Abstract
Backgroud
Daptomycin has been used in bone and joint infections (BJI) and prosthesis joint infections (PJI) considering spectrum of activity and biofilm penetration. However, the current experience is based on case reports, case series, cohorts, and international surveys. The aim of this systematic review was to evaluate studies about daptomycin treatment efficacy in BJI/PJI compared to other antibiotic regimens.
Methods
PubMed, LILACS, Scielo and Web of Science databases were searched for articles about daptomycin and treatment of BJI and PJI from inception to March 2018. Inclusion criteria were any published researches that included patients with BJI treated with daptomycin. Diagnosis of BJI was based on clinical, laboratory and radiological findings according to IDSA guidelines.
Results
From 5107 articles, 12 articles were included. Only three studies described the outcomes of patients with BJI treated with daptomycin with comparator regimen (vancomycin, teicoplanin and oxacillin). Studies presented large heterogeneity regarding device related infections, surgical procedures, and daptomycin regimens (varied from 4 mg/kg to 10 mg/kg). A total of 299 patients have been included in all studies (184 infections associated with orthopedic disposal and 115 osteomyelitis/septic arthritis). Two hundred and thirty-three patients were treated with daptomycin. The clinical cure rates on device related and non-device related infections (i.e. osteomyelitis) were 70% and 78%, respectively. Compared to all regimens evaluated, daptomycin group outcomes were non-inferior.
Conclusion
Although a randomized clinical trial is needed, this systematic review tends to support daptomycin usage for bone and joint infections.
Keywords: Daptomycin, Staphylococcus aureus, Osteomyelitis, Joint infection, Arthritis
Introduction
Daptomycin is an important antibiotic to treat Gram-positive infections.1 Methicillin resistant Staphylococcus aureus (MRSA) has been a global public health concern, becoming endemic in most hospitals, although some studies have shown a tendency to decline from 2005 in some regions.2, 3 Therapeutic option for the treatment of MRSA infection was based on vancomycin and teicoplanin for several decades. However, new options have been developed with several advantages, but lacking evidence of clinical superiority. Some of these drugs include linezolid, telavancin, tigecycline, ceftaroline, and daptomycin.
Daptomycin is a bactericidal antibiotic with pharmacokinetics profile that allows once daily infusion and safety in outpatient parenteral therapy (OPAT) practice.4 OPAT programs are important in bone and joint infections (BJI) and prosthesis joint infections (PJI) because long-term treatments are commonly necessary.5 Staphylococcal BJI and PJI are commonly associated with biofilm formation. Most antibiotics have effect against planktonic microorganisms, but not against those inside and in the base of the biofilm. This is due to poor penetration of most drugs in the biofilm (e.g. vancomycin) and most of them cannot reduce the biofilm burden and lack bactericidal activity against non-growing microorganisms present in the biofilm. Daptomycin is a drug that can penetrate biofilm, although it is no able to reduce the biofilm formation.6, 7
Dose and bone penetration of daptomycin has raised concern and some specialists have being cautious to advocate its routine use.8, 9 Nevertheless, several case reports, case series, cohorts, and international surveys suggest safety of its use. Cubicin Outcomes Registry and Experience (CORE) studies and its sub-analyses represented international surveys from all around the world, which included daptomycin use to treat BJI and PJI. Variable doses, surgery techniques and time of follow-up were used, but results often demonstrated good efficacy.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Considering the importance of daptomycin as an option in the treatment of MRSA-associated bone and joint infections and OPAT, in this systematic review we tried to get the studies on daptomycin treatment efficacy in BJI/PJI compared to other antibiotic regimens.
Methods
Search strategy
Using PubMed, Scielo, LILACS and Web of Science databases we searched for published literature in English and Spanish that evidenced the efficacy of daptomycin in the treatment of BJI. The search included publications from inception to March 2018. The keywords used were "daptomycin" and "bone OR osteomyelitis OR joint OR biofilm OR prosthesis joint infection OR orthopedic infection OR staphylococcus OR staphylococcal infection". This systematic review followed PRISMA statement guidelines.
