Abstract
Purpose
Bladder cancer (BC) is a common malignancy with well-established differences in incidence, clinical manifestation and outcomes between men and women. It is unknown to what extent disparities in outcomes are influenced by differences in treatment approaches. This paper describes treatment patterns among men and women with muscle-invasive BC focusing on curative treatment (radical cystectomy or trimodal therapy).
Methods
A retrospective population-based cohort study was performed with data from the Netherlands Cancer Registry. All patients newly diagnosed with muscle-invasive, non-advanced BC (MIBC, cT2-4a, N0/X, M0/X) in the years 2018, 2019 and 2020 were identified. Patient and tumor characteristics and initial treatment were compared between men and women with descriptive statistics and multivariable logistic regression analyses.
Results
A total of 3484 patients were diagnosed with non-advanced MIBC in 2018–2020 in the Netherlands, of whom 28% were women. Women had higher T-stage and more often non-urothelial histology. Among all strata of clinical T-stage, women less often received treatment with curative intent (radical cystectomy [RC] or trimodality treatment). Among RC-treated patients, women more often received neoadjuvant treatment (except for cT4a disease). After adjustment for pre-treatment factors, odds ratios were indicative of women having lower probability of receiving curative treatment and RC specifically, and higher probability to receive NAC when treated with RC then men, although not statistically significant.
Conclusions
Considerable differences in treatment patterns between men and women with MIBC exist. A more considerate role of the patient’s sex in treatment decisions could help decrease these differences and might mitigate disparities in outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00345-022-04080-6.
Keywords: Sex differences, Gender, Bladder cancer, MIBC, Urothelial carcinoma, Treatment pattern, Treatment allocation, Cancer registry
Introduction
Urothelial carcinoma of the bladder (BC) is a common cancer, ranking tenth in worldwide absolute cancer incidence. It is a particularly illustrative example of the differential impact of both sex and gender in a non-sex-related disease. Men are three to four times more often affected than women with a worldwide age standardized incidence rate per year of 9.6 per 100,000 for men and 2.4 per 100,000 for women [1]. This sex disparity in risk persists even after adjusting for differences in occupational hazards and smoking prevalence and intensity [2, 3], possibly due to biological differences. While cancer risk is higher in men, women present with more advanced disease at the time of diagnosis [4–6]. This is largely due to diagnostic delay, as women with hematuria are more likely to be diagnosed with a urinary tract infection and receive symptomatic treatment and less likely to undergo abdominal imaging [7, 8] and be referred for urological evaluation [8, 9].
Women have a higher cancer-specific mortality risk and lose a greater fraction of their life expectancy to BC [10]. Although women have worse survival rates even after adjusting for disease stage at diagnosis, this excess mortality is present only in the first 2 years after diagnosis, thereafter the mortality rate of women is lower than that of men [4, 6, 11, 12]. According to an analysis of the SEER database between 1990 and 2005, differences in prognostic factors such as age, tumor stage, grade and histological type account only for about 30% of the sex differences in BC mortality [11], suggesting that other factors such as differences in tumor biology, choice and efficacy of treatments and delay in treatment could contribute to this excess female mortality [12, 13].
There are only limited data on potential sex differences in treatment allocation and efficacy with conflicting results. Data from the SEER database from 1992 to 1999 do not show a significant difference in RC rates between men and women [14, 15]. A more contemporary cohort study from the Netherlands is indicative of lower probabilities of RC among women after adjustment for age, stage, socioeconomic status and type of hospital, although not significant [16]. Some single-center analyses have reported that women undergoing RC have longer operative time, more blood loss, and more frequent perioperative complications and a higher 90-day mortality risk. In addition, the rates of pelvic lymph node dissection and continent diversion are lower for women [17, 18]. In a recent analysis of a large US RC cohort, female sex was associated with longer operative time and length of stay, while reoperation, readmission and 30-day mortality rates were similar [19]. Other single-center or multicenter analyses, however, have not found significant sex differences in surgery complications, resection margins or lymph node count but consistently reported a poorer outcome for women [5, 20, 21].
To obtain insight into differences in treatment between male and female patients, we analyzed treatment patterns from a contemporary cohort of BC patients diagnosed in 2018–2020, with a focus on curative treatment, defined as radical cystectomy or trimodal therapy (TMT).
