Abstract
Epithelial ovarian cancer remains the lethal gynecological malignancy in women. The representative histotype is high-grade serous carcinoma (HGSC), and most patients with HGSC present at advanced stages with peritoneal dissemination. Since the peritoneal dissemination is the most important factor for poor prognosis of the patients, complete exploration for its molecular mechanisms is mandatory. In this narrative review, being based on the clinical, pathologic, and genomic findings of HGSC, chromosomal instability and epigenetic dynamics have been discussed as the potential drivers for cancer development in the fallopian tube, acquisition of cancer stem cell (CSC)-like properties, and peritoneal metastasis of HGSC. The natural history of carcinogenesis with clonal evolution, and adaptation to microenvironment of peritoneal dissemination of HGSC should be targeted in the novel development of strategies for prevention, early detection, and precision treatment for patients with HGSC.
Keywords: High-Grade Serous Ovarian Cancer, Peritoneal Dissemination, Chromosomal Instability, Epigenetic Dynamics, Cancer Stem Cell-Like Properties, Clonal Evolution
INTRODUCTION
Epithelial ovarian cancer is the leading cause of death from gynecologic cancer in developed countries, and consists of five main histological types such as high-grade serous carcinoma (HGSC), low-grade serous carcinoma, endometrioid carcinoma, clear cell carcinoma, and mucinous carcinoma [1]. Among them, HGSC is the most common histotype and often occurs in the sixth and seventh decades (mean age 63 years) [1,2]. Nearly all patients present with tumor of advanced stages, International Federation of Gynecology and Obstetrics (FIGO) Stage III or IV. Early detection of HGSC is clinically difficult, as Horiuchi et al. reported in 2003 the natural history of HGSC development based on the sequential clinicopathological study using transvaginal ultrasonography (TVUS). There had been no apparent abnormalities in the adnexal regions 2 to 12 months prior to the diagnosis, but laparotomy showed all cases to have advanced stage of tumor [3]. In addition, a large randomized clinical trial in the United Kingdom (UK Collaborative Trial of Ovarian Cancer Screening; UKCTOCS) has demonstrated that screening program by TVUS with or without preceding CA125 does not increase the rate of early stage of ovarian cancer, and not decrease the mortality rate [4]. Regarding the treatment, primary debulking surgery followed by standard chemotherapy with taxane and platinum is effective in more than 80% of patients, who enjoy the complete remission after the first-line treatment. However, most patients will have recurrence and die due to peritoneal carcinomatosis, and 5-year survival rate of optimally debulked patients is approximately 50% [1,2].
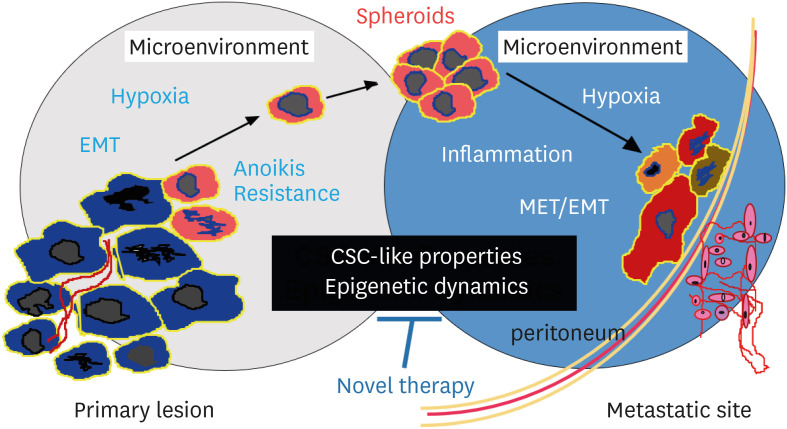
Since the most important factor for poor prognosis of HGSC is peritoneal dissemination, novel development of precision treatment targeting the disease process is needed (Fig. 1). However, the central player responsible for peritoneal dissemination has not so far been identified in the genomic abnormalities. In 1889, Paget proposed first the “seed and soil” theory, and then, its pathogenesis has been discussed with the interactions among cancer cells, peritoneal mesothelial cells, endothelial cells, immune cells, and cancer-associated fibroblasts (CAFs) under the specific microenvironment [5]. Recently, genetic, epigenetic, and chromosomal analyses on HGSC have been disclosing the extreme genomic instability, as well as the transcriptional and epigenetic adaptation of HGSC cells to various microenvironments. Cancer stem cell (CSC)-like properties have been characterized. In addition, the histogenetic origin of HGSC and its clonal evolution have recently been explored. In this narrative review, being based on those studies, we present our hypothesis that chromosomal instability prepares the CSC-like properties, and epigenetic adaptation facilitates the progression of HGSC.
Fig. 1. Peritoneal dissemination of HGSC cells.
Peritoneal dissemination is a unique metastatic process, characterized by the interactions between cancer cells and other cells under the specific microenvironment. HGSC cells acquire CSC-like properties, and metastasize using epigenetic dynamics in the microenvironment. These processes should be targeted by novel therapy (original illustration by Dr. Akiko Horiuchi).
CSC, cancer stem cell; EMT, epithelial-mesenchymal transition; HGSC, high-grade serous carcinoma; MET, mesenchymal-epithelial transition.
Our study was approved by the Ethics Committee at National Hospital Organization Kyoto Medical Center (No. 22-018).
GENOMIC ALTERATION CHARACTERISTICS OF HGSC
In 2011, The Cancer Genome Atlas (TCGA) Research Network reported a comprehensive genetic and epigenetic changes of HGSC [6]. Nearly all cases harbored mutations in TP53, although the frequency of each somatic mutation other than TP53 is less than 5%, confirming that mutation of driver oncogene is extremely rare in HGSC. Elevated DNA methylation and reduced expression implicated 168 genes as epigenetically silenced in HGSC. Aberrations of the homologous recombination (HR) pathway genes include BRCA1 germline mutation (8%), BRCA1 somatic mutation (3%), BRCA2 germline mutation (6%), BRCA2 somatic mutation (3%), BRCA1 promoter methylation (10%), CDK12 mutations (3%), and other HR-related gene mutations (20%). Accordingly, approximately 50% of HGSC cases are characterized by homologous recombination deficiency (HRD). Currently, HRD is used as the scientific basis for clinical application of poly-(ADP-ribose) polymerase (PARP) inhibitors for patients with HGSC (Table 1).
