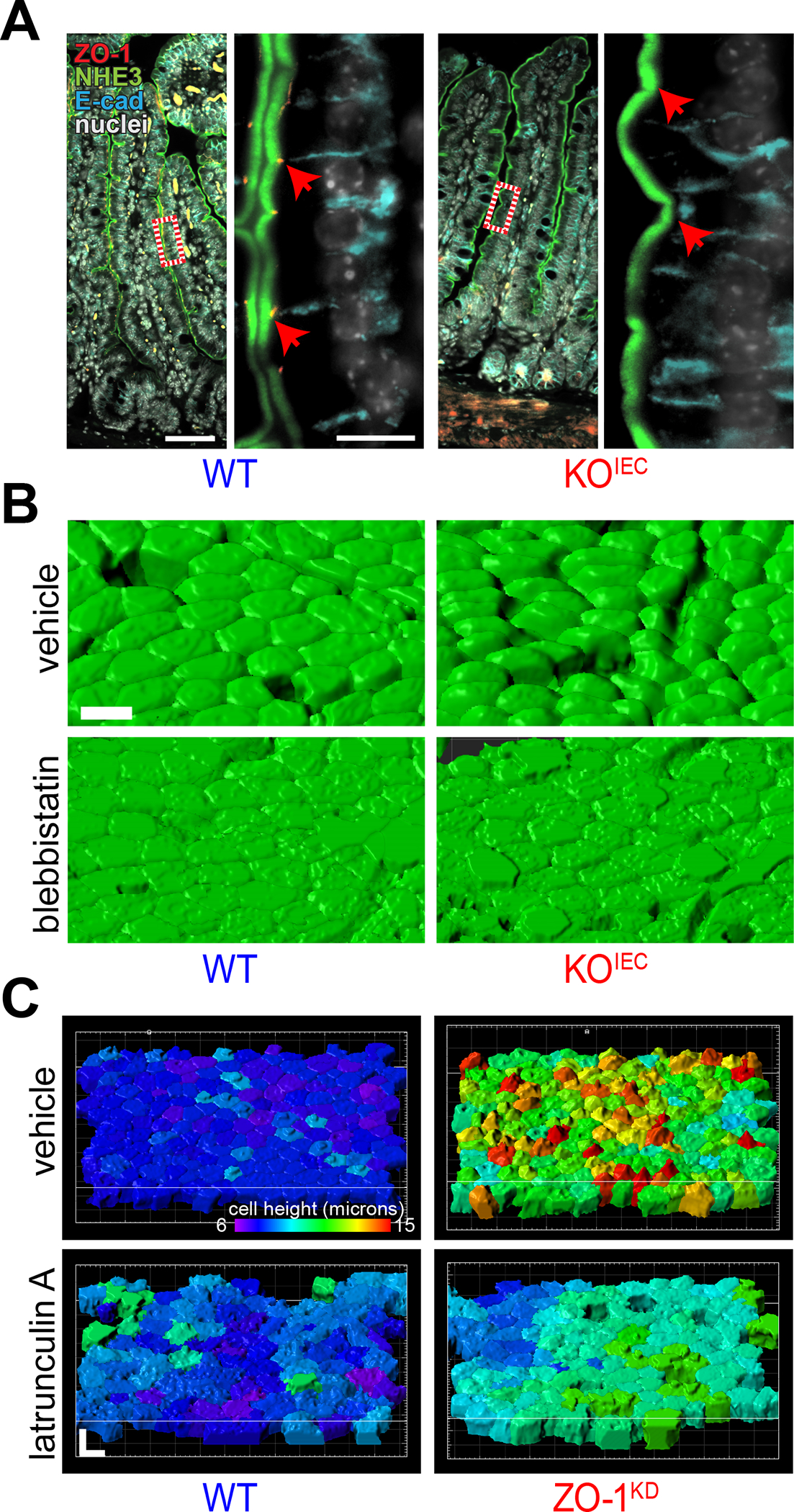
ZO-1 regulates cortical actomyosin and epithelial structure. (A) The smooth apical surface normally seen in wild-type (WT) epithelial monolayers is disrupted by apical distensions in jejunum from intestinal epithelial-specific ZO-1 gene knockout (KOIEC) mice. NHE3 (green), ZO-1 (red), E-cadherin (cyan), and nuclei (white). Bars = 100 μm (low magnification) and 10 μm (high magnification). (B) 3D reconstructions of F-actin–labeled epithelial surfaces highlight the apical distensions of KOIEC epithelium and show that blebbistatin, a myosin II inhibitor, normalizes apical contours. (C) The effect of apical distensions induced by in vitro ZO-1 knockdown (ZO-1KD) is emphasized in reconstructions by pseudocoloring each cell according to height (violet = 6 μm, red = 15 μm). Similar to the in vivo effect of blebbistatin, in vitro application of latrunculin A, which causes F-actin disruption, normalizes the height of ZO-1KD epithelial cells. Bars = 10 μm. Originally published in Odenwald et al.109
Further study of the actin-based structural abnormalities induced by ZO-1 gene deletion showed that inhibition of myosin II ATPase activity and actomyosin contraction or F-actin depolymerization were sufficient to restore apical structure in vivo (Fig. 4B) and in vitro (Fig. 4C).109 In contrast, ROCK inhibition was insufficient to restore structure. Consistent with this, in vitro atomic force microscopy studies showed that deletion of ZO-1 and ZO-2 markedly enhanced apical tension and viscosity and that apical tension could be restored to normal levels by myosin II ATPase, but not ROCK, inhibition.110 In contrast, viscosity could be restored by inhibition of either myosin II ATPase or ROCK, suggesting that apical cytoskeletal tension is regulated by processes distinct from those that modulate viscosity.110
ZO-1 regulates lumen formation in vitro
When cultured in 3D, ZO-1–knockdown MDCK cells failed to form typical single lumen cysts (Fig. 5A).108 Lumen development was delayed and, ultimately, was characterized by septae that divide the lumen into multiple compartments. A transient multilumen stage is typically seen when MDCK cells are grown in collagen gels, but this ultimately resolves. The inability of ZO-1–deficient cells to form single lumen cysts does not, however, represent failure of progression, as results were similar in Matrigel, where there is not an intermediate multilumen phase. The development of septae in cysts formed by ZO-1–deficient cells was, in part, due to impaired contact inhibition and increased proliferation of ZO-1–knockdown cells at later, but not earlier, times after plating. This could be rescued, to a limited degree, by culture in reduced serum media in which proliferation is reduced.108
Figure 5.