Patients and methods
Cohort
For this population-based cohort study, all patients newly diagnosed with muscle-invasive BC (MIBC) defined as cT2-4b, cN0/X, cM0/X (without previous invasive bladder cancer diagnosis, i.e., no earlier T1 diagnosis), diagnosed between 2018 and 2020 were identified from the Netherlands Cancer Registry (NCR). All histologies were included, except for urachus tumors.
This nationwide population-based registry, held by the Netherlands Comprehensive Cancer Organisation (IKNL), includes data on all cancer diagnoses for residents of the Netherlands since 1989.
The Privacy Review Board of the NCR approved the study and waived the need for written informed consent.
Clinical data
Data from the NCR about patient and tumor characteristics, disease stage, initial treatment, comorbidities, performance status, laboratory test results, treatment details (e.g., number of cycles and dose adjustments) were used. These data have been collected by specialized data managers at the NCR directly from electronic health records in all hospitals in the Netherlands. Clinical data were completed approximately 9 months after diagnosis.
The clinical TNM stage was based on physical examination, imaging and transurethral resection of the tumor (TUR). Radical cystectomy with or without neoadjuvant treatment with platinum-based chemotherapy or immune checkpoint inhibitors, and trimodal therapy (maximal TUR with subsequent chemoradiation) were considered treatments with curative intent.
Performance status documented as the Karnofsky performance score (KPS) was converted to Eastern Cooperative Oncology Group (ECOG) score, as follows: KPS 100 = ECOG 0; KPS 80–90 = ECOG 1; KPS 60–70 = ECOG 2; KPS 40–50 = ECOG 3; and KPS 10–30 = ECOG 4 [9]. Age-adjusted Charlson Comorbidity Index (aCCI) was used, excluding the presence of MIBC itself (i.e., the morbidity of interest). Renal function was categorized as 0–39 (cisplatin ineligible), 40–59 (eligible for split-dose cisplatin), and 60–79 or ≥ 80 mL/min/1.73 m2 (cisplatin eligible). Hospital type was categorized into three groups. University hospitals are allied with major Dutch universities, with a specific focus on research and education in addition to patient care, and provide specialized care. Non-university teaching hospitals also work with the university hospitals to aid in the training of professionals, and offer some of the more specialized treatments. General hospitals provide high volumes of standard health care for less specialized issues and generally refer patients to more specialized facilities. In case, more hospitals were involved in the diagnosis and/or treatment, the hospital where the diagnostics were performed was included in the analyses.
Statistical analysis
Data were summarized as frequencies with percentages, or mean values with standard deviation, or median values with interquartile range. Standardized differences between distributions among men and women patients were calculated as a measure of difference between both sexes [22], which are not dependent on sample size and are standardized to values between 0 (no difference) and 1 (maximum difference). This measure was considered appropriate given the descriptive nature and given that all results are based on a population-based cohort or subgroups thereof that were not specifically powered to test a priori defined hypotheses for statistical significance. There is no established manner to interpret the magnitude of standardized differences, as the clinical relevance depends on the context, but the authors considered all standardized differences above 0.1 to be relevant for this study. Multivariable logistic regression was performed to assess the association of sex with treatment patterns adjusted for other pre-treatment factors.
Results
In the years 2018–2020, a total of 3484 patients were diagnosed with MIBC without nodal or distant metastasis, of whom 28% (n = 992) were women. Median age at diagnosis was 74 years for both sexes (Table 1). The performance status at diagnosis was 0 or 1 in 54% of men, and 47% of women, respectively. Most patients were eligible for cisplatin-based therapy based on only their renal function. The ineligible group consisted of 7% of the men and 8% of the women having an eGFR of 0–30 ml/min and 17% and 16% had a eGFR 30–50, respectively. Women were diagnosed with more advanced tumors, with cT2 in 64% and cT4a in 10% of the cases, while men more often presented with a cT2 tumor (72%) and only in 5% with a cT4a tumor (standardized difference: 0.19). Women also had tumors with non-urothelial histology more often (10 vs 6%, standardized difference: 0.22).
Table 1.
