Abstract
Here we reported the outbreak of measles cases caused by the genotype D8 measles virus for the first time in Jiangsu province in China, which was possibly imported by a foreign student from Laos. Throat swab specimens were collected, and used to isolate virus. 634-bp fragment of the N gene and 1854-bp fragment of H gene were amplified by reverse transcription-PCR and sequenced, respectively. Phylogenetic results indicated that they belonged to genotype D8 measles virus. Further epidemiology investigation showed that the adults with D8 measles virus infection did not receive measles vaccine before having measles. In China, almost all D8 genotype MeV only infected those population without receiving measles vaccine immunization. Therefore, it is still necessary to implement the supplement activity of measles immunization target adult with immunity gap.
Keywords: Measles virus, Imported D8 genotype, Outbreak
Herein we report the outbreak of measles caused by the genotype D8 measles virus (MeV) for the first time in Jiangsu province in China, which was possibly imported by a foreign student from Laos. Throat swab specimens were collected and used to isolate the virus. A 634-bp fragment of the N gene and 1854-bp fragment of H gene were amplified by reverse transcription-PCR and sequenced, respectively. Phylogenetic results indicated that they belonged to genotype D8 MeV. Further epidemiological investigation showed that adults with D8 MeV infection had not been vaccinated against measles. In China, almost all cases of D8 genotype MeV infection occurred in patients with no previous measles vaccination. Therefore, it is necessary to offer measles vaccination for adults who are immunized.
Countries all over the world have adopted goals for measles elimination by or before 2020.1 Toward that end, China government implemented measles supplementary immunization activity in the whole country in 2010, and the incidence of measles decreased dramatically, reaching the lowest reported level in 2012.2, 3 However, a nationwide resurgence of measles occurred primarily among young unvaccinated children in 2013.4 Both before and after resurgence of measles, H1 has been the predominant strain in China, although only a few imported D8, D9, and vaccine-associated strains were found.5, 6, 7
Jiangsu Province, located in the east of China, is one of the highly economically developed regions in China. It has nearly 80 million permanent residents and migrants. Over 95% of the children are vaccinated against measles. Nonetheless, measles resurged in 2014, and subsequently declined rapidly to the level before 2013 until 2016. Molecular epidemiology surveillance showed that only H1a genotype was circulating in Jiangsu province.
Between September 22 and October 18, 2017 two measles cases were reported by Nantong Prefecture Center for Disease Control and Prevention (NPCDC). Case 1: on October 15, 2017, a 31-year old female college teacher presented with fever. Two days later, rashes appeared, followed by cough, rhinorrhea, sneezing, and was diagnosed as measles in hospital X.
Case 2: on October 13, 2017, a 36-year old female janitor, working at the outpatient transfusion room in hospital X presented with fever, progressing with rashes and cough on October 17, and was diagnosed as measles.
Throat swab and blood specimens were collected on October 17 and October 18, respectively. IgM antibody and real-time florescence PCR for measles and rubella were performed at NPCDC. IgM antibody in serum and nucleic acid in swab turned out positive for measles virus. Subsequently, swab specimens were transported to Jiangsu Province Center for Disease Control and Prevention (JSCDC) for viral isolation and genotype identification. The Vero/SLAM cell line (African green monkey kidney cells human signaling lymphocyte activation molecule) was used for the isolation of MeV, and the infected cells were harvested when the cytopathic effect (CPE) was visible in more than 75% of the cell layer. The QIAamp® Viral RNA Mini Kit (Qiagen, Hilden Germany) was used to extract MeV nucleic acid according to the manufacturer's instructions. Reverse transcription amplification was performed using previously described primers to amplify a 634-bp fragment of the N gene and a 1854-bp fragment of the H gene,8 which included enough length fragment recommended for genotyping. The PCR products were sequenced using ABI 3730 DNA Sequencer (Applied Biosystems, Foster City, CA, USA) at Sagon Biotech (Shanghai, China) using directional primers. The sequences were edited using Bioedit software, then analyzed using MEGA 7.0 software.
