Abstract
Background
ABL-class fusions including NUP214-ABL1 and EBF1-PDGFRB occur in high risk acute lymphoblastic leukaemia (ALL) with gene expression patterns similar to BCR-ABL-positive ALL. Our aim was to evaluate new DNA-based measurable residual disease (MRD) tests detecting these fusions and IKZF1-deletions in comparison with conventional immunoglobulin/T-cell receptor (Ig/TCR) markers.
Methods
Precise genomic breakpoints were defined from targeted or whole genome next generation sequencing for ABL-fusions and BCR-ABL1. Quantitative PCR assays were designed and used to re-measure MRD in remission bone marrow samples previously tested using Ig/TCR markers. All MRD testing complied with EuroMRD guidelines.
Results
ABL-class patients had 46% 5year event-free survival and 79% 5year overall survival. All had sensitive fusion tests giving high concordance between Ig/TCR and ABL-class fusion results (21 patients, n = 257 samples, r2 = 0.9786, P < 0.0001) and Ig/TCR and IKZF1-deletion results (9 patients, n = 143 samples, r2 = 0.9661, P < 0.0001). In contrast, in BCR-ABL1 patients, Ig/TCR and BCR-ABL1 tests were discordant in 32% (40 patients, n = 346 samples, r2 = 0.4703, P < 0.0001) and IKZF1-deletion results were closer to Ig/TCR (25 patients, n = 176, r2 = 0.8631, P < 0.0001).
Conclusions
MRD monitoring based on patient-specific assays detecting gene fusions or recurrent assays for IKZF1-deletions is feasible and provides good alternatives to Ig/TCR tests to monitor MRD in ABL-class ALL.
Subject terms: Prognostic markers, Acute lymphocytic leukaemia
Introduction
ABL-class fusions are a feature of approximately 3% of paediatric acute lymphoblastic leukaemia (ALL) cases [1, 2] with similar gene expression patterns to Philadelphia chromosome positive (Ph-pos) ALL and with generally poor responses to standard induction chemotherapy. While Ph-pos ALL results from a t(9;22) translocation creating a BCR-ABL1 fusion; this subset of Ph-like ALL cases involve the fusion of another gene expressed during lymphocyte differentiation such as EBF1, SSBP2, ETV6, NUP214 with a gene encoding a tyrosine kinase or a receptor tyrosine kinase such as PDGFRB, CSF1R, ABL1 or ABL2. ALL patients with these ABL-class fusions are generally sensitive to tyrosine kinase inhibitors (TKI) including dasatinib in vitro [3] and adjuvant TKIs in patients [4, 5]. Two recent studies from the AIEOP-BFM and Pont di Legno groups showed the 5-year event-free survival (EFS) for patients with ABL-class fusions in the pre-TKI era was 49% (n = 46) [1] and 59% (n = 122) respectively [2].
The poor outcomes associated with both BCR-ABL1 ALL and Ph-like ALL mean that many treating clinicians request close MRD monitoring for these patients, during initial therapy and particularly for post-remission surveillance, including after HSCT. Moreover, in the TKI era, where HSCT is no longer indicated in many patients with BCR-ABL1 ALL [6], accurate determination of post-treatment MRD is critical to identify patients with sub-optimal TKI response, where HSCT may still offer the best chance of cure. However, in a previous study of BCR-ABL1 ALL [7], we demonstrated that MRD analysis using conventional MRD markers based on immunoglobulin and T-cell receptor (Ig/TCR) rearrangements fails to detect or underestimates MRD compared to qPCR genomic tests detecting the BCR-ABL1 gene fusion itself in some CML-like patients. It is also accepted in KMT2A-rearranged infant ALL, that MRD testing based on detection of this disease related fusion is not only feasible, but also preferable to Ig/TCR markers given the high incidence of oligoclonality and earlier stage of cell of origin that characterises ALL with translocations of the KMT2A gene (previously known as MLL) [8, 9]. This collective knowledge raised the question of the reliability of immunoglobulin and T-cell receptor gene markers (Ig/TCR) in patients with ABL-class fusions.
Both BCR-ABL1 ALL and Ph-like ALL have a high incidence of IKZF1 deletions [10, 11]. Copy number analysis by microarray or by MLPA has revealed a variety of IKZF1 deletions in ALL cases and their poor prognosis in newly diagnosed B-ALL was shown in German, Dutch, Italian and Australian cohorts [12–15]. International collaborations have included IKZF1 deletions in multifactorial risk analysis [16, 17] and provided evidence that most if not all IKZF1 deletions are associated with high risk of relapse [18] including recurrent internal deletions that are amenable to detection by generic qPCR MRD assays [19, 20]. These assays serve a dual purpose, with capacity to rapidly identify a subset of IKZF1 high risk patients as well as to measure MRD without requiring prior sequencing.
This study therefore set out to develop and evaluate patient-specific qPCR MRD assays for paediatric ALL cases with EBF1-PDGFRB, SSBP2-CSF1R, NUP214-ABL1 and other ABL1 gene fusions and to compare the MRD results obtained with those based on IKZF1-deletion and conventional Ig/TCR qPCR MRD measurements. We used two different next generation sequencing (NGS) strategies - targeted and whole genome sequencing - to determine the precise breakpoint sequences needed to design patient-specific qPCR assays for ABL-class fusions.
Methods
Patient samples
This study was conducted on DNA samples from 65 paediatric ALL patients with parental consent and human ethics approval. All bone marrow samples were originally tested for MRD in response to clinical requests and results reported in real-time. The same samples were stored for research including retesting (with technical triplicates) to compare MRD levels obtained using alternate MRD assays.
