Abstract
Despite the beneficial effects of sperm cryopreservation, increased reactive oxygen species (ROS) production during this process can affect spermatozoon structure and function. Moreover, ROS production is associated with elevated DNA damage and alterations in DNA methylation. There is little information about the effects of cryopreservation on epigenetic modulation in sperm and the health of children born with frozen spermatozoa. Considering the potential consequences of cryopreservation in ART-conceived children, it is necessary to assure that cryopreservation does not modify sperm DNA methylation status. This review summarizes reports on epigenetic modifications of spermatozoa during cryopreservation and the probable effects of this process on offspring health. Contradictory results have reported the influence of sperm cryopreservation on DNA methylation in imprinted genes. Multiclinical studies with larger sample sizes under the same conditions of cryopreservation and DNA methylation analysis are needed to make any definitive conclusion about the effect of the cryopreservation process on sperm DNA methylation.
Keywords: Spermatozoa, Cryopreservation, DNA methylation, ROS, ART
Introduction
Since the introduction of human sperm cryopreservation in the 1960s, this procedure has been known as an effective procedure for management of male fertility and to store and preserve donor spermatozoa before cancer therapy, vasectomy, or surgical infertility treatments [1]. Although cryopreservation extends sperm availability, the fertilization potential of cryopreserved sperm is compromised due to the structural and physiological changes in sperm [2]. It is proposed that during the freezing process, several factors, such as sudden changes in temperature, osmotic stress, and ice formation, can lead to low sperm quality after thawing [3]. Furthermore, increased reactive oxygen species (ROS) production during the freezing-thawing process is another reason for the structural and functional alterations in spermatozoa [4].
In addition to structural and physiological changes, it is reported that the expression of genes and proteins, mRNA stability, and epigenetic content in spermatozoa can be affected by the freezing-thawing process. These alterations in frozen-thawed spermatozoa may influence fertility potential and embryo development [3].
Genomic imprinting is an epigenetic phenomenon that affects a subset of mammalian genes, resulting in a parental monoallelic expression pattern [5]. To differentiate the parental alleles, epigenetic marks including DNA methylation and histone modifications are initiated in the germ cells and maintained during the embryonic development and postnatal lifespan [6]. An increased risk of imprinting disorders, such as Angelman syndrome (AS) and Beckwith–Wiedemann syndrome (BWS), in children born after assisted reproductive technologies (ARTs) [7] has raised concerns about the occurrence of epigenetic modifications during the freezing-thawing process. Recent studies have begun to answer the question of whether sperm cryopreservation is associated with epigenetic disorders. However, limited information is available about the effects of cryopreservation on epigenetic modulation in sperm as well as the effects of possible epigenetic changes on the health of children born with frozen spermatozoa. Although some studies reported that cryopreservation could not affect the DNA methylation pattern of human sperm genes [8, 9], the results of other investigations are not consistent with these studies [10]. Therefore, this review summarizes reports on epigenetic modifications of spermatozoa during cryopreservation and the probable effects of this process on offspring health.
ROS and sperm cryopreservation
ROS include free radicals, such as superoxide anions (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH–), that may trigger mitochondrial dysfunction, lipid peroxidation, DNA damage, and apoptosis [11]. Oxidative stress arises when the ROS production exceeds the antioxidant scavenging activity of cell and results in cellular damage. A high content of polyunsaturated fatty acids (PUFA), intracellular antioxidant enzyme deficiency, and limited ability to repair DNA are reasons why spermatozoa are vulnerable to oxidative stress [12]. Remarkably, ROS can affect male fertility through lipid peroxidation in sperm membrane and lead to a decrease in sperm motility and sperm–oolemma fusion [12].
It is indicated that an excessive generation of ROS during sperm cryopreservation has harmful effects on sperm parameters, such as motility, viability, and morphology [4, 13]. Since cryopreservation decreases the antioxidant activity of sperm, it is apparent that sperm is vulnerable to ROS-induced damages [14]. Although the mechanism of decreased sperm motility during cryopreservation has not been thoroughly clarified to date, there is a strong association between the number of immotile sperm cells and mitochondrial defects after cryopreservation [15]. The high sensitivity of the mitochondrial membrane to low temperatures can lead to ROS generation [16]. Also, ROS production can be due to the activation of the mitochondrial apoptotic pathways and electron leakage from the electron transport chain [17, 18]. Changes in the fluidity of the mitochondrial membrane may be another reason for ROS release [16]. Furthermore, mitochondrial membranes contain high levels of PUFA, as favored substrates for ROS. This causes lipid peroxidation and the production of reactive lipid aldehydes, which covalently bind to the electron transport chain and reinforce ROS production in mitochondria [12, 19]. Increased ROS production and decreased antioxidant enzyme activity in sperm can trigger apoptotic pathways that result in decreased sperm viability [1]. Morphological alterations in spermatozoa, including loose head, coiled tails, and looped tails, have been observed after cryopreservation due to an uncontrolled influx of liquid into sperm, altering cellular osmolality and membrane structure [3, 15, 20].
There is a concern that cryopreservation affects sperm DNA integrity. Although some studies demonstrated that cryopreservation has a damaging effect on sperm nuclear DNA and chromatin structure [21–25], other studies found no effect [26, 27]. A change in the properties of the mitochondrial membrane and increased ROS production can lead to DNA oxidation and DNA strand breakage [16]. Moreover, it is suggested that another reason for the vulnerability of sperm DNA to cryopreservation is defects of DNA repair proteins, occurring throughout this process [28]. Nevertheless, further investigation is necessary to clarify the effect of cryopreservation on sperm DNA structure and chromatin integrity [29].
