Abstract
Pesticides are either natural or chemically synthesized compounds that are used to control a variety of pests. These chemical compounds are used in a variety of sectors like food, forestry, agriculture and aquaculture. Pesticides shows their toxicity into the living systems. The World Health Organization (WHO) categorizes them based on their detrimental effects, emphasizing the relevance of public health. The usage can be minimized to a least level by using them sparingly with a complete grasp of their categorization, which is beneficial to both human health and the environment. In this review, we have discussed pesticides with respect to their global scenarios, such as worldwide distribution and environmental impacts. Major literature focused on potential uses of pesticides, classification according to their properties and toxicity and their adverse effect on natural system (soil and aquatic), water, plants (growth, metabolism, genotypic and phenotypic changes and impact on plants defense system), human health (genetic alteration, cancer, allergies, and asthma), and preserve food products. We have also described eco-friendly management strategies for pesticides as a green solution, including bacterial degradation, myco-remediation, phytoremediation, and microalgae-based bioremediation. The microbes, using catabolic enzymes for degradation of pesticides and clean-up from the environment. This review shows the importance of finding potent microbes, novel genes, and biotechnological applications for pesticide waste management to create a sustainable environment.
Keywords: pesticides, water, plants, DNA damage, cancer, allergy, biodegradation
Introduction
Pesticides are chemical compounds that are used to eliminate insects, rodents, fungi, and weeds. They include insecticides, herbicides, nematicides, fungicides, molluscicides, rodenticides, plant growth regulators, and other compounds (Zhan et al., 2020; Bhatt et al., 2021a; Zhang et al., 2021). It is generally used to prevent illnesses spread by vectors, including crop protection, food preservation, and significant roles in commercial as well as food based industrial practices, i.e., aquaculture, agriculture, food processing, and storage (Mieldazys et al., 2015; Sharma et al., 2019). Any living bodies, either animals or plants, which are harmful for human or animals are known as pests. Pesticides are substances that are used to either kill or prevent the growth of pests.
According to the United States Code of Federal Regulations (CFR), a pesticide is any component or mixture of compounds intended for use as a plant regulator, defoliant, or desiccant (United States Environmental Protection Agency, 2004). Pesticides are defined by the Food and Agriculture Organization (FAO) of the United Nations as substance or mixture of substances attended for controlling, preventing, destroying any pest, animal, or human disease causing vectors, undesirable plants, or animal species affecting food production, managing, selling, storage, and transportation (World Health Organization, 2015). Since ancient times, a variety of chemical compounds have been used to control pests. Sulfur compounds are well known example of such insect and mite control pesticides (Gyawali, 2018). Pyrethrum, a plant (Chrysanthemum cinerariaefolium) derived pesticide, has been used for over 2000 years (Unsworth, 2010). Salty water and chemical compounds (organics as well as inorganic) were widely used to control pests’ populations until the introduction of dichloro diphenyl trichloroethane (DDT) by Paul Herman Muller in 1939 as a potent pesticide (Abubakar et al., 2020). However, use of DDT is helpful to increasing the food productivity and shelf-life of food products. Thus, the global demand for DDT increased day by day, which opened the door to synthesizing new chemical substances that act as pesticides. DDT was replaced by organophosphates (OPs) and carbamates (CMs) in the United States in 1975 (Barnhoorn et al., 2009).
The global pesticide consumption in 2019 was approximately 4.19 million metric tons, where China was by far the largest pesticide-consuming country (1.76 million metric tons), followed by the United States (408 thousand tons), Brazil (377 thousand tons), and Argentina (204 thousand tons) (Fernández, 2021). In southeast Asia, WHO reported an annual increase in pesticide usage with 20% of developing countries as pesticide-consumers, including Cambodia, Laos, and Vietnam (Schreinemachers and Tipraqsa, 2012; Schreinemachers et al., 2015). India belongs to one of the major pesticide producing countries in Asia, having 90 thousand tons annual production of organochlorine pesticides including benzene hexachloride and DDT (Khan et al., 2010; Pozo et al., 2011). Between 2010 and 2014, the average cost/benefit ratio was 0.645 g of total pesticides per kilogram of crop yield, with an average yearly consumption of 2.784 kg ha–1. Japan (18.94 kg ha–1) had the greatest average pesticide usage from 2010 to 2014, followed by China (10.45 kg ha–1), Mexico (7.87 kg ha–1), Brazil (6.16 kg ha–1), Germany (5.12 kg ha–1), France (4.85 kg ha–1), United Kingdom (4.03 kg ha–1), United Status (3.88 kg ha–1), and India (0.26 kg ha–1) (Zhang, 2018).
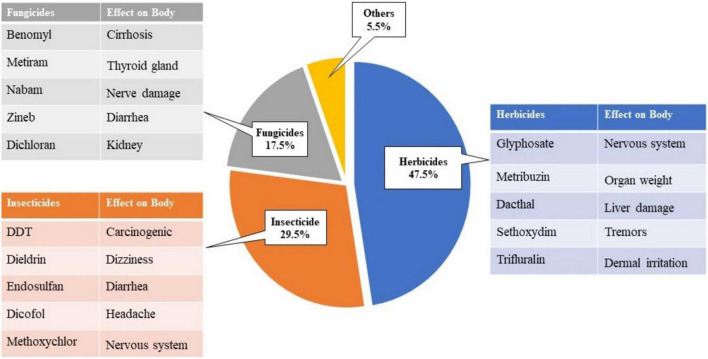
Herbicides account for 47.5% of pesticide contributions, followed by insecticides 29.5%, fungicides 17.5%, and other types of insecticides 5.5%, as shown in Figure 1 (Gill and Garg, 2014; Zhang, 2018; Sharma et al., 2019). Pesticides are classified based on a variety of variables. The most often used criteria for pesticide classification are the mode of entry, chemical makeup, and the target it kills. On the other hand, the WHO and Globally Harmonized System (GHS) classified pesticides based on their toxicity or harmful effects, prioritizing public health.
FIGURE 1.
Percentage distribution of pesticides (Nicolopoulou-Stamati et al., 2016; Alengebawy et al., 2021).
The main advantages of pesticides are the expected immediate gains after application, e.g., eliminating caterpillars, which has the primary benefit of raising cabbage yields and quality. The three major outcomes result in 26 key advantages, ranging from the preservation of recreational grass to the saving of human lives. Secondary benefits are those that arise as a result of the primary advantages but are less obvious or immediate. They might be subtle, less visible at first glance, or long-term in character. As a result, proving cause and effect for secondary benefits is more difficult, although they can still be strong pesticide reasons. Increased productivity of cabbage leads to an increase in economic wealth, which helps to improve children’s health and education systems. Secondary benefits have been identified, including healthier individuals and permanently cultivated land that conserves biodiversity. This accomplishment was aided by the use of high-yield seed types, advanced irrigation technologies, and agricultural herbicides (Bureau, 1993). Similarly, most nations’ productivity and output have increased significantly, such as wheat yields in the United Kingdom and maize yields in the United States. A multitude of factors have been blamed for increased productivity, including better cultivars, machinery use, and fertilizer usage. Pests, insects, diseases, and weeds can substantially reduce the production of harvestable crops; as a result, pesticides have played a crucial role in food production and processing. Warren (1998) also highlighted the huge increase in food production in the United States over the 20th century. Pesticides are used to increase agricultural output and food preservation while ignoring their associated risks. Overuse, exposure, and harmful consequences can all be mitigated by applying it judiciously and utilizing different pesticide categories (World Health Organization, 2009). Many detrimental effects have been seen as a result of widespread pesticide usage, and effective waste management strategies are necessary to address pesticide issues.
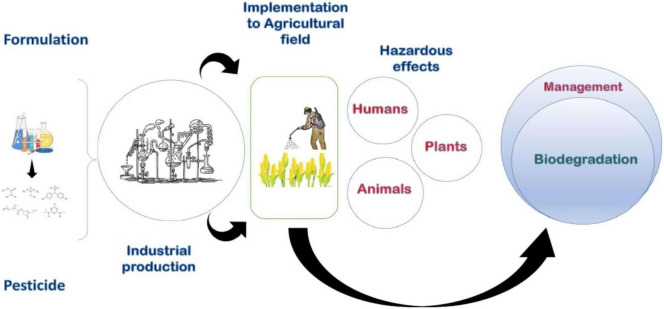
Pesticide biodegradation is a new way of environmentally acceptable pesticide pollution control for a long-term environmental benefit. Microorganisms play a significant role in the breakdown of pesticides and have been recognized for their influence and many uses in human welfare. Several recent studies have demonstrated the potential of microorganisms, isolated from sewage or soil to degrade pesticides. These microbes include several bacterial and fungal strains, actinomycetes, algae, etc. (Kafilzadeh et al., 2015). The process of pesticide biodegradation, which involves bacteria and enzymes, is described in detail in the biodegradation portion of this review. The entire process of pesticide synthesis or formulation, manufacturing or mass industrial production, detrimental effects on the environment and human health, and biodegradation of pesticides has been shown in Figure 2. To date, there is scant information about the detailed classifications, toxicity, and remediation of pesticides in the environment. Therefore, this review article exploring the new dimensions for removal of pesticides from the environment. This review discusses the impact on living systems, bioremediation approaches, and complete residual removal of pesticides from the environment.
FIGURE 2.
Thematic diagram of the synthesis, production, uses, effects, and eco-friendly management of pesticides.
Classification of pesticides
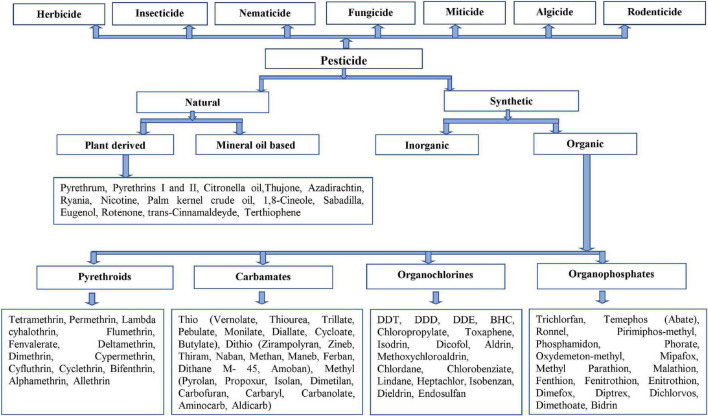
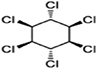
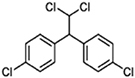
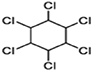
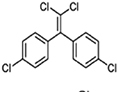
The pesticides show the toxicity in the living systems on the basis of their chemical formulations and quantity in an instance. Pesticides are a broad category of products that include antiseptics, disinfectants, anti-bacterial, fungicides, algicides, rodenticides, and herbicides (Garcia et al., 2012). Pesticides are classified into two major categories based on their physical and chemical properties. Pesticide classification by nature of pesticide (synthetic and natural) and acting on pest type is illustrated in Figure 3. Organic chemicals made up the majority of synthetic pesticides, which were grouped into the following four groups: Organophosphates, organochlorines, carbamates, and pyrethroids. Some widely used pesticides and their structures are shown in Table 1. Naturally occurring pesticides, also known as biopesticides, are formed by living creatures such as plants, bacteria, and fungus (Abubakar et al., 2020; Bhatt et al., 2020a, 2021b).
FIGURE 3.
Classification of pesticides (Jayaraj et al., 2016; Hassaan and El Nemr, 2020; Malhotra et al., 2021; Souto et al., 2021; Parra-Arroyo et al., 2022).
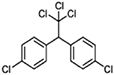
TABLE 1.
Generally used pesticides and their chemical structures.
| Name | Structure | Name | Structure |
| DDT (Dichlorodiphenyltrichloroethane) | Lindane | ||
|
|
||
| DDD (Dichlorodiphenyldichloroethane) | HCH | ||
|
|
||
| DDE (Dichlorodiphenyldichloroethylene) | Chlordecone | ||
|
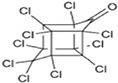
|
||
| Dieldrin | Toxaphene | ||
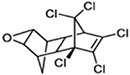
|
|
||
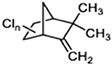
| Aldrin | Mirex | ||
|
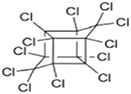
|
||
| Endrin | Endosulfan | ||
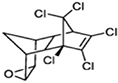
|
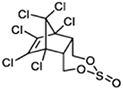
|
||
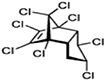
| Heptachlor | Chlordane | ||
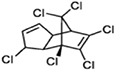
|
|
Classification of pesticides on the basis of toxicity
The amount of pesticides used (dose) and exposure period (time) are the two most important factors for pesticide toxicity that define the acute and chronic toxicity of pesticides. Acute toxicity refers to a pesticide’s toxicity to animals, plants, and humans following a definite short-term exposure of pesticide. A pesticide with a high acute toxicity is fatal, even if only a tiny quantity is absorbed into body. The World Health Organization (WHO) recognizes only acute toxicity for pesticide categorization and based on lethal dosage (LD50) divided into two types, i.e., acute cutaneous (dermal) toxicity (e.g., extremely: less than 50-mg/kg body weight of rat; highly: 50-200-mg/kg body weight of rat; moderately: 200-2,000-mg/kg body weight of rat, etc.) and acute oral toxicity (e.g., extremely: less than 5-mg/kg body weight of rat; highly: 5-50-mg/kg body weight of rat; moderately: 50-2,000-mg/kg body weight of rat, etc.) are shown in Table 2 (World Health Organization, 2009).
TABLE 2.
Pesticides classification according to WHO guidelines (World Health Organization, 2009).
| Class | LD50 of rat | Hazardous level | |
|
|
|||
| Dermal | Oral | ||
| Ia | Less than 50 mg/kg body weight | Less than 5 mg/kg body weight | Extremely |
| Ib | 50–200 mg/kg body weight | 5–50 mg/kg body weight | Highly |
| II | 200–2000 mg/kg body weight | 50–2000 mg/kg body weight | Moderately |
| III | Above 2000 | Above 2000 | Slightly |
| U | 5000 or above | 5000 or above | Unlikely to present acute hazard |
The deadly impact of pesticide exposure that persists over time is known as chronic toxicity. Chronic toxicity of pesticides is a worry for the general population and those who work with pesticides directly because of possible exposure to pesticides. Pesticides are now classified into “WHO Hazard classifications” according to the widely used “WHO Recommended Categorization of Pesticides by Hazard.” Following a change in 2009, such a classification was merged with the “Globally Harmonized System (GHS) Acute Toxicity Hazard Category” shown in Table 3 (Mieldazys et al., 2015). Pesticides are also classified based on pest type, mode of action, and disease management strategies as shown in Table 4. Another type of classification is based on its mode of entry, which is divided into the following five categories: (1) Systemic pesticides (absorbed by animals or plants and transferred to other locations, such as in plants, entering into untreated tissues of roots, stems, or leaves via multidirectional movement through the vascular system), (2) non-systemic or contact pesticides (they require physical contact with the pest for their action), (3) stomach toxicants (it enters the digestive tract and is absorbed inside the insect’s body; such toxicants are effective for vector control and are used for mosquito or black fly management by malathion application), (4) fumigants (these pesticides are used as poisonous gases or vapor that enter the pest respiratory system via spiracles and kill it), and (5) repellents (it is used to keep pests away from treated objects) (Yadav and Devi, 2017).
TABLE 3.
Pesticides classification according to the Globally Harmonized System GHS (World Health Organization, 2009).
| Category | Classification criteria | |||
|
|
||||
| LD50 of rat dermal | Hazardous description | LD50 of rat oral | Hazardous description | |
| 1 | Less than 50 mg/kg body weight | Lethal if come in skin contact | Less than 5 mg/kg body weight | Lethal if consumed |
| 2 | 50–200 mg/kg body weight | Lethal if come in skin contact | 5–50 mg/kg body weight | Lethal if consumed |
| 3 | 200–1000 mg/kg body weight | Toxic if come in skin contact | 50–300 mg/kg body weight | Toxic if consumed |
| 4 | 1000–2000 mg/kg body weight | Harmful if come in skin contact | 300–2000 mg/kg body weight | Harmful if consumed |
| 5 | 2000–5000 mg/kg body weight | Possibly harmful if come in skin contact | 2000–5000 mg/kg body weight | Possibly harmful if consumed |
TABLE 4.
Pesticides classification according to pest type, functions, and management strategies.
| Type of pesticide | Type of pests | Functions | Pests and disease management | References |
| Aldicarb | Nematicides | Inhibit nematodes (plants parasites) | Damage tissue via oxidative stress, and also binds and inhibits acetylcholinesterase (AChE) (controlling acetylcholine neurotransmitter) | Yadav and Devi, 2017; Hassaan and El Nemr, 2020 |
| Insecticides | Inhibit insects and other arthropods also | |||
| Atrazine | Herbicides | Destroy weeds and other plants, photosystem-II (PSII)–inhibiting | Use to control grasses and broadleaf weeds in sorghum, corn, and sugar cane crops | Gupta and Crissman, 2013; Yadav and Devi, 2017 |
| Avitrol | Avicides | Chemicals that lethal to small seed-eating birds | Used for population management of certain birds (crows, gulls, cowbirds, blackbirds, starlings, grackles, pigeons, sparrows, red-winged blackbirds) | Yadav and Devi, 2017; NIPHM, 2018; Hassaan and El Nemr, 2020 |
| Azoxystrobin | Fungicides | Kill fungi (blights, rusts, molds, and mildews), azoxystrobin act fungal mitochondrion, binds to cytochrome bc1 complex and inhibit electron transport thorough oxidative phosphorylation. | Uses to kill Oomycetes, Ascomycetes, Deuteromycetes, BasidiomycetesAnd it controls disease like apple scab rusts, rice blast, powdery and downey mildew. | Yadav and Devi, 2017; Hassaan and El Nemr, 2020 |
| Benzoxazin | Ovicides | Prevention of mites and insects egg growth | In pest managmeent | Yadav and Devi, 2017; Hassaan and El Nemr, 2020 |
| Bifenazate | Acaricides | Control spiders and mites that feed on plants and animals by altering their growth and development. Target site of Bifenazate is mitochondrial, particularly the Qo site of that encoded for cytochrome b | Bifenazate uses as an acaricide on strawberry, flowering plants, and nursery ornamentals | Van Nieuwenhuyse et al., 2012; Hassaan and El Nemr, 2020; Authority et al., 2021 |
| Boric acid | Desiccants | Act on plants by drying their tissues | Use to bed bug control | Hassaan and El Nemr, 2020; United States Environmental Protection Agency [US-EPA], 2022 |
| Copper complexes | Bactericides | Prevent bacteria with greater doses, copper works as a broad-spectrum biocide by interfering with nucleic acids, disrupting enzyme active sites, interfering with the energy transport system, cell membranes integrity disrupted | Copper complexes are used to prevent infection of seedlings from plant pathogens by seed treatment | Yadav and Devi, 2017; Hassaan and El Nemr, 2020 |
| Copper sulfate | Algaecides | Control or kill growth of algae | Alter the algal growth and photosynthesis | Lamichhane et al., 2018; Hassaan and El Nemr, 2020 |
| Dichlorobenzene | Moth balls | Inhibit molds and moth larvae and prevent cloths damage | Commonly used to control moths, molds, and mildew | Yadav and Devi, 2017; Eastmond and Balakrishnan, 2010 |
| Fipronil | Termiticides | Fipronil inhibits termites by acting as a GABA antagonist and leads to excessive CNS excitation and causes death | Used in seed coatings and granular soil treatments to control unwanted arthropods in many kinds of food, horticultural, and turf plants | Cannon and Ruha, 2013; Beasley, 2020; Hassaan and El Nemr, 2020 |
| Methiocarb | Repellents | Repel pest vertebrates and invertebrates by its taste or smell | Use as a seedling bird repellant and also effective against frit fly larvae. | Finch et al., 2014; Yadav and Devi, 2017 |
| Methoprene | Larvicides | Prevents larvae growth | Uses as mosquito larvicide, also effective against horn flies, mushroom flies in compost, dipteran pests of livestocks, nuisance flies, highly selectivity for insects and no acute toxicity is expected in humans | Ramaseshadri et al., 2012; Monteiro and Jurado, 2014; Yadav and Devi, 2017 |
| Metaldehyde | Molluscicides | Prevent mollusk’s (snail’s) usually disturbing growth of plants or crops | Use in vegetables and gardens, to kill slugs, snails, other garden pests | Yadav and Devi, 2017; Hassaan and El Nemr, 2020 |
| Rotenone | Piscicides | Toxic and act on fishes | Uses in fisheries and fish management strategies (where unbalanced population of fish) | Gupta, 2014; Hassaan and El Nemr, 2020 |
| Scytovirin | Virucides | Acts against viruses | Control of viral infections and diseases | Hassaan and El Nemr, 2020 |
| Tebuthiuron | Silvicides | Specific to woody vegetation and act on it | Uses to manage the undesirable plants or unwanted forest species and apply to eliminate trees and brush or “entire forest” | Yadav and Devi, 2017 |
| Trifluromethyl nitrophenol (TFM) | Lampricides | Target larvae of lampreys by uncoupling mitochondrial oxidative phosphorylation and ATP production reduces which ultimately leads to death | TFM used to control invasive sea lamprey (Petromyzon marinus) | Birceanu and Wilkie, 2018; Hassaan and El Nemr, 2020 |
Migration and behavior of pesticides in the ecosystem
When pesticides are administered to a specific area or plant by a farmer, they have the potential to migrate and degrade into the environment and using indigenous microbial strains and physicochemical factors. They show a variety of effects on non-targeted plants as well as kingdom animalia after entering into the ecosystem (Tudi et al., 2021). Pesticides are degraded in our ecosystem by a variety of physical and microbiological processes, including light, temperature, moisture, oxygen, and microorganisms. Pesticides degrade into new chemical entities called metabolites, which can be hazardous or non-toxic depending on their chemical composition (Liu et al., 2015; Marie et al., 2017). Pesticides and their metabolites are transported from a targeted to a non-targeted area via adsorption, leaching, volatilization, or surface runoff (Tudi et al., 2021). Because there is an attraction between soil particles and pesticides in sorption systems (attraction influenced by soil organic matter and soil texture), pesticides linger in the soil for a long period of time and have a harmful effect on the soil and ecosystem (Qin et al., 2014).
