Abstract
The single polar flagellum of Pseudomonas aeruginosa plays an important role in the pathogenesis of infection by this organism. However, regulation of the assembly of this organelle has not been delineated. In analyzing the sequence available at the Pseudomonas genome database, an open reading frame (ORF), flanked by flagellar genes flhF and fliA, that coded for a protein (280 amino acids) with an ATP-binding motif at its N terminus was found. The ORF was inactivated by inserting a gentamicin cassette in P. aeruginosa PAK and PAO1. The resulting mutants were nonmotile on motility agar plates, but under a light microscope they exhibited random movement and tumbling behavior. Electron microscopic studies of the wild-type and mutant strains revealed that the mutants were multiflagellate, with three to six polar flagella per bacterium as rather than one as in the wild type, indicating that this ORF was involved in regulating the number of flagella and chemotactic motility in P. aeruginosa. The ORF was named fleN. An intact copy of fleN on a plasmid complemented the mutant by restoring motility and monoflagellate status. The β-galactosidase activities of eight flagellar operon or gene promoters in the wild-type and fleN mutant strains revealed a direct correlation between six promoters that were upregulated in the fleN mutant (fliLMNOPQ, flgBCDE, fliEFG, fliDS orf126, fleSR, and fliC) and positive regulation by FleQ, an NtrC-like transcriptional regulator for flagellar genes. Based on these results, we propose a model where FleN influences FleQ activity (directly or indirectly) in regulating flagellar number in P. aeruginosa.
Flagella serve primarily as locomotory organelles in flagellated bacterial species. They have also been implicated in biofilm development in Pseudomonas aeruginosa and Escherichia coli (24, 26) and in the pathogenesis of infections by P. aeruginosa, Campylobacter jejuni, Helicobacter pylori, and Vibrio cholerae (23, 25). The bacterial flagellum is a complex structure requiring more than 40 genes for its assembly (18). Over the years, significant progress has been made in identifying various flagellar structural and regulatory genes, elucidating the composition of flagellar substructures, and understanding the mechanisms of its assembly in a number of bacterial species, including E. coli, Salmonella enterica serovar Typhimurium (1, 18, 22), and Caulobacter crescentus (28, 38). Work is in progress to elucidate the pathway of flagellar assembly in the pathogens P. aeruginosa, V. cholerae, and H. pylori.
Most flagellated bacterial species have extracellular flagella; exceptions are spirochetes such as pathogenic Treponema pallidum and Borrelia burgdorferi, which have flagella implanted in the periplasmic space. In some cases, extracellular flagella are covered by a sheath, as in V. cholerae and H. pylori (23). The distribution of flagella may be monotrichous polar as in P. aeruginosa (14) and V. cholerae (39) or peritrichous (5 to 10 flagella) as in E. coli and Salmonella serovar Typhimurium (18). Flagellar number, a characteristic feature of each species, is successfully maintained over the generations, but nothing is known about the genes and the mechanisms which contribute to its regulation. In a recent model proposed for Salmonella, the number of flagellar filaments has been linked to the cell cycle (2).
A major goal of our laboratory is to gain an insight into the biogenesis pathway of the flagellum of P. aeruginosa. The published information from our laboratory and elsewhere (5, 6, 29, 33) indicates that the system deviates from the well-described enteric model (18). It has certain similarities to the Caulobacter (38), Vibrio (17), and Helicobacter (32) systems, which utilize the alternative sigma factor RpoN and an NtrC transcriptional regulator homologue at some stage(s) of flagellar biogenesis.
The availability of the partial genome sequence of P. aeruginosa from strain PAO1 at the Pseudomonas genome database website (www.pseudomonas.com) has simplified our quest to understand the flagellar biogenesis pathway in this organism. In this paper, we report the identification of fleN, a gene that is involved in controlling the number of flagella and chemotactic motility in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. They were grown in Luria-Bertani (LB) broth (30) at 37°C with shaking at 250 rpm or on LB agar plates unless stated otherwise. The following antibiotics were used to maintain the plasmid and chromosomal insertions in P. aeruginosa: gentamicin at 50 μg/ml (100 μg/ml for plates), carbenicillin at 150 μg/ml (300 μg/ml for plates), streptomycin at 300 μg/ml, and tetracycline at 50 μg/ml (100 μg/ml for plates). In E. coli, the concentrations used were 200 μg/ml for ampicillin, 10 μg/ml for gentamicin, and 25 μg/ml for tetracycline.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant information | Source or reference |
|---|---|---|
| E. coli DH5α | hsdR recA lacZYA φ80 lacZΔM15 | GIBCO-BRL |
| P. aeruginosa | ||
| PAK | Wild-type clinical isolate | D. Bradley |
| PAO1 | Wild type | M. Vasil |