Table 1. Approved drugs and possible candidates targeting chromosomal instability, CSC-like properties, and microenvironment of peritoneal dissemination of HGSC.
| Target | Approved drugs | Candidate drugs | |
|---|---|---|---|
| Chromosomal instability | |||
| HRD | PARP inhibitors (olaparib, rucaparib, niraparib) | ||
| CSC-like properties | |||
| Hedgehog signal | Hedgehog inhibitor (sonidegib) | ||
| Wnt signal | Wnt inhibitor (ipafricept) | ||
| ALDH | ALDH inhibitor (disulfilam) | ||
| EpCAM | Anti-EpCAM Ab (catumaxomab) | ||
| Microenvironment of peritoneal dissemination | |||
| VEGF | Anti-VEGF Ab (bevacizumab) | VEGFR inhibitors (apatinib, cediranib) | |
| S100A4/RhoA | Statins (simvastatin) | ||
| Inflammation | Anti-inflammatory drugs (aspirin, NSAIDs) | ||
| Anti-IL-6R Ab (tocilizumab) | |||
| Immune evasion | Immune checkpoint inhibitor (pembrolizumab for MSI-H) | Immune checkpoint inhibitors (nivolumab, pembrolizumab, avelumab) | |
| EMT/Histone deacetylation | HDAC inhibitor (resminostat) | ||
| EMT/TGF-β signal | TGF-β inhibitors (vactosertib, ABBV151) | ||
ALDH, aldehyde dehydrogenase; CSC, cancer stem cell; EMT, epithelial-mesenchymal transition; EpCAM, epithelial cell adhesion molecule; HDAC, histone deacetylase; HGSC, high-grade serous carcinoma; HRD, homologous recombination deficiency; MSI-H, microsatellite instability-high; NSAID, non-steroidal anti-inflammatory drug; PARP, poly-(ADP-ribose) polymerase; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
With regard to mRNA expression profile, TCGA report showed the four subtypes of HGSC, i.e., immunoreactive, differentiated, proliferative, and mesenchymal [6]. Subsequent studies reported that patients with the immunoreactive showed the best survival, then the differentiated, followed by the proliferative, and the mesenchymal one the worst [7,8]. Our analysis on the pathological features along with mRNA expression profile found that the differentiated is histologically well-differentiated grade 1 carcinoma (Fig. S1A), and the proliferative is poorly differentiated grade 3 carcinoma (Fig. S1B). Thus, they represent the conventional histological grades [9]. In contrast, the mesenchymal shows desmoplastic stroma (Fig. S1C), and the immunoreactive is characterized by many lymphocytes surrounding the cancer nest (Fig. S1D). Therefore, the latter two represent the total gene expressions of cancer including those from the microenvironment [9]. These data demonstrated that prognosis of the patients is influenced not only by tumor grade but also by microenvironment of cancer.
Widespread copy number (CN) alterations have occurred in the chromosomes of HGSC, as TCGA analysis identified 8 regional CN gains and 22 losses [6]. There are no specific patterns in either numerical or structural abnormalities. Recently, however, Macintyre et al. [10] reported that CN aberrations can be classified into the seven CN Signatures, being correlated with the biological behavior and the outcome of patients. Signature 1 is correlated with RAS/MAPK signaling. Signature 2 frequently shows CDK12 mutation and poor survival of patients. Signature 3 shows HRD with frequent BRCA1/2 mutations and better survival of patients. Signature 4 is characterized by high CN states and mutation of MYC, CDK12, and CCNE1. Signature 5 suggests chromothriptic-like events. Signature 6 shows extremely high CN states with amplification of CCNE1, CCND1, CDK2, CDK4, or MYC. Signature 7 is related with non-BRCA HRD and good patient survival [10]. Thus, amplification of driver oncogene is present in some cases, but is not the ubiquitous phenomenon, suggesting that loss of tumor suppressor genes may be more important in the development of HGSC.
PATHOLOGY AND EPIGENETICS IN PERITONEAL DISSEMINATION OF HGSC
Peritoneal dissemination is a unique metastatic process, which consists of detachment of cancer cells from the primary lesion, floating in the ascitic fluid, attachment to the peritoneal mesothelium, invasion into the submesothelial space, and remodeling of the tumor microenvironment including angiogenesis (Fig. 1). After colonization in the metastatic sites, cancer cells show both mesenchymal-epithelial transition (MET) and epithelial-mesenchymal transition (EMT). From the first metastatic lesions, cancer cells metastasize again to other peritoneal or retroperitoneal sites. Thus, the peritoneal dissemination is a dynamic process in the peritoneal cavity, and HGSC cells encounter the different microenvironments according to the above sequential steps of the metastasis. Pathological findings of peritoneal dissemination have been reported mainly in advanced cases at Stage IIIC with high-volume disease [11]. In order to observe each step in detail, as tried similarly in a previous report [12], we examined closely the microscopic slides of patients at earlier cases, stage IIIA or IIIB, in which peritoneal dissemination is just starting or under early construction.
1. Detachment of HGSC cells and hypoxia
In HGSC having peritoneal dissemination, there are many spheroids floating in the ascitic fluid and approaching to the peritoneal mesothelial cells (Fig. 2A), indicating that HGSC cells metastasize in the form of spheroids [11,12,13]. HGSC is histologically characterized by papillary or solid proliferation of cancer cells (Fig. S1A and B). HGSC cells at the tip of papillary lesion or in the center of solid growth, being apart from the blood supply, are exposed to hypoxia (Fig. 1), as Imai et al. [14] demonstrated that these cells exhibit an increased expression of hypoxia-inducible factor-1α (HIF-1α). Interestingly, HGSC cells under hypoxia simultaneously show the loss of E-cadherin expression [14]. Hypoxia attenuates the expression of E-cadherin via upregulation of the transcription factor SNAIL, along with the increase in cell motility and invasive capacity of HGSC cells, suggesting that EMT program is working (Fig. 3) [14]. Since ascitic fluids contain half the soluble oxygen as blood [15], HGSC cells continue to be exposed to hypoxia, but survive with anoikis resistance (Fig. 1). Hypoxia stimulates the HIF-1α-responsive signaling of target genes, such as GLUT-1, VEGF, EPO, CXCR4, OCT4, SOX2, NANOG, and KLF, which are crucial regulating glucose metabolism, angiogenesis, inflammation, migration, and self-renewal of cancer cells [15,16]. In 1997, we first reported that elevated expression of vascular endothelial growth factor (VEGF) is an important prognostic factor, and that high levels of VEGF are also found in both serum and ascites of the patients [17]. Since 2014, molecular-targeted therapy with anti-VEGF antibody has been used as the maintenance treatment for better survival and quality of life of patients with advanced and recurrent ovarian cancer (Table 1).
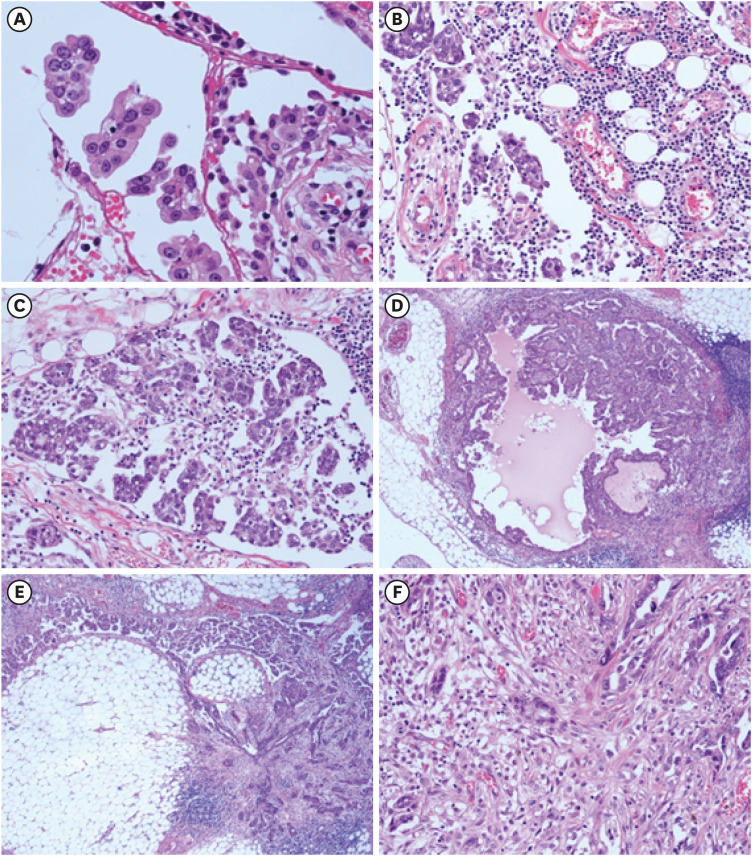
Fig. 2. Phenotypic plasticity of HGSC cells in peritoneal dissemination.
