Summary
The continual evolution of SARS-CoV-2 and the emergence of variants that show resistance to vaccines and neutralizing antibodies threaten to prolong the COVID-19 pandemic. Selection and emergence of SARS-CoV-2 variants are driven in part by mutations within the viral spike protein and in particular the ACE2 receptor-binding domain (RBD), a primary target site for neutralizing antibodies. Here, we develop deep mutational learning (DML), a machine-learning-guided protein engineering technology, which is used to investigate a massive sequence space of combinatorial mutations, representing billions of RBD variants, by accurately predicting their impact on ACE2 binding and antibody escape. A highly diverse landscape of possible SARS-CoV-2 variants is identified that could emerge from a multitude of evolutionary trajectories. DML may be used for predictive profiling on current and prospective variants, including highly mutated variants such as Omicron, thus guiding the development of therapeutic antibody treatments and vaccines for COVID-19.
Keywords: directed evolution, protein engineering, machine learning, deep learning, artificial intelligence, viral escape, deep sequencing, yeast display
Graphical abstract
Highlights
-
•
Millions of combinatorial SARS-Cov-2-RBD variants screened by yeast surface display
-
•
Machine learning models accurately predict ACE2 binding and antibody escape
-
•
Identification of combinatorial mutations that drive escape to multiple antibodies
-
•
Assessment of antibody robustness to billions of prospective RBD variants
A machine-learning-guided, protein engineering method enables the prediction of how SARS-CoV-2 RBD combinatorial mutations will impact therapeutic antibody escape and ACE2 affinity. This method facilitates the identification of multisite mutations that are major drivers of antibody escape and the evaluation of neutralizing antibody efficacy on heavily mutated viral variants.
Introduction
Throughout the course of 2021 and 2022, variants of SARS-CoV-2 associated with higher transmissibility and/or immune evasion (antibody escape) have supplanted the original founder strain (Wu-Hu-1) (Wu et al., 2020a). Such variants often possess at least one mutation in the receptor-binding domain (RBD), which can directly influence binding to angiotensin-converting enzyme 2 (ACE2) receptor (Supasa et al., 2021; Yi et al., 2020). For example, Alpha (B.1.1.7) , Beta (B.1.351), and Gamma (P.1) variants all possess the N501Y mutation, which is associated with higher affinity binding to ACE2 (Han et al., 2021), suggesting that this may represent a possible selective pressure for variant emergence.
Neutralizing antibodies, including monoclonal antibody (mAb) therapeutics and those induced by vaccination (with the original Wu-Hu-1 spike protein), often display reduced binding and neutralization to variants (Garcia-Beltran et al., 2021, McCormick et al., 2021, Zhou et al., 2021). Detailed molecular analysis has revealed that many neutralizing antibodies to SARS-CoV-2 share sequence and structural features (Dejnirattisai et al., 2021, Nielsen et al., 2020; Yang et al., 2021, Zost et al., 2020), which has led to their categorization into four common classes defined by groups of targeted RBD epitopes (Barnes et al., 2020; Harvey et al., 2021). For example, Class 1 antibodies include the previously clinically used REGN10933 (casirivimab) (Baum et al., 2020; Hansen et al., 2020) and LY-CoV16 (etesevimab) (Shi et al., 2020). Circulating variants with mutations in position K417 (e.g., Beta, Gamma, and Delta plus [B.1.617.2 + K417N]) as well as the mink-selected Y453F mutation (Cluster 5) display decreased neutralization by these class 1 antibodies (Hoffmann et al., 2021; Starr et al., 2021a; Wang et al., 2021b, Wibmer et al., 2021). Class 2 neutralizing antibodies including the clinically used LY-CoV555 (bamlanivimab) also strongly inhibit ACE2 binding; however, variants such as Beta, Gamma, eta (B.1.525), Kappa (B.1617.1), and iota (B1.526) all possess the RBD mutation E484K/Q that can lead to a substantial loss of binding and neutralization. Class 3 antibodies, including the clinically used REGN10987 (imdevimab) and S309 (sotrovimab) (Pinto et al., 2020), bind partially conserved epitopes and are resistant to several variants (e.g., Alpha, Beta, and Gamma) (Tzou et al., 2020). Class 4 antibodies such as CR3022 target a highly conserved epitope among sarbecoviruses (ter Meulen et al., 2006; Wu et al., 2020b; Yuan et al., 2020) and have been largely resistant to escape variants but generally lack neutralizing potency since they do not directly inhibit ACE2 binding.
The emergence of Omicron has revealed that variants can evolve with severe immune evasion properties such as escape from several classes of neutralizing antibodies that bind to a diverse set of RBD epitopes (Cao et al., 2022a; Dejnirattisai et al., 2022; Iketani et al., 2022; Liu et al., 2022). Notably, nearly all clinically approved antibody therapies have lost substantial neutralizing activity against Omicron (BA.1/B.1.1.529), including the multi-class antibody cocktails from Eli Lilly (LY-CoV16+LY-CoV555), Regeneron (REGN10933+REGN10987), and Astra Zeneca (AZD8895+AZD1061) (Cao et al., 2022a), all of which have subsequently had their clinical authorization revoked (Eli Lilly and Regeneron) or dosage modified (Astra Zeneca) by the US FDA (FDA, 2022a, 2022c; NIH, 2022). One exception was S309, originally isolated from B cells of a patient infected with SARS-CoV-1 and possessing cross-reactivity to SARS-CoV-2 (Pinto et al., 2020); S309 has reduced but still potent neutralizing activity against Omicron (BA.1) (Cameroni et al., 2022; Sheward et al., 2022), likely because it binds to a highly conserved epitope found across genetically diverse sarbecoviruses. However, the Omicron sublineage BA.2 shows substantial escape from S309 (Iketani et al., 2022) and became widely circulating in early 2022, thus leading to the loss of clinical authorization of S309 (FDA, 2022b). As of August 2022, LY-CoV1404 (bebtelovimab) is the only clinically approved antibody discovered based on binding to the original Wu-Hu-1 RBD (Westendorf et al., 2022) that has maintained strong neutralizing activity to Omicron BA.1 and BA.2 variants (Iketani et al., 2022), as well as emerging sublineages BA.4 and BA.5 (Cao et al., 2022b).
Bloom and colleagues have performed yeast surface display and deep mutational scanning (DMS) (Fowler and Fields, 2014) on the entire 201 amino acid RBD of SARS-CoV-2 to determine the impact of single-position substitutions on binding to ACE2 and escape from monoclonal or serum antibodies (Greaney et al., 2021a, 2021b; Starr et al., 2020, 2021a, 2021b). Although DMS has been very effective at single-mutation profiling of the RBD, several previously circulating variants (e.g., Beta, Gamma, and Delta) possess multiple RBD mutations, and Omicron and its sublineages possess up to 18 RBD mutations (BA.2.75), thus underscoring the urgent need to determine the impact of combinatorial mutations. However, combinatorial sequence space grows exponentially as the number of mutations and amino acid diversity increases, rapidly outpacing the capabilities of experimental screening techniques. For instance, when focusing only on a subset of twenty RBD residues directly involved in ACE2 binding (Lan et al., 2020), theoretical sequence space (2020 = 1 × 1026) far exceeds what can be screened by yeast display libraries (∼109).
Here, we establish deep mutational learning (DML), which integrates experimental yeast display screening of RBD mutagenesis libraries with deep sequencing and machine learning (Figure 1). We perform DML to comprehensively interrogate combinatorial RBD mutations and their impact on ACE2 binding and escape from a panel of neutralizing antibodies, including clinically used therapeutics and other broadly neutralizing and potent antibodies. DML reveals a highly diverse mutational landscape of RBD mutations that can maintain binding to ACE2 while escaping many different classes of neutralizing antibodies. Finally, DML is able to predict antibody robustness to prospective SARS-CoV-2 variants and thus may be a valuable tool in evaluating and selecting the most promising antibody therapeutics for clinical development.
Figure 1.
Overview of deep mutational learning of the RBD for prediction of ACE2 binding and antibody escape
The RBD or the SARS-CoV-2 spike protein is expressed on the surface of yeast, and mutagenesis libraries are designed on the RBM of the RBD (RBM-3, RBM-1, and RBM-2), which are the sites of interaction with ACE2 and neutralizing antibodies (e.g., therapeutic antibody drugs). RBD libraries are screened by FACS for binding to ACE2 and neutralizing antibodies, both binding and non-binding (escape) populations are isolated and subjected to deep sequencing. Machine learning models are trained to predict binding status to ACE2 or antibodies based on RBD sequence. Machine learning models are then used to predict ACE2 binding and antibody escape on current and prospective variants and lineages.
Results
Design and screening of RBD mutagenesis libraries
SARS-CoV-2 RBD mutagenesis libraries were targeted to core regions of the receptor-binding motif (RBM-3: positions 439–452; RBM-1: 453–478; RBM-2: 484–505), which are subregions of the RBD that interface with ACE2 and where mutations are commonly observed in viral genome sequencing data (available on Global Initiative on Sharing Avian Influenza Data, GISAID [www.gisaid.org]). To generate training datasets covering a high mutational sequence space, combinatorial mutagenesis schemes were designed based on DMS data for ACE2 binding, previously published by Starr et al. (2020). Single-mutation fitness values were empirically thresholded and converted to amino acid frequencies, with mutations below the ACE2 binding fitness threshold excluded. For each position, degenerate codons approximating the desired amino acid distribution were selected by minimizing mean-squared error (Mason et al., 2018) (some positions remained fixed due to their inability to tolerate mutations and retain ACE2 binding in the DMS data), resulting in RBM libraries with theoretical amino acid diversities of 3.5 × 107 (library 3C), 1.2 × 1010 (library 1C), and 1.50 × 1010 (library 2C) (Figures 2A and S1A). An extended version of library 2C was also designed, with fully degenerate NNK codons (N = A, G, C, or T; K = G or T) at positions 417 and 439, which are mutated in a number of circulating variants and associated with antibody escape (Thomson et al., 2021; Tsai et al., 2021), resulting in a theoretical amino acid diversity of 5.99 × 1012 (library 2CE). To generate training datasets covering a lower mutational sequence space, we constructed tiling mutagenesis libraries, whereby fully degenerate codons (NNK) were tiled across three of the positions in each RBM, resulting in a theoretical amino acid diversities of 3.94 × 105 (library 3T), 2.53 × 106 (library 1T), and 1.53 × 106 (library 2T) (Figure 2B).
