Abstract
Background
A number of studies have reported on the effectiveness of sulbactam-based therapies for Acinetobacter baumannii infection; however, there is little evidence that sulbactam-based therapies are more or less effective than alternative therapies. Unfortunately, there is a distinct lack of high quality data (i.e., from randomized controlled trials) available on this issue. Therefore, we conducted a systematic review and meta-analysis comparing the efficacy of sulbactam-based and non-sulbactam-based regimens in the treatment of A. baumannii infection.
Methods
We searched PubMed, MEDLINE, Biomedical Central, Google Scholar, the China National Knowledge Infrastructure, the Cochrane library, and the Directory of Open Access using the terms “sulbactam and baumannii” or “maxtam and baumannii”. Randomized controlled trials, controlled clinical studies, and cohort studies were considered for inclusion. The primary outcome was the clinical response rate for sulbactam-based therapy vs comparator therapies.
Results
Four studies (1 prospective, 3 retrospective) were included in the meta-analysis. Sulbactam was given in combination with ampicillin, carbapenem, or cefoperazone (n = 112 participants). Comparator drugs included colistin, cephalosporins, anti-pseudomonas penicillins, fluoroquinolones, minocycline/doxycycline, aminoglycosides, tigecycline, polymyxin, imipenem/cilastatin, and combination therapy (n = 107 participants). The combined clinical response rate odds ratio did not significantly favor sulbactam-based therapy over comparator therapy (odds ratio = 1.054, 95% confidence interval = 0.550–2.019, p = 0.874), nor did any of the individual study odds ratios.
Conclusions
The available evidence suggests that sulbactam-based therapy may be similarly efficacious to alternative antimicrobial therapies for the treatment of A. baumannii infection. Further research on this issue is warranted given the limited availability of data from high quality/randomized controlled trials.
Keywords: Acinetobacter baumannii, Infection, Meta-analysis, Sulbactam, Systematic review
Introduction
Acinetobacter baumannii is a major cause of nosocomial infection, particularly in critically ill patients,1, 2 and has been reported to be associated with significant mortality in this population.3 Common clinical manifestations of A. baumannii infection include pneumonia and bacteremia.2 Worryingly, there is evidence to suggest that the incidence of A. baumannii infection may be increasing.4, 5 Hence, determining the most effective means of treatment is a pressing concern.
A. baumannii has developed resistance to many conventional treatments and can therefore be very challenging to treat.1, 2, 6 Indeed, researchers in the US have reported that 29.3% of A. baumannii isolates examined from January 2004 to September 2005 were multidrug resistant.7 Given this evidence, it is perhaps not unsurprising that there is no available consensus or guidelines outlining the optimal strategy for treating A. baumannii infection. Various antimicrobials have been used to treat A. baumannii infection, including carbapenems, polymyxins, tetracyclines and glycylcyclines, aminoglycosides, fluoroquinolones, and various combination therapies.1, 2 Of these antimicrobial agents, carbapenems have traditionally been used as the first line treatment of choice; however, rates of resistance to this agent are often high, rendering treatment ineffective.1, 2 Polymyxins appear to be effective for treating A. baumannii infection, with low rates of resistance, although data from well-designed clinical trials are lacking.1, 2 Similarly, there is a paucity of clinical data regarding effectiveness and resistance for the other antimicrobials thus mentioned.
Sulbactam is a β-lactamase inhibitor that is typically given in combination with ampicillin. Production of β-lactamase is a common cause of bacterial resistance, rendering β-lactam antibiotics such as penicillin ineffective.8 β-Lactamase inhibitors, including sulbactam, bind to β-lactamase, thereby increasing the susceptibility of the microorganism to co-administered β-lactam antibiotics.8 Indeed, most β-lactamase inhibitors do not exert antimicrobial activity if given alone.8 Sulbactam, however, has been demonstrated to have antimicrobial properties, including against A. baumannii,9, 10 which are thought to be mediated by binding to penicillin binding-proteins.11
A large number of clinical studies have reported on the effectiveness of sulbactam-based therapies for the treatment of A. baumannii infection.11, 12 For instance, Kempf et al. reported that sulbactam given in combination with colistin may offer a significant benefit over colistin monotherapy.13 Most recently, high dose sulbactam/ampicillin was used in combination with rifampicin and fosfomycin to successfully treat a case of postsurgical meningitis caused by A. baumannii.14 In another recent report, a patient with drug-resistant A. baumannii-induced peritonitis was successfully treated with sulbactam/ampicillin in combination with polymyxin B. In vitro studies have also demonstrated the efficacy of sulbactam in combination with various antibiotics including minocycline and cefoperazone15 and fosfomycin.16
Although the available evidence suggests that sulbactam-based therapies can be effective for the treatment of A. baumannii infection, there is little evidence to suggest that sulbactam-based therapies are more or less effective than alternative therapies. Indeed, there is a distinct lack of high quality data (i.e., from randomized controlled trials) available on this issue. In an attempt to overcome this limitation and gain a better understanding as to the effectiveness of sulbactam in the treatment of A. baumannii infection, we conducted a systematic review of the available literature and performed a subsequent meta-analysis to compare the efficacy, taken as the clinical response rate, of sulbactam-based and non-sulbactam-based therapeutic regimens.