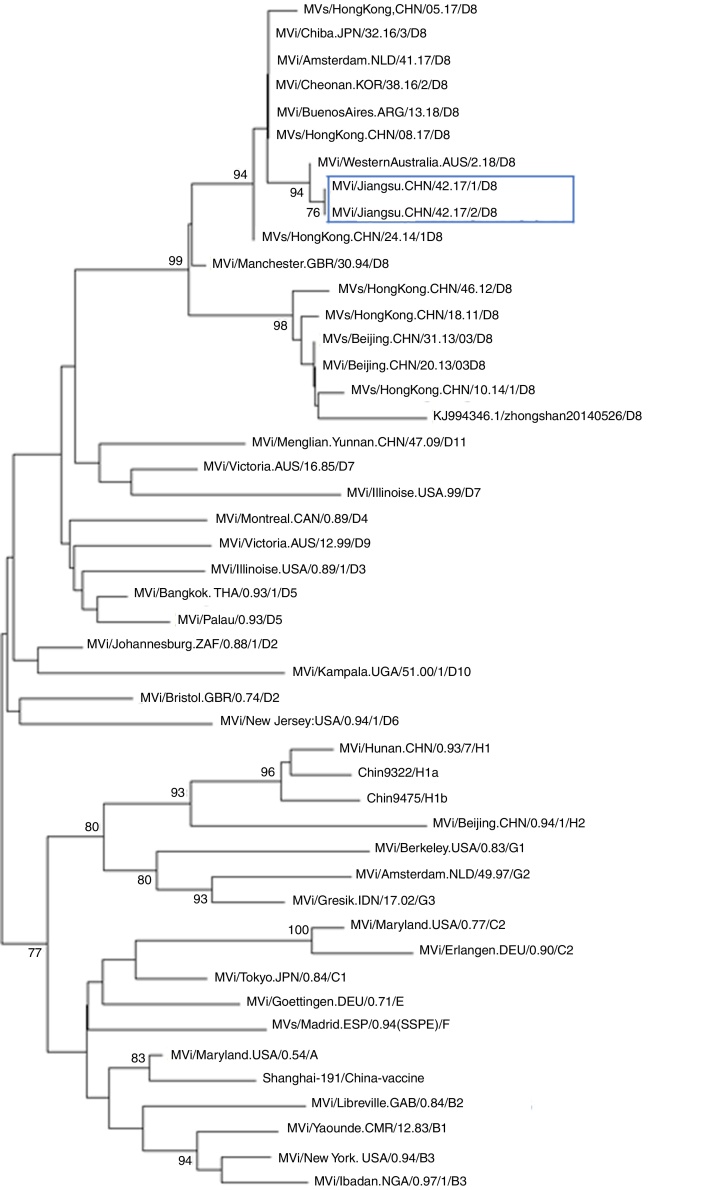
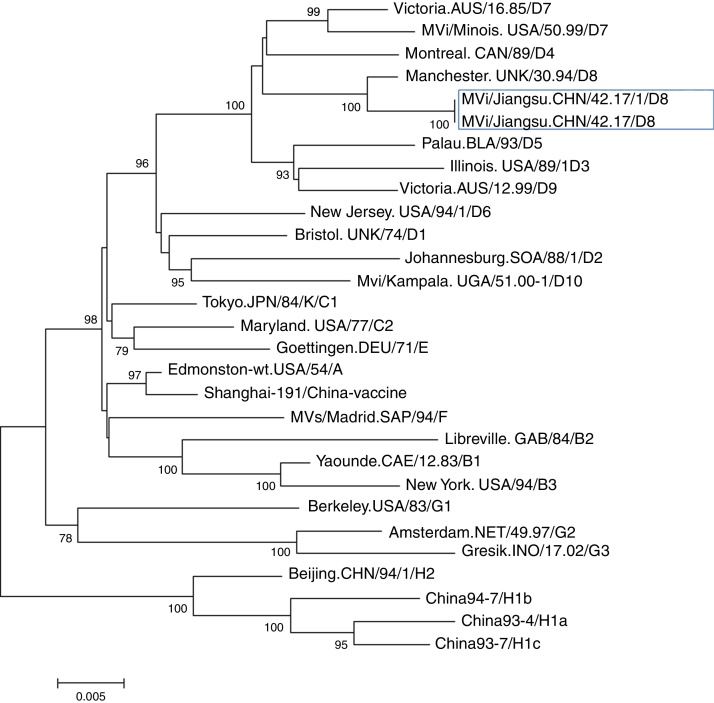
Further epidemiological investigation showed that these two cases had no history of traveling to other countries and had not received measles vaccination. A foreign female student from Vientiane (the capital and largest city of Laos) had been to China on September 12, arriving to Nantong on September 14. As of September 22, she developed fever and rashes and went to Hospital X for care. However, no further information could be obtained. Nonetheless, there was a possibility of measles transmission between this student and the two cases described above. Case 1 was the teacher of this student in college. Case 2 had been working at hospital X when the foreign student went to hospital X for care. The further phylogenetic analysis showed the measles viruses in this event belong to D8 genotype (Fig. 1, Fig. 2). They share 99% identity with the N gene sequences (450 bp) of measles virus from Japan, Australian, Argentina, England, Netherlands, and China Hongkong and with the H gene sequences (1854 bp) of it from the United Kingdom, Canada, USA, and Australia.
Fig. 1.
Phylogenetic tree based on the nucleotide protein (N) gene sequences of various strains of the measles virus. The evolutionary distance was calculated using Kimura's two-parameter method, and the tree was plotted using the neighbor-joining method. Numbers at each branch indicate the bootstrap values of the clusters supported by that branch, only more than 75% of bootstrap value is indicated. Jiangsu cases were circled with rectangle line.
Fig. 2.
