Abstract
Purpose
This study aims to assess whether antibiotic therapy for chronic endometritis (CE) could improve subsequent IVF outcomes in patients with recurrent implantation failure (RIF).
Methods
Studies that explore CE treatment in patients with RIF were retrieved from PubMed, EMBASE, Wanfang, and Google Scholar up to Jan 31, 2022. All retrieved studies were selected according to the inclusion and exclusion criteria. The main outcome measures include implantation rate (IR), clinical pregnancy rate (CPR), ongoing pregnancy rate/live birth rate (OPR/LBR), and miscarriage rate (MR). Odds ratios (ORs) were analyzed for pregnancy outcomes with a 95% confidence interval (CI).
Results
Nine articles were enrolled in this study. Patients receiving oral antibiotic administration (OAA) did not show any advantage over patients without CE with regard to IR, OPR/LBR, and MR, but they showed a higher CPR. Patients with cured CE after OAA therapy had significantly higher CPR, IR, and OPR/LBR compared with patients without CE. Patients with persistent CE after OAA therapy had significantly lower IR, CPR, and OPR/LBR compared with patients without CE. Patients with cured CE had significantly higher IR, CPR, and OPR/LBR compared with persistent CE patients.
Conclusions
Antibiotic treatment may improve the pregnancy outcomes of RIF patients in subsequent IVF cycles only if the condition of CE is confirmed cured in a control biopsy afterwards. Otherwise, no sufficient evidence has shown improvements in clinical outcomes in RIF patients with persistent CE.
Keywords: Chronic endometritis, Antibiotic therapy, In vitro fertilization, Embryo transfer, Implantation failure
Introduction
Recurrent implantation failure (RIF), defined as the failure to achieve a clinical pregnancy after multiple embryo transfers, happens in approximately 20% of women undergoing in vitro fertilization (IVF) treatment [1–4]. Chronic endometritis (CE) is a condition of persistent inflammation characterized by the infiltration of plasma cells in the endometrium and was often overlooked due to a lack of apparent clinical manifestations. Now, CE is a potential adverse factor for fertility, and 14–67.5% of RIF patients are reported to be affected by CE [5, 6]. Multiple pathogens, including Chlamydia trachomatis, Enterococcus, Escherichia coli, Gardnerella vaginalis, and Klebsiella pneumoniae, are associated with the infectious process of CE [7], and dysregulation of lymphocyte subsets and regulatory molecules contributes to compromised endometrial receptivity [8, 9]. Endometrial biopsy and histological analysis remain the golden standard for the diagnosis of CE, and immunohistochemistry for CD138 increases diagnostic sensitivity [3, 10, 11]. However, there is no consensus definition of RIF to date, and various diagnosis criteria of CE in histology are applied in clinical practice and scientific research across the world.
Apart from oral antibiotic administration (OAA), which has been utilized as the gold standard treatment, other antibiotic therapies, including intrauterine antibiotic infusion (IAI), are among the treatment options for CE [12, 13]. The effectiveness rate following OAA was around 58.95–99.15%, while OAA combined with IAI was reported to enhance the treatment effectiveness [2, 14–16]. However, although antibiotic therapy has been proven to eliminate the chronic infection in the endometrium, whether it could improve clinical outcomes in subsequent IVF cycles of RIF patients continues to be controversial [17, 18].
We performed a systematic review and meta-analysis of data published in recent years to validate the impact of antibiotic-treated CE on the subsequent IVF outcomes in RIF patients.
Materials and methods
Data sources and study selection
This study was performed in accordance with the PRISMA guidelines. Electronic databases, including PubMed, EMBASE, Wanfang, and Google Scholar, were searched until 31 January 2022. The following keywords were used: chronic endometritis, antibiotic therapy, in vitro fertilization, infertility, recurrence, embryo implantation, and embryo transfer.
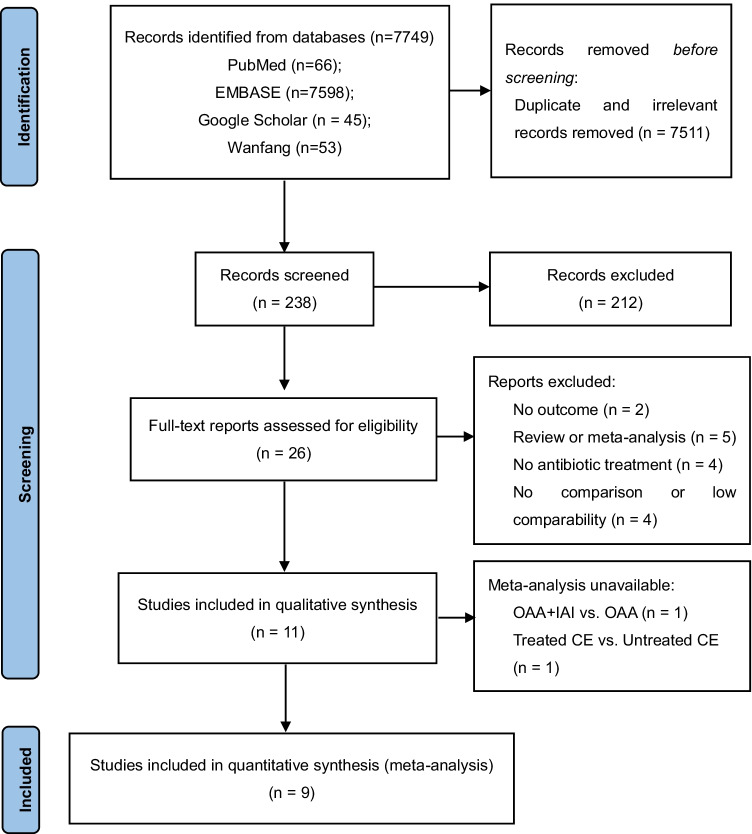
Two of the authors (X.C. and Z.X.) independently checked the titles and abstracts of every record retrieved, read all potentially relevant articles as full texts, and selected the retrieved literature according to the inclusion and exclusion criteria developed collectively. Disagreements were discussed with a third author (Z.H.) to reach a final result (Fig. 1).
Fig. 1.
PRISMA flow diagram for the selection of eligible studies. OAA, oral antibiotic administration; IAI, intrauterine antibiotic infusion; CE, chronic endometritis
Eligibility criteria
Inclusion criteria:
-
(i)
Study types include randomized controlled studies, non-randomized prospective studies, and retrospective studies; (ii) RIF patients (defined as at least two previous failed IVF-ET attempts) with or without histologically confirmed CE (defined as the presence of at least one endometrial stromal plasma cell in the entire section, demonstrated by conventional staining and/or immunohistochemistry); (iii) patients with CE receiving antibiotic treatment and patients without CE who were not treated; and (iv) subsequent IVF cycles after antibiotic treatment.
