Abstract
In Caulobacter crescentus, stalk biosynthesis is regulated by cell cycle cues and by extracellular phosphate concentration. Phosphate-starved cells undergo dramatic stalk elongation to produce stalks as much as 30 times as long as those of cells growing in phosphate-rich medium. To identify genes involved in the control of stalk elongation, transposon mutants were isolated that exhibited a long-stalk phenotype irrespective of extracellular phosphate concentration. The disrupted genes were identified as homologues of the high-affinity phosphate transport genes pstSCAB of Escherichia coli. In E. coli, pst mutants have a constitutively expressed phosphate (Pho) regulon. To determine if stalk elongation is regulated by the Pho regulon, the Caulobacter phoB gene that encodes the transcriptional activator of the Pho regulon was cloned and mutated. While phoB was not required for stalk synthesis or for the cell cycle timing of stalk synthesis initiation, it was required for stalk elongation in response to phosphate starvation. Both pstS and phoB mutants were deficient in phosphate transport. When a phoB mutant was grown with limiting phosphate concentrations, stalks only increased in length by an average of 1.4-fold compared to the average 9-fold increase in stalk length of wild-type cells grown in the same medium. Thus, the phenotypes of phoB and pst mutants were opposite. phoB mutants were unable to elongate stalks during phosphate starvation, whereas pst mutants made long stalks in both high- and low-phosphate media. Analysis of double pst phoB mutants indicated that the long-stalk phenotype of pst mutants was dependent on phoB. In addition, analysis of a pstS-lacZ transcriptional fusion showed that pstS transcription is dependent on phoB. These results suggest that the signal transduction pathway that stimulates stalk elongation in response to phosphate starvation is mediated by the Pst proteins and the response regulator PhoB.
The gram-negative bacterium Caulobacter crescentus undergoes an asymmetric cell division to produce two morphologically and functionally distinct progeny cells (7). The swarmer cell has a polar flagellum, whereas the stalked cell has a polar stalk that is a cylindrical extension of the cell membrane and peptidoglycan layer (19, 30, 39, 41). Stalk synthesis is initiated coincident with the initiation of DNA replication during the differentiation of the swarmer cell. In addition to developmental regulation, the availability of phosphate in the environment influences stalk synthesis. When inorganic phosphate is in excess, stalk length averages 1 to 2 μm, which is approximately the length of the cell body. When Caulobacter is starved for phosphate, stalk synthesis is stimulated, resulting in stalks 30 μm or more in length (38, 40). The increase in surface/volume ratio caused by stalk elongation during phosphate starvation is thought to allow Caulobacter cells to take up phosphate more efficiently (34). The mechanisms by which Caulobacter cells regulate their response to phosphate starvation are unknown.
Caulobacter cells are typically found in aquatic ecosystems, where the most common limiting nutrient is phosphorus (29). Phosphorus is an essential element and is preferentially imported in the form of inorganic phosphate. When starved for phosphate, bacteria increase their ability to take up inorganic phosphate and to utilize organic phosphate sources (37).
The regulatory response to phosphate starvation that results in the induction of the phosphate (Pho) regulon (Fig. 1) is best understood in Escherichia coli (52). The majority of genes of this regulon are involved in the metabolism of phosphorus compounds. The Pho regulon genes induced by phosphate starvation contain a cis-regulatory sequence, the Pho box, which overlaps with the −35 regions of their promoters and is required for activation of these genes under phosphate starvation conditions (25). During phosphate starvation, PhoR, the sensor kinase of the Pho regulon, undergoes autophosphorylation. Phospho-PhoR phosphorylates PhoB, the response regulator of the Pho regulon (46, 51), increasing its affinity for the Pho box (24, 25). Phospho-PhoB activates transcription of the Pho regulon genes by binding to the Pho box and interacting with the ς70 subunit of the RNA polymerase holoenzyme (23). For example, phosphate starvation results in the induction of the Pho box-containing pstSCAB operon that encodes the high-affinity phosphate transport system, and phoA, the alkaline phosphatase gene required for the utilization of organic forms of phosphate. The PstSCAB proteins form a repression complex with PhoR when phosphate is in excess, thereby inhibiting expression of the Pho regulon (50). Null mutations in any of the high-affinity phosphate transport pst genes constitutively activate the Pho regulon (52).
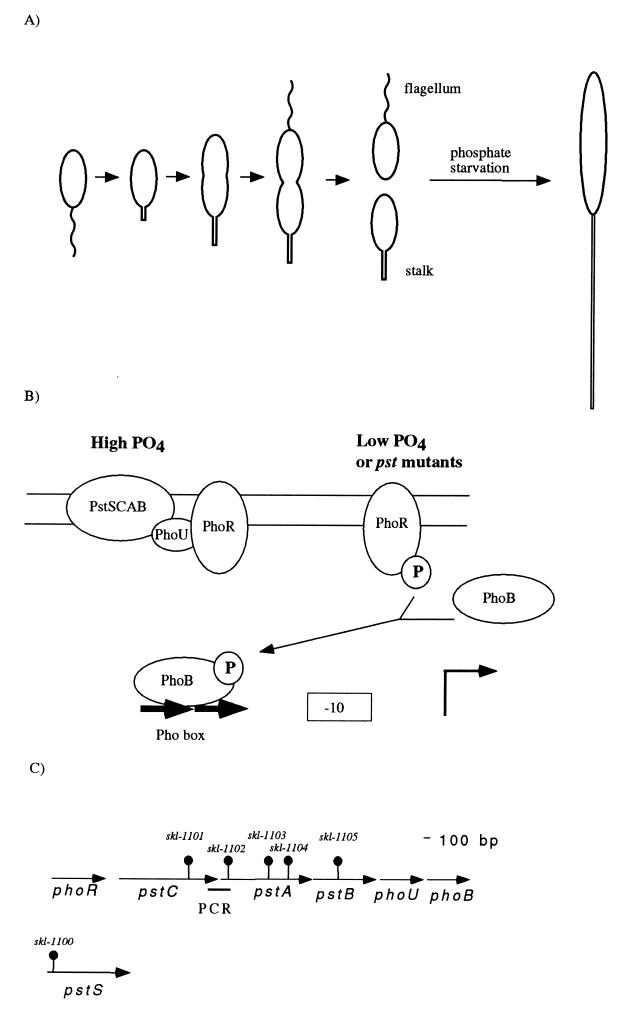
FIG. 1.
Model of the Pho regulon and organization of pst and pho genes of Caulobacter. (A) The Caulobacter life cycle and effect of phosphate starvation. The life cycle of swarmer cells is depicted. The newborn swarmer cell spends an obligatory period of its life cycle as a chemotactically competent polarly flagellated cell unable to initiate DNA replication. Stalk synthesis is initiated at the pole that previously contained the flagellum coincidently with the initiation of DNA replication during the swarmer-to-stalked cell differentiation. The new stalked cell elongates, initiates cell division, and synthesizes a flagellum at the pole opposite the stalk, giving rise to an asymmetric predivisional cell. Cell division yields a stalked cell that can immediately initiate a new cell cycle and a swarmer cell. Phosphate starvation yields elongated cells with long stalks. (B) Model of the Pho regulon. This model is adapted from work with E. coli (52). The PstSCAB proteins form the high-affinity phosphate transport system. When phosphate is in excess, the Pst complex represses the autophosphorylation of the histidine kinase PhoR. PhoU is required to inhibit the expression of the Pho regulon, but is not required for phosphate transport by the Pst system. Deletion of phoU has deleterious effects on growth, and these effects are dependent on phoB (15). When cells are starved for phosphate, the Pst complex releases PhoR, which autophosphorylates and transfers the phosphate residue to PhoB. PhoB∼P binds to the Pho box sequences of promoters (−10 and bent arrow) and activates the transcription of most genes of the Pho regulon. In a few cases, binding of PhoB-∼P represses transcription. We hypothesize that PhoB∼P activates the transcription of a gene or genes whose expression results in an increase in stalk synthesis. (C) Organization of the pst-pho gene cluster. Genes are represented by arrows, and the sites of transposon insertion in the different mutants are represented by “lollipop” structures. The thick line labeled PCR under the region between pstC and pstA indicates the PCR product that was obtained with oligonucleotides from the end of the phoR-pstC sequence contig and the beginning of the pstA-pstB-phoU-phoB sequence contig. The pstS gene is shown below the pst-pho region because it maps to an unlinked locus.