Impact of physical and chemical factors on the transformation of pesticides in soil and water
Physical and chemical properties such as molecular weight, ionizability, lipophilicity, polarizability, and volatility of pesticides decide their behavior and biological activity in soil (Bailey and White, 1970; Pignatello and Xing, 1995; Gevao et al., 2000; Beulke et al., 2004). In general, pesticide fate in a soil ecosystem depends on the abiotic transformation related to its physicochemical properties and also on biological transformation related to the presence of live organisms (Różański, 1992). The physical properties make them resistant, reducing losses while chemical structures determine the persistence of pesticides in soil or the environment. These physical and chemical properties of chemical compounds are linked to their movement in soil and aquatic systems and robustness under adverse conditions (Pereira et al., 2016).
Some crucial processes, including adsorption, degradation, and movement, control the behavior and fate of pesticides in soil. Depending on how the pesticide moves, these processes are further classified into leaching, transmission, runoff, microbial and plant absorption. Pesticide transformations in the soil system may vary. Adsorption processes are based on physical forces such as van der Waals or chemical nature, such as electronic interactions (Gevao et al., 2000). Degradation of the pesticides leads to formation of free and bound residues with some altered molecular structures, which are difficult to extract (Roberts, 1984; Gevao et al., 2000). Through diffusion and volatilization, pesticides can dissipate into the atmosphere and wind or runoff leading to subsequent contamination of water bodies. The physical and chemical properties of soil and pesticides, along with other environmental conditions, are mainly responsible for their adsorption by target and non-target organisms, a phenomenon known as bioaccumulation. Chemical and physical characteristics have an impact on leaching, and vertical downward shifting from soil systems. Through the leaching process, pesticides can reach up to groundwater level, making water vulnerable to pollution. Leaching of pesticide into the groundwater in sufficient quantities can pose a hazardous risk to animal and human health. The soil with a sandy nature and low organic content acted as an unstable holding system and weakly absorbed or persistent compounds were most likely to leach-out easily. The chemical, physical, and biological factors of soil with pesticides applied for agriculture practices may influence the leaching process (Steffens et al., 2013). The various agriculture practices are responsible for pesticides translocation in soil or water and the period of their persistence in that environment can be short or longer for weeks, months, or even years due to a number of factors, which include climate change, texture of soil, pH, temperature, moisture, and the content of mineral and organic compounds (Bailey and White, 1970; Gevao et al., 2000; Gupta and Gajbhiye, 2002). Additionally, the leaching and seepage of chemical compounds depends on their mobility as well as persistence, which increases the risk of water pollution (Pereira et al., 2016).
Pesticide impact on the natural system
Pesticides safeguard around a third of all agricultural goods globally, yet their extensive usage has negative consequences for ecosystems (Zhang et al., 2011). Pesticides harm and accumulate in more other places than crops due to poor management/mishandling, or a lack of information (misuse and overuse). Label instructions on how to use and safety recommendations such as donning rubber gloves and protecting eyeglasses from exposure are not effectively followed by users (EPA Common cause of pesticide incidents) (Qu et al., 2019). Pesticides have a wide range of effects on non-targeted creatures, resulting in environmental issues (Rosell et al., 2008). In the case of air pollution by persistence organic pesticide (POP), is caused by ground and spray. Pesticides that are semi-volatile in nature adsorbed on aerosol particles. The half-lives of these particles are few days to more than a month, it depends on gas-phase reactivity. POP (which are present in the air) undergo a transformation from their native form to a highly toxic form via oxidation and photochemical reactions. The migration of these pesticides (POP) depends on the low solubility in water, climate-weather, temperature and humidity (Woodrow et al., 2018). Current use pesticides (CUPs) are more biodegradable in nature as well as less toxic and persistent as compared to previously used organochlorine pesticides (Chen et al., 2020).
Pesticide impact on the soil system
Pesticides are generally used to protect the crop, but there are several ways in which they can also contaminate the soil. Some of the common reasons include inappropriate use, a lack of information on how to use them in terms of amount, a high amount of runoff into water bodies, and pesticides that are adsorbed, desorb, and broken down during their passage through soil, and these phenomena are dependent on pesticide properties such as persistence, bio-accumulation, and toxicity. Because of this process, the soils become secondary sources of the pollutants with respect to air soil exchange (Pokhrel et al., 2018). According to the report, in European countries, the distribution of 76 pesticide residues was evaluated in 317 agricultural top soil samples, either they contained one pesticide or more than one (Silva et al., 2019).
The bioavailability of pesticides in the food web, pesticide uptake, toxic kinetics, dispersion, metabolism, and excretion all have an impact on species. Pesticides are used excessively and arbitrarily on various crop species, causing harm to beneficial biota such as microorganisms, honey bees, predators, birds, plants, and small animals (Alengebawy et al., 2021).
Pesticide impact on the aquatic system
Persistence organic pesticide and CUP pesticides enter into the water bodies through a variety of mechanisms, including atmospheric precipitation, chemical or pesticide manufacturing industries releasing unprocessed chemical waste into running water sources (rivers) and other water bodies, where these pesticides travel for miles and contaminate aquatic or water bodies, negatively impacting aquatic ecosystems (Socorro et al., 2016). These pesticides accumulate and transmit from lower to higher trophic levels in aquatic systems, affecting aquatic flora and fauna directly, from which these pesticides have an impact on human health through intake or other means (Woodrow et al., 2018). Chen et al. (2020) studied the aquatic system of shanghai, China and reported a high concentration of CUP (napropamide, atrazine, and chlorpyriphos).
Effect of pesticide on water eco system
Water is one of the essential elements for all forms of life on earth. About 71% of the water is covered by the earth’s surface. Groundwater constitutes about 30% of the world’s freshwater resources (Marsala et al., 2020). Groundwater quality is under threat due to fast population growth, urbanization, industrialization agricultural pesticides, and population stress (Jayaraj et al., 2016; Wagh et al., 2020). Pesticides may get into groundwater as a result of agricultural runoff from the field or even direct application. The presence of pesticides in water sources is a cause for worry. Pesticides are a type of hazardous chemical that poses a health risk to humans. In many places in the world, groundwater is the most significant source of drinking water. Pesticide pollution is generated from poorly managed agricultural operations and contaminates the surface and ground water. It reduces the quality of drinking water available (Khatri and Tyagi, 2015).
Among the pesticides, organochlorine pesticides (OCPs) have been widely used across the world to control agricultural pests and vector borne diseases (malaria and dengue). Organochlorine pesticides are non-volatile compounds. The problem with using them is that they linger for a long time in natural systems. The use of these substances in an indiscriminate manner has the potential to affect the environment, drinking water systems, and human health. The OCPs’ exposure over time can result in cancer, birth deformities, neurological impairment, reproductive problems, and immune system disease (Agbeve et al., 2014; Fosu-Mensah et al., 2016).
The entry of pesticides into both ground and surface water should be protected. Surface runoff and leaching carry pesticides into water bodies. These pesticides are taken up by plants in the soil, reduced into different chemical forms, and then leached into groundwater. High rainfall increases the risk of pesticides contaminating water. Pesticides that enter groundwater impair the quality of the water, making it unsafe for human consumption as well as for flora and animals. Eliminating pesticides from groundwater is a challenging process. Pesticides in drinking water have negative consequences for both individuals and the ecosystems. According to WHO, around 1 million people are poisoned acutely because of pesticide contact (Hassaan and El Nemr, 2020). To improve production, pesticides will always be a part of human existence and the environment. For pest management, an Integrated Pest Management (IPM) method should be used, which is meant to cause the least amount of environmental disruption by pesticides.
Effects of pesticides on aquatic animals
Pesticide exposure does not just harm target creatures; it also affects a variety of non-target organisms, with fish being the most notable one. Acute exposure to several pesticides resulted in the mortality of fish in certain cases, whereas lower exposure to the same chemicals resulted in deadly alterations. In many species of fish exposed to various pesticides, changes in hematological parameters such as red blood cells, white blood cells, or plasma and serum level modifications lead to histological abnormalities affecting the liver, kidneys, gills, muscles, brain, and gut (Tahir et al., 2021). Furthermore, genotoxicity has been documented in numerous cases caused by several pesticides. Fish are the lowest rung of the aquatic food chain; thus, they mirror the state of water quality and contamination. Submissive phenomena allow them to collect and store compounds such as heavy metals and pesticides, allowing contaminants in their environment to be recognized. Fish ingest a higher amount of pesticide-infected algae, phytoplankton, and other aquatic plants, causing toxic toxins to progressively accumulate in the tissues and organs of the fish. A small number of these compounds can be regulated by metabolism, while the rest bio-accumulate in the organs and organ systems of fish. Different pollutants are absorbed by the fish’s gills, skin, and alimentary canal, which then disseminate into various organs and tissues, altering physiological and natural phenomena (Banaee et al., 2011). Because the gills are completely exposed to water, they are the most polluted organs. Toxicants enter the body through the gills, increasing oxygen demand. As a result, monitoring any hazardous stress in the aquatic environment is an important metric (Panigrahi et al., 2014).
The following components of a global bicycle should be addressed when determining the principal pathways of pesticide exposure to aquatic systems and biota: (1) The water column, which is frequently the first to be exposed to pesticides, (2) Algae, mosses, vascular hydrophytes, leaf litter, and branches are examples of organic substrates, (3) Inorganic substrates ranging from fine silt to coarse sand particles (Murthy et al., 2013). Pesticide levels in interstitial water and sediments are often lower than in the water column, and lithic biotopes are typically less polluted than the standing waters. Pesticides have toxic effects on aquatic creatures, including fish, at sub-lethal and deadly dosages (Khafaga et al., 2020).
Hematological causes by pesticide in fish
Fish hematological research has grown in importance as a reliable and sensitive index for assessing biological and pathological changes caused by natural or anthropogenic factors such as microbial infection or levels of contamination in aquatic sources. As a result, hematological parameters are regarded as a crucial tool for determining the body’s functioning condition in response to various stresses (Ali and Rani, 2009). Pesticides changed the hematological parameters of fish in a relatively short time (Rezania et al., 2018). As a result, the hematologic index may be used to efficiently monitor the health and reaction of fish and aquatic creatures to various toxicants, displaying the ecological position of the environment and a typical way to determine the contaminant’s sub-lethal effects (Pimpao et al., 2007). According to Rios et al. (2002), the blood parameters of fish were altered by several genetical and environmental factors. Pesticides affect a variety of fish characteristics, with a focus on blood parameters.
Pesticide-induced behavioral changes in fish
In several fish species, including Tor putitora and Cyprinus carpio, pesticides can cause schooling behavior, mucus formation via skin’s goblet cells (sliminess), motionlessness, transformations in migration activities, tumbling toward base, jumping, non-responsiveness with hyperexcitability, irregular activities, greater opercular rate (respiration increases), and modifications in body color. Furthermore, they have the ability to change and disturb aquatic vertebrate swimming behavior, such as that of fish and amphibians, as well as impair their growth rates (Stehle and Schulz, 2015). Pyrethroid exposure, decreased the function of the dopamine active transporter, resulting in unpredictable behavior (Wang et al., 2020).
Malformations and reproductive disorders caused by pesticides in fish
Pesticides may cause reproductive issues in brown trout (Salmo trutta) and in Atlantic salmon (Salmo salar) (Jaensson et al., 2007). In addition, additional studies discovered a range of developmental abnormalities in fish exposed to the herbicide (Dawar et al., 2016). Pyrethroids have been found in various studies to be harmful to fish reproductive and early embryonic stages. Pyrethroids such as bifenthrin and permethrin can cause egg proteins (choriogenin and vitellogenin) to be delayed in juvenile fish (Brander et al., 2012). Deltamethrin [second-generation (type II) pyrethroid neurotoxin insecticide] at concentrations of 20 and 40 g/L was shown to be damaging to the development of the swim bladder in zebrafish embryos reported by Wu et al. (2020).
Common effects of pesticides on fish
Pesticides have been shown to have effects on the activity of acetylcholinesterase (AChE), causing an impact on the neurological system and triggering numerous neurotoxic effects (neurotoxicity) in fish (Sharbidre et al., 2011). Fish species such as Rhamdia quelen, C. carpio, Colisa fasciatus, Oreochromis mossambicus, and Labeo rohita are affected by pesticide exposure and have also shown the alteration in AChE activity (Joseph and Raj, 2011). In addition, cypermethrin (CYP) caused neurotoxicity and apoptosis in the Catla catla brain (Jindal and Sharma, 2019). Pesticides also harm fish’s endocrine systems (Brodeur et al., 2013). When used in large numbers, these chemical compounds may induce molecular toxicity in fish such as Cirrhinus mrigala, Carassius auratus (goldfish), and L. rohita (Ullah et al., 2014). According to histopathological examinations, they have a negative effect on the endocrine systems of Oncorhynchus mykiss and L. rohita (Dey and Saha, 2014). Pesticides also cause oxidative stress in T. putitora, Lepomis macrochirus, Hoplias malabaricus, Oreochromis niloticus, Clarias gariepinus, and L. rohita by affecting antioxidant defense enzyme activities and reducing the lipid peroxidation marker malondialdehyde, glutathione-S-transferase, glutathione reductase, and glutathione level (Muthukumaravel et al., 2013).
Effect of chemical pesticides on plants
Nowadays, chemical pesticides are widely used by farmers on agricultural land to control weeds, insects, bacteria, fungus, mollusks, rodents, etc. To combat their needs, an increasing population is demanding more foods. Pesticides are used for better crop production (Tomer, 2013). The pesticide defends crops in agricultural land and also minimizes the risk of damage during post-harvest storage. It is very effective and successful in controlling a number of diseases in plants as well as humans, such as malaria and typhoid, but on the other hand, it decreases the soil quality of agricultural land, which is the reason that their negative effects are kept in mind. In 1960, most of the technologically advanced countries banned or restricted the use of pesticides. Ideally, a synthetic or chemical pesticide must be toxic or lethal to the targeted or non-target species. Because of extensive use of pesticides, the pests and insects are going to develop resistance to transformed pesticides like DDT and escape from it.
Effect of pesticides on vegetables and fruits
The use of pesticides provides a protective layer against pod infection by other pod-feeding insect pests, but damaged pods may not yield seeds or be of poor quality and unfit for use (Mugo, 1998). The usage of chitosan at an early developmental stage boosted plant growth and development and produced higher seed output in rice and soybeans (Chibu et al., 2002). Similar work has been done by Boonlertnirun et al. (2005) in rice and Rehim et al. (2009) in maize and bean.
Pesticides impact on plant growth and metabolism
Although all pesticides are designed to eliminate or prevent certain plant or animal species, it is a great deal to know about the increasing biological as well as physiological effects of these chemicals on their target organisms. Simultaneously, there are many advantages and potential risks to the use of agrochemicals. Chemically treated seeds are often exposed to substantially greater chemical concentrations than the mature plants during cultivation, so these benefits are countered by the danger of phytotoxicity. Herbicides suppress or control plant weeds by a variety of mechanisms with biological processes such as photosynthesis activity, mitosis cell division, function of enzymes, root and leaf development, DNA and protein synthesis, cell membrane destruction, or encouraging uncontrolled growth. The use of pesticides involves a variety of enzymatic and non-enzymatic alterations in biochemical and physiological antioxidants that can have an initial effect on plant growth from germination and ultimately affect the production of plant yield, e.g., vegetables, fruits, and seeds (Choudhury, 2019; Yengkokpam and Mazumder, 2020).
Effect of pesticides on plant growth and development
Plant (crop) growth and development do not proceed normally and lead to growth due to the life cycle of the crop, which increases seed size, dry matter accumulation, food storage material in leaves, stems, fruits and roots (Jan et al., 2012). Despite the fact that plant development is influenced by a variety of environmental, genetic, exogenous, and endogenous variables, as well as hormonal situations. Plant development, on the other hand, is an essential phase in determining their producing capability. Brecke and Duke (1980) introduced glyphosate to reduce leaf dry matter accumulation in Phaseolus vulgaris L. Basantani et al. (2011) observed an overall decrease in germination rate, dry weight, and root length of Vigna radiata after treatment with glyphosate (10 mm). Mishra et al. found that spraying high quantities of pesticides (dimethoate) shortens root and shoot length. Due to increasing levels, dimethoate concentrations in the root are higher than in the shoot (Mishra et al., 2008). Murthy et al. (2005) conducted similar research on Glycin max L.
Effect of pesticides on plant physiology
In the field of pesticide studies, the plant growth is hampered by pesticide accumulation in plants and causes a variety of metabolic disorders, such as chlortoluron affected the plant photosynthetic electron transport chain mechanism (Fuerst and Norman, 1991; Sharples et al., 1997), and Barry et al. (1990) was observed that the PS II reaction center was disrupted. During the photosynthetic pathway, uracil-type herbicides prevent the hill reaction and photosystem II. Reduction of total chlorophyll as well as chlorophyll a, b, and carotenoid content is increased with the increasing application of fungicide doses to plant leaves (Tort and Turkyilmaz, 2003). Sharma et al. (2018a) stated that employment of herbicide causes noxious effects on plants like necrosis, stunting, burns, chlorosis and twisting of leaves. However, Donald (2004) has observed in his experiment that excessive application of pesticides can cause a major reduction in structural vegetation of diversity. Most scientists have been recorded that use of pesticides adversely affects the plant growth and development (Sharma et al., 2015, 2016a,b,c; Shahzad et al., 2018).
Effect of pesticides on plant defense systems
The use of pesticides causes oxidative stress due to the formation of reactive oxygen species (ROS), which can finally lead to growth deficiency and reduced efficiency of photosynthesis in plants. Plants improve the toxicity because of pesticides by increasing the activity of their antioxidative defense system, which includes non-enzymatic antioxidants and antioxidative enzymes (Xia et al., 2009; Sharma et al., 2015, 2017a,b,2018b). Plant proteins, chlorophyll pigments, and photosynthetic efficacy are all reduced by oxidative stress (Xia et al., 2006).
Effect of pesticides on human health
The human body gets exposure to pesticides either directly or indirectly. By using pesticides on crops, humans come in direct contact with them and they affect the skin, eyes, mouth, and respiratory tract, and cause acute reactions such as headache, irritation, vomiting, sneezing, and rashes on the skin. The severity of these pesticides on humans depends upon exposure time and concentration. Generally, pesticides are released from the body in the form of excretion (urinary, biliary, and secretory gland). The consumption of such vegetables and fruits that are grown in pesticide contaminated soil and water used for long-term, accumulation increase the concentration of toxins inside the body organs and causes chronic diseases such as neurotoxicity, cancer, necrosis, asthma, reproductive disorder, cardiac disease, diabetes, etc. (Kalyabina et al., 2021). The quaternary nitrogen compounds such as paraquat are associated with neurodegenerative diseases like Parkinson’s, but their molecular mechanism are still not well known (Franco et al., 2010). Similarly, pesticide group of carbamates inhibits the acetylcholinesterase (AChE) activity and is used as a biomarker of neurotoxicity (Gupta et al., 2016). The cancer problem is caused by the various pesticides, but breast cancer is the most common in all cancer types and is associated with organophosphorus (malathion and parathion) that affect cellular growth and proliferation (Calaf, 2021). Similarly, autoinhibitory M2 muscarinic receptors on parasympathetic neurons that innervate airway smooth muscle are implicated in the case of asthma by organophosphorus (Calaf, 2021). It also reduces fertility and creates genital tract anomalies in both males and females by affecting the action of endocrine hormones, their release timing, and imitating these hormones. According to several studies, organophosphorus reduces paraoxonase activity and increases the risk of coronary artery disease (Kabir et al., 2015). In several African nations, hunger and undernutrition are the most serious concerns.
Role of pesticides in genetic damage
The DNA is an important biomolecule present in living organisms that carries hereditary information and controls the biological synthesis of proteins and enzymes. It acts as the key molecular target of drugs and environmental chemicals such as pesticides. Pesticides interact with DNA and cause conformational changes that could induce gene mutations and lead to adverse health consequences such as carcinogenesis. The acute effects of such chemically synthesized compounds on human health are generally tested and reported before the market launch of these pesticides (Van der Plaat et al., 2018). However, the long-term effect of chronic exposure to pesticides has become a major concern in the last decade.