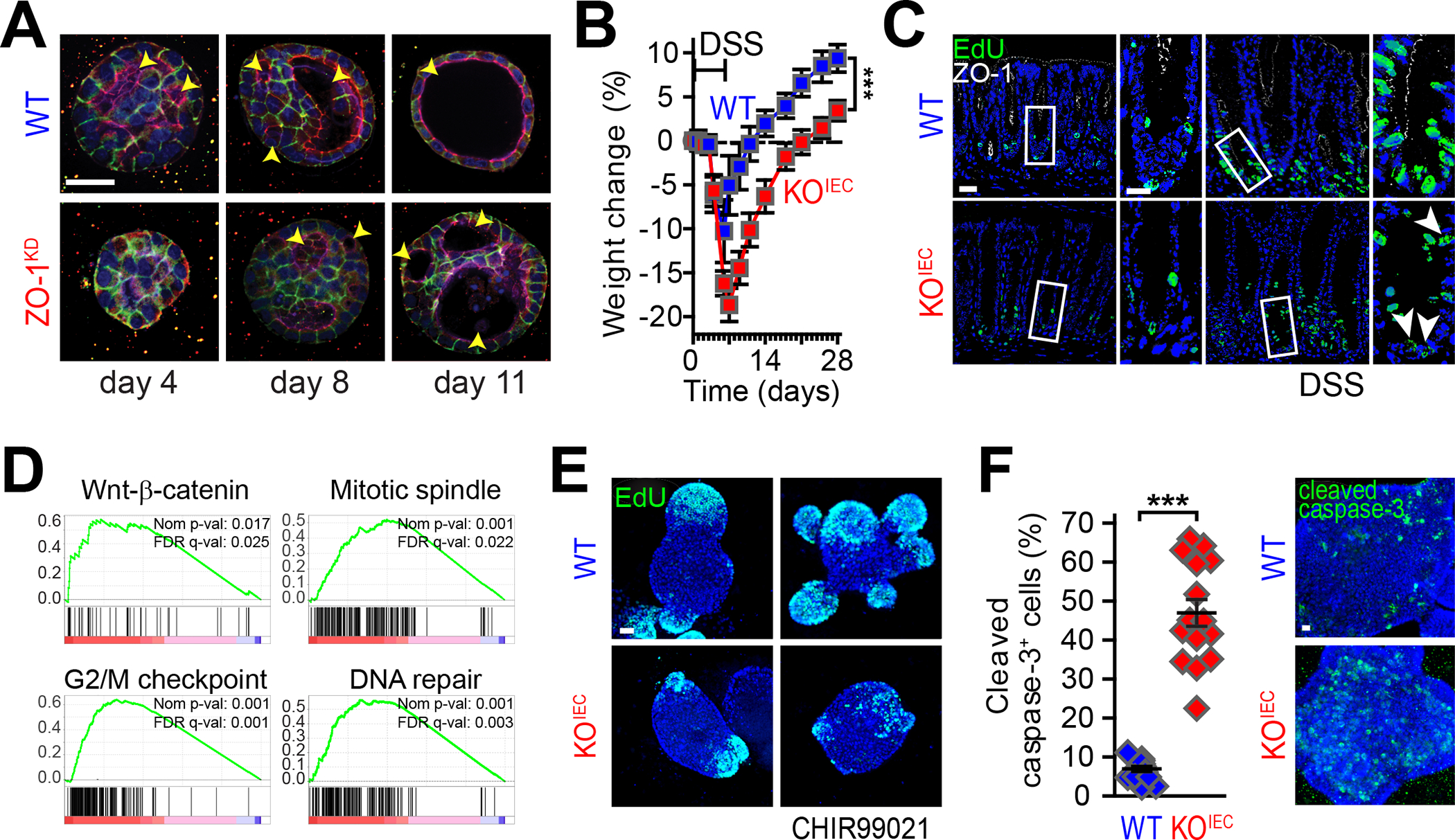
ZO-1 directs epithelial growth and repair. (A) WT and ZO-1 knockdown (ZO-1KD) MDCK epithelial cells were stained for ZO-1 (red), E-cadherin (green), F-actin (purple), and nuclei (blue) at days 4, 8, and 11 after plating in collagen gels. Lumen formation (arrows) is delayed in ZO-1KD epithelial cells, which also fail to resolve the normally transient multilumen phase to form single-lumen cysts. Bars = 20 μm (low magnification) and 10 μm (high magnification). (B) Wildtype (WT, blue) and intestinal epithelial-specific ZO-1 gene knockout (KOIEC, red) mice were treated with DSS for 6 days. Weight loss is exaggerated and recovery is delayed in KOIEC mice. Bar = 50 μm. (C) One day after discontinuing DSS treatment (day 7), mice were injected with EdU (green) and sacrificed 2 hours later in order to track proliferating cells. Despite significant damage, the proliferative response of KOIEC mice is defective. (D) Gene set enrichment analysis (GSEA) of RNA-sequencing data from jejunal epithelia harvested 2 days after induction of cytokine storm (by anti-CD3 antibody injection). Activation of WNT–β-catenin, mitotic spindle, G2/M check point, and DNA repair pathways is deficient in KOIEC mice. (E) Colonoids from WT and KOIEC mice were treated with the GSK3β inhibitor CHIR99021 to potentiate WNT signaling and fixed 2 hours after EdU (green) addition. Numbers of EdU-labeled cells increased markedly in WT, but not KOIEC, colonoids. Bar = 20 μm (F) CHIR99021 induces extensive apoptosis in KOIEC, but not WT, colonoids. Bar = 20 μm. Panel A: Originally published in Odenwald et al.108 Panels B-F: Originally published in Kuo et al.68
Similar to apical structure, single lumen cyst formation was rescued by expression of full-length ZO-1 but not ZO-1ΔU5-GuK. The observation that ZO-1ΔABR was able to rescue single lumen cyst formation when cells were grown in Matrigel, but not when cells were grown in collagen gels, suggests that the strong polarity cues provided by Matrigel were sufficient to compensate for the absence of morphogenetic signals provided by ZO-1–F-actin interactions. 108
Three proteins, shroom2, α-catenin, and occludin, are known to interact with the U5 GuK region of ZO-1. Knockdown of shroom2 had no effect on the ability of MDCK cells to develop single lumen cysts. α-Catenin–knockdown cells took on bizarre, angulated, sheet-like structures but were able to form single-lumen cysts.108 In contrast, occludin knockdown resulted in multi-lumen cysts. This could be rescued by expression of full-length occludin, but not by occludin lacking the C-terminal OCEL domain that mediates interactions with ZO-1.
