Abstract
Backgrounds/Aims
Historically, the incidence and prognosis of patients diagnosed with intrahepatic cholangiocarcinoma (ICC) have been inadequately understood. Survival analysis in ICC has yet to be investigated in a population-based study.
Methods
We conducted a retrospective cohort study using the Surveillance, Epidemiology, and End Results Program (SEER) 18 Registry. Risk ratios were estimated via Poisson regression. Hazard ratios for 5-year survival were estimated using hierarchical Cox regression models.
Results
Males show a higher rate of age-adjusted ICC incidence. Blacks carried a decreased risk of ICC diagnosis than Whites, while Asians revealed a higher risk of ICC diagnosis when compared with Whites. The observed survival rates at 12, 36, and 60 months were 36.3%, 12.8%, and 8.1%, respectively. Compared with Whites, Blacks showed an increased risk of death (p < 0.01). Lymph node resection during surgery was associated with a 64.1% reduced risk of mortality (p < 0.01). A higher T stage at diagnosis was associated with poor survival (p < 0.01). Surgery combined with chemoradiotherapy, radiotherapy, or chemotherapy was associated with a reduced risk of mortality compared with nonsurgical interventions (p < 0.01).
Conclusions
ICC incidence has been increasing since 2000, especially in White males. The risk of ICC rises with age. Lymph node removal is associated with better survival. In recent years, survival had worsened, and surgical intervention improved survival compared with nonsurgical management.
Keywords: Lymphatic metastasis, Prognosis, Cholangiocarcinoma, SEER Program, Survival
INTRODUCTION
Biliary tract cancer is mainly divided into extrahepatic and intrahepatic cholangiocarcinoma (ICC). Although rare, ICC is one of the most common primary hepatic malignancies next to hepatocellular carcinoma (HCC) [1]. ICCs are considered to be liver tumors based on the international classification of the disease since they originate within the bile duct inside the liver [1]. Multiple risk factors have been linked to ICCs, such as inflammatory bowel disease, congenital abnormalities of the bile ducts, Fasciola hepatica (liver fluke) infection, and history of primary sclerosing cholangitis (PSC) [2]. Additional risk factors include smoking, liver cirrhosis, obesity, and hepatitis C virus infection [3]. Symptoms of biliary obstruction include jaundice, pruritus, clay-colored stools, and dark urine. Other common symptoms include abdominal pain, weight loss, and fever [4]. Accurate rates of ICC incidence in the United States are attributed to misclassification and inclusion of other tumors as ICCs, for example, Klatskin tumors resulting in improper reporting and trends [5]. Twenty percent of hepatic tumors can be wrongly diagnosed as HCC rather than ICC [1,6].
The location of ICC is in the liver parenchymal bile duct and can be confused with distal extrahepatic or perihilar tumors leading to inaccurate incidence and survival analysis. Previous studies describe surgical resection as the best modality for improved survival in ICCs [7]. Based on the Surveillance, Epidemiology, and End Results (SEER) registry, we examined the incidence, risk ratios, and survival, focusing on patient demographics and factors affecting the survival. The study utilized SEER registry 18 (2000–2017). We intend to extend the knowledge about ICCs by analyzing factors that improve survival in these patients [8].
MATERIALS AND METHODS
Study population
SEER 18 includes 28% of the United States population as of the 2010 census [9]. The registries included in SEER 18 can be found at https://seer.cancer.gov/data/. SEER 18 encompasses SEER 13, which itself includes SEER 9 in addition to supplementary registries [9]. Within the SEER database, International Classification of Diseases (ICD-0–3/WHO 2008): Intrahepatic bile duct (site C22.1) was used viaSEER*Statprogram to select cases of intrahepatic bile duct cancer alone [10]. Patients without microscopically confirmed diagnosis were excluded.
Patient characteristics
The incidence of disease was categorized into three groups based on the year of diagnosis: 2000–2006, 2007–2011, and above 2012. Based on age, the three groups include: less than 60 years, 60–69 years, and above 70 years. Patients were classified as Whites, Blacks, and other ethnic groups: Asian/Pacific Islander and American Indian/Alaskan Natives [11]. Data regarding cancer stage based on diagnosis and surgical interventions were grouped accordingly. Age, race, and sex were classified as incidence without any change. This is an observational study. The present study was exempt from institutional review board oversight, because the SEER 18 database is accessible to the public and the patients in the database are de-identified.
Statistical analysis
The incidence and age-adjusted rates, grouped by age, sex, ethnicity, and year were calculated using the SEER*Stat software program [10]. Trends associated with sex, age, and race in annual diagnoses (2000–2017) were analyzed. The period-specific rates were plotted utilizing temporal trends and presented as rates per 100,000 [12]. The annual percent changes (APCs) were quantified using annual rates for temporal trends, with 95% confidence intervals (CIs). Incidence rate ratios (IRRs) for sex and ethnicity/race were calculated; 95% CIs for the IRRs were age-adjusted and calculated using the Age-Stratified Poisson Model. Cox regression models were constructed to estimate the hazard ratios (HRs) for survival at five years after adjusting for age, sex, and race using Stata Corp (2017) Stata Statistical Software and Kaplan-Meier curves were generated. Analyses were performed using Statistical software for data science (STATA) version 16.0 software (StataCorp LLC, Station, TX, USA). Our analysis had 0.05 as the threshold for statistical significance, and all p-values were 2-sided.