Pesticide exposure is of the following three types. (1) Direct occupational: Farmworkers who mix and spray the pesticides in agriculture fields; (2) Direct non-occupational: Rural-resident people who live near agriculture fields; (3) Indirect exposure: People who stay far from agriculture areas but get exposed to pesticides through agriculture products, the food chain and contaminated water. Occupational exposure is the most dangerous one as it is linked to a broad range of immediate effects or diseases such as lung disease and airway obstruction. A study conducted in the Dutch population reflects a significant association between the airway obstruction in farmworkers and the corresponding genomic methylation of 31 CpGs (Van der Plaat et al., 2018). Alteration in the genomic methylation pattern affects the expression and repression of genes.
Paredes-Céspedes et al. (2019) reported a notable increase of %5mC in the CpG sites of the WRAP53α gene, “antisense” gene of the p53, in mestizo urban fumigation sprayers who generally use organophosphate insecticides and pyrethroids. Such genetic modifications could act as carcinogenic agents. Differentially methylated CpGs have been found to be unique to the active ingredients of marketed pesticides such as mesotrione, dicamba, acetochlor, picloram atrazine, malathion, glyphosate, and metolachlor (Hoang et al., 2021). Occupational and non-occupational pesticide exposure, as well as chronic and high pesticide exposure in human beings, lead to altered genomic methylation. Various pesticides, including DDT, vinclozolin, methoxychlor, chlorpyrifos methyl, and organochlorine, have been reported to increase or decrease the epigenetic methylation pattern in human beings (Mahna et al., 2021).
The possible genetic damage initiated by occupational pesticide exposure is much greater than that caused by smoking and alcohol consumption (Nascimento et al., 2022). This points to the commonly unacceptable fact that pesticide exposure is much more dangerous than quitting smoking. The random effect of DNA damage in the pesticide-exposed group is roughly 4.63 times more than in the control-exposed group, according to a meta-analytical evaluation addressing probable DNA damage arising from pesticide exposure to farmers (Nascimento et al., 2022). A total of 42 studies were included in the study, with a total number of individuals 2,885 and 2,543 in the exposed and control groups, respectively. In contrast to previous studies, this study found that DNA damage induced by pesticides was not affected by the usage of personal protective equipment, pesticide type, or an individual’s age and gender.
Non-farm employees who reside near agricultural grounds are exposed to pesticides through passive exposure and are thus at risk of pesticide-induced genetic destruction. Non-occupational exposure to pesticides generally corresponds to a high blood concentration of pesticides and increased DNA damage. The pesticides, being oxidizing in nature induces DNA damage via oxidative stress (Doǧanlar et al., 2018). The literature represents that aged people, females, and children are more vulnerable to non-occupational pesticide exposure. Increased micronuclei (MN) numbers, oxidative damage, and strand breaks in DNA were seen in the peripheral blood lymphocytes of toddlers living in pesticide-sprayed areas (Kapka-Skrzypczak et al., 2019).
Non-occupational exposure to pyrethroids, a key pesticide used in agricultural and commercial locations, occurs primarily via residues through contaminated air and diet. The presence of pyrethroids metabolites in the human urine, including CDCCA [cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid], DBCA (cis-2,2-dibromovinyl-2,2-dimethylcyclopropane-carboxylic acid), TDCCA [trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid], and 3PBA (3-phenoxybenzoic acid) provides an indication of non-occupational pesticide exposure. The studies to estimate the effect of non-occupational pesticide exposure on human sperm are generally conducted on men recruited from infertility clinics with normal sperm concentrations. The presence of pyrethroid metabolites in human urine is linked to sperm DNA damage increasing and the quality of semen reduces (Meeker et al., 2008).
A positive association were examined with the medium DNA fragmentation index (M DFI) percentage and CDCCA 450th as well as the percentile of 3PBA 450th and high DNA fragmentation index (H DFI) (Jurewicz et al., 2015). Non-occupational exposure to pyrethroids also increases the risk of sex chromosome disomy in sperm nuclei. Radwan et al. (2015) reported disomy in sperm chromosome YY (3PBA), XY (3PBA, TDCCA), 18 (3PBA, CDCCA), 21 (3PBA), and total disomy (3PBA). Those with higher levels of TDCCA and CDCCA have a consistent increased risk of XY, YY, XX, and disomy in the total sex chromosome (7–30%). Males with higher levels of 3PBA displayed an increased risk of YY disomy (28%), a decreased rate of XY disomy (16%), a decreased total disomy (7%), and an increased chromosome 18 disomy (Young et al., 2013).
In reality, human beings and animals are exposed to multiple pesticides and herbicides simultaneously, which may act independently or interdependently. The pesticides organophosphates (OP) and pyrethroids (PYR) act in synergism to increase the risk of germ cell abnormalities (Figueroa et al., 2019). Earlier, Salazar-Arredondo et al. (2008) also reported the chromatin as well as DNA damage in human spermatozoa caused by in vitro exposure to a mixture of various organophosphorus pesticides including CPO (chlorpyrifos-oxon), CPF (chlorpyrifos), DZO (diazoxon) or DZN (diazinon), and MePO (methyl-paraoxon).
The pesticides cause DNA damage by interacting with the DNA backbone in either of three ways (1) Intercalation, (2) Grove binding, and (3) Methylation. Extensive studies have been reported in the literature that show the type of interaction between DNA and pesticides (Table 5). The genetic damage caused by pesticides is generally studied in animal models such as mice or rats. Dinitroaniline herbicide, pendimethalin (PND), causes significant DNA damage in the liver and kidney cells of treated rats. This damage is shown to disturb the oxidative balance and activate apoptosis genes (Ahmad et al., 2018).
TABLE 5.
Mode of interaction of various pesticides with DNA.
| Pesticide | Pesticide group | Mode of interaction | References |
| Chloridazon or Pyrazon | Organochlorine herbicide | Intercalation via GC region | Ahmadi et al., 2011 |
| Fenitrothion | Organophosphorus insecticide | Partially intercalation via NO2 and the C Form conformation | Ahmadi et al., 2013 |
| Permethrin, deltamethrin | Synthetic pyrethroid insecticides | Groove binding and partial intercalation | Ahmadi and Ghanbari, 2014 |
| Methyl Thiophanate | Fungicide | Non-intercalative groove binding via AT region | Saquib et al., 2010 |
| Propyzamide | Herbicide | Intercalation via AT region | Zhang et al., 2015 |
| Edifenphos | Organophosphate pesticide | Electrostatic binding minor groove binding via AT region | Ahmad and Ahmad, 2018 |
| Tau-fluvalinate, flumethrin | Synthetic pyrethroid pesticide | Hydrogen bonding and Van der Waals forces, minor groove binding via AT region | Tao et al., 2016 |
| Dinitramine | Herbicide | Hydrophobic interactions, major groove binding | Daneshmehr et al., 2016 |
| Resmethrin | Synthetic pyrethroid insecticides | Hydrogen bonds and Van der Waals forces, groove binding via GC region | Tao et al., 2015 |
| Pendimethalin | Herbicide | Intercalation via GC region | Ahmad et al., 2016 |
| Organophosphates | Pesticide | DNA methylation | Paul et al., 2018 |
| Organophosphate, pyrethroids | Fumigation insecticide | DNA methylation | Paredes-Céspedes et al., 2019 |
| Endosulfan | Pesticide | DNA hypomethylation | Mahna et al., 2021 |
| Glyphosate | Pesticide | DNA hypermethylation | Mahna et al., 2021 |
| Diazinon | Pesticide | DNA hypermethylation | Mahna et al., 2021 |
| Fonofos, parathion, terbufos | Pesticide | DNA hypermethylation | Mahna et al., 2021 |
Pesticides’ role in cancer
Several epidemiological and molecular research highlighted a close association between persistent pesticides exposure and increased risk of diseases such as neurodegenerative disorders, endocrine disruptors, respiratory complications, reproductive disorders, and birth defects (García et al., 2017; Larsen et al., 2017; Addissie et al., 2020; Bast et al., 2021; Bhadauriya et al., 2021; Witczak et al., 2021; Gea et al., 2022; Iteire et al., 2022). In addition, the carcinogenic, teratogenic, and mutagenic nature of these compounds are also believed to be a contributing source of cancer development in the human population.
It has been observed that a person with a direct exposure to pesticides is highly susceptible to several human malignancies such as cancer including head, neck, breast, thyroid, brain, colorectal, pancreatic, lung, leukemia, prostate, non-Hodgkin lymphoma and ovarian cancer (Obiri et al., 2013; Pardo et al., 2020; Leonel et al., 2021; Lerro et al., 2021). Several pathways have been discovered to date; however, the major molecular mechanism that is likely to cause pesticide-induced carcinogenesis involves oxidative stress, genetic and epigenetic changes, and endocrine disruptions (Figure 4). For instance, excessive production of ROSs as a result of pesticide exposure can disrupt the cellular equilibrium between pro and anti-oxidant molecules and induce oxidative stress to induce macromolecule damage, leading to dysregulation of several fundamental processes and subsequently stimulating cancer initiation, growth, progression, metastasis, and chemotherapeutic resistance (Pardo et al., 2020; Leonel et al., 2021; Lerro et al., 2021).
FIGURE 4.
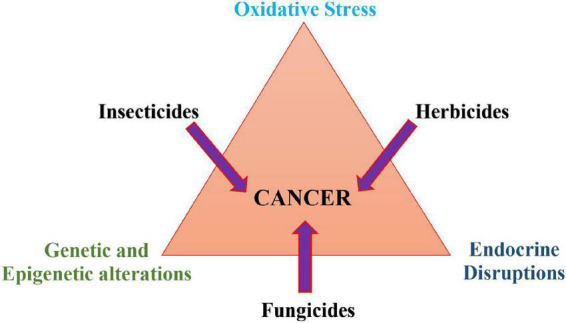
Major molecular mechanisms associated with pesticides-induced carcinogenesis.
In a study by Želježić et al. (2018), herbicide terbuthylazine exposure was reported to form reactive terbuthylazine metabolites, which induce DNA cross-links in both in vitro and in vivo systems. Thakur et al. (2018) reported that oxidative DNA damage induced by two extensively used organophosphate pesticides, monocrotophos and chlorpyrifos, modulate the AP endonuclease 1-dependent base excision repair pathway to promote the proliferation of lung cancer. Similar toxic effects were also observed for widely used insecticide, neonicotinoid (dinotefuran, nitenpyram, and acetamiprid) exposure, which resulted in disturbance of amino acid metabolism, accumulation of lipids, and enhance oxidative stress in ICR mice via decreasing glutathione (GSH) level and increasing superoxide dismutase (SOD) level (Yan et al., 2020). Polymorphism in oxidative stress-related genes (catalase, glutathione peroxidase, glutathione-S-transferases, manganese superoxide dismutase, and paraoxonase) may not be directly linked to cancer; instead, they make people more vulnerable to pesticide-induced oxidative stress (Kaur et al., 2018; Moradi et al., 2018; Costa et al., 2019; Mbah Ntepe et al., 2020).
Endocrine disruptions are caused by agents/EDCs (endocrine disrupting chemicals) that affect the natural function of the endocrine (hormone) systems of a body by disrupting the synthesis, release, binding, specific activity or abolition of normal hormone, which are responsible for the growth, development, fertility, and homeostasis maintenance of a cell. Pesticides are well known for disrupting endocrine function via mimicking or delaying the release of natural hormones, thus, being accountable for decreased fertility, neurological or behavioral dysfunctions, thyroid gland abnormalities, immunosuppression, and carcinogenesis (Kori et al., 2018; Pizzorno, 2018; Requena et al., 2019; Brandt et al., 2020; Montes-Grajales and Olivero-Verbel, 2020). Most of the pesticides work as agonists to activate numerous hormone receptors for instance androgen receptors, estrogen receptors, pregnane X receptors, nuclear hormone receptors, and aryl hydrocarbon receptors (Eve et al., 2020; Lacouture et al., 2022). Low dose of phenolic EDCs upregulated aromatase signaling and thus regulated aromatase-induced 17β-estradiol biosynthesis to support breast cancer cells proliferation (Williams and Darbre, 2019). Furthermore, thiacloprid and imidacloprid exposure stimulates CYP19 promoter activity, which increases estrogen biosynthesis in vitro in a similar manner to hormone-dependent breast cancer (Caron-Beaudoin et al., 2018). Recently, antagonistic effects of pesticides have also come into focus. For example, cypermethrin showed an inhibitory effect on the dihydrotestosterone activated interaction of the androgen receptor with its coactivators ARA70 and ARA55 (Zhen et al., 2020). Zhang et al. (2018) discovered a novel mechanism of endocrine disruption, where 16 pesticides showed anti-mineralocorticoid activity, among which 14 interfere with nuclear translocation of the mineralocorticoid receptor to promote hepatocellular carcinoma. Another novel pathway involves fungicides (Prochloraz, vinclozolin, and M2) competing with the androgen receptor, ZIP9, to block pro-apoptotic signaling in prostate cancer cells (Thomas and Dong, 2019). In another study, glyphosate was reported to inhibit aromatase signaling in a non-competitive manner while imidacloprid and thiacloprid inhibited estrogen receptor activity in MELN cells (Zhang C. et al., 2020). Overall, we observed that pesticides can alter the cellular metabolism in multiple ways to induce cancer risk. It was also observed that a person with direct or occupational exposure along with inherent genetic susceptibilities is more prone to disease.
Pesticide exposure causes allergies and asthma
The salubrious nature of pesticides makes them ideal candidates for modern agriculture techniques and enhanced crop-production. However, extensive usage of pesticides leads to serious health conditions due to their bio-magnification and persistent nature (Sharma et al., 2019). The vapors of pesticides can invade water, soil, air and finally enter the food chain, thereby threatening to human health (Sharma et al., 2017c). It has been found that food contaminated with pesticide residues leads to a higher level of toxicity compared to drinking or inhaling contaminated water or air (Margni et al., 2002). Pesticides can mimic or antagonizes natural hormones, thus disbalancing hormonal homeostasis, reducing immunity, causing cancer and other reproduction-related problems (Yadav et al., 2015).
Studies have reported that acute or chronic exposure to such pesticides leads to airway diseases such as allergic rhinitis or asthma. The population at high-risk of developing health issues due to pesticide exposure includes mainly farm workers, pest control workers, or workers from agricultural industry, and the other environmentally exposed individuals residing near farms or agriculture fields or the individuals exposed to household pesticides (Ernst, 2002; Ndlovu et al., 2011).
More evidence of exposure to pesticides has been reported among farmers and their families along with insecticide producers or applicators across the globe, such as the United States, Canada, France, and Australia, with increased asthmatic conditions (Baldi et al., 2014). Such exposures may lead to decreased FEV 1 (forced expiratory volume in 1 s) of forced breath with exacerbation of asthma and also induction of autonomic function and altered immune response (Osteen and Fernandez-Cornejo, 2013; Henneberger et al., 2014). In relation to the use of domestic pesticides, exposure to insecticides has a particularly important role in the induction and worsening the asthma and asthma-like syndrome (Osteen and Fernandez-Cornejo, 2013). In countries such as the United States, where asthma morbidity is high due to cockroach sensitization, insecticides are used to control exposure, which in turn increases pesticide exposure, and asthma morbidity (Garthwaite et al., 2012).
Another study on farm operators showed a significant association between current asthma and lifetime allergic rhinitis by the use of carbaryl and 2,4-dichlorophenoxyacetic acid. Approximately 40% of 2.1 million farm operators had lifetime allergic rhinitis in 30% farmers and 5.1% has current asthma (Patel et al., 2018). Some synthetic insecticides, such as pyrethroid, used to control mosquitoes are known to cause asthma attacks, while permethrin and Sumithrin are key contributors to headaches, tremors, convulsions, asthmatic attacks, and can be lethal in more serious conditions (EPA et al., 2009; Amaral, 2014). Not much is known about specific pesticides responsible for allergic/asthmatic exposure. Studies from Canada, Spain, India, or South Africa demonstrated that pesticides belonging to class organophosphates and carbamates are particularly involved in causing asthmatic conditions (Hernández, 2015). These studies mainly performed lung function assays such as spirometry, and lung volumes/capacity, but none has involved primary inhalation challenge testing.
Effect of pesticides on asthma
Pesticide use and asthma incidences were reported in the common people as reported by some of the studies performed in the United States population. The US urban population was found to be chemically intolerant to at least three commonly used chemicals such as paints, pesticides, perfumes, or car exhaust. Subjects reported asthmatic and respiratory symptoms such as shortness of breath with wheezing and chest tightness (Baldwin et al., 1997; Amaral, 2014). A cross-sectional study of US National Health and Nutrition showed an association of residential pesticides with respiratory problems in children, mostly used in the kitchen or dining area (Xu et al., 2012). The incidence of such residential exposures has increased in the United States from 1.1 to 4.4 per million (Amaral, 2014; Hudson et al., 2014). Indoor air pollution, caused by pesticide spraying or the use of over-the-counter insecticides, has exacerbated symptoms such as irritation, lower respiratory pain, wheezing, dyspnea, and dry cough. In a randomized investigation of 25 asthmatic participants exposed to modest amounts of aerosols, asthmatic symptoms worsened when compared to a control group (given water). Asthmatic patients had a more than 15% decrease in FEV1 and severe bronchial responsiveness, with symptoms affecting the chest, nose, and eyes (Salome et al., 2000).
Previously, it has been reported that allergic asthma was relatively more common in children than in adults. The risk of environmental exposure to pesticides was higher for school children, especially those living near farms or rural areas (Matthews, 2005; De Barros Rodrigues et al., 2022). Children with acute symptoms have been reported due to pesticide drift near their schools, or they might be at even higher risk because of accidental contact while playing on agriculture farms with empty containers of contaminating materials (Buralli et al., 2020). In a longitudinal study, children living in agricultural communities had higher amounts of the dialkylphosphate (DAP) metabolite in their urine. The DAP metabolites are general to organophosphorus pesticides and are responsible for the temporal pattern of children’s pesticide exposure upon pesticide spraying in an agricultural region (Koch et al., 2002). Other factors for children’s hospitalization related to pesticide exposure are their increased respiratory rate, comparatively larger surface area of skin, and elevated metabolic rate (Sharma et al., 2019).
A few studies investigated the role of allergic asthma as well as other respiratory symptoms due to pesticide exposure among women. The studies were mainly focused on male workers, associated directly or non-directly with agricultural fields, but it was evident that women are also increasingly affected and at high-risk due to pesticide exposure (Ndlovu et al., 2011). In a study, Hoppin et al. (2008) evaluated pesticide and occupational exposures as risk factors for farm women. Out of 25,000 women with atopic and non-atopic asthma, who grew up on farms and used pesticides, were more likely to develop atopic asthma than the non-users. In an infant’s environmental health birth cohort study of 266 mothers in Costa Rica, by performing a survey, they investigated the outcomes of respiratory and allergic conditions in mothers upon exposure to pesticides and other environmental metabolites. The study found significant association of high asthma score and urinary levels of thiabendazole metabolite in women living near waste burning farms and women living in agriculture farms reported eczema and itch rash (Garry, 2004; Alhanti et al., 2021). Another study linked pesticide exposure to changes in the serum metabolome after eating fruits and vegetables (FVs). The study analyzed 171 women under infertility treatment and showed significant associations of metabolic pathways upon the eating of either high or low-to-moderate pesticide residue FVs. Different biological pathways were associated with the intake of high or low pesticide residues, including metabolism (energy, cellular receptor, enzyme, lipid, and vitamin) and intracellular signaling (Hood et al., 2022). There is a need to perform more such unique studies about associations between environmental and occupational pesticide exposures and respiratory and allergic diseases. Such an insightful study related to dietary intake of pesticides might provide information on potential mechanisms associated with human diseases.
A link between food allergies and pesticides
Food allergy affects up to 10% of the world population, with more severity in infants as compared to adults. It has been referred to as the “second wave” of the allergy epidemic, following asthma (Loh and Tang, 2018). In parallel, the use of pesticides such as organophosphates has been increased in agriculture and industries. This increased use of organic agents might prolong the allergic manifestations in atopic individuals by potential mechanisms such as epigenetic control of allergen expression, modifying proteins to make them even more allergenic; or increased polyamine production in stressed condition (Falak et al., 2012; Loh and Tang, 2018).
People who are exposed to chemicals either through chlorinated water or come into contact with foods that contain them or breathe polluted air are more likely to develop food allergies. Chemicals like dichlorophenols can alter the microbiota of the human body and in turn influence the body’s immune system to trigger such reactions. In contrary to hygiene hypothesis, dichlorophenols can kill microbes and clear the environment such that young children become prone to developing allergy risks. In an international survey of the United States (NHANES) in the period 2005–2006, 2,200 children aged 6 were checked for dichlorophenol levels in their urine along with allergies to peanuts, eggs, milk, and shrimp. It was found that children with high levels of urine dichlorophenol were 80% more likely to develop allergies (Jerschow et al., 2012).