ZO-1 regulates epithelial proliferation, mitotic spindle orientation, and mucosal repair
Despite a modest increase in leak pathway permeability, mice with intestinal epithelial-specific ZO-1 gene deletion reproduced, developed, and gained weight normally without spontaneous disease.68 Thus, ZO-1 is not essential for intestinal homeostasis and barrier function. However, when stressed by either chemically-induced or immune-mediated intestinal injury, disease was far more severe and recovery was incomplete in intestinal epithelial ZO-1 gene knockout mice (Fig. 5B). This failure of repair correlated with reduced proliferation of ZO-1 gene knockout intestinal epithelial cells in vivo (Fig. 5C), which was surprising, as previous studies of MDCK cell lines and embryonic stem cells demonstrated enhanced proliferation in the absence of ZO-1.68, 111, 112 System based analysis of RNAseq data demonstrated that, in response to injury, ZO-1–deficient intestinal epithelial cells were defective in WNT–β-catenin signaling, mitotic spindle function, DNA repair, and progression through the G2/M checkpoint (Fig. 5D). Consistent with this, proliferation and DNA synthesis were markedly reduced in ZO-1 gene knockout organoids (Fig. 5E). Potentiation of WNT signaling by inhibiting GSK3β did not expand numbers of proliferating cells (Fig. 5E) but caused a ~10-fold increase in numbers of cleaved-caspase 3–positive apoptotic cells (Fig. 5F).68
To determine how enhanced WNT signaling leads to increased apoptosis, cell division was studied by live imaging.68 ZO-1 gene knockout resulted in aberrant mitotic spindle orientation (Fig. 6A). This resulted in one daughter cell losing contact with the basement membrane and undergoing anoikis, a form of anchorage-independent apoptosis. Thus, ZO-1 gene loss resulted in abortive proliferation that failed to generate additional viable cells.68 Further studies showed that mitotic spindle orientation of ZO-1–deficient intestinal epithelial cells was disturbed in vivo and that ZO-1 associates transiently with spindle poles during mitosis (Fig. 6B). Moreover, close examination identified a very small pool of endogenous ZO-1 that was associated with the centriole. It may be that centriole-associated ZO-1 contributes to WNT signaling (Fig. 6C).
Figure 6.