(A) Spheroids of HGSC cells are floating in the ascitic fluid, and are attaching to the mesothelial layer of greater omentum. (B) Then, spheroids of HGSC cells are embedded in the prominent inflammation with angiogenesis mimicking the granulation tissue of wound healing. (C) Colonization and proliferation of HGSC cells start at the disseminated sites, and (D) cancer cells show less-invasive features resembling those in the ovarian tumors. (E) HGSC cells at the metastatic sites show colonization (left), whereas other cells exhibit the invasive features with EMT (right). (F) In EMT lesions, HGSC cells exhibit spindle-like shape, being associated with desmoplastic reaction. HGSC cells are intermingled with CAFs, immune cells, and endothelial cells in the desmoplastic stroma (original magnification: A, ×200; B, C, F, ×100; D, E, ×40).
CAF, cancer-associated fibroblast; EMT, epithelial-mesenchymal transition; HGSC, high-grade serous carcinoma.
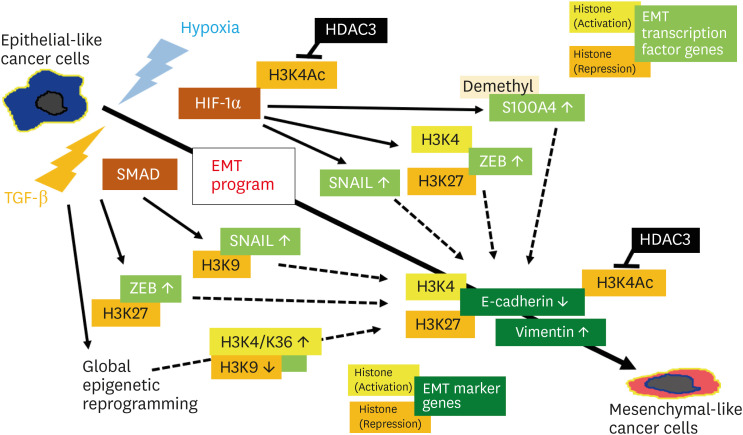
Fig. 3. Epigenetic regulation of EMT program under hypoxia or TGF-β stimulation in cancer.
EMT program proceeds by activation of EMT transcription factors such as SNAIL and ZEB, followed by the change in the expression of EMT marker genes such as E-cadherin and vimentin, mainly via modification of various histones.
EMT, epithelial-mesenchymal transition; TGF-β, transforming growth factor-β.
S100A4 (metastasin) is a small Ca-binding protein of the S100 family, and is secreted in the form of extracellular vesicles, which stimulates invasiveness and upregulates matrix metalloproteinases (MMPs) [18]. In 2006, Kikuchi et al. [19] first demonstrated that an increased expression of S100A4 is significantly associated with poor prognosis of ovarian cancer patients, and that HGSC cells secrete S100A4 as well as express the receptor for S100A4. Transcription of S100A4 gene in HGSC cells is upregulated by hypoxic condition, and is associated with EMT and increased invasive capacity in vitro, suggesting its pivotal role as autocrine/paracrine factor for the tumor progression [19]. Interestingly, elevated expression of S100A4 is correlated with hypomethylation of S100A4 gene in ovarian cancer cases. In addition, chronic hypoxia in vitro is shown to induce the hypomethylation of CpG sites in the first intron of S100A4 gene [20]. Accordingly, the epigenetic mechanism is actually involved in the adaptation to hypoxic microenvironment during progression of HGSC cells (Fig. 3). Since high levels of serum S100A4 transcripts have recently been identified, it could be the useful tumor marker of ovarian cancer [18,21]. Intracellular signaling of S100A4 is transferred via RhoA [22], which mediates the autocrine/paracrine stimulation of not only S100A4 but also insulin-like growth factor (IGF), hepatocyte growth factor (HGF), and lysophosphatidic acid (LPA). Statins have been known to inhibit the signal transduction of RhoA, via inhibition of mevalonate pathway, and is shown to suppress the peritoneal dissemination of ovarian cancer cells in mouse model [23]. Thus, statins have received attention as a promising drug for the suppression of peritoneal dissemination of HGSC (Table 1) [24,25].
2. Implantation of HGSC cells and inflammation
The second step of peritoneal dissemination is an approach of HGSC spheroids to the peritoneum (Figs. 1, 2A), followed by attachment to the mesothelial cells with arrangement of the microenvironment. Just before cell-to-cell attachment, HGSC cells induce the inflammatory reaction leading to the leakage of fibrinogen, followed by the cell anchorage in the fibrin networks [12]. HGSC cells also produce various molecules such as HGF, transforming growth factor-β (TGF-β), and interleukin-1β (IL-1β), and induce the transformation of normal mesothelial cells into the mesenchymal-like mesothelial cells, which promotes the adhesion of cancer cells on the peritoneum, as well as enhances angiogenesis via VEGF production [26]. Interestingly, we found that the spheroids of HGSC are embedded in the inflammatory tissues resembling the granulation of wound healing (Fig. 2B). Malignant ascites have been reported to contain elevated levels of various cytokines, such as tissue factor, tumor necrosis factor-α (TNF-α), IL-6, IL-8, and LPA [11,12,13,26,27]. Pro-inflammatory prostaglandins may also be important, since the expression of COX-1 and COX-2 is reportedly increased in ovarian cancer [28]. Thus, the inflammatory microenvironment produced by various cytokines and prostaglandins seems to be beneficial for the survival of HGSC cells in the disseminated sites [11,12,13,15,16,26,27,28].
Recently accumulating evidence indicates that chronic inflammation plays an important role in the progression of cancer, via inflammation-mediated epigenetic alterations of cancer cells [27,29]. In ovarian cancer, epigenetic modification of histone such as suppression of H2B ubiquitylation has been shown to induce the upregulation of TNF-α and IL-6 along with increased migration of HGSC cells [30]. Inflammation in the peritoneal dissemination might be one of appropriate targets for preventing the metastasis of ovarian cancer, and current or post-diagnosis use of anti-inflammatory drugs like aspirin has been reported to contribute to better survival of patients (Table 1) [31]. Immunologic aspects including the evasion of ovarian cancer cells from the host immune mechanism are also very important, especially in the modern era of use of immune checkpoint inhibitors, and have already been reviewed elsewhere [32].
