Abstract
Background and aims
Hepatitis E virus infection in patients with underlying chronic liver disease is associated with liver decompensation and increased lethality. The seroprevalence of hepatitis E virus in patients with chronic hepatitis C in Brazil is unknown. This study aims to estimate the seroprevalence of hepatitis E virus in patients with chronic hepatitis C and to describe associated risk factors.
Methods
A total of 618 patients chronically infected with hepatitis C virus from three reference centers of São Paulo, Brazil were included. Presence of anti-HEV IgG was assessed by enzyme-linked immunosorbent assay (WANTAI HEV-IgG ELISA).
Results
Out of the 618 patients tested, 10.2% turned out positive for anti-HEV IgG (95% CI 8.0–12.8%). Higher seroprevalence was found independently associated with age over 60 years (OR = 2.04; p = 0.02) and previous contact with pigs (OR = 1.99; p = 0.03).
Conclusions
Patients with chronic hepatitis C are under risk of hepatitis E virus superinfection in São Paulo. Contact with pigs is a risk factor for the infection, suggesting a possible zoonosis with oral transmission.
Keywords: Hepatitis E virus, Hepatitis C virus, Brazil, Seroprevalence
Introduction
Hepatitis E virus (HEV) is the most common hepatotropic virus in the world.1, 2, 3 The World Health Organization estimates the occurrence of 20 million infections per year worldwide, with around 3.3 million symptomatic infections, and 44,000 deaths in 2015.4
HEV includes four main genotypes that infect humans with different epidemiological characteristics. Genotypes 1 and 2, described only in humans, is the etiologic agent of large fecal-oral transmission epidemics, typical of overpopulated regions and with poor sanitary conditions. Genotype 1 has been described in Asia and Africa, and genotype 2 in Mexico and Africa. Genotype 3, described in Europe, the Americas and Japan, and genotype 4, described in Asia, infect a large variety of mammals and occur in the form of sporadic cases. Ingestion of undercooked pork meat is implicated in the transmission of such zoonosis. HEV can also be transmitted alternatively by parenteral route, such as transfusion of blood products.2
Brazil exhibits an intermediate HEV seroprevalence, with rates of 2–9.8% reported in blood donors,5, 6 12% in intravenous drug users,7 and 15% in renal transplant patients.8 Previous study of HEV seroprevalence among pigs in Brazil detected seropositivity in 97.3% of the animals older than 25 weeks of age.9 Genotype 3 was identified in humans and pigs in this country.8, 10, 11
It is estimated that 1.6–3.2 million people are chronically infected with hepatitis C virus (HCV) in Brazil.12, 13 A previous study found high morbidity and mortality rates of HEV infection in patients with underlying liver disease.14
HEV seroprevalence in patients with chronic hepatitis C virus infection is unknown in Brazil. The objective of this study was to estimate the prevalence of anti-HEV antibodies in chronic HCV infected patients in the State of São Paulo, as well as to evaluate associated risk factors.
Material and methods
Study design
A cross-sectional study of seroprevalence was conducted at the hepatitis outpatient services of Universidade Federal de São Paulo, Hospital de Transplante Euryclides Jesus Zerbini, and Instituto de Vacinação e Infectologia de Piracicaba, in the State of São Paulo, Brazil.
Between October 2015 and December 2016 patients of both sexes, older than 18 years, and with chronic HCV infection confirmed by RT-PCR RNA were included.
Sample size calculation
Assuming an expected prevalence of HEV infection of 8%, a confidence level of 99%, and a total width of confidence interval (CI) around the expected proportion of 6%, a total of 576 HCV infected patients would be necessary.
Anti-HEV antibodies detection
The presence of anti-HEV IgG was assessed by enzyme-linked immunosorbent assay (WANTAI HEV-IgG ELISA), strictly according to the manufacturer's recommendations. Patients positive for HEV IgG were further tested for the presence of HEV IgM antibodies using a specific kit from the same manufacturer.
