Abstract
Neurological disorders are one of the leading causes of morbidity and mortality worldwide, and their therapeutic options remain limited. Recent animal and clinical studies have shown the potential of extracorporeal shock wave therapy (ESWT) as an innovative, safe, and cost-effective option to treat neurological disorders. Moreover, the cellular and molecular mechanism of ESWT has been proposed to better understand the regeneration and repairment of neurological disorders by ESWT. In this review, we discuss the principles of ESWT, the animal and clinical studies involving the use of ESWT to treat central and peripheral nervous system diseases, and the proposed cellular and molecular mechanism of ESWT. We also discuss the challenges encountered when applying ESWT to the human brain and spinal cord and the new potential applications of ESWT in treating neurological disorders.
Keywords: extracorporeal shock wave therapy, peripheral nervous system diseases, central nervous system diseases, mechanotransduction, cost-effectiveness, neural tissue regeneration
Introduction
ESWT originates from high-intensity extracorporeal shock wave lithotripsy (ESWL), a well-established treatment method for urolithiasis (1, 2). It was found later that a decrease in the energy of ESWL could produce more beneficial effects on human tissues. As a new disease treatment option, ESWT offers many benefits. First, because ESWT has a lower energy intensity than that of traditional ESWL (3, 4), it is generally considered safer and non-invasive and has fewer side effects such as pain, small and superficial ecchymosis, and mild numbness (5). Second, ESWT is simple and relatively easy to operate. For example, when ESWT is used, physicians just need to first determine the area targeted by ESWT, which can be achieved by examining the lesion location. Then the gel is placed on the skin of the to-be-treated area. Thereafter, physicians can simply select and adjust the treatment according to patients' pain, tolerance, and other factors to achieve the best result. Clearly, the ESWT procedure is much simpler than surgery. Third, in terms of convenience and cost-effectiveness, ESWT has a long treatment interval with short treatment times. Thus, patients do not need to spend much time or money on ESWT. Because of these beneficial characteristics, ESWT has been a therapeutic option for the treatment of many musculoskeletal diseases (6, 7), including plantar fasciitis (8–10), calcific shoulder tendinopathy (11), tennis elbow (12), trigger finger (13), knee osteoarthritis (14), and bone non-union (15–17). ESWT has also been utilized to treat wounds (18–21), urological diseases (22–24), and male erectile rejuvenation (25).
Neurological disorders consist primarily of central nervous system diseases and peripheral nerve injury. Central nervous system diseases can be a significant burden to the family and society because of their severe conditions (26), while peripheral nerve injury is difficult to cure and often leads to pain and functional disorders. Drug therapy and surgery are standard options to treat these conditions, but drug therapy often produces limited clinical benefits, while surgery may necessitate supplementary therapy to achieve better rehabilitation. Clearly, treatment of these diseases remains a challenge and there is a need for new options for their treatment. Recently, considerable animal and clinical research have been carried out to explore the use of ESWT to treat neurological disorders and the results are very encouraging because they demonstrated that ESWT has the potential to become a valuable therapy for neurological disorders. Despite the fact that there is no general medical guide currently available, which can guide physicians in using ESWT to treat neurological disorders, physicians at many hospitals have already utilized ESWT clinically to treat patients with neurological disorders such as limb spasm and peripheral nerve diseases.
To better inform the physicians about the potential of ESWT as a therapeutic option for the treatment of neurological disorders, in this review, we describe the properties of ESWT and the cellular and molecular mechanism proposed to elucidate how the neural tissues respond to ESWT, review the animal and clinical studies involving ESWT, and discuss the challenges encountered and new prospects for the use of ESWT as a therapeutic option for treatment of neurological disorders.
Principles of ESWT
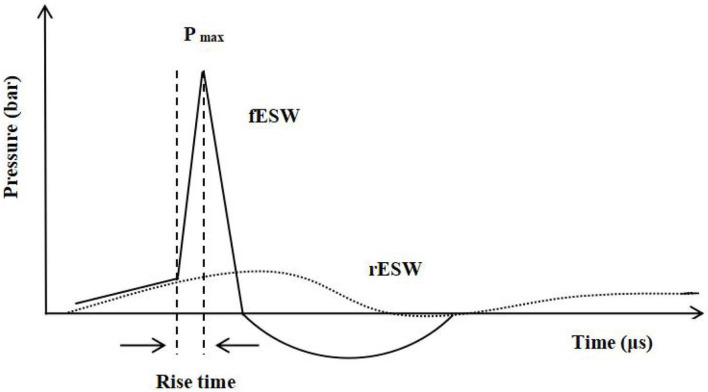
ESWT is a form of mechanotherapy with a peak pressure of about 1,000 times more than ultrasound therapy (27). ESWT can be classified into focused ESWT (fESWT) and radial ESWT (rESWT) based on the wave patterns used (28). In general, a focused extracorporeal shock wave (fESW) is generated electrohydraulically, electromagnetically, or piezoelectrically, followed by converging it into a focal tissue zone (29). As an acoustic wave, fESW is characterized by its high pressure of more than 1,000 bar (100 MPa), an extremely short rise time (<10 ns), a short duration (<10 ms), and a broad frequency spectrum (16–20 MHz) (30–32). Unlike fESW, radial extracorporeal shock wave (rESW) does not possess the shock wave characteristics of a short rise time, a high peak pressure, and non-linearity. Some scholars even call “rESW” “radial pressure waves” because rESW uses the energy generated from compressed gas to drive the bullet body to the treated tissue area in a pulsed manner (29, 33, 34) (Figure 1).
Figure 1.
—Waveform characteristics of shock wave (fESW): a short rise time, high peak pressure, and non-linearity. Pmax, Pressure maximum. ...Waveform characteristics of pressure wave (rESW).
The major parameters that affect the treatment effectiveness of ESWT include air pressure (unit: bar), energy flux density (EFD) (unit: mJ/mm2), number of pulses (unit: pulses), and frequency (unit: Hz) (35, 36). EFD is the parameter that indicates the energy intensity of shock waves in a unit area. It was suggested that ESWT should be classified into low (<0.08 mJ/mm2), medium (<0.28 mJ/mm2), and high (<0.60 mJ/mm2) energy according to the energy (intensity) value of its EFD (37).
The effectiveness of ESWT also depends on the penetration depth of ESWT. The probe of the handpiece used in an ESWT device dictates the penetration depth of ESWT because each probe has a specific penetration depth range. It is noted that the ESWT device makers provide various handpieces of ESWT, sometimes making it difficult for physicians to select which handpieces to be used clinically. Compared with rESWT, fESWT produces a shock wave with a higher EFD and its probe can penetrate deeper treatment areas (38, 39). To illustrate, rESWT is generally safer when used to treat superficial bone fracture non-union like tibia (15). In contrast, when deeper lesions such as femoral head necrosis are treated, fESWT is frequently used because its higher EFD could lead to a better therapeutic result (40). In general, fESWT can reach tissues that are as deep as 12 cm, while the penetration depth of rESWT is only 3–4 cm deep (41).
It is generally believed that ESWT produces two significant physical effects on tissues. The first physical effect is called “mechanotransduction”, which refers to the capability of ESWT to produce the shear and pressure forces (42). Mechanotransduction can reduce and even reverse injury to damaged tissues, and mechanically promote homeostasis in healthy tissues at the molecular, cellular, and tissue levels. Mechanotransduction is generally considered a direct physical effect because it directly acts on tissues (43). It is suggested that ESWT-induced direct mechanical perturbations might be transmitted to tissues, affecting cell membrane polarization, radical formation, cell proliferation, and growth factor production (7, 44). It is also proposed that cells could sense mechanical forces and transmit mechanical stimuli into biochemical signals, which then lead to modulation of the functions of cells in turns, such as migration, proliferation, and differentiation, and even maintaining cytoskeletal structure and homeostasis, in which mechanotransduction plays an essential role (45). It has been suggested that the action of ESWT on the mechanotransduction signaling pathway consists of four major phases (46): the first phase is the mechanocoupling phase, which converts external mechanical signals (shock waves) to mechanical signals near the cell; the second phase is called biochemical coupling, meaning that mechanical signals are transduced into biochemical signals, thus leading to changes in the gene and/or protein level; the third phase is the signal transmission, which sends biochemical signals from sensor cells to the effector cells; the last phase is the responses of the effector cells. It is the cytoskeleton that mainly senses the mechanical stimuli of shock waves during the first phase (47), while certain cell membrane proteins are activated in the second phase, causing a downstream signaling response. For example, it has been reported that the proteins like Piezo serve as mechanical sensitive ion channels (48). Under the impact of ESWT, these mechanical sensitive ion channels become activated to transfer biochemical signals into cells, causing changes in downstream “mechanotransduction” proteins. It is noted that the exact mechanism of mechanotransduction is still not well understood when ESWT is used to treat neurological disorders. More studies are warranted to better understand the treatment mechanism of mechanotransduction for this direct physical effect, which could potentially lead to new approaches to the treatment of neurological disorders by ESWT (43).
The second physical effect is called cavitation, which refers to the rapid formation, expansion, and subsequent implosion of air bubbles due to the negative pressure (49). Shock waves could produce high stress at the boundary interface between the body tissues and generate the tensile forces that lead to cavitation, which is regarded as an indirect mechanical force of shock waves (50). The process in which the growth and collapse of acoustic waves including shock waves induce bubbles in liquid is called acoustic cavitation. Acoustic cavitation not only breaks calcific deposits, but also excites nerves, stimulates axons, and reduces pain (51). There are many small cavitation bubbles in the liquid between the body tissues. When the high-energy shock wave acts on the tissues, it can cause the rapid decline of the bubble surface tension and lead to the elastic deformation between the tissues, thus relaxing the local soft tissue adhesion and reducing the local adhesion symptoms. Although the cavitation effect is likely to damage some normal cells, the cavitation is predictable and regulatable, and thus it can be used for the treatment of certain diseases. For example, in the nervous system, a shock wave with high energy may cause traumatic encephalopathy, but the cavitation effect induced by the appropriate dose of the shock wave can make the drug effectively pass through the blood-brain barrier (52), which is conducive to tumor blood vessel blockage. In addition, when it comes to the ability of the cavitation induced by ESWT to excite nerves, researchers found that cavitation could excite neural tissues through the interaction between shock waves and bubbles to generate the action potential (53).
