Abstract
Microvascular dysfunction describes a varied set of conditions which includes vessel destruction, abnormal vasoreactivity, in situ thrombosis, and fibrosis which ultimately results in tissue damage and progressive organ failure. Microvascular dysfunction has a wide array of clinical presentations, ranging from ischemic heart disease to renal failure, stroke, blindness, pulmonary arterial hypertension, and dementia. An intriguing unifying hypothesis suggests that microvascular dysfunction of specific organs is an expression of a systemic illness that worsens with age and is accelerated by vascular risk factors. Studying relationships across a spectrum of microvascular diseases affecting the brain, retina, kidney, lung, and heart may uncover shared pathologic mechanisms that could inform novel treatment strategies. We review the evidence that supports the notion that MVD represents a global pathologic process. Our focus is on studies reporting concomitant microvascular dysfunction of the heart with that of the brain, kidney, retina, and lung.
Keywords: Microvascular dysfunction, coronary microvascular dysfunction, small-vessel disease
Introduction
The human microcirculation is tasked with the delivery of oxygen and nutrients and removal of cellular waste products in the left-sided circulation, and with gas exchange in the pulmonary circulation.1 Microvascular dysfunction (MVD) describes a varied set of conditions which cause small-vessel obstruction, resulting in tissue damage and organ dysfunction. MVD has a wide array of clinical presentations, ranging from ischemic heart disease, to renal failure, stroke, blindness, pulmonary hypertension, and dementia.2 This concept was introduced more than 30 years ago in a study demonstrating that patients with angina and normal epicardial coronary arteries had a systemic abnormality in their vasodilator reserve.3 An intriguing unifying hypothesis suggests that microvascular dysfunction of specific organs is an expression of a systemic illness that worsens with age and is accelerated by vascular risk factors.2 In support of this hypothesis, MVD of the heart and brain may share a common pathophysiology.4–6 Studying relationships across a spectrum of microvascular diseases affecting the brain, retina, kidney, lung, and heart may uncover shared pathologic mechanisms that could inform novel treatment strategies. We review the evidence supporting MVD as a global pathologic process, specifically focused on concomitant presentation of MVD of the heart and other organs (Figure 1).
Figure 1.
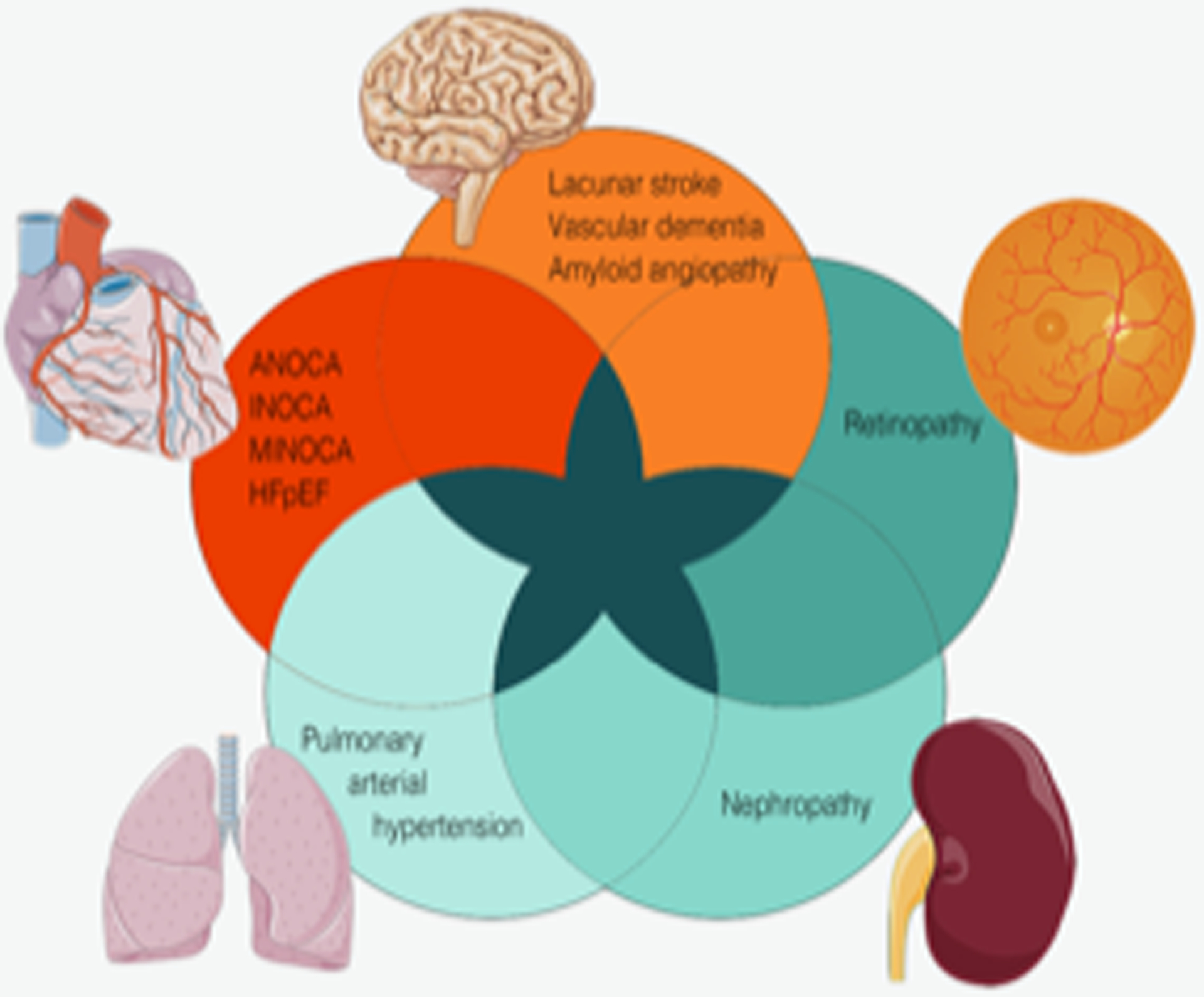
Microvascular dysfunction affecting the heart, brain, retina, lung, and kidney, representing different manifestations of small-vessel disease. They share pathophysiologic mechanisms and can occur concomitantly. ANOCA, angina with no obstructive coronary artery disease; HFpEF, heart failure with preserved ejection fraction; INOCA, ischemia with no obstructive coronary artery disease; MINOCA, myocardial infarction with no obstructive coronary arteries.