Data extraction and quality evaluation
Two reviewers independently screened all studies based on either title or abstract for eligibility. Discrepancies were resolved through discussion. The authors then independently extracted the relevant data from all the publications to include in the review (JT & FT).
Inclusion and exclusion criteria
Inclusion criteria were any published researches that included patients with BJI and/or PJI treated with daptomycin. Publications with less than 10 patients, experimental studies, without clear description of the strategy treatment (i.e. types of surgical procedures) or clinical outcome were excluded.
Definitions and outcomes
Diagnoses of BJI and PJI were based on clinical, laboratory and radiological findings according to Infectious Disease Society of America (IDSA) (i.e. histopathology, sinus tract with prosthesis communication, purulence around prosthesis without another known etiology, cultures form pre or intraoperative or clinical judgment after reviewing pre and intraoperative information).5 The terms “treatment successes” and “favorable outcomes” were defined as clinical improvements at the end of the treatment with daptomycin.
A “clinical cure” was defined as a total resolution of all infections signs and symptoms, or an improvement of signs and symptoms to such an extent that no further antimicrobial therapy was necessary. Secondary outcomes such as mortality, incidence of adverse events, serious adverse events, and discontinuation due to adverse events were not evaluated. The standard form included the following information: first author, study design, country, publication year, number of patients, period of the study, classification of the infection, percentage of MRSA, surgical procedure, daptomycin dose, antimicrobial agents combination or switch therapy, duration of the treatment, follow-up and explanation to use daptomycin.
Statistical analysis was not performed due to absence of randomized clinical studies to perform a meta-analysis. Grading quality of evidence and risk of bias were not accessed.
Results
Selected articles
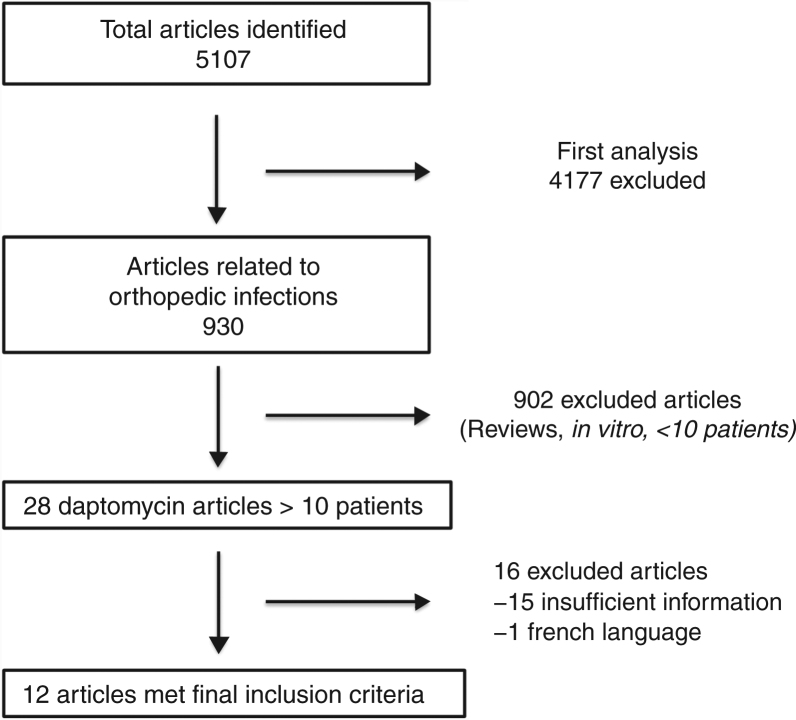
Five thousand one hundred and seven articles were initially found using the search criteria. After first analysis, only 930 articles were related to daptomycin and BJI and/or PJI. Following title and abstract reviews, only 12 articles fulfilled the inclusion criteria (Fig. 1). The first study was published in 2005, and the last in 2017. Daptomycin dose and duration of regimens, device related infection, and clinical outcomes were evaluated (Table 1).
Fig. 1.
Flowchart of selection process for daptomycin and BJI articles included in review.
Table 1.