| PAK-Q | PAK fleQ::Gmr | 5 |
| PAK-N | PAK fleN::Gmr | This study |
| PAO-N | PAO fleN::Gmr | This study |
| Plasmids | ||
| pGEM3Zf(+) | Cloning vector, Ampr, LacZα peptide | Promega Corp., Madison, Wis. |
| pGEM-fleN | pGEM3Zf(+) with a 2.0-kb PCR product (fleN locus) cloned into the EcoRI/BamHI sites | This study |
| pGEM-fleNG | pGEM-fleN with a gentamicin resistance gene inserted in the unique EcoRV site of fleN | This study |
| pPZ375 | oriV in pGEM3Zf(+) | 35 |
| pPZ375-fleN | pPZ375 containing fleN as a 1.0-kb HindIII/SstI PCR fragment | This study |
| pET15bVP | oriV cloned as a PstI fragment in bla of pET15b | 5 |
| pET-fleN | fleN inserted as a PCR product into the NdeI/BamHI sites of pET15bVP | This study |
| pDN19lacΩ | Promoterless lacZ oriV oriT Tetr Strr Ω fragment | 37 |
| placΩQ | pDN19lacΩ containing the fleQ promoter region | 5 |
| placΩS | pDN19lacΩ containing the fleSR promoter region | 5 |
| placΩE | pDN19lacΩ containing the fliEFG promoter region | 4 |
| placΩD | pDN19lacΩ containing the fliDSorf126 promoter region | 6 |
| placΩflgE | pDN19lacΩ containing the flgBCDE promoter region | This study |
| placΩL | pDN19lacΩ containing the fliLMNOPQ promoter region | This study |
| pMS565 | pDN19lacΩ containing the fliA promoter region | 33 |
| pPT269 | pDN19lacΩ containing the fliC promoter region | 37 |
| pMSZ5 | pDN19lacΩ containing the pilA promoter region | 15 |
| Primersa | ||
| pPAO4 | 5′ cccaaagaatTCCCGGCCAGTCGCTGAT 3′, EcoRI site incorporated | |
| pPAO5 | 5′ cccaaaggATCCGCCAGGGCCGCCTGG 3′, BamHI site incorporated | |
| flnHind | 5′ cccaaaaagcttGAGGACGTGGGAAGAAC 3′, HindIII site incorporated | |
| flnSst | 5′ cccaaagagCTCCAGAGGCCGCTGTC 3′, SstI site incorporated | |
| flnPst | 5′ GTGAGCCTGCAGGCACCGGAAGAGCC 3′, PstI naturally present | |
| flnNde | 5′ GACAACACAAcatATGAAGCAGATGGG 3′, NdeI site incorporated | |
| flnBam | 5′ CCTTGCTATACgggaTCCAGAGGCCGCTG 3′, BamHI site incorporated | |
| 5PfliLbgal | 5′ cccaaagaattcCTCGGGCGATGAGGAAC 3′, EcoRI site incorporated | |
| 3PfliLbgal | 5′ cccaaaggattcCTCGCCTCTTTCTTAGCC 3′, BamHI site incorporated | |
| RER 41 | 5′ cccaaagaattcGGGCTTGCCACCCTTGCC 3′, EcoRI site incorporated | |
| RER 42 | 5′ cccaaaggatccCGTTGGCCAGGTTGTTGG 3′, BamHI site incorporated |
In primer sequences, lowercase denotes nucleotides added or modified to facilitate restriction digestion at the sites marked in bold.
Computer analysis.
The nucleotide sequences of earlier contigs and contig 53 from the Pseudomonas genome database (release date, March 15, 1999) were subjected to an open reading frame (ORF) search using the ORF Finder program at the National Center for Biotechnology Information (NCBI) website (www.ncbi.nlm.nih.gov). Later, the deduced amino acid sequence of the uncharacterized ORF, fleN, was subjected to BLASTP (3) searches using the GenBank database entries. TBLASTN (3) was used to search for homologues of FleN in the preliminary sequence data of V. cholerae (obtained from The Institute of Genomic Research [TIGR] website at www.tigr.org) and C. crescentus (www.ncbi.nlm.nih.gov). The deduced amino acid sequence of FleN was subjected to an online PROSITE database search (at www2.ebi.ac.uk).
Transformation and electroporation.
Frozen competent E. coli DH5α cells were prepared and transformed by essentially using the standard procedure (30). Electroporations in P. aeruginosa were performed by using a modification of the protocol of Smith and Iglewski (31). For gene replacement experiments involving chromosomal recombinations, about 1 μg of linearized plasmid was used. For introducing replicative plasmids, 50 to 100 ng of plasmid DNA prepared by the alkaline lysis procedure (8) was electroporated into the strains.
PCR.
PCR was performed in a DNA Thermal Cycler 480 (Perkin-Elmer Cetus, Norwalk, Conn.), using either Taq DNA polymerase or eLongase (GIBCO-BRL Inc., Gaithersburg, Md.) in 100-μl reaction volumes. Briefly, the reaction mixture consisted of 100 ng of template DNA, 1.5 mM MgCl2, 1× polymerase buffer, 0.2 mM deoxynucleoside triphosphates, 0.5 μM concentrations of each primer (Table 1; custom synthesized at GIBCO-BRL), 2% dimethyl sulfoxide, 1 U of Taq DNA polymerase, or 2 U of eLongase. PCR was performed as follows: initial denaturation of 10 min at 94°C, followed by 35 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 55°C (pPAO4-pPAO5), 50°C (flnHind-flnSst and flnPst-flnSst), or 52°C (flnNde-flnBam), and an extension of 1 min/kb at 72°C with Taq or 2 min/kb at 68°C with eLongase. With primer pairs 5PfliLbgal-3PfliLbgal and RER41-RER42, Pfu DNA polymerase (Stratagene, La Jolla, Calif.) was used according to the manufacturer's instructions, with 45°C as the annealing temperature. The template DNA used for PCR was either purified genomic DNA isolated by the cetyltrimethylammonium bromide procedure (7) or a plasmid preparation made by the alkaline lysis method. The PCR products were electrophoresed on a 1% SeaPlaque GTG agarose (FMC Bioproducts, Rockland, Maine) gel and stained with ethidium bromide, and the desired bands were electroeluted for further applications.