In summary, both mechanotransduction and cavitation may play a major role in introducing shock waves to tissues and triggering physiological actions at the molecular and tissue levels, which then produce beneficial biological events such as tissue regeneration and repairment, angiogenesis, pain relief, metabolic activation, and anti-inflammation, leading to beneficial therapeutic results as demonstrated in many animal and clinical studies.
Animal studies of ESWT
Sciatic nerve injury
Sciatic nerve injury is a peripheral nerve disorder. When applied to the animals with sciatic nerve injury, ESWT was found to be able to lead to the recovery of peripheral nerve injury (54–56) (Figure 2A). For example, in an animal study, medium-energy ESWT (a frequency of 3 Hz and energy flux density of 0.09 mJ/mm2, 300 pulses) was employed to treat rats' sciatic nerve damage. This study showed that the rats in the ESWT group had a significantly increased sciatic functional index (SFI) (57). Animal studies also suggested that it is essential to use the appropriate dose of ESWT to treat sciatic nerve injury. Fu et al. (56) established a rat model of chronic nerve compression of sciatic injury by applying ESWT of 1.0, 1.5, 2.0, and 2.5 bar pressure to rats. Their results showed that ESWT with 1.0 bar pressure led to the best improvement in relieving pain in rats, suggesting that there may be an energy saturation effect on the therapeutic effectiveness when ESWT is used to treat nerve compression injury. Another study showed that the pulses of 300 shock waves led to the best therapeutic result, enhancing nerve function recovery when ESWT was used to treat injured sciatic nerve (58).
Figure 2.
Animal studies of the use of ESWT in sciatic nerve (A), brain (B), and spinal cord (C).
Central nervous system diseases
Because of the concerns about the potential damage to the human brain or spinal cord by ESWT, few clinical studies were carried out to explore the clinical use of ESWT in the treatment of human central nervous system diseases. However, animal studies have shown that ESWT was effective when treating central nerve damage. In general, cerebral infarction and spinal cord injury (SCI) were the focus of the animal studies (Figures 2B,C). In one study, ESWT was used to treat stroked rats' brains. The result showed that ESWT could inhibit neurological dysfunction of rats after acute ischemic stroke and was able to reduce brain infarct volume (59). Kang et al. (60) also used ESWT (2.0 bar, 200 pulses, 10 Hz) to treat rats' brains with cerebral infarction. Their result showed that appropriate doses of ESWT could improve cerebral blood flow effectively, increasing the neurological function of rats without side effects. In a study of treating chronic SCI by ESWT, Lee et al. (61) found that rats' behavioral tests were improved when the stem cell therapy was combined with ESWT. In addition, fESWT at three energy levels (level 1, 0.01 mJ/mm2; level 2, 0.04 mJ/mm2; and level 3, 0.11 mJ/mm2) was applied at 1,000 pulses to rats' spinal cords. Histological examination has shown that there were no neurological impairments when fESWT was applied at these energy levels. It was also reported that when ESWT was used to treat spinal cord injury rats, it reduced the neural tissue damage, enhanced the effectiveness of neuroprotection, and improved motor function without any detrimental effect (62–64).
Clinical studies of ESWT
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is an entrapment neuropathy of the median nerve characterized by pain and paresthesia in median nerve innervated areas (65). There are many conservative interventions such as therapeutic ultrasound, wrist splinting, and steroid injections to treat CTS (66), which reduce the intracarpal tunnel pressure by reducing inflammation reactions. However, these treatment approaches generally produce short-term therapeutic efficacy (67). Recently, ESWT was shown to be a promising therapeutic method for the treatment of peripheral nerve injury because of its therapeutic efficacy. Ke et al. (68) used ESWT to treat the area of the median nerve walk from the pea bone level to the proximal end of the carpal tunnel entrance with 2,000 pulses and 4 bar pressure at a frequency of 5 Hz once per week. They found that ESWT was a promising therapeutic strategy for patients with mild to moderate CTS and multiple-session ESWT produced cumulative clinical results. Other studies have shown that ESWT improved pain symptoms in patients with CTS (69) and reduced the sensory latency of the median nerve (70). ESWT can also potentially be an alternative conservative strategy. Compared with other conservative strategies, ESWT demonstrated good short-term treatment efficacy and produced long-term therapeutic effects on CTS. It was reported that the optimal therapeutic result can be achieved if the probe of ESWT is placed perpendicularly on the median nerve and the EFD level is low or medium when CTD is treated by ESWT. In general, both fESWT and rESWT can provide safe and good outcomes when used to treat CTS (71–75) (Table 1).
Table 1.
Summary of the applications of ESWT in CTS.
| Treatment | Group mode | Study design | Reference |
Intervention and ESWT Protocol |
Follow-up | Outcome |
|---|---|---|---|---|---|---|
| rESWT | wrist splint group (n = 47), splint+rESWT group (n = 47), rESWT group (n = 45), splint+ placebo rESWT group (n = 50) | RCT | 71 | – ESWT (1,000 shocks, 0.05 mJ/mm2 at 5 Hz; 3 sessions, 1 week between sessions) – wrist splint |
3 months | Pain and functionality significantly improved in ESWT groups; A more remarkable improvement in the hand function and electrophysiological measures was observed in the group with ESWT and a combined wrist splint. |
| ESWT | ESWT group (n = 30), Local corticosteroid injection (LCI) group (n = 25) | RCT | 72 | – ESWT (1,000 shocks, 1.5 bar at 6 Hz; 3 sessions, 1 week between sessions) – LCI |
3 weeks 9 weeks 12 weeks | Pain, function and median nerve sensory nerve action potential distal latency improved in the 9 and 12-week follow-ups for the ESWT group. |
| rESWT | single dose rESWT group (n = 13), LCI group (n = 12) | RCT | 73 | – ESWT (5,000 shocks, 4 bar at 15 Hz; 1 session) – LCI |
1 week 4 weeks 12 weeks 24 weeks | Significantly greater improvement in symptom severity, functionality and pain at weeks 12 to 24 in the rESWT group. |
| fESWT | 60 patients with CTS were examined in ESWT group and control group | RCT | 74 | – ESWT (800–1,100 shocks, 0.05–0.15 energy; 4 sessions, 1 week between sessions) – Conservative treatment including wrist splint; nonsteroidal anti-inflammatory drugs; Vitamin B1 |
3 months 6 months. | Pain and function significantly improved in the ESWT group after 3 months and 6 months of treatment. |
| fESWT | shock wave group (n = 34), nutraceutical group (n = 26) | RCT | 75 | – ESWT (1,600 shocks, 0.03 mJ/mm2 at 4 Hz; 3 sessions, 1 week between sessions) -Nutraceuticals composed of ALA, GLA, and Echinacea |
6 months | ESWT provided an improvement comparable to nutraceuticals on pain and functional ability in patients with CTS. |
ESWT, extracorporeal shock wave therapy; fESWT, focused extracorporeal shock wave therapy; rESWT, radial extracorporeal shock wave therapy; CTS, Carpal Tunnel Syndrome; RCT, randomized clinical trial; LCI, Local corticosteroid injection; ALA, α-Linolenic acid; GLA, γ-linolenic acid.
Postherpetic neuralgia
Postherpetic neuralgia (PHN) is a common type of neuralgia related to neuronal damage and can seriously affect the quality of life due to the pain and poor response to the currently available treatments (76, 77). The main approaches to PHN management are medication and invasive interventional therapies. However, these methods have many adverse effects (76). Physicians have used non-invasive ESWT to treat the pain area of PHN. It was found that both fESWT and rESWT could reduce pain (78) and produced effective outcomes with few side effects when PHN was treated by ESWT. For example, a clinical study has shown that when ESWT [rESWT: R15 probe (radius of 15 mm), 1–4 bar, 5,000–7,000 pulses, 10 Hz] was used to treat patients with PNH, ESWT reduced pain and improved life quality compared with conventional therapies (76). Another clinical study showed that the medium-energy ESWT with 6 sessions (fESWT, EFD of 0.09–0.16 mJ/mm2, frequency of 5 Hz, and 2,000 pulses) could reduce the numerical rating scale of PNH, suggesting that fESWT could alleviate the skin pain and itch (77).
Trigeminal neuralgia
Trigeminal neuralgia (TN) is a severe type of neuralgia that occurs in the distribution area of the facial trigeminal nerve (79). Persons with TN tend to experience a series of painful symptoms. Zhang et al. (80) reported that a woman with primary TN was treated by ESWT two times a week for 3 years with 3,000–6,000 pulses at a frequency of 10 Hz with an air pressure of 1.4–1.5 bar and the treatment sites were centered on the anterior ear region (the projection area of the trigeminal ganglion surface) and the pain area of the upper and lower jaw. The patient's pain was relieved after the 8-week treatment and the patient's life quality was significantly improved after the 3-month treatment. This study showed that ESWT produced good treatment efficacy, demonstrating the potential and feasibility of applying ESWT to treat diseases involving human facial and peripheral nerves.
Central nervous system diseases
Current clinical studies of ESWT mainly focus on peripheral nervous system diseases and much fewer studies have been carried out to explore the use of ESWT to treat human central nervous system diseases because of the concern about potential injury of the brain and spinal cord by ESWT. However, one study has already shown that ESWT could be applied to the brain of patients with unresponsive wakefulness syndrome to improve consciousness (81). In that study, it was found that the skull absorbs most of the shock wave energy and only about 10% of the total energy actually reached the brain. It was reported that ESWT could be effective if the actual energy that reached brain tissues was in the range of 0.01–0.02 mJ/mm2 (81). Werner et al. (82) also used fESWT to treat brain stem (0.1 mJ/mm2, 4,000 pulses, 6 Hz) three times a week over four weeks with a penetration depth of the focus of approximately 5.5 cm. They reported that ESWT could stimulate vigilance in patients with unresponsive wakefulness syndrome and suggested that the use of ESWT with a relatively low EFD may cause fewer side effects when ESWT is used to treat human brain diseases (81). Moreover, the animal studies discussed above have also demonstrated the feasibility as well as safety of ESWT when it was employed to treat central nervous system diseases. Nevertheless, when ESWT is applied to the human brain or spinal cord, the energy dose of ESWT is still a critical issue for physicians to consider and more studies are needed to examine the potential adverse effects of ESWT on human brain tissues and determine the optimal ESWT energy level to be used so that ESWT can be used effectively as well as safely to treat the diseases involving human brain tissues.