3. Colonization of HGSC cells and EMT
The third step is the colonization of HGSC cells at the disseminated sites after obtaining an appropriate blood supply (Fig. 2C). Here, HGSC cells show the pathologically less-invasive features (Fig. 2D), and a reverse phenomenon of MET occurs in such lesions, as the expression of E-cadherin has reportedly been increased again at metastatic sites [33]. In most cases, however, HGSC cells re-start the invasion into the stroma, being associated with desmoplastic reaction (Fig. 2E), which consists of CAFs, immune cells, endothelial cells, and the extracellular matrix (Fig. 2F). HGSC cells exhibit the pathologic finding of EMT, which is characterized by the transformation into spindle-like shape of cancer cell. Such EMT feature is typically observed in the mesenchymal subtype of HGSC (Fig. S1C), and also prominent in the huge metastasis to the greater omentum, being clinically called as “omental cake” (Fig. S2A) [34]. Our analysis comparing the gene expressions between the ovarian and omental tumors identified the activation of TGF-β pathway in the omental metastasis [35]. This is consistent with that, among a variety of growth factors, TGF-β signaling plays a fundamental role in EMT [36,37].
EMT of cancer cells is known to be regulated by both transcriptional and epigenetic mechanisms (Fig. 3) [38,39]. Various factors such as hypoxia and TGF-β trigger the EMT program via the intracellular signaling cascades including HIF-1α and SMAD, followed by activation of EMT transcription factors such as SNAIL and ZEB. Then, these transcription factors act on the promoter of EMT marker genes such as E-cadherin and vimentin [36,39]. Epigenetic regulation of the EMT program has been investigated mainly on the modifications of chromatin-associated histones, such as H3K27, H3K9, and H3K4, in the promoter of both EMT transcription factor genes and marker genes [38]. For example, hypoxia influences the expression of EMT markers via change of acetylation levels of H3K4Ac by activation of histone deacetylase 3 (HDAC3) [39]. In ovarian cancer, expression of HDAC3 has reportedly been increased, and the suppression of HDAC3 in vitro resulted in the elevation of E-cadherin expression along with reduced cell migration of HGSC cells [40]. In the TGF-β-induced EMT, global epigenetic reprogramming occurs via histone modifications including the methylation of different histones such as H3K9, H3K4, and H3K36 [39,41]. In addition, EMT has been known to play an important role in the chemoresistance, and especially, an enhanced resistance to drugs has been shown in the hybrid EMT phenotype consisting of the mixture of epithelial-like cells and mesenchymal-like cells (Fig. S2B) [16,36]. In mouse model of peritoneal dissemination of ovarian cancer, treatment with inhibitor of TGF-β signaling is effective for better survivals [35]. Therefore, EMT in the peritoneal dissemination of HGSC cells under hypoxia or TGF-β action could be the target of novel treatment strategies (Table 1).
4. Phenotypic plasticity and CSC-like properties
As shown in the pathology of peritoneal dissemination (Fig. 2A-F), HGSC cells exhibit the extreme phenotypic diversity according to different microenvironments. Although genetic alterations had previously been considered as the driver for such plasticity [42], accumulating evidences indicate that epigenetic dynamics plays a pivotal role in adaptation to each microenvironment of the peritoneal dissemination [43]. Such phenotypic plasticity appears to represent the pluripotentiality of HGSC having CSC-like properties, and the microenvironments such as hypoxia, inflammation, and desmoplastic stroma are currently considered to function as niche, that is essential for the survival of CSCs [15,16]. Interestingly, there have been no specific genome alterations known for acquiring the CSC-like properties [44], and the possible role of epigenetics has recently been discussed [45,46]. CSCs are characterized by enrichment in the side population (SP) in the flow cytometry analysis [47], and also by the expression of CSC markers such as CD133, ALDH, CD44, CD117, and CD 326 (EpCAM) [15,16]. In ovarian cancer, the presence CSCs was implicated by successful isolation of a single tumorigenic clone derived from the ascites of a patient with HGSC [48], and have received much attention as the main cause of intratumoral heterogeneity leading to the tumor recurrence with chemoresistance [15,16,48,49,50]. Novel drugs targeting the CSC signaling such as Hedgehog and Wnt have been tried in ovarian cancer patients (Table 1).
CELL ORIGIN AND CLONAL EVOLUTION OF HGSC
Historically, the cell origin of epithelial ovarian cancer was proposed by Scully that it is originated in the ovarian surface epithelium (OSE), and growing toward various directions of mullerian metaplasia [51]. Possible OSE origin has been still under re-examination [52]. Since 2007, however, thorough examinations of the fallopian tubes obtained from women with germline BRCA1/2 pathogenic variants have disclosed the presence of precursor lesion in the tubal fimbria, that is serous tubal intraepithelial carcinoma (STIC) with TP53 mutation [53,54]. In addition, further studies revealed the epithelial foci of tubal secretory cells having TP53 mutation and overexpression without cellular atypia. Such foci are designated as p53 signature, and considered to be the earliest event of carcinogenesis. Serous tubal intraepithelial lesion (STIL) or “dormant STIC” (Ki-67 labeling index <10%) is an intermediate lesion between p53 signature and “proliferative STIC” (Ki-67 labeling index >10%) [55].
Strikingly, we recently identified that, in a case of incidentally found STIC with micro-metastasis to the ovary, there are many spheroids of HGSC floating in the tubal fluid near or apart from the STIC (Fig. 4). There has been another report of spheroids floating in a case of STIC without ovarian or tubal HGSC [56]. These findings suggest that HGSC cells can be shed directly from STIC without the accompanying invasive tumors, and have the capacity of forming spheroids [55,56,57]. Generally, organization of the spheroids is known as one of the characteristic features of CSC [58], which shows the expression of Yamanaka transcription factors, such as OCT4, SOX2, KLF4, and C-MYC [59]. The spheroids derived from ovarian cancer cell lines also exhibit an increased expression of OCT4, SOX2, NANOG, and C-MYC, along with that of CSC markers such as CD133, ALDH, CD44, and CD117 [13,60,61]. Therefore, it is likely that STIC cells have already acquired the CSC-like properties during the precursor phase of HGSC. For the comprehensive understanding of peritoneal dissemination, it is essential to introduce the concept of CSC in the story on the clonal evolution of HGSC.
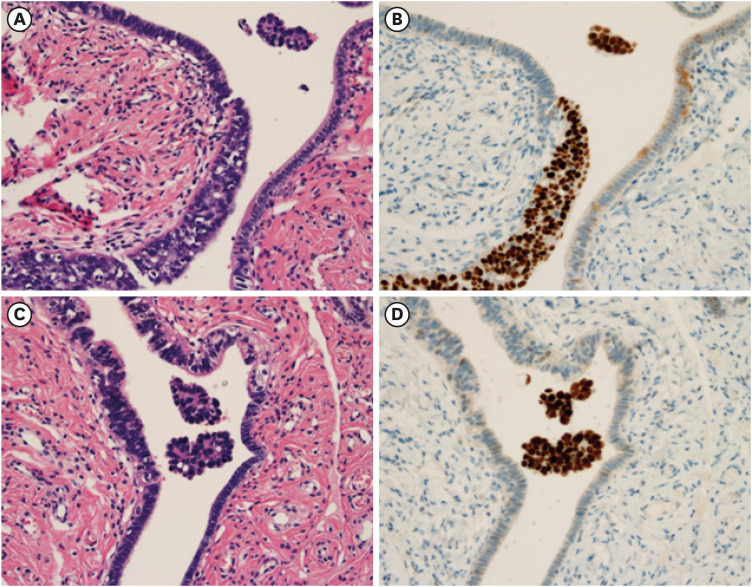
Fig. 4. Spheroids floating in the tubal fluid in the vicinity of STIC.