Exclusion criteria: Posters, conferences, letters, comments, editorials. Publications in languages other than English or Chinese were excluded.
Outcomes include (i) implantation rate (IR): the number of gestational sacs on transvaginal ultrasound per the number of embryos transfers; (ii) clinical pregnancy rate (CRP): the presence of at least one gestational sac determined by ultrasound examination per patient; (iii) ongoing pregnancy (OPR): clinical pregnancies that exceeded 20 weeks’ estimated gestational age per patient; (iv) live birth rate (LBR): delivery of at least one viable fetus per patient; (v) miscarriage rate (MR): the number of pregnancy losses before 20 weeks’ gestation per patient.
Data extraction
Two of the authors (X.C. and Z.X.) performed data extraction. Reference lists of studies were searched manually to avoid missing potentially relevant articles. Additional data about the included studies were obtained by contacting the corresponding authors by e-mail. A third author (Z.H.) reviewed the data extraction process. The information and data were extracted from eligible studies by using piloted screening forms in Microsoft Office Excel.
Statistical analysis
The meta-analysis was conducted using Review Manager (RevMan, version 5.4; Nordic Cochrane StataCorp LLC, USA). The odds ratio (OR) was analyzed for pregnancy outcomes with a 95% confidence interval (CI). We used the I2 value to assess heterogeneity; I2 ≥ 50% indicates statistical heterogeneity. When I2 < 50%, we used the fixed-effects models. Otherwise, random-effects models of analysis were applied. In addition, subgroup analysis was performed based on study type (retrospective vs. prospective study) and study outcome (miscarriage < 12 weeks vs. miscarriage < 28 weeks). Sensitivity analysis was carried out using the leave-one-out approach.
Results
Study inclusion and basic characteristics
A total of 7749 potentially relevant studies were retrieved from electronic databases. After duplicate and irrelevant records were removed, 238 records were screened for titles and abstracts, in which 212 records were excluded. A full-text review of 26 articles was performed. Finally, 11 articles met the inclusion criteria, and 9 articles were enrolled for quantitative synthesis. A flow diagram shows the study inclusion process in Fig. 1.
The 11 studies comprised one randomized controlled trial (RCT) [15], three non-randomized prospective studies [19–21], and seven retrospective cohort studies [22–28]. Antibiotic therapies conducted in diagnosed CE patients included OAA, OAA combined with IAI, and IAI combined with dexamethasone. Tests of cure biopsies were performed after antibiotic therapies to identify the treatment efficacy in 6 out of 11 studies. The specific characteristics of enrolled studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Authors and year | Study design, country, and time of realization | Participants and main inclusion criteria | Methods | Diagnosis of chronic endometritis | Treatment procedure | Groups | Outcomes |
|---|---|---|---|---|---|---|---|
| Demirdag et al. 2021[28] | Retrospective study, Turkey, September 2016 to December 2019 |
202 patients undergoing IVF-ET cycle At least three embryo transfer cycles (fresh or frozen) with a minimum transfer of four good-quality embryos Age < 40 y Normal parental karyotype Normal intrauterine cavity Normal antiphospholipid antibody testing |
EB HIS examination Antibiotic therapy (if necessary) IVF cycle |
CD138 staining Positive: one or more CD138 positive plasma cells identified in a spindle cell alteration in the stroma |
Single course 14 days of ciprofloxacin 500 mg bid + ornidazole 500 mg bid |
Group A: patients with treated CE (n = 129) Group B: patients without CE (n = 103) Group C: patients had the first IVF cycle (n = 932) |
Implantation rate Clinical pregnancy rate Live birth rate |
| Pantos et al. 2021[15] | Randomized controlled trial, Greece, March 2017 to February 2019 |
40 patients undergoing IVF-ET cycle At least three failed previous IVF attempts employing good-quality embryos Age < 35 y No other infertility etiology FSH and LH levels |
Diagnostic HSC EB HIS examination Antibiotic therapy IVF cycle |
HSC (presence of polypoid endometrium, micropolyps, stromal edema, and diffuse hyperemia), H&E staining, CD138 staining Positive: positive in all three methods |
Single course 14 days of doxycycline 100 mg + metronidazole 500 mg bid Intrauterine infusion of 3 ml ciprofloxacin solution (200 mg/100 ml). 1 infusion every 3 days. 10 infusions in total in 30 days |
Group A: patients treated with oral antibiotics only (n = 20) Group B: patients treated with oral antibiotics and intrauterine antibiotics infusion (n = 20) |
Treatment efficiency Adverse effects rate Clinical pregnancy rate Live birth rate |
| Mitter et al. 2021[27] | Retrospective study, Switzerland, January 2014 to December 2018 |
28 patients undergoing HSC and biopsy At least six failed transfer of good-quality cleavage-stage embryos Age < 42 y 18 < BMI < 35 No known conditions associated with RIF |
Diagnostic HSC EB HIS examination Antibiotic therapy IVF cycle |
CD138 staining Positive: one or more plasma cell per whole-slide tissue section |
Single course Doxycycline 100 mg bid for 14 days or ciprofloxacin 400 mg a day for 14 days (if intolerant to doxycycline) |
Group A: patients with treated CE (n = 13) Group B: patients without CE (n = 15) |
Clinical pregnancy rate Live birth rate Chance of pregnancy Live birth over time |
| Li et al. 2021[26] | Retrospective study, China, January 2018 to December 2019 |
326 patients undergoing IVF/ICSI-ET cycle At least two embryo transfer cycles (fresh or frozen) with a minimum transfer of four good-quality embryos Age < 40 y Normal parental karyotype Normal intrauterine cavity No endocrine diseases No blood hypercoagulability tendency |
EB HIS examination Antibiotic therapy (if necessary) Repeated biopsy IVF cycle |
CD138 staining (five or more plasma cells in endometrium stroma per 30HPFs) Strong positive: three or more positive HPFs Weak positive: one to two positive HPFs Negative: one to four plasma cells in endometrium stroma or none |
Single course Doxycycline 100 mg bid for 14 days and metronidazole 500 mg tid for 14 days |
Group A: patients with weak positive CE after treatment (n = 27) Group B: patients turned CE-negative after treatment (n = 82) Group C: patients without CE (n = 217) |
HCG positive rate Clinical pregnancy rate Implantation rate Ectopic pregnancy rate Miscarriage rate Live birth rate |
| Yang et al. 2020[25] | Retrospective study, China, January 2016 to January 2017 |
287 patients undergoing HSC and biopsy At least three embryo transfer cycles (fresh or frozen) with a minimum transfer of four good-quality embryos Age < 40 y |
Diagnostic HSC EB HIS examination Antibiotic therapy (if necessary) IVF cycle |
H&E staining, CD138 staining Positive: five or more plasma cells in endometrium stroma per HPF at × 400 magnification Negative: CD138 staining negative, or less than five plasma cells in endometrium stroma per HPF at × 400 magnification |
Single course Levofloxacin 0.5 g qd for 14 days and tinidazole 0.5 g qd for 14 days |
Group A: patients with treated CE (n = 111) Group B: patients without CE (n = 176) |