ROS and DNA methylation
DNA methylation, as an epigenetic mechanism, involves the addition of methyl groups to cytosine or adenine bases in DNA that usually occurs in CpG (5’—C—phosphate—G—3’) dinucleotide context [30]. Non-CpG methylation is detected in embryonic stem cells and non-dividing cells (such as neurons) and it regulates cell type-specific functions [31]. CpG sites occur with high frequency in certain genomic locations, known as CpG islands [32]. In mammals, CpG islands frequently occur in promoter regions and are usually unmethylated, whereas other CpG sites are frequently methylated [32]. In addition to its role in normal development, DNA methylation is also involved in genomic imprinting, X-chromosome inactivation, suppression of repetitive elements, and carcinogenesis [32.
Increased levels of ROS and DNA methylation are observed in several types of cancer cells [33]. Recently, the ROS induction theory has been suggested to elucidate the mechanisms for the establishment of either hyper- or hypomethylation [33–35]. ROS can induce site-specific hypermethylation through either the up-regulation of DNA methyltransferases (DNMTs) or the formation of new DNMT-including complexes [33].
Furthermore, ROS can induce hypomethylation through 5-hydroxymethylcytosine (5hmC) and 8-oxo-7, 8-dihydro-2′deoxyguanosine (8-OHdG) [33]. The hydroxylation of methylcytosine via ROS attack leads to the generation of 5hmC that can suppress the faithful transmission of genomic methylation patterns [36]. The formation of 8-OHdG, as a biomarker of oxidative stress, can trigger DNA hypomethylation through the inhibition of DNA methylation nearby cytosine bases [33]. Since ROS can affect many cellular processes, other mechanisms may exist for DNA methylome alterations. H2O2 can inactivate Sirtuin-1, which is a NAD+-dependent histone deacetylase [37]. DNMT1, methyl-CpG binding protein 2 (MECP2), methyl-CpG binding domain 2 (MBD2), and MBD3 are linked with histone deacetylase. They can provide new pathways for the possible targeting of DNA methylation [38]. MECP2 is a transcriptional regulator that specifically binds methylated DNA to regulate transcription and organization of chromatin [39]. MECP2 mutations cause Rett syndrome, a neurological disorder that affects almost exclusively females [40]. Non-neuronal cells similarly to neurons begin MECP2 expression at the late stages of differentiation. However, MECP2 is not as a differentiation marker in sperm cells and oocytes [39].
Furthermore, since ROS can stimulate both DNA methylation and histone modifications, it may change the pattern of DNA methylation by the regulation of histone modifications [33]. With all these, further studies are needed to discover the detailed mechanisms by which ROS affect DNA methylation.
Sperm cryopreservation and DNA methylation
Cryopreservation may affect the DNMT proteins and decrease the level of functional enzyme, or the post-translational modifications (PTMs) that regulate intracellular localization of DNMTs [41]. In frozen boar spermatozoa, down-regulation of mRNA expression for DNMT3A and DNMT3B was detected as compared to unfrozen spermatozoa [42]. One study evaluated the expression of DNMTs and histone PTMs, and the progression of spermatogenesis following in vitro culture of fresh or cryopreserved testicular tissues from prepubertal mice. After in vitro maturation, no major changes in DNMT1 and DNMT3a expression were detected in germ cells of fresh or frozen-thawed testicular tissues as compared to those developed in vivo [43]. However, differences in the expression levels of histone modifying enzymes and the distribution of histone marks including H4K8ac, H3K9ac, and H3K4me3 were observed between spermatozoa produced from in vitro culture of frozen-thawed mouse testicular tissues as compared to those developed in vivo [43]. Significant reduction in histone H3 and H4 acetylation was detected in cryopreserved cells [44]. Yan and colleagues showed that methylation of H3K9 and acetylation of H4K5 increase after cryopreservation [45]. Furthermore, cryopreservation reduces the level of H3K4me3, a modification related to actively transcribed genes; and increases the level of H3K27me3, a modification related to transcriptional repression [46].
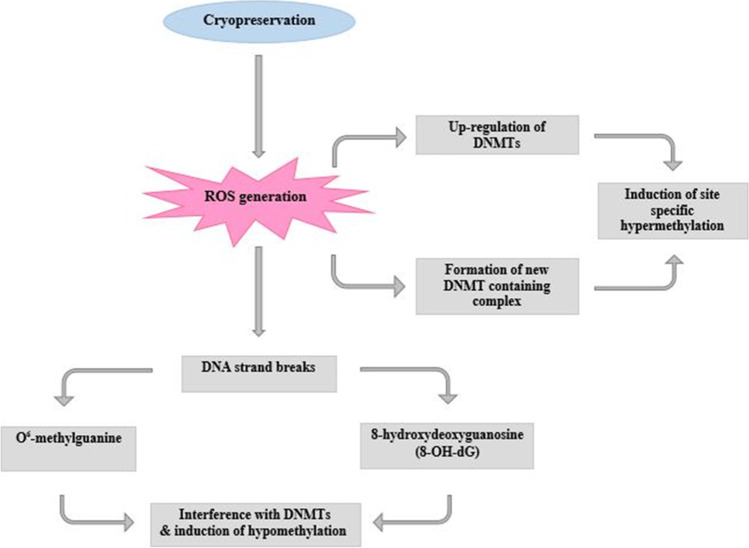
Given the role of ROS in DNA methylation modifications, as well as the production of ROS in the cryopreservation process, it seems that the modifications of DNA methylation may occur during this process (Fig. 1). In recent years, increasing attention has been paid to explain the possible epigenetic changes that may occur in spermatozoa during the freezing-thawing process (Table 1). Although a number of these studies have evaluated the effect of sperm cryopreservation on the level of global DNA methylation, some have assessed the effect of this process on the DNA methylation at individual imprinted genes.
Fig. 1.
Illustration showing the possible role of the ROS in modifications of DNA methylation during cryopreservation
Table 1.
