Abstract
SARS-CoV-2 infection arose in 2019 and has changed life as we know it. With our ever-advancing knowledge, therapies, and vaccines, more functions of the SARS-CoV-2 virus are being investigated outside of its pulmonary invasion. Here, we set out to review the current and pertinent literature on the impact of SARS-CoV-2 on the male genitourinary system including the bladder, lower urinary tract, prostate, testis, and penis. The biggest newsworthy stake was if SARS-CoV-2 could be transmitted through semen. Although initially thought to occur, more recent studies have opposed this hypothesis. Outside of the reproductive spread of SARS-CoV-2, multiple studies in this review highlight where the virus resides and what effect it may be having on this genitourinary system including increased voiding problems, viral persistence months after systemic clearance, and rare penile complications post-infection. Long-term outcomes are still needed to fully understand how SARS-CoV-2 infection can alter the genitourinary system.
Keywords: SARS-CoV-2, male reproduction, prostate, bladder, testes
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first identified in the fall of 2019. Since then, several reports have described the novelty of this virus and characterized the respiratory pathogenesis of this virus. There are few studies however that comment on the virulence in the male genitourinary (GU) tract, highlighting worsening urgency, frequency, nocturia, and gross hematuria. This review sets out to combine the currently known literature in a cohesive manner of the male genitourinary system. The understanding of SARS-CoV-2 in the genitourinary tract is of the utmost importance since the virus has been identified in urine among other common fluids such as respiratory droplets, sweat, tears, and feces.
The male GU system has served as a host for 27 different viruses [1]. Of the 27, 11 viruses were shown to have viability in the testes including Zika, Mumps, and the Ebola virus. Further these viruses have been shown to exist in the infected host’s semen. Similarly, to these aforementioned virus’, initial evidence demonstrated the existence of SARS-CoV-2 in the semen of 6/38 patients [2]. This initial finding has failed to be demonstrated again in the literature [3-6].
To enter the cell, the SARS-CoV2 spike protein must bind to the ACE2 receptor on the host cell [7]. Once bound, human proteases will cleave the virus allowing for entry into the host cell, where it is well characterized that ACE2 may act as target for SARS-CoV-2 invasion [8]. Zou and colleagues used a threshold of 1% ACE2-positive cells (based on ACE2 expression in type 2 alveolar cells) to identify organs at risk for SARS-CoV-2 invasion [9]. Both the renal proximal tubules and bladder urothelial cells expressed ACE2 receptors above the threshold set at 4% and 2.4% respectively, making them high risk targets for SARS-CoV-2 invasion in the setting of viremia [9]. The prostate is uniquely vulnerable to the SARS-CoV-2 virus because it demonstrates TMPRSS2 protease activity and the presence of ACE2 receptors [10]. In addition to its role in the pathogenesis of SARS-CoV-2, The TMPRSS2 gene is frequently implicated in primary prostate cancer where it is constitutively active [11]. Glandular cells of the prostate, hillock, and club, demonstrate both ACE2 and TMPRSS2 expression, which may further the risk of normal prostatic tissue being infected [12,13] (Figure 1).
Figure 1.
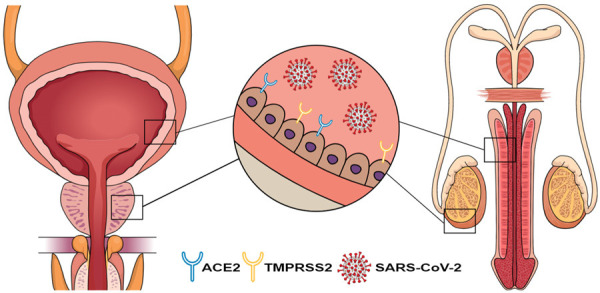
Theorized pathogenesis of COVID-19 infection on male genitourinary organs. ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease, serine 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Due to a paucity of human data investigating the pathophysiology of SARS-CoV-2 infection, Madden and colleagues have published a pre-print manusctipt characterized COVID locations in Rhesus monkeys following infections of different strains of the virus through the GU tract [14]. They found viral uptake throughout the male gential track within a few weeks of infections. They noted a stronger viral load in the prostate, testis, penile tissue, and pampiniform plexus. Further the more advanced the SARS-CoV-2 virus, the more infectious in the male gential tract.
Discussion
All studies have been formatted into Table 1 at the end of the review.
Table 1.
