Abstract
Bladder cancer is a common malignant tumor in the urinary system. Depending on whether bladder cancer invades muscle tissue, it is classified into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). It is crucial to accurately diagnose the muscle invasion of bladder cancer for its clinical management. Although imaging modalities such as CT and multiparametric MRI play an important role in this regard, radiomics has shown great potential with the development and innovation of precision medicine. It features outstanding advantages such as non-invasive and high efficiency, and takes on important significance in tumor assessment and laor liberation. In this article, we provide an overview of radiomics in the prediction of muscle-invasive bladder cancer and reflect on its future trends and challenges.
Keywords: bladder cancer, radiomics, machine learning, muscle-invasive, CT, MRI
1 Introduction
Bladder cancer (BC) is the second most common cancer among urological malignancies, with an estimated 573,200 people diagnosed with BC worldwide in 2020 (1). The rates of bladder cancer increase with age. The risk of BC is multifactorial, with smoking (2) being the most important risk factor. Uroepithelial carcinoma accounts for approximately 90% of bladder cancer cases and typically presents as multifocal and recurrent; other subtypes are squamous cell carcinoma (6-8%) and adenocarcinoma (3).
Determining the invasion of the tumor into the muscle layer of the bladder wall is probably the most critical step in clinical management, as it directly affects the patient’s treatment strategy. Bladder cancers are classified into non-muscle-invasive bladder cancer (NMIBC) (≤ T1 stage) and muscle-invasive bladder cancer (MIBC) (≥ T2 stage) according to whether they invade muscle tissue or not. NMIBC is mostly in the early stages of the disease, with a 5-year probability of recurrence and progression of 78% and 45%, respectively (4), while MIBC has a poor prognosis, with approximately 50% (5) of patients developing metastases within 2 years after radical cystectomy(RC). NMIBC is usually treated by transurethral resection of bladder tumors(TURBT) with or without intravesical chemotherapy (6). Whereas MIBC is usually treated with radical cystectomy(RC), radiotherapy, chemotherapy, or combination therapy (5). Currently, pathological examination of TURBT specimens is the gold standard for identification of MIBC. However, according to previous studies, the error rate is about 20-80% due to problems such as differences in resection (7). Even though the error rate can be reduced by repeating TURBT, underestimation of staging and delayed treatment of the condition may lead to disease progression and worse prognosis, and this invasive operation also carries some safetyoperational risks. Faced with the above problems, scholars have searched for an alternative, non-invasive and efficient diagnostic tool to accurately predict muscle-invasive bladder cancer, so they have turned their attention to “radiomics” - a hot and promising diagnostic technology. Radiomics is the extraction and analysis of quantitative imaging features from imaging tools (CT, MRI, PET-CT, etc.) for the development of descriptive and predictive models (8). Machine learning (ML), a branch of artificial intelligence, is a typical approach used in radiomics model generation (9). Through the inferential training of datasets, ML aids in the development of highly accurate and effective predictive models based on radiomics analysis (10). In this paper, we review the current existing research related to our topic, summarize the results of using machine learning to accurately predict muscle-invasive bladder cancer, and reflect on the future directions and challenges of the topic.
2 Search criteria
A comprehensive review of current literature was performed using the PubMed-Medline and Web of Science database up to April 5, 2022 using “bladder cancer”, combined with one of the following terms: “radiomics”, “machine learning”, and “artificial intelligence” in combination with “muscle invasive”.
The exclusion criteria for the articles were as follows:
(1) Published in a language other than English.
(2) The purpose of the article study was not to predict muscle invasion of bladder cancer.
(3) The article was not studied with imaging tools.
(4) Reviews, conference abstracts, and editorials were excluded.
The inclusion criteria for the article were as follows:
(1) Background introduction of radiomics, machine learning, deep learning or artificial intelligence and bladder cancer.
(2) The purpose of the article study was to predict muscle invasion of bladder cancer.
(3) The article was studied with imaging tools(CT, MRI, PET-CT, SPECT e.g.).