Sequencing.
DNA sequencing was accomplished by using the Taq DyeDeoxy terminator and DyePrimer cycle sequencing protocols developed by Applied Biosystems (Perkin-Elmer Corp., Foster City, Calif.), using fluorescence-labeled dideoxynucleotides. The labeled extension products were analyzed on an Applied Biosystems model373A DNA sequencer.
Generation of fleN chromosomal mutants.
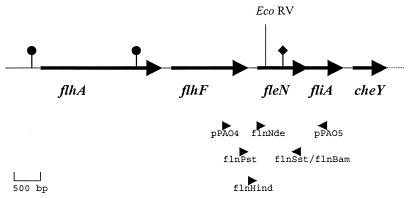
The 2.0-kb PCR product generated by using amplimers pPAO4 and pPAO5 (Fig. 1) from the PAO1 genome was restricted at the EcoRI and BamHI unique sites incorporated in the primers and cloned into pGEM3Zf(+), yielding pGEM-fleN. The cloned insert contained the ∼1.0-kb coding region of the new ORF, fleN, and its flanking regions. A 1.7-kb gentamicin resistance cassette with blunt ends was inserted into the unique EcoRV site of the cloned ORF in pGEM-fleN (Fig. 1). The resulting plasmid, pGEM-fleNG, was linearized and electroporated into both PAK and PAO1 strains of P. aeruginosa for insertional inactivation of fleN by homologous recombination.
FIG. 1.
Schematic representation of the fleN locus based on the P. aeruginosa PAO1 sequence of contig 53 from the Pseudomonas genome database. Bold arrows indicate locations of the ORFs (flhA, flhF, fleN, fliA, and cheY) in this region, and their respective orientations; ● indicates the presence of a ς54 consensus sequence; ♦ denotes the location of the fliA promoter. The position of the unique EcoRV site in fleN, used for inserting the gentamicin resistance gene, is shown. Arrowheads show positions and orientations of primers used in this study.
Construction of plasmids for complementation and promoter activity studies.
fleN was amplified from pGEM-fleN by using primers flnHind and flnSst (Fig. 1). The 1.0-kb PCR product was digested with HindIII and SstI and cloned into pPZ375, a high-copy-number plasmid, yielding pPZ375-fleN. pET-fleN was derived from pET15bVP by cloning an 880-bp PCR product obtained from PAK by using primers flnNde and flnBam (Fig. 1) sequentially digested with NdeI and BamHI.
To clone the putative promoters of fliLMNOPQ and flgBCDE operons (S. K. Arora and R. Ramphal, unpublished data), 340- and 260-bp stretches mapping immediately upstream of the respective operons were amplified by using primer pairs 5PfliLbgal-3PfliLbgal and RER41-RER42 (Table 1), respectively. They were digested at the unique EcoRI/BamHI sites incorporated in the primers and cloned into pDN19lacΩ in E. coli. The resulting plasmids were named placΩL and placΩflgE, respectively.
Electron microscopy (EM).
Static cultures were grown overnight at 37°C with the appropriate antibiotics. A drop of the culture was allowed to adhere to a carbon-coated grid for 10 s and drained off; the grid was then rinsed in a drop of saline, and adherent cells were negatively stained with a 2% aqueous solution of phosphotungstic acid for 10 s. Samples were examined with a Hitachi H-7000 transmission electron microscope.
Video light microscopy.
To test the motility of the various strains under examination by the hanging drop method, a 50-μl drop of the culture was placed in the center of a glass coverslip edged with vacuum grease, and the coverslip was inverted carefully on a concave glass slide. The bacteria were examined with high-dry and oil immersion objectives of a Zeiss light microscope (Carl Zeiss Inc., Thornwood, N.Y.) equipped with a chilled charge-couple device camera (Hammamatsu Photonics K. K., Hamamatsu City, Japan).
β-Galactosidase assay.
The promoter regions of flagellar operons and genes were cloned upstream of a promoterless lacZ in pDN19lacΩ (Table 1). The constructs and vector pDN19lacΩ were electroporated into wild-type PAK and mutants PAK-N and PAK-Q and then tested for β-galactosidase activity (20). The strains were grown to late log phase (A600 of 0.7 to 1.0) in LB medium containing either streptomycin or tetracycline.
Nucleotide sequence accession number.
The P. aeruginosa PAK fleN sequence reported in this paper has been submitted to the GenBank database and assigned accession no. AF133657.
RESULTS
Nucleotide sequence analysis of the fleN locus.
Analysis of the available sequence at the Pseudomonas genome project (www.pseudomonas.com; release date, March 15, 1999) revealed an ORF (Fig. 1), located on contig 53, encoding a protein of 280 amino acids. The ORF was named fleN for reasons described below. It mapped between flhF, encoding the putative homologue of a protein implicated in flagellin-specific export in Bacillus subtilis (11) and fliA, encoding the flagellar sigma factor ς28 in P. aeruginosa (33). flhA, coding for a flagellin export apparatus protein homologue of Salmonella serovar Typhimurium (21), mapped further upstream of flhF. The che locus consisting of genes involved in chemotaxis (13) mapped downstream of fliA. In E. coli and Salmonella serovar Typhimurium, the master regulator operon of the flagellar biogenesis pathway, flhCD, maps upstream of the che locus (18), but in P. aeruginosa we were unable to identify flhCD sequence homologues in the same or different loci of the genomic sequence. Thus, the location of the previously uncharacterized ORF fleN, upstream of the che locus, among flagellar genes tempted us to investigate its possible involvement in flagellar biogenesis.