The effects of cryopreservation on sperm DNA methylation.
| Gene | Species | Cryopreservation method | DNA methylation assay | Result(s) | Reference |
|---|---|---|---|---|---|
| MEG3, H19, LIT1, SNRPN, MEST, ALU, LINE1, VASA, and MTHFR | Human | Slow freezing | Bisulfite pyrosequencing of genomic DNA | No alteration in DNA methylation status regardless of cryopreservation duration. | Kläver et al. 2012 [8] |
| H19 and MEST imprinting control region (ICR) | Human | Vitrification | Bisulfite sequencing PCR (BSP) method | No significant alteration in DNA methylation status between cryoprotectant, cryopreservation process, or duration of cryopreservation groups. | Lu et al. 2015 [9] |
| Global DNA | Horse | Slow freezing | Increased the DNA methylation level. | Aurich et al. 2016 [47] | |
| SNRPN and GRB10 | Human | Vitrification | Sequenom DNA sequencing | Significant changes on sperm DNA methylation. | Zhou et al. 2017 [48] |
| Global DNA | Colossoma macropomum | Vitrification | Enzymatic DNA methylation analysis | Significant changes on sperm DNA methylation caused by all cryoprotectants and exhibition of more delays and abnormal embryonic development. | De Mello et al. 2017 [49] |
| Global DNA | Goldfish, zebrafish | Slow freezing | Luminometric methylation assay | No alteration in global DNA methylation of goldfish spermatozoa after cryopreservation with methanol, reduced DNA methylation with dimethylsulfoxide and 1, 2-propanediol. | Depincé et al. 2020 [50] |
| Increased DNA methylation of zebrafish spermatozoa after cryopreservation with methanol. | |||||
| Promoters of UBE3A and SNURF-SNRPN, PWS-ICR and AS-ICR | Human | Vitrification | Quantitative methylation specific PCR (qMSP) | No alteration in DNA methylation. | Khosravizadeh et al. 2020 [51] |
| Global DNA | Rabbit | Freeze-drying | Immunohistochemistry | No alteration in global DNA methylation. | Mercati et al. 2020 [52] |
| Global DNA | Rooster | Slow freezing | Immunohistochemistry | No difference in DNA methylation between the Lake and Beltsville extenders. | Salehi et al. 2020 [53] |
| Significant decrease in H3K4 methylation and H3K9 acetylation after cryopreservation with Lake extender. | |||||
| Global DNA | Ram | Slow freezing | Immunohistochemistry | Increase DNA methylation during prolonged exposure to capacitating conditions (1–4 h). | Peris-Frau et al. 2021 [54] |
| Global DNA | Ram | Slow freezing | ELISA | Significant increase in global DNA methylation. | Güngör et al. 2021 [55] |
Sperm cryopreservation and global DNA methylation
The level of global sperm DNA methylation is correlated with sperm parameters, including concentration, motility, and also DNA integrity. High levels of DNA methylation may be considered as a marker of normal spermatogenesis, and low levels of DNA methylation levels may show imperfect spermatogenesis [56].
Chromosome compaction in mature spermatozoa protects paternal genetic and epigenetic inheritance during the journey of sperm through the male and female genital tract [54]. Sperm chromatin decondensation at the suitable time of the fertilization process is essential for the formation of male pronucleus and zygote [57]. Cryopreservation can affect the sperm chromatin structure and at the same time accelerate capacitation [58]. One study investigated DNA methylation of ram spermatozoa under capacitating conditions before and after cryopreservation. In the cryopreserved spermatozoa, prolonged exposure to capacitating conditions (1–4 h) could increase DNA methylation [54]. The cryopreservation process could increase DNA cytosine methylation in stallion semen. However, DNA methylation was not affected by the cryopreservation extender [47]. De Mello et al. evaluated the influence of cryoprotectant agents, including methanol, glycerol dimethylsulfoxide, ethyl glycol, and dimethylformamide, on DNA methylation of Colossoma macropomum spermatozoa and subsequent embryo development. Their results showed that all cryoprotectant agents resulted in decreased DNA methylation levels in spermatozoa, and exhibition of more delays and abnormal embryonic development. Although methanol led to lower DNA methylation levels, the rates of fertilization, hatching, and embryonic development were closer to that of the control group [49]. However, one study demonstrated that goldfish sperm cells cryopreserved with methanol did not show any alteration in the global DNA methylation, whereas dimethylsulfoxide and 1, 2-propanediol reduced DNA methylation. In contrast, DNA methylation of zebrafish spermatozoa significantly increased after cryopreservation with methanol [50]. Salehi et al. evaluated DNA methylation and histone modification of rooster semen before and after cryopreservation by the Lake and Beltsville extenders. Although there was no difference in DNA methylation between the extenders used for cryopreservation, the Lake extender resulted in a significant decrease in H3K4 methylation and H3K9 acetylation compared to the Beltsville [53].
Mercati and colleagues demonstrated that the freeze-drying process (lyophilization), drying frozen material by ice sublimation, did not influence the global DNA methylation of rabbit spermatozoa. Furthermore, ethylenediamine tetraacetic acid (EDTA) supplementation, as well as the addition of rosmarinic acid or melatonin to freeze-drying media, did not affect the methylation of spermatozoa [52]. A recent study found that the cryoprotectant and freezing-thawing process significantly increase global DNA methylation levels in ram spermatozoa [55]. It seems that the adverse effects of the freezing-thawing process on spermatozoa can be reduced by choosing the proper cryoprotectant agents composing the cryopreservation solution [49].
