Abstract
This study aimed to evaluate the protective role of statins on the development of sepsis and infection-related organ dysfunction and mortality in a hospitalized older Chinese population with bacterial infections. In this retrospective cohort study, 257 older patients with bacterial infection were divided into two groups: a statin group, those who had received statin therapy for ≥1 month before admission and continued receiving statin during hospitalization; and a non-statin group, those who had never received statin or used statin for <1 month prior to admission. A multivariate logistic regression analysis was performed to identify risk and protective factors for severe sepsis. A significantly lower incidence of organ dysfunction was found in the statin group, as compared with the non-statin group (13.3% vs 31.1%, respectively; p = 0.002), corresponding to adjusted rates ratio of 0.32 (95% confidence interval [CI], 0.13–0.75; p = 0.009). No significant difference was found between statin and non-statin groups in 30-day sepsis-related mortality (4.4% vs 10.2%, respectively; p = 0.109), incidence of intensive care unit admission (13.3% vs 16.8%, respectively; p = 0.469), or length of hospital stay (20.5 vs 25.9 days, respectively; p = 0.61). Statins significantly reduced the development of sepsis and infection-related organ dysfunction in hospitalized older Chinese patients but did not reduce 30-day mortality, ICU admission incidence, or length of hospital stay.
Keywords: Mortality, Organ dysfunction, Older adults, Sepsis, Statins
Introduction
Sepsis occurs in approximately 2% of general hospitalized populations in developed countries, including 6%–30% of all patients admitted to intensive care units (ICU).1, 2 About one-half to three-quarters of patients with sepsis experience organ failure.3 In the United States, approximately 751,000 patients developed severe sepsis in 1995; currently, among the more than 1,000,000 hospitalized patients in the United States diagnosed with sepsis annually, the incidence is projected to increase by 1.5% annually, with only 50%–70% of patients surviving.1, 4 However, a 20-year study showed that sepsis is increasing at a rate of 8.7% per year, which is greater than the population growth rate.5 The incidence of sepsis is also high in China; older patients with comorbidities who are admitted with bacterial infections have a higher risk.6
Although the pathophysiology of sepsis has not been fully elucidated, general consensus indicates that sepsis arises from immunodysregulation and uncontrolled systemic inflammatory responses.7 The inflammatory cascade is multilevel and involves the production of pro-inflammatory cytokines, adhesion molecules, and reactive oxygen species.8 Given this pathophysiology, clinical trials have investigated adjuvant mediator therapy to identify drugs that block specific inflammatory mediators.9, 10, 11 Toward that end, statins are well known as lipid-lowering agents and have also demonstrated anti-inflammatory and immunomodulatory properties.12 Almog et al.13 demonstrated that prior statin therapy might reduce rates of severe sepsis and ICU admissions. This findings have prompted researchers worldwide to study the pleiotrophic effects of statins on sepsis, with largely unconfirmed results.12, 14, 15, 16 A recent meta-analysis of randomized controlled trials (RCTs) suggested that statins should not be recommended to manage severe sepsis in critically ill patients.17 In addition, a recent systematic review reported that statins do not decrease the incidence of sepsis, progression to severe sepsis, or associated mortality rates.18 Nevertheless, patients receiving statins prior to hospital admission due to infection, bacteremia, sepsis, and infection-related organ dysfunction continue to demonstrate reduced risks of sepsis progression/severity, rates of hospital-acquired bacteremia, and hospital mortality.13, 16 Most previous studies were conducted in Western populations except for a retrospective study conducted by Yang et al.19 who studied an Asian population in Taiwan, indicating that statins neither benefited Asian patients with sepsis nor improved short-term survival.
Although RCTs have examined the potential value of statins in sepsis through meta-analysis and systematic review,17, 18 to our knowledge Asian patients or exclusively older patients with bacterial infections have not been studied, and conclusions have not shown a beneficial role of statins in bacterial infections. Therefore, the present study aimed to investigate the protective role of statins on the development of bacterial infections and infection-related organ dysfunction and mortality in older Chinese patients hospitalized due to bacterial infections.
Material and methods
Study design and patients
A retrospective cohort study enrolling older Chinese patients hospitalized due to bacterial infection was conducted between March 2011 and March 2012 in the Geriatric Medicine Department at First Affiliated Hospital, School of Medicine of Zhejiang University, Zhejiang Province, China. This study was approved by the Clinical Research Ethics Committee of First Affiliated Hospital. As patient data were de-identified and patients remained anonymous during the retrospective review, signed informed consent was waived.
For data collection purposes, only the first admission from a series of multiple admissions involving the same patients during the study period was included. For this study, inclusion criteria were: patients aged ≥65 years old with sepsis whose records included complete clinical and laboratory data.