Limb spasticity is one of the common neurological symptoms of central nervous system diseases, which include stroke, spinal cord injury, cerebral palsy (CP), and multiple sclerosis (MS) (83). The existence of spasticity may be associated with hyperexcitation of distraction reflex due to motor neuron lesion (84). Studies showed that ESWT improved upper and lower spasticity in post-stroke patients (85, 86). Wu et al. (87) compared the therapeutic effect of rESWT with that of fESWT on post-stroke spastic equinus and found that both increased the passive motion of the ankle joint and the contact area of the foot. ESWT was also safe and effective when used to treat limb muscle spasms in children with cerebral palsy (88–90). Because rESWT is less painful, cheaper, and more comfortable when used to treat spasticity in comparison with fESWT and rESWT can reach spastic muscles (88), most clinical studies use rESWT to treat spasticity. Leister et al. (91) suggested that ESWT could be used to treat acute traumatic spinal cord injury and they have developed a protocol for using ESWT to treat motor and sensory impairment as well as spasticity caused by spinal cord injury. Since ESWT is non-invasive, it has been reported that ESWT could also be a good option to treat spasticity in people with multiple sclerosis (92). In short, ESWT, especially rESWT, has the potential to reduce spasticity in various central nervous system diseases. Table 2 summarizes various applications of rESWT (88, 90, 93–96).
Table 2.
The application of rESWT to treat spasticity in people with central nervous system diseases.
| Reference | Participants |
Type of diseases |
ESWT Protocol and other intervention |
Treatment area |
Clinical examination of spasticity |
Outcome |
|---|---|---|---|---|---|---|
| 94 | 20 chronic poststroke patients | Stroke | – rESWT (1,500 shocks, 1.5 bar at 4 Hz, 1 session lasted about 6 min) | muscle belly of the wrist flexor hypertonic muscles of the forearm, the flexor carpi radialis, flexor carpi ulnaris. | MAS | A single session of rESWT could be an effective alternative treatment for reducing limb spasticity. |
| 93 | 12 patients | Stroke | – rESWT (2,000 shocks, 1.0 bar at 5 Hz, 1 session) | gastrocnemius (plantar flexor spasm) | MAS, ankle PROM and AROM, PPFT, TUG | The rESWT improved plantar flexor spasticity, and the effects were sustained for 1 h. |
| 90 | rESWT group (n = 43) and the control group (n = 39) | CP | – rESWT (2,000 shocks, 2.0 bar at 10 Hz, probe diameter was 15 mm, 4 sessions, 1 week between sessions) – Both groups underwent routine rehabilitation (physiotherapy, occupational therapy, speech therapy, and orthotic treatment) |
leg tricep hamstring and hamstring muscle belly with a radius of 2.5 cm | MAS, GMFM (88 items) | The rESWT combined with rehabilitation can quickly and effectively relieve paralysis of lower extremities, reduce the tension of hamstrings and calf muscles, relieve muscle spasms, and rapidly improve limb function. |
| 88 | 25 children | CP | – rESWT (1,500 shocks, 1.5 bar at 5 Hz, 1 session) – Before the rESWT, a placebo session was applied, and each child served as its control |
gastrocnemius and soleus muscle of the lower limb, mainly in the middle of the muscle belly | PROM of the ankle joint in degrees, MAS | A significant reduction in the spasticity of plantar flexor muscles after a single session of rESWT and this improvement remains at the 4-week follow-up. |
| 95 | rESWT group(n = 34) and placebo group(n = 34) | MS | – rESWT (2,000 shocks, 1.5 bar at 4 Hz, 4 sessions, 1 week between sessions) – placebo treatment – Both groups underwent drugs (baclofen, benzodiazepines, pregabalin, gabapentin, fampridine) |
ankle extensors muscles | VAS, MAS, 10-MWT | Muscle tone was decreased 1 week after the last session and pain was decreased at all follow-up evaluations in rESWT group. |
| 96 | 16 patients | MS | – rESWT (500 shocks, 1.8 bar at 4 Hz, 4 sessions, 1 week between sessions) – BoNT-A Injection (4 months after BoNT-A injection, the same spastic muscles were subjected to rESWT) |
triceps surae | MAS, MTS, and kinematic analysis of passive and active ankle ROM | The use of rESWT following BoNT-A injection helps avoid some limitations and prolongs the therapeutic effects of BoNT-A therapy (a significant reduction of spasticity and improvement in passive and active ankle ROM). |
rESWT, radial extracorporeal shock wave therapy; MAS, Modified Ashworth Scale; PROM, Passive range of motion; AROM, Active range of motion; CP, cerebral palsy; GMFM, Gross Motor Function Measure; MS, multiple sclerosis; VAS, Visual Analog Scale; 10-MWT, 10-meter walking test; MTS, Modified Tardieu Scale; PPFT, passive plantar flexor torque; TUG, timed up and go test. BoNT-A, Botulinum toxin serotype A.
Cellular and molecular mechanism
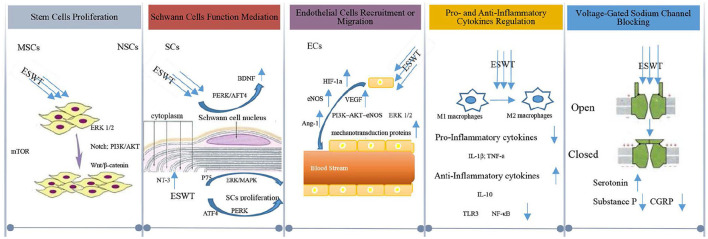
At first, it was suggested that ESWT was likely to first cause microtrauma in nerve tissues and then accelerate the regeneration of neural tissues (97). Later, studies have shown that ESWT promoted neural tissue regeneration and repairment. It is generally believed that the cellular and molecular alterations triggered by ESWT can enhance the regeneration of injured tissues in the central and peripheral nervous systems. Next, we discuss the mechanism proposed to explain how ESWT triggers tissue alternation at the cellular and molecular level after the tissues are subjected to ESWT, and its effects on neural tissues (Figure 3).
Figure 3.
Cellular and molecular mechanisms for the interaction of ESWT with tissue.
Stem cells proliferation
Although the ESWT effect on stem cells has gained increasing attention, the cellular and molecular mechanism of the stem cell growth triggered by ESWT remained poorly understood (98). It was found that ESWT could activate marrow stromal cells (MSCs) in vitro (99–101), causing accelerated proliferation to accomplish angiogenesis and nerve regeneration (102–104). In terms of the shock wave's function on MSCs proliferation, it was found that shock wave-elicited mechanical force triggered the mTORC1-FAK signaling pathway and remodeled the focal adhesion (FA) complex, inducing the MSC proliferation (105). MSCs comprise a heterogeneous group of stem/progenitor cells that can differentiate into mesoderm and non-mesoderm lineages, including epithelial cells and neurons, etc. (106). Moreover, MSCs have the multimodal therapeutic capacity in neural protection as well as the ability to promote angiogenesis in spinal cord injury (107). In the peripheral nerve system, MSCs might promote peripheral nerve regeneration after nerve injury (108).
When it comes to neural stem cells (NSCs), the ESWT's ability to promote the proliferation of NSCs was highly significant, which needs to be studied further. Kisoh et al. (109) suggested that recruitment of NSCs may improve brain dysfunction after cerebral ischemia. Zhang et al. (110) found that ESWT enhanced the proliferation and differentiation of NSCs by Notch, PI3K/AKT, and Wnt/β-catenin Signaling, leading to the repairment of the damaged nerve function in central nervous system diseases. It is known that NSCs play a crucial role in the central and peripheral nervous system, and could replace damaged neural cells (111–114). It is clear that stem cell proliferation induced by ESWT is of great significance in the repair and regeneration of the nervous system.
Schwann cells function mediation
Schwann cells (SCs) exert a crucial function in the nervous system and are also involved in nerve regeneration and repairment (115, 116). It is believed that ESWT could first induce ATP release, stimulate purinergic receptors and activate ERK1/2 signaling, and then enhance SCs proliferation (117, 118), ultimately promoting nerve regeneration. Another study also suggested that low-intensity ESWT played a role in the proliferation of SCs via the activation of ERK/MAPK signaling and p75 neurotrophin receptor, enabling nerve regrowth (119). The proliferation of SCs triggered by ESWT leads to the upregulation of neurotrophic factors that are believed to enhance the process of nerve regeneration and functional recovery. Among neurotrophic factors, brain-derived neurotrophic factor (BDNF) has been shown to upregulate the function of several neurons in the nervous system, which could promote nerve regeneration via SC-dependent Janus kinase (JAK)/signal transducer and activator of transcription (JAK/STAT) pathways (120, 121). It was found that the expression level of BDNF in SCs in vitro increased when low-intensity ESWT was applied to nerve injury because ESWT could activate the protein kinase RNA-like endoplasmic reticulum (ER) kinase (PERK) pathway and enhance activating transcription factor 4 (ATF4) (47, 122). SCs can also maintain the survival of the neurons through the increased production of neurotrophic factor-3 (NT-3) and other neurotrophic factors around the injured tissues (123), ensuring continuous stimulation for the growth of axons in SCs (123).
Endothelial cells recruitment or migration
ESWT can also have a significant impact on the neural tissue repairment and regeneration process by increasing local blood circulation, as ESWT can mechanically lead to vascular endothelial shear stress, generating a frictional force on the surface of the vascular epithelium (124), which can potentially lead to the recruitment or migration of endothelial cells (ECs). Studies of human umbilical vein endothelial cells (HUVECs) treated with ESWT (125) suggested that vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) are two essential angiogenesis mediators for ESWT-induced ECs recruitment and migration. Endothelial cells that are devoid of oxygen can increase the level of hypoxia-inducible factor 1 alpha (HIF-1α), thereby stimulating the release of VEGF. When VEGF binds to endothelial cells' receptors, it enhances the recruitment and migration of endothelial cells (126–128). In the nervous system, studies suggested that the increased expression of VEGF elicited by ESWT can produce a neuroprotective effect to reduce secondary neural tissue damage (62). Moreover, eNOS produces nitric oxide (NO), which contributes to vessel remodeling and vasodilating in the nervous system.