Sperm cryopreservation and sperm DNA methylation at individual imprinted genes
The effect of cryopreservation on sperm DNA methylation at individual imprinted genes is evaluated in a few studies. Vasa and chemokine (C-X-C motif), receptor 4b (cxcr4b) genes are involved in the development of germ cells and the migration of primordial germ cells (PGCs), respectively [59, 60]. An increased methylation level of vasa and cxcr4b promoters was detected after cryopreservation of zebrafish genital ridges that could be associated with their significant down-regulation state [61].
Given the evidence for increased incidence of imprinting disorders, such as AS in ART newborns [7], there is concern that cryopreservation may affect the chromosome 15q11–q13, containing a cluster of both paternally and maternally imprinted genes. These imprinted genes are controlled by a bipartite imprinting center containing the PWS-imprinting control region (PWS-ICR) and the AS-ICR. Epigenetic modifications in the chromosome 15q11–q13 result in imprinting disorders, such as PWS and AS [62]. Valcarce et al. demonstrated that the freezing-thawing process can produce DNA lesions in genome regions related to AS and Prader-Willi syndrome (PWS), including SNORD116/PWSAS and UBE3A [63]. One study examined the impact of cryopreservation on DNA methylation in promoters of UBE3A (paternally imprinted gene) and SNURF-SNRPN (SNURF-small nuclear ribonucleoprotein polypeptide; maternally imprinted gene), PWS-ICR, and AS-ICR in the chromosome 15q11–q13. Although cryopreservation significantly increased the levels of ROS generation and DNA fragmentation in spermatozoa from normozoospermic men, this process did not affect DNA methylation of the selected gene regions [51]. Kläver et al. evaluated the effect of cryopreservation on DNA methylation of nine spermatozoa genes, including SNRPN, maternally expressed gene 3 (MEG3), H19, LIT1 (an imprinted antisense RNA in the human KvLQT1 locus), mesoderm specific transcript (MEST; maternally imprinted gene), ALU, long interspersed element class 1 (LINE1), VASA, and methylenetetrahydrofolate reductase (MTHFR). Analysis of these genes in the semen samples from normozoospermic men showed that cryopreservation duration (short and mid-term) and cryoprotectant have no alteration in the DNA methylation pattern of this examined sub-fraction of the genome [8]. However, the human semen freezing-thawing process affected DNA methylation of SNRPN and growth-factor receptor-bound protein 10 (GRB10) [48].
BWS and Wilms tumor are related to H19 (paternally imprinted gene)-ICR [64]. The DNA methylation of MEST is connected with increased levels of follicle-stimulating hormone (FSH), reduction in testicular volume, and oligozoospermia [65]. Lu and colleague evaluated the influence of cryoprotectant, cryopreservation process, and duration of cryopreservation (2 and 60 days) on DNA methylation of H19 and MEST-ICR in human spermatozoa. Although the level of DNA methylation of H19-ICR showed a declining trend, there was no significant difference between cryoprotectant, cryopreservation process, or duration of cryopreservation groups. Also, the level of DNA methylation of MEST-ICR had no significant difference between the groups, despite a trend of increasing [9]. The discrepancy between findings from these studies may be due to the small sample size, different seminogram parameters, and different methods used for the evaluation of sperm DNA damage, sperm preparation, freezing-thawing, and cryoprotectant agents [1, 29].
It is uncertain whether these modifications in the methylation of sperm genomic DNA imply only hypermethylation, hypomethylation, or both modifications in different regions of the gene, which leads to no difference in global sperm DNA methylation [54].
Sperm cryopreservation, DNA fragmentation, and DNA methylation
A significant increase in 8-OHdG, as a marker of DNA oxidative stress, and DNA fragmentation was reported after human sperm cryopreservation [66]. Although assessment of motility, viability, and sometimes DNA fragmentation of spermatozoa after the freezing-thawing process has been considered sufficient to ensure the safety of this process, even without any DNA fragmentation, significant DNA damage could be present in genomic regions [63].
A recent study reported a positive correlation between DNA fragmentation and DNA methylation of AS-ICR after cryopreservation of human spermatozoa [51]. However, Peris-Frau and colleagues did not observe any significant association between DNA methylation and nuclear variables, including percent DNA, chromatin decondensation, and high DNA stainability in fresh and frozen spermatozoa under capacitating and non-capacitating incubation conditions [54]. Moreover, in a study by Kläver et al., no correlation was observed between DNA fragmentation and DNA methylation of sperm from normozoospermic men [8]. Considering limited research with contradictory results, there is no certainty about the possible association between the extent of sperm DNA fragmentation following cryopreservation and the probable alteration in the pattern of DNA methylation [8].
Spermatozoa from infertile men seem to be more vulnerable to freezing damage than those from fertile men [67]. Tunc and Tremellen showed a negative association between fragmentation and methylation of sperm DNA, as well as seminal ROS generation in sperm from infertile men [68]. Furthermore, in men with structural chromosomal abnormalities and reproductive failure, the low integrity of sperm chromatin might be related to the high levels of DNA hypomethylation [69]. It was detected that the incidence of DNA hypermethylation at maternally differentially methylated regions (DMRs) or hypomethylation at paternally imprinted DMRs increased in sperm from oligozoospermic men [70]. Moreover, in boar, spermatozoa with different degrees of DNA fragmentation displayed similar global DNA methylation. However, these spermatozoa exhibited dissimilar site-specific DNA methylation signatures [71]. Putting these findings together, further studies are needed to evaluate the effect of cryopreservation on DNA methylation of spermatozoa in infertile men.
Sperm cryopreservation, DNA methylation, and health of offspring
Sperm cryopreservation leads to a decrease in the offspring’s growth that is easily neglected due to its manifestation at later developmental stages when several other factors also influence growth [72]. Inseminated hens with frozen-thawed spermatozoa showed a lower fertility rate than inseminated hens with fresh spermatozoa. However, the fertility rate of hens inseminated with frozen-thawed spermatozoa in the Lake extender was significantly higher than that inseminated with frozen-thawed spermatozoa in the Beltsville [53]. The assumed effect of sperm cryopreservation on the next generation can be described through the transgenerational epigenetic information carried by sperm [73].