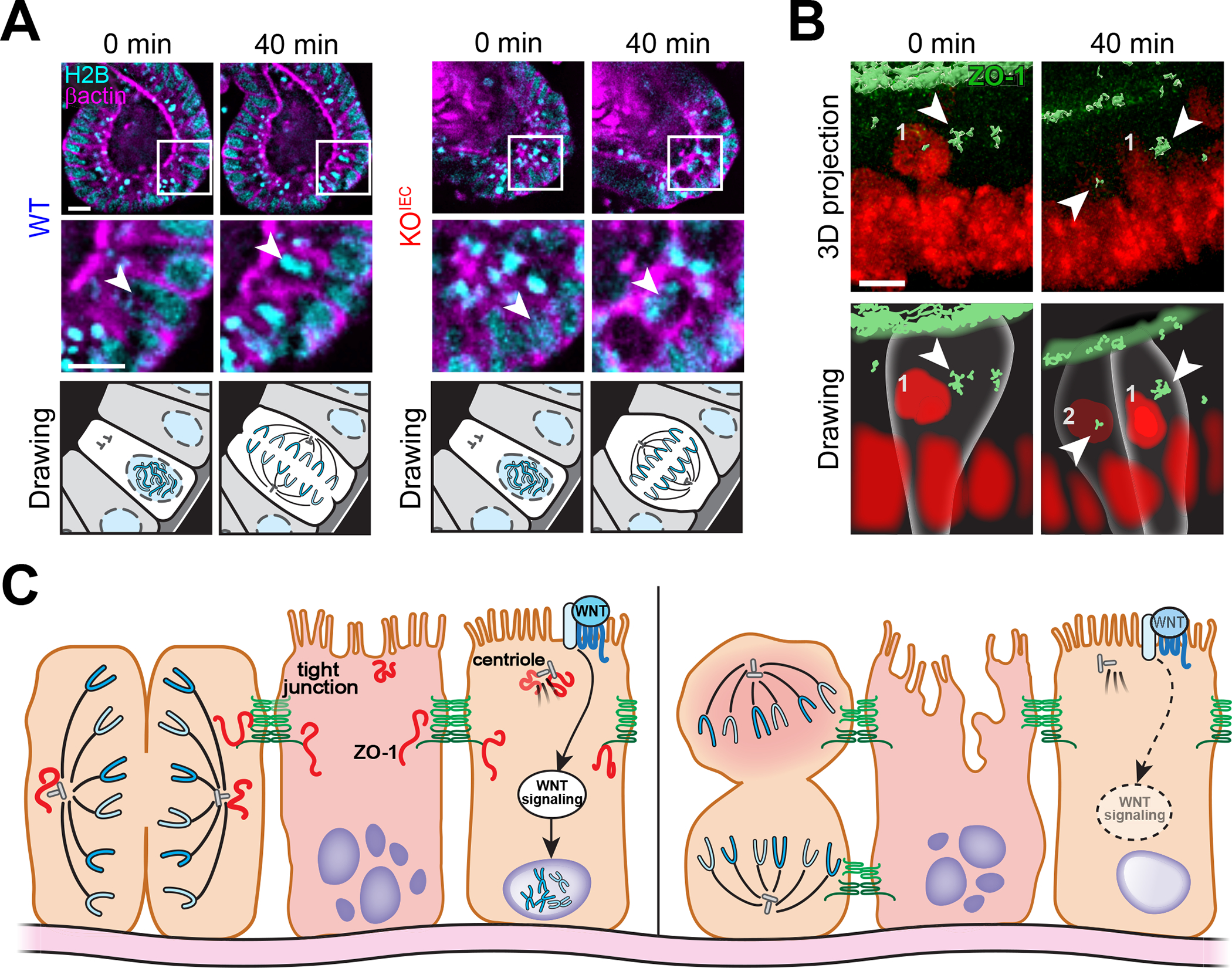
ZO-1 associates with spindle poles facilitating correct mitotic spindle orientation. (A) Live imaging of colonoids expressing EGFP-β-actin (magenta) and H2B-mCherry (cyan) shows that the mitotic spindle (arrows) is correctly oriented perpendicular to the basement membrane in wildtype (WT) but not ZO-1 gene knockout (KOIEC) cells. Drawings are presented to simplify interpretation. Bars = 10 μm. (B) Live imaging of mCherry-ZO-1 (green) expressing colonoids labeled with an intravital DNA stain (red) shows transient ZO-1 association with mitotic spindle poles (arrows). Bar = 5 μm. Drawings are presented to simplify interpretation. (C) Non-canonical functions, including regulation of WNT signaling and mitotic spindle orientation, make ZO-1 critical to mucosal repair. Reduced ZO-1 expression may contribute to ineffective mucosal healing in inflammatory bowel disease. Originally published in Kuo et al.68
In contrast to MDCK cells, neither occludin- nor ZO-1–deficient intestinal organoids formed multilumen cysts. However, similar to organoids, mitotic spindle polarization was defective in MDCK cells lacking either ZO-1 or occludin.108 Consistent with this, studies of immortalized or transformed cultured cells grown in 3D have shown that mitotic spindle misorientation is a common cause of multi-lumen cyst formation. Therefore, one explanation for multilumen cyst formation in immortalized but not non-neoplastic epithelial cells is that immortalized do not undergo anoikis after loss of contact with the basement membrane. Continued growth of these detached cells could result in formation of septae that ultimately create multi-lumen cysts that is prevented in organoids that undergo anoikis. This hypothesis remains to be tested.
Overall, the studies of intestinal epithelial-specific ZO-1 gene knockout mice show that, in the absence of stress, ZO-1 is dispensable for intestinal epithelial function. However, in response to stress, ZO-1 is required for effective WNT–β-catenin signaling, mitotic spindle orientation, and overall epithelial proliferation. These defects may reflect association of ZO-1 with the centriole and mitotic spindle poles. However, they appear to be independent of ZO-1 contributions to tight junction barrier function. Thus, noncanonical, rather than canonical functions are responsible for the severe phenotype of stressed ZO-1 gene knockout mice.
