Abstract
Background:
The development of benign prostatic hyperplasia (BPH) and medication-refractory lower urinary tract symptoms (LUTS) remain poorly understood. This study attempted to characterize the pathways associated with failure of medical therapy for BPH/LUTS.
Methods:
Transitional zone tissue levels of cholesterol and steroids were measured in patients who failed medical therapy for BPH/LUTS and controls. Prostatic gene expression was measured using qPCR and BPH cells were used in organoid culture to study prostatic branching.
Results:
BPH patients on 5-α-reductase inhibitor (5ARI) showed low levels of tissue dihydrotestosterone (DHT), increased levels of steroid 5-α-reductase type II (SRD5A2), and diminished levels of androgen receptor (AR) target genes, prostate-specific antigen (PSA), and transmembrane serine protease 2 (TMPRSS2). 5ARI raised prostatic tissue levels of glucocorticoids (GC), whereas alpha-adrenergic receptor antagonists (α-blockers) did not. Nuclear localization of GR in prostatic epithelium and stroma appeared in all patient samples. Treatment of four BPH organoid cell lines with dexamethasone, a synthetic GC, resulted in budding and branching.
Conclusions:
After failure of medical therapy for BPH/LUTS, 5ARI therapy continued to inhibit androgenesis but a 5ARI-induced pathway increased tissue levels of GC not seen in patients on α-blockers. GC stimulation of organoids indicated that the GC receptors are a trigger for controlling growth of prostate glands. A 5ARI-induced pathway revealed GC activation can serve as a master regulator of prostatic branching and growth.
Keywords: androgen, BPH, glucocorticoid, LUTS, prostate branching, prostate hyperplasia
1 ∣. INTRODUCTION
The Medical Therapy of Prostate Symptoms (MTOPS) randomized trial showed a 66% reduced risk of overall symptomatic progression in patients with benign prostate hyperplasia (BPH) taking combination medical therapy of a steroid 5α-reductase inhibitor (5ARI) plus alpha-adrenergic receptor antagonist (α-blockers)1-3 compared to placebo. The ComBAT study revealed that patients on a combined regimen of α-blocker (tamsulosin) and 5ARI (dutasteride) had a 41% reduced risk of clinical progression compared to tamsulosin monotherapy.4 A recent follow-up analysis of the MTOPS data demonstrated that men with inflammation on baseline prostate biopsy had increased prostate volume and risk of progression compared to men without inflammation.5 The association between inflammation and BPH may be secondary to the growth promoting effects of proinflammatory factors, cytokines, and prostaglandins (PGs). Inflammation,5,6 NF-ϰB activation,7,8 and stress pathways9 are all associated with failure of medical therapy for BPH. We reported previously that proinflammatory PGs are elevated in all BPH patients who failed medical therapy and required surgery to relieve lower urinary tract symptoms (LUTS).10 Although inflammation is associated with BPH,11 the role inflammation plays in the pathogenesis of BPH or in patients who are or become refractory to medical therapy remains unresolved.
Steroid levels were measured in prostatic tissue collected from the transition zone (TZ) of patients who failed medical therapy and underwent surgical (S) intervention to relieve LUTS (S-BPH), and in control TZ (incidental [I]) from patients without BPH who underwent radical prostatectomy for prostate cancer (I-BPH). S-BPH patients on 5ARI or α-blocker plus 5ARI showed high levels of T but low levels of tissue dihydrotestosterone (DHT) and diminished activation of the androgen receptor (AR), which confirmed that the 5ARI inhibited androgenesis. However, 5ARI treatment raised prostatic tissue levels of glucocorticoids (GC) while patients on α-blockers or no therapy showed low levels of GC. GC also induced budding and branching in organoid culture using benign prostatic cell lines. These data suggest that 5ARIs increase GC levels, which can serve as a master regulator of prostatic growth.
2 ∣. PATIENTS AND METHODS
2.1 ∣. Human prostate tissue collection
Patient tissue was collected under written informed consent. S-BPH was transitional zone tissue collected at the time of bladder outlet obstruction surgery (HoLEP or simple prostatectomy) for treatment of LUTS. I-BPH tissue was benign transitional zone tissue from patients undergoing radical prostatectomy for low volume, low grade prostate cancer (Gleason score 6 or 7 [3 + 3 or 3 + 4]) and localized in the peripheral zone (PZ) to minimize any cancer field effects on the TZ. The use of radical prostatectomy patients to collect control samples in any size beyond a biopsy core is an accepted method to obtain TZ.12-15 A pathologist confirmed that that the TZ contained only benign tissue. All samples were snap frozen. Approximately 1 g of TZ was powdered under liquid nitrogen, mixed, and used for mass spectrometry analysis of the cholesterol pathway and steroid levels, and qPCR. IHC was performed on formalin-fixed tissue from the same patients. Patient demographics and disease characteristics including voiding symptoms and medications used were collected in anonymized fashion. Patient were stratified into subgroups depending on medication used for treatment of BPH (α-blockers, 5ARI, combination, or none). The I-BPH population was limited to patients on no medical treatment or only α-blockers, since 5ARI use is rare in men with prostate cancer.
2.2 ∣. Cholesterol and precursors analysis
Precursors to cholesterol synthesis and cholesterol (n = 46) were measured by a TSQ Quantum Ultra mass spectrometer (ThermoFisher) from snap frozen TZ tissue samples from S-BPH and I-BPH patients. For details see Supporting Information: Patients and Methods on cholesterol and precursors analysis.