RESULTS
Incidence
The study population consistent of more than 79.2% Whites and 7.8% Blacks; 48.3% of subjects were aged above 70 years at the time of diagnosis (data not shown). The male and female age-adjusted ICC incidence rates were 1 and 0.8 per 100,000, respectively (Table 1). The ICC age-adjusted incidence rates gradually increased between 2000–2006, 2007–2011, and 2012 onwards (0.6, 0.8 vs. 1.1 per 100,000, respectively).
Table 1.
Number of incident ICC cases, age-specific, and age-adjusted incidence rates per 100,000 persons, standardized to the US Standard Population in 2000, by age, sex, race, income, and year of diagnosis based on SEER registry 18 (2000–2017)
| Variable | Case (n) | Rate |
|---|---|---|
| Sex | ||
| Male | 7,053 | 1.1 |
| Female | 6,833 | 0.8 |
| Race | ||
| White | 10,976 | 0.9 |
| Black | 1,087 | 0.7 |
| Othera) | 1,823 | 1.1 |
| Age group | ||
| < 60 yr | 3,412 | 0.2 |
| 60–69 yr | 3,762 | 2.9 |
| ≥ 70 yr | 6,712 | 5.1 |
| Year | ||
| 2000–2006 | 3,370 | 0.6 |
| 2007–2011 | 3,336 | 0.8 |
| 2012+ | 5,624 | 1.1 |
ICC, intrahepatic cholangiocarcinoma; SEER, Surveillance, Epidemiology, and End Results Program.
a)American Indian/Alaska Native and Asian/Pacific Islander.
Relative risks
Modeled rate ratios were adjusted for sex and age. Adjusted risks ratios showed that females had reduced ICC risk compared with males. Blacks had about a 17% (RR 0.83) lower risk of being diagnosed with ICC relative to Whites, and Asians had a 40% higher risk of being diagnosed with ICC relative to Whites. Compared with the 2000–2006 time period, the risk of ICC diagnosis increased to 80% by 2011. Compared with ages less than 60 years, those aged 60 years and above had a significantly higher risk of ICC diagnosis (Table 2). Interactions between age and year groups were assessed by race and sex. The ICC risk was significantly lower among females in all age and year groups (Table 3). Females aged less than 60 were 29% less likely to be diagnosed with ICC relative to males. Females aged 60–69 were 24% less likely to be diagnosed with ICC than males, and females aged greater than 70 were 23% less likely to be diagnosed with ICC than males. ICC risk was significantly lower among Blacks relative to Whites in all year groups. Based on age group, only Blacks aged above 70 years carried a 25% lower risk than Whites (RR 0.75).
Table 2.
Poisson regression estimates of specific ICC incidence rate ratios by sex, race, age, and year of diagnosis based on SEER registry 18 (2000–2017)
| Variable | Rate ratio |
|---|---|
| Sex | |
| Male | 1 |
| Female | 0.77 (0.75–0.81)* |
| Race | |
| White | 1 |
| Black | 0.83 (0.77–0.88)* |
| American Indian | 0.93 (0.76–1.12) |
| Asian/Pacific Islander | 1.4 (1.32–1.47)* |
| Age group | |
| < 60 yr | 1 |
| 60–69 yr | 11.7 (11.1–12.3)* |
| ≥ 70 yr | 20.8 (19.9–21.7)* |
| Year | |
| 2000–2006 | 1 |
| 2007–2011 | 1.2 (1.1–1.3)* |
| 2011+ | 1.8 (1.7–1.91)* |
ICC, intrahepatic cholangiocarcinoma; SEER, Surveillance, Epidemiology, and End Results Program.
*Statistically significant (p < 0.05).
Table 3.
Incidence rate ratios (RR) (and 95% confidence intervals) by year, age, sex, and race, estimated using poisson regression based on SEER registry 18 (2000–2017)
| Variable | RR Females (relative to males) | RR Blacks (relative to whites) |
|---|---|---|
| Year | ||
| 2000–2006 | 0.75 (0.7–0.81)* | 0.85 (0.74–0.97)* |
| 2007–2011 | 0.81 (0.75–0.86)* | 0.83 (0.72–0.94)* |
| 2012+ | 0.78 (0.74–0.83)* | 0.77 (0.7–0.86)* |
| Age group | ||
| < 60 yr | 0.81 (0.75–0.86)* | 0.90 (0.80–1.01) |
| 60–69 yr | 0.76 (0.71–0.81)* | 0.91 (0.81–1.02) |
| ≥ 70 yr | 0.77 (0.73–0.81)* | 0.75 (0.68–0.83)* |
SEER, Surveillance, Epidemiology, and End Results Program.