Description of published studies evaluating daptomycin use in orthopedic infections.
| Author | Region | Date | N | Infection (n) | Daptomycin dose (mg/kg) | Length of therapy (days) | Clinical cure rates |
|---|---|---|---|---|---|---|---|
| Chang | Taiwan | 2013–2014 | 16 | KA (9) | 8.3 | 14 | KA (80%) |
| HA (7) | HA 91% | ||||||
| Herrera | Espanha | 2008–2015 | 21 | KA (12) | 10 | 24 | KA (83%) |
| HA (9) | HA (88%) | ||||||
| Jugun | Swiss | 2013 | 16 | OM (10) | 8.15 | 21 | OM (90%) |
| KA (3) | KA (100%) | ||||||
| HA (3) | HA (100%) | ||||||
| Kuo | Taiwan | 2010–2012 | 22 | KA (10) | 6 | 14 | KA (100%) |
| HA (12) | HA (100%) | ||||||
| Lora-Tamayo | Espanha | 2010–2012 | 18 | KA (7) | 10 | 42 | KA (42%) |
| HA (11) | HA (50%) | ||||||
| Byren | Multicenter | 2007–2010 | 47 | KA (27) | 6 e 8 | 24 | KA (59%) |
| HA 20) | HA (42-75%) | ||||||
| Lai | Taiwan | 2007–2009 | 12 | OM/SA (12) | 6, 8 e 10 | – | 91% |
| Moenster | EUA | 2003–2009 | 17 | OM (17) | 5.58 | 14 | 71% |
| Pérez-Cardona | Espanha | 2008–2010 | 20 | KA (7) | 6.6 | 45 | KA (45%) |
| HA (13) | HA (65%) | ||||||
| Lalani | Multicenter | 2002–2005 | 21 | OM (10) | 6 | 23 | SA (64%) |
| SA (11) | OM (70%) | ||||||
| Rao | USA | 2004–2005 | 13 | KA (10) | 4 | 240 | KA (50%) |
| HA (2) | HA (50%) | ||||||
| Sh Ar (1) | Sh Ar (0%) | ||||||
| Finney | USA | 2003–2004 | 10 | OM (6) | – | 24 | OM (100%) |
| SA (4) | SA (75%) |
KA, knee arthroplasty; HA, hip arthroplasty; Sh Ar, shoulder arthroplasty; OM, osteomyelitis; SA, septic arthritis.
Characteristics of selected articles
Two hundred ninety-nine patients were included from a total of 12 articles. Two hundred thirty-three patients were treated with daptomycin. Two were multicenter,21, 22 three studies were from USA,23, 24, 25 Spain26, 27, 28 and Taiwan29, 30, 31 each, and one from Switzerland.32 Nine studies described the outcomes of patients with BJI/PJI treated with daptomycin without comparator, and three studies compared the outcomes of daptomycin with other antibiotic therapy (vancomycin, teicoplanin and oxacillin).21, 22, 25, 33 Studies methodology presented large heterogeneity regarding device related infections, surgical procedures and daptomycin regimens.
Device related infections and surgical procedures
A total of 299 patients have been included in all studies, 184 with infections associated with orthopedic disposal and 115 patients with osteomyelitis/septic arthritis. Daptomycin was used to treat 88% (163/184) of device related infections. These were represented by 85 total knee arthroplasty (TKA), 77 hip arthroplasty (HA) and one shoulder arthroplasty. Clinical cure rate of patients with hardware infection was 70%. Overall, variability was observed and median clinical cure rate from studies results was 70% [IQR 50–89]. Clinical cure were observed in 58 TKA infections (68%) and 56 HA infections (73%). Shoulder device infection did not have favorable outcome in one study with few cases (Table 1).
Surgical procedures also had variability. Clinical cure rate of patients submitted to two-stage surgical strategy was 73% (n = 106) and median cure rate from six studies was 76% [IQR 59.5–90]. Clinical cure rate of patients submitted to debridement, antibiotics and implant retention procedure (DAIR) was 63% (n = 43) and median cure rate from six studies was 80% [IQR 57–80.75]. Fusion and one-stage revision was used on two studies each and clinical cure rates varied between 75–100% and 33–100%, respectively.