2.3 ∣. Steroid analysis
Snap frozen human TZ prostate samples (n = 87) were analyzed using an expansion and modification of a high-pressure liquid chromatographic assay with tandem mass spectrometric detection (LC-MS/MS) for androstenedione (ASD), testosterone (T), dehydroepiandrosterone (DHEA), DHT, and androsterone (AND).16 Modifications were made to the validated assay (Guidance for Industry, Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, May 2001) to analyze prostate tissue and incorporate additional metabolites that made the assay a semiquantitative tool to assess the steroidogenesis pathway for GC, mineralocorticoids, and androgen metabolites. Thirty-two authentic standards were acquired from Steraloids or Sigma-Aldrich, dissolved, and infused on the mass spectrometer to find the optimal transitions for detection (Supporting Information: Table 1). An assay previously validated for the measurements of androgen hormones was modified to include the following list of metabolites.16 The modifications include: (1) changing the HPLC column, (2) modifying the gradient, and (3) including a one-point calibration sample for each metabolite being measured. The HPLC column was changed to a CORTECS® C18 + (2.1 × 150 mm; 2.7 μ m; Waters) column and mobile phase A was changed to 40% methanol instead of 65% methanol in the previously published assay to improve chromatographic separation between closely eluting metabolites; column temperature was unchanged. The gradient was extended making the total run time 35 min (starting conditions were 0% B increasing to 100% B over 23 min, held at 100% B for 5 min before returning to 0% B for re-equilibration). To extract tissue, tissue samples were weighed and homogenized in 25% methanol and the same amount of homogenate analyzed as the validated plasma method. The resulting reconstituted residue was centrifuged and the supernatant injected. Single-point calibration samples were prepared and extracted alongside the prostate tissue samples and injected at the beginning and end of the run as well as in the middle of the analytical run to monitor for any retention time drift and stability during the run. Quantitation was performed by regressing the single point calibrator through zero, and samples with a signal-to-noise ratio less than 3 were reported as BLQ.
2.4 ∣. Organoid culture
Prostate cell lines PZ-HPV-7 and RWPE-1 were purchased from ATCC. Both were maintained in keratinocyte serum free media supplemented with bovine pituitary extract (0.05 mg/ml) and human recombinant epidermal growth factor (5 ng/ml). Benign prostate cell lines BHPrE-1 and NHPrE-1 provided by Simon Hayward were maintained in dulbecco's modified eagle medium (DMEM) F12 (1:1) supplemented with 5% FBS, 0.4% bovine pituitary extract, 1% antibiotic/antimycotic, 1% Insulin-Transferrin-Selenium, and 10 ng/ml human recombinant epidermal growth factor. 3D embedded culture was performed as described in Application_Note_CLS-DL-AN-414_Matrigel_Matrix_3D_In_Vitro_Protocol. pdf (Corning Life Sciences) using DMEM F12 (1:1) media described above with the substitution of 5% charcoal stripped FBS. Cultures were treated with ethyl alcohol (1:1000) as vehicle control or dexamethasone (Dex) 10 nM. Harvesting of cells was performed as follows. Each well was washed 3x with cold PBS pH 7.4. Matrigel was solubilized by adding 1 ml 5 mM EDTA/PBS on ice with shaking for 1–2 h. Cells were transferred to 1.5 ml tube, centrifuged at 4° (10,000 rpm) washed 2x with cold PBS and cell pellet frozen for protein and or RNA. Two cell lines, RWPE-1 and PZ-HPV-7, are reported to spontaneously bud/branch in 3D culture. By mass spectrometry, we measured the levels of steroids in the recommended media for RWPE-1 and PZ-HPV-7 and found that this media contained cortisol at 60 ng/ml (0.17 μM) while the recommended media for BHPrE1 and NHPrE1 contained <0.12 ng/ml. We found that in the recommended media, RWPE-1 and PZ-HPV-7 did “spontaneously” bud/branch but no longer would when the media was changed to the same used for BHPrE1 and NHPrE1.
2.5 ∣. Statistical analysis
The cholesterol and steroid pathway or qPCR experimental groups were compared using two-sample t-tests. Significance was defined as p < 0.05. Analysis was performed using SigmaPlot software. In the clinical tables, continuous variables were assessed with a two-tailed, nonpaired student's t-test. Dichotomous variables were assessed using Fisher's exact test. In both cases α was designated as 0.05.
3 ∣. RESULTS
3.1 ∣. Study population
The median age of patients for cholesterol pathway analysis with S-BPH (n = 36) was 67 years and for patients with I-BPH (n = 10) was 60 years (Table 1). For the cohorts used to measure TZ tissue steroid levels, the median age of patients with S-BPH (n = 55) was 69 years and I-BPH (n = 32) was 62 years (Supporting Information: Table 1). At the time of surgery, S-BPH men were either on no medication (none), 5ARI, α-blockers, or combination α-blockers plus 5ARI. At the time of surgery, I-BPH men were either on no medical therapy (none) or on α-blockers. Although BPH nodules do occur in the TZ of I-BPH patients, the overall data supports that the I-BPH patient population are statistically significant to have a lower incidence of treatment for LUTS, a smaller prostate volume, and minor symptoms of BPH/LUTS when compared to the S-BPH group (Supporting Information: Table 1). The same samples were used for all assays when possible; extra samples were added to expand the number of patients in some groups.
TABLE 1.
Demographics of patients included in cholesterol pathway analysis
| Patient type | S-BPH | I-BPH | p Value |
|---|---|---|---|
| Number of patients | 36 | 10 | N/A |
| Median age (range) | 67 (53–82) | 60 (53–74) | 0.02* |
| Body mass index, kg/m2 (range) | 30 (18–44) | 28 (19–33) | 0.3 |
| Comorbidities | |||
| Hypertension | 29 (75%) | 6 (60%) | 0.43 |
| Diabetes | 7 (19%) | 4 (40%) | 0.22 |
| Dyslipidemia | 14 (39%) | 5 (50%) | 1 (NS) |
| Taking glucocorticoids | 1 (3%) | 0 (0%) | 0.72 |
| Medical treatment for BPH/LUTS | |||
| None | 7 | 6 | |
| α-blocker | 13 | 4 | 0.03* |
| 5ARI | 7 | 0 | |
| α-blocker + 5ARI | 9 | 0 | |
| IPSS | |||
| 0–7 | 2 | 1 | |
| 8–19 | 18 | 2 | 0.44 |
| >20 | 15 | 2 | |
| Number of patients stratified by preop prostate volume | |||
| <40 cc | 2 | 3 | |
| 40–59cc | 5 | 2 | 0.01* |
| >60 cc | 26 | 2 | |
| Gleason score | |||
| 3 + 3 | 0 | 10 | N/A |
Note: Surgical BPH (S-BPH) was defined as benign prostatic tissue collected from the transition zone (TZ) of patients who failed medical therapy and underwent surgical intervention to relieve LUTS. Control tissue was termed Incidental BPH (I-BPH). I-BPH was TZ obtained from men undergoing radical prostatectomy for prostate cancer confined to the peripheral zone. Medical treatment at the time of surgery was none; α-blocker (alpha-adrenergic receptor antagonist); 5ARI (5α-reductase inhibitor); or α-blocker + 5ARI.
Abbreviations: BPH, benign prostate hyperplasia; LUTS, lower urinary tract symptoms; N/A, not applicable.
p < 0.05.