Despite concern about damage to sperm nuclear DNA during cryopreservation, no confirmed increase in genetic or phenotypic anomalies in offspring fertilized with frozen sperm has been recognized [74]. Oxidative stress in sperm can influence epigenetic reprogramming during early embryonic development [75]. In a study by Chao and colleagues, DNA methylation and histone modification were evaluated in embryos derived from cryopreserved spermatozoa at −20°C without cryoprotectants. Although fertilization and developmental competence of mouse embryos decreased, the epigenetic reprogramming of embryos derived from cryopreserved spermatozoa was similar to that of embryos derived from fresh spermatozoa. According to these findings, cryopreservation of spermatozoa without cryoprotectant agents is epigenetically harmless for intracytoplasmic sperm injection (ICSI) [76]. However, some studies reported that ICSI itself can potentially result in epigenetic alterations [77, 78]. These heterogeneous findings may warrant further studies regarding the effects of sperm cryopreservation which may affect DNA methylation and consequently the health of offspring.
Conclusion and future perspective
Excessive production of ROS during cryopreservation has harmful effects on sperm quality. Since abnormal sperm is an important source of free radicals, the level of ROS induced by the cryopreservation process could be higher in abnormal sperm than in morphologically normal sperm. Recently, contradictory results have been reported about the influence of sperm cryopreservation on the DNA methylation patterns in imprinted genes [29]. In recent years, more attention has been given to the role of oxidative stress in alterations of DNA methylation [79]. Oxidative stress can influence the epigenetic status of cells by affecting DNA, histones, and histone modifiers [80]. ROS production is associated with elevated DNA damage and chromosomal DNA degradation with methylation alterations (both hypermethylation and hypomethylation) [81].
Although global DNA methylation is a robust factor regarding sperm cryopreservation, it can be changed when sperm manipulation is not performed in the best condition [50]. It has not been specified yet whether cryopreservation-associated changes in DNA methylation are a characteristic of cryopreservation protocols, laboratory methods, or sperm epigenetic defects related to spermatogenesis [51]. Contradictory findings about the effect of sperm cryopreservation on the DNA methylation patterns may be explained by problems with spermatogenesis, the initial quality of semen, sperm cryosensitivity, genetic differences, different techniques used to measure DNA methylation, different protocols used for sperm cryopreservation, a limited number of studies, and sample size [82]. Moreover, a possible explanation for the discrepancy between the results found in DNA methylation after sperm cryopreservation could be the differences between species. Although it is suggested that DNA methylation assessment may be considered as a valuable supplementary endpoint for the evaluation of frozen-thawed sperm quality [47], it is not possible in many fertility clinics since it is time-consuming and expensive. Multiclinical studies with larger sample sizes under the same conditions of the cryopreservation process and DNA methylation analysis are needed to make any definitive conclusion about the effect of cryopreservation on sperm DNA methylation. Also, given the sensitivity of spermatozoa from infertile men to cryopreservation, further studies are needed in order to evaluate the effect of cryopreservation on DNA methylation status of spermatozoa from these patients.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kajal Khodamoradi, Zahra Rashidi, Malihe Jahromi, Elham Shiri, and Ensieh Salehi contributed equally to this work.
References
- 1.Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Ther Adv Urol. 2011;2012:854837. doi: 10.1155/2012/854837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medeiros C, Forell F, Oliveira A, Rodrigues J. Current status of sperm cryopreservation: why isn’t it better? Theriogenology. 2002;57(1):327–344. doi: 10.1016/S0093-691X(01)00674-4. [DOI] [PubMed] [Google Scholar]
- 3.Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, et al. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod BioMed Online. 2018;37(3):327–339. doi: 10.1016/j.rbmo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Valipour J, Nashtaei MS, Khosravizadeh Z, Mahdavinezhad F, Nekoonam S, Esfandyari S, et al. Effect of sulforaphane on apoptosis, reactive oxygen species and lipids peroxidation of human sperm during cryopreservation. Cryobiology. 2020. 10.1016/j.cryobiol.2020.11.012. [DOI] [PubMed]
- 5.Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014;6(2):a018382. doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millership SJ, Van de Pette M, Withers DJ. Genomic imprinting and its effects on postnatal growth and adult metabolism. Cell Mol Life Sci. 2019;76(20):4009–4021. doi: 10.1007/s00018-019-03197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amor DJ, Halliday J. A review of known imprinting syndromes and their association with assisted reproduction technologies. Hum Reprod. 2008;23(12):2826–2834. doi: 10.1093/humrep/den310. [DOI] [PubMed] [Google Scholar]
- 8.Kläver R, Bleiziffer A, Redmann K, Mallidis C, Kliesch S, Gromoll J. Routine cryopreservation of spermatozoa is safe—evidence from the DNA methylation pattern of nine spermatozoa genes. J Assist Reprod Genet. 2012;29(9):943–950. doi: 10.1007/s10815-012-9813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W-H, Yang X-Y, Liang X-W, Gu Y-Q. AB082. Effect of cryopreservation on DNA methylation status of imprinted genes in human sperm. Transl Androl Urol. 2015;4(Suppl 1):402–408. doi: 10.3978/j.issn.2223-4683.2015.s082. [DOI] [Google Scholar]