Patients were divided into two groups based on those who had prior statin use and no statin use. The statin group were those who had used a statin for at least one month prior to the first hospital admission and continued statin treatment during hospitalization. Based on a prior study that indicated that the therapeutic effects of statins are demonstrated after one month of continuous use, the non-statin group was characterized by patients who either had never taken statins or had taken a statin for <1 month prior to admission.13 Although other characteristics between the two groups were different, all patients received treatment for bacterial infection, and the study endpoint was development of sepsis. Patients in the statin group were treated with several different statin drugs and dosages including atorvastatin, 20 mg daily; simvastatin, 40 mg daily; fluvastatin, 40 mg daily; pravastatin, 20 mg daily; and rosuvastatin, 10 mg daily. Data from both groups were reviewed 30 days after admission.
Sepsis and severe sepsis with organ failure were defined by the most recent criteria from the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) written by Singer et al.20 Patients were assessed using Acute Physiology and Chronic Health Evaluation II (APACHE II) criteria, and their APACHE II scores were determined upon admission. Sepsis management was based on SCCM practice parameters for the hemodynamic support of sepsis in adult patients.
Data were obtained from electronic medical records from time of admission and in the following 30 days, with the exception of those with nosocomial infections whose data were collected at the time their sepsis diagnosis was confirmed. Patient characteristics were collected from data on a single admission, including demographics, pre-existing medical conditions, concurrent medications, infection type and site, APACHE II scores, laboratory data, microbiological culture results, ICU admission, length of hospital stay, and sepsis-related mortality. Laboratory values (e.g., albumin, triglycerides, C-reactive protein [CRP]) selected for analysis were a single measure in any 24-h period that most accurately represented patient status (i.e., highest or lowest value depending on specific test). The primary endpoints were the development of sepsis as indicated by the incidence of multiple organ dysfunction syndrome (ICD Code 995.92) and 30-day sepsis-related mortality. Secondary outcomes included incidence of ICU admission and length of hospital stay.
Statistical analysis
Continuous data are presented as median and interquartile range and were compared between groups using the Mann–Whitney U test. Categorical data are presented as number and percentage (%) and were assessed using χ2 test or Fisher's exact test. Logistic regression analysis was conducted to screen for variables independently associated with severe sepsis (defined as sepsis with organ dysfunction). Age, gender, and variables with a p of <0.05 in univariate analysis were included in a model for the multivariate analysis. Associations between the dependent outcome variables (multiple organ dysfunction, ICU admission incidence, length of hospital stay, 30-day mortality) and the variables included in the final model are expressed by odds ratios (OR) and their respective 95% confidence intervals (CIs). The significance level was set at 0.05. All statistics were two-sided and analyzed using SPSS 22.0 (IBM Corp., Armonk, NY).
Results
A total of 257 eligible patients were included, among whom 90 (35%) used statins. Baseline patient characteristics are summarized in Table 1. Comparing the statin and non-statin groups, no significant differences were demonstrated in age, gender distribution, APACHE II scores, smoking and drinking habits, steroid treatment, heart rate, respiratory rate, blood culture results, blood pressure, and most biochemical and hematological parameters. However, a higher percentage of patients not treated with statins had bacterial infections in the biliary/gastrointestinal tract (14.4% vs 4.4%, respectively; p = 0.015) and higher activated partial thromboplastin time (APTT) levels (p = 0.042) than statin users (Table 1). Body temperature was higher in the statin group than the non-statin group (p = 0.001). Patients of the non-statin group were less likely to have nosocomial infections, as compared with statin-group patients (25.7% vs 42.2%, respectively; p = 0.007).
Table 1.
Baseline demographic and clinical characteristics of 257 older patients with bacterial infections.