Implantation rate Clinical pregnancy rate Ongoing pregnancy rate Early miscarriage rate |
| Zhang et al. 2019[20] | Prospective study, China, February 2015 to June 2017 |
109 patients undergoing HSC At least three embryo transfer cycles (fresh or frozen) or a minimum transfer of six good-quality embryos Age < 35 y No abnormality at TVS and at HSG Normal parental peripheral karyotype |
Diagnostic HSC EB HIS examination Endometrial culture Antibiotic therapy Repeated biopsy IVF cycle |
HSC, H&E staining, CD138 staining HSC-positive: micropolyps, polypoid endometrium, stromal edema, and focal or diffuse hyperemia HIS-positive: one or more plasma cell per HPF HIS-negative: less than one plasma cell per HPF |
Repeated course Intrauterine infusion with 20 ml mixture (dexamethasone 5 mg, hyaluronidase 1500 U, and saline added up to 20 ml) once a day for seven consecutive days after menstruation Gentamicin 80 mg (if gram-negative) Clindamycin 60 mg (if gram-positive) Minocycline 6 mg (mycoplasma and urealyticum) Gentamicin 80 mg + clindamycin 60 mg + metronidazole 80 mg (if culture negative) |
Group A: patients with cured CE (n = 85) Group B: patients with persistent CE (n = 24) Group C: patients with no CE at hysteroscopy (n = 126) |
Implantation rate Clinical pregnancy rate Live birth rate Clinical loss rate |
| Kitaya et al. 2017[19] | Prospective study, Japan, November 2011 to July 2014 |
342 patients undergoing IVF-ET cycle Negative pregnancy tests following transfer of three or more morphologically good cleavage-stage embryos and/or blastocysts No intrauterine lesions |
Diagnostic HSC EB HIS examination Endometrial culture Antibiotic therapy (if necessary) Repeated biopsy IVF cycle |
H&E staining, CD138 staining Positive: 0.25 or more endometrial stromal plasmacyte density index evaluated in 20 or more HPFs |
Repeated course (up to two times) Doxycycline 100 mg bid for 14 days; metronidazole 250 mg bid for 14 days and ciprofloxacin hydrochloride 200 mg bid for 14 days (if resistant to doxycycline) |
Group A: patients with cured CE (n = 116) Group B: patients without CE (n = 226) |
Clinical pregnancy rate Miscarriage rate Live birth rate |
| Cicinelli et al. 2015[24] | Retrospective study, Italy, January 2009 to June 2012 |
61 patients undergoing HSC At least six good-quality embryos transferred in three or more previous IVF/ICSI cycles without signs of implantation Age < 40 y No abnormality at TVS and at HSG Normal response with at least six oocytes retrieved with standard induction protocol Normal parental peripheral karyotype |
Diagnostic HSC EB HIS examination Endometrial culture Antibiotic therapy Repeated biopsy IVF cycle |
HSC, H&E staining HSC-positive: presence of focal or diffuse hyperemia, stroma edema, out of phase endometrium, micropolyps and adhesions HIS-positive: superficial stromal edema, increased stromal density, pleomorphic stromal inflammatory infiltrate dominated by lymphocytes and plasma cells |
Repeated course (up to three times) 10 days of ciprofloxacin 500 mg bid (if Gram negative) 8 days of amoxicillin + clavulanate 1 g bid (if gram-positive) 12 days of josamycin 1 g bid (if mycoplasma and U. urealyticum) and 12 days of minocycline 100 mg bid (if persistent) Ceftriaxone 250 mg IM in a single dose + 14 days of doxycycline 100 mg + metronidazole 500 mg bid (if culture negative) |
Group A: patients with cured CE (n = 46) Group B: patients with persistent CE (n = 15) |
Implantation rate Clinical pregnancy rate Live birth rate Number of miscarriages |
| Tersoglio et al. 2015[21] | Prospective study, Argentina, January 2010 to July 2013 | At least two cycles of IVF/ICSI or cryotransfer, with at least two blastocysts with quality 311–511 of Gardner-Schoolcraft |
EB HIS examination Endometrial culture Antibiotic therapy Repeated biopsy IVF cycle |
Histopathology Positive: presence of plasma cells and polymorphonuclear stromal localization with more than one plasma cells per HPF |
Repeated course Specific antibiotics (if culture positive) 14 days of doxycycline 200 mg, 14 days of metronidazole 1 g + ciprofloxacin 1 g (if culture negative) 10 days of linezolid 600 mg (if persistent) |
Group A: patients with cured CE (n = 9) Group B: patients with persistent CE (n = 5) Group C: patients without CE (n = 16) |
Implantation rate Clinical pregnancy rate Abortion rate Ongoing pregnancy rate Live birth rate |
| Yang et al. 2014[23] | Retrospective study, China, May 2010 to April 2012 |
88 patients undergoing HSC After three IVF treatment attempts or after ≥ 6 high-quality embryo transfers |
Diagnostic HSC EB HIS examination Antibiotic therapy (if necessary) IVF cycle |
HSC, H&E staining, CD38 and CD138 staining HSC-positive: hyperemia, mucosal edema, and micropolyps HIS-positive: not specified |
Single course 14 days of levofloxacin 0.5 g qd + metronidazole 1 g qd |
Group A: patients with treated CE (n = 68) Group B: patients with untreated CE (n = 20) |
Implantation rate Intrauterine pregnancy rate Ongoing pregnancy rate |
| Johnston-MacAnanny et al. 2010[22] | Retrospective study, USA, January 2001 to December 2007 |
518 patients undergoing HSC At least two cycles of IVF-ET with at least one good-quality embryo transferred in each cycle Normal karyotypes Negative antiphospholipid antibodies testing Normal uterine cavity |
EB HIS examination Antibiotic therapy (if necessary) Repeated biopsy IVF cycle |
H&E staining, CD138 staining Negative: less than one plasma cell per HPF |
Repeated course 14 days of doxycycline 100 mg bid 14 days of ciprofloxacin 500 mg bid + metronidazole 500 mg bid (if persistent) |
Group A: patients with cured CE (n = 10) Group B: patients without CE (n = 23) Group C: RIF patients without a biopsy (n = 485) |
Implantation rate Clinical pregnancy rate Clinical loss rate Ongoing pregnancy rate |
IVF, in vitro fertilization; IVF-ET, in vitro fertilization and embryo transfer; IVF/ICSI, in vitro fertilization and intracytoplasmic sperm injection; EB, endometrial biopsy; HIS, histology; HSC, hysteroscopy; HSG, hysterosalpingography; H&E, hematoxylin and eosin; HPF, high-power field; TVS, transvaginal ultrasound; RIF, recurrent implantation failure; CE, chronic endometritis; qd, once a day; bid, twice a day; tid, three times a day
Quality assessment of the included studies
In this meta-analysis, we used the Cochrane risk-of-bias tool to assess the risk of bias in one RCT. This one RCT has a low risk of randomization process, deviations from the intended interventions, missing outcomes, measurement of the outcome, and selection of reported results. For the remaining non-randomized prospective studies and retrospective cohort studies, we used the Newcastle–Ottawa scale, and the results are shown in Table 2. Studies presenting groups with similar baseline characteristics other than the existence of CE were considered to be of good comparability, including age, body mass index (BMI), hormone levels, infertility duration, number of previous IVF attempts, number of embryos transferred, and infertility etiology. After the antibiotic treatments, patients were supposed to be followed up and re-examined by hysteroscopy and/or endometrial biopsy in the follicular phase of the subsequent cycle.