Data collection
The following variables possibly associated with HEV infection were assessed: sex, age, socioeconomic class, HIV co-infection, previous history of contact with pigs (have ever lived in a place where pigs were raised), consumption of pork meat (never, seldom, often), previous use of intravenous drugs, prior surgery, transfusion of blood products, presence of tattoos, and hemodialysis.
For the characterization of the socioeconomic class the validated questionnaire of economic classification developed by the Brazilian Association of Research Companies (ABEP), version 201515 was used.
Statistical analysis
Seroprevalence of IgG antibodies is presented with the relevant 95% confidence interval, calculated according to the Wilson method.
Student's t-test was used to compare means. Proportions were compared by the Chi-square test with Yates correction, or by the Fisher's exact test, when appropriate.
Associations with significance level with p < 0.20 in univariate analyses were subsequently included in multiple logistic regression models. Statistically significant independent association was considered if p < 0.05 after multivariate analysis.
All the analyses and charts were drawn using program R, version 3.3.2 (The R Foundation for Statistical Computing).
Ethical aspects
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the Institutional Ethics Review Board of the Federal University of São Paulo (0350/2015). A written informed consent was obtained from each patient included.
Results
A total of 618 patients with chronic HCV infection was included. Table 1 summarizes the distribution of the variables evaluated. Fifty-three percent of the sample were males, mean age of 53.8 years, minimum 22 and maximum of 86 years. History of previous contact with pigs was reported by 30.4% of the assessed patients. Twenty-six cases of HIV infection (4.2%), five hemophiliacs, three pregnant women, and two transplanted patients were included.
Table 1.
Demographic and clinical characteristics of the HCV infected patients.
| Variable | Distribution n (%) *Median [p25–p75] |
N (%) |
|---|---|---|
| Sex | 618 (100) | |
| Male | 326 (52.8) | |
| Female | 292 (47.2) | |
| Age | *54 [46–62] | 618 (100) |
| 22–29 years | 7 (1.1) | |
| 30–39 years | 70 (11.3) | |
| 40–49 years | 130 (21.1) | |
| 50–59 years | 199 (32.3) | |
| 60–69 years | 160 (25.9) | |
| 70–86 years | 51 (8.3) | |
| Socioeconomic class | 449 (72.7) | |
| A | 22 (4.9) | |
| B | 155 (34.5) | |
| C | 249 (55.5) | |
| D–E | 23 (5.1) | |
| Parenteral exposure | 485 (78.5) | |
| Intravenous drug | 94 (19.4) | |
| Tattoo | 109 (22.5) | |
| Transfusion of blood products | 188 (38.9) | |
| Previous surgery | 352 (72.6) | |
| Hemodialysis | 10 (2.1) | |
| Pork meat consumption | 475 (76.9) | |
| Never | 53 (11.2) | |
| Rarely | 207 (43.6) | |
| Often | 215 (45.3) | |
| Contact with pigs | 144 (30.4) | 474 (76.7) |
| HAV IgG+ | 352 (91.7) | 385 (62.3) |
| Toxoplasmosis IgG+ | 95 (70.4) | 135 (21.8) |
| HIV infection | 26 (4.2) | 618 (100) |
A total of 63 cases were anti-HEV IgG reactive, determining seroprevalence of 10.2% (95% CI 8.0–12.8%). Three cases (0.5%) had an indeterminate result and the information was considered as negative for hypothesis testing. Anti-HEV IgM was screened in all reactive and indeterminate cases for IgG and all of them turned out non-reactive.
Table 2 summarizes the results of univariate analyses for the dependent variable anti-HEV IgG and the assessed variables. Only the variables age and history of home contact with pigs were significantly associated with anti-HEV IgG. Hemophilia exhibited a level of borderline significance (Fisher's exact test p = 0.09).
Table 2.