Studies and reported outcomes included in this review
| Studies [ref.] | City and country | No. of COVID-19 patients | Reported outcomes |
|---|---|---|---|
| Reproductive organ involvement | |||
| Pecoraro et al. [15] | Florence, Italy | 10 | No difference in the inflammatory response in the prostate following SARS-CoV-2 infection |
| Chakravarty et al. [16] | New York, New York | 228 | Higher rates of hospitalization and mortality in prostate cancer patients with COVID-19 vs other genitourinary malignancies |
| Yang et al. [34] | Wuhan, China | 12 | Autopsied testes revealed significantly damaged seminiferous tubules, reduced Leydig cells and lymphocytic inflammation; one patient with detectable viral load within testicular tissue |
| Li et al. [35] | Wuhan, China | 6 | Autopsied testes revealed increased apoptotic cells within the seminiferous tubules and interstitial T-lymphocytes and macrophages |
| Ma et al. [36] | Wuhan, China | 5 | Autopsied testes revealed germ cells within the lumen of seminiferous tubules and detectable SARS-CoV-2 viral particles on electron micrograph in two patients |
| Achua et al. [37] | Miami, Florida | 6 | Inverse association between ACE-2 receptor levels in the testes and spermatogenesis, suggesting a potential mechanism for COVID-19 induced infertility |
| Ediz et al. [40] | Istanbul, Turkey | 10 | New onset scrotal or testicular pain in men with concomitant COVID-19 infection |
| Chen et al. [41] | Wuhan, China | 142 | Acute orchitis, epididymitis, or epididymo-orchitis in men with confirmed COVID-19 infection |
| Best et al. [39] | Miami, Florida | 30 | Total sperm in semen decreased from 59.7 million to 12.5 million per ejaculate |
| Alkhatatbeh et al. [42] | Amman, Jordan | 253 | No reports of testicular or scrotal pain in men with COVID-19 infection |
| Yao et al. [43] | Chongqing, China | 3 | COVID-19 spike protein was found to be present on endothelial cells of the seminal vesicles and epididymis, suggesting that the novel virus is able to penetrate the blood-testis barrier |
| Kresch et al. [46] | Miami, Florida | 2 | Detectable viral particles near penile endothelial cells and decreased corporal nitric oxide synthase expression in men with a history of COVID-19 infection undergoing penile prosthesis placement |
| Lessiani et al. [48] | Città Sant’Angelo, Italy | 1 | Acute superficial vein thrombosis of the penis (Mondor’s disease) in a young male with concomitant COVID-19 infection |
| Balawender et al. [49] | Rzeszow, Poland | 1 | 34-year-old post-SARS-CoV-2 infection with diagnosed Mondor Disease |
| Eren et al. [50] | Instanbul, Turkey | 1 | 33-year-old post-SARS-Cov-2 infection with diagnosed Mondor Disease |
| Sarkis et al. [51] | Beirut, Lebanon | 1 | 58-year-old male with an ischemic penis following SARS-CoV-2 infection |
| Ozbey et al. [52] | Malatya, Turkey | 1 | 71-year-old male with digital and penile necrosis 18 days after his diagnosis of SARS-CoV-2 infection |
| Lam et al. [53] | Pembrokeshire, United Kingdom | 1 | 67-year-old male with severe SARS-CoV-2 infection developed priapism on his last day of life. Authors suggest this may have been due to hypercoaguable nature of the virus |
| Grimberg et al. [55] | Durham, NC | 1 | 45-year-old male with an erection two hours after his emergency room presentation. The erection was treated and recurred eight hours later |
| Alsaedi et al. [54] | Makkah, Saudi Arabia | 1 | 66-year-old male with a 3 day history of ischemic priapism that eventually lead to gangrene. He was diagnosed with SARS-CoV-2 5 days prior to his presentation with priapism |
| Urinary tract involvement | |||
| Kaya et al. [18] | Eskişehir, Turkey | 19 M | Significant difference in storage IPSS scores among three time periods (pre-COVID, during hospitalization and post-hospitalization) in patients with COVID-19 |
| 27 F | |||
| Can et al. [20] | Istanbul, Turkey | 94 | Significant increase in IPSS scores among COVID-19 positive men over the age of 50 |
| Karabulut et al. [19] | Erzurum, Turkey | 63 | Men with COVID-19 and severe BPH-related LUTS exhibited longer duration of hospitalization, more frequent intensive care requirements and higher mortality rates when compared to men with mild to moderate BPH-related LUTS |
| Lamb et al. [27] | Royal Oak, Michigan | 8 | De novo urinary symptoms and elevated urinary inflammatory cytokines in COVID-19 patients |
| Mumm et al. [24] | Munich, Germany | 7 | Increased urinary frequency in men hospitalized with COVID-19 in the absence of any other causes |
| Dhar et al. [28] | Detroit, Michigan | 32 M | De novo urinary frequency, urgency, urge incontinence and nocturia in COVID-19 patients |
| 7 F | |||
| Luciana et al. [30] | Trento, Italy | 3 | Severe gross hematuria requiring hospital admission and procedural/operative intervention in men with a history of mild hematuria related to either BPH or radiation cystitis |
| Djafari et al. [31] | Tehran, Iran | 1 | Severe gross hematuria requiring hospital admission and operative intervention in a COVID-19 patient with renal amyloidosis and newly diagnosed bladder amyloidosis |
| Mehraban-Far et al. [32] | New York, New York | 157 | Gross hematuria associated with poor renal function, higher intubation rates, increased mortality and elevated inflammatory biomarkers in COVID-19 patients when compared to patients with no hematuria or microscopic hematuria |
| Costa et al. [44] | Belo Horizonte, Brazil | 11 | SARS-CoV-2 can be found in macrophage and spermatogonial cells where it can replicate in the endoplasmic reticulum |
| Davis et al. [45] | International Survey | 3,762 | A long term study looking at sexual function 7 months following SARS-CoV-2 infection. 15% of patients had sexual dysfunction, 10% had pain in their testicules and 5% with deceased testicular size |
COVID-19, Coronavirus Disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE-2, angiotensin-converting enzyme 2; M, male; F, female; IPSS, International Prostate Symptom Score; BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms.