An ample number of studies have been performed related to pesticide exposure and asthma, but a lot more meticulous studies need to be accomplished. The previous data was generated accordingly self-reported or doctor-diagnosed asthma, which needs to be refurbished with bronchial responsiveness measurements and lung function. To strengthen the data, a detailed molecular and genetic phenotyping must be explored to study the effect of pesticides in different types of asthmatic conditions (Jerschow et al., 2012; Loh and Tang, 2018). Studies on different active and organic ingredients or new formulations along with potent agents might provide important insights, such as between asthma and exposure to pesticides. The recent cohort studies identified certain biomarkers directly linked to pesticide exposure and asthma, thus new biomarkers for the different and generally used pesticides can be considered. More robust measurement of pesticide exposure depending upon the biomarkers should be the focus of the future comprehensive studies. Their metabolic rate, bioactivity, life time, and threshold levels must be recorded to understand the pathophysiology of the underlying asthmatic or atopic conditions. Finally, more longitudinal studies offering a large sample size over a longer period of time can be a big step toward understanding the biological pathways at the gene level that can directly link pesticide exposure to disease development.
Pesticide effects on preserved food
Pesticides play a global role in the protection, preservation, comfort of food, fiber, and human health (Winteringham, 1971). However, the excessive and uncontrolled use and misuse of pesticides, as well as their long-run transportation and volatility, cause widespread environmental damage or contamination. Moreover, the occurrence of many highly toxic, non-patented, and eco-resistant chemicals creates severe health concerns that causes global impact simultaneously (Ecobichon, 2001). In India, the value addition and processing of ready-to-eat (RTE) or ready-to-serve (RTS) packaged products impact a lot on monitoring the levels of pesticide residues during the final consumption. However, during the processing of raw agricultural commodities (RAC), the levels of pesticides are mostly governed by the concentration level and physico–chemical characteristics of the product to be processed (Muralidhara et al., 2022). Researchers reported that pre- or post-processing steps are capable enough of reducing the load of pesticides in the final product. However, in certain specific cases, processing aids in the accumulation of pesticide residues (e.g., extraction of oil from oil seeds) (Kaushik et al., 2009; Muralidhara et al., 2022). Therefore, a maximum residual limit (MRL) of pesticides needs to be established in the case of food products attaining paramount exposure to pesticides during their pre-harvesting phase (Scholz et al., 2017).
Processing factor (Pf) – During the processing of foods, there is a chance that the whole mass of pesticide residues can be assimilated into processed products. Therefore, the effect of pesticide residues on food products can be expressed by a term “processing factor” and can be calculated as follows.
The processing factor is an integral tool to generate data for global regulatory authorities monitoring the residual limits and also helps in assessing the risks by estimating the refined dietary exposure of pesticides in a processed food commodity before consumption (OECD, 2008).
Effect of pesticide residue on processing operations
Processing operations play a significant role in maintaining or lowering the pesticide limit in the final value-added processed products aiding enhanced shelf-life and better product quality; however, certain processing steps impact negatively by enriching the level pesticide residues in the final product by developing toxic metabolites or second- and third-generation derivatives. Post-harvesting operations such as washing, peeling, chopping, etc. help in reducing the pesticides on the surface of fruit and vegetable commodities (Yigit and Velioglu, 2020). The heat treatments such as pasteurization, sterilization, blanching, frying, boiling, cooking, etc. help in the reduction of pesticides by chemical reactions due to oxidation and hydrolysis of chemical compounds. Also, low moisture content, pH, and time–temperature combination during cooking also modulate the residual pesticide limit in the final product. Similarly, unit operations such as drying and grinding of samples, canning of food products, etc. abundantly reduce the residual limits by evaporating water and altering the physico–chemical nature of pesticides (Kaushik et al., 2009). However, the unit processing operations such as cereal grain processing, fruit processing, oil extraction, grape, egg drying, and so on have a high risk of increased levels of residual pesticides and are affected by a variety of factors such as the physico–chemical behavior of pesticide molecules, produced metabolites during the chemical process, their photostability, lipophilicity, thermal stability, and polarity (Scholz et al., 2017).
Determination of pesticide residues in food matrix
The determination of the residual pesticide limit in RTE/RTS foods involves a complex phenomenon and requires some special criteria. The extractability of a pesticide residue depends on the biochemical nature and behavior of food. The complexity of a matrix behavior is often increased by the processing operations involved, which impacts the performance method by decreasing precision as well as accuracy. Therefore, usage of matrix-matched calibrations and selective clean-up practices are necessary to avoid such issues (Law et al., 2019). The worldwide harmonization of maximum residual limits (MRLs) for pesticide residues in raw agricultural commodities has attained a high recognition. Similarly, in India, food technologists and central agency such as Food Safety and Standard Authority of India (FSSAI) are now emphasizing too much toward a sustainable growth in the processed food sector for making and consumption of value-added items with safe or lower residual limits of pesticides (Muralidhara et al., 2022).
Eco-friendly management of pesticides as bioremediation
Physical and chemical cleaning of pesticides release more toxic compounds, and both are harmful as well as costly. To maintain a sustainable environment with a healthy and productive ecosystem, eco-friendly approach as bioremediation methods is available to remove harmful contaminants (Desisa et al., 2022). Since plants, algae, fungi, bacteria, and their interactions are used to remove toxins via bioremediation, which serves as a cost-effective and environmentally benign method. Pesticide remediation today includes a variety of environment friendly techniques, such as phytoremediation, microalgae bioremediation, myco-remediation, and bacterial pesticide degradation (Singh et al., 2020).
Phytoremediation is an economical, solar-powered method that involves the removal or reduction of harmful chemicals from damaged sites using effective plant species. Kochia sp., Triticum spp., Ricinus communis and Ceratophyllum demersum are well-known plant species that have played a significant role in the removal of atrazine, lindane, chlorpyrifos, and endrin, respectively. The absorption of pesticides by plants results in the conversion of hazardous pesticides into less toxic compounds, which helps to remove toxic pollutants from polluted sites. Plants use various mechanisms to remove pollutants, including pollutant transpiration (phytovolatilization), clean-up through the rhizosphere microbiome (rhizo-degradation), enzymatic degradation (phytodegradation), and pesticide accumulation in different plant parts (phytoextraction). Such plants also improve the landscapes, reduce soil erosion, and prevent pollutant seepage. In addition, phytoremediation serves as an economic, safe, and green approach for chemical waste treatment (Subashini et al., 2007; Gill and Garg, 2014; Mishra et al., 2015; Rissato et al., 2015; Kuppusamy et al., 2016; Main et al., 2017; Mir et al., 2017; Koranteng et al., 2018; Perez-Lucas et al., 2018; Singh et al., 2020).
Microalgae are also known as effective biosorbents of heavy metals and pesticides and can remove them from contaminated areas. Chlamydomonas reinhardtii, Chlamydomonas mexicana, and Dunaliella sp. have been reported for the removal of prometryne, atrazine, and mirex pesticides, respectively. Such photoautotrophic organisms exist in different forms in nature and are involved in the conversion of radiant energy (light energy to chemical energy). The use of microalgae results in the production of oxygen, which preserves the environment’s balance. Oxygen generated from microalgae also helps the bacteria during the biodegradation process. Microalgae have been found to use chemical pollutants as an energy alternate and to accelerate the biodegradation process. It can be used to achieve a variety of objectives, including nutrient recovery from wastewater, biomass formation, removal of contaminants (bioaccumulation and biosorption), and being able to grow under stress conditions. In which, bioaccumulation is an energy-dependent active process involving living organisms that metabolize pollutants. Whereas biosorption is an energy-independent process that involves both dead and living organisms for the removal of contaminant form polluted environments. The use of such technology in a two-way manner, such as pesticide accumulation as well as conversion of toxic into less toxic compounds. The degradation is influenced by the introduction of potent microalgae, optimum conditions, and the chemical composition of pesticides. In addition, there are some major factors that alter the degradation process of pesticides, such as molecular weight, functional group, concentration, and water solubility. Under stress conditions, these microalgae act mixotropically and derive their energy from light and organic carbon, which gives them an advantage over bacteria and fungi during biodegradation (Velasquez and Dussan, 2009; Chojnacka, 2010; Mata et al., 2010; John et al., 2011; Subashchandrabose et al., 2011; Monteiro et al., 2012; Rath, 2012; Chekroun et al., 2014; Kabra et al., 2014; Torres et al., 2017; Singh et al., 2020).
Myco-remediation is another type of biological approach to pesticide waste management, where fungi can use such pollutants as a carbon source and convert them into less toxic compounds, thus cleaning them from the water and soil system. Fungi are ideal among microorganisms due to their structural morphology, which contains hyphae, that allows the transfer of small chemical molecules by microscopic pores easily. The mycelium networks have a multi-functional role, in addition to accelerating pesticide degradation, they also improve the plant’s nutrient and water availability. Ligninolytic fungi are known to secrete a variety of extracellular enzymes that aid in the transformation of recalcitrant chemical compounds. While saprotrophic fungi excrete the most enzymes, followed by other fungi (soft rot, white rot, and brown rot). White-rot fungi (P. Pleurotus ostreatus, Trametes hirsutus, and Cyathus bulleri) are widely known for pesticide biodegradation due to their extracellular enzyme complex (e.g., laccase, manganese peroxidase, and lignin peroxidase) acting non-specifically. The consortium of potent fungal species was found to be suitable for chlorpyrifos and DDT biodegradation. The phyla Zygomycota, Ascomycota, and Basidiomycota are reported for biodegradation via attacking on functional groups (dehydrogenation, demethylation, hydroxylation, etc.). This process is also influenced by other factors such as optimal temperature, pH, moisture, nutrient, and water availability, all of which play a significant role in pesticide degradation. Nowadays, many developing countries cannot afford biopesticides or cannot avoid the use of chemical pesticides, so they need to use myco-remediation or other bioremediation approaches to control pesticide pollution in a parallel manner (Tortella et al., 2005; Huang et al., 2008; Sagar and Singh, 2011; Adenipekun and Lawal, 2012; Chen et al., 2012; Wu et al., 2015; Maqbool et al., 2016; Janusz et al., 2017; Singh et al., 2020).
Bacteria have been widely reported to degrade and remove pesticides as compared to other remedial approaches. Pseudomonas, Azotobacter, Flavobacterium, and Arthrobacter are the major bacterial genus involved in the removal of pesticides from polluted environments. The discovery of pollutant-degrading bacteria aided by advances in genetic engineering methods. These microbes use the pesticide for nutrients, generate H2O and CO2, and overcome the environmental risk associated with pesticides. In the soil system, such pesticides accumulate and act as electron donors and carbon sources for soil microorganisms. The environmental conditions, pesticide exposure time, and concentration, bacterial type, and growth factors (such as temperature, pH, moisture, nutrient, and water availability) all are important for efficient biodegradation. However, the presence of sulfate and chloride act as anion and bind strongly to microbes that blocks the microbial action on pesticides. The chemical structure is the first target of microbial degradation and converted into inorganic components that are further utilized by the microorganism. Advanced approaches such as bioaugmentation, bio-stimulation and natural attenuation are employed to increase the pesticide biodegradability, which includes potent bacteria, nutrient addition, and the introduction of native species to the contaminated site respectively. Alcaligenes, Flavobacterium, Acinetobacter are reported as endosulfan degrading bacteria. Similarly, Stenotrophomonas sp. also known for almost 100% removal of diazinon from the contaminated site. The bacterial system is well studied as compared to other bioremediation technologies. The diverse bacterial groups and their corresponding enzymes responsible for degradation are explained in the “Biodegradation of Pesticide Pollutants” section (Gavrilescu, 2005; Singh and Walker, 2006; Arias-Estevez et al., 2008; Huang et al., 2008; Singh et al., 2011, 2020; Laura et al., 2013; Rani and Dhania, 2014; Adams et al., 2015; Deng et al., 2015).
Biodegradation of pesticide pollutants
Biodegradation of pesticides is mainly mediated by using microbial systems. Microbes are able to produce a specific group of enzymes that are able to catalyze the pesticides from contaminated sites. The pure culture and mixed cultures of the bacteria and fungi were found to be effective in the removal of pesticide residues from the water and soil environment. Microbial consortium was found with superior degradation abilities (Bhatt et al., 2021c). Singh et al. (1999) found that microbes have developed a number of metabolic routes to breakdown or detoxify a variety of environmental contaminants, including pesticides. Conde-Avila et al. (2021) reported bacteria from the genera Streptomyces, Flavimonas, Burkholderia, Micrococcus, Sphingomonas, Brevibacterium, Flavobacterium, Pseudomonas, Agrobacterium, Arthrobacter, Enterobacter, and Bacillus are associated with pesticide biodegradation. There is a diverse group of bacteria and fungi that are capable of degrading pesticides. The different phyla include Bacteroidetes, Basidiomycota, Chlorophyta, Cyanobacteria, Actinomycetota, Firmicutes, and Proteobacteria. The bacteria that fall under Actinobacteria have a tremendous capability to degrade several classes of chemical pesticides as most of the strains have high GC content and are actively used for the recycling of complex polymers. Streptomyces, Nocardioides, Arthrobacter, Rhodococcus, Micrococcus, and Microbacterium are members of the Actinomycetota phylum and can metabolize a variety of chemical compounds such as organochlorides, organophosphates, carbamates, triazinones, and others (Kim et al., 2017). Similarly, Firmicutes are also play a critical role in pesticide biodegradation. Among them, several strains possess endospores that are resistant to any adverse condition and are reported as extremophiles. There are a number of firmicutes that are capable of degrading pesticides, including Paenibacillus polymyxa, Bacillus licheniformis, Bacillus thuringiensis, Bacillus pumilus, Bacillus subtilis, and Bacillus cereus (Patil et al., 1970). Moreover, among the proteobacteria, α-, β-, and γ-proteobacteria have also been reported for their pesticide degradation activity.
Among the α-proteobacteria strains that have been reported are Sphingomonas, Rhizobium, Methylobacterium, Azospirillum, Pseudaminobacter, Bosea, Mesorhizobium, Shinella, and Ochrobactrum. Moreover, Ralstonia, Alcaligenes, Burkholderia, Achromobacter, and Cupriavidus are the reported bacterial strains among β-proteobacteria. Furthermore, reported bacterial strains among γ-proteobacteria are Yersinia, Pseudomonas, Klebsiella, Acinetobacter, Serratia, and Xanthomonas (Bhatt et al., 2020b; Kumar et al., 2021). Microbes and their enzymes associated with biodegradation of different types of pesticide are shown in Tables 6, 7.
TABLE 6.
Pesticides degrading microorganisms.
| Type of pesticide | Example | Microorganism | References |
| Organophosphorus | Chlorpyrifos | Bacillusspp., Pseudomonasspp., Arthrobacterspp., Micrococcus, Flavobacterium, Bacillus licheniformis, Cupriavidusspp., Burkholderia caryophylli, Brevundimonas diminuta, Spirulina platensis, Synechocystis | Nandhini et al., 2021; Lourthuraj et al., 2022 |
| Organophosphorus | Parathion | Pseudomonas diminuta, Flavobacteriumspp., Pseudomonas stutzeri, Arthrobacterspp., Agrobacterium radiobacter, Bacillusspp., Xanthomonasspp. | Mali et al., 2022; Saravanan et al., 2022 |
| Organophosphorus | Methyl parathion | Pseudomonasspp., Bacillusspp., Plesiomonasspp., Pseudomonas putida, | Dong et al., 2005; Singh and Walker, 2006; Parakhia et al., 2014 |
| Glyphosate | Pseudomonasspp., Alcaligenespp., Bacillus megaterium, Rhizobiumspp., Agrobacteriumspp., Arthrobacter atrocyaneus, Geobacillus caldoxylosilyticus, | Huch et al., 2022; Zhang et al., 2022 | |
| Organophosphorus | Coumaphos | Nocardiodes simplex, Agrobacterium radiobacter, Pseudomonas diminuta, Pseudomonas monteilli, Flavobacteriumspp., Nocardiodes Strain B-1 | Singh and Walker, 2006; Blatchford et al., 2012 |
| Organophosphorus | Monocrotophos | Pseudomonasspp., Bacillus subtilis, Arthrobacterspp., Peudomonas mendocina, Bacillus megaterium, Arthrobacter atrocyaneus, Pseudomonas aeruginosa, Clavibacter michiganense | Kaur and Goyal, 2019 |
| Organophosphorus | Fenitrothion | Flavobacteriumspp., Arthrobacter aurescenes; Burkholderiaspp. | Singh and Walker, 2006; Hong et al., 2007 |
| Organophosphorus | Fenthion | Bacillusspp. | Aislabie and Lloyd-Jones, 1995 |
| Organophosphorus | Diazinon | Flavobacteriumspp., Pseudomonasspp., Arthrobacterspp. | Aislabie and Lloyd-Jones, 1995; Singh and Walker, 2006 |
| Organophosphorus | DDT | Alcaligene eutrophus | Aislabie and Lloyd-Jones, 1995 |
| Organochlorine | Aldrin | Micrococcus, Bacillus polymyxa, Flavobacteria, Pseudomonas fluorescens, Phlebia aurea, Phlebia acanthocystis, Phlebia brevispora | Bose et al., 2021 |
| Organochlorine | Dieldrin | Pseudomonas fluorescens, Phlebia aurea, Phlebia acanthocystis, Phlebia brevispora | Bose et al., 2021 |
| Organochlorine | Endosulfan | Pseudomonas aeruginosa, Pseudomonas fluorescens, Mortierellasp.,Trametes hirsute, Aspergillus niger | Romero-Aguilar et al., 2014; Bose et al., 2021 |
| Organochlorine | Alpha endosulfan | Fusarium ventricosum, Klebsiella, Acinetobacter | Siddique et al., 2003; Bose et al., 2021 |
| Organochlorine | Beta endosulfan | Fusarium ventricosum | Siddique et al., 2003 |
| Organochlorine | Dichlorodiphenyl-trichloroethane | Trichoderma harzianum, Stenotrophomonassp., Sphingobacteriumsp., Pseudomonassp., Trichoderma hamatum, Rhizopus arrhizus, | Ortiz-Hernández et al., 2013; Russo et al., 2019; Bose et al., 2021 |
| Organochlorine | Lindane | Microbacterium sp. P27, Paracoccus sp. NITDBR1, Streptomycessp. A5,Streptomycessp. M7, Pleurotus eryngii, Pleurotus florida, Pleurotus sajor-caju, Phanerochaete chrysosporium | Rigas et al., 2005; Xiao and Kondo, 2020; Zhang W. et al., 2020 |
| Triazone | Atrazine | Nocardiaspp.Pseudomonasspp., Rhodococcusspp. | Aislabie and Lloyd-Jones, 1995 |
| Carbamate | Carbafuron | Achromobacterspp., Pseudomonasspp., Flavobacteriumspp. | Aislabie and Lloyd-Jones, 1995 |
| EPTC | Arthrobacterspp., Rhodococcusspp. | Aislabie and Lloyd-Jones, 1995 | |
| Carbafuron | Achromobacter spanius, Diaphorobacter polyhydroxybutyrativorans | Rahman et al., 2018 | |
| Avermectin | Emamectin Benzoate | Achromobacter spanius, Diaphorobacter polyhydroxybutyrativorans | Rahman et al., 2018 |
| Neonicotinoid | Thiamethoxam | Achromobacter spanius, Diaphorobacter polyhydroxybutyrativorans | Rahman et al., 2018 |
TABLE 7.
Bacterial enzymes, responsible for the degradation of pesticides (Ortiz-Hernández et al., 2013).
| Pesticide | Enzyme | Bacteria |
| Gylphosate | Oxidoreductase (Gox) | Pseudomonasspp.,Agrobacterium spp. |
| Endosulfan, aldrin, malathion, DDT, endosulfate | Monooxygenases (Esd) | Mycobacteriumspp.,Arthrobacter spp. |
| Hexachlorobenzene, Pentachlorobenzene | P450 | Pseudomonas putida |
| Trifluralin | Dioxygenases (TOD) | Pseudomonas putida |
| Hexachlorocyclohexane | Haloalkane Dehalogenases (Lin B) | Sphingobiumspp. |
| Chloro-S-trazina | AtzA | Pseudomonasspp. |
| Chloro-S-trazina | TrzN | Nocardioidesspp. |
| Hexachlorocyclohexane (Gamma isomer) | Lin A | Sphingobiumspp. |
| 2,4-dichlorophenoxyacetic acid | TfdA | Ralstonia eutropa |
| Pyridyl-oxyacetic acid | TfdA | Ralstonia eutropa |
| Pyridyl-oxyacetic acid | DMO | Pseudomonas maltophilia |
| Phosphotriester | Phosphotriesterases (OPH/OpdA) | Pseudomonas diminuta, Agrobacterium radiobacter, Flavobacteriumspp. |
The basic stages of pesticide conversion were characterized by Kumar et al., 1996 as follows: (1) Mineralization: Carbon dioxide or methane as an end-product of complete degradation; (2) Detoxification: Conversion of toxic to non-toxic compounds; (3) Co-metabolism: Microbes involved in the metabolism process of compounds without benefiting themselves from these compounds; (4) Activation: Activation of compounds. During the beginning of 1064, hydrolases and oxygenases came in knowledge and Singh et al. also reported involvement of these enzymes in pesticide biodegradation (Bollag et al., 1968; Tiedje et al., 1969; Singh et al., 1999). Under both denitrifying and aerobic conditions, hydrolytic dehalogenation (the substitution of a halogen group by a hydroxyl) can occur, but only methanogenic and sulfonic circumstances result in reductive dehalogenation, which involves the substitution of a halogen group by a hydrogen group. Furthermore, biotransformation events such as polymerization and methylation may occur, resulting in more hazardous or recalcitrant compounds. Different methods for converting hazardous pesticides were used, depending on their chemical constituents and the microbes that were used for bioconversion (Singh et al., 1999). Factors such as the microbial culture, cultivation technique, size of inoculum, growth under elevated pesticide percentage, adaptation, rhizosphere interactions, and response against the environmental factors can all affect the pesticide degradation process (Conde-Avila et al., 2021). Research has concentrated on the practice of microbial cellular immobilization (CI) technology in several materials and supports the long-lasting survival of microbes. Now, research has shifted to the use of microbial cells as CI, which protects and allows them to be reused. Such a strategy enhances the possibilities of techniques lasting and succeeding in a pesticide-contaminated environment for a long period and has been found suitable for pesticide biodegradation (Colla et al., 2014; Pradeep and Subbaiah, 2016; Fernández-López et al., 2017; Conde-Avila et al., 2021).