Methods
We performed a literature search using PubMed and EMBASE for articles pertaining to simultaneous MVD of the heart with MVD of another organ specifically the brain, kidney, retina, and lung. Search terms for MVD of the heart (“microvascular angina” or “syndrome X” or “coronary microvascular dysfunction” or “coronary flow reserve” or “myocardial perfusion” or “normal coronary” or “coronary vasoreactivity” or “nonobstructive coronary”) were combined with search terms for MVD of the brain (brain or cerebral or cerebrovascular or “small-vessel disease” or lipohyalinosis or “cerebral amyloid angiopathy” or lacunar; yielded 835 results), kidney (chronic kidney disease or CKD or renal or nephropathy or nephr* or “end-stage kidney disease” or “end-stage renal disease”; yielded 886 results), lung (“pulmonary hypertension” or “PAH” or “pulmonary vascular resistance” or “pulmonary artery pressure” or “Woods units”; yielded 158 results), and retina (retina or retin* or retinopathy or retinitis or vision or eye or ocular; yielded 239 results). Our literature search, including search terms, was guided by the Associate University Librarian of the Health Science Center Library at the University of Florida, Gainesville, Florida. Abstracts of all articles were reviewed for relevancy to the subject (D.S.F. and E.C.K.). Human case-control, cross-sectional, prospective, and descriptive studies presenting evidence of concomitant MVD of the heart and brain, heart and retina, heart and kidney, and heart and lung, were included. Post-mortem/autopsy reports, and case reports were excluded. The search was updated on March 11, 2022, and no additional articles were identified.
Clinical manifestations of MVD of the heart, brain, kidney, and retina
The majority of patients undergoing coronary angiography for anginal symptoms do not have flow-limiting narrowing in their epicardial arteries7, and are diagnosed with angina with no obstructive coronary artery disease (ANOCA) or ischemia with no obstructive coronary artery disease (INOCA).8 Coronary microvascular dysfunction is defined as limited coronary flow reserve and/or endothelial dysfunction that contributes to myocardial ischemia and angina in the absence of obstructive stenosis.9 It is associated with increased morbidity and mortality including myocardial infarction with no obstructive coronary arteries (MINOCA), and heart failure with preserved ejection fraction (HFpEF).10
Endothelial dysfunction, capillary rarefaction, microcirculatory plugging with microthrombi and microemboli, microvascular remodeling, and impaired autoregulation are key pathophysiologic mechanisms shared amongst MVD in different organs5,11, resulting in diverse presentations: Cerebral small vessel disease affects the microvasculature of the leptomeninges and the deep perforating branches of the anterior, middle, and posterior cerebral arteries.12 This form of microvascular dysfunction manifests clinically as stroke, cognitive dysfunction, cerebral amyloid angiopathy, vascular dementia, depression, and anxiety.13–16 It is the primary cause of lacunar stroke, and a contributing factor in up to 45% of cases of dementia.4,13,14 Chronic kidney disease (CKD) is a common condition affecting 20 million Americans.17 Its pathophysiology is characterized by capillary bed destruction, deranged vasoreactivity, and fibrosis leading to progressive renal injury.18 The retina is unique in that blood vessels can be directly visualized allowing considerable insight into the pathophysiologic changes of the microvasculature. Intimal inflammation and fibrosis, edema, neovascularization, and capillary degeneration results in retinopathy and associated vision loss.19 Lastly, in the lungs, pulmonary arterial hypertension caused by vascular remodeling, vasoconstriction, and in situ thrombosis leads to increased pulmonary vascular resistance, progressive right heart failure and death.20
Concomitant MVD of heart and brain
Studies assessing concomitant MVD in the heart and brain are summarized in Table 1. The largest is a descriptive analysis of 95 subjects with coronary microvascular dysfunction who underwent technetium-99m-HMPAO brain SPECT to assess for cerebral perfusion abnormalities.21 The majority of subjects (76%) had evidence of abnormal cerebral perfusion. The relationship between brain and myocardial perfusion defects have been reported in smaller studies.22,23 In one study of patients with slow coronary blood flow on coronary angiography, investigators reported decreased middle cerebral artery peak systolic, end diastolic, and mean flow velocities,24 while in a smaller study no such association was found.25 CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is an inherited cerebral small-vessel disease linked to mutations in NOTCH3 in which patients present with stroke and dementia.26 Two studies assessed concomitant heart involvement in CADASIL. In the first study, coronary flow reserve was found to be decreased in CADASIL patients compared to control subjects.27 In the second study, 25% of CADASIL mutation carriers had evidence of myocardial infarction, whereas none of the nonmutation carriers had such findings.28 Interestingly, the electrocardiographic changes in the CADASIL patients pre-dated the development of major neurologic symptoms.
Table 1.
Evidence supporting common pathophysiology between MVD of the heart and brain
| Reference | Design | Objective | Population | N | Key findings |
|---|---|---|---|---|---|
| Argirò et al 202127 | Descriptive | Determine prevalence and severity of CMD in patients with CADASIL | CADASIL | 32 | CFR and maximal MBF following regadenason infusion were decreased in CADASIL patients compared with controls. Degree of CMD did not correlate with extent of neurologic dysfunction. |
| Pai et al 200322 | Case-control | Assess brain findings in patients with CMD | CMD | 30 | High incidence of brain hypoperfusion lesions on Tc-99m ECD brain SPECT, and coincident with myocardial defects on the thallium-201 myocardial perfusion SPECT |
| Brunelli et al 199625 | Descriptive | Measure cerebral blood flow and cerebrovascular vasodilator reserve in patients with CMD | CMD | 16 | Cerebral blood flow and cerebrovascular vasodilator reserve preserved in patients with CMD, not consistent with the hypothesis of a diffuse smooth‐muscle disorder |
| Sun et al 200123 | Case-control | Assess brain findings in patients with CMD | CMD |
40 | 92% with definite myocardial perfusion defects on thallium‐201 myocardial perfusion SPECT also had multiple hypoperfusion areas in the brain. Parietal lobes were the most common area of hypoperfusion, cerebellum was the least common |
| Karakaya et al 201124 | Cross-sectional | Investigate cerebral blood flow velocity in patients with slow coronary blood flow | Slow coronary blood flow | 32 | Right and left middle cerebral artery peak systolic, end diastolic and mean flow velocities were significantly lower in patients with slow coronary blood flow than those with normal coronary flow |
| Weidmann et al 199721 | Descriptive | Investigate cerebral blood flow in patients with CMD | CMD | 95 | 76% had pathologic findings suggestive of cerebral perfusion abnormalities |
| Lesnik Oberstein et al 200328 | Descriptive | Assess prevalence of myocardial ischemia in CADASIL | CADASIL | 63 | 25% of mutation carriers had ECG evidence of myocardial infarction versus none in the nonmutation carriers (p=0.011). ECG changes pre-dated neurologic symptoms of CADASIL in all patients. |
CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; ECG, electrocardiogram; MBF, myocardial blood flow; MVD, microvascular dysfunction.