| Non-statin group (n = 167) | Statin group (n = 90) | p-Value | |
|---|---|---|---|
| Age, y | 81 (77, 85) | 83 (78, 86) | 0.228 |
| Male | 109 (65.3) | 52 (57.8) | 0.236 |
| APACHE II score | 12.0 (8.0, 15.0) | 10.5 (7.0, 15.0) | 0.238 |
| Site of infection | |||
| Pulmonary | 115 (68.9) | 66 (73.3) | 0.454 |
| Urinary tract | 10 (6.0) | 8 (8.9) | 0.385 |
| Soft tissue/skin | 8 (4.8) | 6 (6.7) | 0.570 |
| Biliary/gastrointestinal tract | 24 (14.4) | 4 (4.4) | 0.015 |
| Catheter-related infection | 6 (3.6) | 2 (2.2) | 0.717 |
| Others | 4 (2.4) | 4 (4.4) | 0.456 |
| Smoking | 16 (9.6) | 11 (12.2) | 0.510 |
| Drinking | 8 (4.8) | 4 (4.4) | 0.999 |
| Steroid treatment for more advanced septicemia | 40 (24) | 21 (23.3) | 0.911 |
| Blood culture (+) | 27 (16.2) | 10 (11.1) | 0.271 |
| Nosocomial infection | 46 (25.7) | 38 (42.2) | 0.007 |
| Body temperature | 37.2 (36.8, 38.2) | 38.0 (36.9, 38.7) | 0.001 |
| Heart rate | 84 (76, 95) | 83.5 (76, 96) | 0.670 |
| Respiratory rate | 19.7 (18, 20.4) | 19 (18, 20) | 0.393 |
| Systolic blood pressure, mmHg | 133 (118, 148) | 130 (119, 140) | 0.177 |
| Diastolic blood pressure, mmHg | 71 (64, 80) | 72 (67, 78) | 0.310 |
| Laboratory tests | |||
| WBC count, 109/L | 8.7 (5.8, 12.9) | 10.1 (7.5, 12.2) | 0.178 |
| Albumin, g/dL | 36.0 (32.7, 38.8) | 36.7 (34.2, 39.4) | 0.185 |
| Alanine aminotransferase, U/L | 22.0 (13.0, 41.0) | 25.5 (17.0, 38.0) | 0.153 |
| T Bil, μmol/L | 11.4 (8.4, 17) | 12 (7.8, 16) | 0.687 |
| TG, mmol/L | 1.0 (0.8, 1.4) | 1.0 (0.8, 1.4) | 0.490 |
| TC, mmol/L | 4.0 (3.2, 4.5) | 3.6 (3.2, 4.5) | 0.604 |
| HDL-C, mmol/L | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.4) | 0.133 |
| LDL-C, mmol/L | 2.0 (1.5, 2.5) | 1.8 (1.5, 2.5) | 0.487 |
| Glucose, mmol/L | 5.2 (4.6, 6.3) | 5.2 (4.7, 6.0) | 0.933 |
| INR | 1.1 (1.0, 1.2) | 1.0 (1.0, 1.2) | 0.696 |
| Fibrinogen, g/L | 3.9 (3, 5) | 3.5 (2.9, 4.7) | 0.349 |
| APTT, g/L | 29.8 (25.6, 34.2) | 28.1 (24.5, 33.0) | 0.042 |
| Thrombin time, s | 18.2 (17.1, 19.6) | 18.2 (17.1, 19.5) | 0.895 |
| Prothrombin time, s | 12.3 (11.3, 13.3) | 12.3 (11.3, 13.3) | 0.900 |
| D-dimer, μg/L | 244.0 (132.0, 455.0) | 237.5 (129.0, 452.0) | 0.957 |
| CRP, mg/L | 43.5 (17.0, 80.2) | 34.0 (13.2, 60.6) | 0.138 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; T Bil, total bilirubin; TG, triglyceride; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; INR, international normalized ratio; APTT, activated partial thromboplastin time; CRP, C-reactive protein.
Continuous variables are presented as median (interquartile range), and categorical variables are expressed as n (%).
Bold values indicate significance between the two groups, p < 0.05.
Blood samples were obtained from all patients for culture, but confirmatory cultures using other biological specimens including urine or sputum were not collected from all patients. As for patients with a positive blood culture, three patients had a positive sputum culture (Acinetobacter spp, Aeromonas spp., and Staphylococcus spp.). Two patients had a positive urine culture (Klebsiella pneumonia and Corynebacterium spp.), and one patient had a positive throat swab culture (Aeromonas spp.).
As for patients with negative blood cultures, 11 patients had positive sputum culture results (all positive, but the species were not reported). Some patients had positive urine culture results (Klebsiella pneumonia and Escherichia coli). One patient had a positive bile culture result (Moraxella osloensis, Candida albicans, Klebsiella pneumonia, Enterococcus hirae); one patient had positive stool culture (Candida albicans) and throat swab culture results (Aeromonas spp.).
Table 2 shows differences in medications and comorbidities between statin users and non-users. Proportions of hypertension (77.7% vs 65.8%, respectively; p = 0.047) and coronary artery disease (52.2% vs 22.1%, respectively; p < 0.001) were higher in the statin group than the non-statin group. Patients in the statin group were more likely to take aspirin (33.3% vs 17.9%, respectively; p = 0.005), β-blockers (24.4% vs 10.1%, respectively; p = 0.002), calcium antagonists (37.7% vs 25.7%, respectively; p = 0.045), other antiplatelet agents (34.4% vs 11.3%, respectively; p < 0.001), nitrate esters (24.4% vs 13.7%, respectively; p = 0.032), oral hypoglycemics (30.0% vs 13.1%, respectively; p = 0.001), trimetazidine (31.1% vs 8.3%, respectively; p < 0.001), and antiarrythmics (8.8% vs 2.9%, respectively; p = 0.040) than the non-statin group.
Table 2.