Prostate & LUTS
The underlying pathophysiology of SARS-CoV-2 on prostatic tissue remains controversial. The pre-print in rhesus monkeys showed that the virus can be found in the prostatic tissue [14]. Further work supported this where SARS-CoV-2 was found in the club and hillock cells of the prostate had positive viral titers [12,13]. However, how long the virus remains in the prostate following infection remains unknown. A recent study demonstrated that there was no SARS-CoV-2-driven inflammation of the prostate among their ten patients who had a COVID infection after prostate biopy but before prostatectomy [15]. This study is severly limited due to sample size, lack of follow-up, and selection bias; whereas each patient had prostate cancer and their cancer may have altered the prostatic tissue microenviroment, which may not be representative of a normal prostatic gland response to SARS-CoV-2 infection. SARS-CoV-2 infection in the prostate will need to be further studied as findings in a non-human primate model demonstrated that the viral spike proteins can be found in the prostate. If both of these findings hold true, the prostate may act as a safeguard for viral replication and immune-evasion.
TMPRSS2 in the prostate plays a major role in the additional symptom burden, including lower urinary tract symptoms (LUTS), found in males diagnosed with SARS-CoV-2. There is research demonstrating that SARS-CoV-2 is particularly harmful in patients with prostate cancer. Chakravarty et al. found that patients with prostate cancer who were also diagnosed with SARS-CoV-2 had higher rates of hospitalization and mortality when compared to patients with other solid tumors. Prostate cancer patients diagnosed with SARS-CoV-2 also had higher rates of hospitalization when compared to patients with other genitourinary cancers, such as bladder and kidney cancer [16]. This research highlights the vulnerability of the prostate to SARS-CoV-2. Patients with pre-existing prostatic disease are more likely to require higher levels of medical intervention. It is necessary to consider the additional risk prostatic disease presents for patients. Additionally, even in patients without pre-existing prostatic pathology, males are more likely to face additional disease burden and increased mortaility from SARS-CoV-2, which may correlate with TMPRSS2 expression [12].
There is no difference in the rates of diagnosis of SARS-CoV-2 between males and females. Males, however, are more likely to require intensive treatment and have higher likelihood of death [17]. This link has prompted further research on the relationship between lower urinary tract symptoms (LUTS) and SARS-CoV-2. LUTS should be used as an early indicator of SARS-CoV-2 infection. Kaya et al. studied the rates of LUTS in patients with SARS-CoV-2. They selected 46 patients who had recovered from SARS-CoV-2 after hospitalization. Patients filled out the International Prostate Symptom Score (IPSS) and Urinary Symptom Profile based on their symptoms before, during, and after having SARS-CoV-2. Within the male patient cohort, there was a significant difference in storage symptoms between the three time periods. There was not, however, any significant difference between IPSS scores for total score, voiding score, or quality of life score, between the three periods. Based on this data, the authors argue that urinary storage symptoms may be an early symptom of SARS-CoV-2 [18].
In addition to the presence or absence of LUTS, LUTS severity should also be an indicator of COVID-19 disease severity. Karabulut et al. found patients who experienced severe LUTS were more likely to also experience extended hospital stays, admission to intensive care, and death, when compared to patients with mild LUTS [19]. There is data, however, that LUTS and disease severity do not correlate. Can et al, using CT lung imaging to determine disease severity, found that severity of LUTS does not correlate with disease severity. The use of CT lung imaging, instead of patient clinical outcomes, may have failed to capture the true patient course. Additionally, only patients who had been in hospitalized for more than three weeks were selected for the study, limiting the generalizability of these results [20].
Together these studies show that novel LUTS or an increase of severity of LUTS, in the context of a possible SARS-CoV2 infection, should be considered an early sign of infection and may have value as a prognostic indicator. More research needs to be completed to gain a better understanding of the relationship between LUTS and SARS-CoV-2.
Bladder
Several investigators have reported on the isolation of SARS-CoV-2 from the urine. Chan et al performed a systematic review examining the detection of SARS-CoV-2 viral RNA in urine. A total of 21 studies with 3,764 SARS-CoV-2 patients were analyzed and 5.74% of patients were noted to have positive viral RNA in urine samples, although the duration of viral shedding in urine was undetermined [21]. Kashi and colleagues performed a similar systematic review and investigated urinary samples of 533 SARS-CoV-2 patients [22]. The crude overall rate of SARS-CoV-2 detection in urinary samples was 4.5%, and the estimated viral shedding frequency was 1.18%. Urinary shedding was most commonly detected in patients with moderate to severe disease [22].
