Abstract
Objective
The aim of this study was to assess the SLN detection rate in presumed early stage, low- and intermediate-risk endometrial cancers, the incidence of SLN metastases, and the negative predictive value of SLN mapping performed with indocyanine green (ICG).
Methods
A systematic review with meta-analyses was conducted. Study inclusion criteria were A) low- and intermediate-risk endometrial cancer, B) the use of ICG per cervical injection; C) a minimum of twenty included patients per study. To assess the negative predictive value of SLN mapping, D) a subsequent lymphadenectomy was an additional inclusion criterion.
Results
Fourteen studies were selected, involving 2,117 patients. The overall and bilateral SLN detection rates were 95.6% (95% confidence interval [CI]=92.4%–97.9%) and 76.5% (95% CI=68.1%–84.0%), respectively. The incidence of SLN metastases was 9.6% (95% CI=5.1%–15.2%) in patients with grade 1–2 endometrial cancer and 11.8% (95% CI=8.1%–16.1%) in patients with grade 1–3 endometrial cancer. The negative predictive value of SLN mapping was 100% (95% CI=98.8%–100%) in studies that included grade 1–2 endometrial cancer and 99.2% (95% CI=97.9%–99.9%) in studies that also included grade 3.
Conclusion
SLN mapping with ICG is feasible with a high detection rate and negative predictive value in low- and intermediate-risk endometrial cancers. Given the incidence of SLN metastases is approximately 10% in those patients, SLN mapping may lead to stage shifting with potential therapeutic consequences. Given the high negative predictive value with SLN mapping, routine lymphadenectomy should be omitted in low- and intermediate-risk endometrial cancer.
Keywords: Endometrial Cancer, Sentinel Lymph Node Mapping, Lymphadenectomy, Indocyanine Green, Systematic Review, Meta-Analysis
Synopsis
Sentinel lymph node mapping with indocyanine green is feasible with a high detection rate and high negative predictive value in low- and intermediate-risk endometrial cancers.
INTRODUCTION
The vast majority of patients (80%) with endometrial cancer are being diagnosed at an early stage [1]. These patients can be divided into a low-, intermediate- or high-risk classification, based on age, International Federation of Gynecology and Obstetrics stage, histological type, grade of the tumor and myometrial invasion with or without lymphovascular space invasion [1]. The presence of lymph node metastasis is the most important prognostic factor [2,3]. Until recently, complete pelvic with or without para-aortic lymphadenectomy was the recommended strategy to assess lymph node status. Lymphadenectomy is associated with a significant risk of morbidity, especially lymphedema [4,5]. In patients with a high-risk classification, the incidence of risk of lymph node metastasis is up to 25% [6,7,8]. It is therefore of great importance to properly assess the lymph node status and to set the indication for adjuvant therapy, since multiple studies show a survival advantage in women with lymph node metastasis who were treated with adjuvant chemotherapy [9,10]. In patients with presumed low- or intermediate-risk endometrial cancers, the incidence of lymph node metastases has been reported up to 15%, depending on immunohistochemical characteristics of the tumor such as hormone receptor loss [7,11,12]. A complete lymphadenectomy or lymph node sampling is often omitted in these patients given the lower risk of lymph node metastasis and the significant risk of morbidity [1]. Sentinel lymph node (SLN) mapping is a proposed alternative to assess lymph node status. It is performed by injecting dye with or without a radiotracer into the cervical stroma which then allows the identification of the SLN(s) usually with laparoscopic or robotic imaging systems [13]. Removal of specific SLNs is associated with a lower risk of complications compared to a complete lymph node dissection. SLN mapping with pathological ultrastaging (i.e., serial sectioning and immunohistochemistry) has been shown to increase the detection of metastases, largely with detection of micrometastasis and isolated tumor cell metastases [14,15]. Recent studies have shown that SLN mapping is feasible, safe, and cost-effective, with the highest detection rate achieved by using indocyanine green (ICG) as cervical injection [14,16,17,18,19]. The added value of SLN mapping in high-risk tumors is inevitable given the a priori chance of lymph node metastasis. More discussion subsists about the need for SLN mapping in low- and intermediate-risk endometrial cancer. However, with the known discrepancy between the preoperative and postoperative tumor grade, leading to upgrading in 25% of patients with preoperative grade 1 and 2 tumors, and the impact of immunohistochemical characteristics in mind, SLN mapping seems justifiable in presumed low- and intermediate-risk endometrial cancer as well [7,12,20]. The European Society of Gynaecological Oncology guideline therefore states that SLN mapping should be performed in patients with presumed early stage high-intermediate-risk and high-risk endometrial cancer and that it can be considered for patients with low- and intermediate-risk endometrial cancer. This is implemented in multiple European countries [16].
Previous reviews on SLN mapping were rather heterogeneous in their inclusion criteria, with the use of multiple tracers such as blue dye with or without technetium, and multiple injection sites (cervical stroma, subserous, fundal) [21,22]. The aim of this systematic review is to assess the detection rate of SLN mapping in presumed early stage, low- and intermediate-risk endometrial cancer by using a cervical injection of ICG and to assess the diagnostic value of SLN mapping compared to a systematic (pelvic) lymph node dissection.
