Abstract
Background
Intracranial abscesses are associated with high mortality. Staphylococcus aureus is one of the main pathogens that cause intracranial infection. Until now, there is no report to identify the key effectors of S. aureus during the intracranial infection.
Methods
The murine intracranial abscesses model induced by S. aureus was constructed. The vital sign and survival rate of mice were observed to evaluate the infection. Histological examination was used to diagnose the pathological alterations of mouse tissues. The sensitivity of S. aureus to whole blood was evaluated by whole-blood killing assay.
Results
In murine intracranial abscesses model, it was shown that the mortality caused by the accessory gene regulator (agr) locus deficient strain was significant decreased compared with its parent strain. Moreover, we found that RNAIII, the effector of agr system, was essential for the intracranial infection caused by S. aureus. In the further investigation, it was shown that restoration the expression of α-toxin in agr deficient strain could partially recover the mortality in the murine intracranial abscesses model.
Conclusion
Our data suggested that the agr system of S. aureus is an important virulence determinant in the induction and mortality of intracranial abscesses in mice.
Keywords: S. aureus, Agr, Intracranial abscesses model
Introduction
Intracranial abscesses frequently occur as a complication of sinusitis or neurosurgery, which are associated with high mortality (19–43%).1 Until now, the incidence, etiology, management and morbidity of intracranial abscesses are not well understood. Staphylococcus aureus is one of the main pathogens that cause intracranial abscesses.1, 2, 3, 4, 5, 6, 7, 8
The accessory gene regulator (agr) was identified as a quorum sensing system in S. aureus. RNAIII is the major effector of agr system. It acts as a small RNA regulating the expression of many virulence factors, including most of those encoding cell-wall-associated and extra-cellular proteins.9, 10 Exotoxins expressed by S. aureus play important roles in the infection. α-Toxin is one of exotoxins, which is regulated by RNAIII. As a pore forming toxin, α-toxin can cause hemolysis and tissue damage. Recently, it has been found that α-toxin can also interact with its receptor ADAM10 to aggravate S. aureus infection.11
Herein we describe an animal model of intracranial abscesses caused by S. aureus that we have constructed. The pathogenic role of agr system is investigated in this model.
Materials and methods
Mice
Adult female C57BL/6J mice (6–8 weeks old), obtained from Vital River Laboratories, were used for all experiments. Mice were housed in group cages, maintained on a 12:12 hour light-dark cycle, in a controlled environment (25 °C) and given unrestricted access to food and water. All animal experimental protocols of the study are in accordance with the national guidelines for the use of animals in scientific research “Regulations for the Administration of Affairs Concerning Experimental Animals”. It is also approved by Animal Care and Use Committee of Beijing Institute of Basic Medical Sciences with the approval number BMS-130112.
Staphylococcus aureus strains and culture conditions
S. aureus cells were grown in 5 mL of brain heart infusion (BHI), and Escherichia coli cells were grown in Luria-Bertani (LB) medium. Bacteria were cultured at 37 °C for 12 h with shaking at 200 rpm in a 25-mL test tube. Cells were transferred from 1 mL of pre-culture to 100 mL of BHI or LB medium in a 500-mL flask. S. aureus cells were routinely grown in BHI and E. coli cells were grown in LB medium either with no antibiotics, or with 40 μg/mL Chloramphenicol, 50 μg/mL tetracycline, 100 μg/mL ampicillin, and 50 μg/mL kanamycin.