Conclusion
Although occludin and ZO-1 contribute to tight junction barrier function, neither is essential for basal intestinal epithelial function in vivo. However, stress unmasked essential contributions of each of these proteins to processes responsible for epithelial proliferation, repair, and survival. In the case of occludin, Ocln knockout resulted in reduced caspase-3 expression and protection from apoptotic stimuli in vivo and in vitro. Conversely, epithelial cells lacking ZO-1 were markedly impaired in their ability to activate effective proliferation in wound healing due to defects in WNT signaling and mitotic spindle orientation. Importantly, discovery of these noncanonical functions required development of new experiments and models, such as tissue-specific knockout mice, that permit analyses in the complex in vivo milieu. Nevertheless, previous work using reductionist in vitro models, including cell lines, created an essential foundation without which it would have been difficult to interpret vivo results. Future studies should continue to use complementary in vitro and in vivo approaches to solve the many remaining mysteries of tight junction biology.
Acknowledgments
We thank Tiffany S. Davanzo (Slaybaugh Studios) for her beautiful illustrations. This work was supported by National Institutes of Health Grants R01DK61931 (JRT), R01DK68271 (JRT), and P30DK034854 (the Harvard Digestive Disease Center), the National Natural Science Foundation of China Grants 81800464 (LZ) and 82070548 (LZ), Ministry of Science and Technology (Taiwan) Grant 110-2320-B-002-080-MY3 (WTK), Ministry of Education (Taiwan) Grant SPROUT 111L7308 (WTK), and National Taiwan University Hospital Grant 111-S0191 (WTK). All authors contributed to writing and editing of this article. All data are used with permission.
Footnotes
Competing interests
J.R.T. is a founder and shareholder of Thelium Therapeutics and has served is a consultant for Entrinsic, Immunic, and Kallyope.
References
- 1.Anderson JM & Van Itallie CM 2009. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 1: a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Itallie CM & Anderson JM 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol 68: 403–429. [DOI] [PubMed] [Google Scholar]
- 3.Turner JR 2009. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 9: 799–809. [DOI] [PubMed] [Google Scholar]
- 4.Shen L, Weber CR, Raleigh DR, Yu D & Turner JR 2011. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol 73: 283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchiando AM, Graham WV & Turner JR 2010. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 5: 119–144. [DOI] [PubMed] [Google Scholar]
- 6.Farquhar M & Palade G 1963. Junctional complexes in various epithelia. J Cell Biol. 17: 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claude P 1978. Morphological factors influencing transepithelial permeability: A model for the resistance of the zonula occludens. J. Membr. Biol 39: 219–232. [DOI] [PubMed] [Google Scholar]
- 8.Clayburgh DR, Musch MW, Leitges M, Fu YX & Turner JR 2006. Coordinated epithelial nhe3 inhibition and barrier dysfunction are required for tnf-mediated diarrhea in vivo. J. Clin. Invest 116: 2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei L, Solis G, Nguyen MT, Kamat N, Magenheimer L, Zhuo M, Li J, Curry J, McDonough AA, Fields TA, Welch WJ & Yu AS 2016. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J. Clin. Invest 126: 2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atisook K, Carlson S & Madara JL 1990. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am. J. Physiol 258: C77–85. [DOI] [PubMed] [Google Scholar]
- 11.Madara JL & Pappenheimer JR 1987. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol 100: 149–164. [DOI] [PubMed] [Google Scholar]
- 12.Meddings JB & Westergaard H 1989. Intestinal glucose transport using perfused rat jejunum in vivo: Model analysis and derivation of corrected kinetic constants. Clin Sci (Lond). 76: 403–413. [DOI] [PubMed] [Google Scholar]
- 13.Pappenheimer JR & Reiss KZ 1987. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membr. Biol 100: 123–136. [DOI] [PubMed] [Google Scholar]
- 14.Fihn BM, Sjoqvist A & Jodal M 2000. Permeability of the rat small intestinal epithelium along the villus-crypt axis: Effects of glucose transport. Gastroenterol. 119: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 15.Furuse M, Fujita K, Hiiragi T, Fujimoto K & Tsukita S 1998. Claudin-1 and −2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 141: 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitic LL, Van Itallie CM & Anderson JM 2000. Molecular physiology and pathophysiology of tight junctions i. Tight junction structure and function: Lessons from mutant animals and proteins. Am. J. Physiol. - Gastrointest. Liver Physiol 279: G250–254. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A & Tsukita S 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol. 156: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K & Tsukita S 2011. Predicted expansion of the claudin multigene family. FEBS Lett. 585: 606–612. [DOI] [PubMed] [Google Scholar]
- 19.Bleich M & Gunzel D 2017. Physiology, pathophysiology, and clinical impact of claudins. Pflugers Arch. 469: 1–2. [DOI] [PubMed] [Google Scholar]
- 20.Gunzel D & Yu AS 2013. Claudins and the modulation of tight junction permeability. Physiol. Rev 93: 525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukita S, Tanaka H & Tamura A 2019. The claudins: From tight junctions to biological systems. Trends Biochem. Sci 44: 141–152. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson BR, Siliciano JD, Mooseker MS & Goodenough DA 1986. Identification of zo-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 103: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Itallie CM, Aponte A, Tietgens AJ, Gucek M, Fredriksson K & Anderson JM 2013. The n and c termini of zo-1 are surrounded by distinct proteins and functional protein networks. J. Biol. Chem 288: 13775–13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanning AS & Anderson JM 2009. Zonula occludens-1 and −2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci 1165: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouaud F, Sluysmans S, Flinois A, Shah J, Vasileva E & Citi S 2020. Scaffolding proteins of vertebrate apical junctions: Structure, functions and biophysics. Biochim Biophys Acta Biomembr. 1862: 183399. [DOI] [PubMed] [Google Scholar]