*Statistically significant (p < 0.05).
Trends
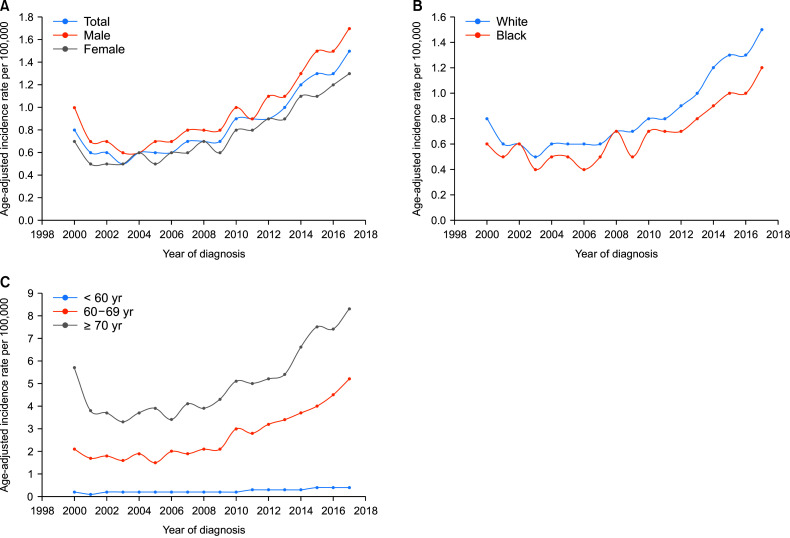
The APC for the ICC incidence rate increased from 2000 to 2017 with a cumulative rate of 5.7 (95% CI, 4.3–7.2; p < 0.05) (Fig. 1). The APC for males was 5.5 (p < 0.05) compared with 5.9 (p < 0.05) for females. The APC in ICC incidence rate for whites was 6.1 (95% CI, 4.5–7.7; p < 0.05) during 2000–2017, compared with 5.4 (95% CI, 3.6–7.2; p < 0.05) for Blacks. The APC for the ICC incidence rate was the highest at 7.4 (95% CI, 6.1–8.8; p < 0.05) in the age group of 60–69 years, followed by 6.7 (95% CI, 5.7–7.7; p < 0.05) for ages < 60 years and 4.6 (95% CI, 2.8–6.3; p < 0.05) for those above 70 years. Despite a higher APC from 2000 to 2017, the age-adjusted incidence rates per 100,000 remained the highest in subjects aged above 70 years (Fig. 1C).
Fig. 1.
Trends of intrahepatic cholangiocarcinoma incidence rates, annual percentage changes. (A) Based on sex (SEER 2000–2017); (B) Based on race (SEER 2000–2017); (C) Based on age group (SEER 2000–2017). SEER, Surveillance, Epidemiology, and End Results Program.
Survival analysis
Intrahepatic bile duct cancer was diagnosed in 50.79% males and 49.21% females from 2000 to 2017. The total mean age of all patients was 67.9 ± 0.11 years (males, 68.8 ± 0.15 years; females, 67.8 ± 0.14 years). The analysis included 3,142 (24.57%) cases aged less than 60 years, 3,762 (27.09%) between ages 60 and 69 years, and 6,712 (48.34%) 70 years or above. Surgical intervention was performed in 2,356 (16.96%) of patients. Out of total 13,866 patients, at the time of diagnosis, 37 (0.26%) cases were at T1 stage; 2,481 (17.86%) were T2; 907 (6.5%) were T3; 1,641 (11.81%) were T4; and 927 (6.6%) were TX.
The overall observed survival at 12, 36, and 60 months was 36.3%, 12.8%, and 8.1%, respectively. The median survival time for all cases was eight months. Patients who underwent surgery combined with chemoradiotherapy had the highest median survival time of 33 months, followed by surgery alone (32 months), surgery along with chemotherapy (30 months), and surgery combined with radiotherapy (25 months). Survival analysis based on adjusted AJCC 6th edition T Stage staging is presented in Table 4. Females and males had a similar median survival time of 8 months. Patients diagnosed between 2000 and 2006 had a median survival time of 12 months, which decreased to 9 months from 2007 to 2011, followed by a decrease to 6 months starting from 2012. Diagnosis at age less than 60 years was associated with a median survival time of 9 months, whereas diagnosis at age 60–69 years with 8 months, and at ages above 70 years it was 6 months (Table 4). A higher number of lymph nodes excisions was also associated with higher median survival time (Table 4).
Table 4.