Concomitant MVD of heart and kidney
Some of the strongest evidence regarding the global nature of MVD is that of cardiovascular disease in patients with CKD. While traditional risk factors are associated with both cardiovascular disease and CKD, cardiovascular disease is more prevalent in patients with CKD, even when adjusted for traditional risk factors.29 Patients with CKD are more likely to die from a cardiac event than from progression to end stage renal disease.30 In addition, kidney dysfunction is associated with increased risk of both acute and chronic forms of cerebral small-vessel disease, including stroke and intracranial hemorrhage.31
While some functional and/or structural aspects of MVD can be directly assessed in the heart, retina, and brain, there is no widely accepted in vivo measurement of MVD in the kidney. However, peritubular capillary flow (renal plasma flow minus glomerular filtration rate) in humans32, and intravital microscopy in a murine sepsis model have been studied.33 Studies reporting a correlation between MVD of the heart and kidney (Table 2), relied on tests of kidney function, such as creatinine, glomerular filtration rate, and albuminuria to quantify renal MVD. Numerous studies have demonstrated that a lower glomerular filtration rate is related to decreased coronary flow reserve in the heart.34–43 Similarly, two studies demonstrated an association between slow coronary contrast flow on coronary angiography and decreased glomerular filtration rate.44,45 One study of 220 subjects with angina but no obstructive epicardial coronary artery disease reported creatinine clearance to be independently associated with the extent of MVD in the heart.46 In hypertensive subjects, plasma levels of asymmetric dimethylarginine, a nitric oxide inhibitor, were highest in those with impaired glomerular filtration rate and coronary flow reserve in one study47, and in a separate study, those with an abnormal stress test but no significant epicardial coronary artery disease, low coronary flow reserve was associated with higher left ventricular mass index and albumin-to-creatinine ratio.48 In a cross-sectional study, diabetics with renal insufficiency had reduced coronary flow velocity reserve compared to control subjects.49 Lastly, in a cohort of women enrolled in the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) study, investigators found a significant inverse correlation between coronary blood flow (determined by invasive coronary function testing) and urine albumin-creatinine ratio, suggesting a systemic process involving both the heart and kidney.50
Table 2.
Evidence supporting common pathophysiology between MVD of the heart and kidney
| Reference | Design | Objective | Population | N | Key findings |
|---|---|---|---|---|---|
| Nelson et al 201934 | Cross-sectional | Measure CFR | ESRD on dialysis and non-obstructive CAD | 30 | CFR was reduced in patients with ESRD |
| Kashioulis et al 202035 | Cross-sectional | Identify abnormalities on echocardiography | Chronic kidney disease | 132 | Patients with CKD stages 3 and had left ventricular diastolic dysfunction and reduced CFR |
| Östlund-Papadogeorgos et al 202046 | Descriptive | Determine predictors of index of microvascular resistance | Chronic angina and non-obstructive LAD disease | 220 | Creatinine clearance was independently associated with index of microvascular resistance |
| Tsiachris et al 201248 | Descriptive study | Identify associations between CFR and cardiac and renal abnormalities | Untreated hypertensives with a positive stress test and no significant CAD | 37 | Never-treated hypertensives with low CFR had larger LV mass index and higher albumin to creatinine ratio compared to those with higher CFR |
| Mohandas et al 201536 | Descriptive study | Assess if eGFR is associated with reduced CFR | Women with signs/symptoms of ischemia referred for coronary angiography | 198 | eGFR significantly correlated with CFR |
| Bozbas et al 200837 | Cross-sectional study | Evaluate degree of CMD | ESRD on dialysis | 86 | CFR was impaired in ESRD compared to renal transplants and controls. On multivariate analysis, creatinine, age, and diastolic dysfunction correlated with CFR |
| Fukushima et al 201238 | Cross-sectional | Determine presence of impaired myocardial perfusion | Referred for myocardial perfusion PET scan without known CAD | 230 | Global MFR is reduced in patients with CKD and no regional perfusion deficits |
| Fujii et al 200847 | Cross-sectional | Identify relationship between ADMA, eGFR and CMD | Hypertensives with normal or mild renal insufficiency | 66 | Plasma ADMA levels were highest in patients with reduced eGFR and CFVR eGFR and CFVR were significantly associated with each other |
| Ragosta et al 200449 | Cross-sectional | Determine prevalence of impaired CVR in diabetics with nephropathy and angiographically normal coronary arteries | Diabetics with nephropathy but no CAD | 64 | Abnormal CVR was associated with patients who had diabetes and nephropathy but not in controls |
| Akin et al 201444 | Cross-sectional | Assess association between eGFR and coronary blood flow | Slow coronary blood flow without obstructive CAD and normal to mildly impaired renal function | 430 | eGFR was significantly correlated with SCF in patients with normal to mildly impaired renal function |
| Chade et al 200639 | Descriptive | Determine association between CKD and CMD | Patients referred for angiography where CAD was excluded | 605 | GFR was significantly associated with CFR Patients with impaired GFR were more likely to be older, hypertensive, and female |
| Sakamoto et al 201240 | Descriptive | Assess if impaired CFR is associated with CKD and cardio-cerebrovascular events | Patients with suspected CAD but no epicardial artery stenosis | 73 | Patients with CKD had a significantly lower CFR compared to those without CKD. In patients with low CFR, cardio-cerebrovascular events were more common compared to patients with normal CFR |
| Tsuda et al 201841 | Cross-sectional | Measure myocardial perfusion reserve | Chronic kidney disease | 92 | Patients with CKD had a significantly lower myocardial perfusion reserve compared to those without CKD |
| Bezante et al 200942 | Descriptive | Characterize changes in CFR and early renal disease | Hypertensives | 26 | Those with impaired CFR also had significantly lower eGFR |
| Imamura et al 201443 | Descriptive | Assess relationship between albuminuria and CMD | Chronic kidney disease | 175 | Worsening renal function was associated with lower CFVR. Albuminuria was the most powerful predictor of abnormal CFVR |
| Yilmaz et al 200945 | Cross-sectional | Determine the presence of slow coronary flow in patients with impaired renal function | Patients with angiographically normal coronary arteries and a GFR < 90 mL/min/1.73 m2 | 207 | Those with impaired renal function had slower coronary flow (assessed by TIMI flow) compared to those with normal renal function |
| Jalnapurkar et al 202150 | Descriptive | Evaluate relationship between UACR and invasive coronary function testing | Women with INOCA enrolled in WISE-CVD | 152 | Coronary endothelial-dependent variables had significant inverse correlations with log UACR |
ADMA, asymmetric dimethylarginine; CAD, coronary artery disease; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CVR, coronary flow velocity reserve; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; INOCA, ischemia with no obstructive coronary artery disease; LV, left ventricular; MFR, myocardial flow reserve; MVD, microvascular dysfunction; UACR, urine albumin-creatinine ratio; WISE-CVD, Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction.