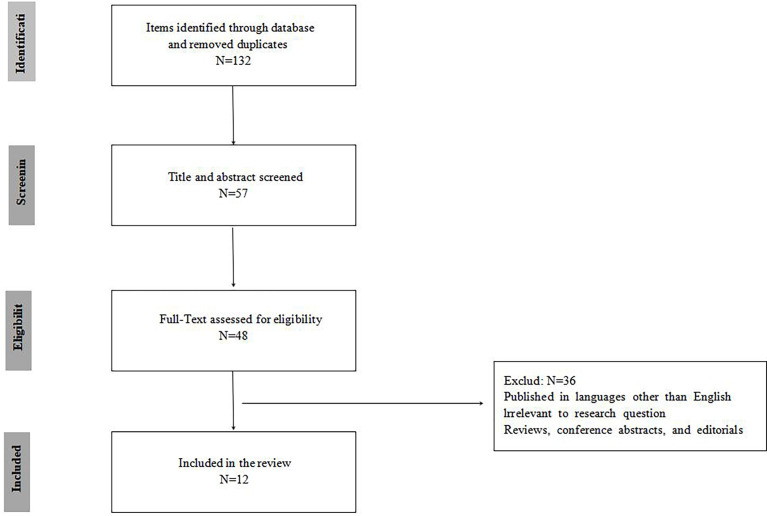
In accordance with the PRISMA criteria, Figure 1 was included to delineate our article
selection process.
Figure 1.
PRISMA flowchart of included studies.
3 Results
The final collection of 12 relevant publications found that the first study started in 2017, reflecting the fact that radiomics is a relatively new concept in the field of BC. The literature related to machine learning for predicting muscle-invasive bladder cancer is summarized in Table 1 (11–22). For studies in this field, four were based on enhanced CT and the remaining eight were related to MRI. Only 16.7% (2/12) of the studies were multi-center studies.
Table 1.
Studies included in the systematic review.
| Study characteristics | Patient characteristics | Imaging characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Study design | Number of cases | Number of selected lesions | Surgical technique | Pathological stage NMIBC: MIBC | Imaging modality | Scanner | |
| 1 | Xu | 2017 | Single-center retrospective | 78 | 118 | NA | 34:84 | T2WI | 3.0T GE |
| 2 | Garapati | 2017 | Single-center retrospective | 76 | 84 | Cystectomy | 43:41 | CTU | NA |
| 3 | Tong | 2018 | Single-center retrospective | 65 | 65 | Cystectomy | 31:34 | T2WI | 1.5-3.0T |
| 4 | Xu | 2019 | Single-center retrospective | 54 | 54 | NA | 24:30 | T2WI、DWI、ADC | 3.0T GE |
| 5 | Zheng | 2019 | Single-center retrospective | 199 | 199 | RC or TURBT | 130:69 | T2WI | 3.0T MR scanner (Intera Achieva, Philips Medical Systems) |
| 6 | Xu | 2020 | Single-center retrospective | 218 | 218 | Both TURBT and RC | 131:87 | DWI | 3.0T MR scanner (Ingenia;Philips Healthcare) |
| 7 | Wang | 2020 | Mult-center retrospective | 106 | 106 | RC or partial cystectomy or TURBT | 64:42 | T2WI、DWI、ADC | 3.0T MR system (MAGNETOM Trio, Siemens Healthineers) |
| 8 | Hammouda | 2021 | Single-center retrospective | 42 | NA | T2WI、DWI、ADC | 3.0T Ingenia Philips MRI scanners | ||
| 9 | Zhang | 2021 | Mult-center retrospective | 441 | 441 | RC or TURBT | 183(development):110(tuning ) :73(internal validation) :75(external validation) |
Enhanced CT | NA |
| 10 | Zheng | 2021 | Single-center retrospective | 185 | 185 | NA | 129:56 | T2WI、DCE | 3.0T MRI scanner(Magnetom Verio: Siemens, Erlangen, Germany) |
| 11 | Zhou | 2021 | Single-center retrospective | 100 | 100 | NA | 70:30 | Enhanced CT | Siemens 64-row spiral CT |
| 12 | Cui | 2022 | Single-center retrospective | 327 | 188 | RC or partial cystectomy or TURBT | 120:68 | CECT | GE Dis covery CT750HD, GE LightSpeed VCT, Philips ICT 256, and Siemens Somatom Definition Flash. |
ADC, apparent diffusion coeffificient; CECT, contrast-enhanced computed tomography; CT, computed tomography; CTU, CT Urography; DCE, dynamic contrast enhanced; DWI, diffusion-weighted imaging; MIBC, muscle-invasive bladder cancer; MR, magnetic resonance; MRI, magnetic resonance imaging; NA, not available; NMIBC, non–muscle-invasive bladder cancer; RC, radical cystectomy; TURBT, transurethral resection of bladder tumor; T2WI, T2-weighted imaging.