In this paper, we report the isolation of Caulobacter mutants that synthesize long stalks in the presence of excess phosphate. These mutants map to genes homologous to the high-affinity phosphate transport genes of enteric bacteria. pst mutants could synthesize long stalks even in low-phosphate medium, whereas a phoB mutant could not. The long-stalk phenotype of the pst mutants and transcription of pstS were dependent on an intact phoB gene. Furthermore, stalk elongation during phosphate starvation was dependent on phoB. These results suggest that pst mutations increase stalk synthesis by activating the Pho regulon through PhoB.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. E. coli strains were grown in Luria-Bertani medium supplemented with 100 μg of ampicillin, 50 μg of kanamycin, or 100 μg of spectinomycin per ml as necessary. C. crescentus strains were grown in peptone-yeast extract (PYE) medium (31) or Hutner base–imidazole-buffered–glucose–glutamate (HIGG) minimal medium (32) supplemented with 20 μg of nalidixic acid, 5 or 20 μg of kanamycin, 1 μg of tetracycline, or 50 μg of spectinomycin per ml as required. Cells were synchronized by the Ludox density centrifugation method (12). β-Galactosidase assays were performed as described previously (26), except that cells were permeabilized with chloroform. Results were expressed in Miller units and represent the average of four independent measurements with a standard deviation of less than 10%.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description or construction | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α F′ | φ80ΔlacZΔM15 endA1 relA1, hsdR17 (r−, m+) supE44 thi-1 λ gyrA relA1 F− Δ(lacZYA-argF)U169 | 16 |
| S17-1 | E. coli 294::RP4-2 (Tc::Mu)(Km::Tn7) | 43 |
| YB1329 | S17-1 λpir pUT::miniTn5lacZ2 | 9; Alley, unpublished results |
| C. crescentus | ||
| NA1000 | Previously called CB15N, a synchronizable derivative of CB15, does not synthesize a holdfast | 12 |
| AE1045 | NA1000 flaN::Tn5lacZ Skl | Alley, unpublished results |
| YB720 | NA1000 phoBΩ12 | This work |
| YB1630 | NA1000 pstS::miniTn5lacZ1100 | 42 |
| YB767 | NA1000 pstS::miniTn5lacZ1100, transduction of Kanr from YB1630 | This work |
| YB770 | NA1000 phoBΩ12 pstS::miniTn5lacZ1100, transduction of Kanr from YB1630 into YB720 | This work |
| YB779 | NA1000 pstC::miniTn5lacZ1101 | This work |
| YB1387 | NA1000 pstA::miniTn5lacZ1104 | This work |
| YB1389 | NA1000 pstA::miniTn5lacZ1102 | This work |
| YB778 | NA1000 pstA::miniTn5lacZ1103 | This work |
| YB783 | NA1000 phoBΩ12 pstA::miniTn5lacZ1103, transduction of Kanr from YB778 into YB720 | This work |
| YB777 | NA1000 pstB::miniTn5lacZ1105 | This work |
| YB784 | NA1000 phoBΩ12 pstB::miniTn5lacZ1105, transduction of Kanr from YB777 into YB720 | This work |
| YB1684 | NA1000 pstB::pBGST18pstB5 (pstB::np) | This work |
| YB1686 | NA1000 phoBΩ12 pstB::pBGST18pstB5 (pstB::np), integration of pBGST18pstB5 into YB720 | This work |
| Plasmids | ||
| pJM290 | 6.5-kb EcoRI fragment containing the C. crescentus pstB, phoU, and phoB genes cloned into pSKII− | This work |
| pXXphoUB19 | 1.0-kb XhoI fragment from pJM290 containing the C. crescentus phoB gene cloned into pKSII+ in the orientation of lacZ | This work |
| pXXphoUB48 | 1.0-kb XhoI fragment from pJM290 containing the C. crescentus phoB gene cloned into pKSII+ in the opposite orientation of lacZ | This work |
| pNPTS138/phoB21 | 1-kb XhoI fragment containing phoB cloned into the SalI site of plasmid pNPTS138 | This work |
| pphoBΩ12 | 2-kb Ω fragment of pHP45Ω cloned into the SmaI site of pNPTS138/phoB21 | This work |
| pGL10phoB | 1.0-kb XhoI fragment from pJM290 containing the C. crescentus phoB gene cloned into the SalI site of plasmid pGL10 | This work |
| pBGST18pstB5 | 400-bp amplified internal fragment of pstB cloned into pBGST18; used to disrupt pstB | This work |
| pGL10 | oriT Kanr | D. Helinski |
| pNPTS138 | Litmus 38-derived vector; oriT sacB Kanr | Alley, unpublished results |
| pHP45Ω | pBR322 derivative, Ampr, contains a 2-kb Ω Specr element | 35 |
| pBGST18 | A derivative of pBGS18 containing an RK2 oriT fragment | 45; Alley, unpublished results |
| pSKII+ | Phagemid, Ampr ColE1 ori f1 ori | 2 |
| pSKII− | Phagemid, Ampr ColE1 ori f1 ori | 2 |
Transposon mutagenesis and screening for long-stalk mutants.
Caulobacter sp. strain NA1000 was mutagenized by conjugation with strain YB1329, an E. coli strain carrying a miniTn5lacZ transposon-bearing plasmid (9; M. R. K. Alley, unpublished results). Two independent screens were subsequently performed to isolate transposon insertion mutants displaying a long-stalk transposon phenotype in PYE.
In the first screen, mutagenized NA1000 cells were separated by density centrifugation in a 17% (vol/vol) Ludox gradient, and the uppermost layer of the gradient was saved. Density centrifugation in a 33% (vol/vol) Ludox gradient facilitates the separation of stalked cells (top of the gradient) from swarmer cells (lower part of the gradient) (12). It is thought that the lower buoyant density of stalked cells is at least in part due to the stalk. Thus, mutants with longer stalks would likely have a lower buoyant density than wild-type-stalked cells. To confirm that cells with long stalks could be separated from wild-type cells, we compared the buoyant density of wild-type cells with that of cells of a spontaneously isolated long-stalk mutant, AE1045. When wild-type and AE1045 cells were mixed, centrifugation in a 17% (vol/vol) Ludox gradient resulted in two bands. The band at the top of the gradient was composed predominantly of long-stalked cells, whereas the band at the bottom of the gradient was composed mainly of cells with no stalk or wild-type-length stalks. Thus, the uppermost band of the mutagenized NA1000 cells would be expected to be enriched for long-stalked mutants. This fraction was washed three times in M2 salts and plated. The resulting colonies were pooled by resuspending them in PYE, and a second round of enrichment was performed after overnight growth. Colonies resulting from this second enrichment were screened for long stalks by phase-contrast microscopy, and a long-stalk mutant, YB767 (pstS1100), was identified. In the second transposon insertion mutant screen, mutagenized NA1000 cells were plated directly onto PYE plates supplemented with 20 μg of kanamycin per ml and screened for a slow-growth phenotype. The resulting colonies were screened for long stalks by phase-contrast microscopy. This screen resulted in the identification of the remainder of the long-stalked mutants described in this paper.
Transducing lysates of the mutants were prepared (11) and used to transduce the mutant phenotype into wild-type cells to confirm that no secondary mutations contributed to the mutant phenotype.
AP-PCR and DNA sequencing.