HEV seroprevalence according to the independent variables assessed among HCV infected patients.
| Variable | IgG+ n (%) |
OR | 95% CI OR | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 37 (11.4) | 1.30 | [0.77–2.21] | 0.39 |
| Female | 26 (9.0) | |||
| Age (years) | ||||
| ≥60 | 30 (14.4) | 1.88 | [1.11–3.19] | 0.002 |
| <60 | 33 (8.1) | |||
| Social class | NS | NS | 0.57 | |
| A | 2 (9.1) | |||
| B | 12 (7.7) | |||
| C | 22 (8.9) | |||
| D-E | 2 (8.7) | |||
| Exposure to pigs | 0.04 | |||
| Contact | 20 (14.0) | 1.99 | [1.06–3.72] | |
| No contact | 25 (7.6) | |||
| Pork meat consumption | NS | NS | 0.88 | |
| Never | 5 (9.4) | |||
| Rarely | 22 (10.2) | |||
| Often | 28 (8.8) | |||
| Intravenous drug | 0.34 | |||
| Yes | 12 (12.9) | 1.50 | [0.74–3.02] | |
| No | 35 (9.0) | |||
| Tattoo | 0.13 | |||
| Yes | 6 (5.6) | 0.48 | [0.19–1.16] | |
| No | 41 (11.0) | |||
| Transfusion of blood products | 1.00 | |||
| Yes | 18 (9.6) | 0.99 | [0.52–1.79] | |
| No | 29 (9.9) | |||
| Previous surgery | 0.84 | |||
| Yes | 33 (9.4) | 0.88 | [0.46–1.71] | |
| No | 14 (10.5) | |||
| Hemodialysis | 1.00 | |||
| Yes | 1 (10.0) | 1.02 | [0.13–8.28] | |
| No | 46 (9.8) | |||
| HIV | 1.00 | |||
| Yes | 3 (11.5) | 1.15 | [0.33–3.94] | |
| No | 60 (10.2) | |||
| Hemophilia | ||||
| Yes | 2 (40.0) | 5.99 | [0.98–36.5] | 0.09 |
| No | 61 (10.0) | |||
Variables with significant association: age and contact with pigs. Hemophilia exhibited level of borderline significance. NS, not significant.
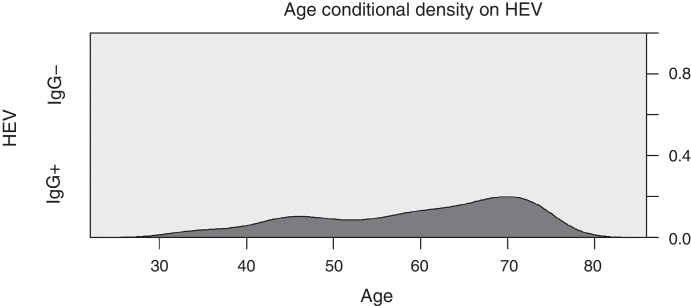
Fig. 1 illustrates the conditional density curve of anti-HEV IgG associated with age, showing a seemingly linear increase from 30 to 70 years, with prevalence ranging from zero up to 20% between these two points. The mean age of patients with positive and negative anti-HEV IgG was 57.98 and 53.36 years (Student's t-test with p < 0.001) and medians 59 and 54 years, respectively. Seroprevalence above 60 years (15.2%) was significantly higher than in those aged less than 60 years of age (8.4%) [OR: 1.96; p = 0.014]. The prevalence of reactive anti-HEV IgG in cases who had contact with pigs was 14.58% and in those with no contact history it was 7.58% (OR: 2.08; p = 0.028). The mean age of the cases with a history of contact with pigs (56.5 years) was significantly higher than the mean age of individuals without a history of contact with pigs (52.81 years) [Student's t-test with p < 0.001].
Fig. 1.
HEV seroprevalence associated with age. Light gray = non-reactive IgG; dark gray = reactive IgG; axis y = proportion of cases.