Medications and comorbidities of the patients by group (n = 257).
| Non-statin group (n = 167) | Statin group (n = 90) | p-Value | |
|---|---|---|---|
| Comorbidities | |||
| COPD | 46 (27.5) | 28 (31.1) | 0.547 |
| Hypertension | 110 (65.8) | 70 (77.7) | 0.047 |
| Diabetes | 49 (29.3) | 37 (41.1) | 0.056 |
| Malignancy | 33 (19.7) | 15 (16.6) | 0.544 |
| Coronary artery disease | 37 (22.1) | 47 (52.2) | <0.001 |
| Cerebrovascular disease | 32 (19.1) | 23 (25.5) | 0.233 |
| Chronic renal failure | 12 (7.1) | 7 (7.7) | 0.863 |
| Chronic heart failure | 21 (12.5) | 17 (18.8) | 0.174 |
| Chronic liver disease | 10 (5.9) | 1 (1.1) | 0.065 |
| Peripheral vascular disease | 6 (3.5) | 6 (6.6) | 0.265 |
| Preadmission medication | |||
| ACE I or angiotensin II antagonists | 28 (16.7) | 24 (26.6) | 0.059 |
| Aspirin | 30 (17.9) | 30 (33.3) | 0.005 |
| Beta blockers | 17 (10.1) | 22 (24.4) | 0.002 |
| Calcium antagonists | 43 (25.7) | 34 (37.7) | 0.045 |
| Antiplatelet agents (other) | 19 (11.3) | 31 (34.4) | <0.001 |
| Spironolactone | 20 (11.9) | 9 (10.0) | 0.633 |
| Furosemide | 17 (10.1) | 9 (10.0) | 0.964 |
| Nitrate esters | 23 (13.7) | 22 (24.4) | 0.032 |
| Oral hypoglycemics | 22 (13.1) | 27 (30.0) | 0.001 |
| Digoxin | 14 (8.3) | 9 (10.0) | 0.665 |
| Trimetazidine | 14 (8.3) | 28 (31.1) | <0.001 |
| Antiarrhythmic agents | 5 (2.9) | 8 (8.8) | 0.040 |
| Insulin | 9 (5.3) | 8 (8.8) | 0.282 |
| Warfarin | 5 (2.9) | 2 (2.2) | 0.717 |
| Donepezil hydrochloride | 8 (4.7) | 4 (4.4) | 0.900 |
| Memantine | 6 (3.5) | 2 (2.2) | 0.546 |
| Steroid use for chronic diseases | 3 (1.7) | 3 (3.3) | 0.436 |
Abbreviations: COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting enzyme inhibitors.
Data are shown as n (%).
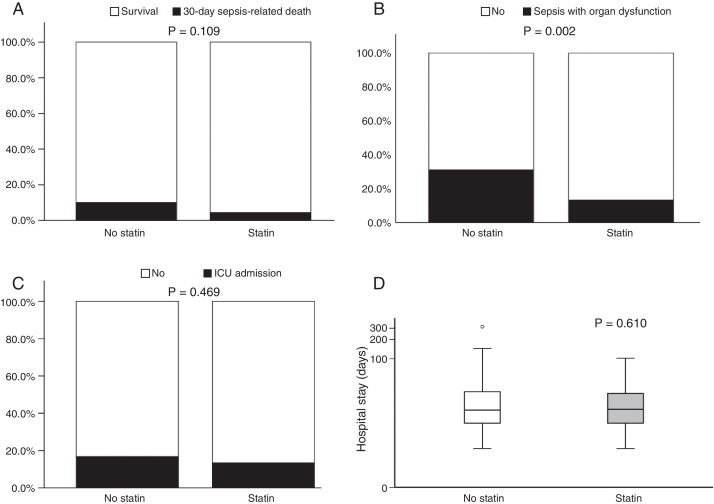
Fig. 1 illustrates 30-day sepsis-related mortality, development of severe sepsis characterized by organ dysfunction, ICU admission, and length of hospital stay between the statin and non-statin groups. Sepsis-related mortality was defined as cause of death directly or indirectly related to infection or defined as cause of death not related to infection but the patient had an uncontrollable infection at the time of death. Non-statin users had higher rates of severe sepsis characterized by organ dysfunction than statin users (31.1% vs 13.3%, respectively; p = 0.002). No difference was found between the two groups in 30-day sepsis-related mortality, ICU admission, or length of hospital stay. However, more patients in the non-statin group (10.2%) died secondary to sepsis-related causes within 30 days of admission for a bacterial infection than those in the statin group (4.4%). The proportion of patients admitted to the ICU was 13.3% for the non-statin group and 16.8% for the statin group, but not significantly different. Regardless of statin use, one-half of all enrolled patients were hospitalized for sepsis treatment for at least 15 days.
Fig. 1.