Patient and disease characteristics of all muscle-invasive, non-advanced bladder cancer patients in the Netherlands diagnosed in 2018–2020, by sex
| Men | Women | Standardized differences | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| All | 2492 | 72 | 992 | 28 | |
| Age at diagnosis | |||||
| 0–50 | 40 | 2 | 25 | 3 | 0.12 |
| 51–60 | 225 | 9 | 103 | 10 | |
| 61–70 | 623 | 25 | 244 | 25 | |
| 71–80 | 957 | 38 | 331 | 33 | |
| > 80 | 647 | 26 | 289 | 29 | |
| Median, IQR | 74 | 67–81 | 74 | 66–82 | |
| Age-adjusted CCI | |||||
| < 2 | 130 | 5 | 70 | 7 | 0.17 |
| 2–3 | 878 | 35 | 398 | 40 | |
| 4 | 570 | 23 | 198 | 20 | |
| 5 | 387 | 16 | 133 | 13 | |
| > 5 | 425 | 17 | 148 | 15 | |
| Unknown | 102 | 4 | 45 | 5 | |
| Performance status at diagnosis | |||||
| ECOG 0 | 965 | 39 | 333 | 34 | 0.15 |
| ECOG 1 | 370 | 15 | 133 | 13 | |
| ECOG 2 | 169 | 7 | 82 | 8 | |
| ECOG 3/4 | 50 | 2 | 31 | 3 | |
| Unknown | 938 | 38 | 413 | 42 | |
| Renal function (mL/min/1.73m2) | |||||
| 0–39 | 342 | 14 | 136 | 14 | 0.05 |
| 40–59 | 533 | 21 | 216 | 22 | |
| 60–79 | 647 | 26 | 253 | 26 | |
| ≥ 80 | 636 | 26 | 251 | 25 | |
| Unknown | 334 | 13 | 136 | 14 | |
| Clinical stage | |||||
| T2, N0, M0 | 1,803 | 72 | 637 | 64 | 0.19 |
| T3, N0, M0 | 552 | 22 | 259 | 26 | |
| T4a, N0, M0 | 137 | 5 | 96 | 10 | |
| Histology | |||||
| Urothelial carcinoma | 2,334 | 94 | 893 | 90 | 0.22 |
| Squamous cell carcinoma | 22 | 1 | 44 | 4 | |
| Adenocarcinoma | 7 | 0 | 2 | 0 | |
| Neuro-endocrine carcinoma | 96 | 4 | 29 | 3 | |
| Undifferentiated carcinoma | 4 | 0 | 4 | 0 | |
| Other/undetermined | 29 | 1 | 20 | 2 | |
IQR interquartile range, CCI Charlson Comorbidity Index, ECOG Eastern Cooperative Oncology Group
For the entire cohort of MIBC patients, women less often received treatment with curative intent (55% vs 59%, standardized difference: 0.08). In addition, within all strata of T-stage, the initial treatment provided was more often with curative intent for men. While for cT2 and cT3 tumors, the difference was small (standardized differences 0.03 and 0.13) between men and women (59% vs 56% and 61% vs 57%), for cT4a tumors only 38% of the women received curative treatment compared to 50% of the men (Table 2), corresponding to a standardized difference of 0.25. The difference in type of treatment administered to men and women with cT4a disease differed the most (standardized difference of 0.37), with lower proportions of women receiving radical cystectomy, trimodal therapy and systemic therapy, and a slightly higher proportion receiving radiotherapy.
Table 2.