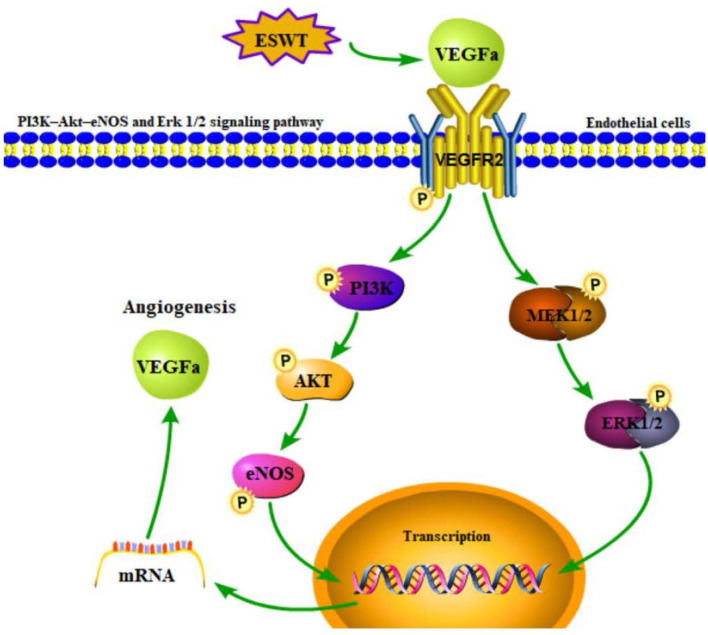
It was suggested that the process of the recruitment or migration of ECs triggered by VEGF involved other proteins or signaling pathways. Hatanaka et al. (45) found that the shock wave treatment exhibited advantageous effects on angiogenesis by upregulating mRNA expression and protein levels of VEGF, eNOS, and mechanotransduction proteins including caveolin-1 and 1-integrin on its downstream pathways (ERK and AKT, et al.). The level of the downstream cytokines such as angiopoietin-1(Ang-1), a regulator of angiogenesis, increased with the level of eNOS due to ESWT. The increase of Ang-1 may also contribute to the recruitment and aggregation of ECs to the local site to form new blood vessels (129). Ha et al. (130) found that the PI3-AKT-eNOS and ERK 1/2 signaling pathways (Figure 4) were activated by shock waves, leading to ECs migration which enhances the process of angiogenesis. Consequently, when ESWT leads to blood supply recovery in ischemic neural tissues, neural tissues are regenerated and repaired.
Figure 4.
PI3K-AKT-eNOS and ERK 1/2 signaling pathway induced by ESWT could promote angiogenesis through endothelial cells migration.
Pro- and anti-inflammatory cytokines regulation
ESWT could also mediate the expression of pro-and anti-inflammatory cytokines in a dose-dependent manner, reducing the pain and inflammation (131, 132). ESWT may exert the function of mediating inflammatory reactions through macrophages. Macrophages play a critical role in tissue regeneration and are shown to be sensitive to ESWT, which does not induce activation of resting macrophages, but reduces the induction of the pro-inflammatory profile in M1 macrophages and increases the anti-inflammatory profile with M2 macrophages (44, 133). Therefore, ESWT can reduce the concentration of pro-inflammatory cytokines such as TNF-α and IL-1β (134), while upregulating the expression of anti-inflammatory cytokines like IL-10 (135).
Macrophages also regulate immune and inflammatory responses by recognizing Toll-like receptors (TLRs), activating myeloid differentiation factor 88 (MyD88) and nuclear factor kappa-B (NF-κB) (136, 137). TLR3 signaling pathway perhaps modulates the early inflammatory response to ESWT in the nervous system and thus represents an innate mechanism of ischemic tissue regeneration and repairment (138–140). In the TLR3 signaling pathway, NF-κB is the downstream factor of TLR3. As a transcription factor, NF-κB represents a key mechanism for regulating the expression of multiple inflammatory genes. Hence, the suppression of NF-κB activation may account for the clinically beneficial action on tissue inflammation (141). It is believed that NF-κB activation in the nervous system could be inhibited by ESWT (142), which subsequently downregulates NF-κB and NF-κB-dependent inflammatory genes such as TNF-α and IL-1, thus leading to the modulation of the whole inflammatory process (132, 143–145). Regulating inflammatory reaction via pro-and anti-inflammatory cytokines balance triggered by ESWT results in a reduction in the inflammation of neural tissue injury and promotes neural repairment and neuroprotection.
Voltage-gated sodium channel blocking
Voltage-gated sodium channels play a crucial role in pain transmission and sensitization pathways in the nervous system (146). ESWT is capable of closing the voltage-gated sodium channels to nociceptive input, which is competitively regulated at the spinal cord dorsal horn laminae (147). ESWT can activate serotonin in the cerebral cortex and reduce the production of substance P and calcitonin gene-related peptide (CGRP) in the dorsal root ganglion (25, 148, 149), which in turn leads to rapid degeneration of the intracutaneous nerve fibers to exert the effects of pain controlling (150). Substance P and CGRP are neuropeptides that are present in small-diameter afferent fibers. They are known to be involved in nociception in both the peripheral and central nervous systems (151). They can be released from nerve endings and play a pro-inflammatory role in the nerve tissues (152, 153). A reduction in their production by ESWT can ultimately prevent central sensitization and control the excessive neurons' excitement (154), thus resulting in pain-controlling effects beneficial to the sensory nerve regeneration processes (155).
Conclusion and future outlook
Clinical areas, in which ESWT can be used, have expanded significantly, ranging from musculoskeletal diseases to regenerative medicine. Because ESWT is non-invasive, it can improve the conditions of many neurological disorders as well. In this review, we have discussed the essential characteristics of ESWT as well as the cellular and molecular mechanism proposed to explain how ESWT improves neural tissue repairment and regeneration in the nervous system. In addition, we reviewed both animal and clinical studies to show that ESWT can produce good therapeutic results when used to treat neurological disorders. For example, the animal studies reviewed show that treating cerebral infarction and spinal cord injury by ESWT could be safe if the optimal ESWT energy has been determined and used. The clinical studies reviewed show that ESWT produces good clinical results when used to treat CTS, TN, and PHN. Moreover, clinical studies have also shown that spasticity after central nervous system diseases such as stroke, cerebral palsy, and multiple sclerosis can be relieved as well by ESWT.
It should be noted that the use of ESWT to treat neurological disorders is promising and its potential is enormous, but there are many issues that are still needed to be addressed to better use ESWT clinically. For example, ESWT is not considered safe when used to treat bleeding disorders and pregnancy problems. In addition, pain, blistering, and hematomas can occur during and after treatment by ESWT although they may disappear within several days. Hence, any possible adverse reactions must be examined and suitable procedures to minimize or even eliminate these adverse effects must be developed before treatment of human diseases.
Another issue is a lack of a uniform standard for selecting the lesion area targeted by ESWT. As a result, the effectiveness of ESWT may vary among physicians. Moreover, when the lesion is deep, imaging such as ultrasound, X-ray, or MRI must be used to assist in locating the targeted area by ESWT. For example, when ESWT is used in the treatment of unresponsive wakefulness syndrome, the brainstem is targeted. Hence, it is necessary to combine body surface anatomical positioning and MRI to locate the targeted site on the body surface.
Moreover, as discussed above, high-energy ESWT can damage neural tissues. Moreover, the effectiveness of ESWT also depends on the penetration depth and the probe of the handpiece used in an ESWT device dictates the penetration depth. There are several ESWT device markers, each of which provides many different types of handpieces, making it difficult for physicians to decide and/or select which handpieces should be used to treat a given disease.
We believe that the biggest issue associated with the use of ESWT is a lack of a medical guideline for using ESWT for the treatment of different neurological disorders. Currently, physicians at different hospitals use ESWT for the treatment of neurological disorders based on their own experiences because of the lack of such a guideline. As a result, the effectiveness of ESWT may vary significantly among hospitals. Therefore, there is an urgent need for medical associations to develop medical guidelines to guide physicians in using ESWT to treat neurological disorders.
When it comes to the future basic research direction, a better understanding of the physical, cellular, and molecular mechanisms of interactions between ESWT and lesion tissues is urgently needed. For example, it has been suggested that ESWT may be able to transfer the mechanical signals to biological signals, thus triggering a series of events at the cellular and molecular levels, but it is still a mystery about how to complete this transition. A better understanding of such mechanisms will help physicians better use ESWT. In fact, the currently proposed cellular and molecular mechanism of the tissue's response to ESWT has already provided new insights into the use of ESWT to treat neurological disorders. It is our strong belief that with a better understanding of the interaction of ESWT with neural tissues in the nervous system, ESWT could, in the future, become a popular treatment option for many patients with central and peripheral nervous system diseases.
Author contributions
JG and YM conceived and wrote the article. HH and YM proofread the data and provided guidance for writing and submission. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81871840).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Bob Guo at Cleveland State University for checking the language of the manuscript.