Bar charts of 30-day sepsis-related mortality: (A), sepsis with organ dysfunction (B), ICU admission (C), and a box plot of length of hospital stay (D) (n = 257). The box plot comprises the 75th percentile (top of box), median (bold line in box), and 25th percentile. Circles and asterisks in the box plot indicate outliers greater than 1.5 and 3 times the interquartile range (IQR). Chi-square test was used to compare differences among 30-day infection-related mortality, bacterial infection with organ dysfunction, and ICU admission between the two groups. Mann–Whitney U test was implemented for hospital stays.
Variables associated with severe sepsis are reported in Table 3. A univariate analysis showed that 10 factors were associated with severe sepsis, but only six remained significant in the final multivariate model. Statin use and albumin were shown to be protective factors for severe sepsis, with ORs of 0.28 (95% CI, 0.13–0.62; p = 0.002) and 0.91 (95% CI, 0.85–0.98; p = 0.013), respectively. Triglycerides (TG), thrombin time, chronic obstructive pulmonary disease (COPD), and chronic heart failure were all associated with severe sepsis and may indicate risk.
Table 3.
Variables associated with severe sepsis.
| Univariate analysisa |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, years | 1.02 (0.98, 1.07) | 0.382 | ||
| Male | 1.08 (0.60, 1.95) | 0.787 | ||
| Statins | 0.34 (0.17, 0.68) | 0.002 | 0.28 (0.13, 0.62) | 0.002 |
| Infection at other sites | 5.37 (1.25, 23.13) | 0.024 | ||
| SBP, mmHg | 0.99 (0.97, 1.01) | 0.090 | ||
| DBP, mmHg | 0.98 (0.95, 1.01) | 0.089 | ||
| Laboratory tests | ||||
| Albumin, g/dL | 0.88 (0.82, 0.94) | <0.001 | 0.91 (0.85, 0.98) | 0.013 |
| T Bil, μmol/L | 1.02 (1.00, 1.03) | 0.039 | ||
| TG, mmol/L | 2.13 (1.41, 3.22) | <0.001 | 2.29 (1.48, 3.55) | <0.001 |
| TC, mmol/L | 1.07 (0.82, 1.4) | 0.612 | ||
| Thrombin time, s | 1.12 (1.01, 1.24) | 0.033 | 1.16 (1.02, 1.30) | 0.018 |
| CRP, mg/L | 1.01 (1.00, 1.01) | 0.017 | 1.01 (0.99, 1.01) | 0.096 |
| Comorbidities | ||||
| COPD | 1.88 (1.04, 3.42) | 0.038 | 2.23 (1.12, 4.45) | 0.022 |
| Chronic heart failure | 2.59 (1.26, 5.32) | 0.009 | 3.07 (1.31, 7.20) | 0.010 |
| Preadmission medication | ||||
| Aspirin | 0.46 (0.21, 0.99) | 0.047 | ||
| Spironolactone | 2.82 (1.27, 6.25) | 0.011 | ||
| Digoxin | 2.56 (1.06, 6.17) | 0.036 | ||
Abbreviations: OR, odds ratio; CI, confidence interval; T Bil, total bilirubin; TG, tryglycerides; CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; DBP diastolic blood pressure; TC, total cholesterol.
Logistic regression was implemented, and a backward procedure was applied to a multivariate analysis model (R2 = 0.315).
Discussion
To our knowledge, this retrospective cohort study was the first to evaluate the effects of statins in older Chinese patients hospitalized due to bacterial infection. Significant decreases were demonstrated in the development of sepsis and infection-related organ dysfunction in older patients treated with statins for >1 month prior to hospitalization and who continued statin treatment during hospitalization infection. Although no differences were found between statin users and non-users in 30-day sepsis-related mortality, ICU admission, or length of hospital stay, the percentage of patients who died because of sepsis-related causes within 30 days of admission due to bacterial infection was lower in the statin group (4.4%) than the non-statin group (10.2%).
Based on a literature review, the results of the present study are congruent with those of previous studies. Almog et al.13 concluded that prior statin treatment might be associated with reduced rates of severe sepsis and ICU admission in patients with acute bacterial infections. Similarly, Martin et al.21 reported that statins appear to curtail the progression of sepsis by preventing sepsis-induced hypotension. Further, patients with sepsis-associated acute respiratory distress syndrome (ARDS) also experienced greater beneficial effects of prior and continuous statin therapy in more severe ARDS, underscoring the potential therapeutic benefits of statins in a high-risk ARDS cohort.22 The present study demonstrated that statins had a protective effect by reducing the rate of sepsis development, as non-statin users had higher rates of severe sepsis characterized by organ dysfunction than statin users (31.1% vs 13.3%, respectively; p = 0.002).