Initial treatment provided for all muscle-invasive, non-advanced bladder cancer patients in the Netherlands diagnosed in 2018–2020, by clinical T-stage and sex
| Men | Women | Standardized differences | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| All stages | 2492 | 100 | 992 | 100 | |
| Treatment | |||||
| RC + NAT | 330 | 13 | 151 | 15 | 0.13 |
| RC−NAT | 804 | 32 | 286 | 29 | |
| Trimodal therapy | 331 | 13 | 106 | 11 | |
| Radiotherapy | 484 | 19 | 207 | 21 | |
| Systemic treatment | 52 | 2 | 24 | 2 | |
| Other/none* | 491 | 20 | 218 | 22 | |
| Curative treatment** | |||||
| Yes (RC/TMT) | 1465 | 59 | 543 | 55 | 0.08 |
| No | 1027 | 41 | 449 | 45 | |
| cT2, N0, M0 | 1803 | 100 | 637 | 100 | |
| Treatment | |||||
| RC + NAT | 182 | 10 | 75 | 12 | 0.12 |
| RC−NAT | 622 | 34 | 201 | 32 | |
| Trimodal therapy | 254 | 14 | 83 | 13 | |
| Radiotherapy | 367 | 20 | 140 | 22 | |
| Systemic treatment | 20 | 1 | 8 | 1 | |
| Other/none* | 358 | 20 | 130 | 20 | |
| Curative treatment** | |||||
| Yes (RC/TMT) | 1058 | 59 | 359 | 56 | 0.03 |
| No | 745 | 41 | 278 | 44 | |
| cT3, N0, M0 | 552 | 100 | 259 | 100 | |
| Treatment | |||||
| RC + NAT | 115 | 21 | 59 | 23 | 0.15 |
| RC−NAT | 156 | 28 | 68 | 26 | |
| Trimodal therapy | 67 | 12 | 21 | 8 | |
| Radiotherapy | 99 | 18 | 52 | 20 | |
| Systemic treatment | 20 | 4 | 9 | 3 | |
| Other/none* | 95 | 17 | 50 | 19 | |
| Curative treatment** | |||||
| Yes (RC/TMT) | 338 | 61 | 148 | 57 | 0.13 |
| No | 214 | 39 | 111 | 43 | |
| cT4a, N0, M0 | 137 | 100 | 96 | 100 | |
| Treatment | |||||
| RC + NAT | 33 | 24 | 17 | 18 | 0.37 |
| RC−NAT | 26 | 19 | 17 | 18 | |
| Trimodal therapy | 10 | 7 | 2 | 2 | |
| Radiotherapy | 18 | 13 | 15 | 16 | |
| Systemic treatment | 12 | 9 | 7 | 7 | |
| Other/none* | 38 | 28 | 38 | 40 | |
| Curative treatment** | |||||
| Yes (RC/TMT) | 69 | 50 | 36 | 38 | 0.25 |
| No | 68 | 50 | 60 | 63 | |
RC radical cystectomy; NAT neoadjuvant treatment, including chemotherapy and immunotherapy; TMT trimodal therapy
*Other/none includes local treatments such as transurethral resection of the tumor, bladder instillations and partial cystectomies
**Curative treatment includes radical cystectomy with or without neoadjuvant treatment and trimodality treatment
The type of treatment administered per cT stratum was assessed by hospital type. The difference of undergoing curative treatment or no tumor-directed treatment between men and women was strongest among patients diagnosed in general hospitals, and weaker or absent in university hospitals (Supplementary Table 1).
Aspects of RC treatment were assessed separately, as the most common treatment modality. Among the patients receiving RC, women more often received neoadjuvant treatment for cT2 and cT3 disease, but less often for cT4a disease. In the vast majority of the cases, neoadjuvant treatment was cisplatin-based chemotherapy. The surgical technique was more often open for women (53% vs 50%) and more often robot-assisted for men (45% vs 42%). The urinary diversion was more often continent for men (6 vs 3%) (Table 3).
Table 3.
Aspects of radical cystectomies for treatment for muscle-invasive, non-advanced bladder cancer by sex
| Men | Women | Standardized differences | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| All | 1134 | 100 | 437 | 100 | |
| Neoadjuvant treatment | 330 | 29 | 151 | 35 | |
| Among cT2 patients | |||||
| Yes | 182 | 23 | 75 | 27 | 0.11 |
| No | 622 | 77 | 201 | 73 | |
| Among cT3 patients | |||||
| Yes | 115 | 42 | 59 | 46 | 0.08 |
| No | 156 | 58 | 68 | 54 | |
| Among cT4a patients | |||||
| Yes | 33 | 56 | 17 | 50 | 0.12 |
| No | 26 | 44 | 17 | 50 | |
| Type (all stages) | |||||
| Cisplatin | 280 | 85 | 127 | 84 | 0.15 |
| Carboplatin | 22 | 7 | 12 | 8 | |
| Cis/carbo cross-over | 11 | 3 | 8 | 5 | |
| Other, incl. immunotherapy | 17 | 5 | 4 | 3 | |
| Surgical approach | |||||
| Surgery technique | |||||
| Open | 571 | 50 | 233 | 53 | 0.06 |
| Scopic (including robot-assisted) | 543 | 48 | 194 | 44 | |
| Other/unknown | 20 | 2 | 10 | 2 | |
| Type of cystectomy | |||||