While there is increasing evidence that SARS-CoV-2 is found within the urine, the exact mechanism behind SARS-CoV-2’s effects on the urinary system is still unknown, although there are two leading theories. Given that SARS-CoV-2 has been isolated in the urine, it can be hypothesized that direct invasion of the luminal urothelial cells via the ACE2 receptor can occur which may lead to lower urinary tract symptomatology. At this time, it is unclear whether the ACE2 receptor is expressed on the basal or luminal urothelial cells, although recent research by Lin and colleagues suggests that ACE2 is expressed amongst all layers of the bladder urothelium with a higher concentration located on the bladder umbrella (luminal) cells when compared to the basal cells [23]. The exact mechanism by which SARS-CoV-2 can cause cystitis (hematogenous invasion from the basal side or urinary invasion from luminal side) remains to be further elucidated. The infrequent detection of urinary viral RNA in SARS-CoV-2 patients with new onset urinary symptoms seems to favor propagation of the virus via the urothelial basal layer to the bladder [24]. Another proposed mechanism of action is via a cytokine-mediated response syndrome leading to systemic inflammation in patients with SARS-CoV-2. This systemic inflammatory response is known to cause intrarenal inflammation, increased vascular permeability and volume depletion, and is clinically characterized by fevers, multiorgan dysfunction and coagulopathy [25]. Interleukin-6 (IL-6) is a key cytokine that has been linked with SARS-CoV-2 disease severity and may play a role in SARS-CoV-2 associated cystitis [26]. One group of researchers found elevated levels of inflammatory cytokines, GRO/CXCL-1, IL-6, IP-10, and CRP in SARS-CoV-2 positive patients with de novo urinary symptoms when compared to SARS-CoV-2 negative patients. This group hypothesized that SARS-CoV-2 associated cystitis may be due to increased inflammatory cytokines that are released into the urine and/or expressed in the bladder [27].
After review of the current literature, involvement of the lower urinary tract was reported in 9 different studies. Urinary symptoms were described as either de novo or deterioration from baseline and consisted of urinary urgency, frequency, incontinence, nocturia or gross hematuria. Mumm et al reported on an increase in urinary frequency in seven SARS-CoV-2 positive male patients in the absence of any other causes and suspected cystitis to be due to active SARS-CoV-2 infection [24]. Interestingly, none of these patients exhibited positive urine PCR tests [24]. Dhar and colleagues reported similar findings and found de novo urinary symptoms in 39 SARS-CoV-2 positive patients (32 males). These researchers utilized a validated bladder health questionnaire (Overactive Bladder (OAB) Assessment Tool) and found the average OAB symptom score in both men and women was 18. Patients described new onset urinary urgency, frequency, urge incontinence and nocturia with urinary frequency and nocturia being the most bothersome symptoms [28]. As previously described, this same group of investigators later analyzed 53 patients with SARS-CoV-2 in a follow up study and found significantly higher OAB symptom scores and increased levels of urinary inflammatory cytokines when compared to SARS-CoV-2 negative patients [27]. In a larger series, Kaya et al analyzed the urinary symptoms of 46 SARS-CoV-2 patients (19 males) during three time periods: pre-SARS-CoV-2, during hospitalization and post-hospitalization [29]. The validated International Prostate Symptom Score (IPSS) and Urinary Symptom Score (UPS) questionnaires were utilized. This group found that storage-IPSS scores for males and overactive bladder scores for females were significantly different between the three different time periods and concluded that lower urinary tract symptoms, especially storage symptoms, may be an early symptom of SARS-CoV-2 infection [29]. Finally, Can et al reported the most recent study analyzing the association between SARS-CoV-2 and lower urinary tract symptoms [20]. In this prospective study, 94 hospitalized male patients with SARS-CoV-2 were assessed for lower urinary tract symptoms via the validated IPSS questionnaire. Patients were divided into two groups based on over or under the age of 50. These investigators found that IPSS scores were significantly increased in men over the age of 50 when compared to their IPSS scores prior to SARS-CoV-2 infection [20].
It is also important to note that there have been case reports linking SARS-CoV-2 infection with symptomatic gross hematuria. Luciani et al described three cases of severe gross hematuria after SARS-CoV-2 infection which required hospital admission and operative/procedural intervention [30]. One case involved a patient with a history of non-severe hematuria related to radiation cystitis for prostate cancer who then experienced severe gross hematuria following SARS-CoV-2 infection which required multiple transfusions and cystoscopy with bladder fulguration. Two other patients with a history of mild hematuria related to benign prostatic hyperplasia were noted to have severe gross hematuria after the onset of symptomatic SARS-CoV-2 infection and required prolonged continuous bladder irrigation with subsequent hypogastric artery embolization [30]. Recently, Djafari and colleagues described a case report of a patient with secondary bladder amyloidosis due to rheumatoid arthritis who then developed acute onset severe gross hematuria after symptomatic SARS-CoV-2 infection [31]. Finally, a more recently analysis from an AUA-accepted abstract examined 157 patients with SARS-CoV-2 infection and found that gross hematuria was significantly associated with poor renal function, higher intubation rates and increased mortality when compared to patients with no hematuria or microscopic hematuria. Gross hematuria was also significantly associated with elevated biomarkers linked to SARS-CoV-2 disease severity, such as CRP and D-dimer [32].