Variables with a level of significance lower than 0.20 in the univariate analysis were included in the multiple logistic regression model. Hemophilia was excluded from the multivariate analysis due to presence in only five cases and, consequently, a large confidence interval. Age was included in the model as a dichotomous variable, with cutoff point at 60 years as indicated by ROC curve analysis. Table 3 shows the final multivariate model with adjusted ORs with 95% CI and their relevant levels of significance. Age and previous contact with pigs were remained independently associated with reactive anti-HEV IgG.
Table 3.
Multiple logistic regression final model for the dependent variable anti-HEV IgG.
| Variable | Coefficienta | OR | IC 95% OR | p-Value |
|---|---|---|---|---|
| Age ≥ 60a | 0.71 | 2.04 | 1.09–3.79 | 0.0237 |
| Contact with pigs | 0.68 | 1.99 | 1.06–3.71 | 0.0298 |
Coefficient value in logit function. Excluding 144 cases of incomplete data.
Discussion
The present study demonstrated 10.2% (95% CI 8.0–12.8%) HEV seroprevalence in patients with chronic HCV infection. Older age and prior contact with pigs were independently associated with reactive anti-HEV IgG.
This study is the first evaluation of HEV seroprevalence in patients with chronic HCV infection in Brazil. It is important to note that the sample studied is not necessarily representative of the HCV-infected population in the country. Only three centers in the State of São Paulo participated in the study and inclusion started in October 2015, a period concomitant with the release of new drugs for the treatment of HCV in Brazil. Thus, the participating centers began to experience a predominant flow of patients with indication of HCV treatment, the majority with advanced liver fibrosis (equivalent to Metavir score F3 or F4). In the sample assessed, 45% of the patients had liver cirrhosis.
In 618 HEVs serologies only three cases with undetermined results were detected, showing a high discriminatory power of the test used. No reactive IgM case was detected thus indicating no recent HEV infection. The HEV seroprevalence (10.2%) in the sample studied was similar to that observed in a recent study in 500 blood donors from the city of São Paulo (9.8%) using the same serologic kit.5 High seroprevalence was also described in certain specific groups, such as professional sex workers (17.7%, N = 214),16 intravenous drug users (11.8%, N = 102),7 cases of acute hepatitis (17.7%, N = 79),16 renal transplant (15.0%, N = 192),8 and HIV infected patients (10.7%, N = 354).17
Temporal, geographical and methodological differences limit comparison between the different surveys. Previous studies have demonstrated significant difference in sensitivity between the different serological kits.18, 19, 20, 21 Therefore, it is possible that previous studies have underestimated HEV seroprevalence in this country. It is important to emphasize that, to date, no serological test for HEV has been released by the Brazilian Food and Drug Administration (ANVISA) for use in Brazil. Therefore, since there is no test available for routine use, the infection is not diagnosed in our country. Existing information was produced using imported kits authorized only for use in clinical research, and pointed at more common HEV infection than clinically suspected. To date, only anecdotal cases of active HEV infection have been reported in Brazil, all of them caused by genotype 3.8, 10, 11 Taking into account the current study and others previously conducted in the country, it may be reckoned that HEV is the second most common hepatotropic virus in Brazil, coming only after hepatitis A virus (HAV). More comprehensive and representative seroprevalence studies are required to confirm this hypothesis.
Few studies have directly evaluated HEV seroprevalence in patients with HCV. A case-control study conducted in Italy, published in 1994, compared samples taken from 100 anti-HCV reactive individuals with 100 non-reactive anti-HCV. Anti-HEV was reactive in 27% and 2%, respectively (p = 0.00012).22 However, the age difference between the groups and the association between age and HEV seroprevalence were also significant (median age 60 versus 42 years). Patients older than 50 years presented anti-HEV seroprevalence of 24.5%, compared to 2.2% of cases under 50 years of age (OR: 14.3; p = 0.0002). The methodology used in this study, where cases and controls were not matched for age, casts doubt about the validity of the higher prevalence of anti-HEV in patients with reactive anti-HCV, with age being an important confounding factor.