Median survival times of patients diagnosed with ICC in SEER registry 18 (2000–2017)
| Variable | Median survival time (mon) |
|---|---|
| Sex | |
| Male | 8 |
| Female | 8 |
| Race | |
| White | 8 |
| Black | 7 |
| Other | 8 |
| Therapy | |
| Surgery with chemoradiotherapy | 33 |
| Surgery with chemotherapy | 25 |
| Surgery with radiotherapy | 30 |
| Surgery alone | 32 |
| TNM staging | |
| T1 | 12 |
| T2 | 13 |
| T3 | 8 |
| T4 | 8 |
| TX | 5 |
| Lymph node removal surgery | |
| None | 7 |
| Biopsy or aspiration of regional lymph node | 13 |
| One to three regional lymph nodes removed | 30 |
| Four or more regional lymph nodes removed | 28 |
| Year | |
| 2000–2006 | 12 |
| 2007–2011 | 9 |
| 2012+ | 6 |
| Age group | |
| < 60 years | 9 |
| 60–69 years | 8 |
| ≥ 70 years | 6 |
ICC, intrahepatic cholangiocarcinoma; SEER, Surveillance, Epidemiology, and End Results Program.
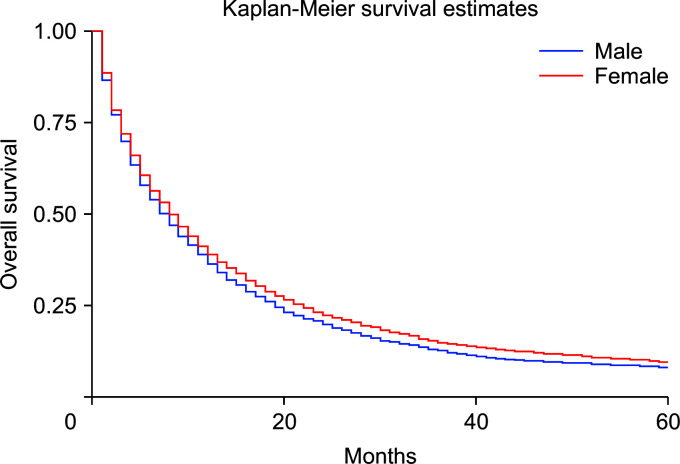
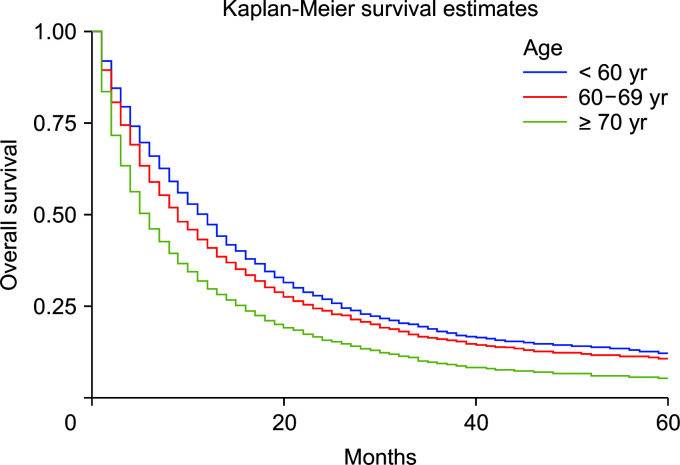
Using the Cox regression model, the HRs were calculated after adjusting for age, sex, and race. The HRs showed slightly better survival in females than in males (adjusted hazard ratio [HRadj]: 0.91, 95% CI 0.87–0.94; p < 0.01) (Fig. 2). Death was 2% more likely with increased age (HRadj: 1.02, 95% CI 1.01–1.019; p < 0.01). Compared with patients below the age of 60 years, aged 60–69 years, and 70 years and above experienced worse survival, with HRadj 1.12 (95% CI, 1.06–1.18; p < 0.01) and HRadj 1.50 (95% CI, 1.43–1.58; p < 0.01), respectively (Fig. 3).
Fig. 2.
Kaplan–Meier curves of survival based on sex (p < 0.01).
Fig. 3.
Kaplan–Meier curves of survival based on age group (p < 0.01).
