Abstract
Objectives
To determine the main predictors of death in multidrug-resistant (MDRTB) patients from Brazil.
Design
Retrospective cohort study, a survival analysis of patients treated between 2005 and 2012.
Results
Of 3802 individuals included in study, 64.7% were men, mean age was 39 (1–93) years, and 70.3% had bilateral pulmonary disease. Prevalence of human immunodeficiency virus (HIV) was 8.3%. There were 479 (12.6%) deaths. Median survival time was 1452 days (4 years). Factors associated with increased risk of death were age greater than or equal to 60 years (hazard rate [HR] = 1.6, confidence interval [CI] = 1.15–2.2), HIV co-infection (HR = 1.46; CI = 1.05–1.96), XDR resistance pattern (HR = 1.74, CI = 1.05–2.9), beginning of treatment after failure (HR = 1.72, CI = 1.27–2.32), drug abuse (HR = 1.64, CI = 1.22–2.2), resistance to ethambutol (HR = 1.30, CI = 1.06–1.6) or streptomycin (HR = 1.24, CI = 1.01–1.51). Mainly protective factors were presence of only pulmonary disease (HR = 0.57, CI = 0.35–0.92), moxifloxacin use (HR = 0.44, CI = 0.25–0.80), and levofloxacin use (HR = 0.75; CI = 0.60–0.94).
Conclusion
A more comprehensive approach is needed to manage MDRTB, addressing early diagnostic, improving adhesion, and comorbidities, mainly HIV infection and drug abuse. The latest generation quinolones have an important effect in improving survival in MDRTB.
Keywords: Survival, Tuberculosis multidrug-resistant, Tuberculosis, Death
Introduction
Tuberculosis (TB), even today, remains a serious public health problem, particularly in developing countries.1 Without treatment TB mortality is high, reaching 70% in ten years in patients without HIV infection.2 Multidrug-resistant tuberculosis (MDRTB), defined as the simultaneous resistance to isoniazid and rifampicin, remains a major challenge for TB control.3, 4 According to World Health Organization (WHO), in 2016, 4.1% of estimated new TB cases and 19% of retreatment cases in the world were caused by MDRTB or rifampicin-resistant (RR-TB) strains. Of these, 490,000 cases were MDRTB.5 Earlier studies indicated that survival of MDRTB cases is poor.6 Treatment outcomes for MDRTB are significantly worse than for drug-susceptible TB. In the 2014 global WHO cohort, 16% MDRTB/RR-TB patients died.5
Brazil is one of the 30 countries with the highest disease burden.5 WHO estimated that in Brazil 1.5% of new TB cases and 8.0% of retreatment cases in 2017 were MDRTB.5 Primary resistance to isoniazid and rifampicin in regional data from Brazil in 2007 was 1.4%.7 In 2017, 583 MDRTB cases were registered in Brazil national surveillance system.8 Rio de Janeiro State registered 166 (28.5%) MDRTB cases in 2017.8 However, WHO estimated that 1900 MDRTB/RRTB cases occurred in Brazil that year. Death rate from 2015 MDRTB national cohort was 8.8%.9
MDRTB treatment in Brazil is standardized in most cases and MDRTB regimen is composed of an injectable drug, one quinolone, ethambutol, pyrazinamide, and terizidon.10, 11 Treatment is usually completed after 18–24 months.10
Factors associated with poor outcomes in MDRTB identified by systematic reviews and observational studies were male sex,12, 13 low body mass index,13, 14 underweight,15 prior treatment with second line drugs,12 extensive resistance pattern (XDR),13 HIV infection,14 no-conversion of sputum culture,16 and alcoholism.13 Regarding the death outcome, the risks factors pointed out in literature were age over 60 years, XDR resistance,12, 17 previous use of second-line drugs,12, 14, 17, 18 higher number of resistant drugs on sensitivity test (ST).19 Better survival is associated to later-generation quinolones use.20 Diabetes is associated with increased risk of death and treatment failure.21 Immunocompromised patients had a nine-fold greater risk of death.6 TB and HIV are the major causes of death worldwide.5 Employment of antiretroviral therapy (ARV) in HIV-infected patients with MDRTB resulted in reduction in mortality, as well as increased survival.22
Objectives
Little is known about the survival of MDRTB patients in Brazil. The aim of the study is to characterize and identify the main predictors of death among MDRTB patients in Brazil.