Materials and methods
Literature search strategy
The following biomedical databases were searched: PubMed; MEDLINE via Medscape; Biomedical Central; Google Scholar; the China National Knowledge Infrastructure; the Cochrane library; and the Directory of Open Access Journals. EMBASE and CINAHL were not searched due to lack of access. Gray literature searched included the abstracts of Annual Meeting of the American Society of Infectious Disease, the American College of Chest Physicians, the American Lung Association, and Clinicaltrials.gov. A loose search strategy using the key words “sulbactam and baumannii” or “maxtam and baumannii” was employed in order to maximize the possibility of identifying all relevant records. For PubMed and other databases, the following search limits were applied where possible: [Clinical Trial], [Randomized Controlled Trial], [Clinical Conference], [Clinical Trial Phase I], [Clinical Trial Phase II], [Clinical Trial Phase III], [Clinical Trial Phase IV], [Consensus Development Conference], [Controlled Clinical Trial], and [English]. The literature was searched from inception to December 2011.
Selection criteria
Studies were eligible for inclusion in the meta-analysis if they were randomized controlled trials, controlled clinical studies, or cohort studies with designs comparing the clinical efficacy of sulbactam-based therapy against other combinations of antimicrobial therapies for the treatment of A. baumannii infection. In vitro studies and studies focusing on the susceptibility of clinical isolates/bacterial strains to antimicrobials without clinical data were excluded from the meta-analysis.
Data extraction and quality assessment
Two independent reviewers extracted the data from eligible studies. A third reviewer resolved any disagreements. The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, type of study, number of participants in each treatment group, participants’ age and gender, name(s) of drug(s) given with sulbactam, name(s) of comparator drug(s), and the clinical response rate.
The primary outcome of interest was the clinical response rate defined as complete or partial resolution (improvement) of the symptoms/signs associated with A. baumannii infection by the end of therapy (72 h). Complete resolution was defined as the eradication of all presenting signs and symptoms of infection, whereas partial resolution was defined as the resolution of some, but not all signs and symptoms of infection. Treatment failure is indicated by lack of obvious improvement in signs and symptoms of infection.
The Newcastle-Ottawa scale (NOS) score17 was determined to assess the quality of each study included in the meta-analysis. Studies with a NOS score <3 were classified as poor quality and were excluded from this meta-analysis.
Data analysis
The clinical response rate was used to evaluate treatment efficacy. Odds ratios (ORs) with 95% confidence intervals were calculated for binary outcomes of patients treated with sulbactam-based therapy and those who were treated with comparator drugs. A chi-square-based test for homogeneity was performed and the inconsistency index (I2) statistic was determined. If I2 was >50% or >75%, the studies were considered to be heterogeneous or highly heterogeneous, respectively. If I2 was below 25%, the studies were considered to be homogeneous. If I2 statistic (>50%) indicated heterogeneity between studies, a random-effects model was calculated. Otherwise, fixed-effects models were calculated. Pooled summary statistics of the ORs for the individual studies are shown. Pooled ORs were calculated and a 2-sided p-value <0.05 was considered to indicate statistical significance. A Funnel plot and the fail-safe n (which indicates whether the observed significance is spurious or not) were used to assess possible publication bias. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Literature search