METHODS
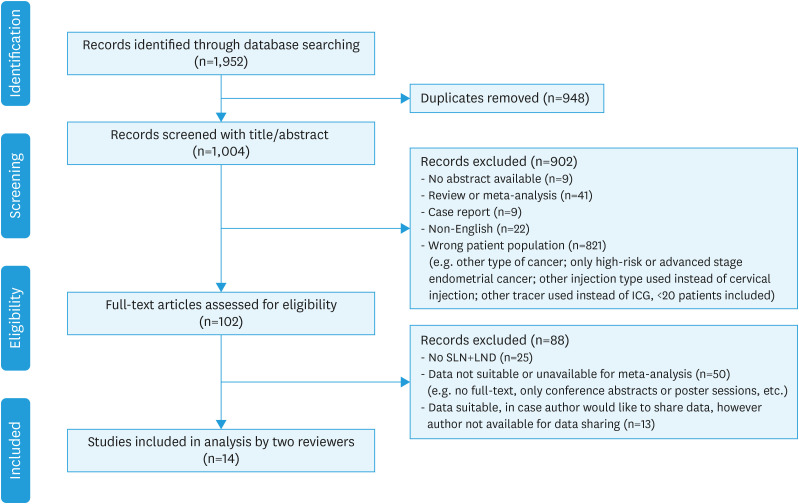
This systematic review was conducted by following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement [23]. A summary of the literature search and study selection is depicted in Fig. 1.
Fig. 1. Summary of the peer-reviewed literature search and selection. All processes were conducted by 2 independent reviewers (LB/SV).
IGC, indocyanine green; LND, lymph node dissection; SLN, sentinel lymph node.
1. Search strategy
A systematic search using electronic databases was performed in February 2021 and repeated in October 2021, using the following search engines: PubMed, Embase, Web of Science, the Cochrane Library and ClinicalTrials.gov. No filter on year of publication was set; all studies published up until the date of the search were taken into account. The search query combined synonyms, including MeSH-terms, for endometrial cancer, SLN mapping and lymph node dissection. Language was restricted to English. Reviews and case reports were excluded. Despite the focus on low- and intermediate-risk endometrial cancer and the use of ICG as marker for SLNs, these terms were not included in the search strategy because of their large limiting effect on the search yield. Furthermore, text words were combined to search for relevant peer-reviewed literature. We also performed a reference and related article search. Duplicate articles were filtered using Rayyan. Titles and abstracts were screened; in case an accurate judgment for inclusion was difficult, the original document was assessed by applying the selection and eligibility criteria.
2. Eligibility criteria
To assess the detection rate of SLN mapping, the following eligibility criteria were used: A) early stage, low- and intermediate-risk endometrial cancer (endometrioid histology, histological grade 1 and 2); B) cervical injection with ICG; C) a minimal number of twenty included patients per study. To assess the diagnostic value of SLN mapping, D) a subsequent (pelvic) lymph node dissection was an additional eligibility criterion. Articles that also included grade 3 endometrioid tumors in addition to grade 1 and 2 tumors were not excluded, as this would greatly lower the yield of articles. In articles in which grade 3 tumors were included as well, it was necessary to distinguish grade 1–2 from grade 3 tumors. The performance of ultrastaging on SLN specimen was not a specific criterion for inclusion or exclusion.
3. Study selection and data extraction
After the full-text assessment for eligibility, all articles that met the eligibility criteria of one or both study aims (i.e., detection rate and/or negative predictive value) were selected. As not all relevant data could be extracted directly from those articles, we contacted the corresponding authors of these articles, and asked them to share their raw data. Articles of authors not responding, not willing, or not able to share their data, were excluded. Eventually, peer-reviewed and published studies were selected that met the eligibility criteria. The eligibility of all articles was independently assessed by 2 reviewers (LB/SV). Data extraction of the remaining fourteen articles included author, year of publication, number of included patients, histological grade of the tumor, results of SLN mapping (bilateral, unilateral or no identification of the SLN), SLN metastasis, and if applicable results of (pelvic) lymph node dissection. As some data were provided by the corresponding authors, not all data can be retrieved directly from the original articles.
4. Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to evaluate the quality of the selected studies [24]. This tool is developed for quality assessment determining the risk of bias and the applicability. This is based on 4 domains: patient selection, index test, reference standard, and flow and timing. Each domain was judged at ‘low risk’, ‘high risk’ or ‘unclear risk’ of bias. The QUADAS-2 assessment was conducted by 2 independent reviewers (LB/SV); in case of not matching opinions, consensus was reached after mutual discussion.
5. Statistical analyses
Random-effects meta-analyses were conducted to estimate the overall detection rate, the bilateral detection rate, the unilateral detection rate, the incidence of SLN metastases, and the negative predictive value, based on the aggregate data as provided in the source papers or by the authors. The meta-analyses were conducted using the inverse variance method, the restricted maximum-likelihood estimator for τ2, the Q-profile method for confidence intervals of τ2 and τ, the Hartung-Knapp-Sidik-Jonkman adjustment for random effects model and an arcsine transformation of the proportions [25,26]. The estimates of the detection rate were based on all studies without making a distinction in histological grade, meaning that all studies including histological grades 1, 2, and 3 were included, as we assumed that the grade of the tumor has no effect on whether or not an SLN is found during surgery. The negative predictive value of SLN mapping, compared to a systematic (pelvic) lymph node dissection, was estimated in 2 ways: for studies including histological grade 1 and 2 only (true low- and intermediate-risk endometrial cancer patients), and for studies including all 3 histological grades. The negative predictive value was based on all cases (both bilateral and unilateral mapping). The individual study proportions in the forest plots were presented with Clopper-Pearson 95% confidence intervals (CIs). Analyses were conducted using the statistical software R version 3.6.2 and package meta version 4.18-2 [27].