Identification of patients with ABL-class fusions
Patients with ABL-class fusions were provisionally identified by several methods: (a) G-banded karyotyping and fluorescent in situ hybridization as performed as standard of care with BCR, ABL1 and PDGFRB at some centres; (b) MLPA analysis performed with SALSA P335 ALL-IKZF1 A4 or B1 kit (MRC-Holland, Amsterdam, the Netherlands) [15] since we discovered that patients with EBF1-PDFGRB have heterozygous loss of EBF1 exon 16; (c) Ph-like TLDA expression pattern in unselected cohort [21] or (d) patients referred on basis of high risk features (defined by ANZCHOG 2014 guidelines). Fusion transcripts were analysed by RT-PCR and Sanger sequencing or RNA-Seq.
Following provisional identification, precise genomic breakpoints were identified in diagnostic DNA using multiplex long-distance PCR for BCR-ABL1 [7] or by analysis of targeted NGS for difficult BCR-ABL1 cases and 12 ABL-class cases.
Analysis of breakpoint sequence for ABL-class fusions from WGS sequence
Two of the ABL-class cases were enroled on the PRISM precision medicine trial (NCT03336931) which used WGS analysis to at least 90x-depth of leukaemia cell DNA and 30x germline DNA [22]. The other seven ABL-class cases were sequenced using WGS to 30x coverage with no matched germline, reasoning that the somatic ABL-class fusions would be readily identifiable. WGS was conducted at the Kinghorn Centre for Clinical Genomics, Garvan Institute of Medical Research (Australia), using the Illumina HiSeq X Ten platform with a paired-end read length of 150 bases. Sequencing libraries were prepared from more than 1 µg of DNA using KAPA PCR-Free v2.1 (Roche). Raw fastq files were aligned to the hs37d5 reference genome using BWA-MEM (v0.17.10-r789) [23] with resulting BAM file reads marked using Novosort (v1.03.01; default settings). For cases with a matched germline, the WGS data were analysed as previously described [22]. For cases without a matched germline, a tumour-only analysis pipeline was adopted, using the following steps: somatic SNVs and short indels (<50 bp) were identified using Sage (v2.2) [24] and germline variants were filtered out using a panel of normals. The panel of normals contains variants identified from 1000 germline controls, sequenced using WGS to 30–40x depth using HiSeq X10, where each variant was observed at least once, with at least three reads and a cumulative base quality of 30. Somatic variants were annotated using SnpEff (v4_3t) [25] and imported into the in-house Glooee platform for filtration and prioritization. Tumour purity, ploidy and somatic copy number variants (CNVs) were identified using PURPLE (v3.0) [24], and structural variants (SVs) were identified using GRIDSS (v2.9.4) [26] and then annotated using Ensembl genes. LINX (v1.16) was used to visualize SV clusters and derivative chromosomes [27].
MRD q-PCR assays to detect ABL fusions, IKZF1 deletions and Ig/TCR rearrangements
MRD tests for ABL-class fusions involved patient specific primers and a Taqman hydrolysis probe spanning the unique breakpoint sequence as reported previously for BCR-ABL1 [7] and testing bone marrow DNA samples usually retrospectively. The unique breakpoint sequences for these patients and custom primers and probes are shown in Supplementary Tables 1 and 2.
Routine PCR-MRD marker screening was performed by 24 single or multiplex PCR reactions on leukaemic DNA to detect rearrangements in immunoglobulin heavy and kappa genes and T-cell receptor gamma, delta, beta and delta-alpha genes (Ig/TCR) followed by heteroduplex analysis and direct Sanger sequencing. Unique breakpoint sequences were identified using the NCBI Nucleotide BLAST database, https://blast.ncbi.nlm.nih.gov/ or hardcopies circulated by the EuroMRD group. The actual MRD testing involved q-PCR assays to detect these markers using one patient-specific primer and a gene segment specific primer and hydrolysis probe performed on an Biorad Icycler or CFX platform in real time [28].
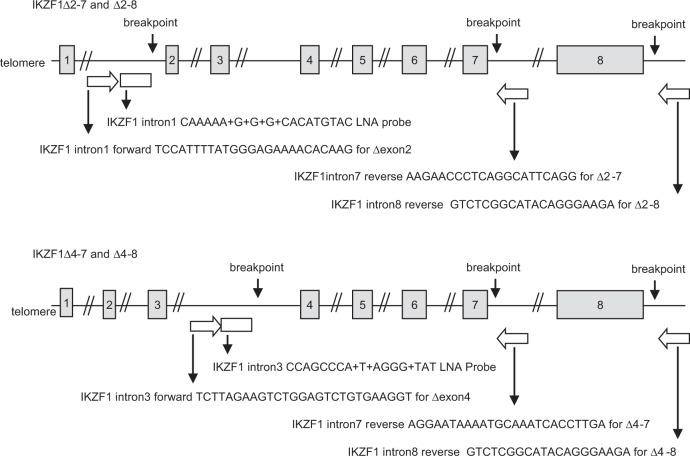
Diagnostic samples for all patients were screened for four IKZF1 MRD markers to detect internal gene deletions specifically IKZF1Δ2-7, IKZF1Δ4-7, IKZF1Δ2-8, IKZF1Δ4-8, using generic qPCR tests with custom made primers and Locked Nucleic Acid (LNA) probes (Integrated DNA Technologies). For patients with a high level of marker (>1 × 10−1 level present in positive controls), the same assay was then used with the patient’s own dilution curve to measure MRD in their remission samples.