The CI technology has served as an environmentally approachable processes for waste management practices. The use of CI of degrading microbes in the elimination and or degradation of pollutants, the CI system has developed as an eco-friendly alternative approach. There are certain disadvantages to CI technology, such as microbial interactions with the immobilization material and its impact on microbial survivability (Conde-Avila et al., 2021). When CI is utilized instead of free cells, the percentage of clearance and efficiency increases for pesticides including chlorpyrifos, atrazine, difenoconazole cypermethrin, carbaryl, endosulfan, and carbofuran. The benefits of utilizing CI are independently supported by the immobilization method or substance employed (Bhadbhade et al., 2002; Pattanasupong et al., 2004; Adinarayana et al., 2005; López-Pérez et al., 2006; Fuentes et al., 2013; Zucca and Sanjust, 2014; Abigail and Das, 2015; Chen et al., 2015; Tallur et al., 2015; Bhatt et al., 2016; Fernández-López et al., 2017). Because pesticides come in such a wide variety of chemical groups, the factors that influence their presence, transit, and mobility are complicated and difficult to anticipate. Extrinsic and intrinsic variables govern adsorption-desorption, biodegradation, volatilization, photodegradation and breakdown phenomena, which mediate pesticide occurrence, and migration (Conde-Avila et al., 2021). Soils with high organic matter reduce pesticide availability through adsorption to a larger percentage than sandy soils (Yanez-Ocampo et al., 2016).
Bioremediation procedures frequently include organic wastes and/or specialist strains with catabolic capabilities against contaminants to assist the breakdown of more persistent pesticides or to reduce their influence on microorganisms. Using genetically engineered strains to breakdown pesticides might be an effective method. Pesticide-exposed native species can develop the capacity to degrade toxic chemicals. Such technology was created to clean up pesticide-related pollutants (Barreiros et al., 2012; Nikel et al., 2014; Castillo et al., 2016; Bhatt et al., 2019a,b,c, 2020c, 2021d).
In comparison to pure cultures, the introduction of consortia or pesticide primed materials has been found to improve pesticide breakdown and mineralization capability in BPSs (bio-purification systems) (Sniegowski and Springael, 2015). Furthermore, Biobed bioremediation systems can be an ideal microcosm for developing specialized microorganisms capable of enhancing pesticide residue metabolization from wastewaters (Dunon et al., 2013). However, the bioaugmentation strategy for various pesticide biodegradations in wastewaters at high concentrations, as occurs in real-world scenarios, is still little known (Sniegowski and Springael, 2015).
Conclusion
Pesticide use has expanded extensively in the recent years, resulting in the environmental damage, particularly water and soil contamination. Pesticides come in a variety of forms, but organophosphates, organochlorine, carbamate, and pyrethroids are the most abundantly uses pesticides and have human and environmental concerns. Refined knowledge of various properties related to the physical and chemical background of pesticides are necessary to determine the impact and behavior of pesticide transformation in that environment. Such pesticides need proper management strategies for converting them to non-toxic compounds before releasing them into the environment. They are the most persistent and generally resistant to degradation under natural conditions. The scientific community has been working hard to come up with creative approaches to pesticide pollution reduction. Environmentally friendly management strategies include several bioremediation approaches and servers to solve pesticide problems or develop alternative green solutions. Bioremediation strategies such as phytoremediation, microalgae bioremediation, myco-remediation, and microbial degradation are also cost-effective and environmentally benign methods. Nowadays, microbial degradation methods are used extensively. Microorganisms and their enzymes play a key role in the breakdown of chemical compounds and synthetic pesticides. Although these methods are environmentally friendly, they have certain limitation such as metabolic routes followed by microbes are highly influenced by external factors. As a result, further study is needed in specific areas before this approach can be declared successful. Enzymatic degradation appears to be a viable method. It is becoming increasingly vital to do significant research to find enzymes capable of degrading synthetic pesticides. Microbial degradation occurs at a considerably slower rate and is not always as efficient or straightforward to carry out as traditional bioremediation technologies. It needed to find more potent microbes, novel genes, and bioremediation approaches for proper waste management of pesticide pollutants. Genetically engineered microorganisms and biotechnology also play a significant role in this area. The above discussion illustrates the utilization of pesticide degrading microorganisms in a constructive way to manage the pesticide pollutants in an eco-friendly manner. Hence, the further studies on the screening of effective microbial strains and enzymes are essential to reduce pesticide risks for the environment and human health.
Author contributions
VMP: conception and design of study and revising the manuscript critically for important intellectual content, approval of the version of the manuscript to be published. VMP and VV: analysis and/or interpretation of data. VMP, VV, BR, BK, NB, AS, SD, MY, RK, SS, AM, VP, NR, and JC: acquisition of data. VMP, BR, and BK: drafting the manuscript. All authors approved the version of the manuscript to be published.
Funding
VP would like to acknowledge the Faculty of Inter-Disciplinary and Applied Sciences, University of Delhi, South Campus, New Delhi, India for providing financial assistance as the V. N. Bakshi Post-Doctoral Fellowship (UDSC/FIAS/VNB-PDF2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abigail M. E. A., Das N. (2015). Removal of atrazine from the aqueous environment using immobilized Pichia kudriavzevii Atz-EN-01 by two different methods. Int. Biodeterior. Biodegrad. 104 53–58. 10.1016/j.ibiod.2015.05.014 [DOI] [Google Scholar]
- Abubakar Y., Tijjani H., Egbuna C., Adetunji C. O., Kala S., Kryeziu T. L., et al. (2020). “Pesticides, history, and classification,” in Natural Remedies for Pest, Disease and Weed Control, eds Egbuna C., Sawicka B. (Amsterdam: Academic Press; ), 29–42. [Google Scholar]
- Adams G. O., Fufeyin P. T., Okoro S. E., Ehinomen I. (2015). Bioremediation, biostimulation and bio-augmention: a review. Int. J. Environ. Bioremed. Biodegrad. 3 28–39. 10.12691/ijebb-3-1-5 [DOI] [Google Scholar]
- Addissie Y. A., Kruszka P., Troia A., Wong Z. C., Everson J. L., Kozel B. A., et al. (2020). Prenatal exposure to pesticides and risk for holoprosencephaly: a case-control study. Environ. Health 19 1–13. 10.1186/s12940-020-00611-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adenipekun C. O., Lawal R. (2012). Uses of mushrooms in bioremediation: a review. Biotechnol. Mol. Biol. Rev. 7 62–68. 10.5897/BMBR12.006 [DOI] [Google Scholar]
- Adinarayana K., Jyothi B., Ellaiah P. (2005). Production of alkaline protease with immobilized cells of Bacillus subtilis PE-11 in various matrices by entrapment technique. AAPS Phar. Sci. Tech. 6 E391–E397. 10.1208/pt060348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbeve S., Osei-Fosu P., Carboo D. (2014). Levels of organochlorine pesticide residues in Mondia whitei, a medicinal plant used in traditional medicine for erectile dysfunction in Ghana. Int. J. Adv. Agric. Res. 1 9–16. [Google Scholar]
- Ahmad A., Ahmad M. (2018). Deciphering the mechanism of interaction of edifenphos with calf thymus DNA. Spectro. Act. Part A: Mol. Biomol. Spectro. 188 244–251. 10.1016/j.saa.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Ahmad I., Ahmad A., Ahmad M. (2016). Binding properties of pendimethalin herbicide to DNA: multispectroscopic and molecular docking approaches. Phys. Chem. Chem. Phys. 18 6476–6485. 10.1039/c5cp07351k [DOI] [PubMed] [Google Scholar]
- Ahmad M., Zafeer M. F., Javed M., Ahmad M. (2018). Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci. Rep. 8 1–9. 10.1038/s41598-018-35484-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi F., Ghanbari K. (2014). Proposed model for binding of permethrin and deltamethrin insecticides with ct-DNA, a structural comparative study. Ecotoxicol. Environ. Saf. 106 136–145. 10.1016/j.ecoenv.2014.02.018 [DOI] [PubMed] [Google Scholar]
- Ahmadi F., Jafari B., Rahimi-Nasrabadi M., Ghasemi S., Ghanbari K. (2013). Proposed model for in vitro interaction between fenitrothion and DNA, by using competitive fluorescence, 31P NMR, 1H NMR, FT-IR, CD and molecular modeling. Toxicol Int. Vit. 27 641–650. 10.1016/j.tiv.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Ahmadi F., Jamali N., Jahangard-Yekta S., Jafari B., Nouri S., Najafi F., et al. (2011). The experimental and theoretical QM/MM study of interaction of chloridazon herbicide with ds-DNA. Spectroch. Act. Part A 79 1004–1012. 10.1016/j.saa.2011.04.012 [DOI] [PubMed] [Google Scholar]
- Aislabie J., Lloyd-Jones G. (1995). A review of bacterial-degradation of pesticides. Soil Res. 33 925–942. [Google Scholar]
- Alengebawy A., Abdelkhalek S. T., Qureshi S. R., Wang M.-Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxicology 9:42. 10.3390/toxics9030042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhanti B., De Joode B. V. W., Martinez M. S., Mora A. M., Gamboa L. C., Reich B., et al. (2021). Environmental exposures contribute to respiratory and allergic symptoms among women living in the banana growing regions of Costa Rica. Occup. Environ. Med. 79 469–476. 10.1136/oemed-2021-107611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H. A. J., Rani V. J. (2009). Effect of phosalone on haematological indices in the tilapia, Oreochromis mossambicus. Tur. J. Vet. Anim. Sci. 33 407–411. [Google Scholar]
- Amaral A. F. (2014). Pesticides and asthma: Challenges for epidemiology. Front. Public Health 2:6. 10.3389/fpubh.2014.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Estevez M., Lopez-Periago E., Martinez-Carballo E., Simal-Gandara J., Mejuto J. C., Garcia-Rio L. (2008). The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 123, 247–260. 10.1016/j.agee.2007.07.011 [DOI] [Google Scholar]
- Authority E. F. S. A., Alvarez F., Arena M., Auteri D., Borroto J., Brancato A., et al. (2021). Updated peer review of the pesticide risk assessment of the active substance bifenazate. EFSA J. 19:e06818. 10.2903/j.efsa.2021.6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey G. W., White J. L. (1970). “Factors influencing the adsorption, desorption, and movement of pesticides in soil,” in Single Pesticide Volume: The Triazine Herbicides, eds Gunther F. A., Gunther J. D. (New York, NY: Springer; ), 29–92. 10.1007/978-1-4615-8464-3_4 [DOI] [PubMed] [Google Scholar]
- Baldi I., Robert C., Piantoni F., Tual S., Bouvier G., Lebailly P., et al. (2014). Agricultural exposure and asthma risk in the AGRICAN French cohort. Int. J. Hygi. Environ. Health 217 435–442. 10.1016/j.ijheh.2013.08.006 [DOI] [PubMed] [Google Scholar]
- Baldwin C., Bell I., O’rourke M., Lebowitz M. (1997). The association of respiratory problems in a community sample with self-reported chemical intolerance. Euro. J. Epidem. 13 547–552. 10.1023/a:1007341813396 [DOI] [PubMed] [Google Scholar]
- Banaee M., Mirvaghefi A., Amiri B. M., Rafiee G., Nematdost B. (2011). Hematological and histopathological effects of diazinon poisoning in common carp (Cyprinus carpio). J. Fish 64 Pe1–Pe12. [Google Scholar]
- Barnhoorn I. E., Bornman M., Van Rensburg C. J., Bouwman H. (2009). DDT residues in water, sediment, domestic and indigenous biota from a currently DDT-sprayed area. Chemo 77 1236–1241. 10.1016/j.chemosphere.2009.08.045 [DOI] [PubMed] [Google Scholar]
- Barreiros L., Peres J., Azevedo N. F., Manaia C. M., Nunes O. C. (2012). Environmental factors influencing molinate biodegradation by a two-member mixed culture in rice paddy field floodwater. Int. Biodeter. Biodegr. 72 52–58. [Google Scholar]
- Barry P., Young A. J., Britton G. (1990). Photodestruction of pigments in higher plants by herbicide action: I. The effect of DCMU (diuron) on isolated chloroplasts. J. Experiment. Bot. 41 123–129. [Google Scholar]
- Basantani M., Srivastava A., Sen S. (2011). Elevated antioxidant response and induction of tau-class glutathione S-transferase after glyphosate treatment in Vigna radiata (L.) Wilczek. Pest. Biochem. Phys. 99 111–117. 10.1016/j.pestbp.2010.11.007 [DOI] [Google Scholar]
- Bast A., Semen K. O., Drent M. (2021). Pulmonary toxicity associated with occupational and environmental exposure to pesticides and herbicides. Curr. Opin. Pulmo. Med. 27 278–283. 10.1097/MCP.0000000000000777 [DOI] [PubMed] [Google Scholar]
- Beasley V. R. (2020). Direct and Indirect Effects of Environmental Contaminants on Amphibians. Amsterdam: Elsevier. 10.1016/b978-0-12-409548-9.11274-6 [DOI] [Google Scholar]
- Beulke S., Brown C. D., Fryer C. J., Van Beinum W. (2004). Influence of kinetic sorption and diffusion on pesticide movement through aggregated soils. Chemo 57 481–490. 10.1016/j.chemosphere.2004.06.026 [DOI] [PubMed] [Google Scholar]
- Bhadauriya P., Parihar R., Ganesh S. (2021). Pesticides DEET, fipronil and maneb induce stress granule assembly and translation arrest in neuronal cells. Biochem. Biophys. Rep. 28:101110. 10.1016/j.bbrep.2021.101110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadbhade B., Sarnaik S., Kanekar P. (2002). Biomineralization of an organophosphorus pesticide, Monocrotophos, by soil bacteria. J. Appl. Microbiol. 93 224–234. 10.1046/j.1365-2672.2002.01680.x [DOI] [PubMed] [Google Scholar]
- Bhatt P., Bhatt K., Huang Y., Lin Z., Chen S. (2020a). Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemo 244:125507. 10.1016/j.chemosphere.2019.125507 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Huang Y., Rene E. R., Kumar A. J., Chen S. (2020b). Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour. Technol. 305:123074. 10.1016/j.biortech.2020.123074 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Huang Y., Zhang W., Sharma A., Chen S. (2020c). Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms 8:223. 10.3390/microorganisms8020223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P., Gangola S., Chaudhary P., Khati P., Kumar G., Sharma A., et al. (2019a). Pesticide induced up-regulation of esterase and aldehyde dehydrogenase in indigenous Bacillus spp. Bioremed. J. 23 42–52. 10.1080/10889868.2019.1569586 [DOI] [Google Scholar]
- Bhatt P., Huang Y., Zhan H., Chen S. (2019b). Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front. Microbiol. 10:1778. 10.3389/fmicb.2019.01778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P., Pal K., Bhandari G., Barh A. (2019c). Modelling of the methyl halide biodegradation in bacteria and its effect on environmental systems. Pest. Biochem. Phys. 158 88–100. 10.1016/j.pestbp.2019.04.015 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Joshi T., Bhatt K., Zhang W., Huang Y., Chen S. (2021a). Binding interaction of glyphosate with glyphosate oxidoreductase and C–P lyase: Molecular docking and molecular dynamics simulation studies. J. Hazar. Mat. 409:124927. 10.1016/j.jhazmat.2020.124927 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Zhou X., Huang Y., Zhang W., Chen S. (2021b). Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazar. Mat. 411:125026. 10.1016/j.jhazmat.2020.125026 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Bhatt K., Sharma A., Zhang W., Mishra S., Chen S. (2021c). Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit. Rev. Biotechnol. 41 317–338. 10.1080/07388551.2020.1853032 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Gangola S., Bhandari G., Zhang W., Maithani D., Mishra S., et al. (2021d). New insights into the degradation of synthetic pollutants in contaminated environments. Chemo 268:128827. 10.1016/j.chemosphere.2020.128827 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Sharma A., Gangola S., Khati P., Kumar G., Srivastava A. (2016). Novel pathway of cypermethrin biodegradation in a Bacillus sp. strain SG2 isolated from cypermethrin-contaminated agriculture field. 3 Bio 6 1–11. 10.1007/s13205-016-0372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birceanu O., Wilkie M. P. (2018). Post-exposure effects of the piscicide 3-trifluoromethyl-4-nitrophenol (TFM) on the stress response and liver metabolic capacity in rainbow trout (Oncorhynchus mykiss). PLoS One 13:e0200782. 10.1371/journal.pone.0200782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatchford P. A., Scott C., French N., Rehm B. H. (2012). Immobilization of organophosphohydrolase OpdA from Agrobacterium radiobacter by overproduction at the surface of polyester inclusions inside engineered Escherichia coli. Biotechnol. Bioeng. 109 1101–1108. 10.1002/bit.24402 [DOI] [PubMed] [Google Scholar]
- Bollag J., Helling C., Alexander M. (1968). 2, 4-D metabolism. Enzymic hydroxylation of chlorinated phenols. J. Agri. Food Chem. 16 826–828. 10.1021/jf60159a037 [DOI] [Google Scholar]
- Boonlertnirun S., Boonlertnirun K., Sooksathan I. (2005). “Effect of chitosan application on growth and yield of rice (Oryza sativa) var. Suphunburi 1,” in Proceedings of 43rd Kasetsart University Annual Conference, (Thailand: ). [Google Scholar]
- Bose S., Kumar P. S., Vo D.-V. N., Rajamohan N., Saravanan R. (2021). Microbial degradation of recalcitrant pesticides: a review. Environ. Chem. Lett. 19 3209–3228. 10.1007/s10311-021-01236-5 [DOI] [Google Scholar]
- Brander S. M., He G., Smalling K. L., Denison M. S., Cherr G. N. (2012). The in vivo estrogenic and in vitro anti-estrogenic activity of permethrin and bifenthrin. Environ. Toxicol. Chem. 31 2848–2855. 10.1002/etc.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A., Hohnheiser B., Sgolastra F., Bosch J., Meixner M. D., Büchler R. (2020). Immunosuppression response to the neonicotinoid insecticide thiacloprid in females and males of the red mason bee Osmia bicornis L. Scientific Reports 10 1–10. 10.1038/s41598-020-61445-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecke B. J., Duke W. B. (1980). Effect of glyphosate on intact bean plants (Phaseolus vulgaris L.) and isolated cells. Plant Physiol. 66 656–659. 10.1104/pp.66.4.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur J. C., Sassone A., Hermida G. N., Codugnello N. (2013). Environmentally-relevant concentrations of atrazine induce non-monotonic acceleration of developmental rate and increased size at metamorphosis in Rhinella arenarum tadpoles. Ecotoxicol. Environ. Saf. 92 10–17. 10.1016/j.ecoenv.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Buralli R. J., Dultra A. F., Ribeiro H. (2020). Respiratory and allergic effects in children exposed to Pesticides—A systematic review. Int. J. Environ Res. Public Health 17:2740. 10.3390/ijerph17082740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau L. (1993). Indian Labour Statistics. India: Government of India. [Google Scholar]
- Calaf G. M. (2021). Role of organophosphorous pesticides and acetylcholine in breast carcinogenesis. Semin. Can. Biol. 76 206–217. 10.1016/j.semcancer.2021.03.016 [DOI] [PubMed] [Google Scholar]
- Cannon R. D., Ruha A. M. (2013). Insecticides, Herbicides, and Rodenticides. Emergency Medicine: Clinical Essentials, 2nd Edn, Vol. 146. Philadelphia, PA: Elsevier Saunders, 1246–1256. [Google Scholar]