References
- 1.Auersperg V, Trieb K. Extracorporeal shock wave therapy: an update. EFORT Open Rev. (2020) 5:584–92. 10.1302/2058-5241.5.190067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson WT, Preminger GM. Extracorporeal shock wave lithotripsy. An update. Urol Clin North Am. (1990) 17:231–42. 10.1016/S0094-0143(21)00324-4 [DOI] [PubMed] [Google Scholar]
- 3.Crevenna R, Mickel M, Keilani M. Extracorporeal shock wave therapy in the supportive care and rehabilitation of cancer patients. Support Care Cancer. (2019) 27:4039–41. 10.1007/s00520-019-05046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramon S, Español A, Yebra M, Morillas JM, Unzurrunzaga R, Freitag K, et al. Current evidences in shockwave treatment SETOC (SpanishSociety of Shockwave Treatment) recommendations. Rehabilitacion (Madr). (2021) 55:291–300. 10.1016/j.rh.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Goldberg D, Andriessen A, Gold M. Radial shockwave therapy for male erectile rejuvenation in a dermatology and/or medical aesthetic practice. J Cosmet Dermatol. (2019) 18:1596–600. 10.1111/jocd.13022 [DOI] [PubMed] [Google Scholar]
- 6.Simplicio CL, Purita J, Murrell W, Santos GS, Dos Santos RG, Lana JFSD. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J Clin Orthop Trauma. (2020) 11:S309–18. 10.1016/j.jcot.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moya D, Ramón S, Schaden W, Wang CJ, Guiloff L, Cheng JH. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J Bone Joint Surg Am. (2018) 100:251–63. 10.2106/JBJS.17.00661 [DOI] [PubMed] [Google Scholar]
- 8.Ilieva EM. Radial shock wave therapy for plantar fasciitis: a one year follow-up study. Folia Med (Plovdiv). (2013) 55:42–8. 10.2478/folmed-2013-0004 [DOI] [PubMed] [Google Scholar]
- 9.Xiong Y, Wu Q, Mi B, Zhou W, Liu Y, Liu J, et al. Comparison of efficacy of shock-wave therapy versus corticosteroids in plantar fasciitis: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. (2019) 139:529–36. 10.1007/s00402-018-3071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melese H, Alamer A, Getie K, Nigussie F, Ayhualem S. Extracorporeal shock wave therapy on pain and foot functions in subjects with chronic plantar fasciitis: systematic review of randomized controlled trials. Disabil Rehabil. (2021) undefined: 1-8. 10.1080/09638288.2021.1928775 [DOI] [PubMed] [Google Scholar]
- 11.Abo Al-Khair MA, El Khouly RM, Khodair SA, Al Sattar Elsergany MA, Hussein MI, Eldin Mowafy ME. Focused, radial and combined shock wave therapy in treatment of calcific shoulder tendinopathy. Phys Sportsmed. (2020) 1–8. 10.1080/00913847.2020.1856633 [DOI] [PubMed] [Google Scholar]
- 12.Król P, Franek A, Durmała J, Błaszczak E, Ficek K, Król B, et al. Focused and radial shock wave therapy in the treatment of tennis elbow: a pilot randomized controlled study. J Hum Kinet. (2015) 47:127–35. 10.1515/hukin-2015-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogru M, Erduran M, Narin S. The effect of radial extracorporeal shock wave therapy in the treatment of trigger finger. Cureus. (2020) 12:e8385. 10.7759/cureus.8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uysal A, Yildizgoren MT, Guler H, Turhanoglu AD. Effects of radial extracorporeal shock wave therapy on clinical variables and isokinetic performance in patients with knee osteoarthritis: a prospective, randomized, single-blind and controlled trial. Int Orthop. (2020) 44:1311–9. 10.1007/s00264-020-04541-w [DOI] [PubMed] [Google Scholar]
- 15.Kertzman P, Császár NBM, Furia JP, Schmitz C. Radial extracorporeal shock wave therapy is efficient and safe in the treatment of fracture nonunions of superficial bones: a retrospective case series. J Orthop Surg Res. (2017) 12:164. 10.1186/s13018-017-0667-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everding J, Stolberg-Stolberg J, Pützler J, Roßlenbroich S, Ochman S, Raschke M. Extracorporal shock wave therapy for the treatment of arthrodesis non-unions. Arch Orthop Trauma Surg. (2020) 140:1191–200. 10.1007/s00402-020-03361-2 [DOI] [PubMed] [Google Scholar]
- 17.Cacchio A, Giordano L, Colafarina O, Rompe JD, Tavernese E, Ioppolo F, et al. Extracorporeal shock-wave therapy compared with surgery for hypertrophic long-bone nonunions. J Bone Joint Surg Am. (2009) 91:2589–97. 10.2106/JBJS.H.00841 [DOI] [PubMed] [Google Scholar]
- 18.Kang N, Yu X, Ma Y. Radial extracorporeal shock wave therapy in a patient with decubitus ulcer after spinal cord injury: a case report. Am J Transl Res. (2020) 12:2093–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Yin C, Han X, Guo A, Chen X, Liu S, et al. Improved healing of diabetic foot ulcer upon oxygenation therapeutics through oxygen-loading nanoperfluorocarbon triggered by radial extracorporeal shock wave. Oxid Med Cell Longev. (2019) 2019:5738368. 10.1155/2019/5738368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao JC, Zhang BR, Shi K, Wang J, Yu QH, Yu JA. Lower energy radial shock wave therapy improves characteristics of hypertrophic scar in a rabbit ear model. Exp Ther Med. (2018) 15:933–9. 10.3892/etm.2017.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CJ, Wu CT, Yang YJ, Liu RT, Kuo YR. Long-term outcomes of extracorporeal shockwave therapy for chronic foot ulcers. J Surg Res. (2014) 189:366–72. 10.1016/j.jss.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Wang YL, Gong DX, Zhang ZX, Yu XT, Ma YW. Radial extracorporeal shock wave therapy as a novel agent for benign prostatic hyperplasia refractory to current medical therapy. Am J Mens Health. (2019) 13:1557988319831899. 10.1177/1557988319831899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang ZX, Zhang D, Yu XT, Ma YW. Efficacy of radial extracorporeal shock wave therapy for chronic pelvic pain syndrome: a nonrandomized controlled trial. Am J Mens Health. (2019) 13:1557988318814663. 10.1177/1557988318814663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Chang D, Chen D, Zhang P, You Y, Huang X, et al. Efficacy of radial extracorporeal shock wave therapy for chronic prostatitis/chronic pelvic pain syndrome: a protocol for systematic review. Medicine (Baltimore). (2020) 99:e22981. 10.1097/MD.0000000000022981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung B, Wiley JP. Extracorporeal shockwave therapy: a review. Sports Med. (2002) 32:851–65. 10.2165/00007256-200232130-00004 [DOI] [PubMed] [Google Scholar]
- 26.Leinenga G, Langton C, Nisbet R, Götz J. Ultrasound treatment of neurological diseases-current and emerging applications. Nat Rev Neurol. (2016) 12:161–74. 10.1038/nrneurol.2016.13 [DOI] [PubMed] [Google Scholar]
- 27.Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. (2003) 26:220–32. [PubMed] [Google Scholar]
- 28.Liu Y, Chen X, Guo A, Liu S, Hu G. Quantitative assessments of mechanical responses upon radial extracorporeal shock wave therapy. Adv Sci (Weinh). (2018) 5:1700797. 10.1002/advs.201700797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwerver J, Waugh C, van der Worp H, Scott A. Can Shockwave Therapy Improve Tendon Metabolism? Adv Exp Med Biol. (2016) 920:275–81. 10.1007/978-3-319-33943-6_26 [DOI] [PubMed] [Google Scholar]
- 30.Cheing GL, Chang H. Extracorporeal shock wave therapy. J Orthop Sports Phys Ther. (2003) 33:337–43. 10.2519/jospt.2003.33.6.337 [DOI] [PubMed] [Google Scholar]
- 31.Patel P, Fode M, Lue T, Ramasamy R. Should Low-intensity Extracorporeal Shockwave Therapy Be the First-line Erectile Dysfunction Treatment for Nonresponders to Phosphodiesterase Type 5 Inhibition? Eur Urol Focus. (2019) 5:526–8. 10.1016/j.euf.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Zelle BA, Gollwitzer H, Zlowodzki M, Bühren V. Extracorporeal shock wave therapy: current evidence. J Orthop Trauma. (2010) null: S66–70. 10.1097/BOT.0b013e3181cad510 [DOI] [PubMed] [Google Scholar]
- 33.Brunelli S, Bonanni C, Traballesi M, Foti C. Radial extracorporeal shock wave therapy: a novel approach for the treatment of Dupuytren's contractures: a case report. Medicine (Baltimore). (2020) 99:e20587. 10.1097/MD.0000000000020587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speed C A. systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med. (2014) 48:1538–42. 10.1136/bjsports-2012-091961 [DOI] [PubMed] [Google Scholar]
- 35.van der Worp H, van den Akker-Scheek I, van Schie H, Zwerver J. ESWT for tendinopathy: technology and clinical implications Knee Surg Sports. Traumatol Arthrosc. (2013) 21:1451–8. 10.1007/s00167-012-2009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi MJ, Kwon O. Temporal and spectral characteristics of the impulsive waves produced by a clinical ballistic shock wave therapy device. Ultrasonics. (2021) 110:106238. 10.1016/j.ultras.2020.106238 [DOI] [PubMed] [Google Scholar]
- 37.Rompe JD, Kirkpatrick CJ, Küllmer K, Schwitalle M, Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis. J Bone Joint Surg Br. (1998) 80-B:546–52. 10.1302/0301-620X.80B3.0800546 [DOI] [PubMed] [Google Scholar]
- 38.Duymaz T, Sindel D. Comparison of radial extracorporeal shock wave therapy and traditional physiotherapy in rotator cuff calcific tendinitis treatment. Arch Rheumatol. (2019) 34:281–7. 10.5606/ArchRheumatol.2019.7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melki E. Endometriosis treatment with shock waves: a novel approach. Med Hypotheses. (2019) 124:114–7. 10.1016/j.mehy.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 40.Sun W, Li Z. Extracorporeal shockwave therapy for osteonecrosis of femoral head: trapsand challenges. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2019) 33:659–61. 10.7507/1002-1892.201905059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dymarek R, Ptaszkowski K, Ptaszkowska L. Shock waves as a treatment modality for spasticity reduction and recovery improvement in post-stroke adults—current evidence and qualitative systematic review. Clin Interv Aging. (2020) 15:9–28. 10.2147/CIA.S221032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura K, Tanaka Y. Facial tightening effects, following focused and radial acoustic wave therapy assessment, using a three-dimensional digital imaging. Lasers Surg Med. (2020) undefined: undefined. 10.1002/lsm.23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.d'Agostino MC, Craig K, Tibalt E, Respizzi S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. (2015) 24:147–53. 10.1016/j.ijsu.2015.11.030 [DOI] [PubMed] [Google Scholar]