Data analysis
All MRD qPCR tests, including the ABL-class fusion and IKZF1 deletion assays, were performed at the Children’s Cancer Institute and analysed according to the guidelines established by EuroMRD (van der Velden et al, 2007). Standard curves met minimum standards of >0.98 correlation coefficient and slope between 3.1 and 3.9. MRD was scored positive or negative according to the definition established for protocols in which therapy intensification is intended and with reference to normal peripheral blood mononuclear cell DNA samples.
When comparing MRD levels determined by different tests concordant results were defined as those with <1.0 log difference in quantitative results or either negative or non-quantifiable results with both marker tests. In contrast discordant results had >1.0 log difference in quantifiable results. When one marker gave a quantifiable result and the other gave non-quantifiable or negative result, the quantitative range was considered. Results were defined as discordant if there was <1.0 log difference between the quantitative result and the quantitative range for the assay used for non-quantifiable positive or negative result. Survival times were measured from date of diagnosis to date of relapse or death, or to last clinic visit for patients without these events. Kaplan-Meier survival curves were generated and log-rank tests applied using GraphPad Prism version 7.04.
Results
Description of patients with ABL-class fusions and BCR-ABL1
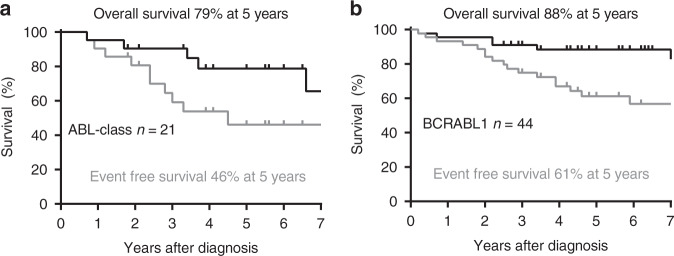
A database search of ALL patients with both qPCR MRD testing and research consent identified 21 paediatric patients with ABL-class fusions including 19 B-ALL and two T-ALL (Table 1). These patients were diagnosed between 2004 and 2018 with a median follow up of 5.5 years for survivors. We also identified an additional 44 children with BCR-ABL1 ALL diagnosed in that timeframe (median follow up of 5.7 years) for comparison. The most frequently identified ABL-class fusions were EBF1-PDGFRB (9 cases, 43%) and NUP214-ABL1 (4 cases, 19%). Two patients had SSBP2-CSF1R, two had other PDGFRB fusions and the remaining four patients had other ABL1 fusions. The patients were predominantly male (71%) with median age of 10 years and 82% had high MRD at end of induction. In contrast to the others, the two patients with ETV6-ABL1 fusion were both one year old females with complete MRD response (MRD negative) (Table 1). As a group, the ABL-class fusions had 5-year EFS of 46% and 5-year OS of 79%. While it appears from the Fig. 1a and b data, that outcomes may be poorer for patients with ABL-class compared to BCR-ABL1, these differences were not statistically significant. It is also worth noting that seven (33%) of the ABL-class patients did not receive a TKI (imatinib or dasatinib) in first remission, compared to one of 44 BCR-ABL1 patients (Table 1).
Table 1.
Characteristics of ALL patients included in ABL fusion marker study.
| ID | ALL type | ABL Fusion | Recurrent IKZF1del | Sex | Age (years) | Front-line therapy | End of Induction MRD (d28-35) | Relapse/SMN (mo) | Later therapy | HSCT (stage) | Death (mo) | Current Status | Discordant MRD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B | EBF1-PDGFRB | IKZF1 4-7 | M | 5 | BFM, no TKI | 6 × 10–2 | 23 | ALLR3, no TKI | CR1 + CR2 | CR2 | No | |
| 2 | B | EBF1-PDGFRB | neg | F | 11 | BFM, no TKI | 1 × 10–0 rel | 37 | ALLR3, no TKI | CR2 | 45 | deceased | No |
| 3 | B | EBF1-PDGFRB | IKZF1 4-7 | M | 14 | BFM, no TKI | 1 × 10–0 | – | CR1 | CR1 | No | ||
| 4 | B | EBF1-PDGFRB | neg | M | 3 | BFM, no TKI | 1 × 10–2 | 30 | ALLR3, imatinib | CR3 | CR3 | No | |
| 5 | B | EBF1-PDGFRB | IKZF1 4-8 | M | 6 | COG, imatinib | 5 × 10–2 | 40 | ALLR3, dasatinib | CR1 + CR2 | CR2 | No | |
| 6 | B | EBF1-PDGFRB | neg | M | 5 | COG, imatinib | 3 × 10–3 | – | – | no | CR1 | No | |
| 7 | B | EBF1-PDGFRB | IKZF1 4-7 | M | 14 | COG, imatinib, dasatinib | 1 × 10–1 | – | – | CR1 | CR1 | No | |
| 8 | B | EBF1-PDGFRB | neg | M | 7 | BFM, imatinib | 3 × 10–1 | – | CR1 | CR1 | No | ||