Methods
A non-concurrent cohort study was performed to analyze the relationship between socio-demographic, clinical, radiological, laboratory aspects, and drug regimens on MDRTB patient survival. The study was conducted at Hélio Fraga Reference Center (ENSP-FIOCRUZ), Rio de Janeiro, and included MDRTB patients from whole country that started treatment between January 1, 2005 to December 31, 2012, and that have been followed until December 31, 2012. Clinical and laboratory data were extracted from the MDRTB surveillance system. Outcomes were assessed at the end of the study.
Patients included in the study were those cases who had sensitivity test showing multidrug-resistance pattern and received regimens that included in its composition the drugs recommended for MDRTB treatment by the National Tuberculosis Program (NTP-MoH).10 Patients excluded from the study were the following: no laboratory confirmation of tuberculosis; patients with changed resistance pattern on consecutive sensitivity tests; no outcome information, or transferred out; use of regimens with less than three second-line drugs, and treatment duration less than 12 months.
Definitions – variables
All the outcomes were defined based on the WHO definitions criteria.3 Therefore death was defined as death from any cause during treatment. Extensive resistance (XDR) is multidrug-resistance pattern plus resistance to fluoroquinolone and second-line injectable drugs (capreomycin, amikacin, kanamycin).3
Demographic variables included were sex (male, female), age group (up to 59 years, 60 years or more), schooling years (<8, +8 years), and ethnicity as self-referred (white, black/brown, others). Clinical presentation of disease, classified as exclusively pulmonary (TB involving only pulmonary parenchyma and tracheobronchial tree), extrapulmonary (pleural TB and disease involving other organs), or both presentations; x-ray was analyzed according to cavity presence and side of pulmonary involvement (unilateral, bilateral). Sensitivity test (ST) results for ethambutol, streptomycin, amikacin, capreomycin, and ofloxacin, pattern of resistance (XDR or MDR), begin treatment after failure of previous TBMDR treatment (yes/no). Drugs included in treatment regimen: injectable drug (amikacin, capreomycin, streptomycin), quinolones (ofloxacin, levofloxacin, moxifloxacin), pyrazinamide, and clofazimine.
Comorbidities and social behaviors studied were the presence of HIV infection, silicosis, hepatitis, use of steroids, neoplasia, organ transplant, presence of renal failure, drug addiction, alcoholism, diabetes, and smoking.
The study was approved by the National School of Public Health Research Ethics Committee (CEP/ENSP), number CAAE47351815.5.0000.5240.
Data analysis was conducted using the statistical software “R” version 3.2.3. It included (a) the estimation of the median survival time to death through Kaplan-Meier method, (b) non-parametric estimation using stratified Kaplan-Meier (KM) method comparing the curves of the strata using Mantel-Haenzel test (log-rank) and Peto test with significance level of 5%, and (c) semi-parametric Cox modelling including covariates that met the proportionality assumptions in order to define the main predictors of death.
Results
Of 3877 cases of MDRTB exported from the surveillance system, 75 cases were excluded from the study, and 3802 individuals were included in the analysis.
There were 2461 (64.7%) men, and the mean age was 39.3 years (SD = 13.1 years). Regarding ethnicity, 2285 (60.1%) were black or brown-skinned (Table 1). Completed seven years of study 55.1% patients. Concerning the type of treatment initiation, 81.7% were new cases and the patients did on average 2.7 (1–10) prior TB treatments. Primary resistance was found in 18.3%. Pulmonary disease was present in 97.4% individuals, having bilateral disease 70.3%. Cavities were present in 81.2% (Table 1). Standardized regimen was employed in 3338 (87.8%) individuals. Amikacin was used in 68.0% of treatments, levofloxacin 43.1%, and moxifloxacin in 2.9% (Table 2). Comorbidities more frequently encountered were alcoholism (18.0%), drug addiction (11.0%), diabetes (10.9%), HIV infection (8.3%). Favorable outcomes occurred in 41.3% of patients, and 12.6% died. At the end of observation period, 965 (25.4%) subjects were still on treatment.
Table 1.