Non-related device bone infection: osteomyelitis (OM) and septic arthritis
Five studies evaluated daptomycin for treating osteomyelitis and/or septic arthritis and totaling 53 patients (Jung et al; Moenster et al; Lalani et al; Lai et al; Finney et al). Clinical cure rate of patients with non-related device infection was 78%. Variability was observed and median clinical cure rate from studies results was 90% [IQR 71–90], highest found by Finney et al. (OM: 100% and septic arthritis 75%) and lowest by Lalani et al. (OM: 64% and septic arthritis: 70%).
Dosing regimens
Daptomycin dosing varied from 4 mg/kg to 10 mg/kg. Three studies used 10 mg/kg,27, 28 including 41 patients with a clinical cure rate of 70%. Doses between 6–8 mg/kg were used in seven studies,21, 22, 26, 30,31, 33, 34 including 151 patients with a clinical cure rate of 74%. Doses between 4–6 mg/kg were used in three studies,23, 24, 25 including 40 patients with a clincal cure rate of 67%. Median clinical cure rates varied from 85% [IQR 67–92] for 10 mg/kg, 85% [IQR 63–95] for 6–8 mg/kg, and 71% [IQR 58–80] for 4–6 mg/kg.
MRSA prevalence
MRSA prevalence varied from 10 to 90%, with a total of 83 patients infected with MRSA and treated with daptomycin. Clinical cure rate was 61.5%. Variability was found and median clinical cure rate from studies results was 43% [IQR 40–83]. Malizos et al. and Forrest et al. observed highest rates, 100 and 89%, respectively.12, 18
Comparator: vancomycin, teicoplanin and oxacillin
Three studies used comparator regimens. Only one was on PJI,22 and two were on osteomyelitis and septic arthritis.21, 25, 33 Vancomycin was used as main comparator regimen, but two studies evaluated teicoplanin or oxacillin as well. In this group of analysis, 85 patients received daptomycin and 66 received comparator regiments (vancomycin, teicoplanin or oxacillin). Daptomycin group outcomes were non-inferior in all studies, showing the same efficacy (Table 2).
Table 2.
Description of published studies evaluating daptomycin compared to other antibiotic therapies in orthopedic infections with cure rates.
| Author | Infection | Daptomycin Doses | Days of therapy | Comparator | Daptomycin Cure rates | Comparator Cure rates |
|---|---|---|---|---|---|---|
| Byren | PJI | 6-8 mg/kg | 24 days | Vancomycin Teicoplanin | 57% | 38% |
| Moenster | OM | 5.58 mg/kg | 14 days | Vancomycin | 71% | 39% |
| Lalani | OM/SA | 6mg/kg | 23 days | Vancomycin Oxacillin | 64-70% | 60% |
Discussion
This review included 12 articles with 299 patients with BJI/PJI. Two hundred and thrity-three patients were treated with daptomycin. However, no randomized controlled trial has been published about BJI/PJI treatment with daptomycin. The cure rate was moderate to high, ranging from 45 and 100%. Daptomycin was approved by FDA for complicated skin infection and S. aureus blood stream infection (except left-sided endocarditis), but not for osteomyelitis without bacteremia. However, several studies evaluating this off-label indication has been published as case series, retrospective analysis of cohorts, subgroup of clinical trial of secondary bacteremia with BJI. In most studies, daptomycin was indicated due to the need of an alternative treatment to currently indicated therapies.
Multicenter international surveys named CORE studies demonstrated daptomycin safety and efficacy on multiple sites of infection.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 BJI and PJI were also evaluated and daptomycin doses varied from 4 to 10 mg/kg. MRSA prevalence varied between 16.9–45%. Although the lowest success clinical rate was 55%,19 usually rates higher than 70%10, 11, 12, 13, 14, 20 were found and even higher than 90%.15, 17, 18 Other non-survey studies with clinical cure rates higher than 70% also demonstrated daptomycin safety and efficacy to bone related infections.20, 21 Unfortunately, these studies were not included on this systematic review due lack of information such as differentiation between prosthesis related infection and osteomyelitis, surgical technique or/and follow-up and clear outcomes.
