Sir,
More than 16,000 cases of monkeypox have been reported globally in 2022, predominately in non-endemic countries [1]. Although transmission in the current outbreak is typically via prolonged direct contact with confirmed cases, infection-competent monkeypox virus (MPXV) has been recovered from contaminated environments multiple days after last occupancy [2], raising the potential for fomite transmission. In addition, prolonged close contact, such as working in an open-plan office, could result in respiratory droplet transmission of MPXV [3,4].
In May 2022, an individual working in a non-clinical role in an administrative office within a hospital acquired MPXV infection following non-occupational exposure. The individual worked in a 15-desk open-plan office for 1 working day following onset of a mild, influenza-like illness, and took steps to reduce mixing and avoid close contact with others. Several coronavirus disease 2019 (COVID-2019) control measures were still implemented within this office, including a requirement to wear medical masks and perform hand hygiene regularly. In addition, this office had permanent desk partitions between desk spaces. The individual reported that skin lesions appeared 2 days after taking sickness absence, at which point the office was closed to all staff pending a risk assessment and risk management plan. Seventeen staff contacts were identified, including six Category 2 contacts and four Category 1 contacts, according to UK Health Security Agency (UKHSA) categorization [5]; four individuals accepted post-exposure prophylaxis with Imvanex vaccine when offered in accordance with UKHSA guidelines. No contacts developed symptoms consistent with monkeypox during their 21-day monitoring period.
A decision to clean and decontaminate the office was made given its location within a healthcare facility, and due to the environmental stability of orthopox viruses. This was performed by professional decontamination staff following a protocol used during previous monkeypox outbreaks [6]. The hospital performed a final decontamination of the office using hydrogen peroxide vapour (Bioquell BQ-50 with 35% hydrogen peroxide solution).
Prior to decontamination, environmental sampling was performed to identify MPXV contamination. Sampling occurred 4 days after the case was last in the office and 2 days after office closure. Surface samples were collected from non-porous surfaces, such as desks and telephones, using Copan UTM swabs, and from porous surfaces, such as carpets and chair seats, using the Sartorius MD8 Airport with gelatine filters. In addition, SKC wearable samplers were utilized during the sample collection process to measure any re-aerosolization of MPXV. All samples were processed as described previously [7], and analysed for the presence of MPXV DNA using quantitative reverse transcription polymerase chain reaction as reported previously [2,8].
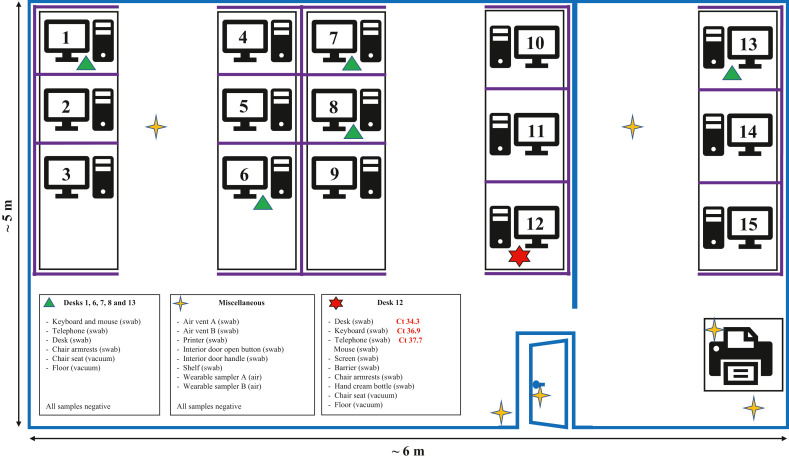
Only three of 34 surface samples were positive for the presence of MPXV DNA, with all positive samples exceeding cycle threshold (Ct) values, indicating low-level contamination (Figure 1 ). All three positive samples were from the case's desk area, including the telephone (Ct 37.7), keyboard (Ct 36.9) and a 10 × 10 cm area of the desk (Ct 34.3). Five other surface samples from the case's desk were negative for MPXV DNA, as were 26 surface samples collected from other desks and high-touch areas throughout the office. All non-porous samples were negative for MPXV DNA, as were both wearable samples.
Figure 1.
Diagrammatic representation of the office environment associated with a confirmed case of monkeypox. Blue lines represent permanent office structures, such as walls and office door; purple lines represent desk partitions (wooden partitions approximately 1.2 m high enclosing desks). Ct, cycle threshold value of monkeypox virus DNA detected in sample.