3.2 ∣. Cholesterol and steroid synthesis are altered in 5ARI-treated patients
TZ patients (Table 1) were used to measure tissue levels of cholesterol, the precursor for steroid synthesis (Figure 1A), and the precursors to cholesterol synthesis (Supporting Information: Figure 1). Cholesterol was lower in S-BPH (n = 36) compared to I-BPH (n = 10) (p < 0.001) (Figure 1B). Cholesterol levels were similar between S-BPH and I-BPH medical therapy subgroups. However, six of nine cholesterol precursors were lower in S-BPH compared to I-BPH: lanosterol (p < 0.05), 24-dehydrolathosterol (p < 0.001), desmosterol (p < 0.05), lathosterol (p < 0.001), 7-dehydrocholesterol (p < 0.05), and 8-dehydrocholesterol (p < 0.05) (Supporting Information: Figure 1). Although three cholesterol precursors were not significantly different between S-BPH and I-BPH patients, two showed a trend toward lower levels in S-BPH patients. There were no significant differences among cholesterol precursors for patients on different medical therapies within S-BPH or I-BPH subgroups (Supporting Information: Figure 1). Thus, not only are the cholesterol levels significantly lower in S-BPH compared to I-BPH, cholesterol precursors are also lower. Therefore, metabolism of cholesterol alone did not account for lower levels in S-BPH; instead, the overall cholesterol synthesis pathway was downregulated in S-BPH compared to I-BPH.
FIGURE 1.
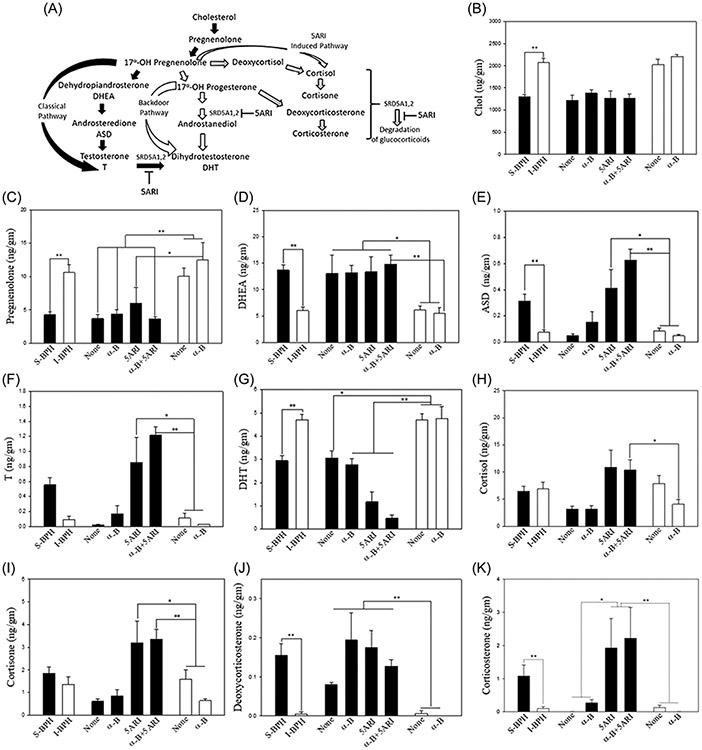
Regulation of prostatic steroid synthesis. Prostaticsteroidogenesis shows 3 pathways that can account for changes in androgens andglucocorticoid. A Testicular synthesis is the prime source of prostatictestosterone (T). T can be synthesized by the classical pathway (black arrows)or the backdoor pathway (white arrows). 5ARIs block both SRD5A1 and SRD5A2 reducing the conversion of T to DHT within the prostate. However, 5ARI also canblock the synthesis of DHT by the backdoor pathway (white arrows). This treatment would divert 17α-OH pregnenolone and SRD5A2 reducing the conversion of T to DHT within the prostate. However, 5ARI also canblock the synthesis of DHT by the backdoor pathway (white arrows). This treatment would divert 17α-OH pregnenolone and 17α-OH progesteroneinto pathways for the synthesis of glucocorticoids (gray gradient arrows). 5ARI also block the degradation of glucocorticoids to dihydrometabolites2; (B).Cholesterol levels (μg/gm tissue), the precursor for all steroid synthesis,were higher in I-BPH (white bars, n = 10) than S-BPH (black bars, n = 36) regardless of treatment; (C). Pregnenolone; (D). DHEA (dehydroepiandrosterone); (E). ASD (androsteredione); (F). T (testosterone); (G). DHT (dihydrotestosterone); (H). cortisol; (I).Cortisone; (J).deoxycorticosterone; (K) corticosterone: Medical treatment at the time of surgery was none; α-B (α-blocker); 5ARI (5α-reductaseinhibitor); or α-B + 5ARI. Steroids levels were expressed as ng/gm tissue with S-BPH (n = 55) treatment groups none (n = 23), α-B (n = 17), 5ARI (n = 8), or α-B+5ARI (n = 7) and I-BPH(n = 32) treatment groups none (n = 7) or α-B (n = 25). Error bars (SD) and Pvalues were shown only when significance was achieved (*p < 0.05,**p < 0.001).
Cholesterol is converted to pregnenolone and from this steroid precursor, pathways diverge to produce T to DHT by the classical route and DHT by the “backdoor route”17 (Figure 1A). Circulating T, mainly of testicular origin, is converted by steroid 5-α-reductase 1 (SRD5A1) and steroid 5-α-reductase 2 (SRD5A2) to produce the intracellular androgen, DHT. A total of 32 steroids were measured using mass spectrometry in prostatic TZ tissue in S-BPH (n = 55) and I-BPH (n = 32) (Supporting Information: Table 2 and Supporting Information: Figure 1). Pregnenolone (Figure 1C) and 17-OH-Pregnenolone (Supporting Information: Figure 1K) were lower (p < 0.001) while DHEA (Figure 1D) was elevated (p < 0.001) in all S-BPH versus I-BPH patients. This pattern was observed regardless of the medical therapy in S-BPH patients. The 5ARI, finasteride, blocks both the classical and backdoor route for DHT synthesis (Figure 1A). As a result, ASD and T (Figure 1E,F) increased in patients on 5ARI (p < 0.05) or 5ARI plus α-blockers (p < 0.001) for each steroid compared to I-BPH patients. 17-OH progesterone (Supporting Information: Figure 1L) and epiandrosterone (Supporting Information: Figure 1M) can serve as precursors to ASD while 17-OH progesterone can follow the backdoor pathway to DHT. Patients on 5ARI have higher levels of 17-OH progesterone (p < 0.05) than I-BPH (Supporting Information: Figure-1L). This difference contributed to an elevation of ASD and T in all S-BPH patients; however, levels in S-BPH medical treatment groups (none and α-blockers) remained low and similar to the I-BPH groups. Consistent with this finding, DHT levels were lowest in S-BPH patients on 5ARI (p < 0.001) or none (p < 0.05) while DHT levels were higher in patients not taking 5ARI (Figure 1G). These results indicated that the 5ARI was effectively blocking DHT synthesis even in S-BPH patients who had failed medical therapy. Furthermore, S-BPH patients not taking 5ARI had lower levels of DHT than I-BPH patients (p < 0.05; Figure 1G). The median age of S-BPH was greater than I-BPH patients, which may have contributed to S-BPH patients having reduced testicular levels of androgens.