- 44.Hsiao CC, Hou YS, Liu YH, Ko JY, Lee CT. Combined melatonin and extracorporeal shock wave therapy enhances podocyte protection and ameliorates kidney function in a diabetic nephropathy rat model. Antioxidants (Basel). (2021) 10:undefined. 10.3390/antiox10050733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatanaka K, Ito K, Shindo T, Kagaya Y, Ogata T, Eguchi K, et al. Molecular mechanisms of the angiogenic effects of low-energy shock wave therapy: roles of mechanotransduction. Am J Physiol Cell Physiol. (2016) 311:C378–385. 10.1152/ajpcell.00152.2016 [DOI] [PubMed] [Google Scholar]
- 46.Huang C, Holfeld J, Schaden W, Orgill D, Ogawa R. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med. (2013) 19:555–64. 10.1016/j.molmed.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 47.Liu T, Shindel AW, Lin G, Lue TF. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Impot Res. (2019) 31:170–6. 10.1038/s41443-019-0113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin P, Jan LY, Jan YN. Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. Annu Rev Neurosci. (2020) 43:207–29. 10.1146/annurev-neuro-070918-050509 [DOI] [PubMed] [Google Scholar]
- 49.Császár NB, Angstman NB, Milz S, Sprecher CM, Kobel P, Farhat M, et al. Radial Shock Wave Devices Generate Cavitation. PLoS ONE. (2015) 10:e0140541. 10.1371/journal.pone.0140541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogden JA, Tóth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. (2001) undefined:8–17. 10.1097/00003086-200106000-00003 [DOI] [PubMed] [Google Scholar]
- 51.López-Marín LM, Rivera AL, Fernández F, Loske AM. Shock wave-induced permeabilization of mammalian cells. Phys Life Rev. (2018) null:1–38. 10.1016/j.plrev.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 52.Adhikari U, Goliaei A, Berkowitz ML. Nanobubbles, cavitation, shock waves and traumatic brain injury. Phys Chem Chem Phys. (2016) 18:32638–52. 10.1039/C6CP06704B [DOI] [PubMed] [Google Scholar]
- 53.Schelling G, Delius M, Gschwender M, Grafe P, Gambihler S. Extracorporeal shock waves stimulate frog sciatic nerves indirectly via a cavitation-mediated mechanism. Biophys J. (1994) 66:133–40. 10.1016/S0006-3495(94)80758-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mense S, Hoheisel U. Shock wave treatment improves nerve regeneration in the rat. Muscle Nerve. (2013) 47:702–10. 10.1002/mus.23631 [DOI] [PubMed] [Google Scholar]
- 55.Schuh CM, Hausner T, Redl HR. A. therapeutic shock propels Schwann cells to proliferate in peripheral nerve injury. Brain Circ. (2016) 2:138–40. 10.4103/2394-8108.192520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu M, Cheng H, Li D, Yu X, Ji N, Luo F. Radial shock wave therapy in the treatment of chronic constriction injury model in rats: a preliminary study. Chin Med J (Engl). (2014) 127:830–4. 10.3760/cma.j.issn.0366-6999.20132619 [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Cho SH. Effect of extracorporeal shock wave therapy on denervation atrophy and function caused by sciatic nerve injury. J Phys Ther Sci. (2013) 25:1067–9. 10.1589/jpts.25.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sağir D, Bereket C, Onger ME, Bakhit N, Keskin M, Ozkan E. Efficacy of Extracorporeal Shockwaves Therapy on Peripheral Nerve Regeneration. J Craniofac Surg. (2019) 30:2635–9. 10.1097/SCS.0000000000005671 [DOI] [PubMed] [Google Scholar]
- 59.Yuen CM, Chung SY, Tsai TH, Sung PH, Huang TH, Chen YL, et al. Extracorporeal shock wave effectively attenuates brain infarct volume and improves neurological function in rat after acute ischemic stroke. Am J Transl Res. (2015) 7:976–94. [PMC free article] [PubMed] [Google Scholar]
- 60.Kang N, Zhang J, Yu X, Ma Y. Radial extracorporeal shock wave therapy improves cerebral blood flow and neurological function in a rat model of cerebral ischemia. Am J Transl Res. (2017) 9:2000–12. [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JY, Ha KY, Kim JW, Seo JY, Kim YH. Does extracorporeal shock wave introduce alteration of microenvironment in cell therapy for chronic spinal cord injury? Spine (Phila Pa 1976). (2014) 39:E1553–9. 10.1097/BRS.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 62.Yamaya S, Ozawa H, Kanno H, Kishimoto KN, Sekiguchi A, Tateda S, et al. Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury. J Neurosurg. (2014) 121:1514–25. 10.3171/2014.8.JNS132562 [DOI] [PubMed] [Google Scholar]
- 63.Matsuda M, Kanno H, Sugaya T, Yamaya S, Yahata K, Handa K, et al. Low-energy extracorporeal shock wave therapy promotes BDNF expression and improves functional recovery after spinal cord injury in rats. Exp Neurol. (2020) 328:113251. 10.1016/j.expneurol.2020.113251 [DOI] [PubMed] [Google Scholar]
- 64.Yahata K, Kanno H, Ozawa H, Yamaya S, Tateda S, Ito K, et al. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J Neurosurg Spine. (2016) 25:745–55. 10.3171/2016.4.SPINE15923 [DOI] [PubMed] [Google Scholar]
- 65.Maeda Y, Kettner N, Holden J, Lee J, Kim J, Cina S, et al. Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain. (2014) 137:1741–52. 10.1093/brain/awu096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi Q, Bobos P, Lalone EA, Warren L, MacDermid JC. Comparison of the short-term and long-term effects of surgery and nonsurgical intervention in treating carpal tunnel syndrome: a systematic review and meta-analysis. Hand (N Y). (2020) 15:13–22. 10.1177/1558944718787892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park GY, Kwon DR, Lee SC. Timing of extracorporeal shock wave therapy in rabbits with carpal tunnel syndrome. J Tissue Eng Regen Med. (2019) 13:1071–8. 10.1002/term.2862 [DOI] [PubMed] [Google Scholar]
- 68.Ke MJ, Chen LC, Chou YC, Li TY, Chu HY, Tsai CK, et al. The dose-dependent efficiency of radial shock wave therapy for patients with carpal tunnel syndrome: a prospective, randomized, single-blind, placebo-controlled trial. Sci Rep. (2016) 6:38344. 10.1038/srep38344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu YT, Ke MJ, Chou YC, Chang CY, Lin CY, Li TY, et al. Effect of radial shock wave therapy for carpal tunnel syndrome: A prospective randomized, double-blind, placebo-controlled trial. J Orthop Res. (2016) 34:977–84. 10.1002/jor.23113 [DOI] [PubMed] [Google Scholar]
- 70.Raissi GR, Ghazaei F, Forogh B, Madani SP, Daghaghzadeh A, Ahadi T. The effectiveness of radial extracorporeal shock waves for treatment of carpal tunnel syndrome: a randomized clinical trial. Ultrasound Med Biol. (2017) 43:453–60. 10.1016/j.ultrasmedbio.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 71.Koçak Ulucaköy R, Yurdakul FG, Bodur H. Extracorporeal shock wave therapy as a conservative treatment option for carpal tunnel syndrome: a double-blind, prospective, randomized, placebo-controlled study. Turk J Phys Med Rehabil. (2020) 66:388–97. 10.5606/tftrd.2020.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu D, Ma W, Jiang W, Hu X, Jiang F, Mao C, et al. A randomized controlled trial: comparing extracorporeal shock wave therapy versus local corticosteroid injection for the treatment of carpal tunnel syndrome. Int Orthop. (2020) 44:141–6. 10.1007/s00264-019-04432-9 [DOI] [PubMed] [Google Scholar]
- 73.Atthakomol P, Manosroi W, Phanphaisarn A, Phrompaet S, Iammatavee S, Tongprasert S. Comparison of single-dose radial extracorporeal shock wave and local corticosteroid injection for treatment of carpal tunnel syndrome including mid-term efficacy: a prospective randomized controlled trial. BMC Musculoskelet Disord. (2018) 19:32. 10.1186/s12891-018-1948-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vahdatpour B, Kiyani A, Dehghan F. Effect of extracorporeal shock wave therapy on the treatment of patients with carpal tunnel syndrome. Adv Biomed Res. (2016) 5:120. 10.4103/2277-9175.186983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Notarnicola A, Maccagnano G, Tafuri S, Fiore A, Pesce V, Moretti B. Comparison of shock wave therapy and nutraceutical composed of Echinacea angustifolia, alpha lipoic acid, conjugated linoleic acid and quercetin (perinerv) in patients with carpal tunnel syndromeInt. J Immunopathol Pharmacol. (2015) 28:256–62. 10.1177/0394632015584501 [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Zhou R, Sun F, Weng Y, Ye L, Yang P. Efficacy and safety of the extracorporeal shockwave therapy in patients with postherpetic neuralgia: study protocol of a randomized controlled trial. Trials. (2020) 21:630. 10.1186/s13063-020-04564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee SH, Ryu KH, Kim PO, Lee HW, Cho EA, Ahn JH, et al. Efficacy of extracorporeal shockwave therapy in the treatment of postherpetic neuralgia: a pilot study. Medicine (Baltimore). (2020) 99:e19516. 10.1097/MD.0000000000019516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolt DM, Burba DJ, Hubert JD, Strain GM, Hosgood GL, Henk WG, et al. Determination of functional and morphologic changes in palmar digital nerves after nonfocused extracorporeal shock wave treatment in horses. Am J Vet Res. (2004) 65:1714–8. 10.2460/ajvr.2004.65.1714 [DOI] [PubMed] [Google Scholar]
- 79.Liao JY, Zhou TH, Chen BK, Liu ZX. Schwann cells and trigeminal neuralgia. Mol Pain. (2020) 16:1744806920963809. 10.1177/1744806920963809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang D, Meng Y, Hai H, Yu XT, Ma YW. Radial extracorporeal shock wave therapy in an individual with primary trigeminal neuralgia: a case report and literature review. Am J Phys Med Rehabil. (2018) 97:e42–5. 10.1097/PHM.0000000000000831 [DOI] [PubMed] [Google Scholar]
- 81.Lohse-Busch H, Reime U, Falland R. Symptomatic treatment of unresponsive wakefulness syndrome with transcranially focused extracorporeal shock waves. NeuroRehabilitation. (2014) 35:235–44. 10.3233/NRE-141115 [DOI] [PubMed] [Google Scholar]