In a case of incidentally found STIC with micrometastasis to the ovary, HGSC spheroids are identified in the vicinity of STIC, floating in the tubal fluid, as shown in (A) H&E and (B) p53 immunostaining. Spheroids are also floating in the tubal fluid apart from STIC lesion, as shown in (C) H&E and (D) p53 immunostaining (original magnification ×200).
H&E, hematoxylin and eosin; HGSC, high-grade serous carcinoma; STIC, serous tubal intraepithelial carcinoma.
Clonal expansion and genomic evolution from p53 signature to STIC, and STIC to invasive HGSC of ovarian and peritoneal lesions has been demonstrated using whole-genome analyses of the microdissected cells from each lesion [62,63,64]. In p53 signatures, there are little somatic mutations other than TP53 mutations. Then, various mutations have accumulated in the phase from “dormant STICs” to “proliferative STICs.” With regard to the allelic imbalance, the frequency of LOH is very rare in the phase of p53 signature or “dormant STICs,” and varies in the “proliferative STICs” without HGSC. In contrast, the “proliferative STICs” having ovarian or peritoneal HGSC tumors have already acquired various chromosomal aberrations, such as deletions and amplifications [62] or LOH in encompassing the tumor suppressor genes like TP53, BRCA1, BRCA2, and PTEN [63]. Interestingly, during or after the progression from STIC to ovarian or peritoneal tumors, additional somatic mutations or chromosomal CN aberrations are little and not ubiquitous [65,66,67,68,69], suggesting that genomic alterations in the later phase do not play an important role in the peritoneal dissemination. Thus, the basic set of genetic and chromosomal alterations necessary for the peritoneal metastasis in each case has already been established during the precursor phase of carcinogenesis (Fig. 5).
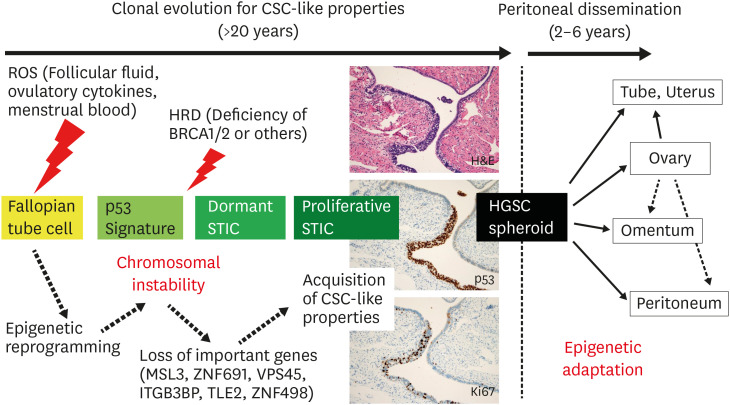
Fig. 5. Hypothetical model for development and progression of HGSC.
Under the influence of ROS from the ovary or menstrual blood, epigenetic reprogramming may occur in the fallopian tube cells. Chromosomal instability, especially loss of important genes, leads to the acquisition of CSC-like properties, during the precursor phase more than 20 years from p53 signature to proliferative STIC. After establishment of metastatic potential, HGSC cells leave the fallopian tube in the form of spheroids, and produce peritoneal disseminations in 2–6 years via epigenetic adaptation to microenvironment.
Histopathological pictures show H&E and immunostainings for p53 and Ki67 in the “proliferative STIC” (original magnification ×100).
CSC, cancer stem cell; H&E, hematoxylin and eosin; HGSC, high-grade serous carcinoma; ROS, reactive oxygen species; STIC, serous tubal intraepithelial carcinoma.
With regard to the timeline from such tubal precursor lesions to HGSC tumors, p53 signature and “dormant STIC” may take a prolonged time (2 decades or more) to develop into “proliferative STIC”, whereas “proliferative STIC” may progress to ovarian carcinoma in a much shorter time (6 years) [64]. Another study also indicated that the average time between STIC and ovarian cancer is 6.5 years (1.4–10.7 years), and the time between the initiation of ovarian cancer and development of other metastases appears to be rather rapid [63]. However, other phylogenic analyses on the clonal evolution of HGSC during the peritoneal dissemination have revealed that, in many cases, HGSC cells metastasize to the peritoneum independently from ovarian carcinomas, indicating the presence of diverse metastatic trajectories [62,65,66,67,68,69]. Thus, peritoneal spreading might occur before or in parallel with ovarian metastases. All of these data indicate that the most important phase of HGSC tumorigenesis is the incubation-like period in the fallopian tube for more than 20 years, during which the precursor cells evolve to HGSC cells having CSC-like properties, and eventually obtained the capability of peritoneal dissemination (Fig. 5) [62,63,64].
CHROMOSOMAL INSTATBILITY AND MACROEVOLUTION OF HGSC
Genomic instability is one of the fundamental features of cancer and contributes to the intratumoral heterogeneity. The events from genomic instability are classified into the two classes, i.e., small range aberrations such as mutation of driver genes and tumor suppressor genes, and large range aberrations due to chromosomal instability leading to the production of aneuploid cells [70]. The latter has been designated as macroevolution of cancer [71], in which various chromosomal changes result in loss of tumor suppressor genes, chimeric fusion of oncogenes, and oncogene amplifications [72,73]. Phenomenon of macroevolution in cancer might be comparable to the creation of new species in animal and plant [74], and large genomic rearrangement is more effective for adaptation to serious change of the environment than single gene mutation [72,73,74]. In normal condition, aneuploid cells cannot survive due to reduced fitness under the selection pressures, although TP53 deficiency has been known to permit the survival of aneuploid cells [75]. This is consistent with the timeline of HGSC tumorigenesis in which TP53 mutation occurs earlier than chromosomal aberrations [55,56]. Thus, HGSC is a typical example of macroevolution, being characterized by the drastic jump from the intraepithelial cancer cells directly into the aggressive cells creating peritoneal disseminations (Fig. 5). Such HGSC tumorigenesis is completely different from that of other solid cancers, showing the stepwise progression from intraepithelial tumor to locally invasive cancer and to aggressive cancer with metastatic potential. This is why early detection of HGSC using the imaging modalities is impossible, and novel development of strategies such as detection of biomarkers produced by HGSC spheroids is mandatory.
A promising hypothesis is that chromosomal instability causing the loss of key genes is responsible for its acquisition of CSC-like properties along with the capability of peritoneal dissemination of HGSC cells. To address this hypothesis, we performed a study of functional genomics screen using a shRNA library targeting 15,000 genes in HGSC cells, which have not yet obtained the capacity of anoikis resistance. A library of 81,000 shRNAs targeting 15,000 genes was transduced into OVCA420 cells, followed by incubation in soft agar and colony selection. shRNAs directed to ABHD2, ELAC2 and CYB5R3 caused reproducible anoikis resistance [76]. Furthermore, we also identified 6 novel genes, such as MSL3, ZNF691, VPS45, ITGB3BP, TLE2, and ZNF498, the suppression of which markedly increased the proportion of SP cells, exhibiting the capacity for spheroids formation, single cell clonogenicity, in vivo tumorigenicity, and chemoresistance [77]. These findings strongly suggests that the loss of such important genes due to chromosomal instability plays a central role in the acquisition of CSC-like properties of HGSC (Fig. 5). Another previous experiment using ovarian cancer cell line also demonstrated that the loss of chromosomal fragments leads to the phenotypic switching to metastasis [78].