Figure 2.
Design of RBD mutagenesis libraries and screening by yeast surface display and deep sequencing
(A) Shown is the amino acid usage in the combinatorial libraries (libraries 3C, 1C, and 2C). Degenerate codons are derived from DMS data for ACE2 binding (Starr et al., 2020).
(B) Representative examples of degenerate codons tiled across RBM-2, which are pooled together to comprise library 2T.
(C) Flow cytometry dot plots depict yeast display screening of combinatorial (1C, 2C, 2CE, and 3C) and tiling (1T, 2T, and 3T) RBD libraries and control RBD (Wu-Hu-1); gating schemes correspond to selection of ACE2-binding and non-binding variants.
(D) Amino acid logo plots of the RBD are based on deep sequencing data from ACE2-binding and non-binding selections.
(E) Flow cytometry dot plots depict yeast display screening of pooled RBD libraries (2C and 2CE) after selection for ACE2 binding; gating schemes correspond to selection of variants for binding and escape (non-binding) to monoclonal antibodies (mAbs).
See also Figures S1 and S2 and Tables S1–S3.
Figure S1.
Design and screening of RBD libraries, related to Figure 2 and Table S1
(A) Amino acid distribution of combinatorial libraries RBM-1 and RBM-3.
(B) Yeast display of RBD libraries pre-selected for ACE2 binding were sorted by flow cytometry for binding and escape to four therapeutic monoclonal antibodies (mAbs): LY-CoV16, LY-CoV555, REGN10933, and REGN10987.
(C) A further nine monoclonal antibodies were screened for binding and escape. Approximately 107 yeast cells were screened for each antibody.
Synthetic oligonucleotides encoding the different libraries and spanning the region of interest were amplified by PCR to produce double-stranded DNA with homology to the full RBD sequence. Co-transformation of yeast (S. cerevisiae EBY100) with library-encoding DNA and linearized plasmid yielded more than 2 × 107 transformants for each library. RBD variants, displayed on the yeast surface as a C-terminal fusion to Aga2 (Boder and Wittrup, 1997), were isolated by fluorescence-activated cell sorting (FACS) based on binding to soluble dimeric human ACE2 receptor (Wu-Hu-1 RBD used as a guide for gating) (Figure 2C). RBD variants which showed a complete loss of binding to ACE2 were also isolated. Importantly, this did not include variants with only partially reduced binding since such an intermediate population could not be assigned as binding or non-binding with sufficient confidence necessary for training supervised machine learning models (Figure 2C; Table S1). Targeted deep sequencing (Illumina) of the RBD gene was performed on all the sorted libraries; protein sequence logos revealed highly similar patterns of amino acid usage between the ACE2-binding and non-binding fractions (Figures 2D and S2; Table S2).
Figure S2.
Combinatorial sequence space of RBD libraries following selection, related to Figure 2 and Table S2
Sequence logo plots of sorted populations for ACE2 binding and antibody escape. For each population, up to the 10,000 most abundant unique amino acid sequences after read count thresholding are shown.
Next, using exclusively the ACE2-binding populations, FACS was performed to isolate variants that maintained binding or showed a complete loss of binding (escape) to a panel of 13 neutralizing antibodies; these include clinically used therapeutic antibodies (REGN10987, REGN10933, LY-CoV16, LY-CoV555, S309, and LY-CoV1404), antibodies demonstrating exceptional sabrecovirus breadth (S2E12, S2H97, and A23-58.1) (Starr et al., 2021c; Wang et al., 2021a) and other potent neutralizers isolated directly from COVID-19 individuals (G32A4, mAb-50, mAb-64, and mAb-82) (Ehling et al., 2022; Tong et al., 2021; Table S3; Figures S2B and S2C). Wu-Hu-1 RBD was again used as a guide for gating antibody binding or escape (Figure 2E). The proportion of binding and escape (non-binding) for each antibody and library was highly variable, for example, with RBM-2 libraries REGN10933 showed a low fraction of escape variants and LY-CoV555 had a very high fraction (Figure 2E; Table S1). Deep sequencing was once again performed on antibody binding and escape fractions of all the sorted RBD libraries, and similar to ACE2, protein sequence logos of the two fractions looked highly similar (Figure S2).
Machine learning models accurately predict ACE2 binding and antibody escape
Deep sequencing data from ACE2 selections underwent pre-processing, quality filtering, and balancing steps to create the final training sets (STAR Methods; Table S4). Following nucleotide to protein translation, amino acid sequences were converted to an input matrix by one-hot encoding (Figure 3A). Supervised machine learning models were trained for classification of ACE2 binding, which is defined as the probability (P) that any given RBD sequence binds to ACE2 (higher P correlates with binding). For initial benchmarking, a range of different baseline models (default parameters) were trained using data derived from RBM-2 libraries (2C, 2CE, and 2T) and evaluated based on their classification performance across several metrics (accuracy, F1, precision, recall). Machine learning models tested included K-nearest neighbor (KNN), logistic regression (Log Reg), naive Bayes (NB), support vector machines (SVMs), and random forests (RFs); long-short term memory recurrent neural networks (RNNs) were also trained, which are a class of deep learning models that have the ability to learn long-range dependencies in sequential data (Akbar et al., 2021; Hochreiter and Schmidhuber, 1997; Mason et al., 2021; Saka et al., 2021). All baseline models trained on RBM-2 libraries performed effectively (i.e., accuracy scores between 0.87 and 0.94), including some of the more simple models such as Log Reg and NB, highlighting the potential importance of training data generated with combinatorial mutagenesis libraries to evaluate to which degree mutations are additive. RF and RNN models were selected for further optimization and application since they showed relatively higher performance metrics and could be trained faster (Figure S3; Table S5).
Figure 3.
Training and testing of machine and deep learning models for prediction of ACE2 binding and antibody escape based on RBD sequence
(A) Deep sequencing data from ACE2 and monoclonal antibody (mAb) selections is encoded by one-hot encoding and used to train supervised machine learning (e.g., random forest [RF]) and deep learning models (e.g., recurrent neural network [RNN]). Models perform classification by predicting a probability (P) of ACE2 binding or non-binding and mAb binding or escape (non-binding) based on the RBD sequence.
(B and C) Performance of RF and RNN models trained on 2T, 2C, or Full ACE2 or LY-CoV16 binding data shown by accuracy, F1, and receiver operating characteristic (ROC) curves. Models are evaluated by rounds of external cross-validation (n = 5), with mean performance displayed and standard deviation indicated by error bars. Low and high distance sequences are defined as those ≤ED5 and ≥ED6 from Wu-Hu-1 RBD, respectively.
(D and E) Accuracy, F1, and AUC of all 13 mAb models trained on RBM-2 and RBM-1 data, evaluated on both low and high distance test sequences.
See also Figures S3 and S4 and Table S4. Detailed sequences used as the training data for individual models, Table S6. Machine and deep learning model predictions compared to susceptibility data from the Stanford Database.
Figure S3.
Performance metrics machine learning models, related to Figure 3 and Table S4. Detailed sequences used as the training data for individual models, Table S6. Machine and deep learning model predictions compared to susceptibility data from the Stanford Database
(A) K-nearest neighbors (KNNs), logistic regression (Log Reg), naive Bayes (NB), random forest (RF), long-short term memory recurrent neural network (RNN), support vector machine with linear kernel (SVM Linear), and support vector machine with radial basis function kernel (SVM RBF) models were trained on the ACE2 deep sequencing data without hyperparameter optimization. Models were then challenged to perform classification by predicting a probability (P) of ACE2 binding on test data. Performance of models was evaluated by accuracy, F1, precision, and recall. All models except RNN were trained using Sci-kit Learn, and the RNN was trained using Keras.
(B) K-nearest neighbors (KNNs), logistic regression (Log Reg), naive Bayes (NB), random forest (RF), long-short term memory recurrent neural network (RNN), support vector machine with linear kernel (SVM Linear), and support vector machine with radial basis function kernel (SVM RBF) models were trained on the ACE2 deep sequencing data without hyperparameter optimization. Models were then challenged to perform classification by predicting a probability (P) of ACE2 binding on test data. Performance of models was evaluated by accuracy, F1, precision, and recall. All models except RNN were trained using Sci-kit Learn, and the RNN was trained using Keras.
(C and D) DMS trained random forest (RF) and long-short term memory recurrent neural network (RNN) models were evaluated on the larger combinatorial ACE2 binding test data shown by accuracy, F1 graphs, and ROC curves.