While patients with SARS-CoV-2 infection typically present with fevers and upper respiratory symptomatology, there are increasing reports of new onset urinary symptoms such as urgency, frequency, incontinence and nocturia, referred to as COVID-19 associated cystitis (CAC) [25]. Clinicians should recognize these symptoms as possible early signs of SARS-CoV-2 infection. There is a paucity of data concerning the long-term effects of SARS-CoV-2 on the lower urinary tract, and clinicians must counsel patients on the uncertainty of urinary symptom duration. Although there are currently no guidelines or specific recommendations on the management of CAC, standard OAB treatment strategies such as behavioral modifications and pharmacological therapies (anti-muscarinics/β-3 agonists) may be utilized. Clinicians may consider procedural interventions (sacral neuromodulation, tibial nerve stimulation, intravesical botulinum toxin) if patients have urinary symptoms refractory to first and second line OAB treatments. It is also crucial for clinicians to recognize the association between SARS-CoV-2 infection and gross hematuria. This is especially important for patients hospitalized with moderate to severe infection as the risk of symptomatic gross hematuria appears to be associated with severity of SARS-CoV-2 infection. These patients can be managed conservatively with large bore urinary catheters, continuous bladder irrigation and supportive care, but may require more invasive measures such as cystoscopy with fulguration, angioembolization or urinary diversion if hematuria persists.
Testes
SARS-CoV-2 has a high affinity for the TMPRSS2 Receptor leading to speculation that the virus can enter into testicular tissue. Currently, 333 patients from 14 studies are found in the literature [33]. Autopsy of 12 SARS-CoV-2 positive patients revealed dramatic damage to the testicular architecture; however, only one patient had a detectable virus load in the testicular tissue itself [34]. This patient additionally has multiple organs with detectable virus, indicative a high viral load. Another autopsy study of six individuals showed increased apoptotic cells within the seminiferous tubules with T-lymphocytes and macrophages present in the interstitium [35]. An additional autopsy study of five SARS-CoV-2 men revealed germ cells in the lumen of seminiferous tubules [36]. Electron micrograph of testicular tissue showed SARS-CoV-2 was present in one living biopsy and one deceased biopsy. This studied totaled three out of six SARS-CoV-2 patients having testicular involvement. Consistent with previous studies by Li et al, interstitial macrophages and leukocyte infiltration were present in the testis tissue [37].
In addition to SARS-CoV-2 existence in the testis, impairment of gametes has been examined. Achua et al concluded that the levels of ACE-2 receptor in a patient were associated with the impairment of spermatogenesis [37]. Supporting evidence was shown in a 44-year-old man who had testicular atrophy at the time of SARS-CoV-2 induced death; however, it should be noted that this patient suffered from myotonic muscular dystrophy and testicular atrophy may be unrelated [38]. Sperm parameters have been found to be impaired in SARS-CoV-2 patients. Best et al reported decreased total sperm numbers within the ejaculate. They reported close to an 80% reduction of total sperm in infected individuals 12.5 million compared to 59.2 million in healthy controls [39].
Two studies looked at scrotal discomfort (scrotal or testicular pain, swelling) across 42 SARS-CoV-2 acute infection patients. Ediz et al enrolled 91 men in a two-month period and found 10/91 (11%) to have scrotal/testicular pain concomitantly with SARS-CoV-2 hospitalization [40]. One studied observed an increase in epididymo-orchitis in their severe SARS-CoV-2 patient cohort. Chen et al conducted a prospective study with 142 patients enrolled with confirmed SARS-CoV-2. Of those 142, 32 (22.5%) had acute orchitis, epididymitis, or epididymo-orchitis [41]. Contradictory evidence is also supported by Alkhatatbeh et al. They reported that 253 with SARS-CoV-2 infection were followed and no patients reported any form of testicular/scrotal pain (age 1-78) [42]. Therefore, more evidence is needed prior to correlating SARS-CoV-2 infection with testicular pain.
Pathological analysis of 26 patients from Wuhan, China was conducted, and SARS-CoV-2 infection was stratified into two groups: localized pulmonary infection and systemic infection [43]. In addition to identifying the number of cases in their autopsy cohort, comparison to the original SARS-CoV was done. The SARS-CoV-2 was present in 3/12 patients where was it was not detected in the original SARS-CoV infection (0/11). Consistent with previous findings of macrophages and lymphocytes in the testes tissue, Yao et al show via immunohistochemistry that the SARS-CoV-2 spike protein is present in endothelial cells in the seminal vesicles, capillaries around the seminal vesicles, epididymis, macrophages, and lymphocytes. Together the authors concluded that the novel SARS-CoV-2 is able to penetrate the blood-testis-barrier [43].
Infected inflammatory cells can migrate into the testicles where SARS-CoV-2 can be taken up into the local macrophages and spermatogonial cells. The virus can then replicate in the endoplasmic reticulum-golgi intermediate complexes. Therefore the patient can retain viral loads long after they have recovered from the acute viral infection [44]. Other studies have shown that SARS-CoV-2 can lasting impacts on ones sexual function. A study by Davis et al found 15% of patients had sexual dysfunction seven months following their infection where they did not previously have these complains. Further they reported that 10% of their patient cohort had pain in their testicles with 5% of the cohort reporting decrease in testicular size [45].