Eight studies presented clinical cure rate above 70%. Highest rates were observed by Kuo et al. and Finney et al. Kuo et al. observed 100% cure rates with systemic and cement impregnation treatment with daptomycin on PJI scenario and 2-stage surgical procedure.31 On another scenario Finney et al. observed 90% cure rates on bone infections with no-device related infections, mainly osteomyelitis.23 Differences cited above as daptomycin impregnation in the orthopedic cement and only patients with 2-stage surgical procedure or no-device related infection (osteomyelitis) might have had an influence on their results. Lower success rate, between 46–60% of clinical cure rate, were demonstrated. Byren et al. conducted a clinical trial with 2-stage surgical procedure and considered therapy failure in those patients with one positive culture on second stage surgery.22 This definition of failure might explain low success rates to vancomycin (38%) and daptomycin (57%), since one positive from multiple negative cultures could represent contamination (e.g. Staphylococcus epidermidis). Lora-Tamayo et al. selected patients who were candidates to DAIR strategy and considered microbiological failure if in a two-year follow-up the patient presented a new infection, even if no culture were positive.27 Thus, different bacterial infection from the beginning could overestimate therapeutic failure. Rao et al. used for all patients 4 mg/kg daptomycin and DAIR strategy success rate (20%) was more affected than 2-stage (62%). Furthermore, they concluded that other studies are needed to support daptomycin usage in PJI, mainly in DAIR strategy. These results reinforce the impact of surgical strategy to explain different results among studies of patients treated with daptomycin.
Daptomycin penetration and activity on bone infections are slowly being understood. in vitro and in vivo studies observed good bone penetration.35, 36 However, it was demonstrated variability on daptomycin concentrations even on those patients with normal renal function and some recommend drug monitoring in case of prolonged use.37 In addition, intracellular activity appears to be an important antimicrobial characteristic if considered intracellular reservoir on bone infection.38, 39, 40 In this context, it was observed that daptomycin limited but did not prevent ofloxacin intracellular bacterial growth41 and presented good intracellular activity against intraosteoblastic S. aureus if combined with oxacillin for MSSA and MRSA.42
Activity on biofilm is still a major problem, nevertheless not only to daptomycin. Studies are different on their conclusions. Reasonable in vitro activity was demonstrated.6, 43 mainly if combined with rifampin.44, 45 However, reliable minimal biofilm eradication concentration (MBEC) of each antibiotic may be difficult to predict. Molina-Manso et al. observed MBEC values as high as 1024 mg/L for most common antibiotics used to treat BJI.46 Therefore, therapeutic failure might be explained by true higher MBEC.
Antibiotic cement loaded is considered an important strategy when 2-stage surgical procedure is needed. Gentamicin is known to be an option with polymethyl-methacrylate since mid 20th century.47 Higher bacterial resistance rates forced to try other options. Since then, vancomycin has been used with gentamicin,48 but has already demonstrated impact on resistance elevating S. aureus MICs.49
This study has several limitations and it is not possible to assume the above results as true. The number of patients is small in all these studies, the duration and doses of treatment with daptomycin varied and most patients completed the treatment with therapies other than daptomycin. The surgical procedures varied according to the group, which is very important in the management of BJI. Clinical cure can only be judged with a follow-up of one year without implant and two years with implant, respectively. Therefore, patients with a shorter follow-up should be excluded from the analysis.
In conclusion, our systematic review tends to supports daptomycin use for bone and joint infections and considers an antimicrobial combination mainly against staphylococci, as rifampin or quinolone, to exert anti-biofilm activity or even prevent possible small-colony resistance appearance during follow-up.
Financial support
None.
Conflicts of interest
Felipe Tuon has received grants from Pfizer and MSD in the last year.
Felipe Tuon conducts research for CNPQ.