SARS-CoV-2 evolves across a range of mutational trajectories, including variants such as Omicron and its sublineages that have accumulated multiple combinatorial mutations in their RBD. Determining the performance of machine learning models across various mutational edit distances (edit distance [ED] from the reference Wu-Hu-1 RBD sequence) is therefore an important criterion. Initially, DMS data consisting of single point mutations (ED1) were used to train baseline models, with binding/non-binding labels assigned based on minimum affinity required to retain ACE2 binding as previously defined in Starr et al. (2020). The resulting models exhibited very low performance metrics for ACE2-binding prediction (i.e., accuracy scores of 0.50 and AUC of 0.56–0.65) (Figure S3). This is likely because single point mutations are not additive at higher distances and therefore are unable to account for the non-linear effects of combinatorial mutations, leading to models that predict nearly all combinatorial escape variants to be ACE2 binders (Figure S3). Next, we examined model performance on RBM-2 test data that was divided into low mutational distances (≤ED5), which corresponds to variants such as Beta and Gamma, and high mutational distances (≥ED6), which corresponds to variants such as Omicron. We found that when models were trained using only the low distance library (2T), their performance on predicting ACE2-binding on high distance data was very poor (accuracy < 0.65 and AUC < 0.83) (Figure 3B). However, models trained using high distance only (RBM-2C/CE) or combined low distance and high distance libraries (RBM-2 Full) resulted in vastly improved performance across all distances, with accuracy scores of >0.94 and >0.92 for low and high distances, respectively, and AUC of >0.97 for both models (Figure 3B).
Similar to the ACE2 selections, deep sequencing data from antibody selections were pre-processed, quality filtered, balanced, and encoded as before. Supervised machine learning models (RF and RNN) were trained to classify antibody escape, which is defined as the probability that a given RBD sequence escapes a defined antibody (lower P correlates with escape). As before, we show that using both low and high distance libraries (RBM-2 Full) for training data resulted in models with better performance for predicting escape from a representative antibody (LY-CoV16) as opposed to training models with only low distance (RBM-2T) or only high distance libraries (RBM-2C/CE) (Figure 3C). RBM-2 models for nearly all antibodies showed high performance metrics, with the only exception being LY-CoV555 exhibiting low F1 scores. Initial machine learning training and benchmarking revealed that balanced classification data (similar number of sequence variants in binding versus non-binding/escape classes) was required for training accurate models (see STAR Methods). Thus, the lower performance of LY-CoV555 models can be explained through its imbalanced classification data (nearly all RBD variants escape LY-CoV555) (Figures 3D and S4B). For RBM-1 libraries, most antibodies produced imbalanced classification data at low ED (very few escape variants at ≤ED5) (Figure S7); therefore, RBM-1 models were trained using only high distance data (≥ED6) and resulted in high performance metrics for most antibodies, with the exception of LY-CoV16, mAb-64, and mAb-82, which had imbalanced classification data (few escape variants) across all distances (Figures 3E and S4A). Finally, for RBM-3 libraries, nearly all antibodies produced imbalanced classification data (mostly all binding or all escape variants) (Figure S4C; Table S5); and thus, RBM-3 machine learning models were excluded for future analysis.
Figure S4.
Distribution of binding and non-binding across RBM regions, related to Figure 3
Count distributions of unique binding/non-binding sequences from the ACE2 and antibody selection library datasets after pre-processing. (A) RBM-1, (B) RBM-2, and (C) RBM-3.
Predictive profiling on synthetic lineage variants
Having established that ACE2 binding and antibody escape machine learning models can make highly accurate predictions on test data, we next evaluated their classification performance on defined variants, followed by experimental validation and structural modeling. First, we identified synthetic RBD variants that had single mutations (ED1 from Wu-Hu-1) predicted to retain binding, whereas the combination of two single mutations (ED2) were predicted to escape a given antibody. Our machine learning models predict that REGN10933 retains binding to the single-mutation variants E484K or F490I or the single-mutation variants G485K and Q493V (Figure S5C), which also correlates with previously published data from DMS (Greaney et al., 2021c). However, the combination of E484K + F490I or G485K + Q493V results in variants predicted to escape REGN10933. We subsequently tested each variant using yeast surface display and evaluated antibody binding and escape; indeed, we observed that single-mutation variants retained binding, whereas the combinatorial variants show substantially reduced binding to REGN10933, thus corresponding with the machine learning predictions (Figure S5D).
Figure S5.
Experimental evaluation of selected RBD variants for antibody escape, related to Figure 4
(A) The 46 selected synthetic variants were individually cloned and expressed for yeast display and ACE2 binding by flow cytometry. 43 variants showed ACE2 binding or non-binding that matched machine learning predictions. The ACE2-binding status for two variants (38 and 42) could not be conclusively determined, while one variant (41) showed was incorrectly predicted by machine learning for ACE2 binding.
(B) RBD sequences at chosen EDs (ED0, ED3, ED5, and ED7) from the Wu-Hu-1 RBD were predicted for ACE2 binding and escape from four therapeutic monoclonal antibodies (mAbs). Accuracy for antibody escape predictions are the following: LY-CoV16 = 31/33 (93.94%), LY-CoV555 = 30/33 (90.91%), REGN10933 = 31/33 (93.94%), and REGN10987 = 32/33 (96.97%).
(C and D) Two double mutants, and their constituent mutations, which were predicted to display epistasis were assayed individually by yeast surface display.
(E and F) Three synthetic RBD variants of ED3 from Wu-Hu-1 RBD that were predicted to escape all four therapeutic antibodies by the consensus machine learning model were expressed as individual clones in yeast and evaluated by flow cytometry for binding to antibody or ACE2.
Next, synthetic lineages were generated in silico to simulate plausible evolutionary paths, where RBD variants without predicted ACE2-binding intermediates at each mutational step were excluded (Figure 4A). We focused our analysis on the RBM-2 region and the four neutralizing antibodies (LY-CoV16, LY-CoV555, REGN10933, and REGN10987) that were used extensively as clinical therapeutics during the peak of the pandemic in 2021. The lineages were designed to include variants at ED3, ED5, and ED7 from the original Wu-Hu-1 RBD sequence (nucleotide and amino acid). Additionally, the sequences were chosen to form lineages containing mutations observed in circulating variants (e.g., Alpha: N501Y, Beta/Gamma: E484K and N501Y, Kappa: E484Q and N501Y). ACE2 binding was predicted based on a consensus model, whereby a given RBD sequence is predicted to bind ACE2 when both RF and RNN models yield P > 0.5, else they were predicted to be non-binders. The 46 synthetic lineage variants were chosen to contain diversity in ACE2 binding prediction (36 predicted binders, 10 predicted non-binders) (Figure 5A). Additionally, predictions for escape from each of the four therapeutic antibodies were made for the synthetic variants using a similar consensus model approach (RBD sequence escapes an antibody when both RF and RNN outputs are P < 0.5) (Figure S5B). After having made all machine learning predictions, each synthetic RBD variant was individually expressed on the surface of yeast cells and assessed for ACE2 binding and antibody escape. The consensus model correctly predicted ACE2 binding for 91.67% (33/36) of the synthetic variants, with an accuracy of non-binding prediction of 100% (10/10), resulting in an overall prediction accuracy of 93.48% (43/46) (Figures 4B and S5A). For the 33 correctly predicted ACE2-binding variants, the combined accuracy of antibody escape predictions across all four therapeutic antibodies was 93.94% (124/132) (LY-CoV16: 31/33, LY-CoV555: 30/33, REGN10933: 31/33, REGN10987: 32/33) (Figures 4C and S5B). Additionally, we identified three variants that were only ED3 (nucleotide and amino acid) from the Wu-Hu-1 RBD and in which consensus models predicted ACE2 binding and escape from all four therapeutic antibodies. One of these variants possessed mutations in positions 493, 498, and 501, which are all mutated in the Omicron variant (Martin et al., 2021). Subsequent yeast display experiments confirmed these machine learning predictions of antibody escape to all four therapeutic antibodies, including escape from the previously mutation-resistant REGN10987 (Figures S5E and S5F). Structural modeling by AlphaFold2 (Jumper et al., 2021) was performed on eight synthetic RBD variants (all variants were accurately classified and experimentally validated for ACE2 binding or non-binding) (Figure 4D). The structural predictions showed that several ACE2 non-binding variants did not differ substantially from the original Wu-Hu-1 RBD. In contrast, the ACE2-binding variants showed a wide diversity of possible structural conformations.
Figure 4.
Prediction and experimental validation of synthetic lineages of RBD variants
(A) Workflow to select and test synthetic variants at chosen edit distances (ED3, ED5, and ED7) from Wu-Hu-1 RBD.
(B) Lineage plot of synthetic variants depicts machine learning predictions and experimental validation (Figure S5) for ACE2 binding and non-binding.
(C) Dot plots of synthetic variants correspond to machine learning model (RF and RNN) predictions and experimental validation for antibody binding or escape.
(D) Structural modeling by AlphaFold2 shows predicted structures of RBD variants that are ACE2 binding (green boxes) or non-binding (red boxes); control is Wu-Hu-1 RBD (black box).
See also Figure S5.