SRD5A1,2 regulate synthesis of steroids other than androgens, such as GC (Figure 1A). Changes in GC levels that showed a distinct profile in prostatic tissues based upon 5ARI treatment was designated as the “5ARI-Induced Pathway” (Figure 1A). Since 5ARI inhibits synthesis of DHT by the classical and backdoor route (Figure 1A), 17α-OH pregnenolone (Supporting Information: Figure 1K) and 17-OH progesterone (Supporting Information: Figure 1L) precursors for androgens could be diverted to synthesis of other products, specifically GCs such as cortisol and corticosterone. Furthermore, inhibition of SRD5A1,2 alters general steroid metabolism by preventing degradation of GC to dihydrometabolites18 (Figure 1A). 17-OH pregnenolone is the precursor of cortisol (active) and cortisone (inactive) while 17-OH progesterone is the precursor for ASD,17 deoxycorticosterone (inactive), and corticosterone (active) (Figure 1A). Cortisol is only significantly changed (p < 0.05) when patients on when S-BPH (α-blocker + 5ARI) was compared to I-BPH (α-blocker) (Figure 1H). Cortisol levels were not significant when compared to I-BPH patients on none (no medical therapy: Figure 1H). However, the downstream inactive cortisone was elevated in S-BPH patients on 5ARI or α-blocker + 5ARI when compared to I-BPH either on none or α-blocker (p < 0.05; Figure 1I). Deoxycorticosterone, the precursor to corticosterone, was elevated in all S-BPH patients, regardless of medical treatment, when compared I-BPH patients on α-blockers or none (p < 0.001; Figure 1J). Importantly, the active corticosterone was only significantly elevated in S-BPH patients on 5ARI or α-blocker + 5ARI when compared to either S-BPH patients on none or α-blockers (p < 0.05; Figure 1K) or I-BPH patients (p < 0.001: Figure 1K) (Note: patients on 5ARI have higher, but not statistically significant, tissue levels of progesterone [Supporting Information: Figure 1N], another downstream product of 17-OH progesterone [Supporting Information: Figure 1L]). In summary, active cortisol is not significantly different and slightly elevated in I-BPH patients on none (no medical therapy) when compared to S-BPH; however, the active corticosterone is only significantly elevated in S-BPH patients on α-blocker + 5-ARI or 5ARI alone and below the limits of detection in all I-BPH patients, regardless of medical treatment. Taken together, the 5ARI induced pathway increased GC levels while blocking the conversion of T to DHT.
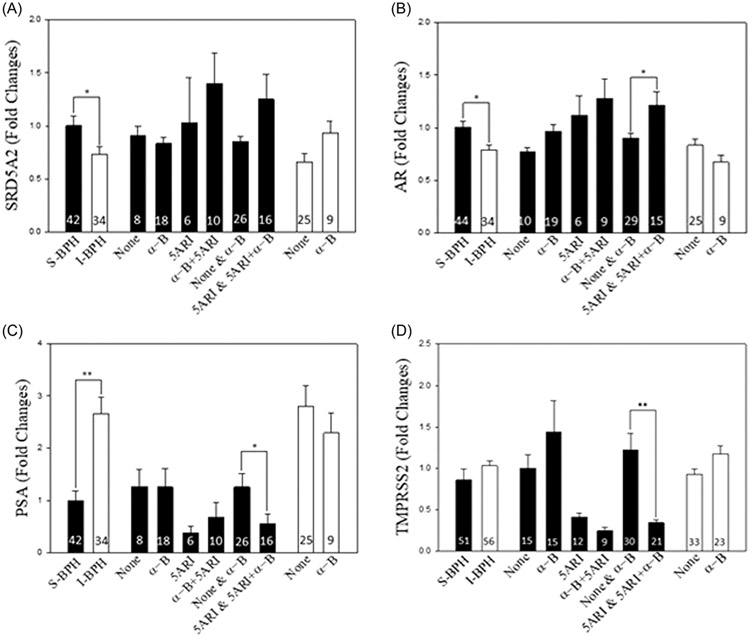
3.3 ∣. Prostate epithelial AR activity is decreased in 5ARI-treated patients that fail therapy
SRD5A2 levels have been reported to increase in patients on 5ARI,8 which may serve as a feedback mechanism to allow for continued production of DHT. Further, although 5ARI continue to reduce DHT levels in BPH patients that fail medical therapy, low levels of DHT have been reported to be sufficient to activate AR in castration-resistant prostate cancer.19 Here, levels of SRD5A2 mRNA were higher in S-BPH compared to I-BPH patients (p < 0.05) and, within the S-BPH group, patients on 5ARI and α-blockers plus 5ARI tended to have higher levels of SRD5A2 mRNA than patients on no medical therapy (none) or α-blockers (Figure 2A). Although AR mRNA levels were higher in S-BPH compared to I-BPH (p < 0.05), patients on 5ARI and 5ARI plus α-blockers had higher levels of AR mRNA than patients on no medical therapy (none) or α-blockers (p < 0.05; Figure 2B). The levels of AR mRNA between I-BPH and S-BPH patients on no medical therapy (none) or α-blockers were similar, which indicated that 5ARI was driving the increase in AR mRNA (Figure 2B). Even though SRD5A2 and AR mRNA increase in S-BPH patients, levels of two androgen target genes, prostate-specific antigen (PSA) (p < 0.05; Figure 2C) and transmembrane serine protease 2 (TMPRSS2) (p < 0.001; Figure 2D) mRNA were lower in S-BPH patients who were on α-blocker + 5ARI or 5ARI when compared to S-BPH on either none or α-blocker. This is consistent with DHT levels being the lowest in patients treated with 5ARI (Figure 1G) when compared either other S-BPH or I-BPH patients. These results indicated that low levels of DHT in patients on 5ARI are not sufficient to activate expression of AR target genes.