Concomitant MVD of heart and retina
Studies assessing the relationship between MVD of the heart and retina are summarized in Table 3. In two studies, coronary reactivity testing was abnormal in diabetics with retinopathy.51,52 In a descriptive study, 60 patients with MVD of the heart underwent optical coherence tomography to assess retinal abnormalities.53 Superficial vascular density was abnormal in 45% of patients with MVD of the heart. Several studies have focused on retinal arteriolar caliber as a marker for MVD in the heart. For example, Wang and colleagues studied 212 subjects with no known cardiovascular disease and found that narrower retinal arterioles were associated impaired hyperemic myocardial perfusion.54 In a separate study of patients with known coronary microvascular dysfunction, higher retinal arteriolar flow was seen in those with slow coronary artery blood flow.55 These investigators concluded that the higher retinal arteriolar flow was likely a product of impaired vasodilation from microvascular dysfunction. Moreover, in a large cross-sectional study, women with small retinal venules were 3-times more likely to have microvascular angina compared to woman with large retinal venules.56 Finally, in a study of 4,593 middle and older age adults with no known cardiovascular disease, the presence of narrow retinal arterioles was associated with left ventricular concentric remodeling independent of atherosclerotic burden.57
Table 3.
Evidence supporting common pathophysiology between MVD of the heart and retina
| Reference | Design | Objective | Population | N | Key findings |
|---|---|---|---|---|---|
| Akasaka et. Al 199751 | Cross-sectional | Assess differences in CFR in patients with and without diabetes | CMD | 44 | CFR was reduced in patients with diabetes and diabetic retinopathy compared to controls. More severe retinopathy was associated with worse coronary flow reserve |
| Eslami et al 202153 | Descriptive | Describe retinal changes in patients with CMD | CMD | 60 | Superficial vascular density was abnormal in ~ half of patients with CMD |
| Sundell et al 200452 | Cross-sectional | Determine whether diabetic retinopathy is associated with reduced coronary vasoreactivity | Diabetic retinopathy | 33 | Dipyridamole-stimulated flow and coronary vascular resistance were blunted in diabetics with retinopathy compared to diabetics without retinopathy and controls |
| Liew et al 201956 | Cross-sectional | Assess for differences in retinal microvasculature between patients with CMD and CAD | Patients with microvascular angina and CAD | 915 | Women (but not men) with small retinal venules were three-fold more likely to have microvascular angina compared to women with large retinal venules |
| Arbel et al 201455 | Cross-sectional | Determine utility of retinal blood flow as a predictor of slow coronary artery blood flow | CMD | 28 | Higher retinal arterial flow was observed in the slow coronary flow group |
| Wang et al 200854 | Cross-sectional | Determine relationship between retinal arteriolar narrowing and myocardial perfusion in patients with and without CAD | Middle and older age adults with no known cardiovascular disease | 212 | Narrower retinal arterioles are associated with lower hyperemic myocardial perfusion in asymptomatic adults with no coronary calcification |
| Cheung et al 200757 | Cross-sectional | Determine relationship between retinal arteriolar narrowing and left ventricular remodeling in patients with and without CAD | Middle and older age adults with no known cardiovascular disease | 4,593 | Narrower retinal arterioles are associated with increased left ventricular concentric remodeling regardless of level of coronary atherosclerosis |
CAD, coronary artery disease; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; MVD, microvascular dysfunction.
Concomitant MVD of heart and lung
Pulmonary hypertension, defined as resting mean pulmonary artery pressure measured by right heart catheterization ≥ 25mmHg, is further delineated into five groups depending on the underlying pathophysiologic mechanism.58 As it pertains to this review, group 1 pulmonary hypertension (pulmonary arterial hypertension) predominately affects the small resistance pulmonary arteries, is associated with a poor prognosis, and can be idiopathic, genetic, or associated with other conditions such as connective tissue diseases.58,59 The concomitant occurrence of pulmonary hypertension and MVD of the heart has been described (Table 4). In a study of patients with systemic sclerosis, investigators reported considerable overlap between cardiac involvement (both epicardial coronary artery disease and coronary microvascular dysfunction) and the presence of pulmonary hypertension.60 For example, about a third of patients with pulmonary hypertension had severely reduced coronary flow reserve on invasive coronary reactivity testing. Other investigators report significantly lower myocardial perfusion on cardiac magnetic resonance testing in subjects with group 1 pulmonary hypertension compared to those without pulmonary hypertension, and the pulmonary artery pressure as an independent predictor of right and left ventricular myocardial perfusion.61 Moreover, in a study of patients with hypertrophic cardiomyopathy, global myocardial flow reserve as measured by positron emission tomography was an independent predictor of concomitant pulmonary hypertension.62 Lastly, studies by Raman colleagues also report evidence coronary microvascular dysfunction of the right and left ventricle on cardiac magnetic resonance testing in patients with pulmonary arterial hypertension.63–65
Table 4.