- 26.Spadaro D, Le S, Laroche T, Mean I, Jond L, Yan J & Citi S 2017. Tension-dependent stretching activates zo-1 to control the junctional localization of its interactors. Curr. Biol 27: 3783–3795 e3788. [DOI] [PubMed] [Google Scholar]
- 27.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M & Tsukita S 2006. Zo-1 and zo-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 126: 741–754. [DOI] [PubMed] [Google Scholar]
- 28.Itoh M, Furuse M, Morita K, Kubota K, Saitou M & Tsukita S 1999. Direct binding of three tight junction-associated maguks, zo-1, zo-2, and zo-3, with the cooh termini of claudins. J Cell Biol. 147: 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugawara T, Furuse K, Otani T, Wakayama T & Furuse M 2021. Angulin-1 seals tricellular contacts independently of tricellulin and claudins. J Cell Biol. 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L & Turner JR 2011. Occludin s408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 193: 565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Itallie CM, Tietgens AJ, Krystofiak E, Kachar B & Anderson JM 2015. A complex of zo-1 and the bar-domain protein toca-1 regulates actin assembly at the tight junction. Mol. Biol. Cell 26: 2769–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Itallie CM, Tietgens AJ & Anderson JM 2017. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through zo-1. Mol. Biol. Cell 28: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasileva E, Rouaud F, Spadaro D, Huang W, Colom A, Flinois A, Shah J, Dugina V, Chaponnier C, Sluysmans S, Méan I, Jond L, Roux A, Yan J & Citi S 2020. Cingulin unfolds zo-1 and organizes myosin-2b and g-actin to mechanoregulate apical and tight junction membranes. BioRxiv. [Google Scholar]
- 34.Schwayer C, Shamipour S, Pranjic-Ferscha K, Schauer A, Balda M, Tada M, Matter K & Heisenberg CP 2019. Mechanosensation of tight junctions depends on zo-1 phase separation and flow. Cell. 179: 937–952 e918. [DOI] [PubMed] [Google Scholar]
- 35.Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C & Honigmann A 2019. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell. 179: 923–936 e911. [DOI] [PubMed] [Google Scholar]
- 36.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S & Tsukita S 1993. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 123: 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapierre LA, Tuma PL, Navarre J, Goldenring JR & Anderson JM 1999. Vap-33 localizes to both an intracellular vesicle population and with occludin at the tight junction. J. Cell Sci 112: 3723–3732. [DOI] [PubMed] [Google Scholar]
- 38.Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C & Mrsny RJ 2000. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem 275: 29816–29822. [DOI] [PubMed] [Google Scholar]
- 39.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M & Piontek J 2006. On the self-association potential of transmembrane tight junction proteins. Cell. Mol. Life Sci 63: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, Breskin LA, Wu L, Anderson K, Turner JR & Weber CR 2013. Occludin ocel-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell 24: 3056–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Fanning AS, Anderson JM & Lavie A 2005. Structure of the conserved cytoplasmic c-terminal domain of occludin: Identification of the zo-1 binding surface. J. Mol. Biol 352: 151–164. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt A, Utepbergenov DI, Mueller SL, Beyermann M, Schneider-Mergener J, Krause G & Blasig IE 2004. Occludin binds to the sh3-hinge-guk unit of zonula occludens protein 1: Potential mechanism of tight junction regulation. Cell. Mol. Life Sci 61: 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walter JK, Castro V, Voss M, Gast K, Rueckert C, Piontek J & Blasig IE 2009. Redox-sensitivity of the dimerization of occludin. Cell. Mol. Life Sci 66: 3655–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T & Tsukita S 2000. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11: 4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitajiri S, Katsuno T, Sasaki H, Ito J, Furuse M & Tsukita S 2014. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biology open 3: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD & Schneeberger EE 1996. Occludin is a functional component of the tight junction. J. Cell Sci 109 (Pt 9): 2287–2298. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Merzdorf C, Paul DL & Goodenough DA 1997. Cooh terminus of occludin is required for tight junction barrier function in early xenopus embryos. J Cell Biol. 138: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito AC, Higashi T, Fukazawa Y, Otani T, Tauchi M, Higashi AY, Furuse M & Chiba H 2021. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 32: 722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH & Turner JR 2010. Caveolin-1-dependent occludin endocytosis is required for tnf-induced tight junction regulation in vivo. J Cell Biol 189: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD & Schneeberger EE 2005. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. - Cell Physiol 288: C1231–1241. [DOI] [PubMed] [Google Scholar]
- 51.Chanez-Paredes SD, Abtahi S, Kuo WT & Turner JR 2021. Differentiating between tight junction-dependent and tight junction-independent intestinal barrier loss in vivo. Methods Mol. Biol 2367: 249–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S & Fromm M 2005. Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta 1669: 34–42. [DOI] [PubMed] [Google Scholar]