RESULTS
1. Study selection
A total of 1,952 publications were identified through database searching. After duplicate removal and title and abstract screening 102 articles remained (Fig. 1). Those articles underwent full-text assessment, which led to 27 articles that met the eligibility criteria. Since not all data could be extracted directly from the articles, corresponding authors were asked to share their raw data. Not all authors responded or were willing or able to share their data, leading to another thirteen exclusions. Eventually, fourteen peer-reviewed and published studies were selected that met all the eligibility criteria, and of which data were available (Fig. 1) [14,15,28,29,30,31,32,33,34,35,36,37,38,39]. The risk of bias, using the QUADAS-2 tool, is depicted in Figs. S1 and S2. Six studies were judged at ‘high-risk’ in the domain ‘reference standard’; in those studies, a subsequent lymph node dissection after SLN mapping was not performed on standard basis. An ‘unclear risk’ of bias or concerns regarding applicability was judged if the patient selection was not quite clear or if the index test did not meet the criteria completely (e.g., SLN mapping with ICG in combination with blue dye, or histological assessment of SLNs did not include ultrastaging).
2. Study characteristics
Fourteen studies were included, and relevant data was extracted in a database (Table 1). More extended information on the included studies is presented in Table S1. Thirteen studies were observational cohort studies of which 4 were prospective and 9 were retrospective. The remaining study was a randomized controlled trial [34]. All patients were diagnosed with presumed early-stage endometrioid endometrial cancer (n=2,620). The majority of patients had histological grade 1 or 2 endometrioid endometrial cancer (7 studies, n=2,267, 87%). Studies that also included patients with grade 3 (n=305 patients, 11%) or unknown grade endometrioid tumors (n=48 patients, 2%) in addition to grade 1 and 2 tumors were not excluded (all patients with grade 3 or unknown tumor grade: 7 studies, n=353 patients, 13%).
Table 1. Baseline information of included studies (n=14) which are included in the analysis of the detection rate of SLN mapping (all studies).
| Author | Year of publication | Study design | Study population | Tumor grade (all endometrioid histology) | Patients with SLN mapping with ICG | SLN detection | SLN meta-stasis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ? | Bilateral | Unilateral | None | ||||||
| Backes et al. [28] | 2019 | P | 204 | 127 | 26 | 51 | 0 | 204 | 138 | 46 | 20 | 32 |
| Bogani et al. [29] | 2020 | R | 62 | 12 | 41 | 9 | 0 | 62 | 59 | 3 | 0 | 9 |
| Buda et al. [30] | 2016 | R | 85 | 35 | 50 | 0 | 0 | 25 | 22 | 3 | 0 | 6 |
| Clinton et al. [31] | 2017 | R | 350 | 212 | 138 | 0 | 0 | 187 | 120 | 65 | 2 | 24 |
| Cusimano et al. [32] | 2021 | P | 156 | 0 | 30 | 126 | 0 | 156 | 121 | 31 | 4 | G1–2: 3 |
| G1–3: 24 | ||||||||||||
| Diniz et al. [33] | 2021 | R | 253 | 167 | 86 | 0 | 0 | 68 | 62 | 3 | 3 | 7 |
| Ditto et al. [34] | 2020 | P | 121 | 60 | 61 | 0 | 0 | 121 | 85 | 19 | 17 | 8 |
| Holloway et al. [15] | 2016 | R | 119 | 46 | 44 | 29 | 0 | 119 | 96 | 21 | 2 | 35 |
| Papadia et al. [35] | 2016 | R | 65 | 24 | 41 | 0 | 0 | 65 | 59 | 4 | 2 | 6 |
| Rossi et al. [14] | 2017 | P | 340 | 152 | 102 | 38 | 48 | 340 | 177 | 116 | 47 | 35 |
| Stephens et al. [36] | 2020 | R | 323 | 212 | 111 | 0 | 0 | 323 | 278 | 33 | 12 | 58 |
| Taskin et al. [37] | 2020 | R | 281 | 161 | 120 | 0 | 0 | 186 | 129 | 41 | 16 | 11 |
| Xue et al. [38] | 2021 | R | 132 | 82 | 21 | 27 | 0 | 130 | 85 | 39 | 6 | 7 |
| Ye et al. [39] | 2019 | P | 131 | 98 | 8 | 25 | 0 | 131 | 81 | 41 | 9 | G1–2: 3 |
| G1–3: 4 | ||||||||||||
G1–2, patients with tumor grade 1 or 2; G1–3, patients with tumor grade 1, 2 of 3; P, prospective; R, retrospective.
All patients who underwent SLN mapping by using ICG per cervical injection (superficial and deep) at 3’ and 9’ o’clock were included in the analyses for the detection rate (n=2,117). Near-infrared imaging was used in all cases. All patients who also underwent a subsequent lymph node dissection were included in the analyses for the negative predictive value (n=1,464, Table 2). Additional information on the included studies is reported in Table S1.
Table 2. Baseline information of included studies (n=8) which are included in the analysis of the negative predictive value of SLN mapping.
| Author | Year of publication | Study design | Study population | Patients underwent SLN mapping with ICG with subsequent lymph node dissection | Metastasis | ||
|---|---|---|---|---|---|---|---|
| In SLN (%) | Not in SLN, but in other lymph node (i.e., false-negative SLN) | ||||||
| Including grade 1 and 2 | |||||||
| Cusimano et al. [32] | 2021 | P | 30 | 30 | 3 (10) | 0 | |
| Stephens et al. [36] | 2020 | R | 323 | 323 | 58 (18) | 0 | |
| Ye et al. [39] | 2019 | P | 106 | 97 | 3 (3) | 0 | |
| Including grade 1–3 | |||||||
| Backes et al. [28] | 2019 | P | 204 | 204 | 32 (16) | 2 | |
| Bogani et al. [29] | 2020 | R | 62 | 62 | 9 (15) | 0 | |
| Cusimano et al. [32] | 2021 | P | 156 | 156 | 24 (15) | 3 | |
| Holloway et al. [15] | 2016 | R | 119 | 119 | 35 (29) | 1 | |
| Rossi et al. [14] | 2017 | P | 340 | 340 | 35 (10) | 1 | |
| Xue et al. [38] | 2021 | R | 130 | 130 | 7 (5) | 2 | |
| Ye et al. [39] | 2019 | P | 131 | 131 | 4 (3) | 4 | |
Note that the diagnostic value was calculated twice: for studies including grade 1–2 endometrial cancer, and for studies including grade 1, 2, and 3 endometrial cancer.