FIGURE 2.
Androgen regulation of target gene expression. Androgen regulation of mRNA levels for (A); SRD5A2 (steroid 5-α-reductase 2); (B) AR (androgen receptor); (C) PSA (prostate specific antigen); and (D) TMPRSS2 (transmembrane serine protease 2). The number of patients in each treatment group appears within the bar. Medical treatment at the time of surgery was none; α-B (α-blocker); 5ARI (5α-reductase inhibitor); or α-B + 5ARI. qPCR levels were expressed as fold change from the mean of S-BPH samples. Error bars (SD) and P values were shown only when significance was achieved (*p < 0.05, **p < 0.001).
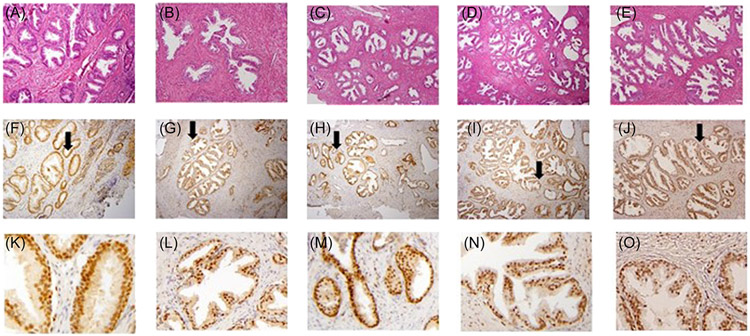
3.4 ∣. Glucocorticoid receptor (GR) activity induces prostate epithelial branching
Nuclear localization of steroid receptors indicates activation. Immunohistochemistry (IHC) was performed for GR (NR3C1) to determine cellular localization in prostatic tissue. Five patients for each medical treatment subgroup in S-BPH and I-BPH were selected for IHC. GR staining was nuclear in basal, luminal, and stromal cells in all S-BPH and I-BPH patients regardless of medical treatment (Figure 3A-O). Histological examination of cytosolic versus nuclear location of GR could not distinguish any difference between S-BPH or I-BPH patients. Also, the level of GR (NR3C1) mRNA was similar between S-BPH and I-BPH or within medical treatment groups (Supporting Information: Figure 2). Although AR drives prostatic growth, information on the functional role of GR in prostatic development is sparse. The function of the high levels of GC and nuclear GR in S-BPH patients on 5ARI was tested using organoid culture of benign prostatic cell lines. In 2D cultures, these cell lines retain basal characteristics (KRT5/KRT14) where they express no or low levels of AR, and no NKX3.1 or PSA.20 In 3D culture, these cell lines form organoids, where the cells organize into basal/luminal cell layers with luminal cells positive for KRT8/18, AR, NKX3.1, and PSA.20 With time, these organoids spontaneously bud and branch to form prostatic glands.20,21
FIGURE 3.
Glucocorticoid receptor expression in prostatic transition zone tissue. Immunohistochemistry of 5 patients in each medical treatment group were stained for GR and representative staining was presented. The first row showed H&E staining, the second row showed IHC on low power where the black arrow showed the region magnified for row 3. S-BPH patients were shown for no medical therapy (none) in (A)/(F)/(K), on α-blockers in (B)/(G)/(L), or on 5ARI in (C)/(H)/(M) (patients on 5ARI + α-blockers were similar to patients on 5ARI alone, not shown). I-BPH patients on none in (D)/(I)/(N) and on α-blockers in (E)/(J)/(O). Nuclear staining for GR was found in both stroma and epithelium (basal and luminal cells) and staining was similar in S-BPH and I-BPH patients.
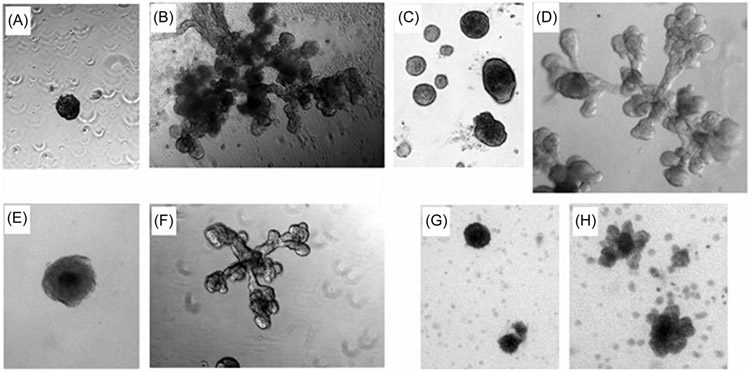
Two cell lines, RWPE-1 and PZ-HPV-7, have been reported to spontaneously bud/branch in 3D culture. The levels of steroids in the recommended media for RWPE-1 and PZ-HPV-7 was measured using mass spectrometry and this media contained 60 ng/ml (0.17 μM) cortisol while the recommended media for BHPrE1 and NHPrE1 contained <0.12 ng/ml. RWPE-1 and PZ-HPV-7 “spontaneously” budded/branched in the recommended media but did not when the media was changed to the same used for BHPrE1 and NHPrE1. Dex, a synthetic glucocorticoid, was used to test the role of GR in organoid culture on BHPrE1, RWPE-1, PZ-HPV-7, and NHPrE1 cell lines. A low power image of BHPrE1 treated cells revealed limited budding/branching in control but a robust response to Dex in treated cells (Supporting Information: Figure 3). In four cell lines, Dex treated organoids showed increased budding and branching compared to controls (Figure 4 and Table 2). Although low levels of steroids such as DHT (0.1–1 nM) can cause a physiological response in LnCaP cells, we found that Dex at 10 nM was optimal. However, we cannot rule out that this higher concentration of Dex can have some off-target effects that may induce budding and branching.
FIGURE 4.