The strains used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids.
| Strain or plasmid | Comments | Source or reference |
|---|---|---|
| Strain | ||
| S. aureus | ||
| 8325-4 | Wild-type, rsbU− | 30 |
| RN6390 | Laboratory virulent strain derived from 8325 | 31 |
| RN6911 | RN6390 with an agr mutation, tetR | 31 |
| RN6911-hla | RN6911 restoring HLA activity, cmrR | This study |
| ΔRNAIII | Restriction-negative strain, 8325 derivative, kanR | 32 |
| ΔRNAIII-R | ΔRNAIII restoring RNAIII activity cmrR | 32 |
| E. coli | ||
| DH5α | A host strain for cloning | Transgene |
Mouse brain infection model
Mice were anesthetized with 1% pentobarbital sodium (3–5 mg/100 gbw i.p.). While under anesthesia, mice were prepared for injection by securing the head in a stereotactic frame and quickly locating appropriate coordinates in the frontal cortex region on the skull. The general location for injection is 1 mm anterior to bregma and 1 mm lateral to the sagittal suture on the right side.12 Parting the fur with a needlepoint along both axes is appropriate for injection. To make an injection, orient the needle perpendicular to the skull and press through the calvarium to approximately 2 mm depth. Inject 5 μL bead suspension (106 CFU) into the brain by pressing the syringe button down and waiting 2 s for the bacteria to enter the brain. Control mice received injections of sterile saline. Carefully remove the needle and place the mouse in a clean, warm cage. All mice survived the injection procedure. After recovery from anesthesia, mice were kept at room temperature (25 °C) and provided with standard chow and watered ad libitum. 48-hour mortality, average time of death and rectal temperature were monitored.
Histological examination of mouse tissues
Mice were killed 12 h after injection and brains were removed immediately. Mouse brain was fixed in 10% neutral-buffered formalin for 48 h. The fixed tissue was processed through graded series of ethanol, followed by xylene, and embedded in paraffin. Tissue blocks through the entire abscess were sectioned at 5 μm and slides stained with hematoxylin–eosin.
Whole-blood killing assays
Bacteria cultures were washed twice in PBS, diluted to an inoculum of 105 CFU in 25 μL PBS, and mixed with 75 μL of freshly drawn mouse blood in heparinized tubes. The tubes were incubated at 37 °C for 4 h with agitation, at which time dilutions were plated on BHI agar for enumeration of surviving CFU.13
Restoring HLA activity in RN6911
The whole HLA coding region was amplified by PCR and cloned into pOS1 vector. The resulting plasmid (pOS1-hla) was transformed into E. coli DH5α. Positive clones were selected on LB plates containing 100 μg/mL ampicillin. The recombinant plasmid was isolated from positive clones and transformed to S. aureus RN4220 cells. Transformants were selected on tryptic soy agar plates containing 40 μg/mL chloramphenicol at 37 °C. The positive clone was selected by colony PCR. Then the plasmid was isolated and transformed to RN6911. The transformants were selected on tryptic soy agar plates containing 40 μg/mL chloramphenicol at 37 °C for 20 h. Plasmids of positive restoring colonies were extracted and confirmed by PCR.
Statistical analysis
Data are expressed as mean ± SEM. The significance of differences between experimental groups was determined by two-tailed t-test assuming a 95% confidence interval.
Results
Construction of murine intracranial abscesses model caused by S. aureus
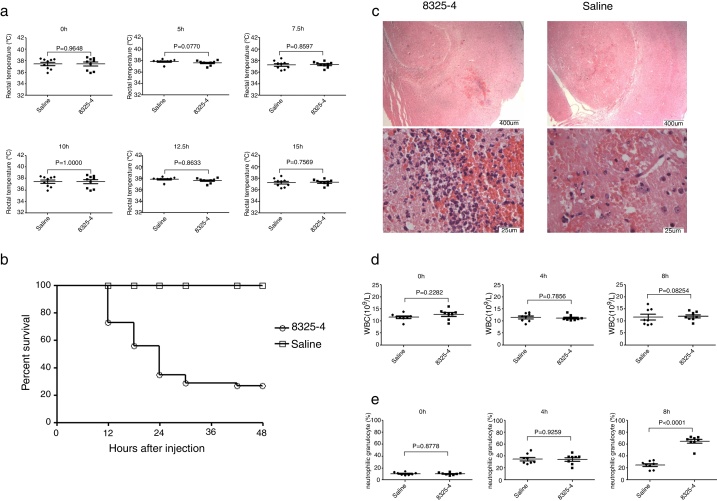
Injection of S. aureus 8325-4 into murine frontal cortex could produce a focal brain abscess with a clinical course similar to that seen in clinical patients with intracranial infection. Within 48 h post bacterial challenge, mice demonstrated clinical signs of illness including hunched posture, ruffled fur, lethargy, spasm, and finally expired. In contrast, any overt changes in posture, grooming, or physical activity of mice injected with sterile saline were not observed. However, challenge with S. aureus could not induce significant changes of rectal temperature (Fig. 1A). More than 50% of mice were dead after 48 h (Fig. 1B). Brains were collected 8 h after injection of Bacteria. A well-circumscribed abscess containing neutrophil and macrophage infiltrate, with significant mass effect was observed in histological examination of brains injected with S. aureus (Fig. 1C). Meanwhile, we also evaluate the hemogram of mice at 4 h and 8 h after infection. The number of white blood cells was not significantly altered (Fig. 1D), but the percentage of neutrophil was significantly increased at different time points in this model (Fig. 1E).