| Sexually preserving | 21 | 2 | 13 | 3 | 0.06 |
| Standard | 1102 | 97 | 418 | 96 | |
| Other/unknown | 11 | 1 | 6 | 1 | |
| Urinary diversion | |||||
| Continent | 65 | 6 | 11 | 3 | 0.15 |
| Incontinent | 1055 | 93 | 418 | 96 | |
| Unknown diversion | 14 | 1 | 8 | 2 | |
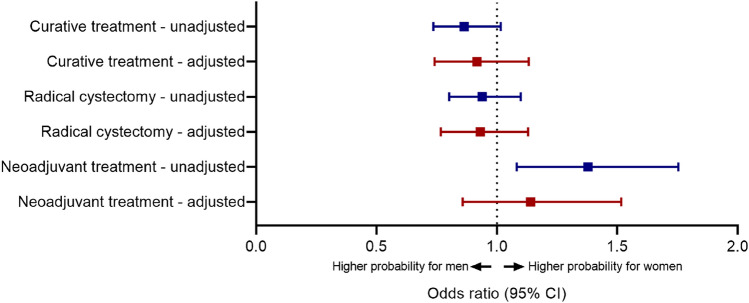
Multivariable logistic regression showed that the probability of undergoing treatment with curative intent, adjusted for age, age-adjusted Charlson comorbidity index, performance status, renal function, cT-stage, histology and hospital type was slightly lower for women, but non-significant (OR 0.92; 95%-CI 0.75–1.13) (Fig. 1). The same was found for radical cystectomy specifically (OR 0.95; 95%-CI 0.78–1.14). Among RC-treated patients, women had slightly higher but non-significant probability of undergoing neoadjuvant treatment after adjustment for pre-treatment factors (OR 1.14; 95%-CI 0.86–1.51).
Fig. 1.
Univariable and multivariable analysis of association between sex and treatment among men and women with muscle-invasive bladder cancer. Adjusted odds ratios are adjusted for age, age-adjusted Charlson comorbidity index, performance status, renal function, cT-stage, and histology and hospital type
Discussion
These analyses among a contemporary nationwide population-based cohort highlights several important differences in treatment patterns among men and women with MIBC. Overall and within strata of clinical stage, women with MIBC were less often treated with curative intent, which was most notable in higher T-stages. In some patients, neoadjuvant treatment with RC may have been planned, but RC was canceled due to change in perceived benefit or feasibility after neoadjuvant treatment was given. This was the case in 26 patients (7 female), so the impact of this on sex differences should be negligible. In addition, physicians’ preferences and patients’ choices may have contributed to the observed differences in treatment patterns, but data for this were not available to determine the extent.
Radical cystectomy comprises the main curative treatment option among MIBC patients, with some aspects of the procedure found to vary between men and women. Women were more likely to be treated with neoadjuvant therapy prior to RC among cT2/3 patients, but less likely among cT4a patients. In addition, among patients undergoing RC, men more often had continent urinary diversions. When considering the type of hospital in which patients were diagnosed, general hospitals seemed to be accounting for more differences in treatment patterns between men and women compared to non-university teaching hospitals and university hospitals.
After taking into account all other pre-treatment factors that may weigh in on treatment decisions in addition to the patient’s sex, the patient’s sex was not significantly associated with receiving curative treatment in general, receiving RC specifically, or receiving neoadjuvant treatment when undergoing RC. Nonetheless, adjusted odds ratios were indicative of women having lower probability of receiving curative treatment and radical cystectomy specifically, and higher probability to receive NAC when treated with RC then men. This suggests that other pre-treatment factors for which the odds ratios were adjusted only account for part of the association of sex with treatment. A SEER-based study in the U.S. found that women were more likely to undergo radical cystectomy, both with and without adjustment for other factors, as compared to any alternative (including only TUR) [23]. In the current cohort, this was not the case, but patient populations seem to differ in relevant aspects including age and nodal status from the SEER cohort. In another US based cohort study using the National Cancer Database that distinguished between different alternatives to RC including non-curative options, the proportion of curatively treated patients was indeed also higher among male patients than female patients [24]. Both these studies included patients back to 2004, when TMT was not often used.