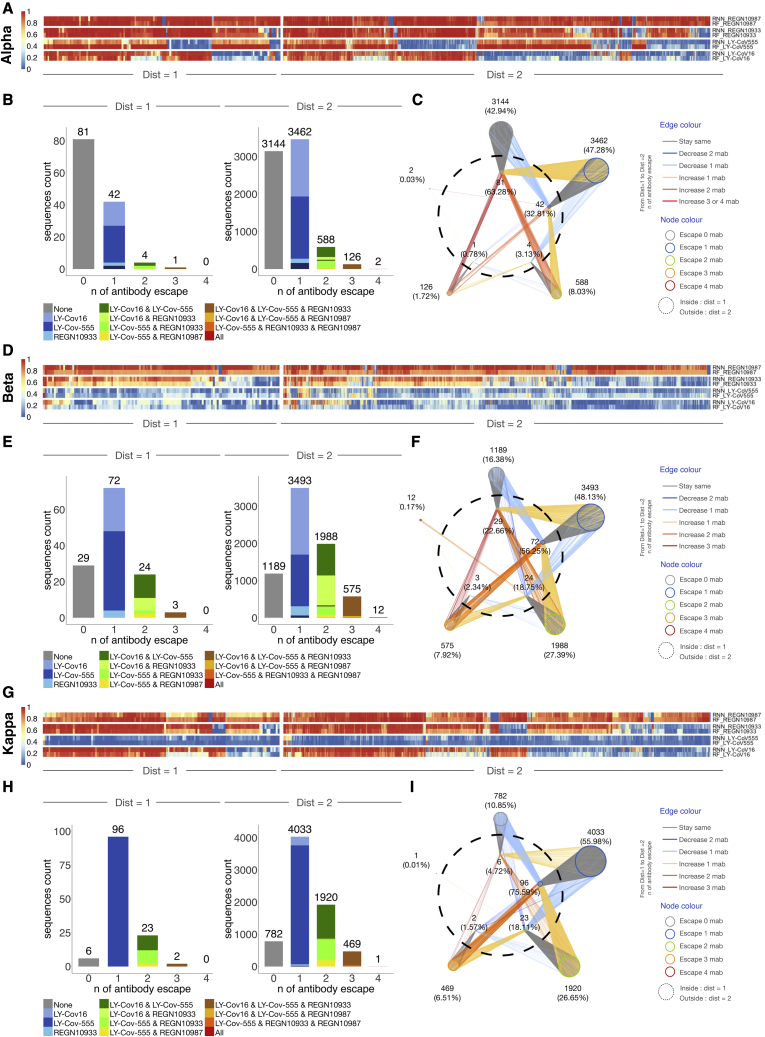
Figure 5.
Predictive profiling of selected RBD variants for antibody escape across low mutational distances
(A, D, and G) Heatmap depicts monoclonal antibody (mAb) binding as assessed by RF and RNN models of ED1 and ED2 variants of Alpha, Beta, and Kappa.
(B, E, and H) The number of sequences escaping a combination of n (number) mAbs for ED1 and ED2 (agreement between models, threshold >0.5).
(C, F, and I) Deep escape networks display possible evolutionary paths between variants and their escape from mAbs.
See also Figure S6.
Predicting antibody escape to current and prospective variants
In addition to the selected synthetic lineages, we also performed machine learning to predict ACE2 binding and antibody escape on a panel of 12 naturally occurring variants of SARS-CoV-2 (Table S6). Once again, we focused our analysis on RBM-2 and four extensively used clinical antibodies (LY-CoV16, LY-CoV555, REGN10933, and REGN10987). We determined the accuracy of machine learning predictions on antibody escape by using the Stanford SARS-CoV-2 Susceptibility Database as a reference (Tzou et al., 2020). Applying the same RBM-2 consensus model approach (RF and RNN) and thresholds as before, the prediction accuracy for ACE2 binding was 100% (12/12) and the prediction accuracy for escape across all four therapeutic antibodies was 85.42% (41/48) (antibody escape is defined here as a reported 30-fold reduced neutralization in the Stanford database). Strikingly, when applying a more stringent threshold for antibody escape prediction that requires both RF and RNN models to have high certainty in their prediction (both models P < 0.25 for escape and P > 0.75 for binding), 100% (30/30) of the machine learning predictions matched the results reported in the Stanford database.
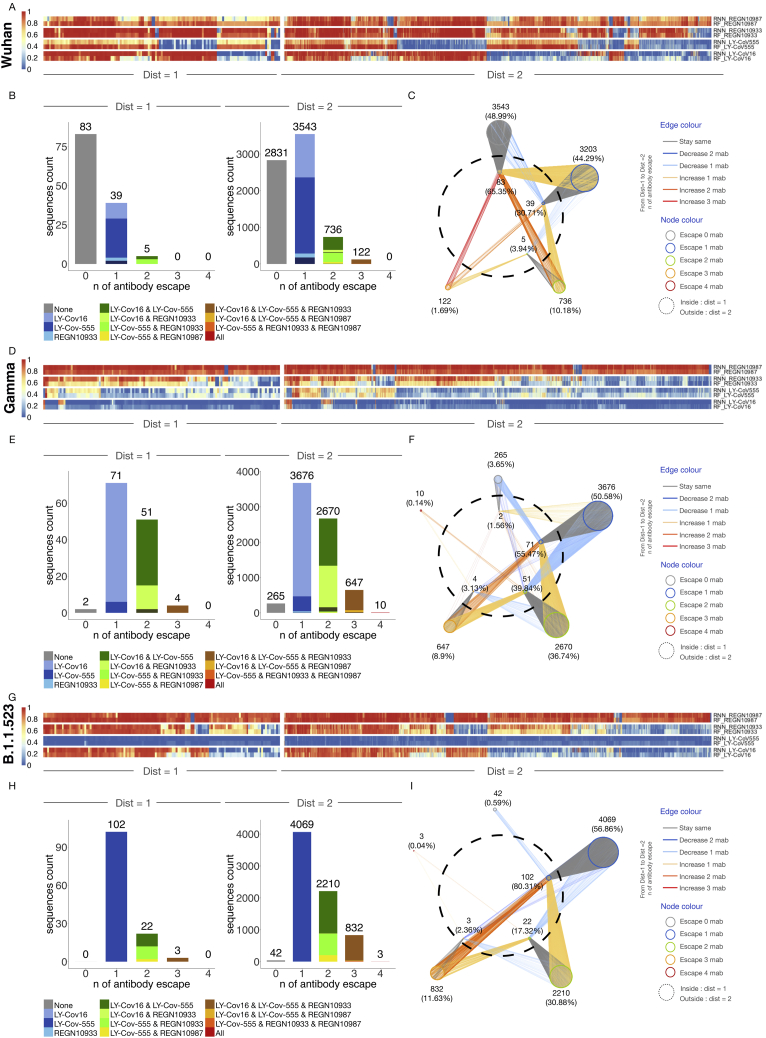
Next, we used machine learning models on RBM-2 to predict antibody escape on prospective ACE2-binding lineages at low mutational distances (ED1 and ED2) from the Wu-Hu-1, Alpha, Beta, Kappa, Gamma, and B.1.1523 RBD sequences (Figures 5 and S6; Table S5). Using a stringent threshold for antibody escape, we identified distinct patterns based on the starting variant. For example, REGN10933 and REGN10987 were largely resilient to escape from ED1 lineages of Wu-Hu-1, Alpha, and Kappa (Figures 5A–5I and S6A–S6I). Although ED1 lineages of Beta and Gamma almost entirely escape both LY-CoV555 and LY-CoV16. A large fraction of ED2 lineages from all variants escaped REGN10933, LY-CoV555, and LY-CoV16, revealing an increasing likelihood of escape with an increasing number of mutations. Notably, a small fraction (0.17%) of Beta ED2 lineages are predicted to escape all four therapeutic antibodies, whereby several of these variants possess mutations in positions 417, 484, 493, and 501, which are all mutated in Omicron variants (Figure 5F). For further visualization, we constructed deep escape networks (Figures 5C, 5F, 5I, S6C, S6F, and S6I), depicting the vulnerability of the four therapeutic antibodies to low distance mutations (ED1 and ED2). Specifically, deep escape networks illustrate the increase in sequence space per mutation while also pointing out the presence of mutations that vastly increase escape from multiple antibodies. For example, there are variants at ED1 from Wu-Hu-1 that are predicted to not escape any of the four antibodies; however, just one additional mutation (ED2) can result in variants predicted to escape up to three antibodies. DML enables rapid in silico evaluation of new variants that appear on genomic databases (GISAID). For example, we performed a similar analysis on B.1.1.523 variant possessing RBD mutations E484K and S494P (Veer et al., 2021), which revealed complete escape from LY-CoV555 and ED1 lineages, as well as substantial escape for other antibodies in ED2 lineages, including three variants that escaped all four of the extensively used therapeutic antibodies (Figures S6G, S6H, and S6I).
Figure S6.
Predictive profiling of additional selected RBD variants for antibody escape across low mutational distances, related to Figure 5
(A, D, and G) Heatmap depicts monoclonal antibody (mAb) binding as assessed by RF and RNN models of ED1 and ED2 variants of Wu-Hu-1, Gamma, and B.1.523.
(B, E, and H) The number of sequences escaping a combination of n (number) mAbs for ED1 and ED2 (agreement between models, threshold > 0.5).
(C, F, and I) Deep escape networks display possible evolutionary paths between variants and their escape from mAbs.