A study conducted in Turkey evaluated the prevalence of anti-HEV in patients with chronic hepatitis B and C and controls. The control group, without HBV or HCV infection and without known liver disease, presented reactive anti-HEV IgG in 15.7% of the cases (28/178). The HBV group did not present a statistically significant difference, whereas the HCV group presented a rate of 54% (94/174). Such a high prevalence of HEV found among HCV reactive individuals suggests a common, probably parenteral, route of transmission.23
Our study shows higher seroprevalence in hemoplilic patients (40% versus 10%; p = 0.09). Because of the limited number of cases with this condition and the consequent large 95% CI, hemophilia was not included in the multivariate model. Taking into account that this is a group of patients with a history of multiple transfusions of blood components, characterizing a high risk of acquisition of blood-borne infections, it can be hypothesized that the infection of these patients occurred by blood transfusion. Similarly, studies conducted in Japan24 and in Tunisia25 identified significantly higher seroprevalence of HEV in hemophiliac patients (7.5% and 16.3%) when compared to blood donors (4.5% and 3.7%), respectively.
A recent study evaluated 500 blood bank samples from São Paulo. No HEV RNA was detected in any sample, but only samples positive for anti-HEV IgG were submitted for RNA detection.5 On the other hand, a study carried out in England with 225,000 blood bank samples detected 79 cases of HEV genotype 3 viremia (1:2848). Follow-up of 43 patients who had received viremic blood identified 18 cases of HEV infection (infection rate = 42%).26 The high seroprevalence (15%) of HEV found in renal transplant patients in São Paulo corroborates the hypothesis of a parenteral transmission route.8
Rivero-Juarez et al. when assessing 894 HIV-infected patients, 461 HIV/HCV co-infected patients and 399 mono-infected HIV patients, found no difference in HEV seroprevalence between HCV infected (9.7%) and HCV non-infected (10%) patients. This study did not evaluate risk variables of parenteral exposure.27 It is possible that both reactive and non-reactive HCV patients had a similar parenteral risk, since in more than 60% of the patients enrolled, all of them HIV infected, there was no risk of sexual exposure. Therefore, it can be hypothesized that similar parenteral exposure between reactive and non-reactive HCV patients would make it impossible to detect the difference in HEV seroprevalence between the two groups.
Passos-Castilho et al. found an HEV seroprevalence of 9.8% (95% CI 7.5–12.7%) using the Wantai kit in 500 blood donors from the city of São Paulo. The prevalence of anti-HEV IgG was significantly increased in older age groups.5 Similarly, our study has demonstrated a significant and independent association between HEV seroprevalence and age increase. Actually, no reactive anti-HEV IgG case was detected in patients under 30 years of age. However, a reactive result was observed in 14.2% of patients over 60 years of age. The difference may be explained as follows: antibodies of the IgG class persist for an undetermined time, making seroprevalence a cumulative phenomenon as the population grows older; some other unassessed risk factor may be associated with both age and HEV infection; and the possibility that in the past, the incidence of HEV infection was higher. Studies carried out in developed countries, with a predominance of genotype 3 HEV, have also described a progressive increase in seroprevalence with aging, particularly in the age group above 50 years.2 Thus, it is possible that most of the patients included in the present study, with a presumed HCV infection for more than two decades, became infected by HEV at a later time.
An important association of contact with pigs rearing history was also detected (adjusted OR = 1.99; 95% CI 1.06–3.70). Studies performed in other countries evidenced pig infection by genotype 3 HEV with high genetic similarity with the virus detected in humans.28, 29 Previous study of HEV seroprevalence among pigs in Brazil detected seropositivity in 63.6% of the animals tested (N = 357). In addition, 97.3% of the pigs older than 25 weeks of age were HEV IgG reactive, demonstrating early infection and the possibility of active infection during the age prior to slaughter for consumption.9 HEV RNA was recovered from 118 liver samples (1.7%) and bile (0.84%) from healthy pigs of a pig farm in the state of Paraná.30 Another study carried out in the state of Rio de Janeiro detected HEV RNA in 11 out of 115 (9.6%) swine bile samples and in three out of six (50%) samples obtained in the drainage pipes of the evaluated breeding sites.31 In both studies, genotype 3 HEV was detected as being the underlying etiologic agent.