- 10.Coll-Sanmartin, Laia; Vidal, Francesca, dir. Effect of cryopreservation in the imprinting pattern of gametes. 2015. 1 pag. (833 Grau en Genètica). https://ddd.uab.cat/record/141371
- 11.Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22(9):4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gualtieri R, Kalthur G, Barbato V, Longobardi S, Di Rella F, Adiga SK, et al. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants. 2021;10(7):1025. doi: 10.3390/antiox10071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiri E, Abolhassani F, Khosravizadeh Z, Najafi A, Khanezad M, Vazirian M, et al. Aqueous Origanum vulgare extract improves the quality of cryopreserved human spermatozoa through its antioxidant effects. Biopreserv Biobank. 2020;18(4):329–336. doi: 10.1089/bio.2020.0008. [DOI] [PubMed] [Google Scholar]
- 14.Lasso JL, Noiles EE, Alvarez JG, Storey BT. Mechanism of superoxide dismutase loss from human sperm cells during cryopreservation. J Androl. 1994;15(3):255–265. doi: 10.1002/j.1939-4640.1994.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 15.Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet. 2008;25(8):403–411. doi: 10.1007/s10815-008-9232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Said TM, Gaglani A, Agarwal A. Implication of apoptosis in sperm cryoinjury. Reprod BioMed Online. 2010;21(4):456–462. doi: 10.1016/j.rbmo.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436(3):687–698. doi: 10.1042/BJ20110114. [DOI] [PubMed] [Google Scholar]
- 18.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aitken RJ, Drevet JR. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: a two-edged sword. Antioxidants. 2020;9(2):111. doi: 10.3390/antiox9020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’connell M, Mcclure N, Lewis S. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod. 2002;17(3):704–709. doi: 10.1093/humrep/17.3.704. [DOI] [PubMed] [Google Scholar]
- 21.Fortunato A, Leo R, Liguori F. Effects of cryostorage on human sperm chromatin integrity. Zygote. 2013;21(4):330–336. doi: 10.1017/S0967199412000032. [DOI] [PubMed] [Google Scholar]
- 22.Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril. 2008;89(6):1723–1727. doi: 10.1016/j.fertnstert.2007.06.087. [DOI] [PubMed] [Google Scholar]
- 23.Lusignan M-F, Li X, Herrero B, Delbès G, Chan PT. Effects of different cryopreservation methods on DNA integrity and sperm chromatin quality in men. Andrology. 2018;6(6):829–835. doi: 10.1111/andr.12529. [DOI] [PubMed] [Google Scholar]
- 24.Chohan K, Griffin J, Carrell D. Evaluation of chromatin integrity in human sperm using acridine orange staining with different fixatives and after cryopreservation. Andrologia. 2004;36(5):321–326. doi: 10.1111/j.1439-0272.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini A, Khalili MA, Talebi AR, Agha-Rahimi A, Ghasemi-Esmailabad S, Woodward B, et al. Cryopreservation of low number of human spermatozoa; which is better: vapor phase or direct submerging in liquid nitrogen? Hum Fertil. 2018. 10.1080/14647273.2018.1456681. [DOI] [PubMed]
- 26.Isachenko E, Isachenko V, Katkov II, Rahimi G, Schondorf T, Mallmann P, et al. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum Reprod. 2004;19(4):932–939. doi: 10.1093/humrep/deh194. [DOI] [PubMed] [Google Scholar]
- 27.Morshedi M, Duru N, Oehninger S. Cryopreservation-thawing does not impair DNA integrity but induces plasma membrane translocation of phosphatidylserine in fractionated human spermatozoa. Fertil Steril. 2000;74(3):S244–S2S5. doi: 10.1016/S0015-0282(00)01445-X. [DOI] [PubMed] [Google Scholar]
- 28.Bogle O, Kumar K, Attardo-Parrinello C, Lewis S, Estanyol J, Ballescà J, et al. Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology. 2017;5(1):10–22. doi: 10.1111/andr.12279. [DOI] [PubMed] [Google Scholar]
- 29.Paoli D, Lombardo F, Lenzi A, Gandini L. Sperm cryopreservation: effects on chromatin structure. Gen Damage Human Spermatozoa. 2014;2014:137–150. doi: 10.1007/978-1-4614-7783-9_9. [DOI] [PubMed] [Google Scholar]
- 30.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J-H, Park S-J, Nakai K. Differential landscape of non-CpG methylation in embryonic stem cells and neurons caused by DNMT3s. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-11800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bird A, Taggart M, Frommer M, Miller OJ, Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated. CpG-rich DNA Cell. 1985;40(1):91–99. doi: 10.1016/0092-8674(85)90312-5. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Ni X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr Drug Targets. 2015;16(1):13–19. doi: 10.2174/1389450116666150113121054. [DOI] [PubMed] [Google Scholar]