ICG, indocyanine green; P, prospective; R, retrospective; SLN, sentinel lymph node.
3. Detection rate
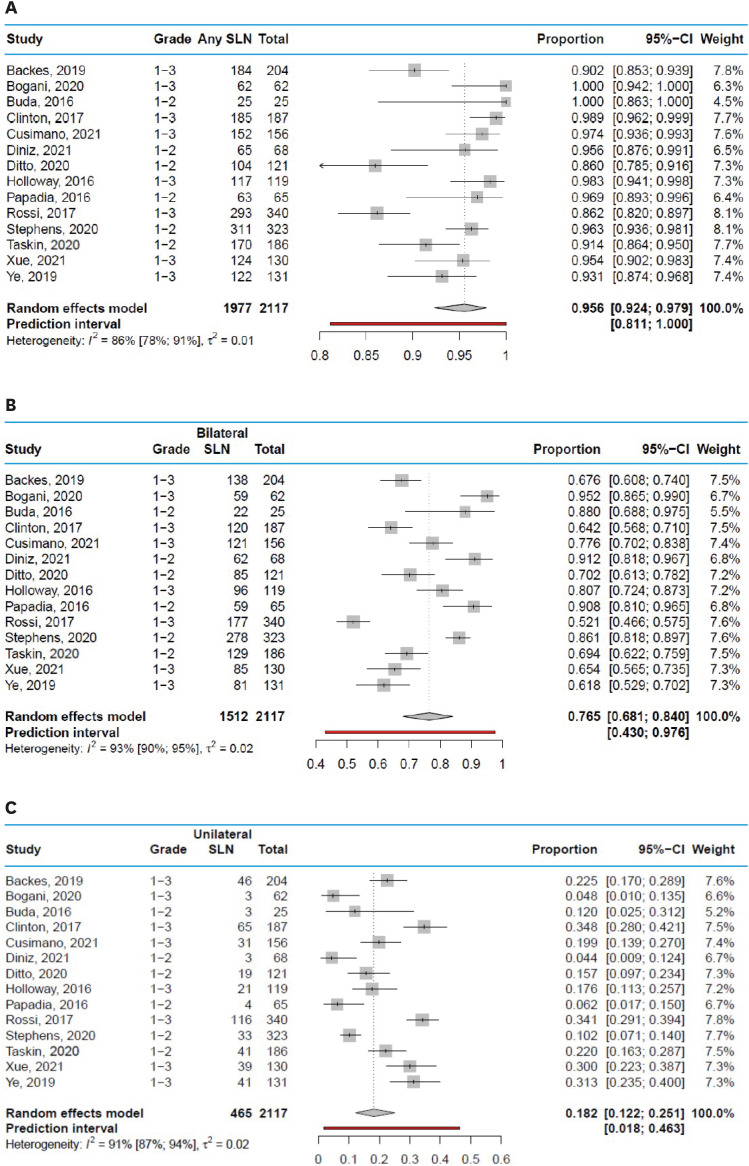
Across studies, the overall SLN detection rate during surgery ranged from 86.0% to 100%, with a pooled average of 95.6% (95% CI=92.4%–97.9%) and a prediction interval from 81.1% to 100% (Fig. 2A). The pooled average of no SLN detection at all is therefore 4.4%. The bilateral detection rate of SLN mapping ranged from 52.1% to 95.2%, with a pooled average of 76.5% (95% CI=68.1%–84.0%) and a prediction interval from 43.0% to 97.6% (Fig. 2B). The unilateral detection rate of SLN mapping ranged from 4.4% to 34.8%, with a pooled average of 18.2% (95% CI=12.2%–25.1%) and a prediction interval from 1.8% to 4.6% (Fig. 2C). Funnel plots are shown in Fig. S3.
Fig. 2. Detection rates of the SLN in all studies. (A) Overall detection rate of the SLN. (B) Bilateral detection rate of the SLN. (C) Unilateral detection rate of the SLN.
CI, confidence interval; SLN, sentinel lymph node.