Although this study detected a strong association of reactive HEV IgG with a history of contact with pigs, it did not detect any association with pork meat consumption. A few reasons may explain this lack of correlation: insufficient power to detect the difference, given the low proportion of cases who reported not consuming pork; possibility of an alternative route of HEV infection, such as inter-human fecal-oral, direct contact with the animals or with their waste; and the semi-quantitative characteristics of the food questionnaire, with inaccurate questions and answers which may have not reflected the actual respondents’ consumption. Previous studies identified HEV genotype 3 in untreated sewage,32 river waters,33 and bivalve molluscs,34 corroborating the hypothesis that HEV infection can be transmitted by contaminated water and that the consumption of infected meat perhaps is not the main form of transmission of this zoonosis.
Previous studies have shown a high risk of severe HAV and HBV superinfection in patients with chronic HCV or liver disease.35, 36 Thus, the indication of immunization against HAV and HBV in such situations is a consensus, when the serological profile is susceptible.37 Similarly, high lethality of HEV genotype 1 infected patients was demonstrated in cirrhotic patients (70% at 12 months).14 However, the routine serological investigation of HEV is not recommended by the current management guidelines for chronic HCV infection. The seroprevalence of HEV found in this and other recent studies conducted in Brazil support the investigation of the serological profile of HEV in patients with chronic hepatitis C and prophylactic measures against HEV infection.
The generalizability of the results of this study is limited as it has included only three centers in the State of São Paulo. In addition, the majority of study subjects had advanced liver fibrosis (equivalent to Metavir score F3 or F4) and therefore a tendency to be older. Therefore, an overrepresentation of older patients might have overestimated the prevalence of HEV infection among HCV infected patients.
Authors’ contribution
Guilherme Bricks: Main Author; study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis; fund raising. Jorge Figueiredo Senise: Acquisition of data; critical review of the manuscript for intellectual content. Henrique Pott Junior, Giuliano Grandi, Dimas Carnaúba Junior and Hamilton Antonio Bonilha de Moraes: Data Acquisition. Amanda Passarini and Débora Bellini Caldeira: Laboratory support. Celso Francisco Hernandes Granato: Laboratory support, critical review of the manuscript for intellectual content. Adauto Castelo Junior: Study supervision, concept and design; acquisition of data; analysis and interpretation of data; statistical analysis; fund raising; critical review of the manuscript for intellectual content.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by the National Council for the Improvement of Higher Education (CAPES).