Sequence analysis of fleN and the adjacent genes revealed stretches of 83 bp between the coding regions of flhA and flhF and 139 bp between those of flhF and fleN, which were devoid of alternative sigma factor consensus sequences for ς54 (CTGGYAYRN4TTGCA) and ς28 (TAAAN15GCCGATAA) that some flagellar genes are known to possess (29, 37). Search for a ς54 promoter consensus sequence further upstream of fleN revealed two candidate sites both having a 14/17 match. One of the sites lay centered 424 bp upstream of the flhF initiation codon, mapping within flhA, and the other was centered 94 bp upstream of the flhA initiation codon (Fig. 1). The fleN stop codon TGA and the initiation codon ATG for the subsequent gene, fliA, overlap, and the promoter of fliA (33) maps within the coding region of fleN (Fig. 1).
fleN regulates the number of polar flagella and motility in P. aeruginosa.
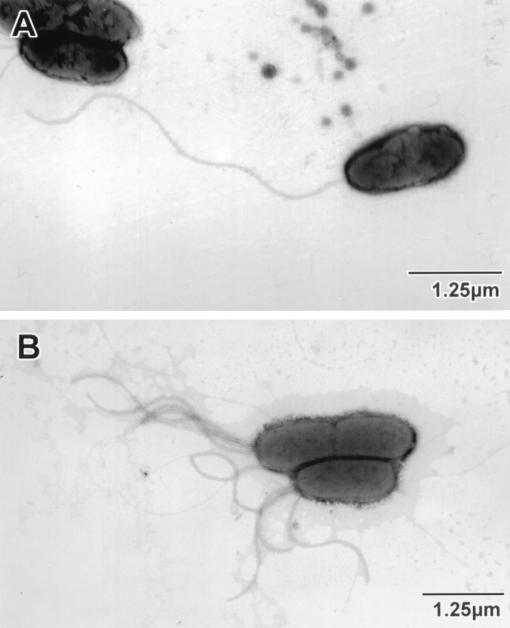
Chromosomal fleN mutants of P. aeruginosa PAK and PAO1 were constructed as described in Materials and Methods. The insertional inactivation of fleN in the Gmr Carbs clones was confirmed by PCR using primers flnHind and flnSst, which yielded a ∼2.7-kb product in the mutants, while the wild type displayed a 1.0-kb product as expected (data not shown). The mutants were tested for their motility phenotypes on motility plates. Both PAK and PAO1 mutants were nonmotile (Fig. 2A). Nonmotility could be attributed to either the absence of flagellum or an impairment in its function when present. EM studies revealed that the nonmotile mutants of both PAK (Fig. 3) and PAO1 (data not shown) had a tuft of three to six polar flagella, as opposed to a single polar flagellum in the wild-type parents. This observation suggested that disruption of the ORF was associated with the loss of flagellar number control. The ORF was thus named fleN, and the PAK and PAO1 mutants were named PAK-N and PAO-N, respectively.
FIG. 2.
(A) Motility phenotype of P. aeruginosa wild-type strains PAK and PAO (PAO1) and their fleN mutants, PAK-N and PAO-N. (B) Motility phenotype of PAK-N containing plasmid constructs pPZ375-fleN, pET-fleN, and their vector controls, pPZ375 and pET15bVP, respectively. The strains were freshly grown on LB plates with appropriate antibiotics; cells were transferred to 0.3% agar plates with a sterile toothpick and incubated at 37°C for 8 h (A) or 12 h (B).
FIG. 3.
Electron micrographs of wild-type PAK (A) and mutant PAK-N (B). The wild-type cell has a long single polar flagellum, as opposed to the polar tuft of shorter flagella present on the mutant bacteria visible in this field.
Direct sequencing of the fleN PCR product obtained from PAK by using primers flnPst and flnSst (Fig. 1) indicated that the sequence of fleN in PAK was identical to that of PAO1 available at the Pseudomonas genome database. Therefore, further studies were restricted to the PAK mutant, PAK-N.
Examination of the multiflagellated PAK-N strain by the hanging drop method (14) revealed that the flagella were not paralyzed, as the majority of the bacteria exhibited random movement, tumbling behavior, and polar spinning but lacked the directional swimming typical of the wild-type PAK strain. This could account for their nonmotile phenotype on motility plates, where chemotactic motility is essential for swarming behavior.
Complementation of PAK-N.