Demographic and clinical characteristics (N = 3802).
| Characteristic | N (%) | Characteristic | N (%) |
|---|---|---|---|
| Sex | Resistance pattern | ||
| Male | 2461 (64.7) | MDR | 3734 (98.2) |
| Female | 1341 (35.3) | XDR | 68 (1.8) |
| Ethnicity | Resistance type | ||
| Brown-skinned | 1641 (43.2) | Acquired | 3106 (81.7) |
| White | 1403 (36.9) | Primary | 696 (18.3) |
| Black | 644 (16.9) | Disease type | |
| Indigenous | 16 (0.4) | Pulmonary | 3704 (97.4) |
| Yellow | 6 (0.2) | Both | 69 (1.8) |
| Ignored | 92 (2.4) | Extrapulmonary | 29 (0.8) |
| Age group | Pulmonary disease | ||
| <60 years | 3530 (92.8) | Bilateral | 2673 (70.3) |
| 60 and more | 272 (7.2) | Unilateral | 1099 (28.9) |
| Schooling (years) | Normal | 30 (0.8) | |
| None | 266 (7.0) | Pulmonary cavity | |
| 1–3 | 718 (18.9) | Yes | 3087 (81.2) |
| 4–7 | 1377 (36.2) | No | 685 (18.8) |
| 8–11 | 854 (22.5) | Regimen | |
| ≥12 | 235 (6.2) | Individualized | 464 (12.2) |
| Ignored | 352 (9.3) | Standardized | 3338 (87.8) |
| MDRTB treatment | DOT | ||
| New case | 3106 (81.7) | Yes | 2761 (72.6) |
| After default | 295 (7.8) | No | 1041 (27.4) |
| After failure | 265 (7.0) | ||
| Relapse | 106(2.8) | ||
| Other | 30 (0.7) | ||
MDR, multidrug-resistance; XDR, extensively drug-resistant; DOT, Directly Observed Treatment.
Table 2.
Sensitivity tests results and medicines employed.
| N (%) | N (%) | ||
|---|---|---|---|
| ST amikacin | Amikacin use | ||
| Resistant | 75 (6.4) | Yes | 2586 (68.0) |
| Sensitive | 295 (3.3) | No | 1216 (32.0) |
| Not done | 3432 (90.3) | Streptomycin use | |
| ST ethambutol | Yes | 1162 (44.0) | |
| Resistant | 1249 (32.8) | No | 2640 (56.0) |
| Sensitive | 2351 (61.8) | Ofloxacin use | |
| Not done | 202 (5.4) | Yes | 2091 (55.0) |
| ST streptomycin | No | 1711 (45.0) | |
| Resistant | 1436 (37.8) | Moxifloxacin use | |
| Sensitive | 2137 (56.2) | Yes | 110 (2.9) |
| Not done | 229 (6.0) | No | 3692 (97.1) |
| ST ofloxacin | Levofloxacin use | ||
| Resistant | 135 (3.6) | Yes | 1639 (43.1) |
| Sensitive | 234 (6.1) | No | 2163 (56.9) |
| Not done | 3433 (90.3) | Clofazimine use | |
| Pyrazinamide use | Yes | 226 (6.0) | |
| Yes | 2658 (69.9) | No | 3576 (94.0) |
| No | 1144 (30.1) | ||
ST, sensitivity test.
Survival analysis
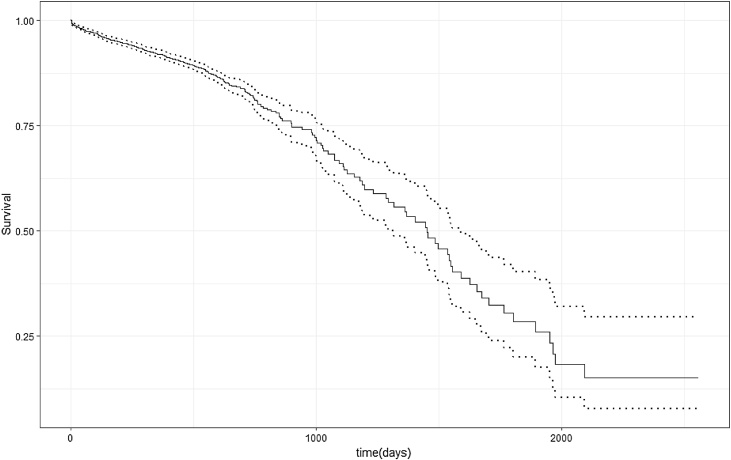
There were 479 deaths (12.7%), and 3323 (87.4%) observations were censored. The median survival time was 1452 days (CI: 1292–1589), or 48.4 months (Fig. 1).