Evidence supporting common pathophysiology between MVD of the heart and lungs
| Reference | Design | Objective | Population | N | Key findings |
|---|---|---|---|---|---|
| Zhao et al 201962 | Retrospective | Assess relationship between CMD and pulmonary HTN | HCM with and without pulmonary HTN | 89 | Global MFR measured by quantitative PET was an independent predictor for pulmonary HTN in patients with HCM |
| Vogel-Claussen et al 201161 | Prospective | Evaluate relationship between LV and RV perfusion with pulmonary hemodynamics | Known or suspected pulmonary HTN and controls | 41 | RV and LV vasoreactivity significantly reduced in subjects with pulmonary HTN Mean PA pressure was an independent predictor of RV and LV myocardial perfusion reserve index on CMR |
| Raman et al 202165 | Prospective | Assess prevalence of RV ischemia in pulmonary HTN without CAD | Pulmonary HTN and controls | 53 | Decreased OS-CMR in pulmonary HTN patients consistent with microvascular dysfunction of the RV |
| Raman et al 202064 | Prospective | Assess prevalence of LV ischemia in patients with pulmonary HTN | Pulmonary HTN, CAD, and controls | 47 | Decreased OS-CMR and T1 reactivity in pulmonary HTN patients without CAD consistent with microvascular dysfunction |
| Raman et al 201963 | Prospective | Determine feasibility of rest/stress OS-CMR in pulmonary HTN | Pulmonary HTN and controls | 29 | Compared to controls, patients with pulmonary HTN had myocardial deoxygenation of the inferior RV on OS-CMR RV deoxygenation correlated with the presence of LV deoxygenation |
| Komocsi et al 200960 | Prospective | Investigate degree of overlap of CAD and pulmonary HTN in systemic sclerosis | Systemic sclerosis patients with suspected pulmonary HTN and suspected CAD | 30 | Angiographic coronary slow flow (quantified by TIMI frame count), was inversely related to CFR Severely reduced CFR (measured invasively at time of coronary angiography) in 35% of patients with pulmonary HTN |
CAD, coronary artery disease; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; HCM, hypertrophic cardiomyopathy; HTN, hypertension; LV, left ventricle; MFR, myocardial flow reserve; MVD, microvascular dysfunction; OS-CMR, oxygen-sensitive cardiac magnetic resonance; PA, pulmonary artery; RV, right ventricle; TIMI, thrombolysis in myocardial infarction.
Discussion
There is a significant body of information supporting the concept of MVD as a systemic and multi-organ pathologic process. A number of potential mechanisms have been proposed to explain these associations: In the heart and brain, MVD is associated with elevated serum levels of homocysteine, serotonin, asymmetric dimethylarginine, and uric acid, all of which perturb the nitric oxide pathway.66–68 Bioavailable nitric oxide is also decreased in CKD which may contribute to both an increased risk of cardiovascular events and progressive renal dysfunction.69 Additional evidence regarding systemic endothelial dysfunction comes from the Coronary Microvascular Angina (CorMicA) study where arterioles isolated from gluteal biopsies in patients with microvascular angina or variant angina exhibited reduced maximum relaxation with acetylcholine and increased sensitivity to vasoconstricting agents70, and the well-known multi-organ involvement seen in systemic sclerosis and systemic lupus erythematosus with abnormalities of the skin, lungs, kidney, heart, and gastrointestinal tract.71 From the diagnostic perspective, investigators identified 16 circulating biomarkers that were found to be common to MVD of the heart, brain, and kidney in a meta-analysis.68 These biomarkers represented multiple mechanistic pathways including inflammation, coagulation/thrombosis, and endothelial dysfunction.
Two genetic illnesses, CADASIL and Fabry disease, may represent inherited forms of MVD. CADASIL, as discussed earlier, has been associated with impaired coronary flow reserve and maximal myocardial blood flow in 32 patients27, and also microvascular angina in several case reports.72,73 Fabry disease, an X-linked lysosomal storage disease caused by a lack of alpha-galactosidase activity, results in the accumulation of globotriaosylceramide in cells and multi-organ failure.74 This disease has been associated with microvascular angina75 as well as cerebral small-vessel disease.76 In one study of 10 patients with Fabry disease and 24 controls, resting and hyperemic myocardial blood flow and coronary flow reserve assessed by positron emission tomography were significantly lower in patients with the disease versus reference subjects.75 Interestingly, female heterozygote patients with Fabry disease are at increased risk of developing small vessel disease of the kidney, heart, and brain despite normal levels of alpha-galactosidase activity.76
We recognize several limitations in this review: First, we focused our review on study level data supporting concomitant MVD in the heart and another organ including the kidney, brain, retina, and lung. There are data supporting concomitant MVD in other combinations not including the heart (such as the brain/retina and the kidney).77 Second, we only included full text articles in English so this may not be a complete list of all available data.
In conclusion, the reported evidence that supports the intriguing notion that MVD is a global pathologic process was reviewed. We contend that the diagnosis of MVD affecting the heart, brain, kidney, lung, or retina should prompt evaluation of other possible affected organs. A number of knowledge gaps need to be addressed to better understand this ubiquitous process and its shared pathophysiology which will ultimately inform novel therapies.
Acknowledgement:
The authors would like to thank Hannah F. Norton (Associate University Librarian and Chair of the Health Science Center Library at the University of Florida, Gainesville, Florida) for her expertise regarding our literature search.