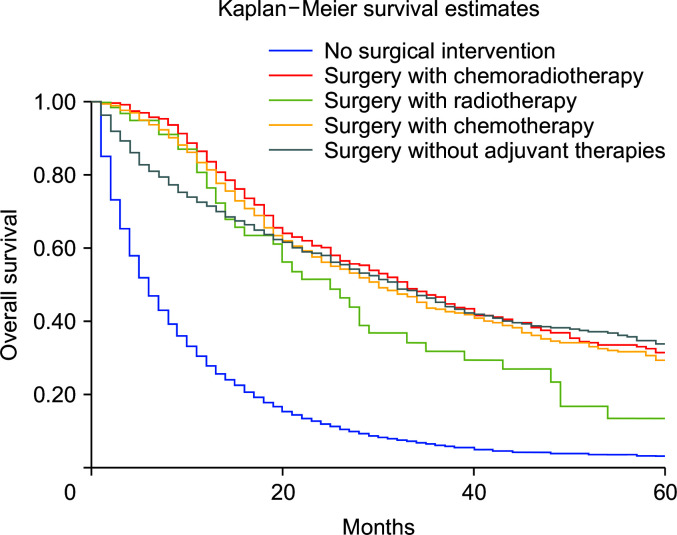
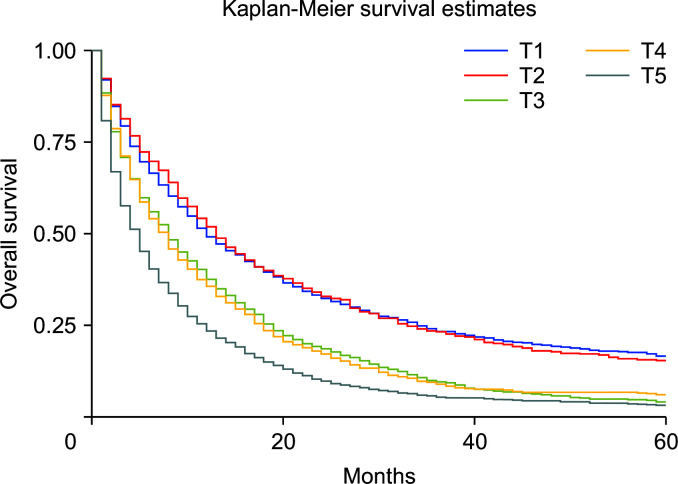
Compared with nonsurgical intervention, surgery alone was associated with improved survival (HRadj: 0.28, 95% CI 0.26–0.31; p < 0.01). Surgery with chemoradiotherapy (HRadj: 0.25, 95% CI 0.22–0.3; p < 0.01), surgery with radiotherapy (HRadj: 0.36, 95% CI 0.26–0.59; p < 0.01) and surgery with chemotherapy (HRadj: 0.27, 95% CI 0.24–0.30; p < 0.01) were also associated with lower risk of death compared with nonsurgical interventions (Fig. 4). Compared with T1 stage at diagnosis, T2 (HRadj: 0.99, 95% CI 0.9–1.1, p = 0.83) did not alter the mortality. T3 (HRadj: 1.51, 95% CI 1.41–1.62; p < 0.01), T4 (HRadj: 1.54, 95% CI 1.41–1.67; p < 0.01) and TX (HRadj: 2.1, 95% CI 1.9–2.2; p < 0.01) were associated with a higher risk of mortality (Fig. 5).
Fig. 4.
Kaplan–Meier curves of survival based on surgical intervention (p < 0.01).
Fig. 5.
Kaplan–Meier curves of survival based on adjusted the sixth edition of the American Joint Committee on Cancer (AJCC) for cancer staging and diagnosis (p < 0.01).
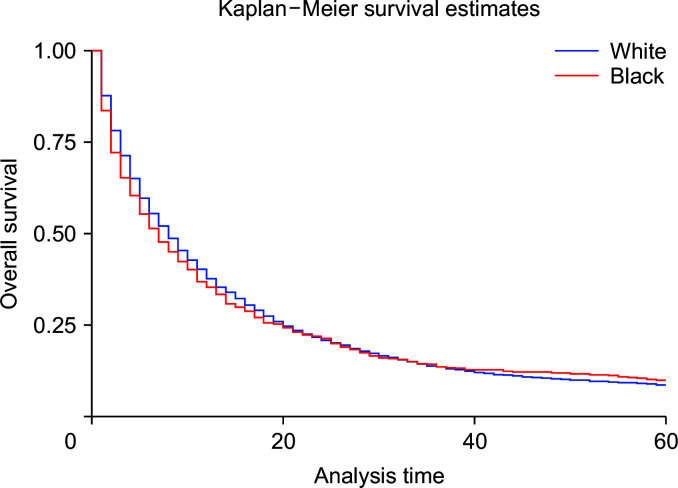
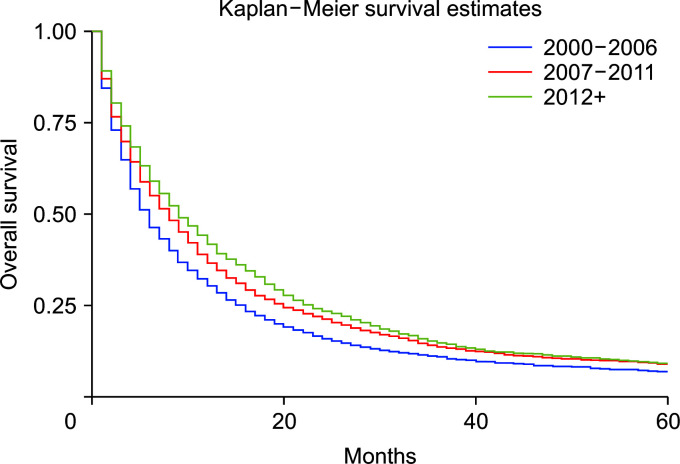
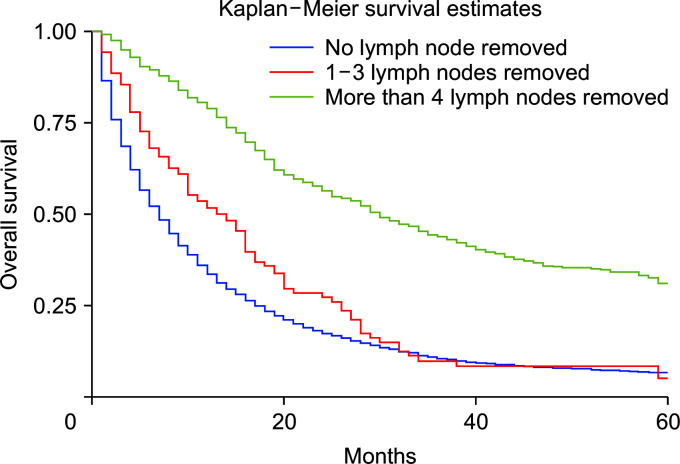
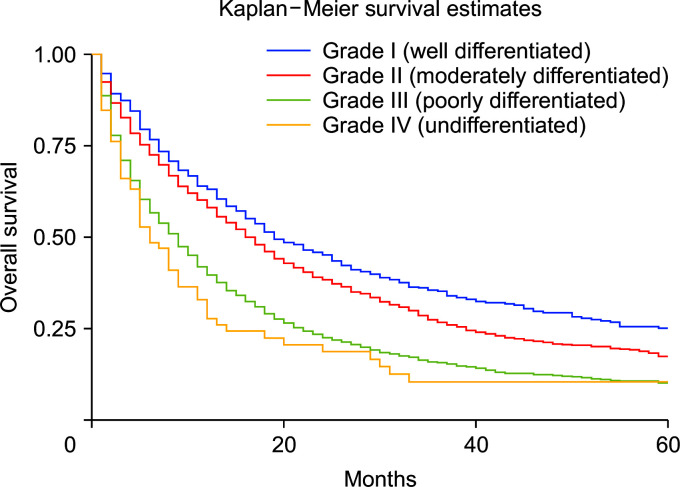
Compared with whites, Blacks carried a 13% higher risk of death (HRadj: 1.13, 95% CI 1.04–1.22; p < 0.01) (Fig. 6). Metastasis at diagnosis was associated with a significant increase in mortality up to 91% (HRadj: 1.90, 95% CI 1.81–2.01; p < 0.01). Compared with 2000–2006, survival increased in 2007–2011 (HRadj: 0.86, 95% CI 0.81–0.91; p < 0.01) and 2012 onwards (HRadj: 0.78, 95% CI 0.74–0.822; p < 0.01) (Fig. 7). Compared with surgery without lymph node removal, surgical excision of one to three regional lymph nodes led to a 64.1% reduced risk of death (HRadj: 0.36, 95% CI 0.32–0.40; p < 0.01), and excision of