4 Discussion
4.1 Traditional diagnostic imaging
In current clinical practice, medical imaging techniques including CT, MRI and other non-invasive and safe diagnostic modalities are increasingly recognized for their performance in predicting muscle invasion and staging of bladder cancer. MRI has mainly been found to play a crucial role in the early localization and invasive diagnosis of BC. T2-weighted imaging(T2WI) is able to illustrate detailed structural information of the lesion and bladder wall, thus potentially reflecting the depth of invasion of the bladder wall of BC. The low signal line of the detrusor muscle is interrupted by MIBC, whereas the detrusor muscle is complete in NMIBC. Diffusion Weighted Imaging(DWI) and Apparent Diffusion Coefficient(ADC) have a good ability to reflect signal intensity differences between muscle, peritumoral inflammation and fibrosis (23, 24). The significance of dynamic contrast enhanced MRI(DCE-MRI) in assessing tumor aggressiveness depends on the neoangiogenesis of the tumor, which is an important factor in tumor growth; the more neovascularization there is, the higher the tumor stage and grade (25). In studies on dynamic enhancement sequences, the tumor, bladder mucosa and submucosa show early enhancement, but the bladder wall muscle maintains its low signal and delays enhancement. As early as 2000, Hayashi et al. observed that image signs of submucosal linear enhancement (SLE) at the base of the tumor were frequently seen on DCE images of NMIBC patients (26). This discovery is unquestionably a watershed moment in imaging-based BC staging and muscle-invasive status (MIS) diagnosis. Takeuchi et al (27) followed up by reporting an important feature found in most NMIBC on DWI, the tumor stalk, which improved the accuracy and robustness of imaging-based BC staging and MIS diagnosis. The accuracy of staging based on tumor stalk was 91.3% in Wang et al. study, while the accuracy of SLE staging was 91.3% (23). Panebianco et al (28) proposed Vesical Imaging-Reporting and Data System (VI-RADS) to quantify these signs on Multi-Parametric Magnetic Resonance Imaging (mpMRI) and to standardize the diagnostic procedure for image-based MIS prediction based on these features. This scoring system has effective diagnostic performance. In the Ueno et al. study, for example, the combined area under the curve(AUC) of five radiologists diagnosing MIBC was as high as 0.90 (29). Another prospective study also demonstrated the high diagnostic reliability of the VI-RADS score (AUC value of 0.94), especially for scores 1-2 and 3-5 (sensitivity 91.9%, 95%; specificity 91.1%, 95%) (30). The VI-RADS scoring method relies on expert visual perception judgment, yet it is still semi-qualitative. As a result, research into the objective and accurate radiomic detection of bladder cancer muscle invasion is required.
4.2 Radiomics
Radiomics is a relatively young concept, and Prof. Lambin originally described it in 2012 (31). Radiomics refers to the high-throughput extraction of image features from the region of interest (ROI) of radiological imaging techniques (CT, MR, but also PET, etc.) for automated analysis, using machine and deep learning techniques to extract critical information for accurate quantitative assessment of lesions, and ultimately for aiding in the diagnosis, classification, or grading of diseases. Radiomics inherits the technological benefits of reproducible, non-invasive radiological imaging over biopsy, making patient status monitoring and prognosis safer and more reliable.
Radiomics techniques can be classified into two groups: those using manual radiomics features and those using deep learning radiomics (32, 33) Traditional manual radiomics has the following four main processing tasks: image acquisition and preprocessing; image segmentation; feature extraction and quantification; and model building. The difference is that segmentation is not a necessity in the automated radiomics pipeline (33).. Radiomics has been increasingly studied in medical field for lung cancer, breast cancer, glioma, prostate cancer and other disorders (34–37). One of the current topics in bladder cancer research is the radiomics prediction of MIBC.
The pertinent radiomics literature is described below in terms of modality selection, volumes of interest (VOIs) segmentation, feature selection, model construction, and integration of clinical features, respectively.