4. Incidence of SLN metastasis
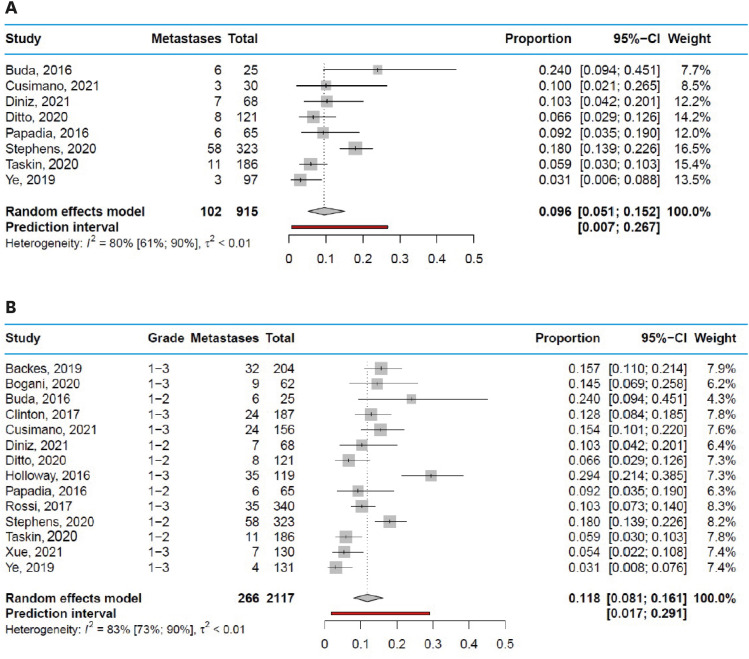
The estimated pooled incidence of SLN metastases in studies including grade 1 and 2 endometrial cancer patients was 9.6% (95% CI=5.1%–15.2%), with a range from 3.1% to 24.0% and a prediction interval from 0.7% to 26.7% (Fig. 3A). In studies including grade 1, 2, and 3 endometrial cancer patients, the estimated pooled incidence of SLN metastases was 11.8% (95% CI=8.1%–16.1%), with a range from 3.1% to 29.4% and a prediction interval from 1.7% to 29.1% (Fig. 3B). Funnel plots are shown in Fig. S4.
Fig. 3. Incidence of SLN metastases in endometrial cancer. (A) Incidence of SLN metastases in grade 1–2 endometrial cancer. (B) Incidence of SLN metastases in grade 1, 2, and 3 endometrial cancer.
CI, confidence interval; SLN, sentinel lymph node.
5. Diagnostic value of SLN mapping
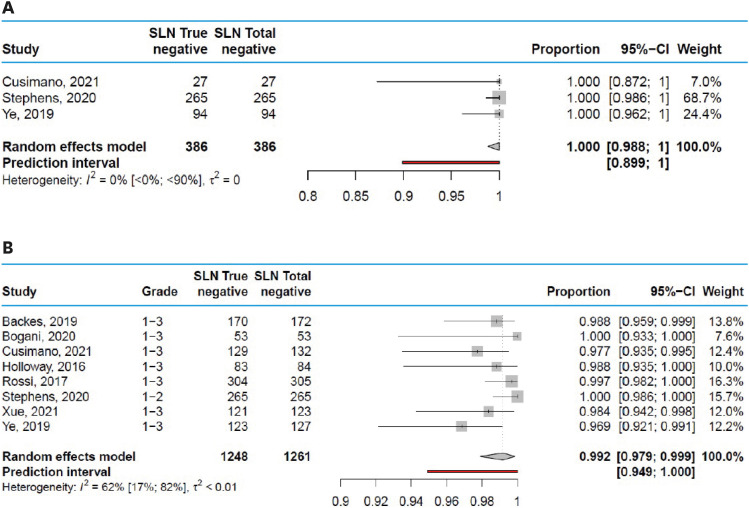
The estimated pooled negative predictive value in studies including only in grade 1 and 2 endometrial cancer patients was 100% (95% CI=98.8%–100%, Fig. 4A), but the prediction interval shows that in a future setting the true negative rate may be as low as 89.9%. The estimated pooled negative predictive value in studies including grade 1, 2, and 3 endometrial cancer patients was 99.2% (95% CI=97.9%–99.9%), with a range from 96.9% to 100% across studies (Fig. 4B). The prediction interval shows that in a future setting the true negative rate may be as low as 94.9%. Funnel plots are shown in Fig. S5.
Fig. 4. Diagnostic value (negative predictive value) of SLN mapping. (A) Negative predictive value of SLN mapping in grade 1–2 endometrial cancer. (B) Negative predictive value of SLN mapping in grade 1–3 endometrial cancer.
CI, confidence interval; SLN, sentinel lymph node.
DISCUSSION
In this systematic review of the literature in combination with meta-analysis, we show that SLN mapping has a high overall detection rate of 95.6% (95% CI=92.4%–97.9%) and a bilateral detection rate of 76.5% (95% CI=68.1–84.0) in low- and intermediate-risk endometrial cancer patients. Lymph node metastases were present in 9.6% (95% CI=5.1%–15.2%) of patients with grade 1 and 2 endometrial cancer, and in 11.8% (95% CI=8.1%–16.1%) in patients with grade 1, 2 of 3 endometrial cancer. The diagnostic value of SLN mapping in low- and intermediate-risk endometrial cancer patients was excellent, with a negative predictive value of 100% (95% CI=98.8%–100%) in grade 1 and 2 tumors, and 99.2% (95% CI=97.9%–99.9%) in studies that included endometrioid grade 3 tumors as well.
Surgical staging with lymph node mapping is standard-of-care in high-risk endometrial cancer in most countries, with SLN mapping increasingly replacing the routine lymph node dissection [16]. This systematic review and meta-analysis supports the importance of SLN mapping in low- and intermediate-risk endometrial cancer patients as well. With lymph node metastasis being the most important risk factor in endometrial cancer, and the presented high overall detection rate, the considerable prevalence of SLN metastasis and the high negative predictive value, SLN mapping turns out to be a feasible technique and a good alternative to lymph node dissection, especially in combination with ultrastaging for detection of micrometastasis [2,3]. SLN mapping in low- and intermediate-risk endometrial cancer is therefore an excellent example of patient-tailored care.