Funding:
This work was supported by Department of Defense-funded WARRIOR trial [W81XWH-17-2-0030] (Pepine), the McJunkin Family Foundation for the WARRIOR Biorepository (Pepine), the American Heart Association [18TPA34170486] (Mehrad), and by contracts from the National Heart, Lung and Blood Institutes nos. R01 HL153500 (Wei), R01 AI135128 (Mehrad), and N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23 HL125941, T32 HL69751, R01 HL090957, 1R03 AG032631, R01 HL146158, R01 HL124649, PR150224P1 (CDMRP-DoD), U54 AG065141, GCRC grant M01-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, the Barbra Streisand Women’s Cardiovascular Research and Education Program, and the Erika J. Glazer Women’s Heart Research Initiative, Cedars-Sinai Medical Center, Los Angeles (Bairey Merz).
Footnotes
Conflict of Interest: Dr. Bairey Merz has disclosures from iRhythm. Dr. Wei has disclosures from Abbott Vascular. The other authors do not have conflict of interest.
References
- 1.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation. Regulation of flow and beyond. Circ Res 2016;118:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson CS, Hakim AM. Living beyond our physiological means. Small vessel disease of the brain is an expression of a sytemic failure in arteriolar function: A unifying hypothesis. Stroke 2009;40:e322–e330. [DOI] [PubMed] [Google Scholar]
- 3.Sax FL, Cannon RO, Hanson C, Epstein SE. Impaired forearm vasodilator reserve in patients with microvascular angina. Evidence of a generalized disorder of vascular function? N Engl J Med 1987;317:1366–70. [DOI] [PubMed] [Google Scholar]
- 4.Berry C, Sidik N, Pereira AC, Ford TJ, Touyz RM, Kaski JC, Hainsworth AH. Small-Vessel Disease in the Heart and Brain: Current Knowledge, Unmet Therapeutic Need, and Future Directions. J Am Heart Assoc 2019;8:e011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejia-Renteria H, Matias-Guiu JA, Lauri F, Yus M, Escaned J. Microcirculatory dysfunction in the heart and brain. Minerva Cardioangiologica 2019;67:318–329. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MA, Hazany S, Ellingson BM, Hu P, Nguyen KL. Pathophysiology, classification, and MRI paralles in microvascular disease of the heart and brain. Microcirculation 2020;27:e12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunadian V, Chieffo A, Camici P, Berry C, Escaned J, Mass AHEM, Prescott E, Karam N, Appelman Y, Fraccaro C, Buchanan GL, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI expert concensus document on ischaemia with non-obstuctive coronary arteries in collaboration with European Society of Cardiology Working Group on coronary pathophysiology & microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chillan WM, Clayton JA, Cooper LS, Crea F, DiCarli M, Douglas PS, Galis ZS, Gurbel P, Handberg EM, Hasan A, Hill JA, Hochman JS, Iturriaga E, Kirby R, Levine GN, Libby P, Lima J, Mehta P, Desvigne-Nickens P, Olive M, Pearson GD, Quyyumi AA, Reynolds H, Robinson B, Sopko G, Taqueto V, Wei J, Wenger N. Ischemia and no obstructive coronary artery disease (INOCA). Developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DelBuono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases. J Am Coll Cardiol 2021;78:1352–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel H, Aggarwal NT, Rao A, Bryant E, Sanghani RM, Byrnes M, Kalra D, Dairaghi L, Braun L, Gabriel S, Volgman AS. Microvascular Disease and Small-Vessel Disease: The Nexus of Multiple Diseases of Women. J Womens Health (Larchmt) 2020;29:770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Yang Y, Reis C, Tao T, Li W, Li X, Zhang JH. Cerebral Small Vessel Disease. Cell Transplant 2018;27:1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology 2019;92:1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y, Qin T, Liu W, Ran L, Yang Y, Huang H, Pan D, Wang M. Cerebral Small-Vessel Disease and Risk of Incidence of Depression: A Meta-Analysis of Longitudinal Cohort Studies. J Am Heart Assoc 2020;9:e016512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatti L, Tinelli F, Scelzo E, Arioli F, Di Fede G, Obici L, Pantoni L, Giaccone G, Caroppo P, Parati EA, Bersano A. Understanding the Pathophysiology of Cerebral Amyloid Angiopathy. Int J Mol Sci 2020;21:3435.doi: 10.3390/ijms21103435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drawz P, Rahman M. Chronic kidney disease. Ann Intern Med 2015;162:ITC1–16. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan S, Suarez-Martinez AD, Bagher P, Gonzalez A, Liu R, Murfee WL, Mohandas R. Micorvascular dysfunction and kidney disease: Challenges and opportunities? Microcirculation 2021;28:e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2017;2:e93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan NSH, Massam BD, Kulkarni SS, Lang CC. Pulmonary arterial hypertension:Pathophysiology and treatment. Diseases 2018;6:38. doi: 10.3390/diseases6020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidmann B, Jansen WC, Bock A, Assheuer J, Tauchert MO. Technetium-99m-HMPAO brain SPECT in patients with syndrome X. Am J Cardiol 1997;79:959–61. [DOI] [PubMed] [Google Scholar]
- 22.Pai PY, Liu FY, Kao A, Lin CC, Lee CC. A higher prevalence of abnormal regional cerebral blood flow in patients with syndrome X and abnormal myocardial perfusion. Jpn Heart J 2003;44:145–52. [DOI] [PubMed] [Google Scholar]
- 23.Sun SS, Shiau YC, Tsai SC, Ho YJ, Wang JJ, Kao CH. Cerebral perfusion in patients with syndrome X: a single photon emission computed tomography study. J Neuroimaging 2001;11:148–52. [DOI] [PubMed] [Google Scholar]
- 24.Karakaya O, Koçer A, Esen AM, Kargin R, Barutcu I. Impaired cerebral circulation in patients with slow coronary flow. Tohoku J Exp Med 2011;225:13–16. [DOI] [PubMed] [Google Scholar]
- 25.Brunelli C, Nobili F, Spallarossa P, Olivotti L, Rossettin P, Rodriguez G, Caponnetto S. Cerebral blood flow reserve in patients with syndrome X. Coron Artery Dis 1996;7:587–90. [DOI] [PubMed] [Google Scholar]
- 26.Wang MM. CADASIL. Handb Clin Neurol 2018;148:733–743. [DOI] [PubMed] [Google Scholar]