Determining antibody robustness to SARS-CoV-2 mutational lineages
Determining antibody robustness (maintenance of binding) to potential SARS-CoV-2 variants, including to high distance variants with many combinatorial RBD mutations such as Omicron, may be a critical parameter when selecting candidate antibodies for therapeutic development. To this end, we applied DML to determine if we could prospectively determine the robustness of several neutralizing antibodies (Table S3). Initially, we focused on synthetic lineage variants that correspond with Omicron (Figure 6A). We determined antibody escape against specific single and combinatorial mutations present in Omicron in RBM-2 (K417N, E484A, Q493R, and N501Y). Machine learning revealed that some antibodies such as LY-CoV16 and LY-CoV555 are predicted to maintain binding to most single variants but lose binding to nearly all combinatorial variants (Figure 6B), whereas in contrast other antibodies such as REGN10987 and LY-CoV1404 are predicted to bind nearly all single and combinatorial variants. Expanding on this approach, we explored the impact of all mutations in a given position or combination of positions, by calculating the average percentage of escape induced by mutations in that position. This allowed us to construct dynamic antibody escape profiles and identify lineages with mutation orders that may lead to increased escape. For example, lineages derived from an initial mutation in position 493 and even at low distances of ED2 are predicted to have very high escape fractions for several antibodies (LY-CoV16, LY-CoV555, REGN10933, mAb-50, and mAb-82) (Figure 6C), whereas lineages from some positions such as 501 require more mutations such as ED3 and ED4 before antibody escape is predicted. Notably, several antibodies such as REGN10987, mAb-50, and LY-CoV1404 showed highly resilient escape profiles across all positional lineages. Determining antibody robustness at high distances is critical, given how most antibodies discovered against the original SARS-CoV-2 (Wu-Hu-1) are unable to neutralize the high distance Omicron variant. Therefore, we used machine learning models to predict antibody binding and escape to high distance combinatorial variants (ED6–ED10) in RBM-2 (Figures 6D and 6E), revealing varying levels of robustness for several antibodies. For example, as expected from low distance predictions, LY-CoV555 showed very little robustness, with nearly all high distance variants predicted to escape. In contrast, antibodies such as S2E12 and S2H97 are predicted to be very robust at high distances, which correlates with the fact they show broad neutralization across a diverse clade of sarbecoviruses (Starr et al., 2021c). Finally, when considering strict thresholds for binding and escape (both RF and RNN models P < 0.25 for escape and P > 0.75 for binding), LY-CoV1404 was determined to be the antibody with the highest robustness, as it is predicted to bind the broadest set of combinatorial RBD variants, thus correlating with the fact that it maintains neutralization to Omicron and its sublineages (BA.2, BA.4, and BA.5) (Cao et al., 2022b).
Figure 6.
Determining antibody robustness to synthetic RBD variants and mutational lineages
(A) Omicron (BA.1) mutations covered by combinatorial library RBM-2.
(B) Binding prediction for single and combinatorial mutations observed in Omicron.
(C) Dynamic escape profile along Omicron lineage with percentage escape sequences across all mutations at distance 1–4 from Wu-Hu-1.
(D) Antibody prediction of ACE2 binding RBDs for each antibody at edit distance 6–10 from Wu-Hu-1 (10,000 sequences simulated in triplicate, only confident predictions shown (i.e., P(ACE2 binding) > 0.5 and either P(antibody binding) > 0.75 or P(antibody escape) < 0.25 for both RNN and RF).
(E) Total count of confident predictions across all distances (mean across triplicates).
Discussion
Eradication of SARS-CoV-2 appears improbable. Instead, an endemic future likely awaits (Antia and Halloran, 2021; Phillips, 2021). An endemic and continually evolving SARS-CoV-2 poses a perpetual risk for the emergence of new variants that escape from vaccine- or infection-induced antibodies. In this study, we develop DML, a machine learning-guided protein engineering method for determining the impact of combinatorial mutations in the SARS-CoV-2 RBD on ACE2 binding and antibody escape. In DML, machine learning models trained on thousands of labeled RBD variants obtained from library screening make highly accurate predictions across a sequence space of billions of RBD variants, several orders of magnitude larger than what is possible from experimental screening alone.
A combination of future library designs, more elaborate screening strategies based on different binding thresholds and improved machine learning models—perhaps incorporating structural knowledge, could improve predictions across longer lengths of the RBD. For example, a recent study performed a machine learning analysis to predict apparent affinities of ACE2 binding on single and multiple mutation RBD variants (Makowski et al., 2022). Another important consideration is that our DML library was based on the original Wu-Hu-1 RBD sequence; however, nearly all of the circulating variants globally (as of August 2022) are Omicron or its sublineages. Bloom and colleagues demonstrated single-amino acid mutations (DMS) result in a shifting of mutational trajectories when on a different background of RBD variants (Starr et al., 2022). Given the extensive mutational changes present in Omicron variants (>15 RBD mutations), future DML (as well as DMS) studies should use mutagenesis libraries based on an Omicron background RBD sequence, with the caveat that other future high distance variants may still yet emerge, requiring continued updating of library designs.
The evolution and emergence of SARS-CoV-2 variants has created a continuously shifting landscape of clinical authorization for antibody therapeutics: several clinically approved antibodies such as REGN10933, REGN10987, LY-CoV555, LY-CoV16, and S309 are no longer authorized for clinical use, in most cases due to loss of activity versus Omicron or its sublineages (Cameroni et al., 2022; Cao et al., 2022b). One exception is LY-CoV1404, which to date has maintained effective binding and neutralization to all SARS-CoV-2 variants, including Omicron BA.1 and its sublineages (BA.2, BA.4, and BA.5) and is therefore still authorized for clinical use (Cao et al., 2022b). By providing accurate predictions of antibody escape across a large mutational landscape, DML may enable researchers to select candidate antibody therapeutics and cocktails with the most robustness: broadest efficacy against the spectrum of possible variants, some of which may occur simultaneously and may be highly mutated such as Omicron. Assessing the robustness of candidate antibodies against future variants puts therapeutic development on a proactive rather than reactive footing, potentially avoiding situations where many clinically approved antibodies are only used for short periods of time. Furthermore, such an approach could be used to guide the development of antibodies and cocktails that maximize breadth and potency (Hastie et al., 2021; Starr et al., 2021c) to both current and prospective variants and therefore extending the lifespan of clinical use. For example, of the 13 antibodies that we profiled, all of which were discovered based on binding to the original Wu-Hu-1 RBD and included several clinically approved therapeutics, DML predicted that LY-CoV1404 is the most robust to prospective RBD variants, which correlates with its broad neutralization and continued clinical use (Cao et al., 2022b). Therefore going forward, in addition to neutralization potency, antibody robustness to combinatorial mutations in the RBD will be a critical parameter to assess for the therapeutic development of antibodies for COVID-19.
Finally, evidence exists that the RBDs of other endemic coronaviruses may be undergoing adaptive evolution to escape from human antibody responses (Eguia et al., 2021; Kistler and Bedford, 2021). Consequently, the application of DML to predict SARS-CoV-2 escape from polyclonal antibodies present in serum of vaccinated or convalescent individuals, combined with phylogenetic models of viral evolution (Worobey et al., 2020), may enable the prospective identification of future variants with the highest likelihood of emergence and thus support vaccine development for COVID-19.
Limitations of the study
To establish DML, we rationally designed our combinatorial mutagenesis libraries using previously published DMS data on the RBD (Starr et al., 2020) to improve the probability of isolating variants that maintain binding to ACE2, which is important for generating sufficient training data for machine learning. This led to us leaving some positions fixed since the single-mutation DMS data suggested that mutations in these positions lead to a complete loss of binding to ACE2 (Figure 2A). Although this approach was largely effective in covering the mutational sequence space of most SARS-CoV-2 variants, it did lead to some limitations, as some of the fixed positions in our library design (e.g., 486, 496) are mutated in Omicron or its sublineages, further highlighting the impact of epistasis or combinatorial mutations in SARS-CoV-2 evolution. Most notable is position 486, which is mutated in Omicron BA.4 and BA.5 variants (F486V) and is strongly associated with antibody escape, including to BA.1-specific antibodies (Cao et al., 2022b). Therefore, future mutagenesis library designs for DML will need to consider the impact of epistatic effects and should not only rely on single-mutation DMS data. Additionally, during library construction, we split the RBD into three distinct regions to build RBM-1, -2, and -3 libraries to constrain the size of our combinatorial libraries. This leaves us unable to explore epistatic effects of mutations across RBM sites (e.g., S477N in RBM-1 and Q498R in RBM-2 present in Omicron).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Flow cytometry staining reagents | ||
| PE anti-DYKDDDDK Tag Antibody | Biolegend | Cat# 637309; |
| anti-human IgG-AlexaFluor647 | Jackson Immunoresearch | 109-605-098; |
| biotinylated human ACE2 | Acro | Cat# AC2-H82E6 |
| streptavidin-AlexaFluor 647 | Biolegend | Cat# 405237 |
| Deposited data | ||
| Raw sequencing data | Starr et al. 2020 | NCBI SRA: BioProject PRJNA639956 |
| Raw and processed sequencing data | This study | |
| Oligonucleotides | ||
| Degenerate Ultramers and oPools for RBD library construction | IDT | https://github.com/LSSI-ETH |
| Recombinant DNA | ||
| pYD1-RBD(wt) | This study | https://github.com/LSSI-ETH; derived from RRID:Addgene_73447 |
| Cell lines | ||
| EBY100 | ATCC | Cat# MYA-4941 |
| Software and algorithms | ||
| bbduk | Joint Genome Institute | https://jgi.doe.gov/data-and-tools/software-tools/bbtools/bb-tools-user-guide/bbduk-guide/ |
| custom scripts for curation, analysis, and visualization | This study | https://github.com/LSSI-ETH |
Resource availability
Lead contact
Further information and requests for reagents and resources should be directed to and will be fulfilled by the lead contact, Sai T. Reddy (sai.reddy@ethz.ch).
Materials availability
SARS-CoV-2 mutagenesis libraries generated in this study will be made available on request to the lead contact with a completed Materials Transfer Agreement.
Experimental model and subject details
Saccharomyces cerevisiae EBY100 harboring the pYD1 plasmid were cultured for 1-2 days at in a 250 rpm shaking incubator at 30C in SD-UT medium (20 g/l glucose, 6.7 g/l yeast nitrogen base without amino acids, 5.4 g/l Na2HPO4, 8.6 g/l NaH2PO4·H2O and 5 g/l casamino acids), or at 23°C for 36 h in SG-UT induction medium (SD-UT with 20 g/l galactose instead of glucose).