| 9 | B | EBF1-PDGFRB | scIKZF1 4-7 | F | 15 | COG, dasatinib | 8 × 10–2 | – | no | CR1 | No | ||
| 10 | B | CD74-PDGFRB | IKZF1 4-7 | F | 2 | COG, dasatinib | N/A | – | CAR-T, HSCT | CR1 | CR1 | No | |
| 11 | B | AT7IP-PDGFRB | neg | M | 9 | BFM, dasatinib | 7 × 10–2 | 11 | blinatumomab, CAR-T | CR1 + CR2 | CR3 | No | |
| 12 | B | ETV6-ABL1 | neg | F | 1 | BFM, no TKI | neg | 55 | ALLR3, no TKI | CR1 | CR2 | No | |
| 13 | B | ETV6-ABL1 | scIKZF1 4-7 | F | 1 | COG, no TKI | neg | – | – | no | CR1 | No | |
| 14 | B | IGSF11-ABL1 | IKZF1 4-7 | M | 14 | COG, dasatinib | 1 × 10–0 | – | vinc/steroid; HSCT | CR1 | CR1 | No | |
| 15 | B | SNX2-ABL1 | IKZF1 2-7 | M | 12 | COG, dasatinib | 3 × 10–2 | – | blinatumomab, HSCT | CR1 | CR1 | No | |
| 16 | B | NUP214-ABL1 | IKZF1 4-7 | M | 16 | BFM, dasatinib | 9 × 10–2 | – | – | CR1 | CR1 | No | |
| 17 | B | NUP214-ABL1 | IKZF1 4-7 | M | 12 | COG, imatinib | 4 × 10–1 | – | blinatumomab | CR1 | CR1 | No | |
| 18 | ETP | NUP214-ABL1 | neg | M | 10 | COG, dasatinib | 2 × 10–2 | 29 (SMN) | AML therapy | CR1 + CR2 | 41 | deceased | No |
| 19 | T | NUP214-ABL1 | neg | M | 14 | COG, dasatinib | 7 × 10–1rel | 15 | IntReALL | CR2 | 21 | deceased | No |
| 20 | B | SSBP2-CSF1R | neg | M | <1 | BFM, no TKI | neg | 34 | ALLR3, no TKI | intent | 79 | deceased | No |
| 21 | B | SSBP2-CSF1R | neg | F | 15 | BFM, dasatinib | 2 × 10–2 | – | – | CR1 | 9 | deceased | No |
| Total | B-ALL | IKZF1del | Male | Age (median, range) | Front-line TKI use % | High EOI MRD > 1 × 10–3 (%) | Relapse or SMN | HSCT % | Death | Current CR1 n, % | Discordant MRD | ||
| n = 21 | 90% | ABL-class fusions | 43% | 71% | 10 (0-16) | 67% | 85% | 43% | 81% | 24% | 52% | 0% | |
| n = 44 | 96% | BCR-ABL1 | 59% | 59% | 8 (0-17) | 98% | 66% | 32% | 68% | 16% | 61% | 33% | |
Each of the 21 ABL-class patients are shown in the top section and then summarised for comparison with the BCR-ABL1 patients in the bottom section. Patient 5 was included in Roberts et al. [3].
Fig. 1. Outcomes for ALL patients included in this study.
a Patients with ABL-class fusions including EBF1-PDGFRB and NUP214-ABL1 (b) patients with BCR-ABL1. The black line in each graph denotes overall survival and grey line event-free survival.
Comparison of ABL-class fusion MRD tests with Ig/TCR tests
In order to determine precise genomic breakpoints and to design fusion-detecting qPCR MRD assays, targeted next generation sequencing (NGS) was performed on diagnostic bone marrow DNA from the 44 BCR-ABL1 cases and 12 of the ABL-class patients. These included all cases with fusions involving ABL1, except one NUP214-ABL1, and five cases with PDGFRB fusions. For the remaining nine patients, whole genome sequencing (WGS) was performed and analysed for breakpoints including five with EBF1-PDGFRB, 1 ATP7IP-PDGFRB, 1 NUP214-ABL1 and 2 SSBP2-CSF1R. These breakpoints were used to design patient-specific qPCR assays composed of two primers specific for each of the genes involved and a hydrolysis probe spanning the precise breakpoint sequence. Each assay was then evaluated for quantitative range and sensitivity according to the widely used EuroMRD guidelines that evaluate amplification of duplicates in a standard curve created from a dilution series of the diagnostic bone marrow DNA sample and 6 normal DNA samples from mononuclear cells. The analysis in Fig. 2a showed that the assays measuring the ABL-class fusion breakpoints had adequate sensitivity (1 × 10-4) in all and were highly sensitive in most patients (1 × 10-5 in 81%), with an acceptable quantitative range (QR at least 1 × 10-4) for all patients except one with QR of 5 × 10-4 and superior quantitation in 71% (QR of 5 × 10-5). These standardised MRD assay metrics compared favourably with conventional Ig/TCR based MRD tests in the same patients and same samples tested earlier and reported in diagnostic MRD reports (Fig. 2a).
Fig. 2. Evaluation of quantitative range and sensitivity of MRD assays specific for gene fusions, clonal Ig/TCR rearrangements and recurrent IKZF1 deletions.