Penis
Currently, there exists only a limited number of studies that discuss SARS-CoV-2 infection in relation to penile tissue and erectile dysfunction. Kresch et al was one of the first that demonstrated the virus within endothelial cells of penile tissue seven months following SARS-CoV-2 infection with electron microscopy [46]. These findings were incidental in patients who underwent penile prosthesis for erectile dysfunction. Histopathologic analysis revealed no differences in architecture compared to non-SARS-CoV-2 patients. They hypothesized that the lower endothelial nitric oxide synthase in SARS-CoV-2 patients may be the driver of subsequent infection related erectile dysfunction. Erectile dysfunction occurred in another patient with SARS-CoV-2 infection 10 after resolution of infection. Eighty days following resolution, the patient presented with a penile plaque and documentation of the first Peyronie’s disease was made. Similar to Kresch et al hypothesis, low endothelial progenitor cells and flow-mediated vasodilation suggest endothelial dysfunction may be the driver of this patient’s erectile dysfunction [47].
SARS-CoV-2 infection has been related a rare phenomenom regarding the vascular supply to the penis. Mondor’s disease is thrombophlebitis of the superficial dorsal vein of the penis where Lessiani et al described the first case of a 28-year-old male single case report following SARS-CoV-2 infection [48]. It is speculated that the SARS-CoV-2 hypercoagulable state resulted in Mordor’s disease. Another case reports recently come out describing the same occurrence of Mordor’s in a 34 year old male [49]. In this case, vasculitis was ruled out for eitology of superficial vein thrombosis. A third and final case of the literature discussed a 33-year-old man who had Mondors disease during the subacute phase of SARS-CoV-2 infection [50]. In each of these three cases, anti-coagulation therapy was given and the thrombosis resolved. It remains unclear where the thrombus originated and whether SARS-CoV-2 was directly involved in superficial dorsal vein or if it’s a systemic effect that happened to manifest within the superficial dorsal vein.
Similarly to thrombophlebitis, there have been two reports of penile necrosis following SARS-CoV-2 infection. The first case reported was published in August of 2021 from a 58-year-old male with several co-morbidities reported penile necrosis after infection. The patient also had necrosis of his fingers, heels, toes [51]. The second reported case came from a 71-year-old male who also had Type 2 diabetes and chronic renal failure who presented 18 days following a positive SARS-CoV-2 infection. On day 18, his glans penis became necrotic along with multiple digits of his right hand [52]. It should be stated that both of these pateints had multiple co-morbidities and other locations of necrosis, decreasing the chance that penile necrosis is specific following SARS-CoV-2 infection but rather general ischemia occurring.
Nine cases of priapism have been reported in the literature. This was first reported in the summer of 2021 where a 67 year old male had ischemic priapism during his SARS-CoV-2 infection [53]. Seven more care reports have come out in 2021 with priapism following COVID. The most recent case described a patient who tested positive in the emergency room with symptoms fo SARS-CoV-2 infection, returning to the emergency room five days later with a three day history of priapism [54]. This study also summarized the the previous case reports. They found all but one case of priapism was ischemic, where that case had stuttering ischemic priapism [54,55]. Similarly to penile necrosis previously described, priapism appears to be more as a secondary consequence to vascular aspects of SARS-CoV-2 infection rather than a direct action of the virus on penile tissue. However, should not completely be discounted as there is some evidence that the virus can invade penile tissue [14]. Therefore health care providers should take any penile concern seriously following a SARS-CoV-2 infection.
Overall, SARS-CoV-2 infection has had effects on penile tissue. It remains unknown if these are direct effects from virus invading the local tissue environment or more of systemic infections where random manifestations occur in the penis. There is strong evidence to support viral prescence in the penile tissue and a multitude of disease processes can occur such as erectile dysfunction, thrombophlebitis and necrosis, as well as priapisms. Regardless of the eitology of these disease, men with SARS-CoV-2 infection should see a health care providor for any concerns regarding their penis as SARS-CoV-2 may be playing a role in the diease pathophysiology.
Conclusion
SARS-CoV-2 is primarily thought about as a respiratory disease. However, a growing body of evidence has revealed how SARS-CoV-2 can affect the male genitourinary system and given the expression of TMPRSS2, this makes for a perfect viral reservoir. Since the tissue provides the necessary proteins for viral entry, the virus is detectible in the urine, but the not in semen. The number of patients experiencing LUTS is also increased relative to the general public. In addition to urinary symptoms, sexual symptoms have also been reported with decreased sperm numbers and erectile dysfunction. Overall, there is a significant burden that SARS-CoV-2 is placing on the genitourinary system and healthcare providers should be aware of how these may present.
Disclosure of conflict of interest
None.