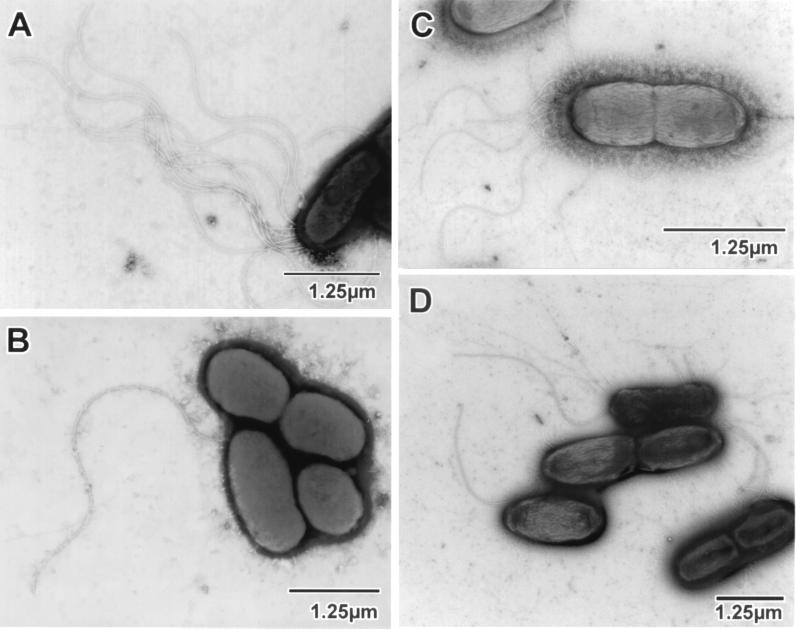
Successful complementation of PAK-N to the wild-type phenotype with an intact copy of fleN on a plasmid was important to demonstrate that fleN was solely responsible for the nonmotility associated with the multiflagellate status. The fleN PCR product generated by using primers flnHind and flnSst was cloned into the broad-host-range plasmid pPZ375 (pPZ375-fleN), allowing the transcription of fleN from the unrepressed strong lac promoter of the vector. The sequence of the PCR product was identical to that of the wild-type parent PAO1 sequence. When examined for complementation, pPZ375-fleN was not able to confer motility to PAK-N (Fig. 2B). However, EM studies showed that the majority of these cells were now devoid of flagella, with few having a single flagellum (Fig. 4B). The mutant strain with pPZ375, the vector control, remained nonmotile (Fig. 2B) and multiflagellate (Fig. 4A). This suggested that FleN was probably being synthesized from pPZ375-fleN, but the excessive amount was inhibiting the formation of the flagellum in the majority of the bacteria. Subsequently, pET15bVP, an expression vector with low basal activity of the T7 promoter, was used in a P. aeruginosa host lacking T7 RNA polymerase to clone fleN downstream of the T7 promoter (pET-fleN) and examined for its ability to complement the fleN mutant. It conferred wild-type motility phenotype to the nonmotile fleN mutant (Fig. 2B), and most of the cells displayed one to two flagella (Fig. 4D).
FIG. 4.
Electron micrographs of mutant PAK-N containing plasmid constructs pPZ375 (A), pPZ375-fleN (B), pET15bVP (C), and pET-fleN (D). The vector controls (A and C) retain the multiple polar flagella of the mutant, whereas most of the cells in panel B, with pPZ375-fleN, are nonflagellated. pET-fleN (D) restores the wild-type monoflagellate status to the mutant, as most of the cells are seen to possess a single polar flagellum.
Flagellar genes that are positively regulated by FleQ are upregulated in the fleN mutant.
The multiflagellate phenotype of the fleN mutant suggested that an excess of flagellar components was being synthesized for the assembly of multiple flagella. Therefore, we compared the β-galactosidase activities of eight flagellar gene promoter fusion constructs available in the laboratory, as well as that of a nonflagellar gene promoter of pilA, in the PAK and PAK-N strains (Table 2). The assay showed a 2- to 27-fold upregulation in the transcriptional activities of six flagellar promoters in PAK-N. These were the promoters of the fliEFG, fliLMNOPQ, fliDS orf126, flgBCDE, and fleSR operons and fliC. The promoter activities of fleQ, fliA, and pilA did not show any appreciable change.
TABLE 2.
Assessment of the transcriptional activities of flagellar gene promoters in strains PAK and PAK-N
| Operon or gene (promoter construct) | β-Galactosidase activity (Miller units; mean ± SD)
|
Fold upregulation of promoter activity in PAK-N | |
|---|---|---|---|
| PAK | PAK-N | ||
| flgBCDE (placΩflgE) | 1,000 ± 100 | 27,000 ± 2,000 | 27 |
| fleSR (placΩS) | 6,300 ± 80 | 80,000 ± 500 | 12.6 |
| fliDS orf126 (placΩD) | 1,900 ± 100 | 18,000 ± 90 | 9.5 |
| fliLMNOPQ (placΩL) | 2,600 ± 40 | 14,800 ± 1,800 | 5.6 |
| fliEFG (placΩE) | 1,000 ± 30 | 5,000 ± 180 | 5 |
| fliC (pPT269) | 3,200 ± 200 | 7,400 ± 300 | 2.3 |
| pilA (pMSZ5) | 10,000 ± 700 | 12,000 ± 500 | 1.2 |
| fleQ (placΩQ) | 2,800 ± 100 | 2,800 ± 300 | 1 |
| fliA (pMS565) | 650 ± 10 | 640 ± 2 | 1 |
| Promoterless lacZ (placΩ) | 289 ± 143 | 238 ± 187 | 0.8 |
Previous studies from our laboratory showed that FleQ, an NtrC-like transcriptional activator of P. aeruginosa that works in concert with RpoN (ς54), positively regulates two of the above six upregulated promoters, namely, fliDSorf126 (6) and fleSR (29), either directly or indirectly. These results were based on β-galactosidase assays performed with wild-type PAK, the rpoN mutant PAK-N1G, and the fleQ mutant PAK-Q. The fleQ promoter is not autoregulated by FleQ (5). To determine whether there was a direct correlation between upregulation of a promoter in the fleN mutant and FleQ dependence, the flagellar promoters of the genes and operons fliC, fliEFG, flgBCDE, fliLMNOPQ, and fliA were tested in an FleQ-deficient background, PAK-Q (Table 3). Reduced activity of the fliC, fliEFG, flgBCDE, and fliLMNOPQ promoters in PAK-Q implied that these genes and operons were positively regulated by FleQ, either directly or indirectly. The fliA promoter was not dependent on FleQ under these conditions, as it did not exhibit an appreciable decrease in its activity in PAK-Q.