- Caron-Beaudoin É, Viau R., Sanderson J. T. (2018). Effects of neonicotinoid pesticides on promoter-specific aromatase (CYP19) expression in Hs578t breast cancer cells and the role of the VEGF pathway. Environ. Health Perspect. 126:047014. 10.1289/EHP2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J. M., Beguet J., Martin-Laurent F., Romero E. (2016). Multidisciplinary assessment of pesticide mitigation in soil amended with vermicomposted agroindustrial wastes. J. Haz. Mat. 304 379–387. 10.1016/j.jhazmat.2015.10.056 [DOI] [PubMed] [Google Scholar]
- Chekroun K. B., Sánchez E., Baghour M. (2014). The role of algae in bioremediation of organic pol- lutants. Int. Res. J. Public. Environ. Health 1 19–32. [Google Scholar]
- Chen C., Wu T.-W., Wang H.-L., Wu S.-H., Tien C.-J. (2015). The ability of immobilized bacterial consortia and strains from river biofilms to degrade the carbamate pesticide methomyl. Int. J. Environ. Sci. Technol. 12 2857–2866. 10.1007/s13762-014-0675-z [DOI] [Google Scholar]
- Chen C., Zou W., Cui G., Tian J., Wang Y., Ma L. (2020). Ecological risk assessment of current-use pesticides in an aquatic system of Shanghai, China. Chemo 257:127222. 10.1016/j.chemosphere.2020.127222 [DOI] [PubMed] [Google Scholar]
- Chen S., Liu C., Peng C., Liu H., Hu M., Zhong G. (2012). Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS One 7:47205. 10.1371/journal.pone.0047205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibu H., Shibayama H., Arima S. (2002). Effects of chitosan application on the shoot growth of rice and soybean. Jpn. J. Crop Sci. 71 206–211. 10.1626/jcs.71.206 [DOI] [Google Scholar]
- Chojnacka K. (2010). Biosorption and bioaccumulation–the prospects for practical applications. Environ. Int. 36 299–307. 10.1016/j.envint.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Choudhury P. P. (2019). “Transformation of Herbicides in the Environment,” in Herbicide Residue Research in India. Environmental Chemistry for a Sustainable World, eds Sondhia S., Choudhury P., Sharma A. (Singapore: Springer; ), 415–442. [Google Scholar]
- Colla T. S., Andreazza R., Bücker F., de Souza M. M., Tramontini L., Prado G. R., et al. (2014). Bioremediation assessment of diesel–biodiesel-contaminated soil using an alternative bioaugmentation strategy. Environ. Sci. Pol. Res. 21 2592–2602. 10.1007/s11356-013-2139-2 [DOI] [PubMed] [Google Scholar]
- Conde-Avila V., Ortega-Martínez L. D., Loera O., El Kassis E. G., Dávila J. G., Valenzuela C. M., et al. (2021). Pesticides degradation by immobilised microorganisms. Int. J. Environ. Anal. Chem. 101 2975–3005. 10.1080/03067319.2020.1715375 [DOI] [Google Scholar]
- Costa C., Miozzi E., Teodoro M., Fenga C. (2019). Influence of genetic polymorphism on pesticide-induced oxidative stress. Curr. Opin. Toxicol. 13 1–7. [Google Scholar]
- Daneshmehr M.-A., Ahmadi F., Ahmadi B., Shakiba E. (2016). Deciphering the binding mode of dinitramine herbicide to ct-DNA, a thermodynamic discussion. Food Agric. Immun. 27 23–39. 10.1080/09540105.2015.1055555 [DOI] [Google Scholar]
- Dawar F. U., Zuberi A., Azizullah A., Khattak M. N. K. (2016). Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemo 144 697–705. 10.1016/j.chemosphere.2015.09.007 [DOI] [PubMed] [Google Scholar]
- De Barros Rodrigues M., De Carvalho D. S., Chong-Silva D. C., de Pereira M. N. E. U., de Albuquerque G. S. C., Cieslak F., et al. (2022). Association between exposure to pesticides and allergic diseases in children and adolescents: a systematic review with meta-analysis. J. Peditr. [Online ahead of print]. 10.1016/j.jped.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Chen Y., Wang D., Shi T., Wu X., Ma X. (2015). Rapid biodegradation of organophos- phorus pesticides by Stenotrophomonas sp. G1. J. Hazard. Mater. 297 17–24. 10.1016/j.jhazmat.2015.04.052 [DOI] [PubMed] [Google Scholar]
- Desisa B., Getahun A., Muleta D. (2022). “Advances in biological treatment technologies for some emerging pesticides,” in Pesticides Bioremediation, eds Siddiqui S., Meghvansi M. K., Chaudhary K. K. (Cham: Springer; ), 10.1007/978-3-030-97000-0_10 [DOI] [Google Scholar]
- Dey C., Saha S. (2014). A comparative study on the acute toxicity bioassay of dimethoate and lambda-cyhalothrin and effects on thyroid hormones of freshwater teleost fish Labeo rohita (Hamilton). Int. J. Environ. Res. 8 1085–1092. 10.22059/IJER.2014.802 [DOI] [Google Scholar]
- Doǧanlar Z. B., Doǧanlar O., Tozkir H., Gökalp F. D., Doǧan A., Yamaç F., et al. (2018). Nonoccupational exposure of agricultural area residents to pesticides: pesticide accumulation and evaluation of genotoxicity. Arch. Environ. Conta. Toxicol. 75 530–544. 10.1007/s00244-018-0545-7 [DOI] [PubMed] [Google Scholar]
- Donald P. F. (2004). Biodiversity impacts of some agricultural commodity production systems. Conserv. Biol. 18 17–37. 10.1111/j.1523-1739.2004.01803.x [DOI] [Google Scholar]
- Dong Y.-J., Bartlam M., Sun L., Zhou Y.-F., Zhang Z.-P., Zhang C.-G., et al. (2005). Crystal structure of methyl parathion hydrolase from Pseudomonas sp. WBC-3. J. Mol. Biol. 353 655–663. 10.1016/j.jmb.2005.08.057 [DOI] [PubMed] [Google Scholar]
- Dunon V., Sniegowski K., Bers K., Lavigne R., Smalla K., Springael D. (2013). High prevalence of IncP-1 plasmids and IS 1071 insertion sequences in on-farm biopurification systems and other pesticide-polluted environments. FEMS Microbiol. Ecol. 86 415–431. 10.1111/1574-6941.12173 [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., Balakrishnan S. (2010). “Genotoxicity of pesticides,” in Hayes’ Handbook of Pesticide Toxicology ed. Krieger R. (Riverside, CA: University of California; ), 357–380. 10.1016/B978-0-12-374367-1.00011-2 [DOI] [Google Scholar]
- Ecobichon D. J. (2001). Pesticide use in developing countries. Toxicology 160 27–33. 10.1016/s0300-483x(00)00452-2 [DOI] [PubMed] [Google Scholar]
- EPA, OPPTS, OPP, and SRRD. (2009). US Environmental Protection Agency Office of Pesticide Programs A Review of the Relationship between Pyrethrins Corrected Version. Washington, DC: United States Environmental Protection Agency [Google Scholar]
- Ernst P. (2002). Pesticide exposure and asthma. Am. J. Respir. Critic. Care Med. 165 563–564. 10.1164/ajrccm.165.5.2201001c [DOI] [PubMed] [Google Scholar]
- Eve L., Fervers B., Le Romancer M., Etienne-Selloum N. (2020). Exposure to endocrine disrupting chemicals and risk of breast cancer. Int. J. Mol. Sci. 21:9139. 10.3390/ijms21239139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falak R., Sankian M., Varasteh A. (2012). The possible role of organophosphorus pesticides in Augmentation of food allergenicity: a putative hypothesis. Res. J. Environ. Toxicol. 6:88. 10.3923/rjet.2012.88.100 [DOI] [Google Scholar]
- Fernández L. (2021). Global pesticide use by country | Statista. Available online at: https://www.statista.com/statistics/1263069/global-pesticide-use-by-country/ (accessed May 31, 2022). [Google Scholar]
- Fernández-López M. G., Popoca-Ursino C., Sánchez-Salinas E., Tinoco-Valencia R., Folch-Mallol J. L., Dantán-González E., et al. (2017). Enhancing methyl parathion degradation by the immobilization of Burkholderia sp. isolated from agricultural soils. Microbiol. Open 6:e00507. 10.1002/mbo3.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa Z. I., Young H. A., Mumford S. L., Meeker J. D., Barr D. B., Gray G. M., et al. (2019). Pesticide interactions and risks of sperm chromosomal abnormalities. Int. J. Hygien. Environ. Health 222 1021–1029. 10.1016/j.ijheh.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch S., Samuel A., Lane G. P. (2014). Lockhart and wiseman’s crop husbandry including grassland. Else 433–453. [Google Scholar]
- Fosu-Mensah B. Y., Okoffo E. D., Darko G., Gordon C. (2016). Assessment of organochlorine pesticide residues in soils and drinking water sources from cocoa farms in Ghana. Springer Plus. 5 1–13. 10.1186/s40064-016-2352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R., Li S., Rodriguez-Rocha H., Burns M., Panayiotidis M. I. (2010). Molecular mechanisms of pesticide-induced neurotoxicity: relevance to Parkinson’s disease. Chem. Biol. Interact. 188 289–300. 10.1016/j.cbi.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes M. S., Briceńo G. E., Saez J. M., Benimeli C. S., Diez M. C., Amoroso M. J. (2013). Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. Biomed. Res. Int. 2013:392573. 10.1155/2013/392573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst E. P., Norman M. A. (1991). Interactions of herbicides with photosynthetic electron transport. Weed Sci. 39 458–464. 10.1017/S0043174500073227 [DOI] [Google Scholar]
- Garcia F. P., Ascencio S. C., Oyarzún J. C. G., Hernandez A. C., Alavarado P. V. (2012). Pesticides: classification, uses and toxicity. Measures of exposure and genotoxic risks. J. Res. Environ. Sci. Toxicol. 1 279–293. [Google Scholar]
- García J., Ventura M. I., Requena M., Hernández A. F., Parrón T., Alarcón R. (2017). Association of reproductive disorders and male congenital anomalies with environmental exposure to endocrine active pesticides. Reprod. Toxicol. 71 95–100. 10.1016/j.reprotox.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Garry V. (2004). Pesticides and children. Toxicol. Appl. Pharmacol. 198 152–163. 10.1016/j.taap.2003.11.027 [DOI] [PubMed] [Google Scholar]
- Garthwaite D., Barker I., Parrish G., Smith L., Hudson S., Pietravalle S. (2012). Pesticide Usage Survey Report 243: Outdoor Vegetable Crops in the United Kingdom 2011. England: Fera Science Ltd. [Google Scholar]
- Gavrilescu M. (2005). Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 5 497–526. 10.1002/elsc.200520098 [DOI] [Google Scholar]
- Gea M., Zhang C., Tota R., Gilardi G., Di Nardo G., Schilirò T. (2022). Assessment of five pesticides as endocrine-disrupting chemicals: effects on estrogen receptors and aromatase. Int. J. Environ. Res. Public Health 19:1959. 10.3390/ijerph19041959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevao B., Semple K. T., Jones K. C. (2000). Bound pesticide residues in soils: a review. Environ. Pol 108 3–14. 10.1016/s0269-7491(99)00197-9 [DOI] [PubMed] [Google Scholar]
- Gill H., Garg H. (2014). Pesticides: Environmental Impacts and Management Strategies, Pesticides-Toxic Aspects. London: IntechOpen, 10.5772/57399 [DOI] [Google Scholar]
- Gupta R. C. (2014). “Rotenone,” in Encyclopedia of Toxicology, 3rd Edn, ed. Philip W. (London: Elsevier; ), 185–187. [Google Scholar]
- Gupta R. C., Crissman J. W. (2013). “Agricultural chemicals,” in Haschek and Rousseaux’s Handbook of Toxicologic Pathology, eds Haschek W., Bolon B., Ochoa R., Rousseaux C., Wallig M. (London: Elsevier; ), 1349–1372. [Google Scholar]
- Gupta S., Gajbhiye V. T. (2002). Effect of concentration, moisture and soil type on the dissipation of flufenacet from soil. Chemo 47 901–906. 10.1016/S0045-6535(02)00017-6 [DOI] [PubMed] [Google Scholar]
- Gupta V. K., Pathak A., Siddiqi N. J., Sharma B. (2016). Carbofuran modulating functions of acetylcholinesterase from rat brain in vitro. Adv. Biol. 2016:3760967. 10.1155/2016/3760967 [DOI] [Google Scholar]
- Gyawali K. (2018). Pesticide uses and its effects on public health and environment. J. Health Promot. 6 28–36. 10.3126/jhp.v6i0.21801 33981099 [DOI] [Google Scholar]
- Hassaan M. A., El Nemr A. (2020). Pesticides pollution: classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 46 207–220. 10.1016/j.ejar.2020.08.007 [DOI] [Google Scholar]
- Henneberger P. K., Liang X., London S. J., Umbach D. M., Sandler D. P., Hoppin J. A. (2014). Exacerbation of symptoms in agricultural pesticide applicators with asthma. Int. Arch. Occupat. Environ. Health 87 423–432. 10.1007/s00420-013-0881-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A. F. (2015). Pesticides and asthma | *Project S E.N.S.O.R, Vol. 26. Cham: Springer. [Google Scholar]
- Hoang T. T., Qi C., Paul K. C., Lee M., White J. D., Richards M., et al. (2021). Epigenome-Wide DNA methylation and pesticide use in the agricultural lung health study. Environ. Health Perspect. 129:097008. 10.1289/EHP8928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Q., Zhang Z., Hong Y., Li S. (2007). A microcosm study on bioremediation of fenitrothion-contaminated soil using Burkholderia sp. FDS-1. Int. Biodeter. Biodegr. 59 55–61. 10.1016/j.ibiod.2006.07.013 [DOI] [Google Scholar]
- Hood R. B., Liang D., Chiu Y. H., Sandoval-Insausti H., Chavarro J. E., Jones D., et al. (2022). Pesticide residue intake from fruits and vegetables and alterations in the serum metabolome of women undergoing infertility treatment. Envir. Inter. 160, 107061. 10.1016/j.envint.2021.107061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin J. A., Umbach D. M., London S. J., Henneberger P. K., Kullman G. J., Alavanja M. C., et al. (2008). Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. Am. J. Respir. Crit. Car. Med. 177, 11–18. 10.1164/rccm.200706-821OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. L., Zeng G. M., Feng C. L., Hu S., Jiang X. Y., Tang L., et al. (2008). Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity. Environ. Sci. Technol. 42 4946–4951. 10.1021/es800072c [DOI] [PubMed] [Google Scholar]
- Huch M., Stoll D. A., Kulling S. E., Soukup S. T. (2022). Metabolism of glyphosate by the human fecal microbiota. Toxicol. Lett. 358 1–5. 10.1016/j.toxlet.2021.12.013 [DOI] [PubMed] [Google Scholar]
- Hudson N. L., Kasner E. J., Beckman J., Mehler L., Schwartz A., Higgins S., et al. (2014). Characteristics and magnitude of acute pesticide-related illnesses and injuries associated with pyrethrin and pyrethroid exposures—11 states, 2000–2008. Am. J Indus. Med. 57 15–30. 10.1002/ajim.22216 [DOI] [PubMed] [Google Scholar]
- Iteire K., Sowole A., Ogunlade B. (2022). Exposure to pyrethroids induces behavioral impairments, neurofibrillary tangles and tau pathology in Alzheimer’s type neurodegeneration in adult Wistar rats. Drug Chem. Toxicol. 45 839–849. 10.1080/01480545.2020.1778020 [DOI] [PubMed] [Google Scholar]
- Jaensson A., Scott A. P., Moore A., Kylin H., Olsén K. H. (2007). Effects of a pyrethroid pesticide on endocrine responses to female odours and reproductive behaviour in male parr of brown trout (Salmo trutta L.). Aquat. Toxicol. 81 1–9. 10.1016/j.aquatox.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Jan S., Parween T., Siddiqi T. (2012). Effect of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ. Rev. 20 17–39. 10.1139/a11-021 [DOI] [Google Scholar]
- Janusz G., Pawlik A., Sulej J., Sìwiderska-Burek U., Jarosz-Wilkołazka A., Paszczyński A. (2017). Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 41 941–962. 10.1093/femsre/fux049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj R., Megha P., Sreedev P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 9:, 90–100. 10.1515/intox-2016-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerschow E., McGinn A. P., De Vos G., Vernon N., Jariwala S., Hudes G., et al. (2012). Dichlorophenol-containing pesticides and allergies: results from the US National Health and Nutrition Examination Survey 2005-2006. Ann. Allergy Asthma Immunol. 109 420–425. 10.1016/j.anai.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal R., Sharma R. (2019). Neurotoxic responses in brain of Catla catla exposed to cypermethrin: a semiquantitative multibiomarker evaluation. Ecol. Indicat. 106:105485. 10.1016/j.ecolind.2019.105485 [DOI] [Google Scholar]
- John R. P., Anisha G. S., Nampoothiri K. M., Pandey A. (2011). Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour. Technol. 102 186–193. 10.1016/j.biortech.2010.06.139 [DOI] [PubMed] [Google Scholar]
- Joseph B., Raj S. J. (2011). Impact of pesticide toxicity on selected biomarkers in fishes. Int. J. Zool. Res. 7 212–222. 10.3923/ijzr.2011.212.222 [DOI] [Google Scholar]
- Jurewicz J., Radwan M., Wielgomas B., Sobala W., Piskunowicz M., Radwan P., et al. (2015). The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Syst. Biol. Reprod. Med. 61 37–43. 10.3109/19396368.2014.981886 [DOI] [PubMed] [Google Scholar]
- Kabir E. R., Rahman M. S., Rahman I. (2015). A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 40 241–258. 10.1016/j.etap.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Kabra A. N., Ji M. K., Choi J., Kim J. R., Govindwar S. P., Jeon B. H. (2014). Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga, Chlamydomonas mexicana. Environ. Sci. Pol. 21 12270–12278. 10.1007/s11356-014-3157-4 [DOI] [PubMed] [Google Scholar]
- Kafilzadeh F., Ebrahimnezhad M., Tahery Y. (2015). Isolation and identification of endosulfan-degrading bacteria and evaluation of their bioremediation in Kor River, Iran. Osong Public Health Res. Perspect. 6, 39–46. 10.1016/j.phrp.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyabina V. P., Esimbekova E. N., Kopylova K. V., Kratasyuk V. A. (2021). Pesticides: formulants, distribution pathways and effects on human health–a review. Toxicol. Rep. 8 1179–1192. 10.1016/j.toxrep.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapka-Skrzypczak L., Czajka M., Sawicki K., Matysiak-Kucharek M., Gabelova A., Sramkova M., et al. (2019). Assessment of DNA damage in Polish children environmentally exposed to pesticides. Mutat. Res. Gen. Toxicol. Environ. Muta. 843 52–56. 10.1016/j.mrgentox.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Kaur G., Dogra N., Singh S. (2018). Health risk assessment of occupationally pesticide-exposed population of cancer prone area of Punjab. Toxicol. Sci. 165 157–169. 10.1093/toxsci/kfy140 [DOI] [PubMed] [Google Scholar]
- Kaur R., Goyal D. (2019). Toxicity and degradation of the insecticide monocrotophos. Environ. Chem. Lett. 17 1299–1324. 10.1007/s10311-019-00884-y [DOI] [Google Scholar]
- Kaushik G., Satya S., Naik S. (2009). Food processing a tool to pesticide residue dissipation–A review. Food Res. Int. 42 26–40. 10.1016/j.foodres.2008.09.009 [DOI] [Google Scholar]
- Khafaga A. F., Naiel M. A., Dawood M. A., Abdel-Latif H. M. (2020). Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquat. Toxicol. 228:105624. 10.1016/j.aquatox.2020.105624 [DOI] [PubMed] [Google Scholar]
- Khan M. J., Zia M. S., Qasim M. (2010). Use of pesticides and their role in environmental pollution. World Acad. Sci. Eng. Technol. 72 122–128. [Google Scholar]
- Khatri N., Tyagi S. (2015). Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 8 23–39. 10.1080/21553769.2014.933716 [DOI] [Google Scholar]
- Kim H., Kim D.-U., Lee H., Yun J., Ka J.-O. (2017). Syntrophic biodegradation of propoxur by Pseudaminobacter sp. SP1a and Nocardioides sp. SP1b isolated from agricultural soil. Int. Biodeteri. Biodegra. 118 1–9. 10.1016/j.ibiod.2017.01.024 [DOI] [Google Scholar]
- Koch D., Lu C., Fisker-Andersen J., Jolley L., Fenske R. A. (2002). Temporal association of children’s pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ. Health Perspect. 110 829–833. 10.1289/ehp.02110829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranteng S. S., Darko D. A., Nukpezah D., Ameka G. K. (2018). Pesticides bioconcentration potential of aquatic plants in the Volta Lake. West. Afr. J. App. Ecol. 26 193–202. [Google Scholar]
- Kori R. K., Singh M. K., Jain A. K., Yadav R. S. (2018). Neurochemical and behavioral dysfunctions in pesticide exposed farm workers: a clinical outcome. Ind. J. Clini. Biochem. 33 372–381. 10.1007/s12291-018-0791-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Yadav A. N., Saxena R., Paul D., Tomar R. S. (2021). Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocata. Agric. Biotechnol. 31:101883. 10.1016/j.bcab.2020.101883 [DOI] [Google Scholar]