Phylogenetic tree based on the hemagglutinin protein (H) gene sequences of various strains of the measles virus. The evolutionary distance was calculated using Kimura's two-parameter method, and the tree was plotted using the neighbor-joining method. Numbers at each branch indicate the bootstrap values of the clusters supported by that branch, only more than 75% of bootstrap value is indicated. Jiangsu cases were circled with rectangle line.
As far as we know, this is the first measles outbreak caused by imported D8 genotype in Jiangsu province, and the fourth in China.2, 5, 9 Further epidemiological investigation showed that neither of the two patients reported herein had received measles vaccination. The first D8 case was an eight-month old child from Shanghai in 2012, with no previous measles vaccination,5 and the second outbreak was in Beijing in 2013, with two adult cases, for whom no information on measles vaccination could be gathered.2 All of these reports suggest that an immunity gap among all imported D8 genotype cases, which is still an important factor for transmitting measles virus. It is therefore necessary to implement supplement activity of measles immunization target adult with immunity gap.
Population density is a major determinant of attack rates and transmission of measles virus.10, 11 In China, almost of all imported measles cases occurred in economically developed regions and border regions.12, 13, 14 Frequent migration and higher population density make these regions more vulnerable than others in China, as all adults got imported measles virus infection in venues with higher population density such as school, hospital, and fabric market. Therefore, more attention should be drawn to measles surveillance in regions and venues with high population migration and density, especially in epidemic seasons.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
We thank all staff at the Department of Expanded Program on Immunization of Nantong Prefecture Center for Disease Control and Prevention for making this investigation possible. This study was partly supported by Jiangsu Provincial High-Level Talents Programs in Health (LGY2016021).
References
- 1.Dabbagh A., Patel M.K., Dumolard L., et al. Progress towards regional measles elimination worldwide, 2000–2016. Wkly Epidemiol Rec. 2017;92:649–660. [Google Scholar]
- 2.Chen M., Zhang Y., Huang F., et al. Endemic and imported measles virus-associated outbreaks among adults, Beijing, China, 2013. Emerg Infect Dis. 2015;21:477–479. doi: 10.3201/eid2103.140646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma C., Li F., Zheng X., et al. Measles vaccine coverage estimates in an outbreak three years after the nation-wide campaign in China: implications for measles elimination, 2013. BMC Infect Dis. 2015;15:1–8. doi: 10.1186/s12879-015-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S., Ma C., Hao L., et al. Demographic transition and the dynamics of measles in six provinces in China: a modeling study. PLoS Med. 2017;14:e1002255. doi: 10.1371/journal.pmed.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S., Qian X., Yuan Z., et al. Molecular epidemiology of measles virus infection in Shanghai in 2000-2012: the first appearance of genotype D8. Braz J Infect Dis. 2014;18:581–590. doi: 10.1016/j.bjid.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S., Zhang Y., Rivailler P., et al. Evolutionary genetics of genotype H1 measles viruses in China from 1993 to 2012. J Gen Virol. 2014;95:1892–1899. doi: 10.1099/vir.0.066746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Wang H., Xu S., et al. Monitoring progress toward measles elimination by genetic diversity analysis of measles viruses in China 2009–2010. Clin Microbiol Infect. 2014;20:O566–O577. doi: 10.1111/1469-0691.12530. [DOI] [PubMed] [Google Scholar]
- 8.Magurano F., Fortuna C., Marchi A., et al. Molecular epidemiology of measles virus in Italy, 2002–2007. Virol J. 2012;9:1–9. doi: 10.1186/1743-422X-9-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan R., He B., Yao F., et al. Investigation of a measles outbreak caused by genotype D8 virus in Pinghu city of Zhejiang province, 2017. Chin J Epidemiol. 2018;39:333–336. doi: 10.3760/cma.j.issn.0254-6450.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Smithson R., Irvine N., Hutton C., et al. Explaining seasonal fluctuations of measles in Niger using nighttime lights imagery. Science. 2011;334:1424–1427. doi: 10.1126/science.1210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk S.J., Guo L.Y., Sekulic N., et al. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348:694–699. doi: 10.1126/science.aaa3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang X., Sun J., Zhang Y., et al. The first measles outbreak caused by imported genotype D9 measles virus in Shandong Province, China, 2013. Jpn J Infect Dis. 2014;67:300–303. doi: 10.7883/yoken.67.300. [DOI] [PubMed] [Google Scholar]
- 13.Pang Y.-K., Li L.-Q., Ding Z.-R., et al. Investigation on an outbreak of measles caused by new virus (d11 genotype) imported from Myanmar. Chin J Epidemiol. 2014;32:17–19. [PubMed] [Google Scholar]
- 14.Zhao Z., Zhou R., Yu W., Li L., Li Q., Hu P. Measles outbreak prevention and control among adults: lessons from an importation outbreak in Yunnan province, China, 2015. Hum Vaccin Immunother. 2018;14:881–886. doi: 10.1080/21645515.2017.1417712. [DOI] [PMC free article] [PubMed] [Google Scholar]