Accumulating evidences indicate that chronic inflammation generating reactive oxygen species (ROS) has been implicated in the chromosomal instability [72,74]. In case of HGSC, its histogenetic origin, distal part of fallopian tubes, in women of reproductive age might be exposed continuously to severe stresses, such as high-level ROS from the follicular fluid [79] or from the menstrual blood regurgitating from the uterine cavity [80], and high-levels of cytokines during ovulation [27,81]. Risk of HGSC increases with increasing number of ovulation in the lifetime [82]. This is consistent with that early carcinogenic process of HGSC starts in young women ranging between 35 and 45 years at latest [64]. ROS often causes the DNA damage with double-strand break, and dysfunction of DNA repair pathway due to HRD may accelerate the chromosomal instability (Fig. 5) [70]. In addition, it has recently been hypothesized that epigenetic reprogramming precedes to various genetic or chromosomal alterations as the earliest step of carcinogenesis [83,84,85]. Initial epigenetic events for the reprogramming consist of global CpG hypomethylation and CpG hypermethylation of the specific genes. Such epigenetic alterations have been reported to occur in the normal-appearing fallopian tubes in women especially with BRCA1/2 pathogenic variants [86], and in the tubal precursors of HGSC [87]. These findings might give us a hint about the novel development of method for the detection of pre-cancerous lesion as well as for the prevention of HGSC in the near future.
CONCLUSIONS
Evidences have been accumulating about the genomic mechanisms in the development and progression of HGSC, and our current hypothetical scheme is shown in Fig. 5. Studies on the clonal evolution of the precursor cells in the fallopian tubes to HGSC cells have been disclosing the natural history of development. Epigenetic reprogramming and chromosomal instability, especially loss of the important genes, might lead to the development of HGSC cells with CSC-like properties via the precursor phase from p53 signature to STIC in the fallopian tube. Then, HGSC cells depart directly from STIC in the form of spheroids, and metastasize to the ovary and peritoneal cavity. During the peritoneal dissemination, addition of genetic mutations and chromosomal aberrations are minimal, whereas the epigenetic dynamics facilitates the phenotypic plasticity of HGSC for adaptation to various microenvironments. Clinical trials for new drugs targeting the peritoneal dissemination of ovarian cancer are in preparation or ongoing [16,88,89,90,91,92,93] (Table 1). Further studies on the dynamic biology of HGSC cells are needed to develop the epoch-making methods for prevention, early detection, and treatment for HGSC.
ACKNOWLEDGEMENTS
We sincerely appreciate Professor Dong Hoon Suh, Editor-in-Chief of Journal of Gynecologic Oncology, and Professor Jae-Weon Kim, President of Asian Society of Gynecologic Oncology, for their kind invitation for submission of this article. We also thank Dr. Akiko Horiuchi, Dr. Kazuko Itoh, Dr. Tsutomu Imai, Dr. Norihiko Kikuchi, and Dr. Toshio Nikaido, for their enthusiastic collaboration for ovarian cancer researches with us.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.I.
- Investigation: A.K., H.T., Y.K., M.R., Y.K., H.J.
- Project administration: K.I.
- Supervision: B.T., M.N., M.M.
- Writing - original draft: K.I.
SUPPLEMENTARY MATERIALS
Histological features of the 4 molecular subtypes of HGSC.
“Omental cake” in laparoscopy and EMT in pathology.
References
- 1.International Agency for Research on Cancer. Female genital tumours. WHO classification of tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2020. Tumours of the ovary; pp. 31–167. [Google Scholar]
- 2.Seidman JD, Ronnett BM, Shih IM, Cho KR, Kurman RJ. In: Blaustein’s pathology of the female genital tract. 7th ed. Kurman RJ, Ellenson LH, Ronnett BM, editors. Cham: Springer; 2019. Epithelial tumors of the ovary; pp. 841–966. [Google Scholar]
- 3.Horiuchi A, Itoh K, Shimizu M, Nakai I, Yamazaki T, Kimura K, et al. Toward understanding the natural history of ovarian carcinoma development: a clinicopathological approach. Gynecol Oncol. 2003;88:309–317. doi: 10.1016/s0090-8258(02)00104-x. [DOI] [PubMed] [Google Scholar]
- 4.Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397:2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikuła-Pietrasik J, Uruski P, Tykarski A, Książek K. The peritoneal “soil” for a cancerous “seed”: a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell Mol Life Sci. 2018;75:509–525. doi: 10.1007/s00018-017-2663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123:517–525. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konecny GE, Wang C, Hamidi H, Winterhoff B, Kalli KR, Dering J, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106:dju249. doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami R, Matsumura N, Mandai M, Yoshihara K, Tanabe H, Nakai H, et al. Establishment of a novel histopathological classification of high-grade serous ovarian carcinoma correlated with prognostically distinct gene expression subtypes. Am J Pathol. 2016;186:1103–1113. doi: 10.1016/j.ajpath.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Macintyre G, Goranova TE, De Silva D, Ennis D, Piskorz AM, Eldridge M, et al. Copy number signatures and mutational processes in ovarian carcinoma. Nat Genet. 2018;50:1262–1270. doi: 10.1038/s41588-018-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Baal JO, van Noorden CJ, Nieuwland R, Van de Vijver KK, Sturk A, van Driel WJ, et al. Development of peritoneal carcinomatosis in epithelial ovarian cancer: a review. J Histochem Cytochem. 2018;66:67–83. doi: 10.1369/0022155417742897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuoka A, Mizumoto Y, Ono M, Kagami K, Obata T, Terakawa J, et al. Novel strategy of ovarian cancer implantation: pre-invasive growth of fibrin-anchored cells with neovascularization. Cancer Sci. 2019;110:2658–2666. doi: 10.1111/cas.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uno K, Iyoshi S, Yoshihara M, Kitami K, Mogi K, Fujimoto H, et al. Metastatic voyage of ovarian cancer cells in ascites with the assistance of various cellular components. Int J Mol Sci. 2022;23:4383. doi: 10.3390/ijms23084383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S, Annett SL, Morgan MP, Robson T. The cancer stem cell niche in ovarian cancer and its impact on immune surveillance. Int J Mol Sci. 2021;22:4091. doi: 10.3390/ijms22084091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terraneo N, Jacob F, Dubrovska A, Grünberg J. Novel therapeutic strategies for ovarian cancer stem cells. Front Oncol. 2020;10:319. doi: 10.3389/fonc.2020.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76:1221–1227. doi: 10.1038/bjc.1997.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei F, Qu J, Zhang M, Li Y, Zhang S. S100A4 in cancer progression and metastasis: a systematic review. Oncotarget. 2017;8:73219–73239. doi: 10.18632/oncotarget.18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi N, Horiuchi A, Osada R, Imai T, Wang C, Chen X, et al. Nuclear expression of S100A4 is associated with aggressive behavior of epithelial ovarian carcinoma: an important autocrine/paracrine factor in tumor progression. Cancer Sci. 2006;97:1061–1069. doi: 10.1111/j.1349-7006.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiuchi A, Hayashi T, Kikuchi N, Hayashi A, Fuseya C, Shiozawa T, et al. Hypoxia upregulates ovarian cancer invasiveness via the binding of HIF-1α to a hypoxia-induced, methylation-free hypoxia response element of S100A4 gene. Int J Cancer. 2012;131:1755–1767. doi: 10.1002/ijc.27448. [DOI] [PubMed] [Google Scholar]