Prospective studies have also demonstrated the beneficial effects of statins. Acute administration of atorvastatin (40 mg/day) reduced sepsis severity in hospitalized patients, suggesting that statins may act acutely to prevent organ dysfunction.9 When atorvastatin was administered to about half (n = 123) of a cohort of 250 critically ill patients, IL-6 levels were not reduced during treatment, but prior statin use was associated with lower baseline IL-6 levels and improved survival.10 We found that statin use and albumin were protective factors for severe sepsis, with ORs of 0.28 (95% CI, 0.13–0.62; p = 0.002) and 0.91 (95% CI, 0.85–0.98; p = 0.013), respectively. Further, in this study, the statin-group patients had received statin treatment prior to their admission and also continued to receive statins after admission. Therefore, we believe continuing statin treatment during hospitalization may positively influence the protective effects of statins on the progression to sepsis, as evidenced by the higher rates of severe sepsis among non-statin users compared with statin users. Nevertheless, our results cannot specifically demonstrate whether prior use or only in-hospital use may be responsible for better clinical outcomes. In several RCTs,10, 11, 13 prior statin use, rather than de novo use, was associated with the biomarkers of sepsis progression.
In contrast to the results of the abovementioned studies, some other studies did not demonstrate any significant benefits of statin use on sepsis outcomes.23, 24 Goodin et al.23 found that prior statin users were typically older and more obese and had a higher prevalence of smoking, diabetes, and ischemic heart disease than non-statin users and benefits of statins were not demonstrated in their primary or secondary outcomes. Leung et al.24 found no significant associations between statin therapy and survival in patients with bloodstream infections. Although the present study found no significant relationship between statin therapy and 30-day mortality in our cohort of only older Chinese patients, fewer patients receiving statins died of sepsis-related causes within 30 days of a bacterial infection than patients who were non-statin users (4.4% vs 10.2%, respectively).
Older patients are characteristically at a greater risk of infection and sepsis and have been shown to have an increased incidence of sepsis, progression to organ dysfunction, and sepsis-related mortality.6 In a population-based cohort analysis of 69,168 older patients with cardiovascular disease,25 statin use was associated with a reduced rate of subsequent sepsis. Mortensen et al.26 also found that current statin use was significantly associated with decreased 30-day mortality in patients aged ≥65 years hospitalized for community-acquired pneumonia. Similarly, reinsure statin use was associated with significantly decreased mortality in older burn patients, although the rate of sepsis development was unaffected.27 These studies demonstrate the beneficial effects of statin use for both the prevention and treatment of sepsis in older patients. Although a significant decrease was noted regarding the progression of sepsis to organ dysfunction among statin users compared with non-users in this study, the lack of significant differences between statin users and non-users in 30-day sepsis-related mortality, ICU admission, and length of hospital stay can possibly be explained by the greater incidence of comorbidities in statin users and the associated weak physical condition of our older patient population that was unaffected by statin use. While we did not focus on comorbidities, the present study results do suggest that the presence of comorbidities such as COPD and chronic heart failure may contribute to the development of sepsis and infection-related organ dysfunction in older patients with bacterial infections.
The exact mechanism as to how statins provide a beneficial effect in patients with bacterial infections remains unclear, but several mechanisms might explain the reported beneficial results. In several animal models, pretreatment with statins or treatment after the onset of sepsis improved the survival of septic animals.28, 29, 30, 31, 32 Statin treatment also altered biodistribution of Tc-99m-sestamibi in rats with abdominal sepsis, interpreted as a protective effect because of statin-mediated increased tissue perfusion.33 The protective effect of statins is attributed to their effects on insulin sensitivity, improving insulin signaling in peripheral tissues,28 preserving cardiac function and hemodynamic status,29, 31 reversing inflammatory cytokines levels,15, 16, 17 and enhancing bacterial clearance.17 Additionally, statins appear to decrease nitric oxide production and restore impaired vascular responsiveness in sepsis.30, 31, 34
A direct antibacterial effect has been reported with decreased bacterial burden of several organisms.35 In clinical studies, statins exerted antioxidant properties in experimental models on sepsis.36 Statins also blunted expression of toll-like receptors 4 and 2 on monocytes in a human model of endotoxemia that decreased the production of inflammatory cytokines.37 Similarly, an association was shown between statin therapy and decreased TNF-α and IL-6 levels in patients with acute bacterial infections.9 Another study reported a 10-fold greater plasma-free cortisol levels versus plasma total cortisol levels in severe sepsis.38 Results of the abovementioned studies suggest that the protective effects of statins may be more related to their expansive anti-inflammatory and immunomodulatory effects, rather than targeting individual inflammatory mediators. A nationwide population-based study in Taiwan also showed associations between statin potency and beneficial effects, indicating that the risk of sepsis progression to organ dysfunction and mortality are lower among high-potency statin users than low-potency statin users.39
The present study had several limitations. First, this study was retrospective and neither randomized nor controlled. Second, potentially uncontrolled confounders including differences between the two studied groups may influence the interpretation of results, and residual confounding cannot be fully ruled out. Third, the sample size was small because of the restrictive inclusion criteria involving older adults with bacterial infections from a single center. Consequently, a sample size calculation was not performed prior to conducting this research, but a power of 0.916 was computed using the group difference results regarding the proportion of patients with multiple organ dysfunction(s). Fourth, because the study objective was to determine effects of statins on the progression of bacterial infections to sepsis, regardless of the specific statin used by the statin group, it was assumed that all statins had the same effect. Other studies10, 39 have also suggested that statin potency is associated with better outcomes, with improved outcomes obtained with high-potency compared to low-potency statins. Fifth, we did not account for the possible influence of glucocorticoid use by some patients. Moreover, examination of many variables may have introduced a type II error. Certain other variables associated with sepsis severity were not measured, including consciousness level, lactate levels, vasoactive drug use, and ventilator use. APACHE II scores and CRP values were relatively low given a sepsis diagnosis in the study population. Although the most representative APACHE II and CRP values during a 24-h period were used for analysis, these values do not typically change immediately in conjunction with disease progression. Therefore, a longer study period is required to obtain values consistent with progressive sepsis. Lastly, the relatively low number of positive microbiological findings may have been because of collection of a single blood sample for culture instead of multiple samples at different times.