- 82.Werner C, Byhahn M, Hesse S. Non-invasive brain stimulation to promote alertness and awareness in chronic patients with disorders of consciousness: Low-level, near-infrared laser stimulation vs. focused shock wave therapy. Restor Neurol Neurosci. (2016) 34:561–9. 10.3233/RNN-150624 [DOI] [PubMed] [Google Scholar]
- 83.Khan F, Amatya B, Bensmail D, Yelnik A. Non-pharmacological interventions for spasticity in adults: An overview of systematic reviews. Ann Phys Rehabil Med. (2019) 62:265–73. 10.1016/j.rehab.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 84.Kenmoku T, Nemoto N, Iwakura N, Ochiai N, Uchida K, Saisu T, et al. Extracorporeal shock wave treatment can selectively destroy end plates in neuromuscular junctions. Muscle Nerve. (2018) 57:466–72. 10.1002/mus.25754 [DOI] [PubMed] [Google Scholar]
- 85.Cabanas-Valdés R, Serra-Llobet P, Rodriguez-Rubio PR, López-de-Celis C, Llauró-Fores M, Calvo-Sanz J. The effectiveness of extracorporeal shock wave therapy for improving upper limb spasticity and functionality in stroke patients: a systematic review and meta-analysis. Clin Rehabil. (2020) 34:1141–56. 10.1177/0269215520932196 [DOI] [PubMed] [Google Scholar]
- 86.Cabanas-Valdés R, Calvo-Sanz J, Urrùtia G, Serra-Llobet P, Pérez-Bellmunt A, Germán-Romero A. The effectiveness of extracorporeal shock wave therapy to reduce lower limb spasticity in stroke patients: a systematic review and meta-analysis. Top Stroke Rehabil. (2020) 27:137–57. 10.1080/10749357.2019.1654242 [DOI] [PubMed] [Google Scholar]
- 87.Wu YT, Chang CN, Chen YM, Hu GC. Comparison of the effect of focused and radial extracorporeal shock waves on spastic equinus in patients with stroke: a randomized controlled trial. Eur J Phys Rehabil Med. (2018) 54:518–25. 10.23736/S1973-9087.17.04801-8 [DOI] [PubMed] [Google Scholar]
- 88.Gonkova MI, Ilieva EM, Ferriero G, Chavdarov I. Effect of radial shock wave therapy on muscle spasticity in children with cerebral palsy. Int J Rehabil Res. (2013) 36:284–90. 10.1097/MRR.0b013e328360e51d [DOI] [PubMed] [Google Scholar]
- 89.Wang T, Du L, Shan L, Dong H, Feng J, Kiessling MC, et al. A prospective case-control study of radial extracorporeal shock wave therapy for spastic plantar flexor muscles in very young children with cerebral palsy. Medicine (Baltimore). (2016) 95:e3649. 10.1097/MD.0000000000003649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin Y, Wang G, Wang B. Rehabilitation treatment of spastic cerebral palsy with radial extracorporeal shock wave therapy and rehabilitation therapy. Medicine (Baltimore). (2018) 97:e13828. 10.1097/MD.0000000000013828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leister I, Mittermayr R, Mattiassich G, Aigner L, Haider T, Machegger L, et al. The effect of extracorporeal shock wave therapy in acute traumatic spinal cord injury on motor and sensory function within 6 months post-injury: a study protocol for a two-arm three-stage adaptive, prospective, multi-center, randomized, blinded, placebo-controlled clinical trial. Trials. (2022) 23:245. 10.1186/s13063-022-06161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Etoom M, Khraiwesh Y, Lena F, Hawamdeh M, Hawamdeh Z, Centonze D, et al. Effectiveness of physiotherapy interventions on spasticity in people with multiple sclerosis: a systematic review and meta-analysis. Am J Phys Med Rehabil. (2018) 97:793–807. 10.1097/PHM.0000000000000970 [DOI] [PubMed] [Google Scholar]
- 93.Radinmehr H, Nakhostin Ansari N, Naghdi S, Olyaei G, Tabatabaei A. Effects of one session radialextracorporeal shockwave therapy on post-stroke plantarflexor spasticity: a single-blin clinical trial. Disabil Rehabil. (2017) 39:483–90. 10.3109/09638288.2016.1148785 [DOI] [PubMed] [Google Scholar]
- 94.Dymarek R, Taradaj J, Rosińczuk J. Extracorporeal shock wave stimulation as alternative treatment modality for wrist and fingers spasticity in poststroke patients: a prospective, open-label, preliminary clinical trial. Evid Based Complement Alternat Med. (2016) 2016:4648101. 10.1155/2016/4648101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marinelli L, Mori L, Solaro C, Uccelli A, Pelosin E, Currà A, et al. Effect of radial shock wave therapy on pain and muscle hypertonia: a double-blind study in patients with multiple sclerosis. Mult Scler. (2015) 21:622–9. 10.1177/1352458514549566 [DOI] [PubMed] [Google Scholar]
- 96.Marinaro C, Costantino C, D'Esposito O, Barletta M, Indino A, De Scorpio G, et al. Synergic use of botulinum toxin injection and radial extracorporeal shockwave therapy in Multiple Sclerosis spasticity. Acta Biomed. (2021) 92:e2021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu YH, Lun JJ, Chen WS, Chong FC. The electrophysiological and functional effect of shock wave on peripheral. nerves Annu Int Conf IEEE Eng Med Biol Soc. (2007) 2007:2369–72. 10.1109/IEMBS.2007.4352803 [DOI] [PubMed] [Google Scholar]
- 98.Zhao Z, Wang Y, Wang Q, Liang J, Hu W, Zhao S, et al. Radial extracorporeal shockwave promotes subchondral bone stem/progenitor cell self-renewal by activating YAP/TAZ and facilitates cartilage repair in vivo. Stem Cell Res Ther. (2021) 12:19. 10.1186/s13287-020-02076-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salcedo-Jiménez R, Koenig JB, Lee OJ, Gibson TWG, Madan P, Koch TG. In vitro extracorporeal shock wave therapy enhances the metabolic activity and differentiation of equine umbilical cord blood mesenchymal stromal cells. Front Vet Sci. (2020) 7:554306. 10.3389/fvets.2020.554306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y, Xu J, Huang Z, Yu M, Zhang Y, Chen H, et al. An innovative approach for enhancing bone defect healing using plga scaffolds seeded with extracorporeal-shock-wave-treated bone marrow mesenchymal stem cells (BMSCs). Sci Rep. (2017) 7:44130. 10.1038/srep44130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suhr F, Delhasse Y, Bungartz G, Schmidt A, Pfannkuche K, Bloch W. Cell biological effects of mechanical stimulations generated by focused extracorporeal shock wave applications on cultured human bone marrow stromal cells. Stem Cell Res. (2013) 11:951–64. 10.1016/j.scr.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 102.Hausner T, Pajer K, Halat G, Hopf R, Schmidhammer R, Redl H, et al. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp Neurol. (2012) 236:363–70. 10.1016/j.expneurol.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Li ZL, Yang F, Zhang Q, Su XZ, Li J, et al. Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing. Stem Cell Res Ther. (2018) 9:54. 10.1186/s13287-018-0805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Y, Wang J, Wang M, Sun P, Chen J, Jin X, et al. Activation of bone marrow-derived mesenchymal stromal cells-a new mechanism of defocused low-energy shock wave in regenerative medicine. Cytotherapy. (2013) 15:1449–57. 10.1016/j.jcyt.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 105.Lee FY, Zhen YY, Yuen CM, Fan R, Chen YT, Sheu JJ, et al. The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am J Transl Res. (2017) 9:1603–17. [PMC free article] [PubMed] [Google Scholar]
- 106.Zhai L, Sun N, Zhang B, Liu ST, Zhao Z, Jin HC, et al. Effects of focused extracorporeal shock waves on bone marrow mesenchymal stem cells in patients with avascular necrosis of the femoral head ultrasound. Med Biol. (2016) 42:753–62. 10.1016/j.ultrasmedbio.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 107.Yao S, He F, Cao Z, Sun Z, Chen Y, Zhao H, et al. Mesenchymal stem cell-laden hydrogel microfibers for promoting nerve fiber regeneration in long-distance spinal cord transection injury. ACS Biomater Sci Eng. (2020) 6:1165–75. 10.1021/acsbiomaterials.9b01557 [DOI] [PubMed] [Google Scholar]
- 108.Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, et al. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci Rep. (2016) 6:31306. 10.1038/srep31306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kisoh K, Hayashi H, Arai M, Orita M, Yuan B, Takagi N. Possible involvement of PI3-K/Akt-dependent GSK-3β signaling in proliferation of neural progenitor cells after hypoxic exposure. Mol Neurobiol. (2019) 56:1946–56. 10.1007/s12035-018-1216-4 [DOI] [PubMed] [Google Scholar]
- 110.Zhang J, Kang N, Yu X, Ma Y, Pang X. Radial extracorporeal shock wave therapy enhances the proliferation and differentiation of neural stem cells by notch, PI3K/AKT, and Wnt/β-catenin signaling. Sci Rep. (2017) 7:15321. 10.1038/s41598-017-15662-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee DC, Chen JH, Hsu TY, Chang LH, Chang H, Chi YH, et al. Neural stem cells promote nerve regeneration through IL12-induced Schwann cell differentiation. Mol Cell Neurosci. (2017) 79:1–11. 10.1016/j.mcn.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 112.Ham TR, Farrag M, Leipzig ND. Covalent growth factor tethering to direct neural stem cell differentiation and self-organization. Acta Biomater. (2017) 53:140–51. 10.1016/j.actbio.2017.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiong LL, Hu Y, Zhang P, Zhang Z, Li LH, Gao GD, et al. Neural stem cell transplantation promotes functional recovery from traumatic brain injury via brain derived neurotrophic factor-mediated neuroplasticity. Mol Neurobiol. (2018) 55:2696–711. 10.1007/s12035-017-0551-1 [DOI] [PubMed] [Google Scholar]
- 114.Richard SA, Sackey M. Elucidating the Pivotal Neuroimmunomodulation of Stem Cells in Spinal Cord Injury Repair. Stem Cells Int. (2021) 2021:9230866. 10.1155/2021/9230866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bolívar S, Navarro X, Udina E. Schwann cell role in selectivity of nerve regeneration. Cells. (2020) 9:undefined. 10.3390/cells9092131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Min Q, Parkinson DB, Dun XP. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia. (2021) 69:235–54. 10.1002/glia.23892 [DOI] [PubMed] [Google Scholar]