4.2.1 Input modality
It mainly based on enhanced CT, MRI, with MRI accounting for (8/12) of the included literature. Since CT is weaker than MRI in discriminating soft tissues and the borders and bases of lesions are rarely distinguishable in discriminating MIS (38), there is a greater preference for MRI, mainly around T2WI, DWI and ADC and DCE sequences. In 2017, Garapati (11) and Xu et al. (12)established a precedent for using radiomics to predict MIS using CT and MRI, respectively, and inspired readers to combine additional MRI sequences to improve the possibility of differentiation task performance. As a result, extensive research on the precise differentiation of NMIBC and MIBC using radiomic methods with multi-parametric MRI images started to be conducted. Xu et al. obtained mean accuracies of 79.63%, 81.37%, and 91.22% for T2WI, DWI, and the combined of both sequences, with AUCs of 0.8828, 0.8884, and 0.9756, respectively (14). The superiority of DWI sequences over T2WI sequences in reflecting heterogeneous differences between NMIBC and MIBC (14, 16) has been repeatedly demonstrated. This might be because muscle-infiltrating tumors have a propensity to impede water molecule diffusion by shrinking extracellular space (39–41), which is better captured by DWI and the related ADC maps. And multi-sequence MRI was more helpful to predict the muscle invasion condition of BC preoperatively compared with single sequence T2WI and DWI, which was consistent with previous knowledge.
4.2.2 Volumes of interest segmentation
The three basic methods of delineating the area of interest are manual, semi-automated, and automatic. Even with computerized techniques, radiologists still need to examine and manually adjust them to assure the correctness of ROI descriptions because the majority of them are still primarily manual, which takes time and is tiresome. Initially, academics mostly concentrated on the overall tumor volume. As research developed, it was generally acknowledged that the information in the region around the tumor also held a lot of relevant information. The body of literature suggests that the determination of muscle invasiveness is related to bladder tumors as well as the tumor’s base (15) and adjacent bladder tissue (13). In addition, most of the relevant experiments have been conducted so far at the 3D level. Compared to 2D system analysis, 3D has higher precision and AUC (95.24% and 0.9864 vs. 92.86% and 0.9705) (18) which reflects the importance of 3D processing as it provides a comprehensive BC assessment with full descriptive information and details.
4.2.3 Feature extraction and quantization
Currently, there are mainly shape and intensity features based on histogram, texture features including gray level co-occurrence matrix (GLCM), gray Level run length matrix (GLRLM), gray-level size zone matrix (GLSZM), gray level dependence matrix (GLDM), neighborhood gray-tone difference matrix (NGTDM), and higher-order feature wavelet features. The global, local and regional distribution features of image grayscale can be comprehensively described. Although there are a large number of features available for analysis, redundancy of features can seriously affect prediction performance. So feature selection is essential for developing optimal prediction models. Combined with other advanced selection strategies for statistical analysis, such as support vector machine (SVM)-based recursive feature elimination (SVM-RFE), the least absolute shrinkage and selection operator
(LASSO), max-relevance and min-redundancy(mRMR), these methods are widely used to reduce the impact of feature redundancy, and other methods such as Boruta are also used. After feature selection, Xu et al. found that the run length matrix (RLM) features accounted for a greater proportion of 13/19 in the optimal subset (14), better reflecting the regional heterogeneity differences between NMIBC and MIBC. The Co-occurrence matrices(CM), RLM and GLSZM features were found to be favorable feature classes for predicting BCa muscle invasion condition by Wang et al. (16).
4.2.4 Model construction
Different machine learning classifiers can be employed with the chosen features to create predictive models. Classifiers that are typically used include LASSO, SVM, random forest (RF), logistic regression, etc. Convolutional neural networks (CNN) are the most commonly used artificial neural networks for deep learning. SVM-RFE was the most commonly used machine learning method (7/12), among all the methods used for the classification task. Table 2 demonstrates how different models’ prediction efficacy varies. NN, SVM, and RF classifier diagnostic performance were tested by Hammouda et al. in descending order (18). Garapati et al. observed that the AUC for morphological and texture features was roughly 0.90 (11); for various other mri-based radiomics models, the AUC ranged from 0.87 to 0.98 (14–17). However, all of the preceding experiments have the disadvantage of lacking independent external validation, so the true validity of the diagnostic performance of these models must be confirmed further. In contrast, so far, the prediction model developed by Zhang et al. is the only experiment with external validation results. But the AUC (0.791-0.936) of the study by Zhang et al. was slightly lower (19). This may be the risk of misclassification of some models influenced by tumor size, which may lead to a decrease in the diagnostic performance of the model, and therefore tumor size is one of the critical features to determine the muscle invasion condition of BC.
Table 2.