References
- 1.Hoofnagle J.H., Nelson K.E., Purcell R.H. Hepatitis E. N Engl J Med. 2012;367:1237–1244. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 2.Kamar N., Bendall R., Legrand-Abravanel F., et al. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 3.Purcell R.H., Emerson S.U. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . 2017. Global Hepatitis Report 2017. [Google Scholar]
- 5.Passos-Castilho A.M., Reinaldo M.R., de Sena A., Granato C.F.H. High prevalence of hepatitis E virus antibodies in Sao Paulo, Southeastern Brazil: analysis of a group of blood donors representative of the general population. Braz J Infect Dis. 2017 doi: 10.1016/j.bjid.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echevarría J.M., González J.E., Lewis-Ximenez L.L., et al. Hepatitis E virus infection in Latin America: a review. J Med Virol. 2013;85:1037–1045. doi: 10.1002/jmv.23526. [DOI] [PubMed] [Google Scholar]
- 7.Trinta K.S., Liberto M.I., de Paula V.S., Yoshida C.F., Gaspar A.M. Hepatitis E virus infection in selected Brazilian populations. Mem Inst Oswaldo Cruz. 2001;96:25–29. doi: 10.1590/s0074-02762001000100004. http://www.ncbi.nlm.nih.gov/pubmed/11285473 [DOI] [PubMed] [Google Scholar]
- 8.Hering T., Passos A.M., Perez R.M., et al. Past and current hepatitis E virus infection in renal transplant patients. J Med Virol. 2014;86:948–953. doi: 10.1002/jmv.23915. [DOI] [PubMed] [Google Scholar]
- 9.Vitral C.L., Pinto M.A., Lewis-Ximenez L.L., Khudyakov Y.E., dos Santos D.R., Gaspar A.M.C. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem Inst Oswaldo Cruz. 2005;100:117–122. doi: 10.1590/s0074-02762005000200003. S0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- 10.Passos A.M., Heringer T.P., Medina-Pestana J.O., Ferraz M.L.G., Granato C.F.H. First report and molecular characterization of hepatitis E virus infection in renal transplant recipients in Brazil. J Med Virol. 2013;85:615–619. doi: 10.1002/jmv.23494. [DOI] [PubMed] [Google Scholar]
- 11.Passos-Castilho A.M., Porta G., Miura I.K., et al. Chronic hepatitis E virus infection in a pediatric female liver transplant recipient. J Clin Microbiol. 2014;52:4425–4427. doi: 10.1128/JCM.02286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amaku M., Burattini M.N., Coutinho F.A.B., et al. Estimating the size of the HCV infection prevalence: a modeling approach using the incidence of cases reported to an official notification system. Bull Math Biol. 2016;78:970–990. doi: 10.1007/s11538-016-0170-4. [DOI] [PubMed] [Google Scholar]
- 13.Petruzziello A., Marigliano S., Loquercio G., Cozzolino A., Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar Acharya S., Kumar Sharma P., Singh R., et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387–394. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 15.ABEP . 2015. Critério de Classificação Econômica Brasil 2015. Associação Brasileira de Empresas de Pesquisa.http://www.abep.org/criterio-brasil [accessed 9.07.17] [Google Scholar]
- 16.Gonçales N.S., Pinho J.R., Moreira R.C., et al. Hepatitis E virus immunoglobulin G antibodies in different populations in Campinas, Brazil. Clin Diagn Lab Immunol. 2000;7:813–816. doi: 10.1128/cdli.7.5.813-816.2000. http://www.ncbi.nlm.nih.gov/pubmed/10973460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira A.C., Gomes-Gouvêa M.S., Lisboa-Neto G., et al. Serological and molecular markers of hepatitis E virus infection in HIV-infected patients in Brazil. Arch Virol. 2017 doi: 10.1007/s00705-017-3562-3. [DOI] [PubMed] [Google Scholar]
- 18.Drobeniuc J., Meng J., Reuter G., et al. Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: pangenotypic evaluation of performances. Clin Infect Dis. 2010;51:e24–e27. doi: 10.1086/654801. [DOI] [PubMed] [Google Scholar]
- 19.Abravanel F., Chapuy-Regaud S., Lhomme S., et al. Performance of anti-HEV assays for diagnosing acute hepatitis E in immunocompromised patients. J Clin Virol. 2013;58:624–628. doi: 10.1016/j.jcv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel J.J., Preiss J., Schemmerer M., Huber B., Jilg W. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis. 2013;207:497–500. doi: 10.1093/infdis/jis688. [DOI] [PubMed] [Google Scholar]