Virus isolation was attempted on the positive desk sample (Ct 34.3) using a previously described method [7]; no evidence of replicating virus or cytopathic effect was observed after 10 days of monitoring, suggesting the absence of infection-competent virus. As sampling was performed 4 days after occupancy by the infected individual, it is possible that some level of DNA or viral degradation occurred prior to sampling, although the office was windowless (minimizing ultraviolet light degradation), was not cleaned prior to sampling, and MPXV is known to be environmentally stable.
It is notable that the patient reported that skin lesions only emerged after they had taken leave from work due to illness, raising the possibility that the MPXV DNA detected may have come from respiratory secretions through droplets or contaminated hands. If so, it is possible that their use of a medical mask may have reduced environmental contamination by respiratory droplets containing virus.
Although this office may be similar to other offices in design, the findings should be seen as context-specific, including that the individual worked only during the early ‘prodromal’ phase of their monkeypox illness, several COVID-19 measures were still in place, and physical partitions were present between desk spaces. The limited detection of MPXV DNA and absence of secondary cases do not demonstrate that cleaning is unnecessary in an office where an infected person has worked, or that focused cleaning of an infected person's desk area is sufficient. In the absence of real-time environmental sampling to inform decontamination, and the fact that the office was within a hospital, the detection of environmental MPXV DNA supports the decision made to remediate the entire office. These data confirm that MPXV contamination can occur in workplace environments occupied by a person with early monkeypox illness and, accordingly, appropriate cleaning and decontamination measures should be considered in such situations.
Acknowledgements
The authors wish to acknowledge Ambipar Response Ltd for providing information on their decontamination process.
Author contributions
Conceptualization and methodology: BA, SG, TF, AMB and JD.
Investigation: BA, SG, T-CB and JD.
Formal analysis: BA, AS, OO, JF, JG and SS.
Writing – original draft: BA, SG, TF, AMB and JD.
Writing – review and editing: All authors.
Disclosure statement
This report contains work supported by UKHSA Grant-in-Aid. The contents of this paper, including any opinions and/or conclusions expressed, are those of the authors alone and do not necessarily reflect UKHSA policy.
Conflict of interest statement
None declared.
Funding sources
This work was funded by UKHSA Grant in Aid and the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. JD is supported by the MoH Foundation.
Ethical approval
The investigations performed were a component of the urgent public health investigation performed as part of UKHSA's public health incident response to cases of a high consequence infectious disease in the UK. UKHSA is the national health security agency for England and an executive agency of the UK Government's Department of Health and Social Care. The study protocol was subject to internal review by the Research Ethics and Governance Group, which is the UKHSA Research Ethics Committee, and was granted full approval.
References
- 1.World Health Organization . WHO; Geneva: 2022. Multi-country outbreak of monkeypox.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1079779/Monkeypox_Guidance__cleaning_decontamination.pdf External situation report #2, 25 July 2022. Available at: [last accessed August 2022] [Google Scholar]
- 2.Atkinson B., Burton C., Pottage T., Thompson K.-A., Ngabo D., Crook A., et al. Infection-competent monkeypox virus contamination identified in domestic settings following an imported case of monkeypox into the UK. Environ Microbiol. 2022 doi: 10.1111/1462-2920.16129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ježek Z., Grab B., Szczeniowski M.V., Paluku K.M., Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- 4.Hutson C.L., Carroll D.S., Gallardo-Romero N., Weiss S., Clemmons C., Hughes C.M., et al. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS One. 2011;6:e28295. doi: 10.1371/journal.pone.0028295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Health Security Agency. Monkeypox: contact tracing. UKHSA; n.d. Available at: https://www.gov.uk/government/publications/monkeypox-contact-tracing [last accessed July 2022].
- 6.Public Health England . PHE; London: 2018. Monkeypox: guidance for environmental cleaning and decontamination – Version 4.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/746086/Monkeypox_Guidance__cleaning_decontamination.pdf Available at: [Google Scholar]
- 7.Gould S., Atkinson B., Onianwa O., Spencer A., Furneaux J., Grieves J., et al. Air and surface sampling for monkeypox virus in UK hospitals. MedRxiv. 2022;2022 doi: 10.1101/2022.07.21.22277864. 07.21.22277864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]