Genomic DNA isolated as previously described (18) from the long-stalked mutants was used as the template for arbitrarily primed PCR (AP-PCR) to determine the location of the transposon insertion (28). The primers for the first round of PCR were ARB1 (5′ GGC CAC GCG TCG ACT AGT ACN NNN NNN NNN GAT AT 3′), ARB6 (5′ GGC CAC GCG TCG ACT AGT ACN NNN NNN NNN ACG CC 3′), and either lacZext1 (5′ GGG TTT TCC CAG TCA CGA CGT TGT 3′) or lacZext2 (5′GAT TAA GTT GGG TAA CGC CAG GGT 3′). The second-round PCR primers were Tn5lacExt (5′ GTT CAC CAA TCA AAT TCA CGC GG 3′) or Tn5lacInt (5′ GGC GCC TGA ATG GTG TGA ATG GCA 3′) and ARB2 (5′GGC CAC GCG TCG ACT AGT AC 3′). Qiaquick purified AP-PCR products (30 ng) and the same miniTn5-specific primer that was used in the second round of AP-PCR were used for sequencing. DNA sequencing was performed on an ABI 373 automated DNA sequencer at the Institute for Molecular and Cellular Biology, Indiana University, Bloomington. Sequencing was done by using a modification of the ABI sequencing protocol that includes an initial denaturation step of 96°C for 1 min prior to cycling and an increase in the number of cycles to 35. Sequences were analyzed as described previously (18). Preliminary Caulobacter genome sequence data were obtained from The Institute for Genomic Research website at http://www.tigr.org.
Generation of gene disruptions.
phoB was disrupted by homologous recombination with a null allele. A 1-kb XhoI fragment from pJM290 containing the entire phoB gene was cloned into the SalI site of plasmid pNPTS138, generating pNPTS138/phoB21. This plasmid cannot replicate in Caulobacter and carries both a kanamycin resistance gene and the sacB gene that confers sucrose sensitivity. A 2-kb SmaI fragment of pHP45Ω containing a spectinomycin-streptomycin resistance cassette (called the Ω fragment) was cloned into pNPTS138/phoB21 to give plasmid pphoBΩ12. The SmaI digest of pNPTS138/phoB21 released a 159-bp internal fragment of phoB that was replaced by the 2-kb Ω fragment. Plasmid pphoBΩ12 was introduced into the wild-type Caulobacter strain, NA1000, by conjugation. Chromosomal integration of the plasmid by homologous recombination was identified by selection on PYE plates supplemented with 20 μg each of nalidixic acid and kanamycin per ml. Selection for the loss of the integrated plasmid by a second recombination event was performed by growing colonies overnight in PYE and plating them on PYE plates containing 3% sucrose. This second recombination event gave rise to strains containing either a wild-type copy of phoB or a disrupted copy. Strains with a disrupted copy of phoB were identified by screening sucrose-resistant colonies for resistance to streptomycin and sensitivity to kanamycin. Southern hybridization was used to confirm the presence of the Ω fragment (3).
pstB was disrupted by amplifying and cloning a 400-bp internal fragment from the gene into plasmid pBGST18, which does not replicate in Caulobacter. The oligonucleotides used for amplifying pstB were pstB5′Eco (5′ GAT CTC GAC GAA TTC GCC AAG TCG3′) and pstB3′Bam (5′GAT CGC GCG GGA TCC GAC GAG ACG3′). The resulting plasmid, pBGST18pstB5, was introduced into wild-type and phoB mutant strains by conjugation. Cells were plated on PYE supplemented with 20 μg of kanamycin per ml to select for chromosomal integrants. Integration created a duplication of the internal pstB fragment with the first copy of the gene missing the C-terminal coding region and the second copy missing the N-terminal coding region. Since the orientation of the lacZ promoter of the plasmid was the same as that of the pstB fragment in pBGST18pstB5, the transcription of the genes downstream from the insertion, phoU and phoB, was controlled by the lacZ promoter.
Microscopy.
Cells were fixed in 1.6% formaldehyde, and phase-contrast microscopy was performed with a Nikon Eclipse E800 light microscope equipped with a Princeton Instruments Cooled charge-coupled device (CCD) camera, model 1317. Images of different fields of view were captured with the CCD camera, and stalk lengths and cell bodies were measured with the Metamorph Imaging Software package, version 3.0. Automated measurements of cell bodies were accomplished by thresholding to highlight cell bodies and measuring them with the “measure object” command. Measurements of background dirt and cells touching each other were excluded from the final measurements by determining the high and low measurements for actual cells by hand. Statistical analysis was performed with GraphPad Prism, version 2.0.
Phosphate uptake assays.
Cells were grown overnight in HIGG medium containing 1 mM phosphate. Cells were chilled on ice and washed three times in ice-cold HIGG medium with no phosphate to remove phosphate. Cells were diluted to an optical density at 600 nm of 0.2 in HIGG with 1 mM phosphate or no phosphate and grown for 5 h. To remove phosphate, cells were chilled, washed, and resuspended in ice-cold HIGG medium with no phosphate. Prior to uptake assays, cells were prewarmed to room temperature. Uptake was initiated by the addition of 32Pi (ICN) to 20 μM (200 mCi/mmol). Aliquots of 50 μl were removed every 20 s for a total of 2 min, filtered through PALL Supor membranes (0.45-μm pore size) presoaked with 100 mM phosphate buffer (pH 7.0), and immediately washed with four 1-ml aliquots of distilled water. Filters were then dried, placed in scintillation liquid, and counted.
Nucleotide sequence accession number.
The pstB phoU phoB sequence has been deposited in GenBank (accession no. AF196490).
RESULTS
Isolation of long-stalk mutants.
We performed two different miniTn5lacZ mutant screens to identify genes involved in the control of stalk elongation. The first screen was based on differences in buoyant density observed between wild-type cells and cells with long stalks (see Materials and Methods). To identify long-stalk mutants, cells that exhibited a lower buoyant density than wild-type cells were enriched by density centrifugation and were plated. The resulting colonies were screened for long stalks by phase-contrast microscopy, and a long-stalk mutant (skl, for stalk length), the skl-1100 mutant, was identified. The skl-1100 mutant also exhibited very slow growth, and colonies of this mutant could be distinguished easily from wild-type colonies by colony size on PYE plates. Mutants affected in stalk synthesis, such as the rpoN (8), pleC (44, 49), mec (42), and skl (38) mutants, exhibit a slow-growth phenotype. To identify additional stalk mutants, we performed a second transposon mutant screen for slowly growing colonies. We screened 12,000 colonies, of which 591 exhibited slow growth. Out of these, 80 had a stable phenotype that included morphological defects; one mutant had short or absent stalks with a mutation that mapped to pleC (not shown), and 15 had stalks longer than those of the wild type. The six mutants that had the longest stalks were analyzed further. The kanamycin resistance encoded by the miniTn5lacZ transposon and the long-stalk phenotype were successfully transduced from five of the long-stalk mutants (skl-1101 to skl-1105 mutants) into wild-type strain NA1000 (Table 1), confirming that the transposon insertion was responsible for the phenotype.
Characterization of the long-stalk mutants.