A previous Vignette study on patients’ views on SLN mapping already showed that patients are in favor of SLN mapping in low- and intermediate-risk endometrial cancer [40]. These preferences should be taken into account when counseling a patient for surgical treatment with presumed early-stage low- and intermediate-risk endometrial cancer, especially since SLN mapping is the most cost-effective strategy to guide the need for adjuvant therapy in patients with low and intermediate-risk endometrioid endometrial cancer [17]. Patient-tailored care and shared decision making are also important in case of unsuccessful SLN mapping (unilateral mapping or no mapping at all), in which the decision must be made whether or not to perform a full lymphadenectomy. For example, in the current Dutch guidelines, no assessment of lymph nodes is performed in low- and intermediate-risk endometrial cancer. The implementation of SLN mapping would therefore be an addition to standard care [1]. In that case, a previous cost-effectiveness analysis showed that only unilateral SLN mapping is already cost-effective to guide the need for adjuvant therapy and thereby labeled as successful. A lymphadenectomy can therefore be omitted [17]. In other countries, different considerations may be made. Furthermore, it is known that a discrepancy exists between the preoperative and postoperative grading of a tumor. Visser et al. [20] showed that 18% of preoperative grade 1–2 tumors is upgraded to grade 3 tumors postoperative, and 25% of preoperative grade 3 tumors is downgraded to grade 2 tumors postoperative. Especially for those patients who are upgraded in the final pathological assessment, SLN mapping is of added value, since their a priori risk of SLN metastasis is increased compared to patients with actual grade 1 and 2 endometrial cancer.
Multiple previous studies already reviewed the value of SLN mapping in endometrial cancer, however they included all histologies and grades, all types of dye with or without radiotracer, and no specific way of injecting the dye [18,19]. A meta-analysis from 2017, including studies with all tracers and methods of injecting the dye, mentioned an overall detection rate of 81% and a bilateral detection rate of 50%. The bilateral detection rate increased to up to 75% if a cervical injection of ICG was used. In only 7 out of 55 studies included in the 2017 review, patients actually received ICG. Furthermore, women with more advanced stage of disease and other histology than endometrioid endometrial cancer were included [21]. Similar results were seen in other reviews [22]. Our systematic review and meta-analysis is the first to specifically examine the added value of SLN mapping by solely using the cervical injection of ICG in low- and intermediate-risk endometrial cancer patients, which is nowadays considered as first choice.
A limitation of our study is that some included articles also included grade 3 patients (13%), while the aim of this systematic review and meta-analysis was to investigate patients with grade 1 and 2 endometrial cancer (i.e., with low- and intermediate-risk profile). Not all articles clearly distinguished between grade 1–2 and grade 3 patients. Therefore, corresponding authors were asked to share their raw data. As not all authors were able or willing to share their data, thirteen studies could not be included in the analyses. Nevertheless, the inclusion of both grade 1–2 and 3 should not impact the analysis on the detection rate, since tumor grade is presumed not to affect the detection of SLNs during surgery, since the detection rate of SLNs during surgery is independent of histological grade. The histological grade does however impact the negative predictive value, since diagnostic values are dependent of a priori risks. Therefore, 2 analyses were performed, including studies with and without grade 3 tumors respectively, leading to comparable results.
It is remarkable that the incidence of SLN metastasis varies so widely among the included articles, from 3% to 24% and 3% to 29% in studies including grade 1 and 2 and including grade 1, 2 and 3 endometrial cancer, respectively. The study of Xue et al. [38] for example reports one of the lowest incidence of SLN metastasis (5.4% in all grades). In their data sharing report, they mention that, since their study is a retrospective analysis, ultrastaging was not performed in all SLN procedures, inevitably leading to missed micrometastasis. Since the majority of the included studies were retrospective in design, it cannot be ruled out that this applies to several studies.
Another limitation of our study might be the difference between the number of included patients in all fourteen articles combined (n=2,620) and the number of patients who actually underwent SLN mapping (n=2,117). There are several reasons for this discrepancy. In some studies, not all patients had ICG as tracer for SLN mapping, thus only a selection of the study population could be included in our study [30,33,37]. In another included study, the decision regarding lymph node dissection was at the discretion of the individual surgeon [31]. Some patients had SLN biopsy with or without subsequent lymph node dissection, and some patients had no lymph node sampling (SLN mapping or lymph node dissection) at all. As the surgeons became more familiar with SLN mapping, more patients underwent SLN mapping. To clarify, the latter article was not included in the analysis of the diagnostic value, only in the analysis of the detection rate.
In conclusion, SLN mapping with ICG is a feasible technique with a high detection rate and negative predictive value in low- and intermediate-risk endometrial cancers. Given the incidence of lymph node metastasis, it therefore should be considered in the treatment of patients with low- and intermediate-risk endometrial cancer, since lymph node metastases are an important prognostic factor and SLN mapping may lead to stage shifting with potential therapeutic consequences. Our results support the opinion that a more rigorous routine lymphadenectomy can be safely omitted in low- and intermediate-risk endometrial cancer patients who undergo successful bilateral SLN mapping procedures.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: B.L.C., B.R.L., K.R.F., Z.P.L.
- Data curation: B.L.C., V.S., B.R.L., I.J., H.R.W., T.S., F.S.E., X.Y., D.A., B.G., P.A., B.G., B.A., K.R.F., Z.P.L.
- Formal analysis: B.L.C., V.S., I.J.
- Investigation: B.L.C.
- Methodology: B.L.C., V.S., I.J.
- Software: I.J.
- Supervision: B.R.L., I.J., K.R.F., Z.P.L.
- Visualization: B.L.C., B.R.L., K.R.F., Z.P.L.
- Writing - original draft: B.L.C.
- Writing - review & editing: B.L.C.