Radiomic characteristics of studies included in the systematic review.
| Author | Segmentation method | Radiomic feature categories | Machine-learning method for feature selection | Number of selected features | Model | AUC of radiomic model with the best performance | clinical factor | AUC of radiomic-clinical model | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Training set | Validation set | Training set | Validation set | |||||||
| Xu | Semi-automatic segmentation(3D) | Signal intensity histogram-based features and 3D ND-Haralick texture features based intensity and its high-order derivative maps | SVM-RFE","SMOTE | 13 | SVM-RFE | 0.861 | NA | NA | NA | NA |
| Garapati | Automatic segmentation(3D) | First-order statistics, shape, contrast, GLRLM, | Stepwise feature selection | 3 subsets of radiomic features | LDA, NN, SVM, RF | 0.97 | NA | NA | NA | NA |
| Tong | Manual segmentation(3D) | LBP、GLCM | An optimal biomarker approach | 9 | SVM | Patient level:0.806,radial sector level:0.813 | NA | NA | NA | NA |
| Xu | Manual segmentation(2D) | Histogram, CM , RLM, | SVM-RFE","SMOTE, | 19 | SVM-RFE | 0.9857 | NA | NA | NA | NA |
| Zheng | Semi-automatic segmentation(3D) | first-order statistics,shape-based,GLCM,GLRLM,GLSZM,NGTDM,and GLDM | LASSO LR | 23 | LASSO | 0.913;Optimism-corrected:0.912 | 0.874 | Tumor size | 0.922;optimism-corrected AUC of 0.921 | 0.876 |
| Xu | Manual segmentation and automatic segmentation(3D) | First-order intensity features,high-order texture features,and shape ,GLCM,GLRLM,GLSZM and NGTDM | Boruta | 21 | RF, AR | .0.907 | 0.904 | RandomForest model and TURBT | NA | NA |
| Wang | Manual segmentation and automatic segmentation(2D) | Histogram , CM, RLM, NGTDM and GLSZM | SVM-RFE | 36 | LR, LASSO | 0.88 | external validation cohort 0.813 | Radscore and tumor stalk | 0.924 | 0.877 |
| Hammouda | Automatic segmentation(3D) | Histogram ,GLCM,GLRLM,and morphological features | NA | NA | NN(best)","RF,SVM | 0.9864 | NA | NA | NA | |
| Zhang | Semi-automatic segmentation(3D) | NA | NA | NA | FGP-Net | development cohort:0.936, tuning cohort:0.891 | internal validation cohort: 0.861,external validation cohort: 0.791 | NA | NA | NA |
| Zheng | manual segmentation (3D) | shape and size-based features, image intensity, textural features and wavelet features | mRMR | 40 | Lasso(best)、SVM、RF | 0.934 | 0.906 | VI-RADS | 0.97 | 0.943 |
| Zhou | semi-automatic segmentation(3D) | GLDM,Shape2D,GLCM,Shape3D,First-order,GLRLM,GLSZM,and NGTDM | SVM-RFE | 6 | LR, Decision Tree, SVM(best), and Adaboost algorithm | 0.898 | 0.702 | Rad-score, albuminuria and metabolic syndrome | 0.8457 | |
AR, all-relevant model; AUC, area under the curve; CM, Co-occurrence matrices; 3D, three dimensional; 2D, two dimensional; FGP-Net, Filter-guided Pyramid Network; GLCM, grey-level co-occurrence matrix; GLDM, gray level dependence matrix; GLRLM, gray-level run length matrix; GLSZM, gray-level size zone matrix; LASSO, least absolute shrinkage and selection operator; LBP, local binary pattern; LDA, linear discriminant analysis; LR, logistic regression; mRMR, min-redundancy; NA, not available; ND, nondirectional Haralic textural features; NN, neural network; NGTDM, neighborhood gray-tone difference matrix; RF, random forest model; RFE, recursive feature elimination; RLM, run length matrix; SMOTE, synthetic minority oversampling technique; SVM, support vector machine classififier; TURBT, transurethral resection of bladder tumor.
4.2.5 Integration of other clinical factors
It has become a trend to include clinical risk factors in the prediction model in order to better predict MIS and improve clinical diagnostic performance and application value. These include tumor size (15), tumor stalk (16), proteinuria and multiple sclerosis (21), as well as VI-RADS (20) and TURBT (14).The radiomic model incorporating clinical factors performed significantly better than the conventional MRI examination and simply radiomic model in terms of calibration and discrimination. Radiomic-clinical nomogram can be used as a reliable and non-invasive adjunct to differentiate MIBC from NMIBC preoperatively (15).