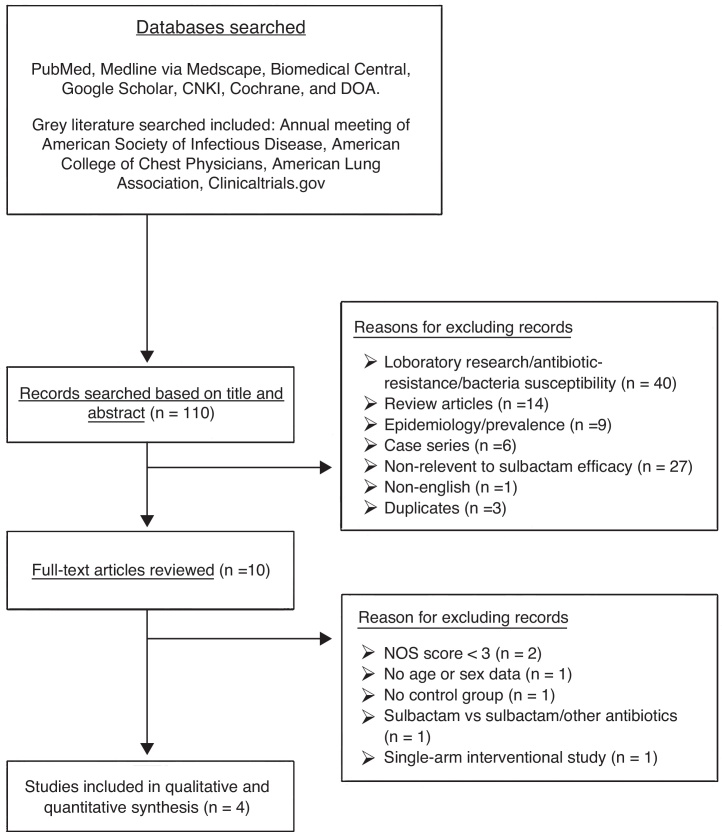
A total of 110 studies were identified by searching the specified databases (Fig. 1). Of these studies, 100 were subsequently excluded because they did not meet the eligibility criteria based on examination of the title and abstract. Full-text review of the remaining 10 studies led to the exclusion of an additional six that did not meet the eligibility criteria. Therefore, a total of four studies18, 19, 20, 21 were included in the meta-analysis.
Fig. 1.
Flow diagram of study selection. CNKI, China National Knowledge Infrastructure; DOAJ, Directory of Open Access Journals.
Study characteristics
The characteristics of the four studies are summarized in Table 1. These studies were published between 2006 and 2010 and included one prospective study20 and three retrospective studies.18, 19, 21 There were no randomized-controlled trials included in the analyses. All studies achieved a NOS score of 3 or above. The studies included a total of 112 participants treated with sulbactam and 107 participants treated with other comparator drugs. All patients had ventilator-associated pneumonia in two of the studies20, 21 and bacteremia in one of the studies.19 The type of infection was not specified in the remaining study.18 Only two studies provided sulbactam dosage information.20, 21 Sulbactam was given in combination with ampicillin,20, 21 carbapenem,18 or cefoperazone.19 Comparator drugs included colistin,20 cephalosporins, anti-pseudomonas penicillins, or fluoroquinolones with aminoglycosides,18 minocycline/doxycycline, aminoglycosides, tigecycline, polymyxin, or combination therapy,21 and imipenem/cilastatin.19
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Study type | Type of infection | Sulbactam dose | Drugs given with sulbactam | Comparator drug(s) | Participants (sulbactam, comparator) | Age (sulbactam vs comparator) | % Male (sulbactam vs comparator) | Response rate |
|---|---|---|---|---|---|---|---|---|---|
| Betrosian et al. (2008) | Randomized prospective cohort study | Ventilator-associated pneumonia | 9 g/8 h (sulbactam/ampicillin) IV | Ampicillin | Colistin | 13, 15 | 72 ± 5 vs 67 ± 9 | 54 vs 47% | 76.8 vs 73.3% |
| Lee et al. (2005) | Retrospective cohort study | Nosocomial pan-drug resistant A. baumanniia | NS | Carbapenem (imipenem or meropenem) | 2nd/3rd generation cephalosporin, anti-pseudomonas penicillin, or fluoroquinolone (all administered with aminoglycoside amikacin) | 59, 30 | 71 ± 14 vs 71 ± 15 | 53 vs 57% | 59 vs 60% |
| Chan et al. (2010) | Retrospective cohort study | Ventilator-associated pneumonia | 1 g/6 h IV | Ampicillin | Minocycline/doxycycline, aminoglycosides, tigecycline and polymyxin (or in combination therapy) | 5, 50 | 40 (15–87) | 72.7% | 60 vs 79.1% |
| Choi et al. (2006) | Retrospective cohort study | Bacteremia | NS | Cefoperazone | Imipenem/cilastatin | 35, 12 | 45 ± 28 vs 55 ± 23 | 69 vs 67% | 77.1 vs 75% |
IV, intravenous; NS, not specified.
No further details provided.