Dexamethasone induction of budding and branching. At the beginning of 3D culture, either control cells (n = 3) were treated with vehicle or experimental cells with Dex (n = 3). BHPrE1: (A) Control, (B). Dex; RWPE-1 cells: (C). Control, (D). Dex; PZ-HPV-7 cells: (E). Control, (F). Dex; NHPrE1 cells: (G). Control, (H). Dex. Cells showed some spontaneous budding and branching and extent of the response to Dex treatment varied with the NHPrE1 showing the least budding. All cultures were maintained for 12-15 days on Matrigel in 3D that allowed them to form organoids and respond to Dex treatment.
TABLE 2.
Analysis of Dex-induced branching
| Cell lines | Treatment | Branched organoids (%) |
|---|---|---|
| RWPE-1 | Control | 5.2 ± 0.86 |
| Dex | 66.3 ± 7.77 | |
| BHPrE1 | Control | 2.8 ± 1.24 |
| Dex | 46.7 ± 3.26 | |
| PZ-HPV-7 | Control | 2.8 ± 0.87 |
| Dex | 19.0 ± 0.09 |
Note: Low power images that would include the complete culture well were taken and the number of organoids in each well and the number of organoids with branching were counted. The number of branched organoids is presented as a percentage of the total organoids. Duplicate wells for each cell type were analyzed + range. To determine statistical significance all control (n = 6) and Dex treatment (n = 6) regardless of cell type were combined (p < 0.002).
Abbreviation: Dex, dexamethasone.
4 ∣. DISCUSSION
In this study, we obtain control tissue to compared to the BPH/LUTS patients from the TZ of patients undergoing a radical prostatectomy from patients with low volume and low grade prostate cancer (Gleason 6 or 7) that is localized to the peripheral zone. This approach is not ideal since the control tissue may be influenced by a field effect caused by the prostate cancer on the TZ; however, collecting control tissue for this study that is not medically warranted would be unethical. Thus, using radical prostatectomy patients to collect control samples in any size beyond a biopsy core is viewed as an accepted method to obtain TZ.12-15 Additionally, clinical studies have shown an association between BPH/LUTS, diabetes, dyslipidemia, and inflammation22 but our study does not find any statistically significant association. This lack of statistical significance could be due, in part, to a relatively small sample size of our study to test for clinical association.
The MTOPS trial was able to use needle biopsy cores to determine the effectiveness of medical therapy for the average BPH/LUTS patient, but the mechanism that causes failure of medical treatment in some patients remains uncertain. Our study measured tissue steroid levels on snap frozen TZ of S-BPH patients compared to I-BPH controls to better understand hormonal changes that may occur with failure of therapy. A previous report by Liu et al.,23 based upon RNA-seq analysis in 5ARI-treated patients, revealed that cholesterol synthesis mRNAs were downregulated in BPH patients. We found that the synthesis of cholesterol was reduced significantly in all S-BPH patients, regardless of whether they were on 5ARI, α-blockers, or no medical treatment. Cholesterol is the building block for all steroids with androgens synthesized by the classic and/or the backdoor pathway (Figure 1A). All S-BPH also had significantly increased levels of DHEA, an early precursor in the synthesis of androgens (Figure 1D). The increased production of DHEA is not sufficient to significantly lower cholesterol levels that are >1000 ug/g tissue since DHEA levels increase only to about 5 ng/g tissue. Thus, the decrease in cholesterol synthesis pathway is specific to BPH/LUTS patients who fail medical therapy and not the type of medical treatment.
Previous reports have shown that 5ARI reduces DHT tissue levels24 and reduces AR-mediated gene expression,23 but we are not aware of any reports that examine tissue DHT levels in 5ARI patients who fail medical therapy for BPH. Although low intracrine tissue synthesis of DHT is sufficient to drive castration-resistant prostate cancer,19 our evidence suggests that the low levels of DHT seen in BPH patients on 5ARI are insufficient to drive prostatic growth since AR target gene mRNAs are reduced by 5ARI. Although we report lower overall DHT in S-BPH (median age 69) vs I-BPH (median age 62) on 5ARI, this lower level is not due to the age difference since T levels are similar in S-BPH patients on no medical treatment or α-blockers to I-BPH patients. Further, treatment with 5ARI lowers DHT and raises T indicating that the drug is pharmacodynamically effective in patients who fail this therapy. SRD5A2 is reported to be significantly increased in S-BPH compared to I-BPH,8 a finding we confirm herein. However, increasing T and decreasing DHT levels in patients on 5ARI show that increased SRD5A2 mRNA, which does not necessarily translate into protein, does not overcome drug inhibition of this enzyme. Although S-BPH levels of AR mRNA are significantly higher than I-BPH, the 5ARI treatment group appears to be responsible for raising the AR mRNA levels (Figure 2B). PSA and TMPRSS2 mRNA, both AR target genes, are lowest in patients on 5ARI treatment, which supports the idea that 5ARI continues to block the AR pathway in patients that fail medical treatment for BPH/LUTS (Figure 2C,D). Again, this raises the same question of the MTOPS trial—why do patients fail or never respond to medical therapy?
Inhibition of 5α-reductase alters steroid synthesis by conversion of T to DHT and direct synthesis of DHT via the backdoor pathway. However, measurements of 32 steroid levels in prostatic tissues revealed a new 5ARI-induced pathway that results in increased prostatic GC levels (Figure 1A). Blocking both the classic and backdoor pathway can shunt 17-α-pregnenolone toward synthesis of cortisol via deoxycortisol and to corticosterone via deoxycorticosterone from 17-α-OH progesterone (Figure 1A). Further, inhibition of 5α-reductase blocks the degradation of GC to dihydrometabolites18 (Figure 1A). Thus, patients on 5ARI show significant increases in prostatic tissue levels of active cortisol (Figure 1H) that is converted to inactive cortisone (Figure 1I) and of inactive deoxycorticosterone (Figure 1J) that is converted to active corticosterone (Figure 1K). Russell and Wilson reported that 5α-reductases (SRD5A1/2) can reduce not only reduce T to DHT but also other steroids such as GC, progestogens, and mineralocorticoids suggesting that the SRD5A enzymes can alter the action of many steroids, not just androgens.25 Further, GC were shown to induce growth of benign prostatic cells in culture resulting in a recommendation that GC should be added to culture media for prostate cell lines.26 Thus, patients on 5ARI show significant increases in prostatic tissue levels of active cortisol (Figure 1H) that is converted to inactive cortisone (Figure 1I) and of inactive deoxycorticosteron (Figure 1J) that is converted to active corticosterone (Figure 1K). The 5ARI-induced pathway (Figure 1A) may alter the steroid synthesis pathway in prostatic tissue and/or possibly change uptake of steroids from the blood.27,28 Further, a recent study shows that BPH patients on dutasteride, a dual inhibitor of SRD5A1/2, alters metabolic function to increase glucose, lipid profiles, and HbA1c (a prediabetic indicator) compared to patients on α-blockers.29 Increase adverse metabolic effects are seen when 5ARI are administrated with the glucocorticoid prednisolone compared to GC alone.30 Also, 5ARI increased serum prednisolone levels,30 likely due to reduction of steroids degradation.2530 Thus, the combination of 5ARI with GC can increase adverse metabolic effects associated with the administration of GC. Regardless of the source of GC from the serum or synthesis/degradation by BPH tissue, 5ARI can induce increase in prostatic GC that can have a major effect on prostate growth.