Table 2.
Quality assessment of the included studies
| Included studies | Selection | Comparability | Outcome | Total quality scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Assessment of outcome | Was follow- up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |||
| Demirdag et al. 2021[28] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Mitter et al. 2021[27] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Li et al. 2021[26] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Yang et al. 2020[25] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Zhang et al. 2019[20] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Kitaya et al. 2017[19] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Cicinelli et al. 2015[24] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Tersoglio et al. 2015[21] | ★ | ★ | ★ | ★ | ★ | 5 | |||
| Yang et al. 2014[23] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Johnston-MacAnanny et al. 2010[22] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for comparability. A study that has five or more total quality scores is considered moderate quality, and a study that has eight or more total quality scores is considered high quality
Meta-analysis
CE treated with OAA vs. non-CE
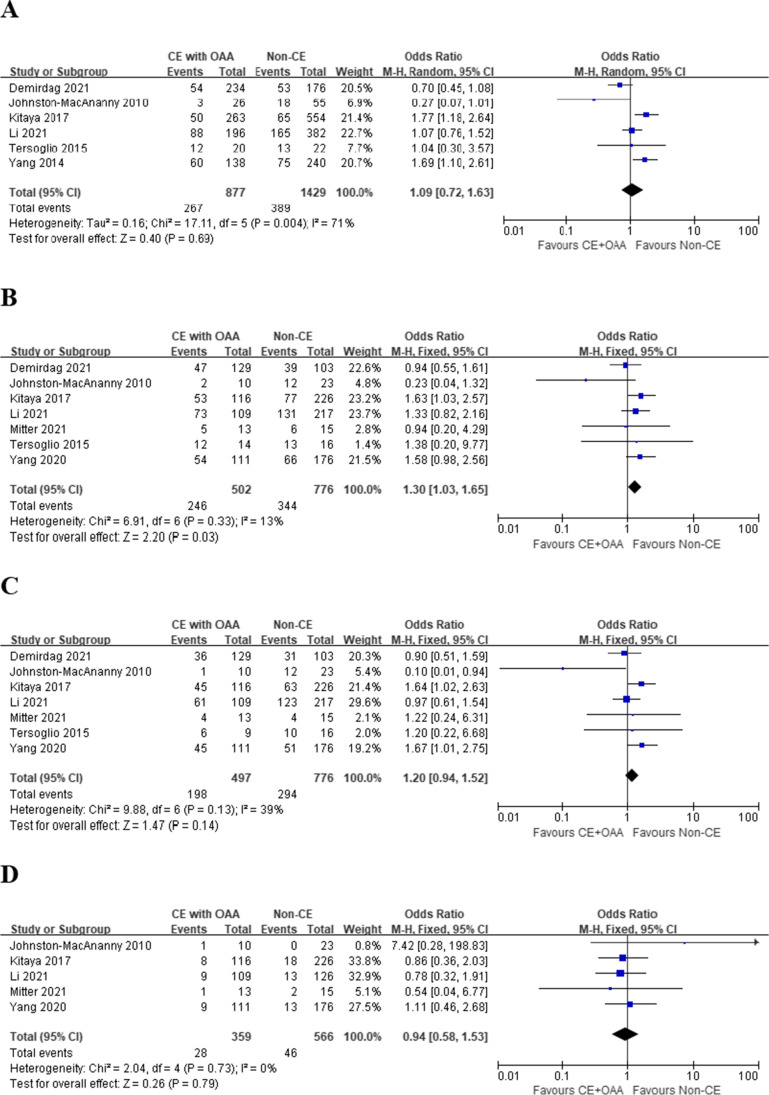
Compared with non-CE patients, no significant differences were detected in terms of IR (OR 1.09, 95% CI 0.72–1.63, I2 = 71%, P = 0.69), OPR/LBR (OR 1.20, 95% CI 0.94–1.52, I2 = 39%, P = 0.14), and MR (OR 0.94, 95% CI 0.58–1.53, I2 = 0%, P = 0.79) (Fig. 2A, C, and D) in CE patients treated with OAA. However, CE patients who received OAA showed significantly higher CPR (OR 1.30, 95% CI 1.03–1.65, I2 = 13%, P = 0.03) compared with non-CE patients (Fig. 2B).
Fig. 2.