Fig. 1.
Construction of murine intracranial abscesses model caused by S. aureus. (A) Measurement of rectal temperature of mice at different time points after challenge with S. aureus 8325-4 (0 h, 5 h, 7.5 h, 10 h, 12.5 h, 15 h) (n = 9 in each group). (B) Measurement of survival curve of mice in the two groups (n = 52 mice in each group). Mice were challenged with S. aureus 8325-4 or saline. The number of dead mice was recorded. The survival rates of the two groups were calculated individually, p < 0.0001. (C) Histological examination of mouse brain tissue. Brain tissues from the two groups were collected 8 h post-challenge with S. aureus 8325-4 and fixed. The fixed tissues were stained by H&E. (D) White blood cell count of the two groups at different time points (0 h, 4 h and 8 h) post-challenge with S. aureus (n = 8 in each group). (E) Percentage of neutrophils in the two groups at different time points (0 h, 4 h and 8 h) post-challenge with S. aureus (n = 8 in each group).
The role of agr system in murine intracranial abscesses model
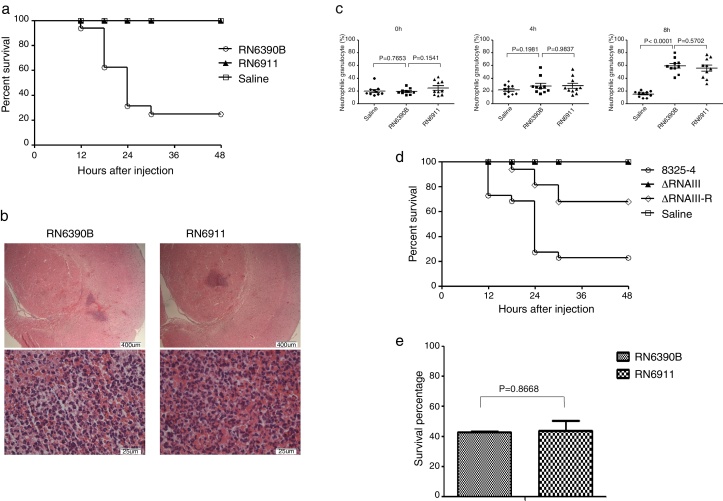
Given that agr system can regulate most of exotoxins expressed by S. aureus, we tried to determine whether agr system was involved in intracranial infection. Mice were injected with S. aureus RN6390B or RN6911 (agr-null strain). Injection with RN6911 could not induce death of mice (Fig. 2A). However, histological examination showed that RN6911 induced similar histological alteration of brain compared to RN6390B (Fig. 2B). The percentage of neutrophil was significantly increased in RN6390B and RN6911 groups, compared to sterile saline (Fig. 2C). It is well known that RNAIII is the key effector of agr system. Further investigating, we determined whether RNAIII deletion as well as agr-null strain was associated with decreased mortality of mice. The RNAIII deletion strains (ΔRNAIII) and the restored strain (ΔRNAIII-R) were previously constructed. Mice were challenged with wild-type S. aureus 8325-4, ΔRNAIII and ΔRNAIII-R, respectively. In line with expectation, ΔRNAIII could not induce the death of mice and challenge with the restoration RNAIII strain recovered virulence and the consequent mortality of mice (Fig. 2D). All these results suggested that agr system was essential for S. aureus induced mortality in the murine intracranial abscesses model.