Method details
Rational design of SARS-CoV-2 RBD mutagenesis libraries
Combinatorial library design
The design of the combinatorial library 2C consisted of mutating residues within the RBM-2 region (positions 484-505 of the RBD) and was based on previously described results from deep mutational scanning (DMS) experiments (Starr et al., 2020). DMS enrichment ratios described by Starr et al. were thresholded to exclude mutations with decreased ACE2-binding fitness and then converted to amino acid frequencies as described previously (Mason et al., 2018). For each position, degenerate codons approximating the amino acid frequency distribution and diversity were selected, resulting in a library with a theoretical diversity of 1.50 x 1010 amino acid sequences. Library 2CE consisted of the same combinatorial design in positions 484-505 but with additional fully degenerate codons (NNK) in positions 417 and 439, resulting in a theoretical amino acid diversity of 5.95 x 1012.
Tiling library design
The tiling library 2T was designed by incorporating three positions with full degenerate codons (NNK) within the RBM-2 (positions 484-505) of the RBD. The degenerate codons are tiled across such that the total sequences of a tiling library, i.e., the number of variants of up to a maximum edit distance (ED) k away from the wild-type sequence is determined by the length of sequence (n, here = 14 non-fixed positions in RBM-2), the number of NNKs (or max ED) introduced (k, here = 3) and the size of the Alphabet (a, here = 20):
Similarly, the number of sequences for a given ED k is given by:
Similarly, the number of sequences for a given ED k is given by:
The resulting total diversity of the library 2T is 1,533,035 sequences.
Cloning and expression of RBD mutagenesis libraries for yeast surface display
For libraries 2C and 2CE, synthetic single-stranded oligonucleotides (ssODNs) (Integrated DNA Technologies ultramers or oPools) were designed with degenerate codons spanning the region of interest and encoding the desired library diversity, with 30 bp overhangs on each end that were homologous to the yeast display plasmid pYD1. For library 2T, pools of ssODN were designed, where each member of the pool contains one combination of the three ‘NNK’ codons; in this case, consisting of 120 unique ssODNs. The ssODNs were amplified by PCR to produce double-stranded DNA. The plasmid pYD1 was modified such that the entire C-terminal fusion to Aga2 was replaced with a cassette encoding the RBD (Wu-Hu-1 sequence), expression tags and stop codon (HA Tag-RBD-FLAG-Stop). The RBM-2 residues 484-505 were replaced with an EcoRI recognition site, allowing production of a linearized vector with homology to mutagenesis ssODNs and with no parental background. Insert and EcoRI-linearized plasmids were concentrated and purified by silica spin columns (Zymo D4013) followed by drop dialysis for 1 hour in nuclease-free H2O (Millipore VSWP02500). The libraries were cloned and expressed in yeast by in vivo homologous recombination, as previously described (Boder and Wittrup, 1997; Chao et al., 2006), using 1 μg each of plasmid and insert DNA per 300 μl of electrocompetent EBY100 cells in a 2 mm electroporation cuvette.
Screening RBD libraries for ACE2-binding and non-binding
Surface expression of SARS-Cov2 RBD was induced by growth in SG-UT medium at 23°C for 16-40 hours, as previously described (Boder and Wittrup, 1997). Approximately 108 library cells were washed once with 1 mL wash buffer (Dulbecco’s PBS+ 0.5% BSA + 0.1% Tween20 + 2 mM EDTA) by centrifugation at 8000 x g for 30 s. Washed cells were stained with 50 nM biotinylated human ACE2 (Acro AC2-H82E6) for 30 minutes at 4 °C, followed by an additional wash. Cells were then stained with 2.5 ng/μl streptavidin-AlexaFluor 647 (Biolegend 405237) and 1 ng/μl PE anti-DYKDDDDK Tag Antibody (Biolegend 637310) for 30 minutes at 4 °C. Cells were subsequently pelleted by centrifugation at 8000 x g for 30s and kept on ice until sorting. Binding (ACE2+/FLAG+) and non-binding (ACE2-/FLAG+) cells were sorted by FACS (BD FACSAria Fusion or Sony MA800 cytometer) (Figure 2). Collected cells were cultured in SD-UT medium for one to two days at 30 °C. Induction and sorting was repeated until the desired populations were pure.
Screening RBD libraries for antibody binding and escape
RBD libraries pre-sorted for ACE2-binding were cultured and induced, as described above. Induced cells were washed once with DPBS wash buffer, followed by incubation with 100 nM monoclonal antibody, or antibody mixtures. In the case of antibody mixtures, 100 nM of each antibody was used. Following an additional wash, cells were resuspended in 5 ng/μl anti-human IgG-AlexaFluor647 (Jackson Immunoresearch 109-605-098) and incubated for 30 minutes at 4°C. Cells were washed once more and resuspended in 1 ng/μl anti-FLAG-PE before 30 minutes of incubation at 4°C. Cells were then pelleted by centrifugation at 8000 x g for 30s and kept on ice until sorting. Cells expressing RBD that maintained antibody-binding (IgG+/FLAG+) or showed a complete loss of antibody binding (escape) (IgG-/FLAG+) were sorted by FACS (BD Aria Fusion or Sony MA800 instrument). Collected cells were cultured in SD-UT medium for 16-40 hours at 30 °C. Induction and sorting was repeated for multiple rounds until the desired populations of RBD variants showed purity for binding and escape (non-binding) to antibodies.
Antibody production and purification
Heavy chain and light chain inserts were cloned into pTwist transient expression vectors by Gibson Assembly. 30 mL cultures of Expi293 cells (Thermo, A14635) were transfected according to the manufacturer’s instructions. After 5-7 days, dense Expi293 cultures were centrifuged at 300 x g for 5 minutes to pellet the cells. Supernatant was filtered using Steriflip® 0.22 μm (Merck, SCGP00525) filter units. Using protein G purification, Expi supernatant was directly loaded onto Protein G Agarose (Pierce, Cat# 20399) gravity columns, washed twice with PBS and eluted using Protein G Elution Buffer (Pierce, Cat# 21004). The eluted fractions were immediately neutralized with 1M TRIS-Buffer (pH 8) to physiological pH and quantified by Nanodrop™ 2000c for A280 nm absorption. Protein containing fractions were pooled and buffer exchanged using SnakeSkin™ dialysis tubing (10 MWCO, Pierce Cat#68100) followed by further dialysis and concentration using Amicon Ultra-4 10kDa centrifugal units (Merck, Cat# UFC801096), as described previously (Vazquez-Lombardi et al., 2018).
Deep sequencing of RBD libraries
Plasmid DNA encoding the RBD variants was isolated following the manufacturer’s instructions (Zymo D2004). Mutagenized regions of the RBD were amplified using custom oligonucleotides. Illumina Nextera barcode sequences were added in a second PCR amplification step, allowing for multiplexed high-throughput sequencing runs. Populations were pooled at the desired ratios and sequenced using Illumina 2 x 250 PE or 2 x 150 PE protocols (MiSeq or NovaSeq instruments).
Experimental validation of selected RBD variants for ACE2-binding and antibody escape
Individual sequences for RBD variants were ordered as complementary forward and reverse primers (Integrated DNA Technologies) in 96-well plates A single round of annealing and extension was used to produce double-stranded DNA with 14-bp of homology at 5’ and 3’ ends to the pYD1-RBD entry vector, followed by Gibson Assembly with EcoRI digested vector. Plasmids were transformed into EBY100 prepared with the Frozen-EZ Yeast Transformation Kit II (Zymo) and plated on SD-UT agar. Individual colonies were picked and grown in SD-UT liquid medium overnight at 30°C, then diluted to OD600 = 0.5 in SG-UT medium and grown for 40-48 hours at 23°C. Cells were stained with biotinylated ACE2 or purified antibody as described above. Flow cytometry analysis was performed on the BD Fortessa cytometer.
Quantification and statistical analysis
Processing of deep sequencing data, statistical analysis and plots
Data preprocessing
Sequencing reads were paired, quality trimmed and assembled using Geneious and BBDuk, with a quality threshold of qphred ≥ 25. Mutagenized regions of interest were then extracted using custom Python scripts, followed by translation to amino acid sequences. The sequences obtained from each of the three libraries (2C, 2CE and 2T) were pre-processed separately before being combined into the final training set used for model training and evaluation. To remove sequencing errors, all libraries were filtered for sequences complying with the initial degenerate codon mutagenesis scheme. Library 2CE was filtered for only those sequences retaining unmutated residues in positions 417/439, to focus on the 484-505 region. Next, library 2T was filtered using a threshold of read counts > 4 and restricted to sequences that were ≤ ED3 from Wu-Hu-1 RBD sequence.
Duplicate sequences in the full dataset were removed and a balanced dataset was created from the remaining data such that equal numbers of positive (binding) and negative class (non-binding) sequences were present for each ED. We observed significant bias in model performance when predictions are separated by ED from the Wu-Hu-1 RBD sequence, which was likely due to class (binding vs. non-binding/escape) imbalance in the training data. Class balancing was thus performed through random subsampling from the majority class at each ED equal to the counts from the minority class. Those that were not sampled from the majority class were then reserved separately as additional “unseen sequences”. These were then used separately for model evaluation, or combined with the held-out test set to ensure that the models could generalize well even to the sequences removed during balancing, as well as perform well with an imbalanced test set.
Statistical analysis and plots
Statistical analysis was performed using R 4.0.1 (R Core Team, 2020) and Python 3.8.5 (Rossum and Drake, 2011) . Graphics were generated using the ggplot2 3.3.3 (Wickham, 2009), ComplexHeatmap 2.4.3 (Gu et al., 2016), pheatmap 1.0.12 (Kolde, 2019), igraph 1.2.6 (Csardi and Nepusz, 2006), RCy3 2.8.1 (Gustavsen et al., 2019), stringr 1.4.0 (Wickham, 2019), dplyr 1.0.6 (Wickham et al., 2020), and RColorBrewer 1.1-2 (Neuwirth, 2014) R package.