Clinical response rate
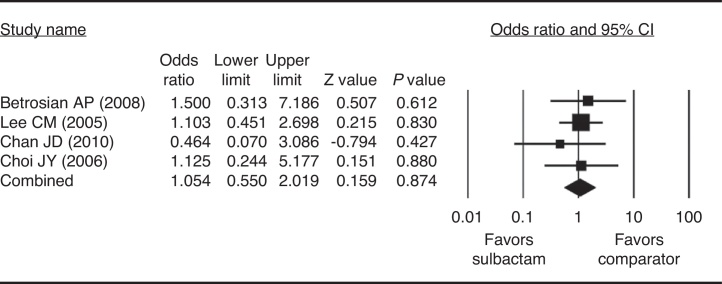
None of the four studies included in the meta-analysis had clinical response rate ORs that significantly favored sulbactam-based therapy over the comparator therapy or vice versa (Fig. 2). There was homogeneity in the response rate among the studies when the data were pooled for analysis (Q = 0.931, I2 = 0.00%, p = 0.810); therefore a fixed-effects model of analysis was used. Further, the combined OR did not significantly favor one form of therapy over the other. ORs ranged from 0.464 to 1.500, with the overall OR being 1.054 (p = 0.874).
Fig. 2.
Clinical response rate for sulbactam vs comparator drugs in the treatment of Acinetobacter baumannii infection.
Publication bias
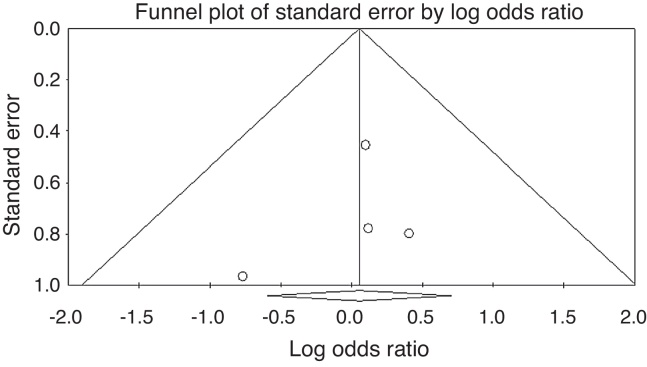
The Funnel plot for publication bias (standard error by log OR for the clinical response rate) demonstrated no marked evidence of asymmetry (Fig. 3), indicating a lack of publication bias. For the clinical response rate, the combined effect size yielded a Z-value of 0.03966, with a corresponding p-value of 0.968. As the overall clinical response rate was not statistically significant, the fail-safe n value was irrelevant.
Fig. 3.
Funnel plot of the standard error by log odds ratio for the clinical response rate. There was no strong evidence of asymmetry and hence publication bias.
Discussion
To our knowledge, this is the first systematic review and subsequent meta-analysis to compare the efficacy (clinical response rate) of sulbactam-based vs non-sulbactam-based therapies for the treatment of A. baumannii infection. Only a small number of studies, involving a relatively small number of participants, met the eligibility criteria and were included in this meta-analysis.18, 19, 20, 21 Analysis of the data extracted from included studies revealed that sulbactam-based therapy was not superior to alternative antimicrobial therapies for the treatment of A. baumannii or vice versa.
None of the individual clinical response rate ORs for the studies included in our analysis favored sulbactam-based therapy over the comparator therapy. There was considerable between study variation in the type of comparator therapy given and, to a lesser extent, the drug given in combination with sulbactam. Unsurprisingly, given the individual study results, the combined clinical response rate OR did not favor one treatment approach over the other. In terms of clinical response, our findings therefore suggest that sulbactam-based therapy is equally effective as non-sulbactam-based therapy for A. baumannii infection.
Our meta-analysis has several limitations, most of which relate to those inherent in the available literature. Notably, only a small number of studies met our inclusion criteria, none of which were randomized controlled trials. Hence, the quality of data extracted from these studies is not optimal. A further limitation is the relatively small number of participants included in the studies and thus in our meta-analysis; this obviously reduces the power of any statistical analysis. Other limitations include a lack of homogeneity in the drugs administered with sulbactam, the comparator drugs, and indeed the baseline characteristics of the participants. The between study differences in the drugs administered with sulbactam and the comparator drugs may be particularly confounding given the inherent pharmacokinetic and pharmacodynamic differences between some of these agents. Additional well-controlled studies are needed. Methodological limitations include the fact that the EMBASE and CINAHL databases were not included in the search and that non-English literature was not considered.
In summary, the findings from our systematic review and meta-analysis suggest that sulbactam-based therapies and non-sulbactam-based therapies may have similar efficacy in the treatment of A. baumannii infection. We must caution, however, that the available evidence is limited in many respects (as previously outlined). Perhaps most importantly, our review highlights the clear need for randomized, well-controlled clinical trials to determine the most effective means of treating A. baumannii infection.