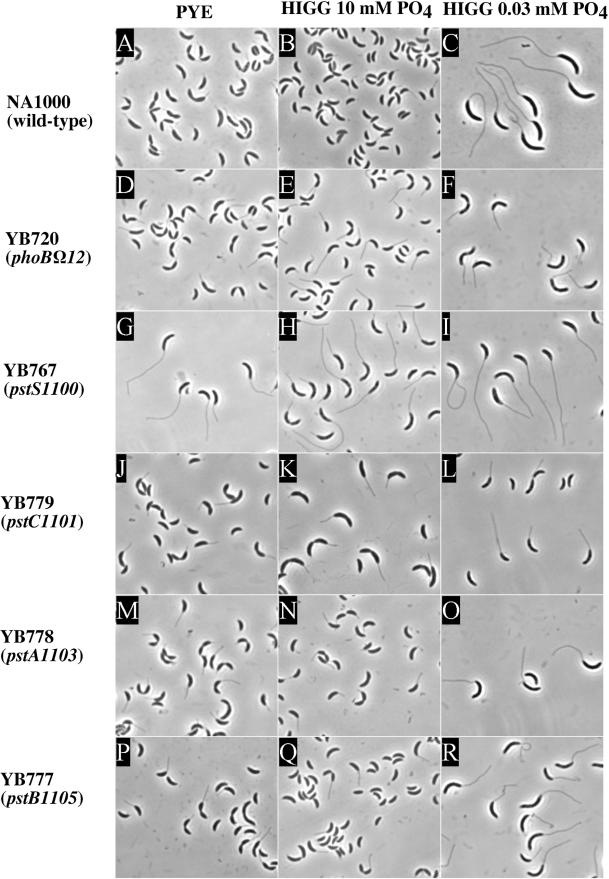
Stalk lengths were measured in fixed populations of cells after growth in PYE medium (Table 2). The relatively large variation in length was probably due to the fact that most of the cells in the population are relatively young stalked cells, since every cell division gives one stalked cell and one nonstalked cell. A one-way analysis of variance was used to determine the statistical significance of the averages. The six long-stalk mutants were divided into two classes based on their stalk length (Fig. 2). The skl-1101 through skl-1105 mutants (Fig. 2D, J, M, and P) all had stalks of approximately the same length, but the skl-1100 mutant (Fig. 2G) had much longer stalks. The skl-1100 mutant had a mean stalk length of 5.2 μm, compared to 1.6 μm for the skl-1105 mutant and 1.0 μm for wild-type cells. The skl-1101 through skl-1104 mutants had mean stalk lengths comparable to that of the skl-1105 mutant (not shown). When starved for phosphate, all of the long-stalk mutants were able to further elongate their stalks (Fig. 2), but the stalks of the skl-1100 mutant were still longer (9.0 μm [Table 2]) than those of the other mutants (5.1 μm for the skl-1105 mutant [Table 2]). The phenotypes of the skl-1101 to skl-1105 mutants were indistinguishable unless otherwise noted, and the skl-1105 mutant was therefore used as a representative of these mutants in subsequent experiments.
TABLE 2.
Average stalk lengths in different strains
| Strain | Genotypea | Stalk length (μm) withb:
|
||
|---|---|---|---|---|
| PYE | HIGG +10 mM PO4 | HIGG +0.03 mM PO4 | ||
| NA1000 | Wild type | 1.0 ± 0.3 | 1.2 ± 0.4 | 10.5 ± 2.4 |
| YB720 | phoBΩ12 | 1.5 ± 0.7 | 2.0 ± 0.8 | 2.8 ± 0.8 |
| YB1684 | pstB::np | 4.8 ± 3.3 | 4.9 ± 3.5 | 12.1 ± 6.8 |
| YB1686 | phoBΩ12 pstB::np | 1.3 ± 0.6 | 1.9 ± 0.7 | 2.8 ± 0.9 |
| YB777 | pstB1105 | 1.6 ± 0.8 | 1.9 ± 0.8 | 5.1 ± 1.6 |
| YB784 | pstB1105 phoBΩ12 | 1.8 ± 0.7 | NDc | ND |
| YB767 | pstS1100 | 5.2 ± 3.3 | 3.6 ± 2.6 | 9.0 ± 5.0 |
| YB770 | pstS110 phoBΩ12 | 1.4 ± 0.3 | 1.5 ± 0.5 | 3.3 ± 0.9 |
pstB::np is a nonpolar insertional disruption in this gene. The pstB1105 and pstS1100 alleles are miniTn5lacZ insertion mutations.
Stalks were measured for at least 80 cells for each strain. Cultures were grown to saturation. The statistical significance of the data was analyzed by one-way analysis of variance with Bonferroni's multiple comparison post test with Prism version 2.0+ from GraphPad Software, Inc. Differences between averages were judged to be extremely significant (P < 0.001), very significant (P > 0.01), and not significant (P > 0.05). All differences that were described as significant or not in the text were validated by this test.
ND, not determined.
FIG. 2.
Morphology of wild-type and stalk mutant cells in different media. Strains were grown in either PYE (A, D, G, J, M, and P), HIGG medium containing 10 mM phosphate (B,E,H,K,N, and Q), or 30 μM phosphate (C,F,I,L,O, and R) until saturation. Phase-contrast micrographs of NA1000 (wild type) (A to C), YB720 (phoBΩ12) (D to F), YB767 (pstS1100) (G to I), YB779 (pstC1101) (J to L), YB778 (pstA1103) (M to O), and YB777 (pstB1105) (P to R) cells are shown. Images of cells were captured with a digital camera and analyzed with the Metamorph Imaging Software package, version 3.0. The cells shown are representative of the population.
The morphology of the skl-1100 mutant was similar to the morphology of wild-type cells that had been starved for phosphate. When wild-type cells are grown to stationary phase with excess phosphate, they arrest at the late predivisional stage, with a deep constriction at the division site and no apparent elongation of the cell body (40) (Fig. 2A and B). When wild-type cells are starved for phosphate, they arrest as elongated stalked cells with no constrictions, and their stalks elongate dramatically (40) (Fig. 2C). Moreover, the cell body area of phosphate-starved wild-type cells increases 2.4-fold compared to that of cells grown in high-phosphate medium (Table 3). The stationary-phase cells of the skl-1100 mutant grown in PYE or in high-phosphate medium had an elongated body, and most cells had not initiated cell division (Fig. 2G and H).
TABLE 3.
Cell body area of different strains
| Strain | Growth condition | Avg cell body area (μm2)a |
|---|---|---|
| NA1000 | HIGG + 10 mM PO4 | 0.9 ± 0.2 |
| NA1000 | HIGG + 0.03 mM PO4 | 2.2 ± 0.4 |
| YB720 (phoBΩ12) | HIGG + 10 mM PO4 | 1.0 ± 0.2 |
| YB720 (phoBΩ12) | HIGG + 0.03 mM PO4 | 1.5 ± 0.4 |
At least 1,000 cells were measured in each case.
The long-stalk mutations map to high-affinity phosphate transport (pst) genes.
To identify the specific genes disrupted in each of the mutants, we obtained the DNA sequence flanking the transposon insertion by performing AP-PCR and sequencing the PCR products. Sequences were compared to preliminary C. crescentus genomic sequence data obtained from The Institute for Genomic Research (TIGR) website at http://www.tigr.org. The sequence from the TIGR database was then used to search GenBank. The six long-stalked mutants were disrupted in genes homologous to the high-affinity phosphate transport system genes (pst) of E. coli. The transposon insertions of the skl-1102, skl-1103, and skl-1104 mutants all mapped to pstA. The transposon insertions of the skl-1101, skl-1105, and skl-1100 mutants mapped to pstC, pstB, and pstS, respectively (Fig. 1). The Caulobacter sequence fragment that contained pstA and pstB overlapped with the pstB-phoU-phoB sequence we had also determined experimentally (see next section), yielding the gene order pstA-pstB-phoU-phoB. The transposon insertion in skl-1102 mapped just downstream of pstC in a sequence fragment that also contained phoR. Because pstA is directly downstream of pstC in E. coli, these genes may also be contiguous in Caulobacter. To test this possibility, PCR was performed with primers that matched the end of the pstC sequence fragment and the beginning of the sequence fragment containing pstA. An approximately 600-bp product resulted, the sequence of which confirmed the gene order phoR-pstC-pstA-pstB-phoU-phoB. The gene organization of this region is summarized in Fig. 1. Results from transduction experiments showed that pstS could not be cotransduced with phoB, indicating that pstS was not linked to the other pst genes as it is in E. coli (not shown). Thus, all long-stalk mutants with stalks approximately 1.5 μm long had mutations that mapped to the pstCAB gene cluster, whereas the one with the longest stalks, the skl-1100 mutant, had a mutation that mapped to the unlinked pstS gene.