Fig. 2.
The role of agr system in murine intracranial abscesses model. (A) Survive curve of mice challenged with different strains (n = 16 mice in each group). Mice were challenged with RN6390B, RN6911, and saline, respectively. The number of dead mice was recorded. The survival rate of the two groups was calculated individually. (B) Histological examination of mouse brain tissue. Brain tissues from RN6390B and RN6911 groups were collected at 12 h post-challenge with bacteria and fixed. The fixed tissues were stained by H&E. (C) Percentage of neutrophils in the three groups (RN6390B, RN6911 and saline) at different time points (0 h, 4 h and 8 h) post-challenge with S. aureus (n = 10 in each group). (D) Survive curve of mice challenged with different strains (n = 22 mice in each group). Mice were challenged with S. aureus 8325-4, ΔRNAIII, ΔRNAIII-R, and saline, respectively. The number of dead mice was recorded. The survival rate of the two groups was calculated individually. (E) Survival percentage of the different strains (RN6390B and RN6911) in murine whole blood. Bacteria (1 × 105 CFU) were incubated with murine whole blood for 4 h. Survival rate of bacteria were determined at BHI plate.
As the alteration of hemogram induced by RN6911 was not different from that of RN6390B, we wondered if a deficient agr system could make the bacteria more sensitive to whole blood killing. RN6911 and RN6390B were incubated with murine whole blood respectively. The bacterial survival rate was assessed 4 h after incubation. The survival rate of RN6911 bacteria was not significantly different when compared to RN6390B bacteria (Fig. 2E). This result suggests that the mortality alteration caused by agr null strain was not related with the sensitivity to whole blood killing.
α-Toxin was involved in the mortality caused by S. aureus in murine intracranial abscesses model
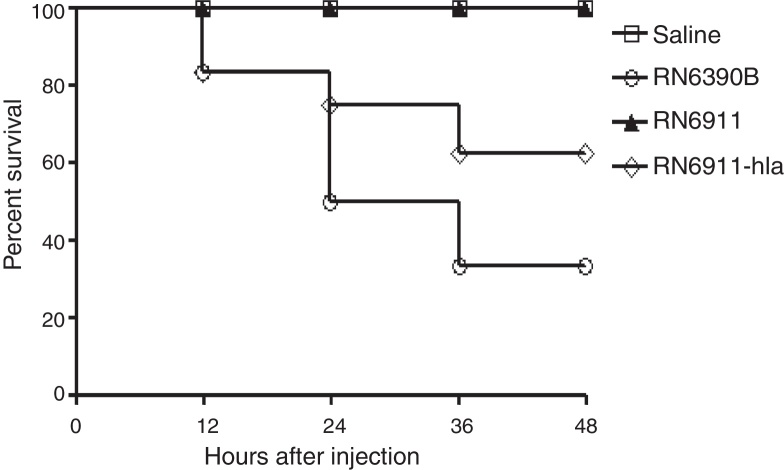
Exotoxins expressed by S. aureus are important for the infection.10, 14, 15 Some exotoxins are regulated by the agr system10, 16 and α-toxin is one of the major exotoxins regulated by agr system. RN6911 do not express α-toxin. In the following study, we investigated whether α-toxin was involved in intracranial abscesses. The expression of α-toxin was recovered in RN6911 by the plasmid pOS1-HLA to generate strain (RN6911-HLA). Mice were challenged with three different strains (RN6390B, RN6911 and RN6911-HLA), respectively. The survival rate of mice from different groups was assessed at the indicated time points. It was shown that restoration of α-toxin could partially recover lethality (Fig. 3). These results suggested that α-toxin played an important role in the intracranial infection causing abscesses.