We examined GR staining in BPH patients and found similar nuclear staining in basal and luminal epithelium and stroma between S-BPH and I-BPH patients. Tissue levels of GR mRNA showed no statistical change between these groups or with 5ARI treatment (Figure 3B). Nuclear localization of a steroid receptor implies transcriptional activity, but the presence of active corticosterone only in patients on 5ARI (Figure 1K) and the presence of active cortisol (Figure 1H) implies specificity of these GC to activate the GR. To test the potential role of elevated tissue GC in patients on 5ARI, we treated four benign prostatic cell lines that form organoids in 3D culture with Dex (a synthetic GC). Dex induced prostatic branching (Figure 4). Budding and branching are required for normal development of the prostate, lung, and mammary gland as well as other organs.31-35 Renewed growth of the prostate with aging requires re-initiation of this developmental process. Morphogenesis of the prostate requires androgen activation of the AR in the stroma but the induction of prostatic branching in 3D by Dex indicates that GR is involved in prostatic regrowth in BPH. Multiple pathways contribute to glandular architecture of the prostate, but the master switch that starts this cascade of events remains unidentified. During lung maturation, GR and Nfib, both transcription factors, have overlapping functions.36 We reported that NFIB is an AR coactivator37 and that knock-out of Nfib in the mouse prostate induces hyperplasia.38 In human BPH, the loss of luminal NFIB correlated with severe disease.38 Further, GC are required for fetal lung maturation but not in postnatal life.39 Recent studies reveal that corticosterone induces prostatic hyperplasia in mice.40 Taken together, these results are consistent with a fundamental role for GR as a master regulator of branching in the prostate and perhaps in other branching organs, such as the lung.
The Prostate Cancer Prevent Trial (PCPT) studied 18,882 men to determine if 5ARIs reduced the risk of prostate cancer. The findings remain controversial since the trial found the overall risk of prostate cancer was reduced but the risk of developing high-grade prostate cancer was increased.41 An 18-year follow-up of PCPT participants found no difference in overall survival.42 Regardless, the PCPT resulted in 5ARI not being approved for use to reduce the risk of prostate cancer. A change in sampling density, because of reduced prostate size, has been hypothesized to have caused the observed change in high grade prostate cancer incidence.43 However, no biologic explanation has been provided from the PCPT as to why 5ARI increased the risk of developing high-grade prostate cancer. Our data that 5ARIs increase GC levels provides a mechanism consistent with new reports on GR action in prostate cancer. Sawyers reported that GR might be driving castration-resistant prostate cancer growth.44 This finding has resulted in new efforts to block GR activity in advanced prostate cancer.45 Although Sawyers reported that GR could replace AR to drive prostate cancer growth.44 our studies in the BPH tissue of patients on 5ARI do not show GR can replace AR, since the AR target genes PSA and TMPSS2 remain suppressed. However, our data that GC induce budding/branching is consistent with a fundamental role for GR in prostatic growth.
5 ∣. CONCLUSIONS
BPH is associated with altered stromal-epithelial communication. MTOPS provided evidence of successful medical treatment of BPH but raised new questions as to why some men progress while others never respond to therapy. Our data show that 5ARIs induce a new pathway that results in the accumulation of GC in prostatic tissue. We propose that GR is a master regulator of prostatic growth. Mechanistically, this may explain why BPH patients fail 5ARI treatment and suggests a novel pathway that could account for the increase in high-grade prostate cancer in participants of the PCPT. However, BPH patients who fail treatment when receiving α-blockers or not under medical therapy do not show a rise in GC levels. This indicates that different mechanisms are responsible for the failure of agents that relax smooth muscle compared to drugs that block AR activity. More extensive analysis of pathways that change and cause failure of treatment in BPH patients is required to improve medical therapy.
Supplementary Material
ACKNOWLEDGMENTS
The use of human tissue is approved by the Vanderbilt University Medical Center Internal Review Board (#120944). Tissue for this study was collected by the Cooperative Human Tissue Network (CHTN) at Vanderbilt University Medical Center. The authors thank the patients for their generously donated tissue for this study. We thank Ms. Shaw Carter and Ms. Darlene Hancock for assistance in the preparation and submission of the manuscript and Dr. John Wilton for guidance on mass spectrometric assay development for tissue steroid levels and data analysis. This study was supported by William L Bray Chair, the Bray Foundation, and NIDDK 5R01 DK111554 to R. J. M., R01 DK115477 to D. W. S. Assay for steroids levels were performed by the Roswell Park Comprehensive Cancer Center Bioanalytical, Metabolomics, and Pharmacokinetics Shared Resource (BMPK) that is supported by the NCI grant P30CA016056 to J. L. M.
Funding information
NIH Clinical Center, Grant/Award Number: R01 DK115477; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: 5R01 DK111554
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data are available for bona fide researchers who request it from the authors.