- 21.Link T, Kuhlmann JD, Kobelt D, Herrmann P, Vassileva YD, Kramer M, et al. Clinical relevance of circulating MACC1 and S100A4 transcripts for ovarian cancer. Mol Oncol. 2019;13:1268–1279. doi: 10.1002/1878-0261.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, et al. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–870. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 23.Horiuchi A, Kikuchi N, Osada R, Wang C, Hayashi A, Nikaido T, et al. Overexpression of RhoA enhances peritoneal dissemination: RhoA suppression with Lovastatin may be useful for ovarian cancer. Cancer Sci. 2008;99:2532–2539. doi: 10.1111/j.1349-7006.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi Y, Kashima H, Rahmanto YS, Banno K, Yu Y, Matoba Y, et al. Drug repositioning of mevalonate pathway inhibitors as antitumor agents for ovarian cancer. Oncotarget. 2017;8:72147–72156. doi: 10.18632/oncotarget.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng JL, Dixon-Suen SC, Jordan SJ, Webb PM. Statin use and survival among women with ovarian cancer: an Australian national data-linkage study. Br J Cancer. 2021;125:766–771. doi: 10.1038/s41416-021-01460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mogi K, Yoshihara M, Iyoshi S, Kitami K, Uno K, Tano S, et al. Ovarian cancer-associated mesothelial cells: transdifferentiation to minions of cancer and orchestrate developing peritoneal dissemination. Cancers (Basel) 2021;13:1352. doi: 10.3390/cancers13061352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savant SS, Sriramkumar S, O’Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel) 2018;10:251. doi: 10.3390/cancers10080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beeghly-Fadiel A, Wilson AJ, Keene S, El Ramahi M, Xu S, Marnett LJ, et al. Differential cyclooxygenase expression levels and survival associations in type I and type II ovarian tumors. J Ovarian Res. 2018;11:17. doi: 10.1186/s13048-018-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vezzani B, Carinci M, Previati M, Giacovazzi S, Della Sala M, Gafà R, et al. Epigenetic regulation: a link between inflammation and carcinogenesis. Cancers (Basel) 2022;14:1221. doi: 10.3390/cancers14051221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooda J, Novak M, Salomon MP, Matsuba C, Ramos RI, MacDuffie E, et al. Early loss of histone H2B monoubiquitylation alters chromatin accessibility and activates key immune pathways that facilitate progression of ovarian cancer. Cancer Res. 2019;79:760–772. doi: 10.1158/0008-5472.CAN-18-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt MA, Rice MS, Barnard ME, Hankinson SE, Matulonis UA, Poole EM, et al. Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study. Lancet Oncol. 2018;19:1107–1116. doi: 10.1016/S1470-2045(18)30373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. In: Frontiers in ovarian cancer science. Katabuchi H, editor. Singapore: Springer; 2017. Immunogy and immunotherapy in ovarian cancer; pp. 225–242. [Google Scholar]
- 33.Imai T, Horiuchi A, Shiozawa T, Osada R, Kikuchi N, Ohira S, et al. Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol. 2004;35:1469–1476. doi: 10.1016/j.humpath.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Motohara T, Masuda K, Morotti M, Zheng Y, El-Sahhar S, Chong KY, et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene. 2019;38:2885–2898. doi: 10.1038/s41388-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamura S, Matsumura N, Mandai M, Huang Z, Oura T, Baba T, et al. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. Int J Cancer. 2012;130:20–28. doi: 10.1002/ijc.25961. [DOI] [PubMed] [Google Scholar]
- 36.Loret N, Denys H, Tummers P, Berx G. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers (Basel) 2019;11:838. doi: 10.3390/cancers11060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshmukh AP, Vasaikar SV, Tomczak K, Tripathi S, den Hollander P, Arslan E, et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc Natl Acad Sci U S A. 2021;118:e2102050118. doi: 10.1073/pnas.2102050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YT, Wu KJ. Epigenetic regulation of epithelial-mesenchymal transition: focusing on hypoxia and TGF-β signaling. J Biomed Sci. 2020;27:39. doi: 10.1186/s12929-020-00632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi A, Horiuchi A, Kikuchi N, Hayashi T, Fuseya C, Suzuki A, et al. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int J Cancer. 2010;127:1332–1346. doi: 10.1002/ijc.25151. [DOI] [PubMed] [Google Scholar]
- 41.Cardenas H, Vieth E, Lee J, Segar M, Liu Y, Nephew KP, et al. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics. 2014;9:1461–1472. doi: 10.4161/15592294.2014.971608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoogstraat M, de Pagter MS, Cirkel GA, van Roosmalen MJ, Harkins TT, Duran K, et al. Genomic and transcriptomic plasticity in treatment-naive ovarian cancer. Genome Res. 2014;24:200–211. doi: 10.1101/gr.161026.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nath A, Cosgrove PA, Mirsafian H, Christie EL, Pflieger L, Copeland B, et al. Evolution of core archetypal phenotypes in progressive high grade serous ovarian cancer. Nat Commun. 2021;12:3039. doi: 10.1038/s41467-021-23171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzoldi EL, Pastò A, Pilotto G, Minuzzo S, Piga I, Palumbo P, et al. Comparison of the genomic profile of cancer stem cells and their non-stem counterpart: the case of ovarian cancer. J Clin Med. 2020;9:368. doi: 10.3390/jcm9020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciriello G, Magnani L. The many faces of cancer evolution. iScience. 2021;24:102403. doi: 10.1016/j.isci.2021.102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 47.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao Y, Li H, Huang R, Mo D, Zeng T, Fang M, et al. Clinicopathological and prognostic significance of cancer stem cell markers in ovarian cancer patients: evidence from 52 studies. Cell Physiol Biochem. 2018;46:1716–1726. doi: 10.1159/000489586. [DOI] [PubMed] [Google Scholar]
- 50.Motohara T, Katabuchi H. Ovarian cancer stemness: biological and clinical implications for metastasis and chemotherapy resistance. Cancers (Basel) 2019;11:907. doi: 10.3390/cancers11070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Washington, D.C.: Armed Forces Institute of Pathology; 1996. Surface epithelial-stromal tumors and serous tumors; pp. 51–79. [Google Scholar]
- 52.Zhang S, Dolgalev I, Zhang T, Ran H, Levine DA, Neel BG. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat Commun. 2019;10:5367. doi: 10.1038/s41467-019-13116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 54.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shih IM, Wang Y, Wang TL. The origin of ovarian cancer species and precancerous landscape. Am J Pathol. 2021;191:26–39. doi: 10.1016/j.ajpath.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bijron JG, Seldenrijk CA, Zweemer RP, Lange JG, Verheijen RH, van Diest PJ. Fallopian tube intraluminal tumor spread from noninvasive precursor lesions: a novel metastatic route in early pelvic carcinogenesis. Am J Surg Pathol. 2013;37:1123–1130. doi: 10.1097/PAS.0b013e318282da7f. [DOI] [PubMed] [Google Scholar]