To our knowledge, this is the first study to evaluate the effects of statins in older Asian patients with bacterial infections. Statin treatment decreased the development of sepsis in older hospitalized Chinese patients with bacterial infections but did not reduce the 30-day mortality, ICU admission incidence, or length of hospital stay. Based on the results of this study, continuing preadmission statin therapy during hospitalization may be associated with a reduced rate of sepsis development and perhaps overall in-hospital mortality in elderly patients with bacterial infections given the lower rate of sepsis development seen in statin users in this study. Given the safety profile of and infrequent adverse effects from statins in older patients, additional prospective controlled trials are needed to elucidate the potential for statin use as a therapeutic strategy in a rigorously selected group of older Asian patients with bacterial infections.
Funding
This study was supported by the Zhejiang Provincial Medical and Health Research Program (2012KYB084) and National Clinical Key Specialty Construction Project of Geriatrics.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Martin G.S. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent J.L., Sakr Y., Sprung C.L., et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J.L., Rello J., Marshall J., et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Balk R.A. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000;16:179–192. doi: 10.1016/s0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 5.Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 6.Chen X.C., Yang Y.F., Wang R., Gou H.F., Chen X.Z. Epidemiology and microbiology of sepsis in mainland China in the first decade of the 21st century. Int J Infect Dis. 2015;31:9–14. doi: 10.1016/j.ijid.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 8.Terblanche M., Almog Y., Rosenson R.S., Smith T.S., Hackam D.G. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 9.Novack V., Eisinger M., Frenkel A., et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009;35:1255–1260. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 10.Patel J.M., Snaith C., Thickett D.R., et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients. (ASEPSIS Trial) Crit Care. 2012;16:R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger P.S., Harward M.L., Jones M.A., et al. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med. 2011;183:774–781. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
- 12.Liao J.K., Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almog Y., Shefer A., Novack V., et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 14.Kavalipati N., Shah J., Ramakrishan A., Vasnawala H. Pleiotropic effects of statins. Indian J Endocrinol Metab. 2015;19:554–562. doi: 10.4103/2230-8210.163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izadpanah R., Schächtele D.J., Pfnür A.B., et al. The impact of statins on biological characteristics of stem cells provides a novel explanation for their pleotropic beneficial and adverse clinical effects. Am J Physiol Cell Physiol. 2015;309:C522–C531. doi: 10.1152/ajpcell.00406.2014. [DOI] [PubMed] [Google Scholar]
- 16.Kouroumichakis I., Papanas N., Proikaki S., Zarogoulidis P., Maltezos E. Statins in prevention and treatment of severe sepsis and septic shock. Eur J Intern Med. 2011;22:125–133. doi: 10.1016/j.ejim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Thomas G., Hraiech S., Loundou A., et al. Statin therapy in critically-ill patients with severe sepsis: a review and meta-analysis of randomized clinical trials. Minerva Anestesiol. 2015;81:921–930. [PubMed] [Google Scholar]
- 18.Deshpande A., Pasupuleti V., Rothberg M.B. Statin therapy and mortality from sepsis: a meta-analysis of randomized trials. Am J Med. 2015;128:410–417. doi: 10.1016/j.amjmed.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 19.Yang K.C., Chien J.Y., Tseng W.K., Hsueh P.R., Yu C.J., Wu C.C. Statins do not improve short-term survival in an oriental population with sepsis. Am J Emerg Med. 2007;25:494–501. doi: 10.1016/j.ajem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Singer M., Deutschman C.S., Seymour C.W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin C.P., Talbert R.L., Burgess D.S., Peters J.I. Effectiveness of statins in reducing the rate of severe sepsis: a retrospective evaluation. Pharmacotherapy. 2007;27:20–26. doi: 10.1592/phco.27.1.20. [DOI] [PubMed] [Google Scholar]