REFERENCES
- 1.Bautista OM, Kusek JW, Nyberg LM, et al. Study design of the medical therapy of prostatic symptoms (MTOPS) trial. Controlled Clin Trials. 2003;24:224–243. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SA, Lee JY, Meehan AG, Kusek JW, Group MR. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol. 2011;185:1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roehrborn CG, Siami P, Barkin J, et al. Comb ATSG: the effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–131. [DOI] [PubMed] [Google Scholar]
- 5.Torkko KC, Wilson RS, Smith EE, Kusek JW, van Bokhoven A, Lucia MS. Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: findings from the MTOPS study. J Urol. 2015;194:454–461. [DOI] [PubMed] [Google Scholar]
- 6.Lucia MS, Torkko KC. Inflammation as a target for prostate cancer chemoprevention: pathological and laboratory rationale. J Urol. 2004;171:S30–S35. [DOI] [PubMed] [Google Scholar]
- 7.Austin D, Strand DW, Love HL, et al. NF-kappaB and androgen receptor variant expression correlate with human BPH progression. Prostate. 2016;76:491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin D, Strand DW, Love HD, et al. NF-kappaB and androgen receptor variant 7 induce expression of SRD5A isoforms and confer 5ARI resistance. Prostate. 2016;76:1004–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin-Tsai O, Clark PE, Miller NL, et al. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate. 2014;74:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin RJ, Strand DW, Forbes CM, et al. The prostaglandin pathway is activated in patients that fail medical therapy for benign prostatic hyperplasia (BPH) with lower urinary tract symptoms (LUTS). Prostate. 2021;81:944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickel JC, Roehrborn CG, Castro-Santamaria R, Freedland SJ, Moreira DM. Chronic prostate inflammation is associated with severity and progression of benign prostatic hyperplasia, lower urinary tract symptoms and risk of acute urinary retention. J Urol. 2016;196:1493–1498. [DOI] [PubMed] [Google Scholar]
- 12.Cantiello F, Cicione A, Salonia A, et al. Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: a prospective cohort study. Urology. 2013;81: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 13.Giri D, Ittmann M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2001;159:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Gharaee-Kermani M, Kunju L, et al. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol. 2012;188: 1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madigan AA, Sobek KM, Cummings JL, Green WR, Bacich DJ, O'Keefe DS. Activation of innate anti-viral immune response genes in symptomatic benign prostatic hyperplasia. Genes Immun. 2012;13: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilton JH, Titus MA, Efstathiou E, Fetterly GJ, Mohler JL. Androgenic biomarker prof∣ling in human matrices and cell culture samples using high throughput, electrospray tandem mass spectrometry. Prostate. 2014;74:722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armandari I, Hamid AR, Verhaegh G, Schalken J. Intratumoral steroidogenesis in castration-resistant prostate cancer: a target for therapy. Prostate Int. 2014;2:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasiri M, Nikolaou N, Parajes S, et al. 5 alpha-reductase type 2 regulates glucocorticoid action and metabolic phenotype in human hepatocytes. Endocrinology. 2015;156:2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. [DOI] [PubMed] [Google Scholar]
- 20.Strand DW, Degraff DJ, Jiang M, et al. Deficiency in metabolic regulators PPARgamma and PTEN cooperates to drive keratinizing squamous metaplasia in novel models of human tissue regeneration. Am J Pathol. 2013;182:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergthorsson JT, Magnusson MK, Gudjonsson T. Endothelial-rich microenvironment supports growth and branching morphogenesis of prostate epithelial cells. Prostate. 2013;73:884–896. [DOI] [PubMed] [Google Scholar]
- 22.Jiang M, Strand DW, Franco OE, Clark PE, Hayward SW. PPARgamma: a molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation. 2011;82:220–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Shoag JE, Poliak D, et al. Integrative multiplatform molecular profiling of benign prostatic hyperplasia identifies distinct subtypes. Nat Commun. 2020;11:1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzouni F, Mohler J. Role of 5alpha-reductase inhibitors in benign prostatic diseases. Prostate Cancer Prostatic Dis. 2012;15:222–230. [DOI] [PubMed] [Google Scholar]
- 25.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. [DOI] [PubMed] [Google Scholar]
- 26.Peehl DM, Stamey TA. Growth responses of normal, benign hyperplastic, and malignant human prostatic epithelial cells in vitro to cholera toxin, pituitary extract, and hydrocortisone. Prostate. 1986;8:51–61. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Godoy A, Azzouni F, Wilton JH, Ip C, Mohler JL. Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5alpha-reductase inhibitors. Prostate. 2013;73:1470–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Tang L, Azabdaftari G, Pop E, Smith GJ. Adrenal androgens rescue prostatic dihydrotestosterone production and growth of prostate cancer cells after castration. Mol Cell Endocrinol. 2019;486:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traish A, Haider KS, Doros G, Haider A. Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction. Horm Mol Biol Clin Investig. 2017;30:20170015. [DOI] [PubMed] [Google Scholar]
- 30.Othonos N, Marjot T, Woods C, et al. Co-administration of 5alpha-reductase inhibitors worsens the adverse metabolic effects of prescribed glucocorticoids. J Clin Endocrinol Metab. 2020;105: e3316–e3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donjacour AA, Cunha GR. The effect of androgen deprivation on branching morphogenesis in the mouse prostate. DevBiol. 1988;128:1–14. [DOI] [PubMed] [Google Scholar]
- 32.Reginensi A, Enderle L, Gregorieff A, Johnson RL, Wrana JL, McNeill H. A critical role for NF2 and the Hippo pathway in branching morphogenesis. Nat Commun. 2016;7:12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freestone SH, Marker P, Grace OC, et al. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264:352–362. [DOI] [PubMed] [Google Scholar]
- 35.Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol. 2005;288:334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lajoie M, Hsu YC, Gronostajski RM, Bailey TL. An overlapping set of genes is regulated by both NFIB and the glucocorticoid receptor during lung maturation. BMC Genomics. 2014;15:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabowska M, Elliott AD, DeGraff DJ, et al. Nuclear factor one transcription factors interact with FOXA1 to regulate prostate specific gene expression. Mol Endocrinol. 2014;6:949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grabowska M, Kelly SM, Reese AL, et al. Nfib regulates transcriptional networks that control the development of prostatic hyperplasia. Endocrinology. 2016;157:1094–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. [DOI] [PubMed] [Google Scholar]
- 40.Simanainen U, Lampinen A, Henneicke H, et al. Long-term corticosterone treatment induced lobe-specific pathology in mouse prostate. Prostate. 2011;71:289–297. [DOI] [PubMed] [Google Scholar]
- 41.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. [DOI] [PubMed] [Google Scholar]
- 42.Thompson IM Jr., Goodman PJ, Tangen CM, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan SA, Roehrborn CG, Meehan AG, et al. PCPT: evidence that finasteride reduces risk of most frequently detected intermediate- and high-grade (gleason score 6 and 7) cancer. Urology. 2009;73: 935–939. [DOI] [PubMed] [Google Scholar]
- 44.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valle S, Sharifi N. Targeting glucocorticoid metabolism in prostate cancer. Endocrinology. 2021;162:162, bqab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available for bona fide researchers who request it from the authors.