Pregnancy outcomes in RIF patients, CE treated with OAA vs. Non-CE. A Implantation rate; B clinical pregnancy rate; C ongoing pregnancy/live birth rate; D miscarriage rate
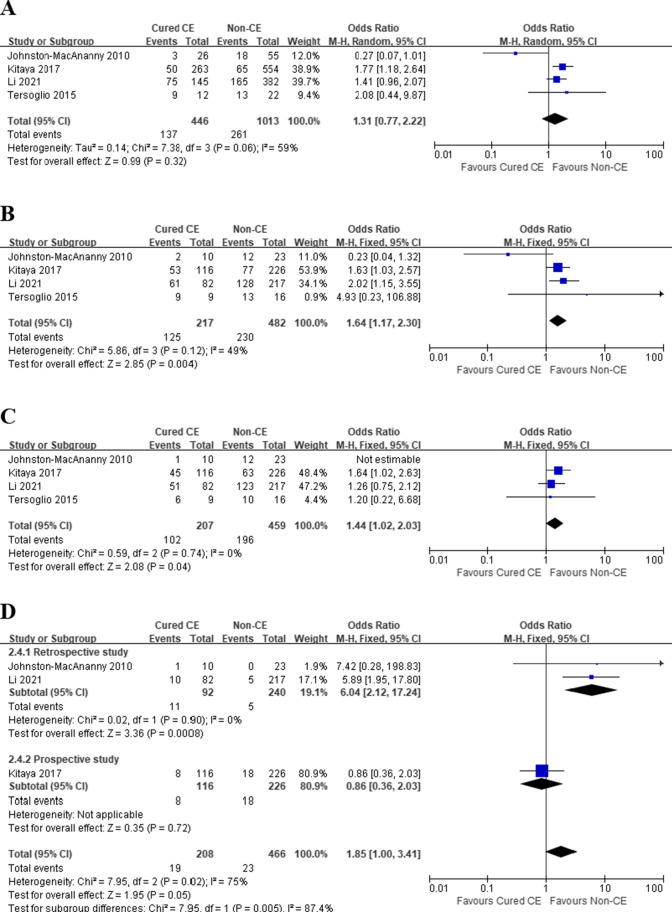
Cured CE after OAA vs. non-CE
With regard to the therapeutic effect of antibiotic treatment, patients with cured CE had significantly higher CPR (OR 1.64, 95% CI 1.17–2.30, I2 = 49%, P = 0.004) (Fig. 3B), and no statistical significance was observed in IR (OR 1.31, 95% CI 0.77–2.22, I2 = 59%, P = 0.32) and OPR/LBR (OR 1.19, 95% CI 0.65–2.17, I2 = 50%, P = 0.57) (Fig. 3A and C). However, after the exclusion of the work of Johnston-MacAnanny et al., which had the smallest sample size in pooled studies, the sensitivity analysis found a significant advantage of cured CE over non-CE patients in terms of IR (OR 1.58, 95% CI 1.20–2.08, I2 = 0%, P = 0.001) and OPR/LBR (OR 1.44, 95% CI 1.02–2.03, I2 = 0%, P = 0.04). No significant difference was observed in MR (OR 2.64, 95% CI 0.53–13.14, I2 = 75%, P = 0.24) with high heterogeneity (Fig. 3D). Subgroup analysis was performed based on their study design: retrospective study and prospective study. Analysis of retrospective study revealed that MR was significantly higher in patients with cured CE (OR 6.04, 95% CI 2.12–17.24, I2 = 0%, P = 0.0008).
Fig. 3.
Pregnancy outcomes in RIF patients, Cured CE vs. Non-CE. A Implantation rate; B clinical pregnancy rate; C ongoing pregnancy/live birth rate; D miscarriage rate
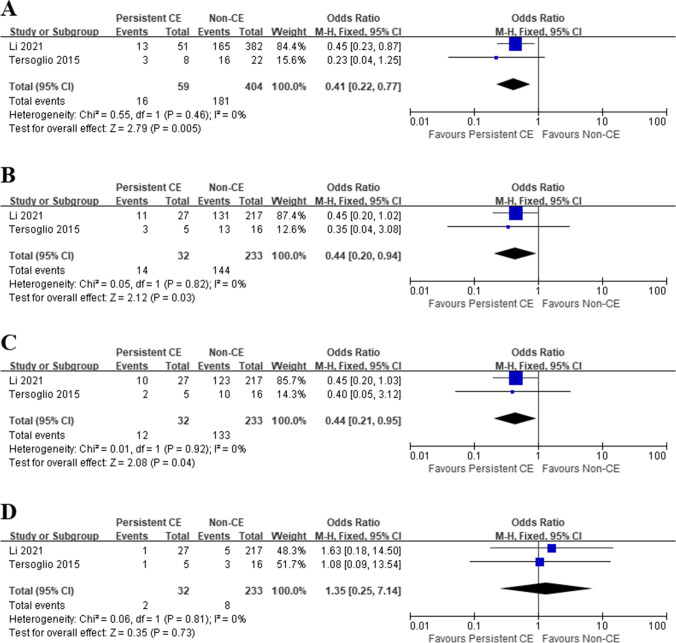
Persistent CE after OAA vs. non-CE
Conversely, patients with persistent CE had significantly lower IR (OR 0.41, 95% CI 0.22–0.77, I2 = 0%, P = 0.005), CPR (OR 0.44, 95% CI 0.20–0.94, I2 = 0%, P = 0.03), and OPR/LBR (OR 0.44, 95% CI 0.21–0.95, I2 = 0%, P = 0.04) than patients without CE (Fig. 4A−C). No significant difference was observed in comparing MR (OR 1.35, 95% CI 0.25–7.14, I2 = 0%, P = 0.73) between patients with persistent CE and non-CE patients (Fig. 4D).
Fig. 4.