SUPPLEMENTARY MATERIALS
Additional information on included studies
Graphical display of the Quality Assessment of Diagnostic Accuracy Studies-2 results showing the proportion of studies with low-, unclear, or high-risk levels of (A) bias, and (B) applicability concerns.
Graphical display of the Quality Assessment of Diagnostic Accuracy Studies-2, showing the risk of bias per study.
Funnel plots, in addition to Fig. 2. Detection rates of the SLN in all studies, with (A) overall detection rate of the SLN; (B) bilateral detection rate of the SLN; and (C) unilateral detection rate of the SLN.
Funnel plots, in addition to Fig. 3. Incidence of SLN metastases in endometrial cancer, with (A) incidence of SLN metastases in grade 1–2 endometrial cancer; and (B) incidence of SLN metastases in grade 1, 2, and 3 endometrial cancer.
Funnel plots, in addition to Fig. 4. Diagnostic value (negative predictive value) of SLN mapping, with (A) negative predictive value of SLN mapping in grade 1–2 endometrial cancer; and (B) negative predictive value of SLN mapping in grade 1–3 endometrial cancer.
References
- 1.Federatie Medisch Specialisten. Guidelines database: endometrial carcinoma guideline [Internet] Utrecht: Federatie Medisch Specialisten; 2011. [cited 2021 Nov 15]. Available from: https://richtlijnendatabase.nl/richtlijn/endometriumcarcinoom/algemeen.html. [Google Scholar]
- 2.Koskas M, Rouzier R, Amant F. Staging for endometrial cancer: the controversy around lymphadenectomy – Can this be resolved? Best Pract Res Clin Obstet Gynaecol. 2015;29:845–857. doi: 10.1016/j.bpobgyn.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Abdullah NA, Huang KG, Casanova J, Artazcoz S, Jarruwale P, Benavides DR, et al. Sentinel lymph node in endometrial cancer: a systematic review on laparoscopic detection. Gynecol Minim Invasive Ther. 2013;2:75–78. [Google Scholar]
- 4.Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124:307–315. doi: 10.1097/AOG.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helgers RJ, Winkens B, Slangen BF, Werner HM. Lymphedema and post-operative complications after sentinel lymph node biopsy versus lymphadenectomy in endometrial carcinomas—A systematic review and meta-analysis. J Clin Med. 2020;10:120. doi: 10.3390/jcm10010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer. 2013;49:3431–3441. doi: 10.1016/j.ejca.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 9.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273–1285. doi: 10.1016/S1470-2045(19)30395-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317–2326. doi: 10.1056/NEJMoa1813181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, Shan B, Xue X, Wang H, Shan W, Ning C, et al. Predicting lymph node metastasis in endometrial cancer using serum CA125 combined with immunohistochemical markers PR and Ki67, and a comparison with other prediction models. PLoS One. 2016;11:e0155145. doi: 10.1371/journal.pone.0155145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah M, Jain SR, Oprea G, Shafi S. Prognostic significance of hormone receptor (ER/PR) status in endometrial carcinoma in black women: Implications with lymph node metastasis. J Clin Oncol. 2020;38:e18099 [Google Scholar]
- 13.Leitao MM, Jr, Khoury-Collado F, Gardner G, Sonoda Y, Brown CL, Alektiar KM, et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129:38–41. doi: 10.1016/j.ygyno.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384–392. doi: 10.1016/S1470-2045(17)30068-2. [DOI] [PubMed] [Google Scholar]
- 15.Holloway RW, Gupta S, Stavitzski NM, Zhu X, Takimoto EL, Gubbi A, et al. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol Oncol. 2016;141:206–210. doi: 10.1016/j.ygyno.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 17.Burg LC, Vermeulen RJ, Bekkers RL, Wijn SR, Rovers MM, Govers TM, et al. A cost-effectiveness analysis of three approaches for lymph node assessment in patients with low- and intermediate-risk endometrial cancer. Gynecol Oncol. 2021;161:251–260. doi: 10.1016/j.ygyno.2021.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Ruscito I, Gasparri ML, Braicu EI, Bellati F, Raio L, Sehouli J, et al. Sentinel node mapping in cervical and endometrial cancer: indocyanine green versus other conventional dyes-a meta-analysis. Ann Surg Oncol. 2016;23:3749–3756. doi: 10.1245/s10434-016-5236-x. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Rustum NR, Angioli R, Bailey AE, Broach V, Buda A, Coriddi MR, et al. IGCS Intraoperative Technology Taskforce. Update on near infrared imaging technology: beyond white light and the naked eye, indocyanine green and near infrared technology in the treatment of gynecologic cancers. Int J Gynecol Cancer. 2020;30:670–683. doi: 10.1136/ijgc-2019-001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visser NC, Reijnen C, Massuger LF, Nagtegaal ID, Bulten J, Pijnenborg JM. Accuracy of endometrial sampling in endometrial carcinoma: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:803–813. doi: 10.1097/AOG.0000000000002261. [DOI] [PubMed] [Google Scholar]
- 21.Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216:459–476.e10. doi: 10.1016/j.ajog.2016.11.1033. [DOI] [PubMed] [Google Scholar]