four or more regional lymph nodes reduced the risk of death by 62% (HRadj: 0.38, 95% CI 0.33–0.43; p < 0.01) (Fig. 8). Compared with grade I (well differentiated) tumor, grade II (HRadj: 1.22, 95% CI 1.1–1.4; p < 0.01), grade III (HRadj: 1.82, 95% CI 0.1.6–2.1; p < 0.01) and grade IV (HRadj: 2.03, 95% CI 1.53–2.69; p < 0.01) tumors were associated with poor survival (Fig. 9).
Fig. 6.
Kaplan–Meier curves of survival based on race (p < 0.01).
Fig. 7.
Kaplan–Meier curves of survival based on year groups (p < 0.01).
Fig. 8.
Kaplan–Meier curves of survival based on lymph node resection during surgery (p < 0.01).
Fig. 9.
Kaplan–Meier curves of survival based on tumor grade on presentation (p < 0.01).
DISCUSSION
Race has been used to report disease heterogeneity in terms of African Americans (Blacks) versus Caucasians (Whites). Studies have explored trends in incidence of intrahepatic and extrahepatic cholangiocarcinoma using the SEER database [13]. More recently, racial disparities in carcinoma have been reported [14]. Our results report the latest incidence and survival across a comprehensive age continuum for the first time. They suggest periods of increased mortality and incident risk of ICCs in the USA. This is also the first study, to our knowledge, examining risk interactions by age, sex, race, and therapeutic interventions. Analysis of ICCs by age revealed that advanced age (70 years and above) was associated with increased incidence and poor survival. Females and males differed slightly in terms of median survival. Previous reports suggest that the incidence of ICC was 1–2 per 100,000 individuals. Our recent analysis is consistent with this report with an incidence ranging between 1 and 0.8 per 100,000 for male and female, respectively [15]. This incidence is reportedly higher in the Asian population, up to 10 per 100,000 persons [15].