- 53.Murakami T, Felinski EA & Antonetti DA 2009. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem 284: 21036–21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordenonsi M, Mazzon E, De Rigo L, Baraldo S, Meggio F & Citi S 1997. Occludin dephosphorylation in early development of xenopus laevis. J. Cell Sci 110 (Pt 24): 3131–3139. [DOI] [PubMed] [Google Scholar]
- 55.Wong V 1997. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am. J. Physiol 273: C1859–1867. [DOI] [PubMed] [Google Scholar]
- 56.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y & Tsukita S 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 137: 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dorfel MJ & Huber O 2012. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. Journal of biomedicine & biotechnology. 2012: 807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dorfel MJ & Huber O 2012. A phosphorylation hotspot within the occludin c-terminal domain. Ann. N. Y. Acad. Sci 1257: 38–44. [DOI] [PubMed] [Google Scholar]
- 59.Dorfel MJ, Westphal JK & Huber O 2009. Differential phosphorylation of occludin and tricellulin by ck2 and ck1. Ann. N. Y. Acad. Sci 1165: 69–73. [DOI] [PubMed] [Google Scholar]
- 60.Smales C, Ellis M, Baumber R, Hussain N, Desmond H & Staddon JM 2003. Occludin phosphorylation: Identification of an occludin kinase in brain and cell extracts as ck2. FEBS Lett. 545: 161–166. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R & Rao R 2009. Pkc eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc. Natl. Acad. Sci. U.S.A 106: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kale G, Naren AP, Sheth P & Rao RK 2003. Tyrosine phosphorylation of occludin attenuates its interactions with zo-1, zo-2, and zo-3. Biochem. Biophys. Res. Commun 302: 324–329. [DOI] [PubMed] [Google Scholar]
- 63.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM & Rao R 2009. Phosphorylation of tyr-398 and tyr-402 in occludin prevents its interaction with zo-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem 284: 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manda B, Mir H, Gangwar R, Meena AS, Amin S, Shukla PK, Dalal K, Suzuki T & Rao R 2018. Phosphorylation hotspot in the c-terminal domain of occludin regulates the dynamics of epithelial junctional complexes. J. Cell Sci 131: jcs206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raju P, Shashikanth N, Tsai PY, Pongkorpsakol P, Chanez-Paredes S, Steinhagen PR, Kuo WT, Singh G, Tsukita S & Turner JR 2020. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J. Clin. Invest 130: 5197–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S & Tsukita S 2008. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol. Biol. Cell 19: 2465–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phua DC, Xu J, Ali SM, Boey A, Gounko NV & Hunziker W 2014. Zo-1 and zo-2 are required for extra-embryonic endoderm integrity, primitive ectoderm survival and normal cavitation in embryoid bodies derived from mouse embryonic stem cells. PLoS One. 9: e99532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, Abraham C & Turner JR 2021. The tight junction protein zo-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterol. 161: 1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Itallie CM, Fanning AS, Bridges A & Anderson JM 2009. Zo-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 20: 3930–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L & Turner JR 2010. Mlck-dependent exchange and actin binding region-dependent anchoring of zo-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. U.S.A 107: 8237–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Itallie CM & Anderson JM 1997. Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci 110 (Pt 9): 1113–1121. [DOI] [PubMed] [Google Scholar]
- 72.Du D, Xu F, Yu L, Zhang C, Lu X, Yuan H, Huang Q, Zhang F, Bao H, Jia L, Wu X, Zhu X, Zhang X, Zhang Z & Chen Z 2010. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell. 18: 52–63. [DOI] [PubMed] [Google Scholar]
- 73.Gu JM, Lim SO, Park YM & Jung G 2008. A novel splice variant of occludin deleted in exon 9 and its role in cell apoptosis and invasion. FEBS J. 275: 3145–3156. [DOI] [PubMed] [Google Scholar]
- 74.Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI & Turner JR 2012. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc. Natl. Acad. Sci. U.S.A 109: 7097–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edelblum KL, Singh G, Odenwald MA, Lingaraju A, El Bissati K, McLeod R, Sperling AI & Turner JR 2015. Gammadelta intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterol. 148: 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nash S, Stafford J & Madara JL 1987. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest 80: 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huber D, Balda MS & Matter K 2000. Occludin modulates transepithelial migration of neutrophils. J. Biol. Chem 275: 5773–5778. [DOI] [PubMed] [Google Scholar]
- 78.Oshima T, Blaschuk O, Gour B, Symonds M, Elrod JW, Sasaki M, Jackson TH & Alexander JS 2003. Tight junction peptide antagonists enhance neutrophil trans-endothelial chemotaxis. Life Sci 73: 1729–1740. [DOI] [PubMed] [Google Scholar]
- 79.Kucharzik T, Walsh SV, Chen J, Parkos CA & Nusrat A 2001. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol 159: 2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W & Schulzke JD 2005. Interleukin-13 is the key effector th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol. 129: 550–564. [DOI] [PubMed] [Google Scholar]
- 81.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M & Schulzke JD 2007. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active crohn’s disease. Gut. 56: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR & Watson AJ 2011. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterol. 140: 1208–1218 e1201–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pentecost M, Otto G, Theriot JA & Amieva MR 2006. Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog. 2: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torres-Flores JM & Arias CF 2015. Tight junctions go viral! Viruses. 7: 5145–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA & Dermody TS 2001. Junction adhesion molecule is a receptor for reovirus. Cell. 104: 441–451. [DOI] [PubMed] [Google Scholar]