- 34.Campos AC, Molognoni F, Melo FH, Galdieri LC, Carneiro CR, D’Almeida V, et al. Oxidative stress modulates DNA methylation during melanocyte anchorage blockade associated with malignant transformation. Neoplasia. 2007;9(12):1111–1121. doi: 10.1593/neo.07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)––induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res-Fund Mol Mutagen. 2011;711(1-2):167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Valinluck V, Sowers LC. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67(3):946–950. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 37.Shao D, Fry JL, Han J, Hou X, Pimentel DR, Matsui R, et al. A redox-resistant sirtuin-1 mutant protects against hepatic metabolic and oxidant stress. J Biol Chem. 2014;289(11):7293–7306. doi: 10.1074/jbc.M113.520403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Osta A, Wolffe AP. DNA methylation and histone deacetylation in the control of gene expression: basic biochemistry to human development and disease. Gene Expr J Liver Res. 2001;9(1-2):63–75. doi: 10.3727/000000001783992731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song C, Feodorova Y, Guy J, Peichl L, Jost KL, Kimura H, et al. DNA methylation reader MECP2: cell type- and differentiation stage-specific protein distribution. Epigenetics Chromatin. 2014;7(1):1–16. doi: 10.1186/1756-8935-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng S-M, Bailey ME, Cobb SR. Rett syndrome: from bed to bench. Pediatr Neonatol. 2011;52(6):309–316. doi: 10.1016/j.pedneo.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Petrussa L, Van de Velde H, De Rycke M. Dynamic regulation of DNA methyltransferases in human oocytes and preimplantation embryos after assisted reproductive technologies. Mol Hum Reprod. 2014;20(9):861–874. doi: 10.1093/molehr/gau049. [DOI] [PubMed] [Google Scholar]
- 42.Zeng C, Peng W, Ding L, He L, Zhang Y, Fang D, et al. A preliminary study on epigenetic changes during boar spermatozoa cryopreservation. Cryobiology. 2014;69(1):119–127. doi: 10.1016/j.cryobiol.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Oblette A, Rondeaux J, Dumont L, Delessard M, Saulnier J, Rives A, et al. DNA methylation and histone post-translational modifications in the mouse germline following in-vitro maturation of fresh or cryopreserved prepubertal testicular tissue. Reprod BioMed Online. 2019;39(3):383–401. doi: 10.1016/j.rbmo.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Li L, Yu Y, Huang H, Uygun BE, Yarmush ML. RNA-based dCas9–VP64 system improves the viability of cryopreserved mammalian cells. Nano Life. 2018;8(03):1850004. doi: 10.1142/S1793984418500046. [DOI] [Google Scholar]
- 45.Yan L-Y, Yan J, Qiao J, Zhao P-L, Liu P. Effects of oocyte vitrification on histone modifications. Reprod Fertil Dev. 2010;22(6):920–925. doi: 10.1071/RD09312. [DOI] [PubMed] [Google Scholar]
- 46.Maldonado MBC, Penteado JCT, Faccio BMC, Lopes FL, Arnold DR. Changes in tri-methylation profile of lysines 4 and 27 of histone H3 in bovine blastocysts after cryopreservation. Cryobiology. 2015;71(3):481–485. doi: 10.1016/j.cryobiol.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Aurich C, Schreiner B, Ille N, Alvarenga M, Scarlet D. Cytosine methylation of sperm DNA in horse semen after cryopreservation. Theriogenology. 2016;86(5):1347–1352. doi: 10.1016/j.theriogenology.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 48.Zhou XWYTL, Ma L, Yan B, Tian J, Zhang F, Zhou Y, Wang H. DNA methylation and expression of SNRPN and GRB10 imprinted genes in human semen freezing-thawing process. J Shandong Univ. 2017;152(2–3):373–380. doi: 10.6040/j.issn.1671-7554.0.2016.1065. [DOI] [Google Scholar]
- 49.de Mello F, Garcia JS, Godoy LC, Depincé A, Labbé C, Streit DP., Jr The effect of cryoprotectant agents on DNA methylation patterns and progeny development in the spermatozoa of Colossoma macropomum. Gen Comp Endocrinol. 2017;245:94–101. doi: 10.1016/j.ygcen.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Depincé A, Gabory A, Dziewulska K, Le Bail PY, Jammes H, Labbé C. DNA methylation stability in fish spermatozoa upon external constraint: Impact of fish hormonal stimulation and sperm cryopreservation. Mol Reprod Dev. 2020;87(1):124–134. doi: 10.1002/mrd.23297. [DOI] [PubMed] [Google Scholar]
- 51.Khosravizadeh Z, Hassanzadeh G, Bazzaz JT, Alizadeh F, Totonchi M, Salehi E, et al. The effect of cryopreservation on DNA methylation patterns of the chromosome 15q11–q13 region in human spermatozoa. Cell Tissue Bank. 2020;21(3):433–445. doi: 10.1007/s10561-020-09828-1. [DOI] [PubMed] [Google Scholar]
- 52.Mercati F, Domingo P, Pasquariello R, Dall’Aglio C, Di Michele A, Forti K, et al. Effect of chelating and antioxidant agents on morphology and DNA methylation in freeze-drying rabbit (Oryctolagus cuniculus) spermatozoa. Reprod Domest Anim. 2020;55(1):29–37. doi: 10.1111/rda.13577. [DOI] [PubMed] [Google Scholar]
- 53.Salehi M, Mahdavi AH, Sharafi M, Shahverdi A. Cryopreservation of rooster semen: evidence for the epigenetic modifications of thawed sperm. Theriogenology. 2020;142:15–25. doi: 10.1016/j.theriogenology.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 54.Peris-Frau P, Álvarez-Rodríguez M, Martín-Maestro A, Iniesta-Cuerda M, Sánchez-Ajofrín I, Medina-Chávez DA, et al. Unravelling how in vitro capacitation alters ram sperm chromatin before and after cryopreservation. Andrology. 2021;9(1):414–425. doi: 10.1111/andr.12900. [DOI] [PubMed] [Google Scholar]
- 55.Güngör İH, Tektemur A, Arkali G, Cinkara SD, Acisu TC, Koca RH, et al. Effect of freeze–thawing process on lipid peroxidation, miRNAs, ion channels, apoptosis and global DNA methylation in ram spermatozoa. Reprod Fertil Dev. 2021;33(14):747–759. doi: 10.1071/RD21091. [DOI] [PubMed] [Google Scholar]
- 56.Montjean D, Zini A, Ravel C, Belloc S, Dalleac A, Copin H, et al. Sperm global DNA methylation level: association with semen parameters and genome integrity. Andrology. 2015;3(2):235–240. doi: 10.1111/andr.12001. [DOI] [PubMed] [Google Scholar]
- 57.Gopalkrishnan K, Hinduja I, Anand KT. In vitro decondensation of nuclear chromatin of human spermatozoa: assessing fertilizing potential. Arch Androl. 1991;27(1):43–50. doi: 10.3109/01485019108987650. [DOI] [PubMed] [Google Scholar]