- 22.How JA, O’Farrell P, Amajoud Z, Lau S, Salvador S, How E, et al. Sentinel lymph node mapping in endometrial cancer: a systematic review and meta-analysis. Minerva Ginecol. 2018;70:194–214. doi: 10.23736/S0026-4784.17.04179-X. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 25.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rücker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28:721–738. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 27.R-Core-Team. A language and environment for statistical computing [Internet] Vienna: R Foundation; 2019. [cited 2021 Nov 23]. Available from: https://www.R-project.org/ [Google Scholar]
- 28.Backes FJ, Cohen D, Salani R, Cohn DE, O’Malley DM, Fanning E, et al. Prospective clinical trial of robotic sentinel lymph node assessment with isosulfane blue (ISB) and indocyanine green (ICG) in endometrial cancer and the impact of ultrastaging ( NCT01818739) Gynecol Oncol. 2019;153:496–499. doi: 10.1016/j.ygyno.2019.03.252. [DOI] [PubMed] [Google Scholar]
- 29.Bogani G, Casarin J, Leone Roberti Maggiore U, Ditto A, Pinelli C, Dell’acqua A, et al. Survival outcomes in endometrial cancer patients having lymphadenectomy, sentinel node mapping followed by lymphadectomy and sentinel node mapping alone: long-term results of a propensity-matched analysis. Gynecol Oncol. 2020;158:77–83. doi: 10.1016/j.ygyno.2020.04.691. [DOI] [PubMed] [Google Scholar]
- 30.Buda A, Crivellaro C, Elisei F, Di Martino G, Guerra L, De Ponti E, et al. Impact of indocyanine green for sentinel lymph node mapping in early stage endometrial and cervical cancer: comparison with conventional radiotracer 99mTc and/or blue dye. Ann Surg Oncol. 2016;23:2183–2191. doi: 10.1245/s10434-015-5022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinton LK, Kondo J, Carney ME, Tauchi-Nishi P, Terada K, Shimizu D. Low-volume lymph node metastases in endometrial carcinoma. Int J Gynecol Cancer. 2017;27:1165–1170. doi: 10.1097/IGC.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 32.Cusimano MC, Vicus D, Pulman K, Maganti M, Bernardini MQ, Bouchard-Fortier G, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157–164. doi: 10.1001/jamasurg.2020.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diniz TP, Drizlionoks E, Faloppa CC, Menezes JN, Mantoan H, Gonçalves BT, et al. Impact of sentinel node mapping in decreasing the risk of lymphocele in endometrial cancer. Ann Surg Oncol. 2021;28:3293–3299. doi: 10.1245/s10434-020-09282-z. [DOI] [PubMed] [Google Scholar]
- 34.Ditto A, Casarin I, Pinelli C, Perrone AM, Scollo P, Martinelli F, et al. Hysteroscopic versus cervical injection for sentinel node detection in endometrial cancer: a multicenter prospective randomised controlled trial from the Multicenter Italian Trials in Ovarian cancer (MITO) study group. Eur J Cancer. 2020;140:1–10. doi: 10.1016/j.ejca.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Papadia A, Imboden S, Siegenthaler F, Gasparri ML, Mohr S, Lanz S, et al. Laparoscopic indocyanine green sentinel lymph node mapping in endometrial cancer. Ann Surg Oncol. 2016;23:2206–2211. doi: 10.1245/s10434-016-5090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens AJ, Kennard JA, Fitzsimmons CK, Manyam M, Kendrick JE, Singh C, et al. Robotic sentinel lymph node (SLN) mapping in endometrial cancer: SLN symmetry and implications of mapping failure. Int J Gynecol Cancer. 2020;30:305–310. doi: 10.1136/ijgc-2019-000915. [DOI] [PubMed] [Google Scholar]
- 37.Taşkın S, Altin D, Vatansever D, Tokgozoglu N, Karabük E, Turan H, et al. Sentinel lymph node biopsy in early stage endometrial cancer: a Turkish gynecologic oncology group study (TRSGO-SLN-001) Int J Gynecol Cancer. 2020;30:299–304. doi: 10.1136/ijgc-2019-000847. [DOI] [PubMed] [Google Scholar]
- 38.Xue Y, Shan WW, Wang Q, Wang C, Luo XZ, Chen XJ. Efficacy of sentinel lymph node mapping in endometrial cancer with low- or high-intermediate risk. J Surg Oncol. 2022;125:256–263. doi: 10.1002/jso.26694. [DOI] [PubMed] [Google Scholar]
- 39.Ye L, Li S, Lu W, He Q, Li Y, Li B, et al. A prospective study of sentinel lymph node mapping for endometrial cancer: Is it effective in high-risk subtypes? Oncologist. 2019;24:e1381–e1387. doi: 10.1634/theoncologist.2019-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aarts JW, Burg LC, Kasius JC, Groenewoud H, Kraayenbrink AA, Stalmeier P, et al. Patients’ and gynecologists’ views on sentinel lymph node mapping in low- and intermediate-risk endometrial cancer: a Dutch vignette study. Int J Gynecol Cancer. 2020;30:813–818. doi: 10.1136/ijgc-2019-001138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on included studies
Graphical display of the Quality Assessment of Diagnostic Accuracy Studies-2 results showing the proportion of studies with low-, unclear, or high-risk levels of (A) bias, and (B) applicability concerns.
Graphical display of the Quality Assessment of Diagnostic Accuracy Studies-2, showing the risk of bias per study.
Funnel plots, in addition to Fig. 2. Detection rates of the SLN in all studies, with (A) overall detection rate of the SLN; (B) bilateral detection rate of the SLN; and (C) unilateral detection rate of the SLN.
Funnel plots, in addition to Fig. 3. Incidence of SLN metastases in endometrial cancer, with (A) incidence of SLN metastases in grade 1–2 endometrial cancer; and (B) incidence of SLN metastases in grade 1, 2, and 3 endometrial cancer.
Funnel plots, in addition to Fig. 4. Diagnostic value (negative predictive value) of SLN mapping, with (A) negative predictive value of SLN mapping in grade 1–2 endometrial cancer; and (B) negative predictive value of SLN mapping in grade 1–3 endometrial cancer.