Escape Networks
Network plots were generated using the igraph package (Csardi and Nepusz, 2006) and Cytoscape software 3.8.2 (Shannon et al., 2003) with edges drawn between every pair of two amino acid sequences from ED 1 and 2, when the pair of sequences share a common mutation on amino acid level. Edges were colored according to the change in number of antibodies that escape. Nodes representing RBD variant sequences were clustered and colored according to the number of antibodies that escape, and the mutational distance from the reference sequence.
Machine learning model training and evaluation
All machine learning (ML) classifier models were built in Python (3.8.5) (Rossum and Drake, 2011). Data was prepared and visualized using numpy (1.19.2), matplotlib (3.3.4), and pandas (1.2.4). Random Forest (RF) and other benchmarking ML models were built using Scikit-Learn (0.24.2), a 80/20 train-test data split (random split) to train baseline models, and a 90/10 train-test data split (random split) for final RF and RNN models. Keras libraries (2.4.3) from Tensorflow (v2.5) were used to build the long-short-term-memory recurrent neural networks (RNN) models.
RBD sequences were one-hot encoded prior to being used as inputs into the models. For the RNN, the 2D one-hot encoded matrix was used as the input, while for all other models, the matrix was flattened into a single dimensional vector beforehand. After selecting the best models, hyperparameter optimization was performed to further improve the performance of the chosen RF and RNN models using 50 rounds of Random Search with 5-fold cross-validation while scoring based on precision (Table S5). All RF models were further calibrated using both the “isotonic” and Platt scaling (Platt, 2000; Boström, 2008; Niculescu-Mizil and Caruana, 2005), and the best model from the three was selected by calculating the overall mean-square error (MSE) from the true labels, with the RF model with the lowest MSE selected as the final model. Models were evaluated on the full test set which is unbalanced and includes the sequences removed during balancing to create the training set. Models were evaluated on the basis of Accuracy, F1, MCC, and AUC-ROC curve using the full test set, over 5 rounds of external cross-validation using different train-test splits. For further detailed evaluation, the test data was separated into two distances: low and high distance sequence sets, which consisted only of sequences ≤ ED5 or ≥ ED6 from Wu-Hu-1 RBD sequence, respectively. These two sets were then used to evaluate the accuracy, F1, MCC, and AUC-ROC of models to investigate any performance bias at different distances.
In silico sequence generation and evaluation
Synthetic RBD variant sequences were generated in silico using custom Python scripts for selected edit distances (ED) from the Wu-Hu-1 RBD sequence. The ED was defined on both the nucleotide and amino acid level, such that each generated nucleotide sequence was categorized by an ED pair (distance_nt, distance_aa). The synthetic variants (in silico generated sequences) were evaluated for their probability of ACE2-binding and non-binding using a consensus model (RF and RNN) approach. For a given RBD sequence, ACE2-binding prediction was defined as the case where both models output P > 0.5, else the sequence was considered as ACE2 non-binding. Similarly, the sequences were evaluated for binding and escape (non-binding) from monoclonal antibodies. Here the sequences were categorized into one of four categories: escape (both models P <0.25), antibody binding (both models P >0.75), unsure (at least one model gives P between 0.25 and 0.75), and disagree (one model outputs P <0.25 while the other model outputs a P >0.75).
Structural Prediction of RBD variants by AlphaFold2
Structural predictions were generated with the Alphafold v2.1.0 public iPython notebook using residues 331-530 of the spike protein. (https://colab.research.google.com/github/deepmind/Alphafold/blob/main/notebooks/AlphaFold.ipynb) (Jumper et al., 2021). Results were visualized and aligned in PyMol v2.2.3 (Schrödinger and DeLano, 2020).
Acknowledgments
We thank the ETH Zurich D-BSSE Single Cell Unit and the Genomics Facility Basel for support. This work was supported by the Botnar Research Centre for Child Health (FTC COVID-19, to S.T.R.) and the European Research Council (H2020-SC1-PHE-Coronavirus-2020, CoroNab, to S.T.R.).
Author contributions
Conceptualization, J.M.T., C.R.W., B.G., R.A.E., and S.T.R. Experimental methodology, J.M.T., R.A.E., M.D.O., and L.F. Computational methodology, J.M.T., C.R.W., B.G., S.W.M., J.H., and A.Y. Supervision, J.M.T., C.R.W., and S.T.R. Writing – original draft, J.M.T., C.R.W., B.G., R.A.E., and S.T.R. Writing – review & editing, all authors.
Declaration of interests
ETH Zurich has filed for patent protection on the technology described herein, and J.M.T., C.R.W., B.G., R.A.E., and S.T.R. are named as co-inventors. C.R.W. is an employee of Alloy Therapeutics (Switzerland) AG. C.R.W. and S.T.R. may hold shares of Alloy Therapeutics. S.T.R. is on the scientific advisory board of Alloy Therapeutics.
Published: August 31, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cell.2022.08.024.
Supplemental information
Each dataset combines sequences from all RBM libraries after preprocessing, filtering, and removing duplicates.
See Figure 3.
(https://covdb.stanford.edu/page/susceptibility-data/, 2021-10-19)
See Figure 3.
Data and code availability
-
•
The main data supporting the results in this study are available within the paper and its supplemental information.
-
•
Raw and processed data and code (scripts) used for data curation, analysis and visualization are available at: https://github.com/LSSI-ETH.
-
•
Additional data files and code that supports the findings of this study is available from the corresponding authors upon reasonable request.
References
- Akbar R., Robert P.A., Weber C.R., Widrich M., Frank R., Pavlović M., Scheffer L., Chernigovskaya M., Snapkov I., Slabodkin A., et al. In silico proof of principle of machine learning-based antibody design at unconstrained scale. bioRxiv. 2021 doi: 10.1101/2021.07.08.451480. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antia R., Halloran M.E. Transition to endemicity: understanding COVID-19. Immunity. 2021;54:2172–2176. doi: 10.1016/j.immuni.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.O., West A.P., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F., et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842.e16. doi: 10.1016/j.cell.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boder E.T., Wittrup K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- Boström H. Seventh International Conference on Machine Learning and Applications. IEEE Publications; 2008. Calibrating random forests; pp. 121–126. [Google Scholar]
- Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., De Marco A., di Iulio J., et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:1–3. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao G., Lau W.L., Hackel B.J., Sazinsky S.L., Lippow S.M., Wittrup K.D. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 2006;1:755–768. doi: 10.1038/nprot.2006.94. [DOI] [PubMed] [Google Scholar]
- Csardi G., Nepusz T. The igraph software package for complex network research. InterJournal Complex Systems. 2006;1695:1–9. [Google Scholar]
- Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., Duyvesteyn H.M.E., Ginn H.M., Mentzer A.J., Tuekprakhon A., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Zhou D., Ginn H.M., Duyvesteyn H.M.E., Supasa P., Case J.B., Zhao Y., Walter T.S., Mentzer A.J., Liu C., et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183–2200.e22. doi: 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguia R.T., Crawford K.H.D., Stevens-Ayers T., Kelnhofer-Millevolte L., Greninger A.L., Englund J.A., Boeckh M.J., Bloom J.D. A human coronavirus evolves antigenically to escape antibody immunity. PLoS Pathog. 2021;17:e1009453. doi: 10.1371/journal.ppat.1009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling R.A., Weber C.R., Mason D.M., Friedensohn S., Wagner B., Bieberich F., Kapetanovic E., Vazquez-Lombardi R., Di Roberto R.B., Hong K.-L., et al. SARS-CoV-2 reactive and neutralizing antibodies discovered by single-cell sequencing of plasma cells and mammalian display. Cell Rep. 2022;38:110242. doi: 10.1016/j.celrep.2021.110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, C. for D.E. FDA; 2022. FDA Authorizes Revisions to Evusheld Dosing. [Google Scholar]
- FDA, C. for D.E. FDA; 2022. FDA Updates Sotrovimab Emergency Use Authorization. [Google Scholar]
- FDA, O. of the C. FDA; 2022. Emergency Use Authorization. [Google Scholar]
- Fowler D.M., Fields S. Deep mutational scanning: a new style of protein science. Nat. Methods. 2014;11:801–807. doi: 10.1038/nmeth.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., St Denis K.S., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Loes A.N., Crawford K.H.D., Starr T.N., Malone K.D., Chu H.Y., Bloom J.D. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29:463–476.e6. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Starr T.N., Barnes C.O., Weisblum Y., Schmidt F., Caskey M., Gaebler C., Cho A., Agudelo M., Finkin S., et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat. Commun. 2021;12:4196. doi: 10.1038/s41467-021-24435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K.H.D., et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29:44–57.e9. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Eils R., Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinform. Oxf. Engl. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- Gustavsen J.A., Pai S., Isserlin R., Demchak B., Pico A.R. RCy3: network biology using cytoscape from within R. F1000Res. 2019;8:1774. doi: 10.12688/f1000research.20887.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Su C., Zhang Y., Bai C., Zheng A., Qiao C., Wang Q., Niu S., Chen Q., Zhang Y., et al. Molecular insights into receptor binding of recent emerging SARS-CoV-2 variants. Nat. Commun. 2021;12:6103. doi: 10.1038/s41467-021-26401-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie K.M., Li H., Bedinger D., Schendel S.L., Dennison S.M., Li K., Rayaprolu V., Yu X., Mann C., Zandonatti M., et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021;374:472–478. doi: 10.1126/science.abh2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter S., Schmidhuber J. Long short-term memory. Neural Comput. 