4.2.6 Method for validating results
83.3 percent (10/12) of the retrieved literature were single-center studies (11–15, 17, 18, 20–22), and the internal validation method was primarily used for model validation. Only two paper performing external validation of the results (16, 19). Because of the lack of externally validated results, the reliability of the remaining articles’ results in terms of diagnostic efficacy is questionable. The sensitivity, specificity, and AUC of the internal validation cohort in Zhang’s prediction model were 0.733, 0.810, and 0.861, respectively, while those of the external validation cohort were 0.710, 0.773, and 0.791, respectively (19).
5 Future and prospects
Of these 12 studies, all were retrospective, subject to selection bias and prone to data loss. Because the sample size was insufficient, cross-validation was essentially required to make up for it. Additionally, only two of the results were externally validated using radiomics models, with the rest being single-center, internally validated results that were not convincing. The current radiomics models are mainly based on single-modality or dual-modality MRI, and there is no multi-modality study combining the three sequences of “T2WI, DWI and DCE”, which needs to be further validated to improve the differentiation performance. Therefore, investigations should be planned in a more thorough and subtle manner for a variety of therapeutic applications to increase the reliability of the results. To completely understand the diagnostic usefulness of machine learning in predicting MIBC, more prospective multi-center and various machine trials will be required in the future. In addition, for future optimization of this new approach, more studies are needed to test the potential of optimizing predictive models by combining imaging biomarkers with other non-imaging biomarkers, such as urine and serum biomarkers. Although there have been significant advances in a number of studies, from fundamental tumor identification to precise staging and grading, recent research has also been gradually moving toward the prediction of treatment outcomes. The needs of the clinical market can no longer be met by illness diagnosis alone. After a bladder cancer diagnosis, increasing focus will be placed on how well machine learning predicts the response to treatment and prognosis outcome of the disease In the future.
Author contributions
FL and YL contributed to the conception of the study. XW contributed significantly to analysis and manuscript preparation. XH performed the data analyses and wrote the manuscript. XL and JD organized and drew tables. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by China International Medical Foundation (No. Z-2014-07-2101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol (2014) 66(1):59–73. doi: 10.1016/j.eururo.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 3. Verma S, Rajesh A, Prasad SR, Gaitonde K, Lall CG, Mouraviev V, et al. Urinary bladder cancer: Role of MR imaging. Radiographics (2012) 32(2):371–87. doi: 10.1148/rg.322115125 [DOI] [PubMed] [Google Scholar]
- 4. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol (2006) 49(3):466–5; discussion 475-7. doi: 10.1016/j.eururo.2005.12.031 [DOI] [PubMed] [Google Scholar]
- 5. Sherif A, Jonsson MN, Wiklund NP. Treatment of muscle-invasive bladder cancer. Expert Rev Anticancer Ther (2007) 7(9):1279–83. doi: 10.1586/14737140.7.9.1279 [DOI] [PubMed] [Google Scholar]
- 6. Josephson D, Pasin E, Stein JP. Superficial bladder cancer: Part 2. management. Expert Rev Anticancer Ther (2007) 7(4):567–81. doi: 10.1586/14737140.7.4.567 [DOI] [PubMed] [Google Scholar]
- 7. Turker P, Bostrom PJ, Wroclawski ML, van Rhijn B, Kortekangas H, Kuk C, et al. Upstaging of urothelial cancer at the time of radical cystectomy: Factors associated with upstaging and its effect on outcome. BJU Int (2012) 110(6):804–11. doi: 10.1111/j.1464-410X.2012.10939.x [DOI] [PubMed] [Google Scholar]