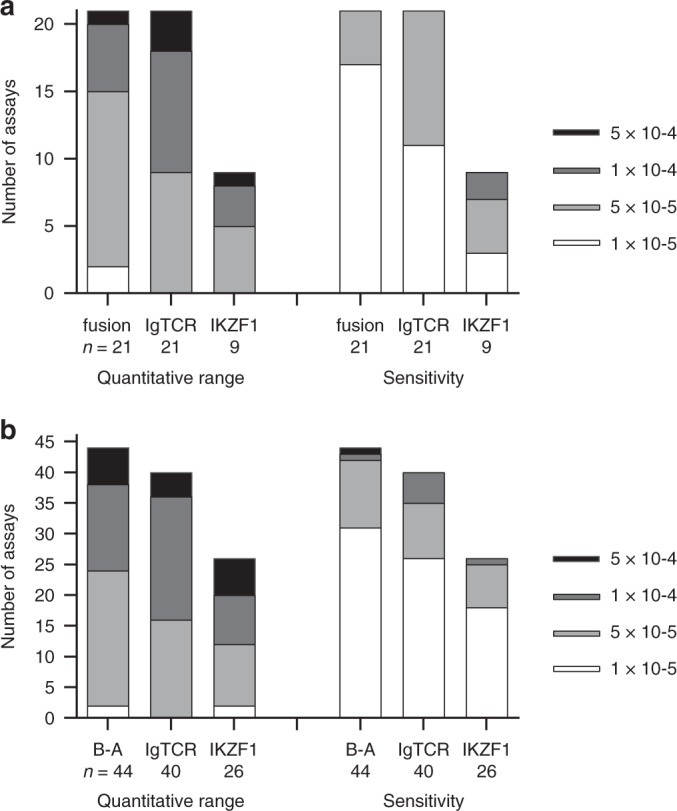
a 21 patients with the ABL-class fusions shown in Table 1 and (b) 44 BCR-ABL1 patients. B-A denotes data for BCR-ABL1 fusion assays.
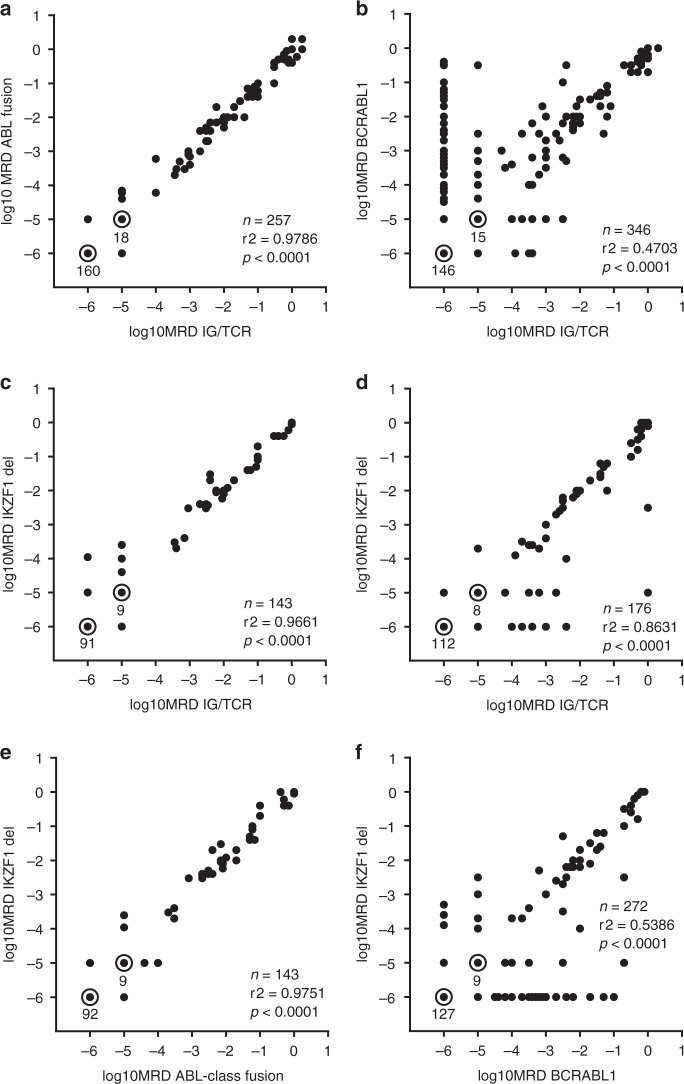
These new MRD assays were then utilized to re-measure MRD levels in 257 bone marrow samples from the 21 ABL-class patients previously tested using Ig/TCR marker tests (Fig. 3a). The MRD data obtained were highly correlated with Ig/TCR results (Pearson correlation coefficient r2 of 0.9123, P < 0.0001). All of the patients had concordant results, defined by <1.0 log difference in quantifiable results or non-quantifiable or negative results for both samples.
Fig. 3. Relationships between MRD levels measured by quantitative real-time PCR on the same DNA samples with different types of MRD markers.
a ABL fusion versus Ig/TCR tests for 21 patients. All assays were patient-specific using primers and probes shown in the supplement. Samples collected from Patient 18 after an AML secondary malignancy were excluded. b BCR-ABL1 versus Ig/TCR tests for 40 patients. All assays were patient-specific. c IKZF1 versus Ig/TCR tests in nine ABL-class patients. Three different IKZF1 generic tests were used. d IKZF1 versus Ig/TCR tests in 23 BCR-ABL1 patients. Four different IKZF1 tests were used. e IKZF1 versus ABL-class tests in nine ABL-class patients. f IKZF1 versus BCR-ABL1 tests in 26 BCR-ABL1 patients.
A similar comparison was performed for 40 BCR-ABL1 patients whose MRD had been evaluated with MRD assays designed to detect the genomic breakpoint as well as an Ig/TCR clonal marker (Fig. 3b). In contrast to ABL-class patients, 13 BCR-ABL1 patients (33%) had discordant MRD results with higher MRD results obtained with the BCR-ABL1 marker in 12 cases and higher Ig/TCR MRD levels in the remaining patient. These marked differences in some patients contributed to a much lower correlation coefficient for the results of the parallel MRD testing of the same samples using 2 different marker types in BCR-ABL1 patients (40 cases, r2 = 0.4703, P < 0.0001, Fig. 3b).