Fig. 1.
Survival curve of MDRTB patients. Dotted line - confidence interval.
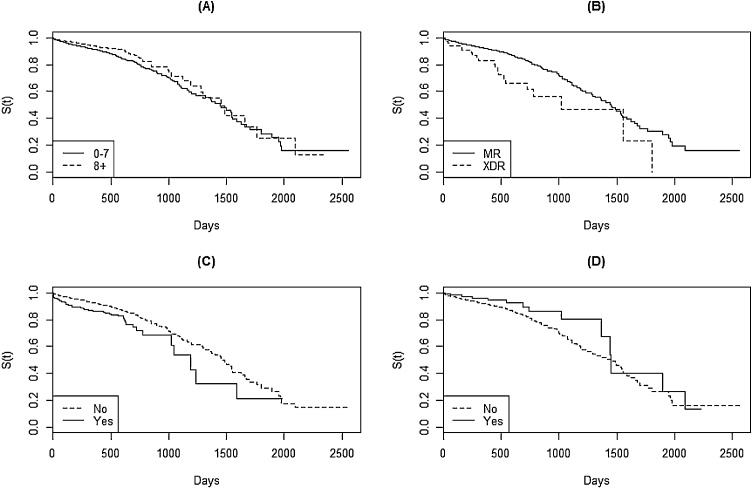
Variables that met the proportionality assumptions were: age group (<60/60+), schooling years, resistance pattern (MDR/XDR), pulmonary or extrapulmonary disease, treatment after failure, amikacin resistance, streptomycin resistance, ofloxacin resistance, ethambutol resistance, bilateral disease on thorax radiography, drug addiction, alcoholism, AIDS, streptomycin use, amikacin use, moxifloxacin use, levofloxacin use (Fig. 2). After running the backwards modelling, the final model was selected by the likelihood ratio (LR), yielding the hazard-rate (HR) for each variable.
Fig. 2.
Stratified Kaplan–Meier survival curves. (A): schooling years; (B): resistance pattern; (C): HIV coinfection; (D): moxifloxacin use; S(t): probability of survival.
Characteristics that did not meet the defined proportionality assumptions were sex, race, type of schema, cavities on X-ray, capreomycin resistance, presence of silicosis, hepatitis, smoking, diabetes, neoplasia, corticosteroid use, organ transplant, renal failure, ofloxacin use, pyrazinamide use, clofazimine use.
According to the final model, the characteristics associated with higher risk of death were age group greater than or equal to 60 years (HR = 1.6), XDR resistance pattern (HR = 1.74), begin of treatment after failure (HR = 1.72), resistance to streptomycin (HR = 1.24), resistance to ethambutol (HR = 1.30), drug abuse (HR = 1.64), presence of AIDS (HR = 1.46). The characteristics associated with lower risk of death were eight or more years of study (HR = 0.8), presentation of disease as exclusively pulmonary type (HR = 0.57), use of moxifloxacin (HR = 0.44), and use of levofloxacin in the treatment regimen (HR = 0.75) (Table 3).
Table 3.
Risk factors for death (Cox model).