1997;9:1735–1780. doi: 10.1162/neco.1997.9.8.1735. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Arora P., Groß R., Seidel A., Hörnich B.F., Hahn A.S., Krüger N., Graichen L., Hofmann-Winkler H., Kempf A., et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384–2393.e12. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S., Liu L., Guo Y., Liu L., Chan J.F.-W., Huang Y., Wang M., Luo Y., Yu J., Chu H., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler K.E., Bedford T. Evidence for adaptive evolution in the receptor-binding domain of seasonal coronaviruses OC43 and 229e. eLife. 2021;10:e64509. doi: 10.7554/eLife.64509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde R. R Package Version; 2019. pheatmap: Pretty Heatmaps. p. 1.0.12.https://rdrr.io/cran/pheatmap/ [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Liu L., Iketani S., Guo Y., Chan J.F.-W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- Makowski E.K., Schardt J.S., Smith M.D., Tessier P.M. Mutational analysis of SARS-CoV-2 variants of concern reveals key tradeoffs between receptor affinity and antibody escape. PLoS Comput. Biol. 2022;18:e1010160. doi: 10.1371/journal.pcbi.1010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D.P., Lyrtas S., Lucaci A.G., Maier W., Grüning B., Shank S.D., Weaver S., Maclean O.A., Orton R.J., Lemey P., et al. Selection analysis identifies significant mutational changes in Omicron that are likely to influence both antibody neutralization and Spike function. bioRxiv. 2022 doi: 10.1101/2022.01.14.476382. Preprint at. [DOI] [Google Scholar]
- Mason D.M., Friedensohn S., Weber C.R., Jordi C., Wagner B., Meng S.M., Ehling R.A., Bonati L., Dahinden J., Gainza P., et al. Optimization of therapeutic antibodies by predicting antigen specificity from antibody sequence via deep learning. Nat. Biomed. Eng. 2021;5:600–612. doi: 10.1038/s41551-021-00699-9. [DOI] [PubMed] [Google Scholar]
- Mason D.M., Weber C.R., Parola C., Meng S.M., Greiff V., Kelton W.J., Reddy S.T. High-throughput antibody engineering in mammalian cells by CRISPR/Cas9-mediated homology-directed mutagenesis. Nucleic Acids Res. 2018;46:7436–7449. doi: 10.1093/nar/gky550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K.D., Jacobs J.L., Mellors J.W. The emerging plasticity of SARS-CoV-2. Science. 2021;371:1306–1308. doi: 10.1126/science.abg4493. [DOI] [PubMed] [Google Scholar]
- Neuwirth E. R package version 1.1-2 (http://colorbrewer2.org); 2014. RColorBrewer: ColorBrewer palettes. [Google Scholar]
- Niculescu-Mizil A., Caruana R. Proceedings of the 22nd International Conference on Machine Learning – ICML ’05. ACM Press; 2005. Predicting good probabilities with supervised learning; pp. 625–632. [Google Scholar]
- Nielsen S.C.A., Yang F., Jackson K.J.L., Hoh R.A., Röltgen K., Jean G.H., Stevens B.A., Lee J.-Y., Rustagi A., Rogers A.J., et al. Human B cell clonal expansion and convergent antibody responses to SARS-CoV-2. Cell Host Microbe. 2020;28:516–525.e5. doi: 10.1016/j.chom.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH . 2022. Anti-SARS-CoV-2 monoclonal antibodies.https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ [Google Scholar]
- Phillips N. The coronavirus is here to stay—here’s what that means. Nature. 2021;590:382–384. doi: 10.1038/d41586-021-00396-2. [DOI] [PubMed] [Google Scholar]
- Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Platt J. Advances in Large Margin Classifiers. MIT Press; 2000. Probabilistic outputs for support vector machines and comparisons to regularized likelihood methods. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; 2020. R: A language and environment for statistical computing. [Google Scholar]
- Rossum G.V., Drake F.L., Jr. Network Theory Ltd; 2011. The Python Language Reference Manual. [Google Scholar]
- Saka K., Kakuzaki T., Metsugi S., Kashiwagi D., Yoshida K., Wada M., Tsunoda H., Teramoto R. Antibody design using LSTM based deep generative model from phage display library for affinity maturation. Sci. Rep. 2021;11:5852. doi: 10.1038/s41598-021-85274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger L., DeLano W. 2020. PyMOL.http://www.pymol.org/pymol [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward D.J., Kim C., Ehling R.A., Pankow A., Castro Dopico X., Dyrdak R., Martin D.P., Reddy S.T., Dillner J., Karlsson Hedestam G.B., et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: a cross-sectional study. Lancet Infect. Dis. 2022;22:813–820. doi: 10.1016/S1473-3099(22)00129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Starr T.N., Czudnochowski N., Liu Z., Zatta F., Park Y.J., Addetia A., Pinto D., Beltramello M., Hernandez P., Greaney A.J., et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature. 2021;597:97–102. doi: 10.1038/s41586-021-03807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Addetia A., Hannon W.W., Choudhary M.C., Dingens A.S., Li J.Z., Bloom J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science. 2021;371:850–854. doi: 10.1126/science.abf9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Dingens A.S., Bloom J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep. Med. 2021;2:100255. doi: 10.1016/j.xcrm.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Hannon W.W., Loes A.N., Hauser K., Dillen J.R., Ferri E., Farrell A.G., Dadonaite B., McCallum M., et al. Shifting mutational constraints in the SARS-CoV-2 receptor-binding domain during viral evolution. Science. 2022;377:420–424. doi: 10.1126/science.abo7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., Navarro M.J., Bowen J.E., Tortorici M.A., Walls A.C., et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295–1310.e20. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Nutalai R., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–2211.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J., van den Brink E.N., Poon L.L.M., Marissen W.E., Leung C.S.W., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E., et al. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E.C., Rosen L.E., Shepherd J.G., Spreafico R., da Silva Filipe A., Wojcechowskyj J.A., Davis C., Piccoli L., Pascall D.J., Dillen J., et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184:1171–1187.e20. doi: 10.1016/j.cell.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong P., Gautam A., Windsor I.W., Travers M., Chen Y., Garcia N., Whiteman N.B., McKay L.G.A., Storm N., Malsick L.E., et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell. 2021;184:4969–4980.e15. doi: 10.1016/j.cell.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.-C., Lee Y.-C., Tseng T.-S. Comprehensive deep mutational scanning reveals the immune-escaping hotspots of SARS-CoV-2 receptor-binding domain targeting neutralizing antibodies. Front. Microbiol. 2021;12:698365. doi: 10.3389/fmicb.2021.698365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzou P.L., Tao K., Nouhin J., Rhee S.-Y., Hu B.D., Pai S., Parkin N., Shafer R.W. Coronavirus antiviral research database (CoV-RDB): an online database designed to facilitate comparisons between candidate anti-coronavirus compounds. Viruses. 2020;12:1006. doi: 10.3390/v12091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Lombardi R., Nevoltris D., Luthra A., Schofield P., Zimmermann C., Christ D. Transient expression of human antibodies in mammalian cells. Nat. Protoc. 2018;13:99–117. doi: 10.1038/nprot.2017.126. [DOI] [PubMed] [Google Scholar]
- Veer B.M.J.W. van der, Dingemans J., Alphen L.B. van, Hoebe C.J.P.A., Savelkoul P.H.M. A novel B.1.1.523 SARS-CoV-2 variant that combines many spike mutations linked to immune evasion with current variants of concern. bioRxiv. 2021 doi: 10.1101/2021.09.16.460616. Preprint at. [DOI] [Google Scholar]
- Wang L., Zhou T., Zhang Y., Yang E.S., Schramm C.A., Shi W., Pegu A., Oloniniyi O.K., Henry A.R., Darko S., et al. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants. Science. 2021;373:eabh1766. doi: 10.1126/science.abh1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Westendorf K., Žentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., Lovett E., van der Lee R., Hendle J., Pustilnik A., et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39:110812. doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., de Oliveira T., Vermeulen M., van der Berg K., et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; 2009. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Wickham H. R package version 1.4.0; 2019. stringr: Simple, Consistent Wrappers for Common String Operations.https://CRAN.R-project.org/package=stringr [Google Scholar]
- Wickham H., François R., Henry L., Müller K. R package dplyr version 1.0.2; 2020. dplyr: A Grammar of Data Manipulation. [Google Scholar]
- Worobey M., Pekar J., Larsen B.B., Nelson M.I., Hill V., Joy J.B., Rambaut A., Suchard M.A., Wertheim J.O., Lemey P. The emergence of SARS-CoV-2 in Europe and North America. Science. 2020;370:564–570. doi: 10.1126/science.abc8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Nielsen S.C.A., Hoh R.A., Röltgen K., Wirz O.F., Haraguchi E., Jean G.H., Lee J.-Y., Pham T.D., Jackson K.J.L., et al. Shared B cell memory to coronaviruses and other pathogens varies in human age groups and tissues. Science. 2021;372:738–741. doi: 10.1126/science.abf6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Sun X., Ye J., Ding L., Liu M., Yang Z., Lu X., Zhang Y., Ma L., Gu W., et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell. Mol. Immunol. 2020;17:621–630. doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Wu N.C., Zhu X., Lee C.-C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C., et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 2020;26:1422–1427. doi: 10.1038/s41591-020-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each dataset combines sequences from all RBM libraries after preprocessing, filtering, and removing duplicates.
See Figure 3.
(https://covdb.stanford.edu/page/susceptibility-data/, 2021-10-19)
See Figure 3.
Data Availability Statement
-
•
The main data supporting the results in this study are available within the paper and its supplemental information.
-
•
Raw and processed data and code (scripts) used for data curation, analysis and visualization are available at: https://github.com/LSSI-ETH.
-
•
Additional data files and code that supports the findings of this study is available from the corresponding authors upon reasonable request.