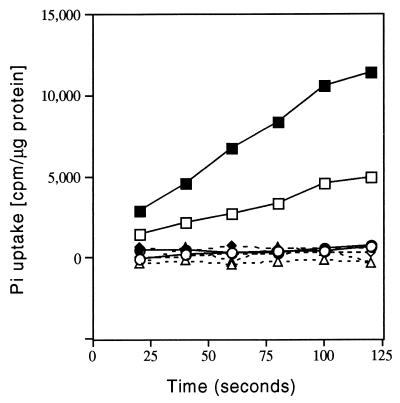
The sequence similarity of the pst genes to E. coli high-affinity phosphate transport genes suggested that they were involved in phosphate transport. To test this, we measured the rates of phosphate transport of wild-type and mutant strains grown in medium containing either 1 mM phosphate or no phosphate. Wild-type strain NA1000 had phosphate uptake rates of 8.7 nmol min−1 per mg of protein when grown with 1 mM phosphate and 21.7 nmol min−1 per mg of protein when grown with no phosphate (Fig. 3). In contrast, the pstS1100 mutant had a rate of 0.2 nmol min−1 per mg of protein under both conditions (Fig. 3), indicating that pstS is necessary for phosphate transport.
FIG. 3.
Phosphate uptake in different Caulobacter strains. 32Pi was added to a final concentration of 20 μM. Phosphate uptake was measured on cells grown in HIGG medium containing 10 mM phosphate (open symbols) or no phosphate (solid symbols) for 5 h. Results are shown for NA1000 (wild type) (squares), YB720 (phoBΩ12) (circles), YB767 (pstS1100) (triangles), and YB770 (phoBΩ12 pstS1100) (diamonds).
phoB is required for stalk elongation during phosphate starvation.
In E. coli, pst mutations result in the constitutive activation of the Pho regulon independent of phosphate levels by activating the phosphorylation of PhoB. The long-stalk Caulobacter mutants, especially the pstS1100 mutant, had a morphology similar to that of phosphate-starved wild-type cells. This raised the possibility that the pst mutants of Caulobacter caused the synthesis of long stalks by inducing the pho regulon in a phoB-dependent fashion. Furthermore, this suggested that phoB could be required for stalk elongation in response to phosphate starvation. Sequences with similarity to phoB had been located approximately 20 kb downstream of the cgtA gene (22; J. Maddock, unpublished data). Cloning and sequencing of a 2.3-kb EcoRI-XhoI fragment containing the sequence similar to that of phoB revealed the 3′ end of pstB, phoU, and phoB, respectively (Fig. 1).
To determine the role of PhoB in Caulobacter, we replaced the chromosomal copy of phoB with a phoB insertion deletion allele. Plasmid pphoBΩ12 was integrated at the phoB locus by homologous recombination, resulting in one wild-type allele and one null allele of phoB. Cells were plated on PYE-sucrose to select for either restoration of the wild-type chromosome (Sucr Spcs Kans) or the replacement of the wild-type allele of phoB with phoBΩ12 (Sucr Spcr Kans). A total of 192 Sucr colonies were screened, and 3 were Sucr Spcr Kans. These recombinants were verified by Southern hybridization to confirm the phoB gene disruption (not shown).
When examined in PYE medium, phoBΩ12 mutant cells were morphologically indistinguishable from wild-type cells, except that their stalks were slightly longer (Table 2 and Fig. 2). Growth rate experiments indicated that phoBΩ12 cells grew approximately 1.5 times slower than wild-type cells in PYE and HIGG minimal medium containing either a low (0.03 mM) or a high (10 mM) concentration of phosphate (not shown). Thus, phoB is required for optimal growth of Caulobacter under both excess and limiting phosphate conditions. Phosphate uptake measurements indicated that the phoBΩ12 mutant was deficient in phosphate uptake (Fig. 3).
We quantitated the effect of phosphate starvation on wild-type and phoBΩ12 mutant cells by growing both strains to saturation in HIGG medium containing an excess (10 mM) or a limiting amount (0.03 mM) of phosphate. The average stalk length for wild-type strain NA1000 growing with excess phosphate was 1.9 μm, with a maximum stalk length of 3.6 μm (Table 2). When NA1000 was grown with limiting phosphate, stalk length averaged 10.5 μm, with a maximum of 30 μm (Table 2). The phoBΩ12 mutant had stalks with an average length of 2.0 μm, with a maximum of 5.5 μm when grown in excess phosphate (Table 2). Unlike those of NA1000, the phoBΩ12 stalks did not undergo extensive elongation when cells were limited for phosphate (compare Fig. 2E and F); their average length was 2.8 μm, with a maximum of 7.6 μm (Table 2). Thus, phosphate starvation caused an average stalk length increase of 1.4-fold in the phoBΩ12 mutant, compared to 9-fold in wild-type cells.
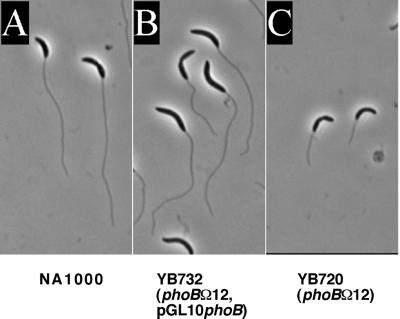
To ensure that the phenotype of the phoB insertion deletion was not due to a polar effect of the mutation, we introduced a plasmid, pGL10phoB, that contains the phoB gene under the control of the lac promoter into the phoBΩ12 mutant. This plasmid was able to rescue the ability of the phoBΩ12 mutant to elongate stalks when starved for phosphate (Fig. 4), indicating that the inability of this mutant to elongate stalks during phosphate starvation is directly due to the disruption of phoB. We conclude that phoB is required for the elongation of stalks in response to phosphate starvation.
FIG. 4.
Complementation of the phoBΩ12 mutant. Cells were grown for 48 h in HIGG medium containing 0.03 mM phosphate. (A) NA1000 (wild type). (B) YB732 (phoBΩ12/pGL10phoB). (C) YB720 (phoBΩ12). The cells shown are representative of the population.
phoBΩ12 cells grown in low phosphate were not uniformly arrested, as seen with wild-type cells. Many cells of the phoBΩ12 mutant arrested with a visible constriction after phosphate starvation, whereas only a few wild-type NA1000 cells arrested at this stage during phosphate starvation. Thus, the inhibition of cell division caused by phosphate starvation seemed to be only partially dependent on phoB. In addition, phosphate-starved phoBΩ12 cells elongated less than wild-type cells (1.5- and 2.4-fold, respectively [Table 3]). This indicates that the increase in surface area of cells during phosphate starvation is also partially dependent on phoB.
As shown in the next section, phoB is required for transcription of the high-affinity phosphate transport gene pstS. One interpretation of the inability of the phoB mutant to elongate stalks during phosphate starvation could be that phoB mutant cells cannot take up sufficient phosphate to elongate stalks when starved. Both phoB and pstS mutants are deficient in phosphate transport, yet the pstS mutant can synthesize long stalks in low-phosphate medium (Fig. 1I and Table 2). Thus, the stalk elongation defect of the phoB mutant cannot be explained by its inability to transport phosphate.
The long-stalk phenotype of the pstS mutant and the transcription of pstS are dependent on phoB.
In E. coli, the proteins of the Pst high-affinity phosphate transporter are thought to form a repression complex that maintains the PhoB kinase, PhoR, in an inactive state when phosphate is in excess (52). Mutations that disrupt the repression complex cause constitutive activation of the Pho regulon. The phenotypes of Caulobacter pst and phoB mutants support a model in which inactivation of pst genes would lead to the constitutive activation of PhoB and consequently of the Pho regulon. This model predicts that phoB would be required for the long-stalk phenotype of the pst mutants. We tested this hypothesis by examining the phenotype of a pstS1100 phoBΩ12 double mutant. Cells of the pstS1100 mutant grown in PYE had a mean stalk length of 5.2 μm (Table 2), whereas cells of a pstS1100 phoBΩ12 double mutant had a mean stalk length similar to that of the phoBΩ12 mutant grown in the same medium (1.4 and 1.5 μm, respectively). This indicates that the long-stalk phenotype of the pstS1100 mutant grown in PYE requires active PhoB. In addition, the pstS1100 phoBΩ12 double mutant grew faster than the pstSΩ1100 mutant, suggesting that the slow-growth phenotype of the pstSΩ1100 mutant was also due to the activation of the Pho regulon.