- 27.Argirò A, Sciagrà R, Marchi A, Beltrami M, Spinelli E, Salvadori E, Bianchi A, Mascalchi M, Poggesi A, Olivotto I, Pescini F. Coronary microvascular function is impaired in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Eur J Neurol 2021;28:3809–3813. [DOI] [PubMed] [Google Scholar]
- 28.Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, Ferrari MD, Haan J. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore) 2003;82:251–256. [DOI] [PubMed] [Google Scholar]
- 29.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 2007;50:217–24. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, Harms HJ, Osborne MT, Bravo P, Andrikopolou E, Divakaran S, Bibbo CF, Hainer J, Skali H, Taqueti V, Steigner M, Dorbala S, Charytan DM, Prabhu SD, Blankstein R, Deo RC, Solomon SD, Di Carli MF. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation 2020;141:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marini S, Georgakis MK, Anderson CD. Interactions Between Kidney Function and Cerebrovascular Disease: Vessel Pathology That Fires Together Wires Together. Front Neurol 2021;12:785273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Futrakul N, Futrakul P. Renal microvascular disease predicts renal function in diabetes. Renal Failure 1012;34:126–129. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogrn species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. JASN 2007;19:1807–1815. [DOI] [PubMed] [Google Scholar]
- 34.Nelson AJ, Dundon BK, Worthley SG, Richardson JD, Puri R, Wong DTL, Coates PT, Faull RJ, Worthley MI. End-stage renal failure is associated with impaired coronary microvascular function. Coron Artery Dis 2019;30:520–527. [DOI] [PubMed] [Google Scholar]
- 35.Kashioulis P, Guron CW, Svensson MK, Hammarsten O, Saeed A, Guron G. Patients with moderate chronic kidney disease without heart disease have reduced coronary flow velocity reserve. ESC Heart Fail 2020;7:2797–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohandas R, Segal MS, Huo T, Handberg EM, Petersen JW, Johnson BD, Sopko G, Bairey Merz CN, Pepine CJ. Renal function and coronary microvascular dysfunction in women with symptoms/signs of ischemia. PLoS One 2015;10:e0125374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bozbas H, Pirat B, Demirtas S, Simsek V, Yildirir A, Sade E, Sayin B, Sezer S, Karakayali H, Muderrisoglu. Evaluation of coronary microvascular function in patients with end-stage renal disease, and renal allograft recipients. Atherosclerosis 2009;202:498–504. [DOI] [PubMed] [Google Scholar]
- 38.Fukushima K, Javadi MS, Higuchi T, Bravo PE, Chien D, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Impaired global myocardial flow dynamics despite normal left ventricular function and regional perfusion in chronic kidney disease: a quantitative analysis of clinical 82Rb PET/CT studies. J Nucl Med 2012;53:887–93. [DOI] [PubMed] [Google Scholar]
- 39.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int 2006;69:266–71. [DOI] [PubMed] [Google Scholar]
- 40.Sakamoto N, Iwaya S, Owada T, Nakamura Y, Yamauchi H, Hoshino Y, Mizukami H, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. A reduction of coronary flow reserve is associated with chronic kidney disease and long-term cardio-cerebrovascular events in patients with non-obstructive coronary artery disease and vasospasm. Fukushima J Med Sci 2012;58:136–43. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda N, Shiraishi S, Sakamoto F, Yuki H, Ogasawara K, Yoshida M, Tomiguchi S, Tsujita K, Yamashita Y. Quantification of myocardial perfusion reserve using dynamic SPECT images of patients with chronic kidney disease. J Cardiol 2018;71:174–180. [DOI] [PubMed] [Google Scholar]
- 42.Bezante GP, Viazzi F, Leoncini G, Ratto E, Conti N, Balbi M, Agosti S, Deferrari L, Deferrari G, Pontremoli R. Coronary flow reserve is impaired in hypertensive patients with subclinical renal damage. Am J Hypertens 2009;22:191–6. [DOI] [PubMed] [Google Scholar]
- 43.Imamura S, Hirata K, Orii M, Shimamura K, Shiono Y, Ishibashi K, Tanimoto T, Yamano T, Ino Y, Kitabata H, Yamaguchi T, Kubo T, Tanaka A, Imanishi T, Akasaka T. Relation of albuminuria to coronary microvascular function in patients with chronic kidney disease. Am J Cardiol 2014;113:779–85. [DOI] [PubMed] [Google Scholar]
- 44.Akin F, Celik O, Altun I, Ayça B. Association of glomerular filtration rate with slow coronary flow in patients with normal to mildly impaired renal function. Angiology 2014;65:850. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz MB, Yalta K. Coronary flow slows as renal function worsens. Clin Cardiol 2009;32:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Östlund-Papadogeorgos N, Ekenbäck C, Jokhaji F, Mir-Akbari H, Witt N, Jernberg T, Wallen H, Linder R, Tornerud M, Samad BA, Persson J. Blood haemoglobin, renal insufficiency, fractional flow reserve and plasma NT-proBNP is associated with index of microcirculatory resistance in chronic coronary syndrome. Int J Cardiol 2020;317:1–6. [DOI] [PubMed] [Google Scholar]
- 47.Fujii H, Takiuchi S, Kawano Y, Fukagawa M. Putative role of asymmetric dimethylarginine in microvascular disease of kidney and heart in hypertensive patients. Am J Hypertens 2008;21:650–6. [DOI] [PubMed] [Google Scholar]
- 48.Tsiachris D, Tsioufis C, Dimitriadis K, Syrseloudis D, Rousos D, Kasiakogias A, Papademetriou V, Tousoulis D, Stefanadis C. Relation of impaired coronary microcirculation to increased urine albumin excretion in patients with systemic hypertension and no epicardial coronary arterial narrowing. Am J Cardiol 2012;109:1026–30. [DOI] [PubMed] [Google Scholar]
- 49.Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J 2004;147:1017–23. [DOI] [PubMed] [Google Scholar]
- 50.Jalnapurkar S, Landes S, Wei J, Mehta PK, Shufelt C, Minissian M, Pepine CJ, Handberg E, Coof-Wiens G, Sopko G, Bairy Merz CN. Coronary endothelial dysfunction appears to be a manifestation of a systemic process: A report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) study. PLoS ONE 2021;19:e0257184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akasaka T, Yoshida K, Hozumi T, Takagi T, Kaji S, Kawamoto T, Morioka S, Yoshikawa J. Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol 1997;30:935–41. [DOI] [PubMed] [Google Scholar]