- 117.Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, Mittermayr R, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. (2014) 289:27090–104. 10.1074/jbc.M114.580936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin G, Reed-Maldonado AB, Wang B, Lee YC, Zhou J, Lu Z, et al. In situ activation of penile progenitor cells with low-intensity extracorporeal shockwave therapy. J Sex Med. (2017) 14:493–501. 10.1016/j.jsxm.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 119.Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. (2016) 13:22–32. 10.1016/j.jsxm.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 120.Bella AJ, Lin G, Tantiwongse K, Garcia M, Lin CS, Brant W, et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J Sex Med. (2006) 3:815–20. 10.1111/j.1743-6109.2006.00291.x [DOI] [PubMed] [Google Scholar]
- 121.Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. (2006) 3:821–9. 10.1111/j.1743-6109.2006.00292.x [DOI] [PubMed] [Google Scholar]
- 122.Wang B, Ning H, Reed-Maldonado AB, Zhou J, Ruan Y, Zhou T, et al. Low-intensity extracorporeal shock wave therapy enhances brain-derived neurotrophic factor expression through PERK/ATF4 signaling pathway. Int J Mol Sci. (2017) 18:undefined. 10.3390/ijms18020433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JH, Kim SG. Effects of extracorporeal shock wave therapy on functional recovery and neurotrophin-3 expression in the spinal cord after crushed sciatic nerve injury in rats. Ultrasound Med Biol. (2015) 41:790–6. 10.1016/j.ultrasmedbio.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 124.Modena DAO, Soares CD, Candido EC, Chaim FDM, Cazzo E, Chaim EA. Effect of extracorporeal shock waves on inflammation and angiogenesis of integumentary tissue in obese individuals: stimulating repair and regeneration. Lasers Med Sci. (2021) undefined:undefined. 10.1007/s10103-021-03387-x [DOI] [PubMed] [Google Scholar]
- 125.Huang TH, Sun CK, Chen YL, Wang CJ, Yin TC, Lee MS, et al. Shock wave enhances angiogenesis through VEGFR2 activation and recycling. Mol Med. (2017) 22: 850–862. 10.2119/molmed.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tepeköylü C, Wang FS, Kozaryn R, Albrecht-Schgoer K, Theurl M, Schaden W, et al. Shock wave treatment induces angiogenesis and mobilizes endogenous CD31/CD34-positive endothelial cells in a hindlimb ischemia model: implications for angiogenesis and vasculogenesis. J Thorac Cardiovasc Surg. (2013) 146:971–8. 10.1016/j.jtcvs.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 127.Gollmann-Tepeköylü C, Lobenwein D, Theurl M, Primessnig U, Lener D, Kirchmair E, et al. Shock wave therapy improves cardiac function in a model of chronic ischemic heart failure: evidence for a mechanism involving vegf signaling and the extracellular matrix. J Am Heart Assoc. (2018) 7:e010025. 10.1161/JAHA.118.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pfaff JA, Boelck B, Bloch W, Nentwig GH. Growth factors in bone marrow blood of the mandible with application of extracorporeal shock wave therapy. Implant Dent. (2016) 25:606–12. 10.1097/ID.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 129.Zhang X, Yan X, Wang C, Tang T, Chai Y. The dose-effect relationship in extracorporeal shock wave therapy: the optimal parameter for extracorporeal shock wave therapy. J Surg Res. (2014) 186:484–92. 10.1016/j.jss.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 130.Ha CH, Kim S, Chung J, An SH, Kwon K. Extracorporeal shock wave stimulates expression of the angiogenic genes via mechanosensory complex in endothelial cells: mimetic effect of fluid shear stress in endothelial cells. Int J Cardiol. (2013) 168: 4168–77. 10.1016/j.ijcard.2013.07.112 [DOI] [PubMed] [Google Scholar]
- 131.Haake M, Thon A, Bette M. No influence of low-energy extracorporeal shock wave therapy (ESWT) on spinal nociceptive systems. J Orthop Sci. (2002) 7:97–101. 10.1007/s776-002-8429-0 [DOI] [PubMed] [Google Scholar]
- 132.Chen KH, Hsiao HY, Glenn Wallace C, Lin KC, Li YC, Huang TH, et al. Combined Adipose-Derived Mesenchymal Stem Cells and Low-Energy Extracorporeal Shock Wave Therapy Protect the Brain From Brain Death-Induced Injury in Rat. J Neuropathol Exp Neurol. (2019) 78:65–77. 10.1093/jnen/nly108 [DOI] [PubMed] [Google Scholar]
- 133.Sukubo NG, Tibalt E, Respizzi S, Locati M, d'Agostino MC. Effect of shock waves on macrophages: a possible role in tissue regeneration and remodeling. Int J Surg. (2015) 24:124–30. 10.1016/j.ijsu.2015.07.719 [DOI] [PubMed] [Google Scholar]
- 134.Chen JW, Stefanovski D, Haughan J, Jiang Z, Boston R, Soma LR, et al. Inflammatory mediators are potential biomarkers for extracorporeal shockwave therapy in horses. Equine Vet J. (2020) 52:449–54. 10.1111/evj.13183 [DOI] [PubMed] [Google Scholar]
- 135.Iannone F, Moretti B, Notarnicola A, Moretti L, Patella S, Patella V, et al. Extracorporeal shock waves increase interleukin-10 expression by human osteoarthritic and healthy osteoblasts in vitro. Clin Exp Rheumatol. (2009) 27:794–9. [PubMed] [Google Scholar]
- 136.Kim MJ, Yun JM. Molecular mechanism of the protective effect of zerumbone on lipopolysaccharide-induced inflammation of THP-1 cell-derived macrophages. J Med Food. (2019) 22:62–73. 10.1089/jmf.2018.4253 [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Wu Y, Sailike J, Sun X, Abuduwaili N, Tuoliuhan H, et al. Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol Res. (2020) 161:105150. 10.1016/j.phrs.2020.105150 [DOI] [PubMed] [Google Scholar]
- 138.Lobenwein D, Tepeköylü C, Kozaryn R, Pechriggl EJ, Bitsche M, Graber M, et al. Shock wave treatment protects from neuronal degeneration via a toll-like receptor 3 dependent mechanism: implications of a first-ever causal treatment for ischemic spinal cord injury. J Am Heart Assoc. (2015) 4:e002440. 10.1161/JAHA.115.002440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gollmann-Tepeköylü C, Nägele F, Graber M, Pölzl L, Lobenwein D, Hirsch J, et al. Shock waves promote spinal cord repair via TLR3. JCI Insight. (2020) 5:undefined. 10.1172/jci.insight.134552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holfeld J, Tepeköylü C, Reissig C, Lobenwein D, Scheller B, Kirchmair E, et al. Toll-like receptor 3 signalling mediates angiogenic response upon shock wave treatment of ischaemic muscle. Cardiovasc Res. (2016) 109:331–43. 10.1093/cvr/cvv272 [DOI] [PubMed] [Google Scholar]
- 141.Sugioka K, Nakagawa K, Murata R, Ochiai N, Sasho T, Arai M, et al. Radial shock waves effectively introduced NF-kappa B decoy into rat achilles tendon cells in vitro. J Orthop Res. (2010) 28:1078–83. 10.1002/jor.21081 [DOI] [PubMed] [Google Scholar]
- 142.Chai HT, Chen KH, Wallace CG, Chen CH, Sung PH, Chen YL, et al. Extracorporeal shock wave therapy effectively protects brain against chronic cerebral hypo-perfusion-induced neuropathological changes. Am J Transl Res. (2017) 9:5074–93. [PMC free article] [PubMed] [Google Scholar]
- 143.Liu S, Zhai L, Shi Z, Jing R, Zhao B, Xing G. Radial extracorporeal pressure pulse therapy for the primary long bicipital tenosynovitis a prospective randomized controlled study. Ultrasound Med Biol. (2012) 38:727–35. 10.1016/j.ultrasmedbio.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 144.Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. mCurr Med Chem. (2009) 16:2366–72. 10.2174/092986709788682119 [DOI] [PubMed] [Google Scholar]
- 145.Wang HJ, Lee WC, Tyagi P, Huang CC, Chuang YC. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol Urodyn. (2017) 36:1440–7. 10.1002/nau.23141 [DOI] [PubMed] [Google Scholar]
- 146.Yang CH, Yip HK, Chen HF, Yin TC, Chiang JY, Sung PH, et al. Long-term Therapeutic Effects of Extracorporeal Shock Wave-Assisted Melatonin Therapy on Mononeuropathic Pain in Rats. Neurochem Res. (2019) 44:796–810. 10.1007/s11064-018-02713-0 [DOI] [PubMed] [Google Scholar]
- 147.Li H, Zhang Z, Peng J, Xin Z, Li M, Yang B, et al. Treatment with low-energy shock wave alleviates pain in an animal model of uroplakin 3A-induced autoimmune interstitial cystitis/painful bladder syndrome Investig. Clin Urol. (2019) 60:359–66. 10.4111/icu.2019.60.5.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luan S, Zhu ZM, Ruan JL, Lin CN, Ke SJ, Xin WJ, et al. Randomized trial on comparison of the efficacy of extracorporeal shock wave therapy and dry needling in myofascial trigger points. Am J Phys Med Rehabil. (2019) 98:677–84. 10.1097/PHM.0000000000001173 [DOI] [PubMed] [Google Scholar]
- 149.Takahashi N, Wada Y, Ohtori S, Saisu T, Moriya H. Application of shock waves to rat skin decreases calcitonin gene-related peptide immunoreactivity in dorsal root ganglion neurons. Auton Neurosci. (2003) 107:81–4. 10.1016/S1566-0702(03)00134-6 [DOI] [PubMed] [Google Scholar]
- 150.Ohtori S, Inoue G, Mannoji C, Saisu T, Takahashi K, Mitsuhashi S, et al. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neurosci Lett. (2001) 315:57–60. 10.1016/S0304-3940(01)02320-5 [DOI] [PubMed] [Google Scholar]
- 151.Abed JM, McClure SR, Yaeger MJ, Evans RB. Immunohistochemical evaluation of substance P and calcitonin gene-related peptide in skin and periosteum after extracorporeal shock wave therapy and radial pressure wave therapy in sheep. Am J Vet Res. (2007) 68:323–8. 10.2460/ajvr.68.3.323 [DOI] [PubMed] [Google Scholar]
- 152.McClure SR, Sonea IM, Evans RB, Yaeger MJ. Evaluation of analgesia resulting from extracorporeal shock wave therapy and radial pressure wave therapy in the limbs of horses and sheep. Am J Vet Res. (2005) 66:1702–8. 10.2460/ajvr.2005.66.1702 [DOI] [PubMed] [Google Scholar]
- 153.Wang HJ, Tyagi P, Chen YM, Chancellor MB, Chuang YC. Low energy shock wave therapy inhibits inflammatory molecules and suppresses prostatic pain and hypersensitivity in a capsaicin induced prostatitis model in rats. Int J Mol Sci. (2019) 20:undefined. 10.3390/ijms20194777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jung YJ, Park WY, Jeon JH, Mun JH, Cho YS, Jun AY, et al. Outcomes of ultrasound-guided extracorporeal shock wave therapy for painful stump neuroma. Ann Rehabil Med. (2014) 38:523–33. 10.5535/arm.2014.38.4.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Casals-Díaz L, Casas C, Navarro X. Changes of voltage-gated sodium channels in sensory nerve regeneration and neuropathic pain models. Restor Neurol Neurosci. (2015) 33:321–34. 10.3233/RNN-140444 [DOI] [PubMed] [Google Scholar]