Fig. 3.
Determination of the role of α-toxin in murine intracranial abscesses model. Survive curve of mice challenged with different strains (n = 12 mice in each group). Mice were challenged with RN6390B, RN6911, RN6911-hla, and saline, respectively. The number of dead mice was recorded. The survival rate of the two groups was calculated individually.
Discussion
Intracranial abscesses caused by bacteria are usually associated with high mortality rate. S. aureus is a common cause of various kinds of infections, including intracranial abscesses. However, there is no report about animal model of intracranial abscesses caused by S. aureus. Moreover, the effect of a known regulator in S. aureus in intracranial infection has not been determined. In our study, we constructed a murine model of intracranial abscesses caused by S. aureus. We also demonstrated that agr, the quorum sensing system, in S. aureus was essential for causing intracranial infection. Furthermore, our results revealed that α-toxin regulated by agr system played an important role in the process of intracranial abscesses.
There are some constructed S. aureus infection models including arthritis, pneumonia, acute peritonitis, endocarditis, and skin infection.17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Until now, there are few reports about intracranial infection model caused by S. aureus. In this study, we constructed a murine model by injecting S. aureus 8325-4 into the frontal cortex. Mice challenged with S. aureus showed some clinical signs of illness. The number of white blood cells and the rectal temperature were not significant altered after injecting S. aureus suggesting that the sign of murine intracranial abscess was not exactly the same as human intracranial abscess. This may be due to difference in resistance to bacteria between mice and humans. However, the percentage of neutrophil in mice challenged with S. aureus was significantly higher than that of control group. This result is in accordance with the clinical sign seen in patients.
Agr system is well studied in S. aureus and is considered essential for infection.10, 16, 27 In line with expectations, we found that the mortality associated with agr-null strain was significantly decreased when compared to the wild type on intracranial abscesses model. Moreover, we also found that deletion of RNAIII suppressed virulence of S. aureus infection.
S. aureus can express various kinds of exotoxins which are necessary for causing infection. α-Toxin is the major exotoxin of S. aureus, regulated by RNAIII.10, 16, 28, 29 Our results showed that restoration of the level of α-toxin in agr-null strain partially recovered the mortality associated with of S. aureus on the intracranial abscesses model. This result suggested that α-toxin should play an important role in intracranial infection. Moreover, some other molecules regulated by the agr system might also be involved in intracranial infection. The role of α-toxin and other molecules expressed by S. aureus in intracranial infection should be further investigated in the future.
Our study presents a murine intracranial abscesses model that can be used to investigate S. aureus infection in brain tissue. Further investigation on the role of agr system will increase our understanding of the intracranial abscesses caused by S. aureus.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by National Natural Science Funding (31271119, 81072074), Beijing Educational Committee Key Funding (KZ201110025033) and Beijing Organization Department of Municipal Committee Talent Funding (3034000004).
Contributor Information
Tao Sun, Email: suntao6699@163.com.
Guang Yang, Email: yanggg@hotmail.com.