Pregnancy outcomes in RIF patients, Persistent CE vs. Non-CE. A Implantation rate; B clinical pregnancy rate; C ongoing pregnancy/live birth rate; D miscarriage rate
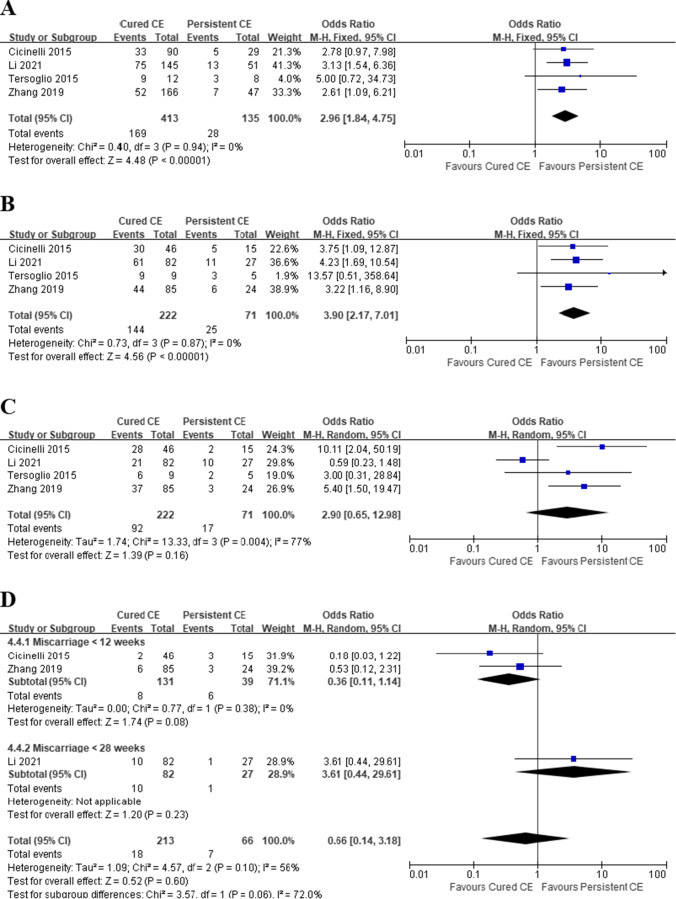
Cured CE vs. persistent CE
Four studies reported pregnancy outcomes in 71 and 222 patients with persistent and cured CE, respectively. The IR, CPR, and OPR/LBR were 20.7% (28/135), 35.2% (25/71), and 23.9% (17/71) in patients with persistent CE, and 40.9% (169/413), 64.9% (144/222), and 41.4% (92/222) in patients with cured CE, respectively. Although patients with cured CE had significantly higher IR (OR 2.96, 95% CI 1.84–4.75, I2 = 0%, P < 0.00001) and CPR (OR 3.90, 95% CI 2.17–7.01, I2 = 0%, P < 0.00001), no statistical significance was discovered in OPR/LBR between the two groups (OR 2.90, 95% CI 0.65–12.98, I2 = 77%, P = 0.16) (Fig. 5A−C). However, after the exclusion of the study by Li et al., which had the largest sample size in pooled studies, the sensitivity analysis found that cured CE had a significant advantage over persistent CE patients (OR 6.02, 95% CI 2.41–15.04, I2 = 0%, P = 0.0001). The heterogeneity decreased to 0 afterwards, indicating that the study of Li et al. might be the source of heterogeneity. There was no significant difference in MR (OR 0.66, 95% CI 0.14–3.18, I2 = 56%, P = 0.60) between patients with cured and persistent CE in three pooled studies, and considerable heterogeneity was detected (Fig. 5D). Subgroup analysis was performed based on the time when a pregnancy loss happened, and the heterogeneity decreased to 0 in studies reporting miscarriages before 12 weeks. However, we found no significant difference in MR between patients with cured and persistent CE (OR 0.36, 95% CI 0.11–1.14, I2 = 0%, P = 0.08).
Fig. 5.
Pregnancy outcomes in RIF patients, Cured CE vs. Persistent CE. A Implantation rate; B clinical pregnancy rate; C ongoing pregnancy/live birth rate; D miscarriage rate
CE receiving OAA combined with IAI vs. CE receiving OAA
Only one study compared the pregnancy outcomes between the two treatment strategies. In spite of the enhanced treatment effectiveness rate (OR 4.20, 95% CI 1.35–13.06, P = 0.01), however, no significant improvements were observed in CPR (P = 0.86), OPR/LBR (P = 0.54), and MR (P = 0.63).
OAA-treated CE vs. untreated CE
Only one study compared the pregnancy outcomes between patients with treated CE and those with untreated CE. OAA treatment did not have a significant impact on IR (P = 0.82), CPR (P = 0.66), and OPR/LBR (P = 0.70).
Discussion
RIF is a complex clinical scenario; yet, it is not perfectly defined. In 2014, Coughlan et al. [4] defined RIF as the failure to achieve a clinical pregnancy after the transfer of at least four good-quality embryos over a minimum of three fresh or frozen cycles in a woman under the age of 40. However, 76 variant definitions of RIF were still deployed in relevant studies [1]. Pirtea et al. [29] studied 4429 women who underwent up to three consecutive frozen euploid single-embryo transfers, and only less than 5% of participants failed to achieve a clinical pregnancy, which contradicted the previously reported prevalence and implied that past studies may have overestimated the existence of RIF in infertile women. The variation of diagnostic criteria in RIF has hindered the treatment of RIF and also introduced great heterogeneity in scientific research [30].
On the other hand, despite the histological detection of plasma cells remaining the golden standard for CE diagnosis, no widely accepted criteria have been determined currently [6, 31]. Johnston-MacAnanny et al. considered it CE-positive on the condition of more than one CD138 + cell in one high-power field (HPF), while Bouet et al. preferred at least five CD138 + cells/10 HPFs [3, 22]. And according to Li et al., more than five plasma cells in at least one out of 30 HPFs would be reliable to diagnose CE [32]. Persistent CE is another poorly defined situation and has only been detailedly characterized in limited literature. For example, Xiong et al. reported persistent CE as more than five CD138 + cells/HPF after two courses of OAA [33].
Although the incidence of CE was believed to be associated with poor prognosis in infertile women [6], opinions about the benefits of antibiotic therapies to reproductive outcomes varied. Based on the infectious nature of CE, adequate antibiotic therapy has proven to be effective in curing CE [34]. Different treatment regimens, including empiric, broad-spectrum, and sensitive antibiotics, were administrated orally and/or intrauterinely in the management of CE, in which OAA was the most commonly used. Recent studies also reported the application of IAI for CE and demonstrated that local delivery of antibiotics might improve the therapeutic efficacy and therefore restore endometrial receptivity; yet, few studies have been conducted to explore the impact of IAI treatment on reproductive outcomes of RIF patients. Sfakianoudis et al. reported successful treatment of persistent CE through IAI in three infertile women with previous failed IVF attempts, and all patients achieved pregnancy via natural conception afterwards [12]. Zhang et al. introduced the combination of IAI and dexamethasone to treat CE in patients with RIF, and patients with cured CE after treatment achieved significantly higher IR and CPR compared with patients with persistent CE [20]. Apart from different routes of antibiotics delivery, a longer course of antibiotic treatment was reported to increase the cure rate of CE [16]. More large-scale and well-designed studies are needed to further assess other antimicrobial options as well as course duration in terms of their efficacy in curing CE and improving reproductive prognosis.