- 8. Ferro M, de Cobelli O, Musi G, Del Giudice F, Carrieri G, Busetto GM, et al. Radiomics in prostate cancer: An up-to-date review. Ther Adv Urol (2022) 14:17562872221109020. doi: 10.1177/17562872221109020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tătaru OS, Vartolomei MD, Rassweiler JJ, Virgil O, Lucarelli G, Porpiglia F, et al. Artificial intelligence and machine learning in prostate cancer patient management-current trends and future perspectives. Diagnostics (Basel) (2021) 11(2):354. doi: 10.3390/diagnostics11020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ge L, Chen Y, Yan C, Zhao P, Zhang P, A R, et al. Study progress of radiomics with machine learning for precision medicine in bladder cancer management. Front Oncol (2019) 9:1296. doi: 10.3389/fonc.2019.01296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garapati SS, Hadjiiski L, Cha KH, Chan HP, Caoili EM, Cohan RH, et al. Urinary bladder cancer staging in CT urography using machine learning. Med Phys (2017) 44(11):5814–23. doi: 10.1002/mp.12510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X, Liu Y, Zhang X, Tian Q, Wu Y, Zhang G, et al. Preoperative prediction of muscular invasiveness of bladder cancer with radiomic features on conventional MRI and its high-order derivative maps. Abdom Radiol (NY) (2017) 42(7):1896–905. doi: 10.1007/s00261-017-1079-6 [DOI] [PubMed] [Google Scholar]
- 13. Tong Y, Udupa JK, Wang C, Chen J, Venigalla S, Guzzo TJ, et al. Radiomics-guided therapy for bladder cancer: Using an optimal biomarker approach to determine extent of bladder cancer invasion from t2-weighted magnetic resonance images. Adv Radiat Oncol (2018) 3(3):331–8. doi: 10.1016/j.adro.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Zhang X, Tian Q, Wang H, Cui LB, Li S, et al. Quantitative identification of nonmuscle-invasive and muscle-invasive bladder carcinomas: A multiparametric MRI radiomics analysis. J Magn Reson Imaging (2019) 49(5):1489–98. doi: 10.1002/jmri.26327 [DOI] [PubMed] [Google Scholar]
- 15. Zheng J, Kong J, Wu S, Li Y, Cai J, Yu H, et al. Development of a noninvasive tool to preoperatively evaluate the muscular invasiveness of bladder cancer using a radiomics approach. Cancer (2019) 125(24):4388–98. doi: 10.1002/cncr.32490 [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Xu X, Zhang X, Liu Y, Ouyang L, Du P, et al. Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: a double-center study. Eur Radiol (2020) 30(9):4816–27. doi: 10.1007/s00330-020-06796-8 [DOI] [PubMed] [Google Scholar]
- 17. Xu S, Yao Q, Liu G, Jin D, Chen H, Xu J, et al. Combining DWI radiomics features with transurethral resection promotes the differentiation between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Eur Radiol (2020) 30(3):1804–12. doi: 10.1007/s00330-019-06484-2 [DOI] [PubMed] [Google Scholar]
- 18. Hammouda K, Khalifa F, Soliman A, Ghazal M, El-Ghar MA, Badawy MA, et al. A multiparametric MRI-based CAD system for accurate diagnosis of bladder cancer staging. Comput Med Imaging Graph (2021) 90:101911. doi: 10.1016/j.compmedimag.2021.101911 [DOI] [PubMed] [Google Scholar]
- 19. Zhang G, Wu Z, Xu L, Zhang X, Zhang D, Mao L, et al. Deep learning on enhanced CT images can predict the muscular invasiveness of bladder cancer. Front Oncol (2021) 11:654685. doi: 10.3389/fonc.2021.654685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng Z, Xu F, Gu Z, Yan Y, Xu T, Liu S, et al. Combining multiparametric MRI radiomics signature with the vesical imaging-reporting and data system (VI-RADS) score to preoperatively differentiate muscle invasion of bladder cancer. Front Oncol (2021) 11:619893. doi: 10.3389/fonc.2021.619893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Q, Zhang Z, Ang X, Zhang H, Ouyang J. A nomogram combined with radiomics features, albuminuria, and metabolic syndrome to predict the risk of myometrial invasion of bladder cancer. Transl Cancer Res (2021) 10(7):3177–91. doi: 10.21037/tcr-21-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui Y, Sun Z, Liu X, Zhang X, Wang X. CT-based radiomics for the preoperative prediction of the muscle-invasive status of bladder cancer and comparison to radiologists' assessment. Clin Radiol (2022) 77(6):e473–82. doi: 10.1016/j.crad.2022.02.019 [DOI] [PubMed] [Google Scholar]
- 23. Wang HJ, Pui MH, Guo Y, Yang D, Pan BT, Zhou XH, et al. Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdom Imaging (2014) 39(1):135–41. doi: 10.1007/s00261-013-0038-0 [DOI] [PubMed] [Google Scholar]