TABLE 3.
Assessment of flagellar gene promoters for their dependence on FleQ, a transcriptional activator
| Operon or gene (promoter construct) | β-Galactosidase activity (Miller units; mean ± SD)
|
|
|---|---|---|
| PAK | PAK-Q | |
| flgBCDE (placΩflgE) | 1,000 ± 120 | 290 ± 40 |
| fliLMNOPQ (placΩL) | 2,500 ± 20 | 640 ± 20 |
| fliEFG (placΩE) | 760 ± 10 | 250 ± 10 |
| fliC (pPT269) | 2,100 ± 200 | 330 ± 7 |
| fliA (pMS565) | 400 ± 20 | 360 ± 20 |
| Promoterless lacZ (placΩ) | 170 ± 50 | 190 ± 70 |
In summary, among the eight flagellar gene promoters tested, the six promoters that were positively regulated by FleQ, namely, fliEFG, fliLMNOPQ, fliDS orf126, flgBCDE, fliC, and fleSR, were upregulated in the fleN mutant. This finding suggests that the absence of functional FleN in PAK-N enhances the transcriptional activator abilities of FleQ by an unknown mechanism.
FleN homologues from other microbial genomes share an N-terminal ATP-binding motif.
Computer-based searches of the various databases revealed that FleN homologues were also present in other microbial genomes (Fig. 5). The majority of the top scorers from the annotated entries were found to be positional homologues of FleN, as respective genes mapped downstream of the flhF counterparts, similar to that of fleN. They included OrfC of P. putida (90% identity) (13), Orf304 of T. pallidum (35% identity (J. M. Hardham, J. G. Frye, N. R. Young, and L. V. Stamm, GenBank accession no. U36839 [unpublished data]), YlxH of B. burgdorferi (34% identity) (J. J. Dunn, L. Butler-Loffredo, J. Kieleczawa, J. Medalle, and B. J. Luft, GenBank accession no. U43739 [unpublished data]), Orf298/YlxH of Bacillus subtilis (33% identity) (16), YlxH of H. pylori (31% identity) (36), and MinD-1 of Aquifex aeolicus (31% identity) (12). ctrA of P. aeruginosa W51D (accession no. AF052586) displayed 94 and 60% identities to fleN at the deduced amino acid and nucleic acid sequence levels, respectively. It maps upstream of rhlG, a gene involved in rhamnolopid synthesis (10). The function of ctrA has not been reported. Neither FleN nor CtrA of P. aeruginosa displayed sequence homology to the C. crescentus master regulator of flagellar biogenesis, also named CtrA (27).
FIG. 5.
PILEUP- and PRETTYBOX-generated alignment of the deduced amino acid sequence of FleN from P. aeruginosa (paeflen) and those of its positional homologues OrfC of P. putida (ppuorfc), Orf313 of V. cholerae (vchorf313), YlxH of B. burgdorferi (bboylxh), Orf304 of T. pallidum (tpaorf304), YlxH of H. pylori (hpyylxh), Orf298 of B. subtilis (bsuorf298), and MinD-1 of A. aeolicus (aaemind-1), using Wisconsin Package (version 10.0; Genetics Computer Group, Madison, Wis.). Dark shading denotes identical amino acid residues, and shades of gray represent conserved substitutions; ∼ represents gaps introduced at the beginning or end of the sequence, and dots denote gaps introduced within the sequence for optimal alignment. A consensus sequence was generated based on the presence of the same amino acid in at least four sequences. The ATP-binding motif corresponding to residues 18 to 25 of paeflen is conserved except in ppuorfc.
Searches with the unannotated partial genome sequences of V. cholerae, a monoflagellate, revealed an ORF on contig asm913, coding for a protein of 313 amino acids, that displayed 60% deduced amino acid sequence identity with FleN (Fig. 5). We refer to it as Orf313 in this study. It mapped downstream of the V. cholerae putative flhF as does fleN in P. aeruginosa, classifying it as another positional homologue of FleN.
The striking similarity among all of the proteins described above was that they all have an ATP-binding motif {[AG]-X4-G-K-[ST]} (PROSITE PS000017) at their N termini (Fig. 5) except for OrfC of P. putida. A closer inspection of the nucleotide sequence of this region in P. putida (accession no. AF031898) (13) revealed that an unannotated ORF mapping upstream of orfC coded for a polypeptide with 76 amino acid residues that bore the missing ATP-binding motif. This finding indicated the possibility of either a sequence error or an inherent mutation(s) leading to a reading frame shift in this locus in P. putida. The available genomic sequences of C. crescentus, E. coli, and Salmonella serovar Typhimurium did not have a FleN homologue.