- 21.Bendall R., Ellis V., Ijaz S., Ali R., Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 22.Pisanti F.A., Coppola A., Galli C. Association between hepatitis C and hepatitis E viruses in southern Italy. Lancet (London, Engl) 1994;344:746–747. doi: 10.1016/s0140-6736(94)92233-0. http://www.ncbi.nlm.nih.gov/pubmed/7915787 [DOI] [PubMed] [Google Scholar]
- 23.Bayram A., Eksi F., Mehli M., Sözen E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology. 2007;50:281–286. doi: 10.1159/000103916. [DOI] [PubMed] [Google Scholar]
- 24.Toyoda H., Honda T., Hayashi K., et al. Prevalence of hepatitis E virus IgG antibody in Japanese patients with hemophilia. Intervirology. 2008;51:21–25. doi: 10.1159/000118792. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Ayed Y., Hannachi H., Ben-Alaya-Bouafif N., Gouider E., Triki H., Bahri O. Hepatitis E virus seroprevalence among hemodialysis and hemophiliac patients in Tunisia (North Africa) J Med Virol. 2015;87:441–445. doi: 10.1002/jmv.24082. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt P.E., Ijaz S., Brailsford S.R., et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet (London, Engl) 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 27.Rivero-Juarez A., Martinez-Dueñas L., Martinez-Peinado A., et al. CROI. 2015. Incidence of hepatitis E virus in HIV-infected patients: a longitudinal prospective study. Abstract 709. [Google Scholar]
- 28.Fu H., Li L., Zhu Y., et al. Hepatitis E virus infection among animals and humans in Xinjiang, China: possibility of swine to human transmission of sporadic hepatitis E in an endemic area. Am J Trop Med Hyg. 2010;82:961–966. doi: 10.4269/ajtmh.2010.09-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norder H., Sundqvist L., Magnusson L., et al. Endemic hepatitis E in two Nordic countries. Euro Surveill. 2009;14 doi: 10.2807/ese.14.19.19211-en. http://www.ncbi.nlm.nih.gov/pubmed/19442399 [DOI] [PubMed] [Google Scholar]
- 30.Gardinali N.R., Barry A.F., Otonel R.A.A., Alfieri A.F., Alfieri A.A. Hepatitis E virus in liver and bile samples from slaughtered pigs of Brazil. Mem Inst Oswaldo Cruz. 2012;107:935–939. doi: 10.1590/s0074-02762012000700016. http://www.ncbi.nlm.nih.gov/pubmed/23147152 [DOI] [PubMed] [Google Scholar]
- 31.dos Santos D.R.L., Vitral C.L., de Paula V.S., et al. Serological and molecular evidence of hepatitis E virus in swine in Brazil. Vet J. 2009;182:474–480. doi: 10.1016/j.tvjl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Steyer A., Naglič T., Močilnik T., Poljšak-Prijatelj M., Poljak M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: prevalence and molecular characterization of a novel genotype 3 lineage. Infect Genet Evol. 2011;11:1732–1737. doi: 10.1016/j.meegid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Iaconelli M., Purpari G., Della Libera S., et al. Hepatitis A and E viruses in wastewaters, in river waters, and in bivalve molluscs in Italy. Food Environ Virol. 2015;7:316–324. doi: 10.1007/s12560-015-9207-3. [DOI] [PubMed] [Google Scholar]
- 34.Mesquita J.R., Oliveira D., Rivadulla E., et al. Hepatitis E virus genotype 3 in mussels (Mytilus galloprovinciallis), Spain. Food Microbiol. 2016;58:13–15. doi: 10.1016/j.fm.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Vento S. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. J Viral Hepat. 2000;7(Suppl. 1):7–8. doi: 10.1046/j.1365-2893.2000.00019.x. http://www.ncbi.nlm.nih.gov/pubmed/10866837 [DOI] [PubMed] [Google Scholar]
- 36.Sagnelli E., Coppola N., Messina V., et al. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology. 2002;36:1285–1291. doi: 10.1053/jhep.2002.36509. [DOI] [PubMed] [Google Scholar]
- 37.Kim D., Riley L., Harriman K., Hunter P., Bridges C. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older. MMWR Morb Mortal Wkly Rep. 2017;66:136–138. doi: 10.15585/mmwr.mm6605e2. https://www.cdc.gov/mmwr/volumes/66/wr/mm6605e2.htm [DOI] [PMC free article] [PubMed] [Google Scholar]