References
- 1.Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23:1922–1924. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O, Antonelli G, Lenzi A, Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43:1819–1822. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayaaslan B, Korukluoglu G, Hasanoglu I, Kalem AK, Eser F, Akinci E, Guner R. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol Int. 2020;104:678–683. doi: 10.1159/000510531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song C, Wang Y, Li W, Hu B, Chen G, Xia P, Wang W, Li C, Diao F, Hu Z, Yang X, Yao B, Liu Y. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients†. Biol Reprod. 2020;103:4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) JAMA. 2020;324:782. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 8.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed MS, Moulin TC, Schiöth HB. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021;71:3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 2020;78:296–298. doi: 10.1016/j.eururo.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren X, Wang S, Chen X, Wei X, Li G, Ren S, Zhang T, Zhang X, Lu Z, You Z, Wang Z, Song N, Qin C. Multiple expression assessments of ACE2 and TMPRSS2 SARS-CoV-2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID-19. Infect Drug Resist. 2020;13:3977–3990. doi: 10.2147/IDR.S270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madden P, Thomas Y, Blair R, Samer S, Doyle M, Midkiff C, Doyle-Meyers L, Becker M, Arif S, McRaven M, Simons L, Carias A, Martinelli E, Lorenzo-Redondo R, Hultquist J, Villinger F, Veazey R, Hope T. An immunoPET probe to SARS-CoV-2 reveals early infection of the male genital tract in rhesus macaques. Res Sq. 2022 [Google Scholar]
- 15.Pecoraro A, Morselli S, Raspollini MR, Sebastianelli A, Nicoletti R, Manera A, Campi R, Liaci A, Serni S, Gacci M. The role of COVID-19 in prostate tissue inflammation: first pathological evidence. Prostate Cancer Prostatic Dis. 2022;25:370–372. doi: 10.1038/s41391-022-00542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarty D, Ratnani P, Sobotka S, Lundon D, Wiklund P, Nair SS, Tewari AK. Increased hospitalization and mortality from COVID-19 in prostate cancer patients. Cancers (Basel) 2021;13:1630. doi: 10.3390/cancers13071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaya Y, Kaya C, Kartal T, Tahta T, Tokgöz VY. Could LUTS be early symptoms of COVID-19. Int J Clin Pract. 2021;75:e13850. doi: 10.1111/ijcp.13850. [DOI] [PubMed] [Google Scholar]
- 19.Karabulut I, Cinislioglu AE, Cinislioglu N, Yilmazel FK, Utlu M, Alay H, Celik EC, Adanur S. The effect of the presence of lower urinary system symptoms on the prognosis of COVID-19: preliminary results of a prospective study. Urol Int. 2020;104:853–858. doi: 10.1159/000510761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Can O, Erkoç M, Ozer M, Umeyir Karakanli M, Otunctemur A. The effect of COVID-19 on lower urinary tract symptoms in elderly men. Int J Clin Pract. 2021;75:e14110. doi: 10.1111/ijcp.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan VW, Chiu PK, Yee CH, Yuan Y, Ng CF, Teoh JY. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J Urol. 2021;39:3127–3138. doi: 10.1007/s00345-020-03246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashi AH, De la Rosette J, Amini E, Abdi H, Fallah-karkan M, Vaezjalali M. Urinary viral shedding of COVID-19 and its clinical associations: a systematic review and meta-analysis of observational studies. Urol J. 2020;17:433–441. doi: 10.22037/uj.v16i7.6248. [DOI] [PubMed] [Google Scholar]
- 23.Lin W, Fan J, Hu LF, Zhang Y, Ooi JD, Meng T, Jin P, Ding X, Peng LK, Song L, Tang R, Xiao Z, Ao X, Xiao XC, Zhou QL, Xiao P, Zhong Y. Single-cell analysis of ACE2 expression in human kidneys and bladders reveals a potential route of 2019-nCoV infection. Cold Spring Harbor Laboratory. 2020 doi: 10.1097/CM9.0000000000001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mumm JN, Osterman A, Ruzicka M, Stihl C, Vilsmaier T, Munker D, Khatamzas E, Giessen-Jung C, Stief C, Staehler M, Rodler S. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao YM, Xu G, Wang B, Liu BC. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J Intern Med. 2021;289:147–161. doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, We X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC) Med Hypotheses. 2020;145:110375. doi: 10.1016/j.mehy.2020.110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhar N, Dhar S, Timar R, Lucas S, Lamb LE, Chancellor MB. De Novo urinary symptoms associated with COVID-19: COVID-19-associated cystitis. J Clin Med Res. 2020;12:681–682. doi: 10.14740/jocmr4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaya Y, Kaya C, Kartal T, Tahta T, Tokgöz VY. Could LUTS be early symptoms of COVID-19. Int J Clin Pract. 2021;75:e13850. doi: 10.1111/ijcp.13850. [DOI] [PubMed] [Google Scholar]
- 30.Luciani LG, Gallo F, Malossini G. Re: Jan-Niclas Mumm, Andreas Osterman, Michael Ruzicka, et al. Urinary frequency as a possible overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:e129–e130. doi: 10.1016/j.eururo.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djafari AA, Hasanzadeh K, Masrour H, Ahadi M, Dargahi M, Rahavian A. Is corona virus infection a risk factor for hematuria in secondary bladder amyloidosis? The first case report. Urol Case Rep. 2021;38:101642. doi: 10.1016/j.eucr.2021.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehraban-Far S, Anderson R, Lee E, Chandra M, Liang X, Hwang K, Kapur A, Chen A, Kim J, Adler H. MP29-14 gross hematuria is associated with more severe disease and poorer clinical outcomes in COVID-19 patients. J Urol. 2021;206:e495–e496. [Google Scholar]