Funding
This work was supported by grant no. 2010118 from the Shanghai Municipal Health Bureau.
Conflict of interest
The authors declare to have no conflict of interest.
References
- 1.Garnacho-Montero J., Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23:332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 2.Karageorgopoulos D.E., Falagas M.E. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8:751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes R., Edwards J.R., National Nosocomial Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 5.Paul M., Weinberger M., Siegman-Igra Y., et al. Acinetobacter baumannii: emergence and spread in Israeli hospitals 1997–2002. J Hosp Infect. 2005;60:256–260. doi: 10.1016/j.jhin.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vila J., Pachon J. Therapeutic options for Acinetobacter baumannii infections: an update. Expert Opin Pharmacother. 2012;13:2319–2336. doi: 10.1517/14656566.2012.729820. [DOI] [PubMed] [Google Scholar]
- 7.Halstead D.C., Abid J., Dowzicky M.J. Antimicrobial susceptibility among Acinetobacter calcoaceticus-baumannii complex and Enterobacteriaceae collected as part of the Tigecycline Evaluation and Surveillance. Trial J Infect. 2007;55:49–57. doi: 10.1016/j.jinf.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Lode H.M. Rational antibiotic therapy and the position of ampicillin/sulbactam. Int J Antimicrob Agents. 2008;32:10–28. doi: 10.1016/j.ijantimicag.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Williams J.D. Beta-lactamase inhibition and in vitro activity of sulbactam and sulbactam/cefoperazone. Clin Infect Dis. 1997;24:494–497. doi: 10.1093/clinids/24.3.494. [DOI] [PubMed] [Google Scholar]
- 10.Corbella X., Ariza J., Ardanuy C., et al. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother. 1998;42:793–802. doi: 10.1093/jac/42.6.793. [DOI] [PubMed] [Google Scholar]
- 11.Rafailidis P.I., Ioannidou E.N., Falagas M.E. Ampicillin/sulbactam: current status in severe bacterial infections. Drugs. 2007;67:1829–1849. doi: 10.2165/00003495-200767130-00003. [DOI] [PubMed] [Google Scholar]
- 12.Betrosian A.P., Douzinas E.E. Ampicillin–sulbactam: an update on the use of parenteral and oral forms in bacterial infections. Expert Opin Drug Metab Toxicol. 2009;5:1099–1112. doi: 10.1517/17425250903145251. [DOI] [PubMed] [Google Scholar]
- 13.Kempf M., Djouhri-Bouktab L., Brunel J.M., Raoult D., Rolain J.M. Synergistic activity of sulbactam combined with colistin against colistin-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2012;39:180–181. doi: 10.1016/j.ijantimicag.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Mellon G., Clec’h C., Picard B., Cohen Y., Jaureguy F. Postsurgical meningitis due to multiresistant Acinetobacter baumannii successfully treated with high doses of ampicillin/sulbactam combined with rifampicin and fosfomycin. J Infect Chemother. 2012;18:958–960. doi: 10.1007/s10156-012-0404-9. [DOI] [PubMed] [Google Scholar]
- 15.Pei G., Mao Y., Sun Y. In vitro activity of minocycline alone and in combination with cefoperazone–sulbactam against carbapenem-resistant Acinetobacter baumannii. Microb Drug Resist. 2012;18:574–577. doi: 10.1089/mdr.2012.0076. [DOI] [PubMed] [Google Scholar]
- 16.Santimaleeworagun W., Wongpoowarak P., Chayakul P., Pattharachayakul S., Tansakul P., Garey K.W. In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. Southeast Asian J Trop Med Public Health. 2011;42:890–900. [PubMed] [Google Scholar]
- 17.Wells G.A., Shea B., O’Connell D., et al. 2012. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 02.08.12] [Google Scholar]
- 18.Lee C.M., Lim H.K., Liu C.P., Tseng H.K. Treatment of pan-drug resistant Acinetobacter baumannii. Scand J Infect Dis. 2005;37:195–199. doi: 10.1080/00365540510026869. [DOI] [PubMed] [Google Scholar]
- 19.Choi J.Y., Kim C.O., Park Y.S., et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med J. 2006;47:63–69. doi: 10.3349/ymj.2006.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betrosian A.P., Frantzeskaki F., Xanthaki A., Douzinas E.E. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 2008;56:432–436. doi: 10.1016/j.jinf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Chan J.D., Graves J.A., Dellit T.H. Antimicrobial treatment and clinical outcomes of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care Med. 2010;25:343–348. doi: 10.1177/0885066610377975. [DOI] [PubMed] [Google Scholar]