| Variable | Coefficient | HR | CI | p-Value |
|---|---|---|---|---|
| Age group (60+) | 0.465 | 1.6 | 1.15–2.20 | 0.005 |
| Schooling (8+) | −0.22 | 0.8 | 0.63–1.01 | 0.068 |
| Resistance pattern (XDR) | 0.55 | 1.74 | 1.05–2.90 | 0.036 |
| Only pulmonary disease (yes) | −0.56 | 0.57 | 0.35–0.92 | 0.021 |
| Retreatment after failure (yes) | 0.54 | 1.72 | 1.27–2.32 | 0.0005 |
| Streptomycin resistance (yes) | 0.21 | 1.24 | 1.01–1.51 | 0.034 |
| Ethambutol resistance (yes) | 0.26 | 1.3 | 1.06–1.60 | 0.0105 |
| Drug addiction (yes) | 0.5 | 1.64 | 1.22–2.20 | 0.0008 |
| HIV infection (yes) | 0.36 | 1.46 | 1.05–1.96 | 0.024 |
| Moxifloxacin use (yes) | −0.82 | 0.44 | 0.25–0.80 | 0.0065 |
| Levofloxacin use (yes) | −0.28 | 0.75 | 0.60–0.94 | 0.016 |
| Likelihood ratio Log: 84.14 on 11 DF. p = 2.327e−13 | ||||
| Wald test: 90.33 em 11 DF p = 1.443e−14 | ||||
CI, confidence interval; HR, hazard ratio; DF, degrees of freedom.
Discussion
The long survival time may indicate the chronic evolution of this disease. In the studied population, median survival time was 3.97 years, similar to that found in other studies, between 3.8 and 4.1 years.6, 23 Beyond the first year, 91.2% were still alive. However, after two years the probability of survival greatly decreased, and could reflect progression of pulmonary damage. Survival time in MDRTB would be similar to that of tuberculosis in pre-antibiotic era.6
There was no difference in survival time related to sex, as found in other survival studies.6, 14, 17 Disease severity presented in both sexes could help explaining the similar survival. Like sex, ethnicity did not influence survival, perhaps for the same reason. Being ill would be influenced by biological and social factors, whereas evolution to death would be more related to disease severity.14
Older patients have greater risk of death. Similar findings were described by others, where the risk of death due to MDRTB increases as patient age increases.6, 12, 14, 23 Risk of death could almost double for each 10 years of increase in age.6 Life expectancy of Brazilian population is increasing steadily over the years.24 Elderly people may suffer from reactivation of TB infection acquired years earlier, or they could acquire it in hospitals or nursing homes.25 Patients that have more than eight schooling years have less risk of dying. Others have found similar association, being survival better in people with more schooling years.23, 26 Less education yield less access to information, which could make access to health services more difficult.26
Regarding the retreatment after MDRTB treatment failure, the risk of dying is 74% greater, although new cases comprise 81.7% of the studied population. Risk of death is higher in patients who have had prior treatment with second-line drugs.12, 14, 17, 18 Failure to a tuberculosis treatment mainly occurs due to the presence of resistance to prescribed medications, often associated with irregular medication taking.27
Better survival in patients with only pulmonary disease may be related to the finding of extrapulmonary or disseminated TB disease in HIV co-infection. Most patients had significant lung involvement suggesting delay in resistance detection and initiation of specific treatment.
Individuals with XDR resistance pattern die more frequently and sooner than MDRTB patients. The median survival time was one year less. The association of the XDR pattern with higher risk of death was found in other studies, being XDR resistance pattern an important predictor of death.12, 17 Particularly in XDR, if there are not many drug options to employ, patient may remain for a long time spreading the bacillus in the community until he evolves to death.27
Resistance to ethambutol or streptomycin, in addition to the multiresistance pattern is associated with a higher risk of death. The lowest survival of MDRTB patients was related to more number of resistances on sensitivity test (ST).19 Resistance to streptomycin would be a predictor of shorter survival time in patients with XDR pattern.17 As the mean of previous treatments for TB of the patients studied was 2.6 treatments, it is quite plausible to find resistance to other drugs in this population.
Use of moxifloxacin or levofloxacin is associated with lower risk of death. In Brazil moxifloxacin is employed in XDR regimens and in patients who failed therapy, both of them usually having more severe disease. In our country there is a widespread use of quinolones. Cross-resistance between quinolones may occur, and levofloxacin use could mask the diagnosis of pulmonary TB, yielding further resistance to this drug. Use of quinolones prior to TB treatment was associated with a higher risk of death, suggesting that someone who is taking quinolones should be screened for tuberculosis.14 High prevalence of ofloxacin resistance in South Korea was reported in patients who have never received TB treatment.18
HIV coinfected patient has a 46% higher risk of dying. Risk of death in HIV patients ranged from 2.7 to 4.2 fold in HIV-infected patients.14, 19, 23 Anderson et al. found a 60% lower chance of favorable outcome in the HIV co-infected patient.28 This variability could be explained, among other factors, by the number of individuals who underwent anti-HIV testing and different prevalences of HIV co-infection between studies, from 1.4%23 to 29.0%.6 Prevalence of HIV infection in studied population was 8.3%. Although ART use could positively influence MDRTB outcomes in HIV coinfection, death could occur due to other comorbidities. We did not find any differences in diabetes, smoking, and other comorbidities on KM curves. This could also be related to advanced disease found in most patients, and missing information on the database.