References
- 1.Humphries R.M., Pollett S., Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26:759–780. doi: 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefani S., Chung D.R., Lindsay J.A., et al. Meticillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents. 2012;39:273–282. doi: 10.1016/j.ijantimicag.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Liebowitz L.D. MRSA burden and interventions. Int J Antimicrob Agents. 2009;34 Suppl 3:S11–S13. doi: 10.1016/S0924-8579(09)70551-5. [DOI] [PubMed] [Google Scholar]
- 4.Nathwani D. Developments in outpatient parenteral antimicrobial therapy (OPAT) for Gram-positive infections in Europe, and the potential impact of daptomycin. J Antimicrob Chemother. 2009;64:447–453. doi: 10.1093/jac/dkp245. [DOI] [PubMed] [Google Scholar]
- 5.Osmon D.R., Berbari E.F., Berendt A.R., et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 6.Ozturk B., Gunay N., Ertugrul B.M., Sakarya S. Effects of vancomycin, daptomycin, and tigecycline on coagulase-negative staphylococcus biofilm and bacterial viability within biofilm: an in vitro biofilm model. Can J Microbiol. 2016;62:735–743. doi: 10.1139/cjm-2015-0855. [DOI] [PubMed] [Google Scholar]
- 7.Boudjemaa R., Briandet R., Revest M., et al. New insight into daptomycin bioavailability and localization in Staphylococcus aureus biofilms by dynamic fluorescence imaging. Antimicrob Agents Chemother. 2016;60:4983–4990. doi: 10.1128/AAC.00735-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sendi P., Zimmerli W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin Microbiol Infect. 2012;18:1176–1184. doi: 10.1111/1469-0691.12003. [DOI] [PubMed] [Google Scholar]
- 9.Kosmidis C., Levine D.P. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother. 2010;11:615–625. doi: 10.1517/14656561003598893. [DOI] [PubMed] [Google Scholar]
- 10.Hermsen E.D., Mendez-Vigo L., Berbari E.F., Chung T., Yoon M., Lamp K.C. A retrospective study of outcomes of device-associated osteomyelitis treated with daptomycin. BMC Infect Dis. 2016;16:310. doi: 10.1186/s12879-016-1590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seaton R.A., Gonzalez-Ruiz A., Cleveland K.O., Couch K.A., Pathan R., Hamed K. Real-world daptomycin use across wide geographical regions: results from a pooled analysis of CORE and EU-CORE. Ann Clin Microbiol Antimicrob. 2016;15:18. doi: 10.1186/s12941-016-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malizos K., Sarma J., Seaton R.A., et al. Daptomycin for the treatment of osteomyelitis and orthopaedic device infections: real-world clinical experience from a European registry. Eur J Clin Microbiol Infect Dis. 2016;35:111–118. doi: 10.1007/s10096-015-2515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Ruiz A., Gargalianos-Kakolyris P., Timerman A., et al. Daptomycin in the clinical setting: 8-year experience with Gram-positive bacterial infections from the EU-CORE(SM) registry. Adv Ther. 2015;32:496–509. doi: 10.1007/s12325-015-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaton R.A., Malizos K.N., Viale P., et al. Daptomycin use in patients with osteomyelitis: a preliminary report from the EU-CORE(SM) database. J Antimicrob Chemother. 2013;68:1642–1649. doi: 10.1093/jac/dkt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher J.C., Huntington J.A., Culshaw D., McConnell S.A., Yoon M., Berbari E. Daptomycin therapy for osteomyelitis: a retrospective study. BMC Infect Dis. 2012;12:133. doi: 10.1186/1471-2334-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Ruiz A., Beiras-Fernandez A., Lehmkuhl H., et al. Clinical experience with daptomycin in Europe: the first 2.5 years. J Antimicrob Chemother. 2011;66:912–919. doi: 10.1093/jac/dkq528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crompton J.A., North D.S., McConnell S.A., Lamp K.C. Safety and efficacy of daptomycin in the treatment of osteomyelitis: results from the CORE registry. J Chemother. 2009;21:414–420. doi: 10.1179/joc.2009.21.4.414. [DOI] [PubMed] [Google Scholar]