Risk factors for ICC are widespread and overlap with those of HCC. Diseases that accelerate hepatic fibrosis and cause inflammation of hepatic tissues increase the odds of ICC [16]. These include diseases such as primary sclerosing cholangitis and primary biliary cirrhosis. Other acquired disorders that cause hepatic inflammation include hepatitis B and C infections, alcoholic liver disease, tobacco use, and congenital bile duct abnormalities [16]. Additionally, parasites including Clonorchis sinensis and Opisthorchis viverrini associated with hepatic anatomy lead to inflammation and contribute to ICC etiology. These parasites are more prevalent in the eastern hemisphere (Asia) than in the United States and account for the ten-fold increased incidence in the population [17]. The incidence rate has been the highest in the last decade after 2011, which could be attributed to increased hepatitis infection secondary to opioid and heroin epidemics that have gripped communities across the United States [18]. Further, the rising incidence could be attributed to increasing rates of liver cirrhosis, non-alcoholic fatty liver disease, and obesity in the United States, which are established risk factors for ICC [18].
Our analysis shows that females carry a reduced risk of ICC compared with males after adjusting for age and race (p < 0.03), which could be due to a higher incidence of disorders that cause hepatic inflammation like hepatitis C, liver cirrhosis, and PSC in males compared with females [19]. Another SEER database analysis of cases up to 2003 reported an increased incidence of ICC in patients younger than 50 years of age. Our recent analysis indicated that the ICC incidence rate was the highest in the age group of 70 plus years despite the higher APC in the 60–69-year group (p < 0.05). The increased APC in the younger population could be related to acquired factors such as exercise, obesity, alcohol abuse, and smoking [20]. Obesity leading to hepatic inflammation-causing non-alcoholic fatty liver disease may like contribute to the rise in incidence. Data associating the risk of ICC with lifestyle factors including physical activity and processed foods will be explored in the future [21].
We also conducted a survival analysis based on various variables, including age, year of diagnosis, American Joint Committee on Cancer (AJCC) 6th edition T stages, and therapeutic intervention. The five-year survival of ICC was only 8.1%, which may be explained by the increased rate in the older population (75% of cases were above age 60 years). Younger patients aged less than 60 years had a better median survival time of 9 months than those above 70 years (6 months) in our analysis. Males had similar median survival compared with females (8 months), and females had a slightly better 5-year survival (HRadj: 0.91, 95% CI 0.87–0.94; p < 0.01).
This study is consistent with previous findings of poor survival in males compared with females in the population during 1990–2003 [21]. Moreover, the survival time was less in Blacks than in Whites, with up to 13% higher risk of death in survival analysis as reported previously [21]; however, a reasonable explanation is still unavailable to explain this racial disparity. Factors such as insurance and socioeconomic status, in addition to a willingness to undergo surgery, may play a role [22].
Our analysis based on disease staging primarily was based on the sixth edition of the AJCC Cancer Staging Manual. Inadequate number of cases with T0 stage during the analysis and the higher T stages had a significantly poor outcome, with mortality up to twice higher than in T1 disease. Five-year survival was best in T1 disease, followed by T2 and T3. Survival of unstaged disease (TX) was also poor, presumably because of the presence of advanced disease. Treatment-based survival was compared between patients who did not undergo surgery compared with surgical intervention with or without adjuvant chemoradiotherapy. Survival outcomes based on surgical interventions have not been reported previously [21]. Surgery alone was associated with improved survival, with the risk of death 72.1% lower than in nonsurgical interventions. Surgery combined with either chemoradiotherapy (75.3%), radiotherapy (64.2%), or chemotherapy (73.1%) was also associated with a lower risk of death compared with nonsurgical interventions. Surgery is the primary curative treatment for ICC. The surgical intervention significantly improved survival outcomes in patients with ICC. However, it might not be an option for metastatic disease [4]. Despite surgery, the long-term probability of cure in ICC is only up to 10% [23]. Surgical resection is effective if negative resection margins are reported, in addition to ensuring adequate liver of size and functionality [6]. Surgery with curative intent is contraindicated for extrahepatic disease. We also compared survival based on lymph node removal during surgery. However, palliative surgery may still be performed. Compared with surgery without lymph node removal, surgical excision of one to three regional lymph nodes reduced the risk of death by 64.1%, and removal of four or more regional lymph nodes reduced the risk of death by 62.3% (p < 0.01). Data regarding survival based on lymph node resection for ICC have yet to be reported. Previous studies reported poor 5-year overall survival of 20% after surgical intervention for ICC. Our analysis revealed increased 5-year overall survival to 31.5% (results not shown). Median survival time of patients who underwent surgery was substantially higher than in nonsurgical cases. Another contraindication for surgical intervention in advanced ICC is the invasion of the main and bilateral hepatic arteries. Reconstruction of these vessels is associated with a high risk of postoperative mortality [24]. Interventions that reduce central venous pressure, restrict fluid resuscitation and enhance recovery decreased the risk of complications [25]. However, postoperative mortality was not analyzed due to limitations of the SEER database. Further studies are needed to address this limitation.
The strength of our study is the use of the SEER database, which includes an extensive population-based data set. It represents the racial-ethnic makeup of the current US population with maximum representation, as SEER 18 registry includes recent cases and covers almost 30% of the US population. Incidence rates, risk ratios, trends, and five-year survival of multiple patient subgroups can explain factors contributing to the variability of ICC outcomes.