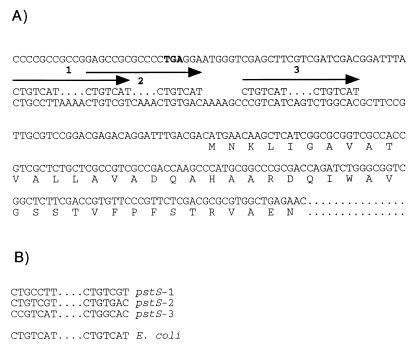
Analysis of the intergenic sequence between the pstS gene and the upstream gene revealed the presence of three putative Pho boxes, each with 10 or 11 out of 14 matches to the consensus Pho box sequence of E. coli (Fig. 5). The two upstream Pho boxes share one 7-bp repeat, and the third Pho box is located 6 bp downstream of the second. We hypothesize that PhoB binds to the Pho box of pstS and activates its transcription in a manner similar to that in E. coli. We used the pstS-lacZ transcriptional fusion created by the miniTn5lacZ transposon to determine whether transcription of pstS is dependent on phoB. In a phoB+ background, the pstS-lacZ fusion produced 2,800 Miller units of β-galactosidase activity. In the phoBΩ12 background, only 300 U were obtained. Thus, high-level pstS transcription was dependent on phoB.
FIG. 5.
Promoter region of pstS and comparison of putative Pho box sequences. (A) Sequence upstream of the pstS gene. Pho box-like sequences are indicated by numbered arrows, with the consensus E. coli Pho box sequence shown above the Caulobacter DNA sequence for comparison. The stop codon (TGA) of the upstream PBP1A gene is shown in boldface. The predicted N-terminal amino acid sequence of PstS is indicated. The sequence shown is to the site of miniTn5lacZ insertion. (B) Comparison of Pho box-like sequences found upstream of pstS and pstC with the consensus Pho box sequence of E. coli.
Effect of a nonpolar mutation in pstB.
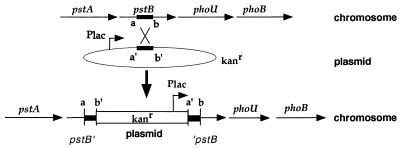
The genetic organization of the phoB region suggested that phoB could be part of an operon with the pstCAB and phoU genes. The stalks of the pstCAB mutants were not as long as the stalks of the unlinked pstS mutant. One possible explanation for the less severe phenotype of the pstCAB mutants compared to that of pstS is that the miniTn5lacZ insertions in pstCAB could prevent full expression of phoB because of a polar effect of the insertions. This possibility was tested by constructing a nonpolar pstB insertion mutant, pstB::np (YB1684), that still allowed the transcription of phoU and phoB (Fig. 6).
FIG. 6.
Construction of a nonpolar disruption in pstB. An internal fragment of the pstB gene (a and b) was cloned into pBGST18 (a′ and b′) in the same orientation as the lacZ promoter of the plasmid (Plac). Integration of the plasmid by a single crossover generates two truncated copies of pstB. pstB′ is missing C-terminal coding sequences, and ′pstB is missing N-terminal coding sequences. The Plac promoter of the plasmid ensures transcription of the downstream genes.
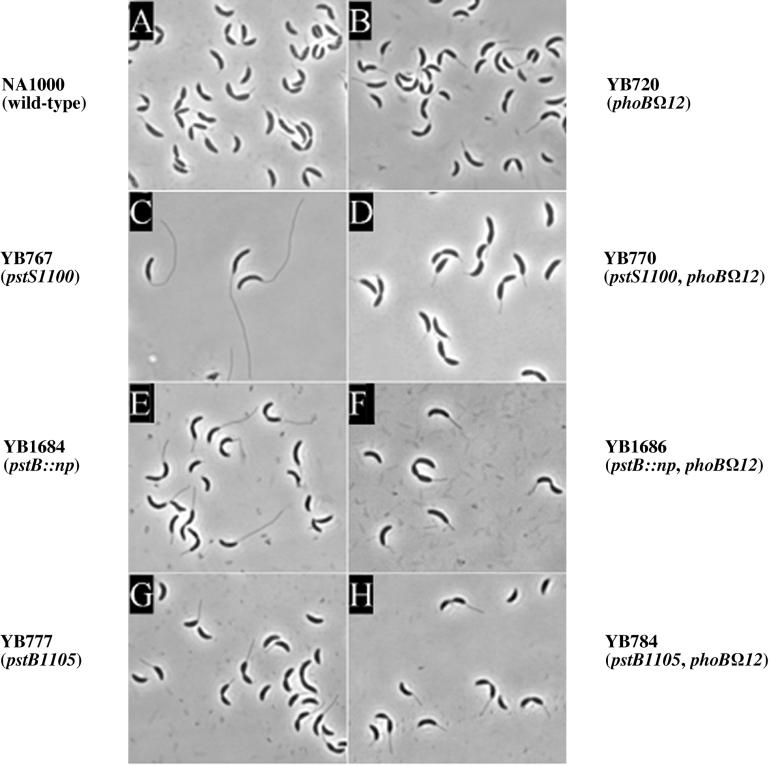
When grown in PYE, cells with the nonpolar mutation in pstB had stalks with an average length of 4.8 μm (Fig. 7 and Table 2), compared to 5.2 μm for the pstS mutant. The pstB1105 mutant had stalks with an average length of 1.6 μm (Fig. 7 and Table 2). When the pstB::np mutation was combined with the phoBΩ12 mutation (YB1686), cells had a stalk length similar to that of a pstS1100 phoB mutant (1.3 and 1.4 μm, respectively) (Fig. 7, Table 2). This was comparable to the length of pstB1105 phoBΩ12 stalks (1.8 μm) (Table 2). These data indicate that the phenotype of the miniTn5lacZ pstCAB mutants was attenuated due to polar effects on the transcription of phoB, preventing full stalk elongation.
FIG. 7.
Comparison of polar and nonpolar disruptions in pstB and a polar disruption in the unlinked pstS gene in a wild-type background and a phoB mutant background. Phase-contrast micrographs of representative cells of NA1000 (wild type) (A), YB720 (phoBΩ12) (B), YB767 (pstS1100) (C), YB770 (pstS1100 phoBΩ12) (D), YB1684 (pstB::np) (E), YB1686 (pstB::np phoBΩ12) (F), YB777 (pstB1105) (G), and YB784 (pstB1105 phoBΩ12) (H) are shown. Strains in the left panels have the phoB+ allele (A, C, E, and G), and strains in the right panels (B, D, F, and H) have the phoBΩ12 allele.
The timing of stalk synthesis is not dependent on phoB.
To determine if phoB is involved in the timing of stalk synthesis, we compared the synthesis of stalks in synchronized cultures of NA1000 and of the phoBΩ12 mutant. Swarmer cells grown in minimal-glucose medium were isolated by density centrifugation, and aliquots of cells were fixed in formaldehyde at 15-min intervals over a period of 3 h for subsequent microscopic examination. In both strains, stalks became visible after 45 min. We measured stalk lengths at different stages of the cell cycle. Because the growth rates of the two strains differ, we used the initiation of cell division and the completion of cell division as morphological landmarks to define similar stages of the cell cycle. For NA1000, initiation of cell division occurred at 75 min and the mean stalk length of cells was 0.6 μm. The phoBΩ12 mutant initiated cell division at 120 min, and cells had a mean stalk length of 0.7 μm. Cell division was completed by NA1000 cells at 165 min, and the mean stalk length was 0.7 μm, whereas the phoBΩ12 mutant completed division at 210 min, with a mean stalk length of 0.9 μm. Thus, both the proper timing of stalk synthesis and stalk elongation occur independently of PhoB.