The correlation between persistent CE and poorer reproductive outcomes was also discovered by Xiong et al. in a cohort study of 640 embryo transfer cycles [33]. Compared with patients with less than four CD138 + cells per high-power field, patients with persistent CE exhibited significantly lower IR, CPR, LBR, and cumulative LBR. Eliminating the proportion of persistent CE using adequate antimicrobial might be the key to improving reproductive outcomes in women with RIF. The etiology and pathophysiology of persistent CE warrant further investigation.
According to CDC 2021 Guidelines for sexually transmitted infections (STI) treatment, intramuscular or oral antibiotic regimens consisting of ceftriaxone, doxycycline, and metronidazole were recommended in the management of PID [35]. However, after 14 days of broad-spectrum oral antibiotic therapy as per the guideline’s suggestion, approximately 10 to 37.5% of patients still presented features of CE in repeated hysteroscopy and endometrial biopsy. And studies using different antibiotics recorded even higher rates of treatment failure after a single course of antibiotic treatment, ranging from 7.2 to 72% [14, 15]. Considering the discrepancy in pregnancy outcomes exhibited by patients with cured and persistent CE, the lack of a test of cure biopsy could be one of the confounding factors in past studies reporting opposite results.
We performed a sensitivity analysis of comparisons that show significant heterogeneity. Our study found no significant differences between IR and OPR/LBR in comparisons between cured CE and non-CE according to random-effects models, and great heterogeneity was identified. After the study of Johnston-MacAnanny et al. was removed, the OR became 1.58 (1.20–2.08), I2 = 0%, P = 0.001 and 1.44 (1.02–2.03), I2 = 0%, P = 0.04 respectively. This result might have been caused by the relatively small number of included patients. Similarly, the removal of the study by Li et al. changed the OR of OPR/LBR from insignificant to 6.02 (2.41–15.04), I2 = 0%, P = 0.0001 in the comparator of cured vs. persistent CE. The results of the study by Li et al. might be compromised by the limited representativeness of patients with persistent CE because only patients showing weak positive features of CE after antibiotic treatment were included in the comparisons, and patients showing strong positive CE were excluded. We also tried to exclude the study of Mitter et al. from the comparator OAA-treated CE vs. non-CE for its application of a stricter CE diagnosis criterion, but no significant changes were identified either in heterogeneity or OR value afterwards.
Notably, a high level of heterogeneity appeared in the statistical analysis of MR in two of the comparators. The sources of heterogeneity were mainly attributed to study number (7 out of 9 studies reported MR), study design (non-randomized study), and outcome (a pregnancy loss before different gestational weeks was defined and calculated as MR). Subgroup analyses were performed and no significant difference was found in MR between patients with cured and persistent CE. Great heterogeneity (I2 = 87.4%) was identified between subgroups of retrospective study and prospective study in comparator of cured CE vs. non-CE.
Our study has several limitations that should be taken into careful consideration before the results are interpreted. Initially, only one RCT compared the reproductive outcomes between patients using OAA and patients using OAA combined with IAI, and only non-randomized prospective studies and retrospective cohort studies were available in the quantitative synthesis. Secondly, great heterogeneity was observed in the definitions and diagnosis of RIF and CE, as well as antimicrobial regimens and IVF procedures. In addition, measurements and reports of outcomes, including CPR, LBR, and MR, were inconsistent among pooled studies. A pregnancy loss before different gestational weeks was defined and calculated as MR. None of the studies specified whether twin gestations should be counted once or twice in the calculation of CRP and LBR. These factors together contributed to the extreme heterogeneity in the meta-analysis. Clear and consensus definitions of pregnancy outcomes should be made, and the type of pregnancy/birth (singletons or multiple pregnancies) should be discussed in future research. Thirdly, although preterm birth was acknowledged as one of the main pathologies related to the IVF population and intrauterine infection, it was not included in the study outcomes of most studies and therefore unavailable for meta-analysis. Finally, instead of directly comparing the pregnancy outcomes of treated and untreated CE, most pooled studies in the meta-analysis chose non-CE patients as the control group due to ethical considerations.
In conclusion, the results of our study indicated that antibiotic therapies may improve the clinical outcomes of women with RIF in subsequent IVF cycles only if the condition of CE is confirmed cured in a repeated biopsy afterwards. Otherwise, no sufficient evidence has shown improvements in patients with persistent CE. Hence, a test of cure biopsy could be beneficial in predicting patients’ reproductive prognosis and helping clinicians make wiser decisions before subsequent IVF cycles.
Acknowledgements
We would like to thank Dr. Demirdag for kindly providing us the data.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81200453), and Chengdu Science and Technology Bureau (2019-YF09-00210-SN).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pirtea P, de Ziegler D, Ayoubi JM. Recurrent Implantation Failure—Is It the Egg or the Chicken? Life. 2021; 12(1):39. 10.3390/life12010039 [DOI] [PMC free article] [PubMed]
- 2.McQueen DB, Bernardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil Steril. 2014;101(4):1026–1030. doi: 10.1016/j.fertnstert.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Bouet PE, El Hachem H, Monceau E, Gariépy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106–110. doi: 10.1016/j.fertnstert.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28(1):14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Park HJ, Kim YS, Yoon TK, Lee WS. Chronic endometritis and infertility. Clin Exp Reprod Med. 2016;43(4):185–192. doi: 10.5653/cerm.2016.43.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura F, Takebayashi A, Ishida M, Nakamura A, Kitazawa J, Morimune A, et al. Review: Chronic endometritis and its effect on reproduction. J Obstet Gynaecol Res. 2019;45(5):951–960. doi: 10.1111/jog.13937. [DOI] [PubMed] [Google Scholar]