References
- 1.Saito N., Aoki K., Sakurai T., et al. Linezolid treatment for intracranial abscesses caused by methicillin-resistant Staphylococcus aureus. Neurol Med Chir. 2010;50:515–517. doi: 10.2176/nmc.50.515. [DOI] [PubMed] [Google Scholar]
- 2.Kono Y., Prevedello D.M., Snyderman C.H., et al. One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hosp Epidemiol. 2011;32:77–83. doi: 10.1086/657635. [DOI] [PubMed] [Google Scholar]
- 3.Dashti S.R., Baharvahdat H., Spetzler R.F., et al. Operative intracranial infection following craniotomy. Neurosurg Focus. 2008;24:E10. doi: 10.3171/FOC/2008/24/6/E10. [DOI] [PubMed] [Google Scholar]
- 4.Fulkerson D.H., Sivaganesan A., Hill J.D., et al. Progression of cerebrospinal fluid cell count and differential over a treatment course of shunt infection. J Neurosurg Pediatr. 2011;8:613–619. doi: 10.3171/2011.8.PEDS11236. [DOI] [PubMed] [Google Scholar]
- 5.Mathisen G.E., Johnson J.P. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.H., Desai N.S., Ricci J., et al. Factors contributing to ventriculostomy infection. World Neurosurg. 2012;77:135–140. doi: 10.1016/j.wneu.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Sifri C.D., Park J., Helm G.A., Stemper M.E., Shukla S.K. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clin Infect Dis. 2007;45:e113–e117. doi: 10.1086/522171. [DOI] [PubMed] [Google Scholar]
- 8.Wang K.W., Chang W.N., Shih T.Y., et al. Infection of cerebrospinal fluid shunts: causative pathogens, clinical features, and outcomes. Jpn J Infect Dis. 2004;57:44–48. [PubMed] [Google Scholar]
- 9.Thoendel M., Kavanaugh J.S., Flack C.E., Horswill A.R. Peptide signaling in the staphylococci. Chem Rev. 2010;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarwood J.M., Schlievert P.M. Quorum sensing in Staphylococcus infections. J Clin Invest. 2003;112:1620–1625. doi: 10.1172/JCI20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoshima I., Inoshima N., Wilke G.A., et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaFrance-Corey R.G., Howe C.L. Isolation of brain-infiltrating leukocytes. J Vis Exp. 2011:52. doi: 10.3791/2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G.Y., Essex A., Buchanan J.T., et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinges M.M., Orwin P.M., Schlievert P.M. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labandeira-Rey M., Couzon F., Boisset S., et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 16.Yarwood J.M., Bartels D.J., Volper E.M., Greenberg E.P. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keffer J., Probert L., Cazlaris H., et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J.H., Ratkay L.G., Waterfield J.D., Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Feng G., Guo Q., et al. Transcriptional events during the recovery from MRSA lung infection: a mouse pneumonia model. PLoS ONE. 2013;8:e70176. doi: 10.1371/journal.pone.0070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginsberg H.S., Moldawer L.L., Sehgal P.B., et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leypoldt J.K., Kamerath C.D., Gilson J.F. Acute peritonitis in a C57BL/6 mouse model of peritoneal dialysis. Adv Perit Dial. 2007;23:66–70. [PubMed] [Google Scholar]
- 22.Takahashi K., Gordon J., Liu H., et al. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–784. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Y.Q., Willard J., Kadurugamuwa J.L., Yu J., Francis K.P., Bayer A.S. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother. 2005;49:380–387. doi: 10.1128/AAC.49.1.380-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson G.W., Kreuser S.C., Riley J.M., et al. Development of a mouse model of induced Staphylococcus aureus infective endocarditis. Comp Med. 2007;57:563–569. [PubMed] [Google Scholar]
- 25.Kennedy A.D., Bubeck Wardenburg J., Gardner D.J., et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown E.L., Dumitrescu O., Thomas D., et al. The Panton–Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuong C., Saenz H.L., Götz F., Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 28.Queck S.Y., Jameson-Lee M., Villaruz A.E., et al. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung A.L., Bayer A.S., Zhang G., Gresham H., Xiong Y.Q. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40:1–9. doi: 10.1016/S0928-8244(03)00309-2. [DOI] [PubMed] [Google Scholar]
- 30.Novick R.P. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 31.Peng H.L., Novick R.P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988;170:4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Mu C., Ying X., et al. RNAIII activates map expression by forming an RNA–RNA complex in Staphylococcus aureus. FEBS Lett. 2011;585:899–905. doi: 10.1016/j.febslet.2011.02.021. [DOI] [PubMed] [Google Scholar]