DISCUSSION
Insertional inactivation of fleN, an ORF of P. aeruginosa flanked by the flagellar genes, flhF and fliA, conferred a multiflagellate, nonmotile phenotype to strains PAK and PAO1 (Fig. 2A). Though nonmotile in the motility plate assay, the fleN mutant displayed tumbling, spinning, and poor swimming abilities when examined under the light microscope. This defect in motility could not be solely attributed to the newly acquired multiflagellated phenotype of the P. aeruginosa fleN mutants, because a closely related species, P. putida, is characteristically known to possess a tuft of polar flagella (three or more) and simultaneously retain its motility (14). Moreover, the fleN mutant seemed distinct from a previously described (34) P. aeruginosa multiflagellate mutant which bore multiple polar flagella but possessed better swarming ability than its wild-type parent. The latter mutant was generated by chemical mutagenesis, and the mutation was mapped to a fla locus, but the exact gene was not identified. These observations suggest that the phenotypes of multiflagellation and nonmotility are not necessarily linked.
For a flagellated cell to chemotactically respond to an environmental stimulus, proper transmission of the message to the flagellar switch is necessary to determine the rotational direction of the flagella. In E. coli and Salmonella serovar Typhimurium, this transmission proceeds via a phosphorylation cascade involving CheA, CheW, CheY, CheZ, CheR, and CheB. Counterclockwise rotation forces the flagellar filaments into a bundle that propels the cell smoothly forward. Reversal of flagellar rotation to clockwise causes the bundle to fly apart and the cell to tumble (19). Unusually high tumbling frequencies have been reported for strains carrying certain mutations in either CheZ or the flagellar switch proteins FliG, FliM, and FliN and in wild-type strains overproducing CheY (9). In the P. aeruginosa multiflagellate fleN mutant, a defect in motility associated with tumbling behavior could arise if the proteins involved in flagellar rotation and chemotaxis were unable to function normally due to abnormal signal transduction in the absence of FleN. Alternatively, the probable lack of an equivalent increment in the number of transducing elements involved in chemotaxis relative to that of the flagellar structural components could explain the associated lack of motility. Thus, FleN could have a dual role in maintaining the single polar flagellum and chemotactic motility in P. aeruginosa.
Efforts to complement the fleN mutant revealed that the flagellar assembly of P. aeruginosa was sensitive to the amounts of FleN being expressed. Two constructs, pPZ375-fleN and pET-fleN, had distinctly different effects on the phenotype of the fleN mutant, though they had essentially the same region of fleN cloned in them. Most of the pPZ375-fleN containing cells were nonflagellate and remained nonmotile, whereas pET-fleN complemented the mutant to its wild-type motility phenotype with one to two polar flagella. Such a phenomenon can be explained based on the amounts of FleN being expressed as a function of the promoter activities of these two constructs. Expression of FleN from pET-fleN, with a low basal activity of the T7 promoter, was probably closer to the normal physiological level, allowing assembly of the flagellum, whereas in pPZ375-fleN, with an unrepressed lac promoter, the amount of FleN in the majority of the cells was high enough to signal complete cessation of flagellar assembly. Such an effect of FleN on flagellar assembly suggested that the chromosomal fleN locus is tightly regulated to control the amount of FleN that is made. The ability to complement the defect in PAK-N with a copy of fleN alone proved that the disruption of the genomic fleN did not jeopardize the functioning of the downstream fliA and che genes.
Multiflagellation in the fleN mutant could be attributed to the upregulation of FleQ-dependent flagellar operons and genes (Tables 2 and 3), which code for homologues of both structural proteins known to be involved in the formation of the flagellar motor and switch (FliM, FliN, and FliG), the basal body (FliE and FliF), the basal body rod (FlgB and FlgC), the hook (FlgD and FlgE), the cap (FliD), and the filament (FliC), and regulatory proteins (FleS and FleR), which are all essential for flagellar assembly. The fleQ promoter is itself not upregulated in the fleN mutant, which rules out the possibility of FleQ overexpression leading to upregulation of its dependent promoters. Further, the observed promoter upregulation suggests that in the absence of functional FleN, FleQ exhibits enhanced transcriptional activator properties allowing elevated expression of the flagellar genes. Therefore, FleN appears to exert an antagonistic effect on FleQ-dependent transcriptional activation. The deduced amino acid sequence of FleN does not have a recognizable DNA-binding domain, which reduces the probability of FleN directly binding to DNA to cause transcriptional repression of fleQ and other FleQ-dependent flagellar genes. We speculate that FleN influences FleQ activity either through direct protein-protein interactions with FleQ or through intermediates.
Except for the indicating that FleN is an ATP-binding protein (Fig. 5), the database searches did not provide clues to the types of interactions in which FleN and its homologues may be involved. Among the homologues of FleN described earlier, Orf298 of B. subtilis is the only one that has been characterized partially (16). A disruption of this ORF did not lead to a defect in chemotactic motility. Of note, another pathogen, V. cholerae, also possesses a FleN homologue, Orf313 (Fig. 5) (TIGR database), in addition to a FleQ homologue, FlrA (17). It is likely that Orf313 and FlrA are involved, in an interaction similar to that of FleN and FleQ of P. aeruginosa, in regulating the number of flagella in the monoflagellate V. cholerae.
With respect to the distribution of flagella, the multiple flagella in the fleN mutants of P. aeruginosa were found to be invariably polar. In contrast, wild-type E. coli and Salmonella serovar Typhimurium have peritrichous flagella. This observation suggests that the polar placement of flagella in P. aeruginosa may involve a factor(s) probably located in the cell wall.