Comparison of IKZF1 MRD tests with Ig/TCR tests
All ABL-class and BCR-ABL1 patients were also screened with four qPCR assays designed to detect IKZF1Δ4-7, IKZF1Δ2-7, IKZF1Δ2-8 and IKZF1Δ4-8 respectively (Fig. 4). Of the 21 ABL-class fusion cases, nine (43%) showed a high level of at least one of these deletions gauged to be suitable for an effective MRD test because the level of deletion was greater than 1 × 10-1 compared to our positive control DNA for the deletion derived from primary patient cells or patient-derived xenograft. An additional two cases had a deletion at a sub-clonal level (Table 1). In comparison, screening for the four IKZF1 deletion assays identified 26 (59%) of the BCR-ABL1 patients with a high level for one of these dual-purpose markers. The sensitivity and QR levels for these generic IKZF1 qPCR tests were assessed using a dilution series of each patient’s diagnostic DNA to create standard curves that were also used to measure the remission samples in the same assay. The sensitivity and QR levels for generic IKZF1 assays were similar to the Ig/TCR assays for both ALL subtypes (Fig. 2a, b).
Fig. 4. Sites and sequences of primers and probes used for q-PCR analysis to detect and measure recurrent deletions in the IKZF1 gene.
Each of the four assays requires a forward primer, a fluorescent Locked Nucleic Acid (LNA) hydrolysis probe and a reverse primer.
When the MRD levels measured by IKZF1 markers were compared with the results of Ig/TCR assays for the same 143 samples from ABL-class patients there was a > 1 log difference for only a single sample (from Patient 14) Fig. 3c. For BCR-ABL1 patients, three patients had discordant results with samples giving different MRD levels (>1 log difference). The overall correlation for MRD levels measured by the IKZF1 compared with Ig/TCR markers in ABL-class patients were better for patients with ABL-class fusions (r2 = 0.9661, Fig. 3c) than BCR-ABL1 (r2 = 0.8631, Fig. 3d).
Comparison of IKZF1 MRD tests with fusion MRD tests
To improve our understanding of the variations in MRD level that are sometimes observed with different markers in particular patients, further comparisons were made between the MRD results obtained with the fusion-based versus IKZF1 deletion-based markers (Fig. 3e, f). The results of these two types of MRD tests on the same samples from patients with BCR-ABL1 fusions showed relatively low Pearson’s correlation coefficient (0.5386, Fig. 3f). This reflects the observation that at the patient level, 6/26 (23%) patients had discordant MRD results. We were able to compare MRD determined by all three marker types for 23 BCR-ABL1 patients –16 patients (70%) were concordant in all samples.
Again, higher concordance was observed for cases with ABL-class fusions compared to BCR-ABL1 patients with none of the nine ABL-class patients showing discordant results with IKZF1 markers (>1 log difference). In fact only 3 of 143 comparisons showed a difference >0.5 log (the 3 all from Patient 14) leading to a high Pearson’s coefficient (Fig. 3e, r2 = 0.9751, P < 0.0001).
Discussion
This study demonstrated the feasibility of using targeted NGS or WGS to define genomic breakpoints and to design satisfactory qPCR MRD assays to monitor disease in ALL patients with ABL-class fusions. These 21 patients had poor outcomes, comparable to larger studies [1, 2], although we would expect this to improve with earlier diagnosis of the fusions and consistent intervention with TKIs [5]. The study also found a high incidence of recurrent IKZF1 deletions in ABL-class patients consistent with a previous report of 40% IKZF1 deletions in Ph-like ALL [11] and demonstrated effective use of these markers to monitor disease. Both the fusion and deletion MRD tests showed generally highly comparable results versus conventional Ig/TCR markers, thus providing additional scope to monitor MRD in these patients. MRD tests based on IKZF1-deletions were of particular interest since these deletions occurred in 23% of relapsed B-ALL [29] as well as Ph-like ALL and approximately half are recurrent and can be detected in multiple patients by generic assays [19, 20]. In this study, it was feasible to use these dual-purpose markers which do not require any prior sequencing to perform MRD testing in 43% of ABL-class patients and 59% of BCR-ABL1 patients.
The analyses in this paper complement previous studies showing the potential to use genomic breakpoints for BCR-ABL1 [7]; KMT2A-rearrangements [8, 9]; and CDKN2A/B deletions [30] as MRD markers in ALL. This is in line with our hypothesis that disease-drivers will make more reliable MRD markers than disease-passengers such as clonal Ig/TCR markers. While Ig/TCR markers have served as effective MRD markers for ALL, particularly when two markers are used as recommended by most trials [31], their use is restricted to lymphoid disease, they can underestimate MRD in KMT2A-rearranged infant ALL [9] and they can be subject to clonal evolution or selection at relapse [32].
However, each new genomic fusion or deletion marker should be considered on its own merits by careful evaluation and comparison with other methodologies before general diagnostic use for ALL or other diseases. In our laboratory, recurrent ALL genomic deletions in BTLA and SLX4IP genes did not appear to provide stable MRD markers (unpublished data). The approach used herein has the advantage that the well-established EuroMRD guidelines for evaluating the quantitative range and sensitivity of MRD tests in ALL can be readily applied to new genomic breakpoint assays and their MRD results, allowing fair comparisons to be made with measurement of Ig/TCR markers by either q-PCR [33] or by ddPCR [30].