- Kumar S., Mukerji K., Lai R. (1996). Molecular aspects of pesticide degradation by microorganisms. Crit. Rev. Microbiol. 22 1–26. 10.3109/10408419609106454 [DOI] [PubMed] [Google Scholar]
- Kuppusamy S., Palanisami T., Megharaj M., Venkateswarlu K., Naidu R. (2016). In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. Rev. Environ. Contam. Toxicol. 236 1–115. 10.1007/978-3-319-20013-2_1 [DOI] [PubMed] [Google Scholar]
- Lacouture A., Lafront C., Peillex C., Pelletier M., Audet-Walsh E. (2022). Impacts of endocrine-disrupting chemicals on prostate function and cancer. Environ. Res. 204:112085. 10.1016/j.envres.2021.112085 [DOI] [PubMed] [Google Scholar]
- Lamichhane J. R., Osdaghi E., Behlau F., Köhl J., Jones J. B., Aubertot J.-N. (2018). Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sust. Dev. 38 1–18. 10.1007/s13593-018-0503-9 [DOI] [Google Scholar]
- Larsen A. E., Gaines S. D., Deschênes O. (2017). Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 8 1–9. 10.1038/s41467-017-00349-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laura M., Snchez-Salinas E., Dantn Gonzlez E., Luisa M. (2013). “Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process,” in Biodegradation: Life of Science, ed. Chamy R. (London: IntechOpen; ). 10.5772/56098 [DOI] [Google Scholar]
- Law C., Green R., Suneetha Kadiyala B. S., Knai C., Brown K. A., Dangour A. D., et al. (2019). Purchase trends of processed foods and beverages in urban India. Glob. Food Secur. 23 191–204. 10.1016/j.gfs.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonel A. C., Bonan R. F., Pinto M. B., Kowalski L. P., Perez D. E. (2021). The pesticides use and the risk for head and neck cancer: a review of case-control studies’. Med. Oral. Patol. Oral. Cir. Bucal. 26 e56–e63. 10.4317/medoral.23962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerro C. C., Freeman L. E. B., DellaValle C. T., Andreotti G., Hofmann J. N., Koutros S., et al. (2021). Pesticide exposure and incident thyroid cancer among male pesticide applicators in agricultural health study. Environ. Int. 146:106187. 10.1016/j.envint.2020.106187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mo R., Tang F., Fu Y., Guo Y. (2015). Influence of different formulations on chlorpyrifos behavior and risk assessment in bamboo forest of China. Environ. Sci. Pol. Res. 22 20245–20254. 10.1007/s11356-015-5272-2 [DOI] [PubMed] [Google Scholar]
- Loh W., Tang M. L. (2018). The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 15:2043. 10.3390/ijerph15092043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pérez G. C., Arias-Estévez M., López-Periago E., Soto-González B., Cancho-Grande B., Simal-Gándara J. (2006). Dynamics of pesticides in potato crops. J. Agric. Food Chem. 54 1797–1803. 10.1016/j.envadv.2021.100114 [DOI] [PubMed] [Google Scholar]
- Lourthuraj A. A., Hatshan M. R., Hussein D. S. (2022). Biocatalytic degradation of organophosphate pesticide from the wastewater and hydrolytic enzyme properties of consortium isolated from the pesticide contaminated water. Environ. Res. 205:112553. 10.1016/j.envres.2021.112553 [DOI] [PubMed] [Google Scholar]
- Mahna D., Puri S., Sharma S. (2021). DNA methylation modifications: mediation to stipulate pesticide toxicity. Int. J. Environ. Sci. Technol. 18 531–544. 10.1007/s13762-020-02807-9 [DOI] [Google Scholar]
- Main A. R., Fehr J., Liber K., Headley J. V., Peru K. M., Morrissey C. A. (2017). Reduction of neonicotinoid insecticide residues in Prairie wetlands by common wetland plants. Sci. Total Environ. 579 1193–1202. 10.1016/j.scitotenv.2016.11.102 [DOI] [PubMed] [Google Scholar]
- Malhotra H., Kaur S., Phale P. S. (2021). Conserved metabolic and evolutionary themes in microbial degradation of carbamate pesticides. Front. Microbiol. 12:648868 10.3389/fmicb.2021.648868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali H., Shah C., Rudakiya D. M., Patel D. H., Trivedi U., Subramanian R. (2022). A novel organophosphate hydrolase from Arthrobacter sp. HM01: characterization and applications. Bioresour. Technol. 349:126870. 10.1016/j.biortech.2022.126870 [DOI] [PubMed] [Google Scholar]
- Maqbool Z., Hussain S., Imran M., Mahmood F., Shahzad T., Ahmed Z., et al. (2016). Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ. Sci. Pol. Res. 23 16904–16925. 10.1007/s11356-016-7003-8 [DOI] [PubMed] [Google Scholar]
- Margni M., Rossier D., Crettaz P., Jolliet O. (2002). Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst Environ. 93 379–392. 10.1016/S0167-8809(01)00336-X [DOI] [Google Scholar]
- Marie L., Sylvain P., Benoit G., Maurice M., Gwenaël I. (2017). Degradation and transport of the chiral herbicide s-metolachlor at the catchment scale: combining observation scales and analytical approaches. Environ. Sci. Technol. 51 13231–13240. 10.1021/acs.est.7b02297 [DOI] [PubMed] [Google Scholar]
- Marsala R. Z., Capri E., Russo E., Bisagni M., Colla R., Lucini L., et al. (2020). First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Tot. Environ. 736:139730. 10.1016/j.scitotenv.2020.139730 [DOI] [PubMed] [Google Scholar]
- Mata T. M., Martins A. A., Caetano N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renew. Sust. Energ. Rev. 14 217–232. 10.1016/j.rser.2009.07.020 [DOI] [Google Scholar]
- Matthews D. (2005). Asthma. Child. Pest. 25 1–10. [Google Scholar]
- Mbah Ntepe L. J., Habib R., Judith Laure N., Raza S., Nepovimova E., Kuca K., et al. (2020). Oxidative Stress and Analysis of Selected SNPs of ACHE (rs 2571598). BCHE (rs 3495), CAT (rs 7943316), SIRT1 (rs 10823108), GSTP1 (rs 1695), and Gene GSTM1, GSTT1 in Chronic Organophosphates Exposed Groups from Cameroon and Pakistan. Int. J. Mol. Sci. 21:6432. 10.3390/ijms21176432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Barr D. B., Hauser R. (2008). Human semen quality and sperm DNA damage in relation to urinary metabolites of pyrethroid insecticides. Hum. Reprod. 23 1932–1940. 10.1093/humrep/den242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieldazys A., Mieldazys R., Vilkevicius G., Stulginskis A. (2015). Agriculture-Use of Pesticides/Plant Protection Products. Bilbao: EU-OSHA. [Google Scholar]
- Mir Z. A., Bharose R., Lone A. H., Malik Z. A. (2017). Review on phytoremediation: an eco-friendly and green technology for removal of heavy metals. Crop. Res. 52 74–82. [Google Scholar]
- Mishra R. K., Mohammad N., Roychoudhury N. (2015). Soil pollution: causes, effects and control. Trop. For. Res. Instruct. 3 20–30. [Google Scholar]
- Mishra V., Srivastava G., Prasad S. M., Abraham G. (2008). Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pest. Biochem. Phys. 92 30–37. [Google Scholar]
- Monteiro C. M., Castro P. M. L., Malcata F. W. (2012). Metal uptake by microalgae biotechnology progress: underlying mechanisms and practical applications. Biotechnol. Prog. 28 299–311. 10.1002/btpr.1504 [DOI] [PubMed] [Google Scholar]
- Monteiro J. P., Jurado A. S. (2014). “Methoprene,” in Encyclopedia of Toxicology, 3rd. Edn, ed. Wexler P. (), 246–249. [Google Scholar]
- Montes-Grajales D., Olivero-Verbel J. (2020). Structure-based identification of endocrine disrupting pesticides targeting breast cancer proteins. Toxicology 439:152459. 10.1016/j.tox.2020.152459 [DOI] [PubMed] [Google Scholar]
- Moradi M.-T., Khazaei M., Khazaei M. (2018). The effect of catalase C262T gene polymorphism in susceptibility to ovarian cancer in Kermanshah province, Western Iran. J. Obstet. Gynaecol. 38 562–566. 10.1080/01443615.2017.1381672 [DOI] [PubMed] [Google Scholar]
- Mugo H. M. (1998). Studies of Insect Pests of Pigeonpea, Cajanus cajan (l) Millsp, During the Flowering and Post-Flowering Stages and their Impact on Seed Yield in Kenya. Kenya: University of Nairobi [Google Scholar]
- Muralidhara M., Mithyantha S., Rajendran T., Banerjee K. (2022). Regulatory landscape of risk assessment of pesticide residues in processed foods in India: a perspective. J. Food Sci. Technol. 1–11. 10.1007/s13197-022-05388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy G. P., Prasad G. M., Sudarshana M. (2005). Toxicity of different imbibition periods of dimethoate on germination, chlorophyll a/b and dry matter of Glycine max (L) Merrill. cv. KHSB-2, during early seedling growth. J. Phytol. Res. 18 199–201. [Google Scholar]
- Murthy K. S., Kiran B., Venkateshwarlu M. (2013). A review on toxicity of pesticides in Fish. Int. J. Open Sci. Res. 1 15–36. [Google Scholar]
- Muthukumaravel K., Sivakumar B., Kumarasamy P., Govindarajan M. (2013). Studies on the toxicity of pesticide monocrotophos on the biochemical constituents of the freshwater fish Labeo rohita. Int. J. Curr. Biochem. Biotechnol. 2 20–26. [Google Scholar]
- Nandhini A., Muthukumar H., Gummadi S. N. (2021). Chlorpyrifos in environment and foods: a critical review of detection methods and degradation pathways. Environ. Sci. Pro. Imp. 23, 1255–1277. 10.1371/journal.pone.0038137 [DOI] [PubMed] [Google Scholar]
- Nascimento F. D. A., Pedroso T. M. A., Ramos J. S. A., Parise M. R. (2022). Farmers exposed to pesticides have almost five times more DNA damage: a meta-analysis study. Environ. Sci. Pol. Res. 29 805–816. 10.1007/s11356-021-15573-z [DOI] [PubMed] [Google Scholar]
- Ndlovu V., Dalvie M. A., Jeebhay M. F. (2011). Pesticides and the airways-a review of the literature: allergies in the workplace. Curr. Allergy Clin. Immun. 24 212–217. [Google Scholar]
- Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health. 4:148. 10.3389/fpubh.2016.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikel P. I., Martínez-García E., De Lorenzo V. (2014). Biotechnological domestication of Pseudomonads using synthetic biology. Nat. Rev. Microbiol. 12 368–379. [DOI] [PubMed] [Google Scholar]
- NIPHM (2018). Pesticide management division, syllabus, pesticide classification on use, chemical nature, formulation toxicity and action etc. Hyderabad: NIPHM, 1–17. [Google Scholar]
- OECD (2008). Magnitude of the pesticide residues in processed commodities, OECD guidelines for the testing of chemicals, section 5. OECD Test Guideline No. 508. Paris: OECD Publishing. [Google Scholar]
- Obiri S., Cobbina S. J., Armah F. A., Luginaah I. (2013). Assessment of cancer and noncancer health risks from exposure to PAHs in street dust in the Tamale Metropolis, Ghana. J. Environ. Sci. Health Part A 48 408–416. 10.1080/10934529.2013.728914 [DOI] [PubMed] [Google Scholar]
- Ortiz-Hernández M. L., Sánchez-Salinas E., Dantán-González E., Castrejón-Godínez M. L. (2013). Pesticide biodegradation: mechanisms, genetics and strategies to enhance the process. Biodegr. Life Sci. 251–287. 10.5772/56098 [DOI] [Google Scholar]
- Osteen C. D., Fernandez-Cornejo J. (2013). Economic and policy issues of US agricultural pesticide use trends. Pest. Manag. Sci. 69 1001–1025. 10.1002/ps.3529 [DOI] [PubMed] [Google Scholar]
- Panigrahi A. K., Choudhury N., Tarafdar J. (2014). Pollutional impact of some selective agricultural pesticides on fish Cyprinus carpio. IMPACT 2 71–76. [Google Scholar]
- Parakhia M. V., Tomar R. S., Vadukia M. R., Malviya B. J., Rathod V. M., Thakkar J. R., et al. (2014). Draft genome sequence of the methyl parathion (pesticide) degrading bacterium Pseudomonas spp. MR3. Ind. J. Microbiol. 54 120–121. 10.1007/s12088-013-0433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L. A., Beane Freeman L. E., Lerro C. C., Andreotti G., Hofmann J. N., Parks C. G., et al. (2020). Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ. Health 19 1–12. 10.1186/s12940-020-00583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Céspedes D. M., Herrera-Moreno J. F., Bernal-Hernández Y. Y., Medina-Díaz I. M., Salazar A. M., Ostrosky-Wegman P., et al. (2019). Pesticide exposure modifies DNA methylation of coding region of WRAP53α, an antisense sequence of p53, in a Mexican population. Chem. Res. Toxicol. 32 1441–1448. 10.1021/acs.chemrestox.9b00153 [DOI] [PubMed] [Google Scholar]
- Parra-Arroyo L., González-González R. B., Castillo-Zacarías C., Martínez E. M. M., Sosa-Hernández J. E., Bilal M., et al. (2022). Highly hazardous pesticides and related pollutants: toxicological, regulatory, and analytical aspects. Sci. Total Environ. 807:151879. 10.1016/j.scitotenv.2021.151879 [DOI] [PubMed] [Google Scholar]
- Patel O., Syamlal G., Henneberger P. K., Alarcon W. A., Mazurek J. M. (2018). Pesticide use, allergic rhinitis, and asthma among US farm operators. J. Agromed. 23 327–335. 10.1080/1059924X.2018.1501451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil K., Matsumura F., Boush G. (1970). Degradation of endrin, aldrin, and DDT by soil microorganisms. Appl. Microbiol. 19 879–881. 10.1128/am.19.5.879-881.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanasupong A., Nagase H., Sugimoto E., Hori Y., Hirata K., Tani K., et al. (2004). Degradation of carbendazim and 2, 4-dichlorophenoxyacetic acid by immobilized consortium on loofa sponge. J. Biosci. Bioeng. 98 28–33. 10.1016/S1389-1723(04)70238-8 [DOI] [PubMed] [Google Scholar]
- Paul K. C., Chuang Y.-H., Cockburn M., Bronstein J. M., Horvath S., Ritz B. (2018). Organophosphate pesticide exposure and differential genome-wide DNA methylation. Sci. Total Environ. 645 1135–1143. 10.1016/j.scitotenv.2018.07.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira V. J., da Cunha J. P. A. R., de Morais T. P., Ribeiro-Oliveira J. P., de Morais J. B. (2016). Physical-chemical properties of pesticides: concepts, applications, and interactions with the environment. Biosci. J. 32 627–641. [Google Scholar]
- Perez-Lucas G., Vela N., El Aatik A., Navarro S. (2018). “Environmental risk of groundwater pollution by pesticide leaching through the soil profile,” in Pesticides, Anthropogenic Activities and the Health of our Environment, (London: IntechOpen; ), 10.5772/intechopen.82418 [DOI] [Google Scholar]
- Pignatello J. J., Xing B. (1995). Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 30 1–11. 10.1021/es940683g [DOI] [Google Scholar]
- Pimpao C., Zampronio A., De Assis H. S. (2007). Effects of deltamethrin on hematological parameters and enzymatic activity in Ancistrus multispinis (Pisces, Teleostei). Pest. Biochem. Phys. 88 122–127. 10.1016/j.pestbp.2006.10.002 [DOI] [Google Scholar]
- Pizzorno J. (2018). Environmental toxins and infertility. Integr. Med. 17:8. [PMC free article] [PubMed] [Google Scholar]
- Pokhrel B., Gong P., Wang X., Chen M., Wang C., Gao S. (2018). Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemo 200 532–541. 10.1016/j.chemosphere.2018.01.119 [DOI] [PubMed] [Google Scholar]
- Pozo K., Harner T., Lee S. C., Sinha R. K., Sengupta B., Loewen M., et al. (2011). Assessing seasonal and spatial trends of persistent organic pollutants (POPs) in Indian agricultural regions using PUF disk passive air samplers. Environ. Pol. 159 646–653. 10.1016/j.envpol.2010.09.025 [DOI] [PubMed] [Google Scholar]
- Pradeep V., Subbaiah U. M. (2016). Use of Ca-alginate immobilized Pseudomonas aeruginosa for repeated batch and continuous degradation of Endosulfan. 3 Bio 6 1–13. 10.1007/s13205-016-0438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F., Gao Y. X., Guo B. Y., Xu P., Li J. Z., Wang H. L. (2014). Environmental behavior of benalaxyl and furalaxyl enantiomers in agricultural soils. J. Environ. Sci. Health Part B 49 738–746. 10.1080/03601234.2014.929482 [DOI] [PubMed] [Google Scholar]
- Qu C., Albanese S., Lima A., Hope D., Pond P., Fortelli A., et al. (2019). The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: implications for sources and environmental processes. Environ. Int. 124 89–97. 10.1016/j.envint.2018.12.031 [DOI] [PubMed] [Google Scholar]
- Radwan M., Jurewicz J., Wielgomas B., Piskunowicz M., Sobala W., Radwan P., et al. (2015). The association between environmental exposure to pyrethroids and sperm aneuploidy. Chemo 128 42–48. 10.1016/j.chemosphere.2014.12.077 [DOI] [PubMed] [Google Scholar]
- Rahman M. A., Arefin A. S., Saha O., Rahaman M. M. (2018). Isolation and identification of pesticides degrading bacteria from farmland soil. Bang. J. Microbiol. 35 90–94. 10.3329/bjm.v35i2.42635 [DOI] [Google Scholar]
- Ramaseshadri P., Farkaš R., Palli S. R. (2012). Recent progress in juvenile hormone analogs (JHA) research. Adv. Ins. Phys. 43 353–436. [Google Scholar]
- Rani K., Dhania G. (2014). Bioremediation and biodegradation of pesticide from contaminated soil and water - a noval approach. Int. J. Curr. Microbiol. App. Sci. 3 23–33. [Google Scholar]
- Rath B. (2012). Microalgal bioremediation: current practices and perspectives. J. Biochem. Technol. 3 299–304. [Google Scholar]
- Rehim H., Hegazy E., El-Barbary A. (2009). Radiation modification of natural polysaccharides for applacation in agriculture. Poly. 50 1952–1957. [Google Scholar]
- Requena M., López-Villén A., Hernández A. F., Parrón T., Navarro Á, Alarcón R. (2019). Environmental exposure to pesticides and risk of thyroid diseases. Toxicol. Lett. 315 55–63. 10.1016/j.toxlet.2019.08.017 [DOI] [PubMed] [Google Scholar]
- Rezania S., Park J., Din M. F. M., Taib S. M., Talaiekhozani A., Yadav K. K., et al. (2018). Microplastics pollution in different aquatic environments and biota: a review of recent studies. Mar. Pol. Bull. 133 191–208. 10.1016/j.marpolbul.2018.05.022 [DOI] [PubMed] [Google Scholar]
- Rigas F., Dritsa V., Marchant R., Papadopoulou K., Avramides E., Hatzianestis I. (2005). Biodegradation of lindane by Pleurotus ostreatus via central composite design. Environ. Int. 31, 191–196. 10.1016/j.envint.2004.09.024 [DOI] [PubMed] [Google Scholar]
- Rios F., Kalinin A., Rantin F. (2002). The effects of long-term food deprivation on respiration and haematology of the neotropical fish Hoplias malabaricus. J. Fish Biol. 61 85–95. 10.1111/j.1095-8649.2002.tb01738.x [DOI] [Google Scholar]
- Rissato S. R., Galhiane M. S., Fernandes J. R., Gerenutti M., Gomes H. M., Ribeiro R., et al. (2015). Evaluation of Ricinus communis L. for the phytoremediation of polluted soil with organochlorine pesticides. Biomed. Res. Int. 8:549863. 10.1155/2015/549863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. R. (1984). Non-extractable pesticide residues in soils and plants. Pur. Appl. Chem. 56 945–956. 10.1351/pac198456070945 [DOI] [Google Scholar]
- Romero-Aguilar M., Tovar-Sánchez E., Sánchez-Salinas E., Mussali-Galante P., Sánchez-Meza J. C., Castrejón-Godínez M. L., et al. (2014). Penicillium sp. as an organism that degrades endosulfan and reduces its genotoxic effects. Springer Plus 3 1–11. 10.1186/2193-1801-3-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell G., Quero C., Coll J., Guerrero A. (2008). Biorational insecticides in pest management. J. Pest. Sci. 33 103–121. 10.1584/jpestics.R08-01 27476087 [DOI] [Google Scholar]
- Różański L. (1992). Przemiany pestycydów w organizmach żywych i środowisku. Warszawa: PWRiL. [Google Scholar]
- Russo F., Ceci A., Pinzari F., Siciliano A., Guida M., Malusà E., et al. (2019). Bioremediation of dichlorodiphenyltrichloroethane (DDT)-contaminated agricultural soils: potential of two autochthonous saprotrophic fungal strains. Appl. Environ. Microbiol. 85 e1720–e1719. 10.1128/AEM.01720-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar V., Singh D. P. (2011). Biodegradation of lindane pesticide by non white-rots soil fungus Fusarium sp. World J. Microbiol. Biotechnol. 27 1747–1754. 10.1007/s11274-010-0628-8 [DOI] [Google Scholar]
- Salazar-Arredondo E., De Jesús Solís-Heredia M., Rojas-García E., Hernández-Ochoa I., Quintanilla-Vega B. (2008). Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reproduct. Toxicol. 25 455–460. 10.1016/j.reprotox.2008.05.055 [DOI] [PubMed] [Google Scholar]
- Salome C., Marks G., Savides P., Xuan W., Woolcock A. (2000). The effect of insecticide aerosols on lung function, airway responsiveness and symptoms in asthmatic subjects. Eur. Respir. J. 16 38–43. 10.1034/j.1399-3003.2000.16a07.x [DOI] [PubMed] [Google Scholar]