Drug addiction is an important predictor of death, with drug users having more risk of dying. Findings regarding drug addiction are not very consistent. Some authors evaluate substance abuse and social habits together. One study did not find association of 'social characteristics’ with favorable outcomes.28 Balabanova et al. also found no effect of drug abuse on the survival of MDRTB patients, but found unfavorable outcomes related to alcoholism.23 Notwithstanding another study has reported an association between alcohol, drug abuse, and unfavorable outcomes.29 Drug abuse has increased in Brazil since the 1990s, mainly crack addiction. There is a plausible existence of information bias in the evaluation of drug addiction in non-concurrent studies, as well as difficulties in obtaining this kind of information from patients.
Study limitations
Information bias cannot be ruled out. Some characteristics that may influence the evolution to death may not have been registered in the database. Comorbidity registration is not mandatory, as hospitalization, use of antiretroviral therapy (ART) and any other disease associated with HIV infection. Adverse effects of medications and their effect on patient survival were not evaluated. Adherence to treatment was not accurately assessed, because registration of daily drugs administration is not mandatory in the database.
Conclusions
The protective effect of latest generation quinolones corroborates the WHO recommendations for MDRTB treatment. Alongside with AIDS, drug addiction was the comorbidity that showed an important effect in increasing the risk of death. Drug addiction is a serious social problem that needs a more effective approach by health systems. Ideally patients must be followed by a multidisciplinary team, including social, financial, and psychological support.
It is extremely important that TB control programmes give more attention to factors related to individual behavior and social environment, which, together with the chronic evolution of the disease and its prolonged treatment, may adversely affect MDRTB outcomes.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Glaziou P., Falzon D., Floyd K., Raviglione M. Global epidemiology of tuberculosis. Semin Respir Crit Care Med. 2013;34:3–16. doi: 10.1055/s-0032-1333467. [DOI] [PubMed] [Google Scholar]
- 2.Tiemersma E.W., van der Werf M.J., Borgdorff M.W., Williams B.G., Nagelkerke N.J. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; 2013. Definitions and reporting framework for tuberculosis – 2013 Revision. [Google Scholar]
- 4.Caminero J.A. Multidrug-resistant tuberculosis: epidemiology, risk factors and case finding. Int J Tuberc Lung Dis. 2010;14:382–390. [PubMed] [Google Scholar]
- 5.WHO . World Health Organization; 2017. Global Tuberculosis Report 2017. [Google Scholar]
- 6.Drobniewski F., Eltringham I., Graham C., Magee J.G., Smith E.G., Watt B. A national study of clinical and laboratory factors affecting the survival of patients with multiple drug resistant tuberculosis in the UK. Thorax. 2002;57:810–816. doi: 10.1136/thorax.57.9.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micheletti V.C., Moreira Jda S., Ribeiro M.O., Kritski A.L., Braga J.U. Drug-resistant tuberculosis in subjects included in the Second National Survey on Antituberculosis Drug Resistance in Porto Alegre, Brazil. J Bras Pneumol. 2014;40:155–163. doi: 10.1590/S1806-37132014000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasil-Ministério da Saúde Sistema de Informação de Tratamentos Especiais de Tuberculose. 2010. http://sitetb.saude.gov.br/index.html Retrieved from: [20.01.17]
- 9.Brasil-Ministério da Saúde Detectar, tratar e curar: desafios e estratégias brasileiras frente à tuberculose. Bol Epidemiol. 2015;46 [Google Scholar]
- 10.Brasil-Ministério da Saúde . Ministério da Saúde; Brasil: 2011. Manual de Recomendações para o Controle da Tuberculose no Brasil. [Google Scholar]