- 58.Peris-Frau P, Martín-Maestro A, Iniesta-Cuerda M, Sánchez-Ajofrín I, Cesari A, Garde JJ, et al. Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology. 2020;145:100–108. doi: 10.1016/j.theriogenology.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 59.Raz E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000;1(3):1–6. doi: 10.1186/gb-2000-1-3-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stürchler D, Tanner M, Hanck A, Betschart B, Gautschi K, Weiss N, et al. A longitudinal study on relations of retinol with parasitic infections and the immune response in children of Kikwawila village, Tanzania. Acta Trop. 1987;44(2):213–227. [PubMed] [Google Scholar]
- 61.Riesco MF, Robles V. Cryopreservation causes genetic and epigenetic changes in zebrafish genital ridges. PLoS One. 2013;8(6):e67614. doi: 10.1371/annotation/3265139d-64c7-4c4c-83d3-1e139031e7df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al Ageeli E, Drunat S, Delanoë C, Perrin L, Baumann C, Capri Y, et al. Duplication of the 15q11-q13 region: clinical and genetic study of 30 new cases. Eur J Med Genet. 2014;57(1):5–14. doi: 10.1016/j.ejmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Valcarce D, Cartón-García F, Riesco M, Herráez M, Robles V. Analysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo development. Andrology. 2013;1(5):723–730. doi: 10.1111/j.2047-2927.2013.00116.x. [DOI] [PubMed] [Google Scholar]
- 64.Yoshimizu T, Miroglio A, Ripoche M-A, Gabory A, Vernucci M, Riccio A, et al. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci. 2008;105(34):12417–12422. doi: 10.1073/pnas.0801540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kläver R, Tüttelmann F, Bleiziffer A, Haaf T, Kliesch S, Gromoll J. DNA methylation in spermatozoa as a prospective marker in andrology. Andrology. 2013;1(5):731–740. doi: 10.1111/j.2047-2927.2013.00118.x. [DOI] [PubMed] [Google Scholar]
- 66.Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang J-A, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24(9):2061–2070. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 67.Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76(5):892–900. doi: 10.1016/S0015-0282(01)02834-5. [DOI] [PubMed] [Google Scholar]
- 68.Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009;26(9):537–544. doi: 10.1007/s10815-009-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olszewska M, Barciszewska MZ, Fraczek M, Huleyuk N, Chernykh VB, Zastavna D, et al. Global methylation status of sperm DNA in carriers of chromosome structural aberrations. Asian J Androl. 2017;19(1):117. doi: 10.4103/1008-682X.168684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiura H, Okae H, Chiba H, Miyauchi N, Sato F, Sato A, et al. Imprinting methylation errors in ART. Reprod Med Biol. 2014;13(4):193–202. doi: 10.1007/s12522-014-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khezri A, Narud B, Stenseth E-B, Johannisson A, Myromslien FD, Gaustad AH, et al. DNA methylation patterns vary in boar sperm cells with different levels of DNA fragmentation. BMC Genomics. 2019;20(1):1–15. doi: 10.1186/s12864-019-6307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nusbaumer D, Marques da Cunha L, Wedekind C. Sperm cryopreservation reduces offspring growth. Proc R Soc B. 2019;286(1911):20191644. doi: 10.1098/rspb.2019.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai J, Liu L, Chen J, Liu Z, Wang W, Jiang X, et al. The effect of epididymal sperm cryopreservation on neonatal birthweight following PESA-ICSI. Arch Gynecol Obstet. 2021;305:1233–1239. doi: 10.1007/s00404-021-06350-x. [DOI] [PubMed] [Google Scholar]
- 74.Anger JT, Gilbert BR, Goldstein M. Cryopreservation of sperm: indications, methods and results. J Urol. 2003;170(4):1079–1084. doi: 10.1097/01.ju.0000084820.98430.b8. [DOI] [PubMed] [Google Scholar]
- 75.Wyck S, Herrera C, Requena CE, Bittner L, Hajkova P, Bollwein H, et al. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin. 2018;11(1):1–17. doi: 10.1186/s13072-018-0224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chao S, Li J, Jin X, Tang H, Wang G, Gao G. Epigenetic reprogramming of embryos derived from sperm frozen at −20 °C. Sci China Life Sci. 2012;55(4):349–357. doi: 10.1007/s11427-012-4309-8. [DOI] [PubMed] [Google Scholar]
- 77.Choux C, Petazzi P, Sanchez-Delgado M, Hernandez Mora JR, Monteagudo A, Sagot P, et al. The hypomethylation of imprinted genes in IVF/ICSI placenta samples is associated with concomitant changes in histone modifications. Epigenetics. 2020;15(12):1386–1395. doi: 10.1080/15592294.2020.1783168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Hajj N, Haertle L, Dittrich M, Denk S, Lehnen H, Hahn T, et al. DNA methylation signatures in cord blood of ICSI children. Hum Reprod. 2017;32(8):1761–1769. doi: 10.1093/humrep/dex209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donkena KV, Young CY, Tindall DJ. Oxidative stress and DNA methylation in prostate cancer. Obstet Gynecol Int. 2010. 10.1155/2010/302051. [DOI] [PMC free article] [PubMed]
- 80.Guillaumet-Adkins A, Yañez Y, Peris-Diaz MD, Calabria I, Palanca-Ballester C, Sandoval J. Epigenetics and oxidative stress in aging. Oxidative Med Cell Longev. 2017. 10.1155/2017/9175806. [DOI] [PMC free article] [PubMed]
- 81.Lim S-O, Gu J-M, Kim MS, Kim H-S, Park YN, Park CK, et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology. 2008;135(6):2128–40.e8. doi: 10.1053/j.gastro.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 82.Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21(2):209–227. doi: 10.1093/humupd/dmu063. [DOI] [PubMed] [Google Scholar]