Monitoring residual disease in patients with BCR-ABL1 is challenging. Our previous study identified that a subset of ALL patients with BCR-ABL1 and CML-like features had discordant MRD results measured by qRT-PCR or qPCR for BCR-ABL1 and Ig/TCR [7]. This study used an extended series of BCR-ABL1 patients and found discordancy in qPCR results of >1 log difference in 33% of patients between BCR-ABL1 and Ig/TCR compared to none of the 21 ABL-class ALL cases. The additional use of qPCR IKZF1 deletion markers available in 59% of the BCR-ABL1 patients did not progress our understanding although it served to illustrate further the inherent clonal instability in some of these patients. We have no definitive answer on the best way to monitor residual disease in BCR-ABL1 ALL patients although the results of on-going trials such as EsPhALL trial will be informative.
Finally, as whole genome sequencing becomes more commonly used for ALL patients, this study shows it provides a viable alternative to multiple PCRs followed by heteroduplex analysis and Sanger sequencing for the detection of MRD markers in ALL. The ability to define the precise patient-specific genomic breakpoint sequences for key disease-related fusions and deletions that drive disease also suggests significant opportunity to develop in liquid biopsy assays for MRD in other cancers [30].
Supplementary information
Acknowledgements
We thank Emma McCormack for assisting with ethics applications; staff of the Children’s Cancer Institute Tumour Bank and Queensland Cell and Tissue bank for sample processing and banking and Erika Ong, Amy Smalley and Lynda Saunders who assisted in data collection. Children’s Cancer Institute is affiliated with Sydney Children’s Hospital and UNSW.
Author contributions
RS, TNT, and JZ designed the study, obtained ethics and wrote the manuscript. All authors reviewed and approved the manuscript. RS, MDN, DLW, PJS, TNT, MJC, JZ obtained essential research grants. NCV, LHu, WM, TL designed qPCR assays and performed MRD tests. RS, MH, TL assessed and reported MRD tests. LH and JZ performed targeted NGS and breakpoint analyses. ME and MJC designed and performed WGS analyses. NCV, SH and DLW performed MLPA and RNA seq and KB analysed sequences. SLK, TR, LDP, PJS, CF, ASM, SC and TNT all provided clinical data.
Funding
This study was supported by Cancer Australia PdCCRS1128727 funding awarded to RS, TNT, PS and DLW which provided salary for TL and supported MRD testing, RNA and WGS sequencing. The Czech Health Research Council NU21-03-00128 funding (CI Jan Zuna) supported targeted breakpoint analysis. Salary for LHu was provided by The Cancer Council NSW Program Grant (CI Murray Norris) and Kids Cancer Alliance grant (CI Mark Cowley). Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
Data are securely stored at Children’s Cancer Institute with access limited by ethical considerations.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and the NHMRC Australia National Statement on Ethical Conduct in Human Research (2007). It used leftover samples from paediatric ALL patients, all with signed parental consent for MRD testing and future research. approved Both the research on new MRD markers to improve stratification (2019/ETH06161) and the parental information and consent forms (LNR/13/SCHN/392) were approved by the Sydney Children’s Hospital Network Human Research Ethics Committee.
Consent for publication
Parents of all patients had signed consent for publication of future research in a format that does not identify the patients.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01806-6.
References
- 1.Cario G, Leoni V, Conter V, Attarbaschi A, Zaliova M, Sramkova L, et al. Relapses and treatment-related events contributed equally to poor prognosis in children with ABL-class fusion positive B-cell acute lymphoblastic leukemia treated according to AIEOP-BFM protocols. Haematologica. 2020;105:1887–94. doi: 10.3324/haematol.2019.231720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.den Boer ML, Cario G, Moorman AV, Boer JM, de Groot-Kruseman HA, Fiocco M, et al. Outcomes of paediatric patients with B-cell acute lymphocytic leukaemia with ABL-class fusion in the pre-tyrosine-kinase inhibitor era: a multicentre, retrospective, cohort study. Lancet Haematol. 2021;8:e55–e66. doi: 10.1016/S2352-3026(20)30353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371:1005–15. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanasi I, Ba I, Sirvent N, Braun T, Cuccuini W, Ballerini P, et al. Efficacy of tyrosine kinase inhibitors in Ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood. 2019;134:1351–5. doi: 10.1182/blood.2019001244. [DOI] [PubMed] [Google Scholar]
- 5.Moorman AV, Schwab C, Winterman E, Hancock J, Castleton A, Cummins M, et al. Adjuvant tyrosine kinase inhibitor therapy improves outcome for children and adolescents with acute lymphoblastic leukaemia who have an ABL-class fusion. Br J Haematol. 2020;191:844–51. doi: 10.1111/bjh.17093. [DOI] [PubMed] [Google Scholar]
- 6.Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0031. Leukemia. 2014;28:1467–71. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hovorkova L, Zaliova M, Venn NC, Bleckmann K, Trkova M, Potuckova E, et al. Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood. 2017;129:2771–81. doi: 10.1182/blood-2016-11-749978. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister T, Marschalek R, Schneider B, Meyer C, Gokbuget N, Schwartz S, et al. Monitoring minimal residual disease by quantification of genomic chromosomal breakpoint sequences in acute leukemias with MLL aberrations. Leukemia. 2006;20:451–7. doi: 10.1038/sj.leu.2404082. [DOI] [PubMed] [Google Scholar]
- 9.Van der Velden VH, Corral L, Valsecchi MG, Jansen MW, De Lorenzo P, Cazzaniga G, et al. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23:1073–9. doi: 10.1038/leu.2009.17. [DOI] [PubMed] [Google Scholar]