- 18.Forrest G.N., Donovan B.J., Lamp K.C., Friedrich L.V. Clinical experience with daptomycin for the treatment of patients with documented Gram-positive septic arthritis. Ann Pharmacother. 2008;42:213–217. doi: 10.1345/aph.1K535. [DOI] [PubMed] [Google Scholar]
- 19.Lamp K.C., Friedrich L.V., Mendez-Vigo L., Russo R. Clinical experience with daptomycin for the treatment of patients with osteomyelitis. Am J Med. 2007;120:S13–20. doi: 10.1016/j.amjmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Holtom P.D., Zalavras C.G., Lamp K.C., Park N., Friedrich L.V. Clinical experience with daptomycin treatment of foot or ankle osteomyelitis: a preliminary study. Clin Orthop Relat Res. 2007;461:35–39. doi: 10.1097/BLO.0b013e3181123bc5. [DOI] [PubMed] [Google Scholar]
- 21.Lalani T., Boucher H.W., Cosgrove S.E., et al. Outcomes with daptomycin versus standard therapy for osteoarticular infections associated with Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61:177–182. doi: 10.1093/jac/dkm437. [DOI] [PubMed] [Google Scholar]
- 22.Byren I., Rege S., Campanaro E., et al. Randomized controlled trial of the safety and efficacy of daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob Agents Chemother. 2012;56:5626–5632. doi: 10.1128/AAC.00038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finney M.S., Crank C.W., Segreti J. Use of daptomycin to treat drug-resistant Gram-positive bone and joint infections. Curr Med Res Opin. 2005;21:1923–1926. doi: 10.1185/030079905X74961. [DOI] [PubMed] [Google Scholar]
- 24.Rao N., Regalla D.M. Uncertain efficacy of daptomycin for prosthetic joint infections: a prospective case series. Clin Orthop Relat Res. 2006;451:34–37. doi: 10.1097/01.blo.0000224021.73163.61. [DOI] [PubMed] [Google Scholar]
- 25.Moenster R.P., Linneman T.W., Finnegan P.M., McDonald J.R. Daptomycin compared to vancomycin for the treatment of osteomyelitis: a single-center, retrospective cohort study. Clin Ther. 2012;34:1521–1527. doi: 10.1016/j.clinthera.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Corona Perez-Cardona P.S., Barro Ojeda V., Rodriguez Pardo D., et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother. 2012;67:1749–1754. doi: 10.1093/jac/dks119. [DOI] [PubMed] [Google Scholar]
- 27.Lora-Tamayo J., Parra-Ruiz J., Rodriguez-Pardo D., et al. High doses of daptomycin (10 mg/kg/d) plus rifampin for the treatment of staphylococcal prosthetic joint infection managed with implant retention: a comparative study. Diagn Microbiol Infect Dis. 2014;80:66–71. doi: 10.1016/j.diagmicrobio.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Herrera S., Sorli L., Horcajada J.P. High-dose daptomycin together with rifampin as salvage therapy for prosthetic joint infections. Med Clin (Barc) 2017;149:223–224. doi: 10.1016/j.medcli.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Lai C.C., Sheng W.H., Wang J.T., et al. Safety and efficacy of daptomycin for the treatment of hospitalized adult patients in Taiwan with severe staphylococcal infections. J Microbiol Immunol Infect. 2012;45:52–57. doi: 10.1016/j.jmii.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y.J., Lee M.S., Lee C.H., Lin P.C., Kuo F.C. Daptomycin treatment in patients with resistant staphylococcal periprosthetic joint infection. BMC Infect Dis. 2017;17:736. doi: 10.1186/s12879-017-2842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo F.C., Yen S.H., Peng K.T., Wang J.W., Lee M.S. Methicillin-resistant Staphylococcal periprosthetic joint infections can be effectively controlled by systemic and local daptomycin. BMC Infect Dis. 2016;16:48. doi: 10.1186/s12879-016-1366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jugun K., Vaudaux P., Garbino J., et al. The safety and efficacy of high-dose daptomycin combined with rifampicin for the treatment of Gram-positive osteoarticular infections. Int Orthop. 