- 11.Dalcolmo M.P., Andrade M.K., Picon P.D. Multiresistant tuberculosis in Brazil: history and control. Rev Saude Publica. 2007;41(Suppl 1):34–42. doi: 10.1590/s0034-89102007000800006. [DOI] [PubMed] [Google Scholar]
- 12.Jeon D.S., Shin D.O., Park S.K., et al. Treatment outcome and mortality among patients with multidrug-resistant tuberculosis in tuberculosis hospitals of the public sector. J Korean Med Sci. 2011;26:33–41. doi: 10.3346/jkms.2011.26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston J.C., Shahidi N., Sadatsafavi M., Fitzgerald J.M. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurbatova E.V., Taylor A., Gammino V.M., et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 2012;92:397–403. doi: 10.1016/j.tube.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung-Delgado K., Revilla-Montag A., Guillén-Bravo S., Bernabe-Ortiz A. Weight variation over time and its relevance among multidrug-resistant tuberculosis patients. Int J Infect Dis. 2014;23:20. doi: 10.1016/j.ijid.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Pefura-Yone E.W., Kengne A.P., Kuaban C. Non-conversion of sputum culture among patients with smear positive pulmonary tuberculosis in Cameroon: a prospective cohort study. BMC Infect Dis. 2014;14:138. doi: 10.1186/1471-2334-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.H., Kim H.J., Park S., et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:113–119. doi: 10.1164/rccm.200911-1656OC. [DOI] [PubMed] [Google Scholar]
- 18.Jeon C.Y., Hwang S.H., Min J.H., et al. Extensively drug-resistant tuberculosis in South Korea: risk factors and treatment outcomes among patients at a tertiary referral hospital. Clin Infect Dis. 2008;46:42–49. doi: 10.1086/524017. [DOI] [PubMed] [Google Scholar]
- 19.Mitnick C.D., Franke M.F., Rich M.L., et al. Aggressive regimens for multidrug-resistant tuberculosis decrease all-cause mortality. PLoS ONE. 2013 doi: 10.1371/journal.pone.0058664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahuja S.D., Ashkin D., Avendano M., et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker M.A., Harries A.D., Jeon C.Y., et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padayatchi N., Abdool Karim S.S., Naidoo K., Grobler A., Friedland G. Improved survival in multidrug-resistant tuberculosis patients receiving integrated tuberculosis and antiretroviral treatment in the SAPiT Trial. Int J Tuberc Lung Dis. 2014;18:147–154. doi: 10.5588/ijtld.13.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balabanova Y., Radiulyte B., Davidaviciene E., et al. Survival of drug resistant tuberculosis patients in Lithuania: retrospective national cohort study. BMJ Open. 2011;1 doi: 10.1136/bmjopen-2011-000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IBGE Instituto Brasileiro de Geografia e Estatística – Séries históricas e estatísticas. 2016. http://seriesestatisticas.ibge.gov.br/series.aspx?vcodigo=POP106&t=populacao-presente-residente-cor-raca-dados Retrieved from: [07.01.16]
- 25.Schaaf S., Zumla A., Grange J.M., et al. Elsevier; 2009. Tuberculosis – a comprehensive clinical reference. ISBN: 978-1416039884. [Google Scholar]
- 26.San Pedro A., Oliveira R.M. Tuberculose e indicadores socioeconômicos: revisão sistemática da literatura. Rev Panam Salud Publica. 2013;33:294–301. doi: 10.1590/s1020-49892013000400009. [DOI] [PubMed] [Google Scholar]
- 27.Raviglione M.C., Smith I.M. XDR tuberculosis – implications for global public health. N Engl J Med. 2007;356:656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 28.Anderson L.F., Tamne S., Watson J.P., et al. Treatment outcome of multi-drug resistant tuberculosis in the United Kingdom: retrospective-prospective cohort study from 2004 to 2007. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.40.20601. pii:20601. [DOI] [PubMed] [Google Scholar]
- 29.Cavanaugh J.S., Kazennyy B., Nguyen M.L., et al. Outcomes and follow-up of patients treated for multidrug-resistant tuberculosis in Orel, Russia, 2002–2005. Int J Tuberc Lung Dis. 2012;16:1069–1074. doi: 10.5588/ijtld.11.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]