- 10.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LAA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–80. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Veer A, Waanders E, Pieters R, Willemse ME, Van Reijmersdal SV, Russell LJ, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122:2622–9. doi: 10.1182/blood-2012-10-462358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuiper RP, Waanders E, van der Velden VHJ, van Reijmersdal SV, Venkatachalam R, Scheijen B, et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24:1258–64. doi: 10.1038/leu.2010.87. [DOI] [PubMed] [Google Scholar]
- 13.Dorge P, Meissner B, Zimmermann M, Moricke A, Schrauder A, Bouquin JP, et al. IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica. 2013;98:428–32. doi: 10.3324/haematol.2011.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmi C, Valsecchi MG, Longinotti G, Silvestri D, Carrino V, Conter V, et al. What is the relevance of Ikaros gene deletions as a prognostic marker in pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia? Haematologica. 2013;98:1226–31. doi: 10.3324/haematol.2012.075432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton R, Venn NC, Law T, Boer JM, Trahair TN, Ng A, et al. A risk score including microdeletions improves relapse prediction for standard and medium risk precursor B-cell acute lymphoblastic leukaemia in children. Br J Haematol. 2018;180:550–62. doi: 10.1111/bjh.15056. [DOI] [PubMed] [Google Scholar]
- 16.Hamadeh L, Enshaei A, Schwab C, Alonso CN, Attarbaschi A, Barbany G, et al. Validation of the United Kingdom copy-number alteration classifier in 3239 children with B-cell precursor ALL. Blood Adv. 2019;3:148–57. doi: 10.1182/bloodadvances.2018025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanulla M, Cave H, Moorman AV. IKZF1 deletions in pediatric acute lymphoblastic leukemia: still a poor prognostic marker? Blood. 2020;135:252–60. doi: 10.1182/blood.2019000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boer JM, van der Veer A, Rizopoulos D, Fiocco M, Sonneveld E, de Groot-Kruseman HA, et al. Prognostic value of rare IKZF1 deletion in childhood B-cell precursor acute lymphoblastic leukemia: an international collaborative study. Leukemia. 2016;30:32–8. doi: 10.1038/leu.2015.199. [DOI] [PubMed] [Google Scholar]
- 19.Venn NC, van der Velden VH, de Bie M, Waanders E, Giles JE, Law T, et al. Highly sensitive MRD tests for ALL based on the IKZF1 Delta3-6 microdeletion. Leukemia. 2012;26:1414–6. doi: 10.1038/leu.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caye A, Beldjord K, Mass-Malo K, Drunat S, Soulier J, Gandemer V, et al. Breakpoint-specific multiplex polymerase chain reaction allows the detection of IKZF1 intragenic deletions and minimal residual disease monitoring in B-cell precursor acute lymphoblastic leukemia. Haematologica. 2013;98:597–601. doi: 10.3324/haematol.2012.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heatley SL, Sadras T, Kok CH, Nievergall E, Quek K, Dang P, et al. High prevalence of relapse in children with Philadelphia-like acute lymphoblastic leukemia despite risk-adapted treatment. Haematologica. 2017;102:e490–e3. doi: 10.3324/haematol.2016.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong M, Mayoh C, Lau LMS, Khuong-Quang DA, Pinese M, Kumar A, et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. 2020;26:1742–53. doi: 10.1038/s41591-020-1072-4. [DOI] [PubMed] [Google Scholar]
- 23.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at arXiv. 2013; https://arxiv.org/abs/1303.3997.
- 24.Cameron DL, Baber J, Shale C, Papenfuss AT, Valle-Inclan JE, Besselink N, et al. GRIDSS, PURPLE, LINX: unscrambling the tumor genome via integrated analysis of structural variation and copy number. Preprint at bioRxiv. 2019; https://www.biorxiv.org/content/10.1101/781013v1.
- 25.Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron DL, Schroder J, Penington JS, Do H, Molania R, Dobrovic A, et al. GRIDSS: sensitive and specific genomic rearrangement detection using positional de Bruijn graph assembly. Genome Res. 2017;27:2050–60.. doi: 10.1101/gr.222109.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shale C, Baber C, Cameron DL, Wong M, Cowley MJ, Papenfuss AT, et al. Unscrambling cancer genomes via integrated analysis of structural variation and copy number. Preprint for BioRxiv. 2020; https://www.biorxiv.org/content/10.1101/2020.12.03.410860v1. [DOI] [PMC free article] [PubMed]
- 28.Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. 2015;168:395–404. doi: 10.1111/bjh.13142. [DOI] [PubMed] [Google Scholar]
- 29.Irving JA, Enshaei A, Parker CA, Sutton R, Kuiper RP, Erhorn A, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128:911–22. doi: 10.1182/blood-2016-03-704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subhash VV, Huang L, Kamili A, Wong M, Chen D, Venn NC, et al. Whole genome sequencing facilitates patient-specific quantitative PCR-based minimal residual disease monitoring in acute lymphoblastic leukaemia, neuroblastoma and Ewing sarcoma. British J Cancer. 2022;126;482–91. [DOI] [PMC free article] [PubMed]
- 31.Flohr T, Schrauder A, Cazzaniga G, Panzer-Grümayer R, van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–82. doi: 10.1038/leu.2008.5. [DOI] [PubMed] [Google Scholar]
- 32.Choi S, Henderson M, Kwan EN, Sutton R, Giles J, Venn NC, et al. Relapse in children with acute lymphoblastic leukaemia involving selection of a pre-existing drug resistant subclone. Blood. 2007;110:632–9. doi: 10.1182/blood-2007-01-067785. [DOI] [PubMed] [Google Scholar]
- 33.van der Velden V, Cazzaniga G, Schrauder A, Hancock JF, Bader P, Panzer E, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: Guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are securely stored at Children’s Cancer Institute with access limited by ethical considerations.