The observed sex disparity in treatment allocation is particularly striking in cT4a tumors, with 50% of the men undergoing RC compared to only 38% of the women, although this was based on limited numbers of patients. The staging of cT4a tumors takes into account gender-specific anatomy, with T4 disease defining tumor extension into the prostatic stroma in men and extension into the vagina or uterus in women [25]. In a single-center analysis of 176 patient with pT4a disease, the 1-year cancer-specific mortality rate was higher for invasion of the vagina (71%) or uterus (65%) compared to the invasion of the prostate only (24%) or with concomitant invasion of the seminal vesicles (50%) [26]. With regard to pT4a stage, a significantly lower 5-year cancer-specific survival was found in women (15%) compared to men (35%) [27] and female sex was an adverse prognostic factor for cancer-specific mortality (CSM) [28]. It is, therefore, conceivable that in light of these data and the anatomic differences, RC is considered a less appropriate treatment approach in women than in men with cT4a tumors and is, therefore, less often applied to women. With the TMT not considered an appropriate treatment option for higher T-stages, this could explain (part of) the discrepancy in proportion of curatively treated women with T4a tumors.
The following limitations of this study should be considered. At the individual patient level, several factors that can influence treatment options and choice, including patient preference, were not represented in our data. In addition, although it is likely that higher proportions of treatments that have been established to yield inferior outcomes among women are also associated with worse survival, this has not been directly demonstrated by the current study.
In the era of precision oncology, the selection of patient who might benefit from curative treatment approaches needs to be improved. The sex of the patients is one of the most obvious and important factors affecting the efficacy and toxicity of cytotoxic treatments, with women in general having higher response rates as well as toxicity rates for a variety of anticancer treatments [29]. Indeed, in a recent meta-analysis, women benefited more from neoadjuvant chemotherapy prior to RC, improved disease recurrence and CSM rates compared to men [30]. These data indicate that increasing rates of curative treatment approaches for women could improve the outcome of patients.
To conclude, considerable differences in treatment patterns between men and women with MIBC exist. A more considerate role of the patient’s sex in treatment decisions could help decrease these differences and might mitigate disparities in outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Study concept and design: AR, KA, and BÖ. Acquisition of data: AR. Analysis and interpretation of data: AR, AL, CG, KA, and BÖ. Drafting of the manuscript: AR and BÖ. Critical revision of the manuscript for important intellectual content: KA, CG, and AL. Statistical analysis: AR. Obtaining funding: None. Administrative, technical, or material support: None. Supervision: None. Other: None.
Funding
No funding was obtained for this study.
Declarations
Conflict of interest
All the authors declare no conflicts of interest.
Research involving human participants
This retrospective study with data from the Netherlands Cancer Registry (NCR) does not require approval from an ethics committee in the Netherlands, according to the Central Committee on Research involving Human Subjects.
Informed consent
Based on Dutch legislation, it is not required to obtain informed consent from patients for collection of their data in the NCR. The privacy review board of the NCR reviews all data requests for studies with data of the NCR regarding privacy issues and approved this study (K22.058).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemelt M, Yamamoto H, Cheng KK, Zeegers MP. The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer. 2009;124:412–419. doi: 10.1002/ijc.23856. [DOI] [PubMed] [Google Scholar]
- 3.Krabbe LM, Svatek RS, Shariat SF, et al. Bladder cancer risk: Use of the PLCO and NLST to identify a suitable screening cohort. Urol Oncol. 2015;33(65):e19–25. doi: 10.1016/j.urolonc.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Mungan NA, Kiemeney LA, van Dijck JA, et al. Gender differences in stage distribution of bladder cancer. Urology. 2000;55:368–371. doi: 10.1016/S0090-4295(99)00481-1. [DOI] [PubMed] [Google Scholar]