Maintaining the characteristic number of flagella and its distribution is important for motile bacterial species. fleN of P. aeruginosa is the first gene to be partially characterized as having a dual role in controlling the number of flagellar filaments and motility in flagellated microbial species.
ACKNOWLEDGMENTS
We thank S. Lory for helpful discussions and acknowledge the electron microscopy and sequencing core laboratories of the Interdisciplinary Center for Biotechnology Research at the University of Florida for assistance with the EM and sequencing projects, respectively. W. Zeile's help with the video microscopy is thankfully acknowledged. B. W. Ritchings is acknowledged for technical assistance in constructing placΩL.
This work was supported by NIH grants HL33622 and AI45014 to R.R.
REFERENCES
- 1.Aizawa S-I. Flagellar assembly in Salmonella typhimurium. Mol Microbiol. 1996;19:1–5. doi: 10.1046/j.1365-2958.1996.344874.x. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa S, Kubori T. Bacterial flagellation and cell division. Genes Cells. 1998;3:625–634. doi: 10.1046/j.1365-2443.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and characterization of Pseudomonas aeruginosa fliF necessary for flagellar assembly and bacterial adherence to mucin. Infect Immun. 1996;64:2130–2136. doi: 10.1128/iai.64.6.2130-2136.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J Bacteriol. 1997;179:5574–5581. doi: 10.1128/jb.179.17.5574-5581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun. 1998;66:1000–1007. doi: 10.1128/iai.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1995. [Google Scholar]
- 8.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourret R B, Borkovich K A, Simon M I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–411. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- 10.Campos-Garcia J, Caro A D, Najera R, Miller-Maier R M, Al-Tahhan R A, Soberon-Chavez G. The Pseudomonas aeruginosa rhlG gene encodes an NADPH-dependent beta-ketoacyl reductase which is specifically involved in rhamnolipid synthesis. J Bacteriol. 1998;180:4442–4451. doi: 10.1128/jb.180.17.4442-4451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter P B, Hanlon D W, Ordal G W. flhF, a Bacillus subtilis flagellar gene that encodes a putative GTP-binding protein. Mol Microbiol. 1992;6:2705–2713. doi: 10.1111/j.1365-2958.1992.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 12.Deckert G, Warren P V, Gaasterland T, Young W G, Lenox A L, Graham D E, Overbeek R, Snead M A, Keller M, Aujay M, Huber R, Feldman R A, Short J M, Olsen G J, Swanson R V. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 13.Ditty J L, Grimm A C, Harwood C S. Identification of a chemotaxis gene region from Pseudomonas putida. FEMS Microbiol Lett. 1998;159:267–273. doi: 10.1111/j.1574-6968.1998.tb12871.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilardi G I. Pseudomonas. In: Lennette E H, Balows A, Hausler W J Jr, Jean Shadomy H, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society of Microbiology; 1985. pp. 350–372. [Google Scholar]
- 15.Ishimoto K S, Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci USA. 1989;86:1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch M L, Carpenter P B, Ordal G W. A putative ATP-binding protein from the che/fla locus of Bacillus subtilis. DNA Sequence. 1994;4:217–275. doi: 10.3109/10425179409020852. [DOI] [PubMed] [Google Scholar]
- 17.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibro cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 18.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 19.Macnab R M, Ornston M K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977;112:1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 21.Minamino T, Ino T, Kutsukake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minamino T, Macnab R M. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moens S, Vanderleyden J. Functions of bacterial flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 24.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 25.Ottemann K M, Miller J F. Roles of motility in bacterial-host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 26.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: defining the roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 27.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 28.Ramakrishnan G, Zhao J, Newton A. Multiple structural proteins are required for both transcriptional activation and negative autoregulation of Caulobacter crescentus flagellar genes. J Bacteriol. 1994;176:7587–7600. doi: 10.1128/jb.176.24.7587-7600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect Immun. 1995;63:4868–4876. doi: 10.1128/iai.63.12.4868-4876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Smith A W, Iglewski B H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989;17:10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spohn G, Scarlato V. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J Bacteriol. 1999;181:593–599. doi: 10.1128/jb.181.2.593-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starnbach M N, Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992;6:459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Iino T. Isolation and characterization of multiflagellate mutants of Pseudomonas aeruginosa. J Bacteriol. 1980;143:1471–1479. doi: 10.1128/jb.143.3.1471-1479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temple L, Sage A, Christie G E, Phibbs P V., Jr Two genes for carbohydrate catabolism are divergently transcribed from a region of DNA containing the hexC locus in Pseudomonas aeruginosa PAO1. J Bacteriol. 1994;176:4700–4709. doi: 10.1128/jb.176.15.4700-4709.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 37.Totten P A, Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Newton A. Regulation of the Caulabacter flagellar gene hierarchy; not just for motility. Mol Microbiol. 1997;24:233–239. doi: 10.1046/j.1365-2958.1997.3281691.x. [DOI] [PubMed] [Google Scholar]
- 39.Zwadyk P. Vibrionaceae. In: Joklik W K, Willett H P, Amos D B, Wilfert C M, editors. Zinsser microbiology. 20th ed. Norwalk, Conn: Appleton & Lange; 1992. pp. 566–575. [Google Scholar]