- 22.Mansur A., Steinau M., Popov A.F., et al. Impact of statin therapy on mortality in patients with sepsis-associate respiratory distress syndrome (ARDS) depends on ARDS severity: an observational cohort study. BMC Med. 2015;13:128. doi: 10.1186/s12916-015-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodin J., Manrique C., Dulohery M., Sampson J., Saettele M., Dabbagh O. Effect of statins on the clinical outcomes of patients with sepsis. Anaesth Intensive Care. 2011;39:1051–1055. doi: 10.1177/0310057X1103900611. [DOI] [PubMed] [Google Scholar]
- 24.Leung S., Pokharel R., Gong M.N. Statins and outcomes in patients with bloodstream infection: a propensity-matched analysis. Crit Care Med. 2012;40:1064–1071. doi: 10.1097/CCM.0b013e31823bc9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackam D.G., Mamdani M., Li P., Redelmeier D.A. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367:413–418. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- 26.Mortensen E.M., Pugh M.J., Copeland L.A., et al. Impact of statins and angiotensin-converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J. 2008;31:611–617. doi: 10.1183/09031936.00162006. [DOI] [PubMed] [Google Scholar]
- 27.Fogerty M.D., Efron D., Morandi A., Guy J.S., Abumrad N.N., Barbul A. Effect of preinjury statin use on mortality and septic shock in elderly burn patients. J Trauma. 2010;69:99–103. doi: 10.1097/TA.0b013e3181df61b1. [DOI] [PubMed] [Google Scholar]
- 28.Calisto K.L., Carvalho B.M., Ropelle E.R., et al. Atorvastatin improves survival in septic rats: effect on tissue inflammatory pathway and on insulin signaling. PLoS One. 2010;5:e14232. doi: 10.1371/journal.pone.0014232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Merx M.W., Liehn E.A., Graf J., et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 30.Ando H., Takamura T., Ota T., Nagai Y., Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–1046. [PubMed] [Google Scholar]
- 31.Merx M.W., Liehn E.A., Janssens U., et al. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry M.Z., Wang J.H., Blankson S., Redmond H.P. Statin (cerivastatin) protects mice against sepsis-related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surg Infect (Larchmt) 2008;9:183–194. doi: 10.1089/sur.2006.077. [DOI] [PubMed] [Google Scholar]
- 33.Macedo R., Javadi S.M., Higuchi T., et al. Heart and systemic effects of statin pretreatment in a rat model of sepsis. Assessment by Tc99m-sestamibi biodistribution. Acta Cir Bras. 2015;30:388–393. doi: 10.1590/S0102-865020150060000003. [DOI] [PubMed] [Google Scholar]
- 34.Subramani J., Kathirvel K., Leo M.D., Kuntamallappanavar G., Uttam Singh T., Mishra S.K. Atorvastatin restores the impaired vascular endothelium-dependent relaxations mediated by nitric oxide and endothelium-derived hyperpolarizing factors but not hypotension in sepsis. J Cardiovasc Pharmacol. 2009;54:526–534. doi: 10.1097/FJC.0b013e3181bfafd6. [DOI] [PubMed] [Google Scholar]
- 35.Boyd A.R., Hinojosa C.A., Rodriguez P.J., Orihuela C.J. Impact of oral simvastatin therapy on acute lung injury in mice during pneumococcal pneumonia. BMC Microbiol. 2012;12:73. doi: 10.1186/1471-2180-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durant R., Klouche K., Delbosc S., et al. Superoxide anion overproduction in sepsis: effects of vitamin e and simvastatin. Shock. 2004;22:34–39. doi: 10.1097/01.shk.0000129197.46212.7e. [DOI] [PubMed] [Google Scholar]
- 37.Niessner A., Steiner S., Speidl W.S., et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. 2006;189:408–413. doi: 10.1016/j.atherosclerosis.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesh B., Imeson L., Kruger P., et al. Australian and New Zealand Intensive Care Society Clinical Trials Group; STATInS Trial Investigators Elevated plasma-free cortisol concentrations and ratios are associated with increased mortality even in the presence of statin therapy in patients with sepsis. Crit Care Med. 2015;43:630–635. doi: 10.1097/CCM.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 39.Ou S.Y., Chu H., Chao P.W., et al. Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Med. 2014;40:1509–1517. doi: 10.1007/s00134-014-3418-1. [DOI] [PubMed] [Google Scholar]