2013;37:1375–1380. doi: 10.1007/s00264-013-1856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang S.Y., Khair H.N., McDonald J.R., Babcock H.M., Marschall J. Daptomycin versus vancomycin for osteoarticular infections due to methicillin-resistant Staphylococcus aureus (MRSA): a nested case-control study. Eur J Clin Microbiol Infect Dis. 2014;33:659–664. doi: 10.1007/s10096-013-2001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roux S., Valour F., Karsenty J., et al. Daptomycin & 6 mg/kg/day as salvage therapy in patients with complex bone and joint infection: cohort study in a regional reference center. BMC Infect Dis. 2016;16:83. doi: 10.1186/s12879-016-1420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montange D., Berthier F., Leclerc G., et al. Penetration of daptomycin into bone and synovial fluid in joint replacement. Antimicrob Agents Chemother. 2014;58:3991–3996. doi: 10.1128/AAC.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traunmuller F., Schintler M.V., Metzler J., et al. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J Antimicrob Chemother. 2010;65:1252–1257. doi: 10.1093/jac/dkq109. [DOI] [PubMed] [Google Scholar]
- 37.Goutelle S., Roux S., Gagnieu M.C., et al. Pharmacokinetic variability of daptomycin during prolonged therapy for bone and joint infections. Antimicrob Agents Chemother. 2016;60:3148–3151. doi: 10.1128/AAC.02597-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellington J.K., Harris M., Hudson M.C., Vishin S., Webb L.X., Sherertz R. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J Orthop Res. 2006;24:87–93. doi: 10.1002/jor.20003. [DOI] [PubMed] [Google Scholar]
- 39.Reilly S.S., Hudson M.C., Kellam J.F., Ramp W.K. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone. 2000;26:63–70. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 40.Bosse M.J., Gruber H.E., Ramp W.K. Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis. A case report. J Bone Joint Surg Am. 2005;87:1343–1347. doi: 10.2106/JBJS.D.02649. [DOI] [PubMed] [Google Scholar]
- 41.Valour F., Trouillet-Assant S., Riffard N., et al. Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrob Agents Chemother. 2015;59:2029–2036. doi: 10.1128/AAC.04359-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupieux C., Trouillet-Assant S., Camus C., et al. Intraosteoblastic activity of daptomycin in combination with oxacillin and ceftaroline against MSSA and MRSA. J Antimicrob Chemother. 2017;72:3353–3356. doi: 10.1093/jac/dkx314. [DOI] [PubMed] [Google Scholar]
- 43.Edmiston C.E., Jr, Goheen M.P., Seabrook G.R., et al. Impact of selective antimicrobial agents on staphylococcal adherence to biomedical devices. Am J Surg. 2006;192:344–354. doi: 10.1016/j.amjsurg.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Stein C., Makarewicz O., Forstner C., et al. Should daptomycin-rifampin combinations for MSSA/MRSA isolates be avoided because of antagonism? Infection. 2016;44:499–504. doi: 10.1007/s15010-016-0874-2. [DOI] [PubMed] [Google Scholar]
- 45.El Haj C., Murillo O., Ribera A., et al. Daptomycin combinations as alternative therapies in experimental foreign-body infection caused by meticillin-susceptible Staphylococcus aureus. Int J Antimicrob Agents. 2015;46:189–195. doi: 10.1016/j.ijantimicag.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Molina-Manso D., del Prado G., Ortiz-Perez A., et al. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int J Antimicrob Agents. 2013;41:521–523. doi: 10.1016/j.ijantimicag.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Wahlig H., Hameister W., Grieben A. Release of gentamicin from polymethyl methacrylate. I. Experimental in-vitro tests. Langenbecks Arch Chir. 1972;331:169–192. doi: 10.1007/BF01232226. [DOI] [PubMed] [Google Scholar]
- 48.Bertazzoni Minelli E., Benini A., Magnan B., Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53:329–334. doi: 10.1093/jac/dkh032. [DOI] [PubMed] [Google Scholar]
- 49.George J., Newman J.M., Klika A.K., et al. Changes in antibiotic susceptibility of Staphylococcus aureus between the stages of 2-stage revision arthroplasty. J Arthroplasty. 2018;33:1844–1849. doi: 10.1016/j.arth.2018.01.056. [DOI] [PubMed] [Google Scholar]