DISCUSSION
Caulobacter cells exhibit a remarkable response to phosphate starvation. Stalks, usually similar in length to the cell body (∼1 μm), attain lengths of 20 to 30 μm when cells are starved for phosphate (40). The increase in stalk length results in an increased surface/volume ratio that is thought to enhance nutrient uptake (34). Here we report the isolation and characterization of mutants that have increased stalk elongation in phosphate-rich medium (Skl). skl mutations map to homologues of pst genes (pstSCAB) that encode the high-affinity phosphate transport proteins of E. coli and other bacteria. The Skl mutants that permit expression of phoB in Caulobacter are phenotypically similar to previously isolated but genetically uncharacterized long-stalk mutants (38). We show that the phoB gene, which encodes the transcriptional activator of the Pho regulon, is required for stalk elongation during phosphate starvation. PhoB was required for the long-stalk phenotype of Skl mutants and for the transcription of the pstS gene.
E. coli PhoB binds to Pho boxes located in the promoters of Pho regulon genes, activating or repressing the transcription of these genes (47). In E. coli, pst mutations induce the Pho regulon because PhoR is no longer repressed and phosphorylates PhoB (Fig. 1). pst mutations also induce the Pho regulon in Pseudomonas aeruginosa (20, 27). Our data suggest that the Caulobacter pst mutations induce the Pho regulon as well. The morphology of pst mutant cells is similar to the morphology of phosphate-starved wild-type cells: stalks are long, the cell body is elongated, and stationary-phase cells arrest before the initiation of cell division. The phenotype of pst mutants and the morphological changes caused by phosphate starvation are dependent on phoB.
The requirement of PhoB for stalk elongation during phosphate starvation does not result from a phosphate deficiency preventing the synthesis of stalk biomass. Since PhoB is required for the expression of PstS, phoB mutants might not have been able to take up sufficient phosphate to synthesize stalk biomass because of a deficiency in the Pst high-affinity phosphate transport system. This is not the case, because pstS and pstB mutants can make long stalks in low-phosphate medium. Thus, an active Pst system is not required for stalk synthesis. The precise role of PhoB in stalk elongation remains to be determined. Stalk synthesis involves the synthesis of both cell wall and membrane components and is likely to require the action of many genes. None of the genes directly involved in the synthesis of the stalk has been identified. Thus, PhoB could act directly by binding to the promoters of stalk genes and by stimulating their transcription, or it could indirectly regulate those genes by regulating other regulatory genes. The presence of a PhoB-controlled Pho regulon in Caulobacter was initially suggested by experiments which showed that the expression of oprP from P. aeruginosa, a phosphate-regulated gene whose promoter contains a Pho box, was induced by phosphate starvation in Caulobacter (48). Examination of the promoter regions of the Caulobacter pst genes suggests that Caulobacter PhoB also exerts its regulatory effect by binding to Pho boxes. The promoter region of pstS contains three sequences homologous to the E. coli Pho box consensus sequence, and phoB is required for pstS transcription. Pho box-like sequences are also present upstream of pstC. The Pho box sequences in both promoters have 10 or 11 out of 14 conserved residues of the E. coli Pho box consensus. Pho box-like sequences are found in the promoter region of phoB-regulated genes in another alpha-purple bacterium, Rhizobium meliloti (4, 5). The identification and characterization of Pho box-containing genes in the Caulobacter genomic sequence should help determine the role of PhoB in stalk elongation and aid in the identification of genes in the stalk elongation regulatory pathway.
The analysis of synchronized populations indicated that phoB is not required for the timing of stalk synthesis initiation. Thus, phoB is the first known regulator of stalk synthesis whose only developmental function seems to be the control of stalk length. Mutations in other known regulators of stalk synthesis cause defects in developmental events other than stalk synthesis. Mutants with mutation of the rpoN gene that encodes the ς54 subunit of RNA polymerase lack flagella and stalks. Mutants with mutation of the pleC histidine protein kinase gene are resistant to phage ΦCbK, have inactive flagella, and lack stalks and pili (10, 14). Mutants of the putative response regulator gene pleD are motile throughout the cell cycle and fail to elongate stalks properly (1, 17). The global response regulator gene ctrA is required for stalk synthesis, cell division, and the regulation of flagellum synthesis (36).
Our results indicate that while phoB is required for stalk elongation during phosphate starvation, stalk synthesis is also slightly stimulated in a phoB mutant grown with excess phosphate. This suggests that PhoB negatively regulates stalk synthesis when phosphate is in excess. This hypothesis is supported by the observation that even though rpoN mutants do not synthesize stalks when phosphate is in excess, a double rpoN phoB mutant synthesizes short stalks under the same conditions (M.G., unpublished results). How can PhoB have both positive and negative regulatory effects on stalk synthesis? One possibility is that the stimulation of stalk synthesis in a phoB mutant grown in a high-phosphate medium is indirectly caused by the inability of the mutant cells to transport phosphate efficiently. Alternatively, phospho-PhoB could directly activate transcription of a stalk synthesis gene or genes during phosphate starvation, and unphosphorylated PhoB could repress their transcription when phosphate is in excess. There is precedence for a negative regulatory role for PhoB. In E. coli, the synthesis of at least 19 proteins is repressed by phosphate starvation, and at least three of the genes encoding these proteins contain Pho boxes in their promoter (47). In R. meliloti, phoB genetically acts as a repressor of the pit gene, which encodes a low-affinity phosphate transport protein (6). It is not known whether Caulobacter has a low-affinity phosphate transport system analogous to the Pit system of E. coli. In B. subtilis, the phosphate starvation response regulator PhoP is required for the repression of the teichoic acid synthesis tagAB and tagDEF operons (21).
Recent studies have demonstrated that the rate of phosphate uptake (per cell, unit dry weight, or protein) and the specific activity of alkaline phosphatase increased at higher carbon/phosphorus ratios (13). The rate of phosphate uptake was higher in stalked cells than in swarmer cells when calculated per cell or per unit of protein, but the rates were similar between the two cell types when calculated as activity per cell surface area, including the surface area of the stalk (13). Stalk elongation may have additional roles in the environment. The reduced buoyant density of stalked cells may help keep them at the air-water interface, an obvious advantage for an obligate aerobe (33). In addition, stalked cells are often found attached to surfaces in aquatic environments, where phosphorus is the most common limiting nutrient. Increased stalk elongation under these conditions would allow cells to extend away from the surface, thus benefiting from more nutrient flow and avoiding the competition with other bacteria in a nascent biofilm (33). Thus, the morphogenetic response of Caulobacter to phosphate starvation may have many advantages for this bacterium. Our results indicate that the Pho regulon is of critical importance for this morphological response. The challenge will be to determine how PhoB-regulated genes are involved in stimulating stalk elongation.
ACKNOWLEDGMENTS
We thank Barry Wanner for helpful discussions, Susan Sullivan and members of the Brun laboratory for comments on the manuscript, Ben Gold for developing the buoyant density enrichment procedure, and Marta Lipinski for help with the original density screen. Preliminary sequence data were obtained from The Institute for Genomic Research (TIGR) website at http://www.tigr.org. Sequencing of the Caulobacter genome by TIGR was accomplished with support from the U.S. Department of Energy.
This work was supported by Undergraduate Research Fellowships from the American Society for Microbiology, an Indiana University RUGS Fellowship, and a McClung Fellowship to M.G.; an Indiana University RUGS Fellowship to D.O.; and National Institutes of Health grant GM51986 to Y.V.B.