- 33.He Y, Wang J, Ren J, Zhao Y, Chen J, Chen X. Effect of COVID-19 on male reproductive system - a systematic review. Front Endocrinol (Lausanne) 2021;12:677701. doi: 10.3389/fendo.2021.677701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF, Li X, Zhou JJ, Fan J, Luo DJ, Chang XN, Arkun K, Zhou M, Nie X. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus. 2020;6:1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K, Zhou L, Shen S, Liu L, Liu Q, Xiong C. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Guan C, Chen R, Wang Y, Feng S, Wang R, Qu G, Zhao S, Wang F, Wang X, Zhang D, Liu L, Liao A, Yuan S. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol. 2021;18:487–489. doi: 10.1038/s41423-020-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achua JK, Chu KY, Ibrahim E, Khodamoradi K, Delma KS, Iakymenko OA, Kryvenko ON, Arora H, Ramasamy R. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health. 2021;39:65–74. doi: 10.5534/wjmh.200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, Rosete O, Mora B, Arora H, Ibrahim E, Ramasamy R. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health. 2021;39:489–495. doi: 10.5534/wjmh.200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ediz C, Tavukcu HH, Akan S, Emre Kizilkan Y, Alcin A, Oz K, Yilmaz O. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int J Clin Pract. 2021;75:e13753. doi: 10.1111/ijcp.13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Huang X, Yi Z, Deng Q, Jiang N, Feng C, Zhou Q, Sun B, Chen W, Guo R. Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019: a single-center-based study in Wuhan, China. J Ultrasound Med. 2021;40:1787–1794. doi: 10.1002/jum.15558. [DOI] [PubMed] [Google Scholar]
- 42.Alkhatatbeh H, Alzaghari D, Alkhashman A, Azab M, Edwan GMA, Abufaraj M. Does severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) cause orchitis in patients with coronavirus disease 2019 (COVID-19)? Arab J Urol. 2020;18:129–133. doi: 10.1080/2090598X.2020.1798862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang PP, Cai J, Zhou XD, Jiang DP, Fei XC, Huang XQ, Zhao L, Zhang H, Wu HB, Ren Y, Liu ZH, Zhang HR, Chen C, Fu WJ, Li H, Xia XY, Chen R, Wang Y, Liu XD, Yin CL, Yan ZX, Wang J, Jing R, Li TS, Li WQ, Wang CF, Ding YQ, Mao Q, Zhang DY, Zhang SY, Ping YF, Bian XW. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31:836–846. doi: 10.1038/s41422-021-00523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa GMJ, Lacerda SMSN, Figueiredo AFA, Wnuk N, Brener M, Campolina-silva G, Kauffmann-zeh A, Pacifico L, Versiani A, Andrade L, Antunes M, Souza F, Cassali G, Caldeira-Brant A, Chiarini-Garcia H, Costa V, da Fonseca F, Nogueira M, Campos G, Kangussu L, Martins E, Antonio L, Bittar C, Rahal P, Aguiar R, Mendes G, Procopio M, Furtado T, Guimaraes Y, Menezes G, Martinez-Marchal A, Brieno-Enriquez M, Orwig K, Furtado M. SARS-CoV-2 infects, replicates, elevates angiotensin II and activates immune cells in human testes. medRxiv [Google Scholar]
- 45.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, Redfield S, Austin JP, Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kresch E, Achua J, Saltzman R, Khodamoradi K, Arora H, Ibrahim E, Kryvenko O, Almedia V, Firdaus F, Hare J, Ramasamy R. COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human Penis. World J Mens Health. 2021;39:466–469. doi: 10.5534/wjmh.210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rainer Q, Molina M, Ibrahim E, Saltzman R, Masterson T, Ramasamy R. Peyronie’s disease in a patient after COVID-19 infection: a case report. Andrologia. 2021;53:e14219. doi: 10.1111/and.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lessiani G, Boccatonda A, D’Ardes D, Cocco G, Di Marco G, Schiavone C. Mondor’s disease in SARS-CoV-2 infection: a case of superficial vein thrombosis in the era of COVID-19. Eur J Case Rep Intern Med. 2020;7:001803. doi: 10.12890/2020_001803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balawender K, Pliszka A, Surowiec A, Rajda S. COVID-19 infection as a new risk factor for penile Mondor disease. BMC Urol. 2022;22:57. doi: 10.1186/s12894-022-01002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eren MT, Özveri H, Kurtoğlu H. Penile Mondor’s in a Covid-19 patient on prophylactic anti-thrombosis with rivaroxaban: a case report. Afr J Urol. 2021;27:97. doi: 10.1186/s12301-021-00200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkis P, Sarkis J, Alkassis M, Assaf J, El Gharib K. Penile ischaemia secondary to COVID-19: why should the dermatologist be concerned? J Eur Acad Dermatol Venereol. 2021;35:e487–e489. doi: 10.1111/jdv.17287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozbey R, Algan MF. Acro-ischemic lesions in COVID-19 patients: a case series. J Cosmet Dermatol. 2022;21:1822–1829. doi: 10.1111/jocd.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7:001779. doi: 10.12890/2020_001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alsaedi SM, Alsarwani RM, Ali AI, Aladhrai SA. Ischemic priapism progressing to penile Gangrene in a patient with COVID-19 infection: a case report with literature review. Case Rep Med. 2022;2022:8408216. doi: 10.1155/2022/8408216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimberg DC, Tejwani R, Allkanjari A, Forrester MT, Kraft BD, Kaye DR. Ischemic priapism due to coagulopathy of severe COVID-19 infection. J Clin Urol. 2021:20514158211025914. [Google Scholar]