- 86.Torres-Flores JM, Silva-Ayala D, Espinoza MA, Lopez S & Arias CF 2015. The tight junction protein jam-a functions as coreceptor for rotavirus entry into ma104 cells. Virology. 475: 172–178. [DOI] [PubMed] [Google Scholar]
- 87.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT & Bergelson JM 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. U.S.A 98: 15191–15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coyne CB & Bergelson JM 2006. Virus-induced abl and fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 124: 119–131. [DOI] [PubMed] [Google Scholar]
- 89.Coyne CB, Shen L, Turner JR & Bergelson JM 2007. Coxsackievirus entry across epithelial tight junctions requires occludin and the small gtpases rab34 and rab5. Cell Host Microbe. 2: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD & Rice CM 2007. Claudin-1 is a hepatitis c virus co-receptor required for a late step in entry. Nature. 446: 801–805. [DOI] [PubMed] [Google Scholar]
- 91.Che P, Tang H & Li Q 2013. The interaction between claudin-1 and dengue viral prm/m protein for its entry. Virology. 446: 303–313. [DOI] [PubMed] [Google Scholar]
- 92.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP & Rice CM 2009. Human occludin is a hepatitis c virus entry factor required for infection of mouse cells. Nature. 457: 882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen L & Turner JR 2005. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell 16: 3919–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li D & Mrsny RJ 2000. Oncogenic raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 148: 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li D, Lin HH, McMahon M, Ma H & Ann DK 1997. Oncogenic raf-1 induces the expression of non-histone chromosomal architectural protein hmgi-c via a p44/p42 mitogen-activated protein kinase-dependent pathway in salivary epithelial cells. J. Biol. Chem 272: 25062–25070. [DOI] [PubMed] [Google Scholar]
- 96.Lan M, Kojima T, Osanai M, Chiba H & Sawada N 2004. Oncogenic raf-1 regulates epithelial to mesenchymal transition via distinct signal transduction pathways in an immortalized mouse hepatic cell line. Carcinogenesis. 25: 2385–2395. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z, Mandell KJ, Parkos CA, Mrsny RJ & Nusrat A 2005. The second loop of occludin is required for suppression of raf1-induced tumor growth. Oncogene. 24: 4412–4420. [DOI] [PubMed] [Google Scholar]
- 98.Wang Z, Wade P, Mandell KJ, Akyildiz A, Parkos CA, Mrsny RJ & Nusrat A 2007. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 26: 1222–1230. [DOI] [PubMed] [Google Scholar]
- 99.Beeman N, Webb PG & Baumgartner HK 2012. Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell death & disease. 3: e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beeman NE, Baumgartner HK, Webb PG, Schaack JB & Neville MC 2009. Disruption of occludin function in polarized epithelial cells activates the extrinsic pathway of apoptosis leading to cell extrusion without loss of transepithelial resistance. BMC Cell Biol. 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo WT, Shen L, Zuo L, Shashikanth N, Ong M, Wu L, Zha J, Edelblum KL, Wang Y, Wang Y, Nilsen SP & Turner JR 2019. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterol. 157: 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S & Takai Y 1997. Afadin: A novel actin filament-binding protein with one pdz domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 139: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi W, Acharya BR, Peyret G, Fardin MA, Mege RM, Ladoux B, Yap AS, Fanning AS & Peifer M 2016. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J Cell Biol. 213: 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lockwood C, Zaidel-Bar R & Hardin J 2008. The c. Elegans zonula occludens ortholog cooperates with the cadherin complex to recruit actin during morphogenesis. Curr. Biol 18: 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Otani T, Nguyen TP, Tokuda S, Sugihara K, Sugawara T, Furuse K, Miura T, Ebnet K & Furuse M 2019. Claudins and jam-a coordinately regulate tight junction formation and epithelial polarity. J Cell Biol. 218: 3372–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ikenouchi J, Umeda K, Tsukita S, Furuse M & Tsukita S 2007. Requirement of zo-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 176: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodgers LS, Beam MT, Anderson JM & Fanning AS 2013. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein zo-1. J. Cell Sci 126: 1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Odenwald MA, Choi W, Buckley A, Shashikanth N, Joseph NE, Wang Y, Warren MH, Buschmann MM, Pavlyuk R, Hildebrand J, Margolis B, Fanning AS & Turner JR 2017. Zo-1 interactions with f-actin and occludin direct epithelial polarization and single lumen specification in 3d culture. J. Cell Sci 130: 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Odenwald MA, Choi W, Kuo WT, Singh G, Sailer A, Wang Y, Shen L, Fanning AS & Turner JR 2018. The scaffolding protein zo-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem 293: 17317–17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cartagena-Rivera AX, Van Itallie CM, Anderson JM & Chadwick RS 2017. Apical surface supracellular mechanical properties in polarized epithelium using noninvasive acoustic force spectroscopy. Nature communications. 8: 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balda MS, Garrett MD & Matter K 2003. The zo-1-associated y-box factor zonab regulates epithelial cell proliferation and cell density. J Cell Biol. 160: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu J, Lim SB, Ng MY, Ali SM, Kausalya JP, Limviphuvadh V, Maurer-Stroh S & Hunziker W 2012. Zo-1 regulates erk, smad1/5/8, smad2, and rhoa activities to modulate self-renewal and differentiation of mouse embryonic stem cells. Stem Cells. 30: 1885–1900. [DOI] [PubMed] [Google Scholar]