REFERENCES
- 1.Aldridge P, Jenal U. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol Microbiol. 1999;32:379–392. doi: 10.1046/j.1365-2958.1999.01358.x. [DOI] [PubMed] [Google Scholar]
- 2.Altin-Mees M A, Short J M. pBluescript II: gene mapping vectors. Nucleic Acids Res. 1989;17:9494. doi: 10.1093/nar/17.22.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley/Greene; 1989. [Google Scholar]
- 4.Bardin S, Dan S, Osteras M, Finan T M. A phosphate transport system is required for symbiotic nitrogen fixation by Rhizobium meliloti. J Bacteriol. 1996;178:4540–4547. doi: 10.1128/jb.178.15.4540-4547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardin S D, Finan T M. Regulation of phosphate assimilation in Rhizobium (Sinorhizobium) meliloti. Genetics. 1998;148:1689–1700. doi: 10.1093/genetics/148.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardin S D, Voegele R T, Finan T M. Phosphate assimilation in Rhizobium (Sinorhizobium) meliloti: identification of a pit-like gene. J Bacteriol. 1998;180:4219–4226. doi: 10.1128/jb.180.16.4219-4226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun Y, Marczynski G, Shapiro L. The expression of asymmetry during cell differentiation. Annu Rev Biochem. 1994;63:419–450. doi: 10.1146/annurev.bi.63.070194.002223. [DOI] [PubMed] [Google Scholar]
- 8.Brun Y V, Shapiro L. A temporally controlled sigma factor is required for cell-cycle dependent polar morphogenesis in Caulobacter. Genes Dev. 1992;6:2395–2408. doi: 10.1101/gad.6.12a.2395. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo V, Hererro M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely B, Croft R H, Gerardot C J. Genetic mapping of genes required for motility in Caulobacter crescentus. Genetics. 1984;108:523–532. doi: 10.1093/genetics/108.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ely B, Johnson R C. Generalized transduction in Caulobacter crescentus. Genetics. 1977;87:391–399. doi: 10.1093/genetics/87.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felzenberg E R, Yang G A, Hagenzieker J G, Poindexter J S. Physiologic, morphologic and behavioral responses of perpetual cultures of Caulobacter crescentus to carbon, nitrogen, and phosphorus limitations. J Ind Microbiol. 1996;17:235–252. [Google Scholar]
- 14.Fukuda A, Asada M, Koyasu S, Yoshida H, Yaginuma K, Okada Y. Regulation of polar morphogenesis in Caulobacter crescentus. J Bacteriol. 1981;145:559–572. doi: 10.1128/jb.145.1.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldimann A, Daniels L L, Wanner B L. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J Bacteriol. 1998;180:1277–1286. doi: 10.1128/jb.180.5.1277-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D. Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning: a practical approach. Oxford, United Kingdom: IRL Press; 1985. p. 109. [Google Scholar]
- 17.Hecht G B, Newton A. Identification of a novel response regulator required for the swarmer-to-stalked-cell transition in Caulobacter crescentus. J Bacteriol. 1995;177:6223–6229. doi: 10.1128/jb.177.21.6223-6229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janakiraman R S, Brun Y V. Transcriptional and mutational analyses of the rpoN operon in Caulobacter crescentus. J Bacteriol. 1997;179:5138–5147. doi: 10.1128/jb.179.16.5138-5147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones H C, Schmidt J M. Ultrastructural study of crossbands occurring in the stalks of Caulobacter crescentus. J Bacteriol. 1973;116:466–470. doi: 10.1128/jb.116.1.466-470.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato J, Sakai Y, Nikata T, Ohtake H. Cloning and characterization of a Pseudomonas aeruginosa gene involved in the negative regulation of phosphate taxis. J Bacteriol. 1994;176:5874–5877. doi: 10.1128/jb.176.18.5874-5877.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Eder S, Hulett F M. Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP∼P. J Bacteriol. 1998;180:753–758. doi: 10.1128/jb.180.3.753-758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddock J, Bhatt A, Koch M, Skidmore J. Identification of an essential Caulobacter crescentus gene encoding a member of the Obg family of GTP-binding proteins. J Bacteriol. 1997;179:6426–6431. doi: 10.1128/jb.179.20.6426-6431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino K, Amemura M, Kim S-K, Nataka A, Shinagawa H. Role of the ς70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev. 1993;7:149–160. doi: 10.1101/gad.7.1.149. [DOI] [PubMed] [Google Scholar]
- 24.Makino K, Shinagawa H, Amemura M, Kawamoto T, Yamada M, Nataka A. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol. 1989;210:551–559. doi: 10.1016/0022-2836(89)90131-9. [DOI] [PubMed] [Google Scholar]
- 25.Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, Ishihama A. Regulation of the phosphate regulon of Escherichia coli: activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988;203:85–95. doi: 10.1016/0022-2836(88)90093-9. [DOI] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 27.Nikata T, Sakai Y, Shibata K, Kato J, Kuroda A, Ohtake H. Molecular analysis of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol Gen Genet. 1996;250:692–698. doi: 10.1007/BF02172980. [DOI] [PubMed] [Google Scholar]
- 28.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 29.Paerl H W. Factors limiting productivity of freshwater ecosystems. In: Marshal K C, editor. Advances in microbial ecology. New York, N.Y: Plenum Press; 1982. pp. 75–110. [Google Scholar]
- 30.Pate J L, Ordal E J. The fine structure of two unusual stalked bacteria. J Cell Biol. 1965;27:133–150. doi: 10.1083/jcb.27.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poindexter J S. Biological properties and classification of the Caulobacter group. Bacteriol Rev. 1964;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poindexter J S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978;135:1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poindexter J S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981;45:123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poindexter J S. Role of prostheca development in oligotrophic aquatic bacteria. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C.: ASM Press; 1984. pp. 33–40. [Google Scholar]
- 35.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 36.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 37.Rao N N, Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990;4:1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt J M. Stalk elongation in mutants of Caulobacter crescentus. J Gen Microbiol. 1968;53:291–298. [Google Scholar]
- 39.Schmidt J M. Effect of lysozyme on crossbands in stalks of Caulobacter crescentus. Arch Mikrobiol. 1973;89:33–40. [Google Scholar]
- 40.Schmidt J M, Stanier R Y. The development of cellular stalks in bacteria. J Cell Biol. 1966;28:423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt J M, Swafford J R. Ultrastructure of crossbands in prosthecae of Asticcacaulis species. J Bacteriol. 1975;124:1601–1603. doi: 10.1128/jb.124.3.1601-1603.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seitz L C, Brun Y V. Genetic analysis of mecillinam-resistant mutants of Caulobacter crescentus deficient in stalk biosynthesis. J Bacteriol. 1998;180:5235–5239. doi: 10.1128/jb.180.19.5235-5239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon R, Prieffer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 44.Sommer J M, Newton A. Pseudoreversion analysis indicates a direct role of cell division genes in polar morphogenesis and differentiation in Caulobacter crescentus. Genetics. 1991;129:623–630. doi: 10.1093/genetics/129.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spratt B G, Hedge P J, te Heesen S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 46.Steed P M, Wanner B L. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol. 1993;175:6797–6809. doi: 10.1128/jb.175.21.6797-6809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Bogelen R A, Olson E R, Wanner B L, Neidhardt F C. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J Biochem. 1996;178:4344–4366. doi: 10.1128/jb.178.15.4344-4366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker S G, Hancock R E W, Smit J. Expression in Caulobacter crescentus of the phosphate-starvation-inducible porin OprP of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1991;77:217–222. doi: 10.1016/0378-1097(91)90555-o. [DOI] [PubMed] [Google Scholar]
- 49.Wang S P, Sharma P L, Schoenlein P V, Ely B. A histidine protein kinase is involved in polar organelle development in Caulobacter crescentus. Proc Natl Acad Sci USA. 1993;90:630–634. doi: 10.1073/pnas.90.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wanner B L. Phosphorus assimilation and its control of gene expression in Escherichia coli. In: Hauska G, Thauer R, editors. The molecular basis of bacterial metabolism. Heidelberg, Germany: Springer-Verlag; 1990. pp. 152–163. [Google Scholar]
- 51.Wanner B L. Gene regulation by phosphate in enteric bacteria. J Biol Chem. 1993;51:47–54. doi: 10.1002/jcb.240510110. [DOI] [PubMed] [Google Scholar]
- 52.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1357–1381. [Google Scholar]