- 5.Mitra AP, Skinner EC, Schuckman AK, et al. Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: a critical analysis of 1,994 patients. Urol Oncol. 2014;32(52):e51–59. doi: 10.1016/j.urolonc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Richters A, Dickman PW, Witjes JA, et al. Bladder cancer survival: women only fare worse in the first two years after diagnosis. Urol Oncol. 2019;37:853–861. doi: 10.1016/j.urolonc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JA, Vekhter B, Lyttle C, et al. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: a nationwide claims-based investigation. Cancer. 2014;120:555–561. doi: 10.1002/cncr.28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henning A, Wehrberger M, Madersbacher S, et al. Do differences in clinical symptoms and referral patterns contribute to the gender gap in bladder cancer? BJU Int. 2013;112:68–73. doi: 10.1111/j.1464-410X.2012.11661.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: does a gender disparity exist? Urology. 2008;72:498–502. doi: 10.1016/j.urology.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 10.Scosyrev E, Golijanin D, Wu G, Messing E. The burden of bladder cancer in men and women: analysis of the years of life lost. BJU Int. 2012;109:57–62. doi: 10.1111/j.1464-410X.2011.10318.x. [DOI] [PubMed] [Google Scholar]
- 11.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 12.Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69:300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Lutz CT, Livas L, Presnell SR, et al. Gender differences in urothelial bladder cancer: effects of natural killer lymphocyte immunity. J Clin Med. 2021;10:5163. doi: 10.3390/jcm10215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder C, Harlan L, Knopf K, et al. Patterns of care for the treatment of bladder cancer. J Urol. 2003;169:1697–1701. doi: 10.1097/01.ju.0000056727.30546.b7. [DOI] [PubMed] [Google Scholar]
- 15.Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J Urol. 2003;170:1765–1771. doi: 10.1097/01.ju.0000091620.86778.2e. [DOI] [PubMed] [Google Scholar]
- 16.Ripping TM, Witjes JA, Meijer RP, et al. Hospital-specific probability of cystectomy affects survival from muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2020;935:e939–935.e916. doi: 10.1016/j.urolonc.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Siegrist T, Savage C, Shabsigh A, et al. Analysis of gender differences in early perioperative complications following radical cystectomy at a tertiary cancer center using a standardized reporting methodology. Urol Oncol. 2010;28:112–117. doi: 10.1016/j.urolonc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Gschliesser T, Eredics K, Berger I, et al. The impact of gender on tumour stage in in-house complications and choice of urinary diversion: results of the Austrian cystectomy registry. Urol Int. 2017;99:429–435. doi: 10.1159/000477672. [DOI] [PubMed] [Google Scholar]
- 19.Kotamarti S, et al. Do females have worse surgical outcomes after radical cystectomy? Impact of gender on 30-day complications in a national cohort. J Clin Oncol. 2021 doi: 10.1200/JCO.2021.39.6_suppl.402. [DOI] [Google Scholar]
- 20.Kluth LA, Rieken M, Xylinas E, et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: an international multi-institutional study of more than 8000 patients. Eur Urol. 2014;66:913–919. doi: 10.1016/j.eururo.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Soave A, Dahlem R, Hansen J, et al. Gender-specific outcomes of bladder cancer patients: a stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur J Surg Oncol. 2015;41:368–377. doi: 10.1016/j.ejso.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. SAS global forum. 2012; 1-6
- 23.Grajales V, Bandari J, Hale NE, et al. Associations between female sex and treatment patterns and outcomes for muscle-invasive bladder cancer. Urology. 2021;151:169–175. doi: 10.1016/j.urology.2020.06.058. [DOI] [PubMed] [Google Scholar]
- 24.Krimphove MJ, Szymaniak J, Marchese M, et al. Sex-specific differences in the quality of treatment of muscle-invasive bladder cancer do not explain the overall survival discrepancy. Eur Urol Focus. 2021;7:124–131. doi: 10.1016/j.euf.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Paner GP, Stadler WM, Hansel DE, et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018;73:560–569. doi: 10.1016/j.eururo.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Moschini M, Zamboni S, Mattei A, et al. Radical cystectomy in pathological T4a and T4b bladder cancer patients: is there any space for sub stratification? Urol Int. 2019;102:269–276. doi: 10.1159/000493899. [DOI] [PubMed] [Google Scholar]
- 27.May M, Bastian PJ, Brookman-May S, et al. Gender-specific differences in cancer-specific survival after radical cystectomy for patients with urothelial carcinoma of the urinary bladder in pathologic tumor stage T4a. Urol Oncol. 2013;31:1141–1147. doi: 10.1016/j.urolonc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Rosiello G, Palumbo C, Pecoraro A, et al. The effect of sex on disease stage and survival after radical cystectomy: a population-based analysis. Urol Oncol. 2021;39:236 e231–236 e237. doi: 10.1016/j.urolonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Ozdemir BC, Csajka C, Dotto GP, Wagner AD. Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of precision oncology. J Clin Oncol. 2018 doi: 10.1200/JCO.2018.78.3290. [DOI] [PubMed] [Google Scholar]
- 30.Kimura S, Iwata T, Abufaraj M, et al. Impact of gender on chemotherapeutic response and oncologic outcomes in patients treated with radical cystectomy and perioperative chemotherapy for bladder cancer: a systematic review and meta-analysis. Clin Genitourin Cancer. 2020;18:78–87. doi: 10.1016/j.clgc.2019.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.