Our study has limitations, especially from an etiological perspective, as the non-Medicare-SEER database lacks individual comorbidities and risk factors, which can influence these trends. Additionally, patients migrating in and out of areas registered in the SEER database and selection bias are as concern. We acknowledge the limitation of cancer registries such as SEER reporting only the month and year, and the number of months of survival in efforts to de-identify patients and ensure their privacy. Therefore, limitations exist regarding inaccurate survival dates and times in SEER data.
In conclusion, our study confirms the continued increase in ICC rates in recent years, particularly in the older population. Surgery remains the treatment modality with a proven increase in 5-year survival. Blacks experience worse mortality than the White population. Lymph node removal during surgery is associated with significantly better outcomes. New information regarding ICCincidence and survival based on race, sex, and age will have a positive impact on resource planning and management of this disease.
Footnotes
FUNDING
None.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: HA. Data curation: HA. Methodology: HA, BT. Visualization: HA. Writing - original draft: HA, BT, SHW, RM, ELC. Writing - review & editing: BT, SHW, RM, ELC, EA.
REFERENCES
- 1.Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379:198–205. doi: 10.1016/j.canlet.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10 Suppl 4:308–311. doi: 10.1023/A:1008313809752. [DOI] [PubMed] [Google Scholar]
- 3.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–750.e4. doi: 10.1016/j.jamcollsurg.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 6.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, et al. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–563. doi: 10.1067/msy.2002.127555. discission 563-564. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto N, Yachida S, Okano K, Wakabayashi H, Imaida K, Kurokohchi K, et al. Immunohistochemically detected expression of p27(Kip1) and Skp2 predicts survival in patients with intrahepatic cholangiocarcinomas. Ann Surg Oncol. 2009;16:395–403. doi: 10.1245/s10434-008-0236-0. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance Research Program, NCIs Division of Cancer Control, Population Sciences, author. SEER Registries [Internet] Washington, D.C.: National Cancer Institute; 2021. [cited 2021 Sep 29]. Available from: https://seer.cancer.gov/ [Google Scholar]
- 10.Surveillance Research Program, National Cancer Institute, author. SEER*Stat software version 8.3.9.2 [Internet] Washington, D.C.: National Cancer Institute; 2021. [cited 2021 Sep 29]. Available from: https://seer.cancer.gov/seerstat/ [Google Scholar]
- 11.Deadmond MA, Smith-Gagen JA. Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973-2011. J Cancer Res Clin Oncol. 2015;141:2131–2138. doi: 10.1007/s00432-015-1983-5. [DOI] [PubMed] [Google Scholar]
- 12.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 13.Tyson GL, Ilyas JA, Duan Z, Green LK, Younes M, El-Serag HB, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59:3103–3110. doi: 10.1007/s10620-014-3276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ailawadhi S, Aldoss IT, Yang D, Razavi P, Cozen W, Sher T, et al. Outcome disparities in multiple myeloma: a SEER-based comparative analysis of ethnic subgroups. Br J Haematol. 2012;158:91–98. doi: 10.1111/j.1365-2141.2012.09124.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, et al. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev. 2010;11:1159–1166. [PubMed] [Google Scholar]
- 16.Brito AF, Abrantes AM, Encarnação JC, Tralhão JG, Botelho MF. Cholangiocarcinoma: from molecular biology to treatment. Med Oncol. 2015;32:245. doi: 10.1007/s12032-015-0692-x. [DOI] [PubMed] [Google Scholar]
- 17.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnie RJ, Ford MA, Phillips JK. Trends in opioid use, harms, and treatment. In: Bonnie RJ, Ford MA, Phillips JK, editors. Pain management and the opioid epidemic: balancing societal and individual benefits and risks of prescription opioid use. Washington, D.C: National Academies Press; 2017. pp. 187–266. [DOI] [PubMed] [Google Scholar]
- 19.Toy E, Balasubramanian S, Selmi C, Li CS, Bowlus CL. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. doi: 10.1186/1471-230X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101–104. doi: 10.21037/hbsn.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mody K, Antwi SO, Hodge DO, Ailawadhi S, Roberts L, Bekaii-Saab T. A SEER-based multi-ethnic picture of advanced intrahepatic cholangiocarcinoma in the United States pre- and post-the advent of gemcitabine/cisplatin. J Gastrointest Oncol. 2018;9:1063–1073. doi: 10.21037/jgo.2018.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haider AH, Scott VK, Rehman KA, Velopulos C, Bentley JM, Cornwell EE, 3rd, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216:482–492.e12. doi: 10.1016/j.jamcollsurg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121:3998–4006. doi: 10.1002/cncr.29619. [DOI] [PubMed] [Google Scholar]
- 24.Hartog H, Ijzermans JN, van Gulik TM, Groot Koerkamp B. Resection of perihilar cholangiocarcinoma. Surg Clin North Am. 2016;96:247–267. doi: 10.1016/j.suc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Gurusamy KS, Li J, Vaughan J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev. 2012;2012:CD007338. doi: 10.1002/14651858.CD007338.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]