- 7.Moreno I, Cicinelli E, Garcia-Grau I, Gonzalez-Monfort M, Bau D, Vilella F, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218(6):602.e1–e16. doi: 10.1016/j.ajog.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang WJ, Zhang H, Chen ZQ, Zhang W, Liu XM, Fang JY, et al. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17(1):2. doi: 10.1186/s12958-018-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitaya K, Yamada H. Pathophysiological roles of chemokines in human reproduction: an overview. Am J Reprod Immunol. 2011;65(5):449–459. doi: 10.1111/j.1600-0897.2010.00928.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Chen X, Huang J, Wang CC, Yu MY, Laird S, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018;109(5):832–839. doi: 10.1016/j.fertnstert.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Zargar M, Ghafourian M, Nikbakht R, Mir Hosseini V, Moradi CP. Evaluating chronic endometritis in women with recurrent implantation failure and recurrent pregnancy loss by hysteroscopy and immunohistochemistry. J Minim Invasive Gynecol. 2020;27(1):116–121. doi: 10.1016/j.jmig.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Sfakianoudis K, Simopoulou M, Nikas Y, Rapani A, Nitsos N, Pierouli K, et al. Efficient treatment of chronic endometritis through a novel approach of intrauterine antibiotic infusion: a case series. BMC Womens Health. 2018;18(1):197. doi: 10.1186/s12905-018-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashiri A, Halper KI, Orvieto R. Recurrent Implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(1):121. doi: 10.1186/s12958-018-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song D, He Y, Wang Y, Liu Z, Xia E, Huang X, et al. Impact of antibiotic therapy on the rate of negative test results for chronic endometritis: a prospective randomized control trial. Fertil Steril. 2021;115(6):1549–1556. doi: 10.1016/j.fertnstert.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Pantos K, Simopoulou M, Maziotis E, Rapani A, Grigoriadis S, Tsioulou P, et al. Introducing intrauterine antibiotic infusion as a novel approach in effectively treating chronic endometritis and restoring reproductive dynamics: a randomized pilot study. Sci Rep. 2021;11(1):15581. doi: 10.1038/s41598-021-95072-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Liu B, He Y, Xie Y, Liang T, Bi Y, et al. Variation of diagnostic criteria in women with chronic endometritis and its effect on reproductive outcomes: a systematic review and meta-analysis. J Reprod Immunol. 2020;140:103146. doi: 10.1016/j.jri.2020.103146. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, Yamagishi Y, Hagihara M, Hirai J, Asai N, Shibata Y, et al. Systematic review and meta-analysis for impacts of oral antibiotic treatment on pregnancy outcomes in chronic endometritis patients. J Infect Chemother. 2022 doi: 10.1016/j.jiac.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Vitagliano A, Saccardi C, Noventa M, Di Spiezio SA, Saccone G, Cicinelli E, et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil Steril. 2018;110(1):103–12.e1. doi: 10.1016/j.fertnstert.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Kitaya K, Matsubayashi H, Takaya Y, Nishiyama R, Yamaguchi K, Takeuchi T, et al. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol. 2017;78:e12719. 10.1111/aji.12719 [DOI] [PubMed]
- 20.Zhang Y, Xu H, Liu Y, Zheng S, Zhao W, Wu D, et al. Confirmation of chronic endometritis in repeated implantation failure and success outcome in IVF-ET after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am J Reprod Immunol. 2019;82(5):e13177. doi: 10.1111/aji.13177. [DOI] [PubMed] [Google Scholar]
- 21.Tersoglio AE, Salatino DR, Reinchisi G, Gonzalez A, Tersoglio S, Marlia C. Repeated implantation failure in oocyte donation. What to do to improve the endometrial receptivity? JBRA Assist Reprod. 2015;19(2):44–52. doi: 10.5935/1518-0557.20150012. [DOI] [PubMed] [Google Scholar]
- 22.Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437–441. doi: 10.1016/j.fertnstert.2008.12.131. [DOI] [PubMed] [Google Scholar]
- 23.Yang R, Du X, Wang Y, Song X, Yang Y, Qiao J. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstet. 2014;289(6):1363–1369. doi: 10.1007/s00404-013-3131-2. [DOI] [PubMed] [Google Scholar]
- 24.Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323–330. doi: 10.1093/humrep/deu292. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Ma C, Tao L, Wang Y. Analysis on improvement of pregnancy outcome after treatment of chronic endometritis in patients with repeated implantation failure. Chin J Clin Obstet Gynecol. 2020;21(2):115–9. doi: 10.13390/j.issn.1672-1861.2020.02.002. [DOI] [Google Scholar]
- 26.Li J, Xiao Z, Li X, Ding J, Zhao J, Guan F, et al. Effects of antibiotic treatment on pregnancy outcomes in woman with repeated implantation failure. J Reprod Med. 2021;30(9):1152–1157. doi: 10.3969/j.issn.1004-3845.2021.09.006. [DOI] [Google Scholar]
- 27.Mitter VR, Meier S, Rau TT, Gillon T, Mueller MD, Zwahlen M, et al. Treatment following hysteroscopy and endometrial diagnostic biopsy increases the chance for live birth in women with chronic endometritis. Am J Reprod Immunol. 2021;86(5):e13482. doi: 10.1111/aji.13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demirdag E, Guler I, Cevher Akdulum MF, Sahin E, Erdem O, Erdem A, et al. Subsequent IVF outcomes following antibiotic therapy for chronic endometritis in patients with recurrent implantation failure. J Obstet Gynaecol Res. 2021;47(12):4350–4356. doi: 10.1111/jog.15037. [DOI] [PubMed] [Google Scholar]
- 29.Pirtea P, De Ziegler D, Tao X, Sun L, Zhan Y, Ayoubi JM, et al. Rate of true recurrent implantation failure is low: results of three successive frozen euploid single embryo transfers. Fertil Steril. 2021;115(1):45–53. doi: 10.1016/j.fertnstert.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Garneau AS, Young SL. Defining recurrent implantation failure: a profusion of confusion or simply an illusion? Fertil Steril. 2021;116(6):1432–1435. doi: 10.1016/j.fertnstert.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margulies SL, Dhingra I, Flores V, Hecht JL, Fadare O, Pal L, et al. The diagnostic criteria for chronic endometritis: a survey of pathologists. Int J Gynecol Pathol. 2021;40(6):556–562. doi: 10.1097/PGP.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Xu S, Yu S, Huang C, Lin S, Chen W, et al. Diagnosis of chronic endometritis: how many CD138(+) cells/HPF in endometrial stroma affect pregnancy outcome of infertile women? Am J Reprod Immunol. 2021;85(5):e13369. doi: 10.1111/aji.13369. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Y, Chen Q, Chen C, Tan J, Wang Z, Gu F, et al. Impact of oral antibiotic treatment for chronic endometritis on pregnancy outcomes in the following frozen-thawed embryo transfer cycles of infertile women: a cohort study of 640 embryo transfer cycles. Fertil Steril. 2021;116(2):413–421. doi: 10.1016/j.fertnstert.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Cicinelli E, Resta L, Loizzi V, Pinto V, Santarsiero C, Cicinelli R, et al. Antibiotic therapy versus no treatment for chronic endometritis: a case-control study. Fertil Steril. 2021;115(6):1541–1548. doi: 10.1016/j.fertnstert.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]