- 52.Sundell J, Janatuinen T, Rönnemaa T, Raitakari OT, Toikka J, Nuutila P, Knuuti J. Diabetic background retinopathy is associated with impaired coronary vasoreactivity in people with Type 1 diabetes. Diabetologia 2004;47:725–31. [DOI] [PubMed] [Google Scholar]
- 53.Eslami V, Mojahedin S, Nourinia R, Tabary M, Khaheshi I. Retinal changes in patients with angina pectoris and anginal equivalents: a study of patients with normal coronary angiography. Rom J Intern Med 2021;59:174–179. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion. Multi-Ethnic study of atherosclerosis. Hypertension 2008;51:119–126. [DOI] [PubMed] [Google Scholar]
- 55.Arbel Y, Sternfeld A, Barak A, Burgansky-Eliash Z, Halkin A, Berliner S, Herz I, Keren G, Rubinstein A, Banai S, Finkelstein A. Inverse correlation between coronary and retinal blood flows in patients with normal coronary arteries and slow coronary blood flow. Atherosclerosis 2014;232:149–54. [DOI] [PubMed] [Google Scholar]
- 56.Liew G, Mitchell P, Chiha J, Plant AJH, White A, Joachim N, Wang S, Burlutsky G, Kovoor P, Thiagalingam A, Gopinath B. Retinal microvascular changes in microvascular angina: Findings from the Australian Heart Eye Study. Microcirculation 2019;26:e12536. [DOI] [PubMed] [Google Scholar]
- 57.Cheung N, Bluemke DA, Klein R, Sharrett AR, Islam FM, Cotch MF, Klein BE, Criqui MH, Wong TY. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol 2007;50:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prins KW, Thenappan T. WHO group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin 2016;34:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haque A, Kiely DG, Kovacs G, Thompson AAR, Condliffe R. Pulmonary hypertension phenotypes in patients with systemic sclerosis. Eur Respir Rev 2021;30:210053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komocsi A, Pinter T, Faludi R, Magyari B, Bozo J, Kumanovics G, Minier T, Radics J, Czirjak L. Overlap of coronary disease and pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis 2010;69:202–205. [DOI] [PubMed] [Google Scholar]
- 61.Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, Lechtzin N, Girgis RE, Mathai SC, Goldstein TA, Zheng J, Lima JAC, Bluemke DA, Hassoun PM. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology 2011;258:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao M, Leal JP, Tsui BMW, Wong DF, Pomper MG, Zhou Y. Association of PET-measured myocardial flow reserve with echocardiography-estimated pulmonary artery systolic pressure in patients with hypertrophic cardiomyopathy. PLoS ONE 2019;14:e0212573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raman KS, Stokes M, Walls A, Perry R, Steele PM, Burdeniuk C, De Pasquale CG, Celermajer DS, Selvanayagam JB. Feasibility of oxygen sensitive cardiac magnetic resonance of the right ventricle in pulmonary hypertension. Cardiovasc Diagn Ther 2019;9:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raman KS, Shah R, Stokes M, Walls A, Woodman RJ, Ananthakrishna R, Walker JG, Proudman S, Steele PM, De Pasquale CG, Celermajer DS, Selvanayagam JB. Left ventricular ischemia in pre-capillary pulmonary hypertension: a cardiovascular magnetic resonance study. Cardiovasc Diagn Ther 2020;10:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raman KS, Shad R, Stokes M, Walls A, Woodman RJ, Perry R, Walker JG, Proudman S, De Pasquale CG, Celermajer DS, Selvanayagam JB. Right ventricular myocardial deoxygenation in patients with pulmonary artery hypertension. J Cardiovacs Magn Reson 2021;23:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad A, Corban MT, Toya T, Sara JD, Lerman B, Park JY, Lerman LO, Lerman A. Coronary Microvascular Endothelial Dysfunction in Patients With Angina and Nonobstructive Coronary Artery Disease Is Associated With Elevated Serum Homocysteine Levels. J Am Heart Assoc 2020;9:e017746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odaka Y, Takahashi J, Tsuburaya R, Nishimiya K, Hao K, Matsumoto Y, Iot K, Sakata Y, Miyata S, Manita D, Hirowatari Y, Shimokawa H. Plasma concentration of serotonin is a novel biomarker for coronary microvascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur Heart J 2017;38:489–496. [DOI] [PubMed] [Google Scholar]
- 68.Nowroozpoor A, Gutterman D, Safdar B. Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys? - A scoping review. Microvasc Res 2021;134:104123. [DOI] [PubMed] [Google Scholar]
- 69.Baylis C Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 2008;294:F1–9. [DOI] [PubMed] [Google Scholar]
- 70.Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, Yii E, Sidik N, Harvey A, Montezano AC, Beattie E, Haddow L, Oldroyd KG, Youyz RM, Berry C. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J 2018;39:4086–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saygin D, Highland K, Tonelli AR. Microvascular involvement in systemic sclerosis and systemic lupus erythematosus. Microcirculation 2019;26:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral Autosomal Dominant Arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr 2020;14:e1–e6. [DOI] [PubMed] [Google Scholar]
- 73.Rubin CB, Hahn V, Kobayashi T, Litwack A. A Report of Accelerated Coronary Artery Disease Associated with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. Case Rep Cardiol 2015;2015:167513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan B, Adam DN. A Review of Fabry Disease. Skin Therapy Lett 2018;23(2):4–6. [PubMed] [Google Scholar]
- 75.Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, Tome MT, McKenna WJ, Lee P, Camici PG. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 2006;92:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Søndergaard CB, Nielsen JE, Hansen CK, Christensen H. Hereditary cerebral small vessel disease and stroke. Clin Neurol Neurosurg 2017;155:45–57. [DOI] [PubMed] [Google Scholar]
- 77.Toyoda K Cerebral small vessel disease and chronic kidney disease. J Stroke 2015;17:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