- 24. Wang HJ, Pui MH, Guan J, Li SR, Lin JH, Pan B, et al. Comparison of early submucosal enhancement and tumor stalk in staging bladder urothelial carcinoma. AJR Am J Roentgenol (2016) 207(4):797–803. doi: 10.2214/AJR.16.16283 [DOI] [PubMed] [Google Scholar]
- 25. Abouelkheir RT, Abdelhamid A, Abou El-Ghar M, El-Diasty T. Imaging of bladder cancer: Standard applications and future trends. Medicina (Kaunas) (2021) 57(3):220. doi: 10.3390/medicina57030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayashi N, Tochigi H, Shiraishi T, Takeda K, Kawamura J. A new staging criterion for bladder carcinoma using gadolinium-enhanced magnetic resonance imaging with an endorectal surface coil: A comparison with ultrasonography. BJU Int (2000) 85(1):32–6. doi: 10.1046/j.1464-410x.2000.00358.x [DOI] [PubMed] [Google Scholar]
- 27. Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: Diffusion-weighted MR imaging–accuracy for diagnosing T stage and estimating histologic grade. Radiology (2009) 251(1):112–21. doi: 10.1148/radiol.2511080873 [DOI] [PubMed] [Google Scholar]
- 28. Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, et al. Multiparametric magnetic resonance imaging for bladder cancer: Development of VI-RADS (Vesical imaging-reporting and data system). Eur Urol (2018) 74(3):294–306. doi: 10.1016/j.eururo.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueno Y, Takeuchi M, Tamada T, Sofue K, Takahashi S, Kamishima Y, et al. Diagnostic accuracy and interobserver agreement for the vesical imaging-reporting and data system for muscle-invasive bladder cancer: A multireader validation study. Eur Urol (2019) 76(1):54–6. doi: 10.1016/j.eururo.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 30. Del Giudice F, Barchetti G, De Berardinis E, Pecoraro M, Salvo V, Simone G, et al. Prospective assessment of vesical imaging reporting and data system (VI-RADS) and its clinical impact on the management of high-risk non-muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur Urol (2020) 77(1):101–9. doi: 10.1016/j.eururo.2019.09.029 [DOI] [PubMed] [Google Scholar]
- 31. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer (2012) 48(4):441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol (2022) 19(2):132–46. doi: 10.1038/s41571-021-00560-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldenberg SL, Nir G, Salcudean SE. A new era: artificial intelligence and machine learning in prostate cancer. Nat Rev Urol (2019) 16(7):391–403. doi: 10.1038/s41585-019-0193-3 [DOI] [PubMed] [Google Scholar]
- 34. Smith CP, Czarniecki M, Mehralivand S, Stoyanova R, Choyke PL, Harmon S, et al. Radiomics and radiogenomics of prostate cancer. Abdom Radiol (NY) (2019) 44(6):2021–9. doi: 10.1007/s00261-018-1660-7 [DOI] [PubMed] [Google Scholar]
- 35. Tagliafico AS, Piana M, Schenone D, Lai R, Massone AM, Houssami N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast (2020) 49:74–80. doi: 10.1016/j.breast.2019.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi YS, Bae S, Chang JH, Kang SG, Kim SH, Kim J, et al. Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro Oncol (2021) 23(2):304–13. doi: 10.1093/neuonc/noaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolinger GD, García DV, Kramer GM, Frings V, Zwezerijnen GJC, Smit EF, et al. Effects of tracer uptake time in non-small cell lung cancer (18)F-FDG PET radiomics. J Nucl Med (2022) 63(6):919–24. doi: 10.2967/jnumed.121.262660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu X, et al. Study progress of noninvasive imaging and radiomics for decoding the phenotypes and recurrence risk of bladder cancer. Front Oncol (2021) 11:704039. doi: 10.3389/fonc.2021.704039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hafeez S, Huddart R. Advances in bladder cancer imaging. BMC Med (2013) 11:104. doi: 10.1186/1741-7015-11-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mannelli L, Nougaret S, Vargas HA, Do RK. Advances in diffusion-weighted imaging. Radiol Clin North Am (2015) 53(3):569–81. doi: 10.1016/j.rcl.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X, Xu X, Tian Q, Li B, Wu Y, Yang Z, et al. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J Magn Reson Imaging (2017) 46(5):1281–8. doi: 10.1002/jmri.25669 [DOI] [PMC free article] [PubMed] [Google Scholar]