- Saquib Q., Al-Khedhairy A. A., Alarifi S. A., Dutta S., Dasgupta S., Musarrat J. (2010). Methyl thiophanate as a DNA minor groove binder produces MT–Cu (II)–DNA ternary complex preferably with AT rich region for initiation of DNA damage. Int. J. Bio. Macromol. 47 68–75. 10.1016/j.ijbiomac.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Saravanan A., Kumar P. S., Jeevanantham S., Harikumar P., Bhuvaneswari V., Indraganti S. (2022). Identification and sequencing of bacteria from crop field: Application of bacteria—agro-waste biosorbent for rapid pesticide removal. Environ. Technol. Innovat. 25:102116. 10.1016/j.eti.2021.102116 [DOI] [Google Scholar]
- Scholz R., Herrmann M., Michalski B. (2017). Compilation of processing factors and evaluation of quality controlled data of food processing studies. J. Consum. Protect. Food Saf. 12 3–14. 10.1007/s00003-016-1043-3 [DOI] [Google Scholar]
- Schreinemachers P., Afari-Sefa V., Heng C. H., Dung P. T. M., Praneetvatakul S., Srinivasan R. (2015). Safe and sustainable crop protection in Southeast Asia: status, challenges and policy options. Environ. Sci. Pol. 54 357–366. 10.1016/J.ENVSCI.2015.07.017 [DOI] [Google Scholar]
- Schreinemachers P., Tipraqsa P. (2012). Agricultural pesticides and land use intensification in high, middle and low income countries. Food Pol. 37 616–626. 10.1016/j.foodpol.2012.06.003 [DOI] [Google Scholar]
- Shahzad B., Tanveer M., Che Z., Rehman A., Cheema S. A., Sharma A., et al. (2018). Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol. Environ. Saf 147 935–944. 10.1016/j.ecoenv.2017.09.066 [DOI] [PubMed] [Google Scholar]
- Sharbidre A. A., Metkari V., Ka Patode P. (2011). Effect of diazinon on acetylcholinesterase activity and lipid. Res. J. Environ. Toxicol. 5 152–161. 10.3923/rject.2011.152.161 [DOI] [Google Scholar]
- Sharma A., Kumar V., Kanwar M., Thukral A., Bhardwaj R. (2017a). Ameliorating imidacloprid induced oxidative stress by 24-epibrassinolide in Brassica juncea L. Rus. J. Plant Phys. 64 509–517. 10.1134/S1021443717040124 [DOI] [Google Scholar]
- Sharma A., Thakur S., Kumar V., Kesavan A. K., Thukral A. K., Bhardwaj R. (2017b). 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol. 17:56. 10.1186/s12870-017-1003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Kumar V., Bhardwaj R., Thukral A. K. (2017c). Seed pre-soaking with 24-epibrassinolide reduces the imidacloprid pesticide residues in green pods of Brassica juncea L. Toxicol. Environ. Chem. 99 95–103. 10.1080/02772248.2016.1146955 [DOI] [Google Scholar]
- Sharma A., Kumar V., Kumar R., Shahzad B., Thukral A. K., Bhardwaj R. (2018a). Brassinosteroid-mediated pesticide detoxification in plants: a mini-review. Cogn. Food Agric. 4:1436212. 10.1080/23311932.2018.1436212 [DOI] [Google Scholar]
- Sharma A., Kumar V., Yuan H., Kanwar M. K., Bhardwaj R., Thukral A. K., et al. (2018b). Jasmonic acid seed treatment stimulates insecticide detoxification in Brassica juncea L. Front. Plant Sci. 9:1609. 10.3389/fpls.2018.01609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Kumar V., Shahzad B., Tanveer M., Sidhu G. P. S., Handa N., et al. (2019). Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1 1–16. 10.1007/s42452-019-1485-1 [DOI] [Google Scholar]
- Sharma A., Kumar V., Singh R., Thukral A. K., Bhardwaj R. (2016a). Effect of seed pre-soaking with 24-epibrassinolide on growth and photosynthetic parameters of Brassica juncea L. in imidacloprid soil. Ecotoxicol. Environ. Saf. 133 195–201. 10.1016/j.ecoenv.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Sharma A., Bhardwaj R., Kumar V., Thukral A. K. (2016b). GC-MS studies reveal stimulated pesticide detoxification by brassinolide application in Brassica juncea L. plants. Environ. Sci. Pol. Res. 23 14518–14525. 10.1007/s11356-016-6650-0 [DOI] [PubMed] [Google Scholar]
- Sharma A., Thakur S., Kumar V., Kanwar M. K., Kesavan A. K., Thukral A. K., et al. (2016c). Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front. Plant Sci. 7:1569. 10.3389/fpls.2016.01569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma I., Bhardwaj R., Pati P. K. (2015). Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J. Plant Growth Regul. 34 509–518. [Google Scholar]
- Sharples C. R., Hull M. R., Cobb A. H. (1997). Growth and photosynthetic characteristics of two biotypes of the weed black-grass (Alopecurus myosuroidesHuds.) resistant and susceptible to the herbicide chlorotoluron. Ann. Bot. 79 455–461. [Google Scholar]
- Siddique T., Okeke B. C., Arshad M., Frankenberger W. T. (2003). Enrichment and isolation of endosulfan-degrading microorganisms. J. Environ. Qual. 32 47–54. 10.2134/jeq2003.4700 [DOI] [PubMed] [Google Scholar]
- Silva V., Mol H. G., Zomer P., Tienstra M., Ritsema C. J., Geissen V. (2019). Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total. Environ. 653 1532–1545. 10.1016/j.scitotenv.2018.10.441 [DOI] [PubMed] [Google Scholar]
- Singh B. K., Kuhad R. C., Singh A., Lal R., Tripathi K. (1999). Biochemical and molecular basis of pesticide degradation by microorganisms. Crit. Rev. Biotechnol. 19 197–225. 10.1080/0738-859991229242 [DOI] [PubMed] [Google Scholar]
- Singh B. K., Walker A. (2006). Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 30 428–471. 10.1111/j.1574-6976.2006.00018.x [DOI] [PubMed] [Google Scholar]
- Singh D. V., Ali R., Kulsum M., Bhat R. A. (2020). “Ecofriendly approaches for remediation of pesticides in contaminated environs,” in Bioremediation and Biotechnology, eds Bhat R., Hakeem K., Saud Al-Saud N. (Cham: Springer; ), 10.1007/978-3-030-46075-4_8 [DOI] [Google Scholar]
- Singh J. S., Pandey V. C., Singh D. P. (2011). Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 140 339–353. 10.1016/j.agee.2011.01.017 [DOI] [Google Scholar]
- Sniegowski K., Springael D. (2015). Establishment of multiple pesticide biodegradation capacities from pesticide-primed materials in on-farm biopurification system microcosms treating complex pesticide-contaminated wastewater. Pest Manag. Sci. 71 986–995. 10.1002/ps.3876 [DOI] [PubMed] [Google Scholar]
- Socorro J., Durand A., Temime-Roussel B., Gligorovski S., Wortham H., Quivet E. (2016). The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci. Rep. 6 1–7. 10.1038/srep33456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto A. L., Sylvestre M., Tölke E. D., Tavares J. F., Barbosa-Filho J. M., Cebrián-Torrejón G. (2021). Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: prospects, applications and challenges. Molecules 26:4835. 10.3390/molecules26164835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens K., Larsbo M., Moeys J., Jarvis N., Lewan E. (2013). Predicting pesticide leaching under climate change: Importance of model structure and parameter uncertainty. Agri. Ecol. Environ. 172 24–34. 10.1016/j.agee.2013.03.018 [DOI] [Google Scholar]
- Stehle S., Schulz R. (2015). Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. 112 5750–5755. 10.1073/pnas.1500232112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subashchandrabose S. R., Ramakrishnan B., Megharaj M., Venkateswarlu K., Naidu R. (2011). Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol. Adv. 29 896–907. 10.1016/j.biotechadv.2011.07.009 [DOI] [PubMed] [Google Scholar]
- Subashini H. D., Sekar S., Devi V. R. S., Rajam A., Malarvannan S. (2007). Biodegradation of pesticidal residue using traditional plants with medicinal properties and Trichoderma. Res. J. Environ. Toxicol. 1 124–130. 10.3923/rjet.2007.124.130 [DOI] [Google Scholar]
- Tahir R., Ghaffar A., Abbas G., Turabi T. H., Kausar S., Xiaoxia D., et al. (2021). Pesticide induced hematological, biochemical and genotoxic changes in fish: a review. Agrobiol. Rec. 3 41–57. [Google Scholar]
- Tallur P. N., Mulla S. I., Megadi V. B., Talwar M. P., Ninnekar H. Z. (2015). Biodegradation of cypermethrin by immobilized cells of Micrococcus sp. strain CPN 1. Braz. J. Microbiol. 46 667–672. 10.1590/S1517-838246320130557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Zhang G., Pan J., Xiong C. (2016). Deciphering the groove binding modes of tau-fluvalinate and flumethrin with calf thymus DNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 155 28–37. 10.1016/j.saa.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Tao M., Zhang G., Xiong C., Pan J. (2015). Characterization of the interaction between resmethrin and calf thymus DNA in vitro. New J. Chem. 39 3665–3674. [Google Scholar]
- Thakur S., Dhiman M., Mantha A. K. (2018). APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells. Mol. Cell Biochem. 441 201–216. 10.1007/s11010-017-3186-7 [DOI] [PubMed] [Google Scholar]
- Thomas P., Dong J. (2019). Novel mechanism of endocrine disruption by fungicides through binding to the membrane androgen receptor, ZIP9 (SLC39A9), and antagonizing rapid testosterone induction of the intrinsic apoptotic pathway. Steroids 149:108415. 10.1016/j.steroids.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Tiedje J. M., Duxbury J., Alexander M., Dawson J. (1969). 2, 4-D metabolism: pathway of degradation of chlorocatechols by Arthrobacter sp. J. Agric. Food Chem. 17 1021–1026. 10.1021/jf60165a037 [DOI] [PubMed] [Google Scholar]
- Tomer N. (2013). Determination of chlorinated pesticide in vegetables, cereals and pulses by gas chromatography in east national capital region, Delhi, India. Res. J. Agric. Sci. 1 27–28. [Google Scholar]
- Torres E. M., Hess D., McNeil B. T., Guy T., Quinn J. C. (2017). Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol. Environ. Saf. 139 367–376. 10.1016/j.ecoenv.2017.01.034 [DOI] [PubMed] [Google Scholar]
- Tort N., Turkyilmaz B. (2003). Physiological effects of captan fungicide on pepper (Capsicum annuum L.) plant. Pak. J. Bio. Sci. 6 2026–2029. 10.3923/pjbs.2003.2026.2029 [DOI] [Google Scholar]
- Tortella G. R., Diez M. C., Durán N. (2005). Fungal diversity and use in decomposition of environmental pollutants. Crit. Rev. Microbiol 31 197–212. 10.1080/10408410500304066 [DOI] [PubMed] [Google Scholar]
- Tudi M., Daniel Ruan H., Wang L., Lyu J., Sadler R., Connell D., et al. (2021). Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 18:1112. 10.3390/ijerph18031112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah S., Hasan Z., Zuberi A., Younus N., Rauf S. (2014). Comparative study on body composition of two chinese carps, common carp (Cyprinus carpio) and silver carp (Hypophthalmichthys molitrix). Glob. Vet. 13 867–876. 10.5829/idosi.gv.2014.13.05.86234 [DOI] [Google Scholar]
- United States. Environmental Protection Agency (2004). Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs, US Environmental Protection Agency: Endangered and Threatened Species Effects Determinations. Collingdale, PA: DIANE Pub. [Google Scholar]
- Unsworth J. (2010). History of pesticide use. International Union of pure and applied chemistry (IUPAC). North Carolina: IUPAC [Google Scholar]
- United States Environmental Protection Agency [US-EPA] (2022). Pesticide to Control Bed Bug. Available online at: https://www.epa.gov/bedbugs/pesticides-control-bed-bugs#desiccants (accessed April 4, 2022). [Google Scholar]
- Van der Plaat D. A., de Jong K., de Vries M., van Diemen C. C., Nedeljković I., Amin N., et al. (2018). Occupational exposure to pesticides is associated with differential DNA methylation. Occup. Environ. Med. 75, 427–435. 10.1136/oemed-2017-104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwenhuyse P., Demaeght P., Dermauw W., Khalighi M., Stevens C., Vanholme B., et al. (2012). On the mode of action of bifenazate: new evidence for a mitochondrial target site. Pest. Biochem. Phys. 104 88–95. 10.1016/j.pestbp.2012.05.013 [DOI] [Google Scholar]
- Velasquez L., Dussan J. (2009). Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Haz. Mater. 167 713–716. 10.1016/j.jhazmat.2009.01.044 [DOI] [PubMed] [Google Scholar]
- Wagh V., Mukate S., Muley A., Kadam A., Panaskar D., Varade A. (2020). Study of groundwater contamination and drinking suitability in basaltic terrain of Maharashtra, India through PIG and multivariate statistical techniques. J. Water Sup. Res. Technol. Aquat. 69 398–414. 10.2166/aqua.2020.108 [DOI] [Google Scholar]
- Wang H., Meng Z., Liu F., Zhou L., Su M., Meng Y., et al. (2020). Characterization of boscalid-induced oxidative stress and neurodevelopmental toxicity in zebrafish embryos. Chem. 238:124753. 10.1016/j.chemosphere.2019.124753 [DOI] [PubMed] [Google Scholar]
- Warren G. (1998). Spectacular increases in crop yields in the United States in the twentieth century. Weed Technol. 12 752–760. [Google Scholar]
- Williams G. P., Darbre P. D. (2019). Low-dose environmental endocrine disruptors, increase aromatase activity, estradiol biosynthesis and cell proliferation in human breast cells. Mol. Cell Endocrinol. 486 55–64. 10.1016/j.mce.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Winteringham F. (1971). Some global aspects of pesticide residue problems. Isr. J. Entomol. 6 171–181. [Google Scholar]
- Witczak A., Pohoryło A., Abdel-Gawad H. (2021). Endocrine-disrupting organochlorine pesticides in human breast milk: changes during lactation. Nutrition. 13:229. 10.3390/nu13010229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow J. E., Gibson K. A., Seiber J. N. (2018). Pesticides and related toxicants in the atmosphere. Rev. Environ. Conta. Toxicol. 247 147–196. 10.1007/398_2018_19 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2009). Children’s Health and the Environment. WHO Training Package for the Health Sector-World Health Organization. Geneva: World Health Organization [Google Scholar]
- World Health Organization (2015). International Code of Conduct on Pesticide Management: Guidelines on Pesticide Legislation. Geneva: World Health Organization [Google Scholar]
- Wu S., Zhang X., Sun Y., Wu Z., Li T., Hu Y., et al. (2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS. TEM–EDS, and XAFS. Environ. Sci. Technol. 49 14036–14047. 10.1021/acs.est.5b03659 [DOI] [PubMed] [Google Scholar]
- Wu Y., Li W., Yuan M., Liu X. (2020). The synthetic pyrethroid deltamethrin impairs zebrafish (Danio rerio) swim bladder development. Sci. Total Environ. 701:134870. 10.1016/j.scitotenv.2019.134870 [DOI] [PubMed] [Google Scholar]
- Xia X. J., Huang Y. Y., Wang L., Huang L. F., Yu Y. L., Zhou Y. H., et al. (2006). Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pest. Biochem Phys. 86 42–48. 10.1016/j.pestbp.2006.01.005 [DOI] [Google Scholar]
- Xia X. J., Zhang Y., Wu J. X., Wang J. T., Zhou Y. H., Shi K., et al. (2009). Brassinosteroids promote metabolism of pesticides in cucumber. J. Agric. Food Chem. 57 8406–8413. 10.1021/jf901915a [DOI] [PubMed] [Google Scholar]
- Xiao P., Kondo R. (2020). Potency of Phlebia species of white rot fungi for the aerobic degradation, transformation and mineralization of lindane. J. Microbiol. 58 395–404. 10.1007/s12275-020-9492-x [DOI] [PubMed] [Google Scholar]
- Xu X., Nembhard W. N., Kan H., Becker A., Talbott E. O. (2012). Residential pesticide use is associated with children’s respiratory symptoms. J. Occup. Environ. Med. 54 1281–1287. 10.1097/JOM.0b013e31825cb6ae [DOI] [PubMed] [Google Scholar]
- Yadav I. C., Devi N. L. (2017). Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 6 140–158. [Google Scholar]
- Yadav I. C., Devi N. L., Syed J. H., Cheng Z., Li J., Zhang G., et al. (2015). Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: a comprehensive review of India. Sci. Total Environ. 511 123–137. 10.1016/j.scitotenv.2014.12.041 [DOI] [PubMed] [Google Scholar]
- Yan S., Meng Z., Tian S., Teng M., Yan J., Jia M., et al. (2020). Neonicotinoid insecticides exposure cause amino acid metabolism disorders, lipid accumulation and oxidative stress in ICR mice. Chemo 246:125661. 10.1016/j.chemosphere.2019.125661 [DOI] [PubMed] [Google Scholar]
- Yanez-Ocampo G., Wong-Villarreal A., Del Aguila-Juarez P., Lugo-de la Fuente J., Vaca-Paulin R. (2016). Composting of soils polluted with pesticides: a microbial approach and methods for monitoring. JSM Environ. Sci. Ecol. 4:1032. 10.1016/j.envint.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Yengkokpam P., Mazumder P. B. (2020). Phytotoxicity of malathion (PM) and tatafen (PTF) towards Solanum melongena L. cv. Longai: a case study. Plant Phys. Rep. 25 149–156. 10.1007/s40502-019-00498-0 [DOI] [Google Scholar]
- Yigit N., Velioglu Y. S. (2020). Effects of processing and storage on pesticide residues in foods. Crit. Rev. Food Sci. Nutr. 60 3622–3641. 10.1080/10408398.2019.1702501 [DOI] [PubMed] [Google Scholar]
- Young H. A., Meeker J. D., Martenies S. E., Figueroa Z. I., Barr D. B., Perry M. J. (2013). Environmental exposure to pyrethroids and sperm sex chromosome disomy: a cross-sectional study. Environ. Health 12 1–9. 10.1186/1476-069X-12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Želježić D., Žunec S., Bjeliš M., Benković V., Mladinić M., Lovaković Tariba B., et al. (2018). Effects of the chloro-s-triazine herbicide terbuthylazine on DNA integrity in human and mouse cells. Environ. Sci. Pol. Res. 25 19065–19081. 10.1007/s11356-018-2046-7 [DOI] [PubMed] [Google Scholar]
- Zhan H., Huang Y., Lin Z., Bhatt P., Chen S. (2020). New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 182:109138. 10.1016/j.envres.2020.109138 [DOI] [PubMed] [Google Scholar]
- Zhang C., Schilirò T., Gea M., Bianchi S., Spinello A., Magistrato A., et al. (2020). Molecular basis for endocrine disruption by pesticides targeting aromatase and estrogen receptor. Int. J. Environ. Res. Public Health 17:5664. 10.3390/ijerph17165664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Lin Z., Pang S., Bhatt P., Chen S. (2020). Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system. Front. Microbiol. 11:522. 10.3389/fmicb.2020.00522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Huang X., Liu H., Liu W., Liu J. (2018). Novel pathways of endocrine disruption through pesticides interference with human mineralocorticoid receptors. Toxicol. Sci. 162 53–63. 10.1093/toxsci/kfx244 [DOI] [PubMed] [Google Scholar]
- Zhang W. (2018). Global pesticide use: profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8:1. [Google Scholar]
- Zhang W., Li J., Zhang Y., Wu X., Zhou Z., Huang Y., et al. (2022). Characterization of a novel glyphosate-degrading bacterial species, chryseobacterium sp. Y16C, and evaluation of its effects on microbial communities in glyphosate-contaminated soil. J. Haz. Mat. 432:128689. 10.1016/j.jhazmat.2022.128689 [DOI] [PubMed] [Google Scholar]
- Zhang W., Pang S., Lin Z., Mishra S., Bhatt P., Chen S. (2021). Biotransformation of perfluoroalkyl acid precursors from various environmental systems: Advances and perspectives. Environ. Pollut. 272:115908. 10.1016/j.envpol.2020.115908 [DOI] [PubMed] [Google Scholar]
- Zhang W.-J., van der Werf W., Pang Y. (2011). A simulation model for vegetable-insect pest-insect nucleopolyhedrovirus epidemic system. J. Environ. Entomol. 33 283–301. [Google Scholar]
- Zhang Y., Pan J., Zhang G., Zhou X. (2015). Intercalation of herbicide propyzamide into DNA using acridine orange as a fluorescence probe. Sensors Actuators B Chem. 206 630–639. 10.1016/j.snb.2014.09.114 [DOI] [Google Scholar]
- Zhen D., Shen J. Y., Hong J. W., Zhang R., Zheng L., Qi W., et al. (2020). Inhibitory Effects of Cypermethrin on Interactions of the Androgen Receptor with Coactivators ARA70 and ARA55. Biomed. Environ. Sci. 33 158–164. 10.3967/bes2020.022 [DOI] [PubMed] [Google Scholar]
- Zucca P., Sanjust E. (2014). Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules 19 14139–14194. 10.3390/molecules190914139 [DOI] [PMC free article] [PubMed] [Google Scholar]