- 57.Mei J, Tian H, Huang HS, Hsu CF, Liou Y, Wu N, et al. Cellular models of development of ovarian high-grade serous carcinoma: a review of cell of origin and mechanisms of carcinogenesis. Cell Prolif. 2021;54:e13029. doi: 10.1111/cpr.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci. 2017;108:283–289. doi: 10.1111/cas.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gheytanchi E, Naseri M, Karimi-Busheri F, Atyabi F, Mirsharif ES, Bozorgmehr M, et al. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021;21:204. doi: 10.1186/s12935-021-01898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson M, Gilbert SF, Waters JA, Lujano-Olazaba O, Lara J, Alexander LJ, et al. Characterization of SOX2, OCT4 and NANOG in ovarian cancer tumor-initiating cells. Cancers (Basel) 2021;13:262. doi: 10.3390/cancers13020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eckert MA, Pan S, Hernandez KM, Loth RM, Andrade J, Volchenboum SL, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6:1342–1351. doi: 10.1158/2159-8290.CD-16-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8:1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu RC, Wang P, Lin SF, Zhang M, Song Q, Chu T, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. 2019;248:41–50. doi: 10.1002/path.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bashashati A, Ha G, Tone A, Ding J, Prentice LM, Roth A, et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J Pathol. 2013;231:21–34. doi: 10.1002/path.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwarz RF, Ng CK, Cooke SL, Newman S, Temple J, Piskorz AM, et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 2015;12:e1001789. doi: 10.1371/journal.pmed.1001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JY, Yoon JK, Kim B, Kim S, Kim MA, Lim H, et al. Tumor evolution and intratumor heterogeneity of an epithelial ovarian cancer investigated using next-generation sequencing. BMC Cancer. 2015;15:85. doi: 10.1186/s12885-015-1077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McPherson A, Roth A, Laks E, Masud T, Bashashati A, Zhang AW, et al. Divergent modes of clonal spread and intraperitoneal mixing in high-grade serous ovarian cancer. Nat Genet. 2016;48:758–767. doi: 10.1038/ng.3573. [DOI] [PubMed] [Google Scholar]
- 69.Chien J, Neums L, Powell AF, Torres M, Kalli KR, Multinu F, et al. Genetic evidence for early peritoneal spreading in pelvic high-grade serous cancer. Front Oncol. 2018;8:58. doi: 10.3389/fonc.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou J, Zhou XA, Zhang N, Wang J. Evolving insights: how DNA repair pathways impact cancer evolution. Cancer Biol Med. 2020;17:805–827. doi: 10.20892/j.issn.2095-3941.2020.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vendramin R, Litchfield K, Swanton C. Cancer evolution: Darwin and beyond. EMBO J. 2021;40:e108389. doi: 10.15252/embj.2021108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tijhuis AE, Johnson SC, McClelland SE. The emerging links between chromosomal instability (CIN), metastasis, inflammation and tumour immunity. Mol Cytogenet. 2019;12:17. doi: 10.1186/s13039-019-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bach DH, Zhang W, Sood AK. Chromosomal instability in tumor initiation and development. Cancer Res. 2019;79:3995–4002. doi: 10.1158/0008-5472.CAN-18-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye CJ, Sharpe Z, Heng HH. Origins and consequences of chromosomal instability: From cellular adaptation to genome chaos-mediated system survival. Genes (Basel) 2020;11:1162. doi: 10.3390/genes11101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamanoi K, Matsumura N, Murphy SK, Baba T, Abiko K, Hamanishi J, et al. Suppression of ABHD2, identified through a functional genomics screen, causes anoikis resistance, chemoresistance and poor prognosis in ovarian cancer. Oncotarget. 2016;7:47620–47636. doi: 10.18632/oncotarget.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamanoi K, Baba T, Abiko K, Hamanishi J, Yamaguchi K, Murakami R, et al. Acquisition of a side population fraction augments malignant phenotype in ovarian cancer. Sci Rep. 2019;9:14215. doi: 10.1038/s41598-019-50794-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao C, Su Y, Koeman J, Haak E, Dykema K, Essenberg C, et al. Chromosome instability drives phenotypic switching to metastasis. Proc Natl Acad Sci U S A. 2016;113:14793–14798. doi: 10.1073/pnas.1618215113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bahar-Shany K, Brand H, Sapoznik S, Jacob-Hirsch J, Yung Y, Korach J, et al. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecol Oncol. 2014;132:322–327. doi: 10.1016/j.ygyno.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int J Clin Oncol. 2009;14:383–391. doi: 10.1007/s10147-009-0935-y. [DOI] [PubMed] [Google Scholar]
- 81.Sapoznik S, Bahar-Shany K, Brand H, Pinto Y, Gabay O, Glick-Saar E, et al. Activation-induced cytidine deaminase links ovulation-induced inflammation and serous carcinogenesis. Neoplasia. 2016;18:90–99. doi: 10.1016/j.neo.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trabert B, Tworoger SS, O’Brien KM, Townsend MK, Fortner RT, Iversen ES, et al. The risk of ovarian cancer increases with an increase in the lifetime number of ovulatory cycles: an analysis from the Ovarian Cancer Cohort Consortium (OC3) Cancer Res. 2020;80:1210–1218. doi: 10.1158/0008-5472.CAN-19-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8:a019505. doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matei D, Nephew KP. Epigenetic attire in ovarian cancer: the emperor’s new clothes. Cancer Res. 2020;80:3775–3785. doi: 10.1158/0008-5472.CAN-19-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartlett TE, Chindera K, McDermott J, Breeze CE, Cooke WR, Jones A, et al. Epigenetic reprogramming of fallopian tube fimbriae in BRCA mutation carriers defines early ovarian cancer evolution. Nat Commun. 2016;7:11620. doi: 10.1038/ncomms11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pisanic TR, 2nd, Asaka S, Lin SF, Yen TT, Sun H, Bahadirli-Talbott A, et al. Long interspersed nuclear element 1 retrotransposons become deregulated during the development of ovarian cancer precursor lesions. Am J Pathol. 2019;189:513–520. doi: 10.1016/j.ajpath.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y, Yang Y, Yang J, Zhao X, Wei X. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front Cell Dev Biol. 2020;8:758. doi: 10.3389/fcell.2020.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arildsen NS, Hedenfalk I. Simvastatin is a potential candidate drug in ovarian clear cell carcinomas. Oncotarget. 2020;11:3660–3674. doi: 10.18632/oncotarget.27747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo X, Xu J, Yu J, Yi P. Shaping immune responses in the tumor microenvironment of ovarian cancer. Front Immunol. 2021;12:692360. doi: 10.3389/fimmu.2021.692360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caminear MW, Harrington BS, Kamdar RD, Kruhlak MJ, Annunziata CM. Disulfiram transcends ALDH inhibitory activity when targeting ovarian cancer tumor-initiating cells. Front Oncol. 2022;12:762820. doi: 10.3389/fonc.2022.762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin C, Yuan M, Bu H, Jin C. Antiangiogenic strategies in epithelial ovarian cancer: mechanism, resistance, and combination therapy. J Oncol. 2022;2022:4880355. doi: 10.1155/2022/4880355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang T, Tang J, Yang H, Yin R, Zhang J, Zhou Q, et al. Effect of apatinib plus pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin alone on platinum-resistant recurrent ovarian cancer: the APPROVE randomized clinical trial. JAMA Oncol. 2022;8:1169–1176. doi: 10.1001/jamaoncol.2022.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological features of the 4 molecular subtypes of HGSC.
“Omental cake” in laparoscopy and EMT in pathology.