Abstract
Intracellular survival plays a central role in the pathogenesis of Mycobacterium tuberculosis. To identify M. tuberculosis genes required for intracellular survival within macrophages, an M. tuberculosis H37Rv plasmid library was constructed by using the shuttle vector pOLYG. This plasmid library was electroporated into Mycobacterium smegmatis 1-2c, and the transformants were used to infect the human macrophage-like cell line U-937. Because M. smegmatis does not readily survive within macrophages, any increased intracellular survival is likely due to cloned M. tuberculosis H37Rv DNA. After six sequential passages of M. smegmatis transformants through U-937 cells, one clone (p69) was enriched more than 70% as determined by both restriction enzyme and PCR analyses. p69 demonstrated significantly enhanced survival compared to that of the vector control, ranging from 2.4- to 5.3-fold at both 24 and 48 h after infection. DNA sequence analysis revealed three open reading frames (ORFs) in the insert of p69. ORF2 (1.2 kb) was the only one which contained a putative promoter region and a ribosome-binding site. Deletion analysis of the p69 insert DNA showed that disruption of ORF2 resulted in complete loss of the enhanced intracellular survival phenotype. This gene was named the enhanced intracellular survival (eis) gene. By using an internal region of eis as a probe for Southern analysis, eis was found in the genomic DNA of various M. tuberculosis strains and of Mycobacterium bovis BCG but not in that of M. smegmatis or 10 other nonpathogenic mycobacterial species. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis showed that all M. smegmatis eis-containing constructs expressed a unique protein of 42 kDa, the predicted size of Eis. The expression of this 42-kDa protein directly correlated to the enhanced survival of M. smegmatis p69 in U-937 cells. These results suggest a possible role for eis and its protein product in the intracellular survival of M. tuberculosis.
Mycobacterium tuberculosis is an important human pathogen responsible for 3.1 million deaths worldwide per year (9). Although both virulent and avirulent mycobacteria are internalized by monocytes and macrophages (35, 43), only pathogenic mycobacteria survive and replicate intracellularly (28). M. tuberculosis is resistant to macrophage killing, and its survival during phagocytosis and its subsequent multiplication within these professional phagocytes are critical to its pathogenesis. A variety of mechanisms have been suggested to contribute to the survival of M. tuberculosis within macrophages (15, 39), including inhibition of phagosome-lysosome fusion (2), inhibition of the acidification of phagosomes (41), resistance to killing by reactive oxygen intermediates (26) and reactive nitrogen intermediates (12, 27), and modification of the lipid composition of the mycobacterial cell membrane, thereby altering its capacity to interact with immune or inflammatory cells (19). However, little progress has been made in identifying the genes and their corresponding products responsible for these properties. Because they represent potentially interesting targets for novel drugs and vaccines, the identification of the mycobacterial products that promote intracellular survival remains a priority.
Recent development of genetic techniques applicable to studying mycobacteria is advancing our understanding of how mycobacteria survive in phagocytic cells (3, 7, 29, 31, 33). Several groups have recently used Escherichia coli to express M. tuberculosis and Mycobacterium leprae genes which may be involved in entry and survival within mammalian cells (3, 22, 35, 36). However, because of the diverse genetic and structural differences between mycobacteria and enterobacteria, E. coli systems may be limited in the number of tuberculosis virulence gene products which can be successfully expressed, processed, and exported or transported to appropriate functional sites.
New vectors and methodologies for the transformation of mycobacteria have been developed that allow for the study of virulent mycobacterial genes in their homologous hosts. Mycobacterium smegmatis was selected for use in these studies because it grows rapidly in the laboratory, readily expresses genes from other mycobacteria (20, 40, 44), and can be genetically manipulated by various techniques (21). Numerous genes from virulent mycobacteria have been expressed in M. smegmatis, including the superoxide dismutase gene from M. tuberculosis (46), genes for the production of glycopeptidolipid antigens and the Mig protein from Mycobacterium avium (8, 34), a gene expressing a 19-kDa glycosylated antigen from M. tuberculosis (16), the noxR1 gene from M. tuberculosis (14), and the tr-trx gene from M. leprae (44).
In the present study, the vector pOLYG was used to construct a genomic plasmid library from the DNA of the virulent M. tuberculosis strain H37Rv. The library was then introduced into M. smegmatis. A human histocytic macrophage-like cell line, U-937 (18, 42), was used for selecting transformants with enhanced intracellular survival. U-937 cells may be converted from a nonadherent, weakly phagocytic form to an adherent, actively phagocytic state with phorbol esters and other agents. This cell line has been widely used and accepted as a model system for the study of macrophage interactions with a variety of intracellular pathogens (10, 11, 22, 30, 32, 35, 45) (C. Jagannath, E. Sepulveda, L. Srinivasan, R. M. Emanuele, and R. L. Hunter, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. U-97, p. 560, 1997). M. tuberculosis H37Rv replicates and is not killed in U-937 cells (Jagannath et al., Abstr. 97th Gen. Meet. Am. Soc. Microbiol.), while M. smegmatis does not multiply and is readily killed. By serial passage through U-937 cells, an M. smegmatis transformant clone that showed enhanced intracellular survival over M. smegmatis containing the vector alone was isolated. Evidence that this enhanced intracellular survival phenotype is due to an M. tuberculosis H37Rv gene which we have named eis is presented.
MATERIALS AND METHODS
Strains and growth media.
M. smegmatis 1-2c, a derivative of strain mc26 selected for improved transformation efficiency (46), was grown in Middlebrook 7H9 broth (Difco) supplemented with 0.2% glycerol or plated on 7H10 agar (Difco) with 0.2% glucose at 37°C. M. tuberculosis H37Rv was grown in Middlebrook 7H9 broth as previously described (47). Hygromycin B at 50 μg/ml (Boehringer Mannheim) was used in mycobacterial media to maintain the presence of pOLYG. Luria-Bertani (LB) broth or agar with 200 μg of hygromycin B/ml or 100 μg of ampicillin/ml was used for growing E. coli transformants. Human macrophage-like U-937 cells (ATCC 1593.2 CRL) were grown in RPMI 1640 medium (Sigma) containing 10% fetal calf serum (FCS) (Atlanta Biologicals) under 5% CO2 at 37°C.
Construction of an M. tuberculosis H37Rv genomic DNA library.
Genomic DNA was isolated from M. tuberculosis H37Rv as previously described (47). Briefly, fresh liquid cultures of H37Rv were inoculated with a 3-week starter culture and then grown for 7 days before harvesting. M. tuberculosis H37Rv DNA was partially digested with the restriction enzyme Sau3AI, and 4- to 12-kb fragments were recovered after electrophoresis on a 0.7% agarose gel. DNA was extracted from the gel and cloned into the BamHI site of the plasmid pOLYG (17). The library was transformed into Max Efficiency E. coli DH5α (Gibco BRL) competent cells, plated on LB agar containing 200 μg of hygromycin B/ml, and incubated overnight at 37°C. Approximately 7,000 independent E. coli transformants were generated by using this plasmid library. Restriction analysis of plasmids isolated from E. coli transformants demonstrated that 81% had insert DNA with an average size of 5.4 kb. The library pool was mixed in LB–30% glycerol, and aliquots were frozen at −70°C. E. coli DH5α containing the M. tuberculosis plasmid library was amplified by growth on LB plates, and plasmids were isolated by following the Qiagen Maxi-plasmid purification protocol. The isolated plasmid library DNA was then used to transform M. smegmatis.
Electroporation of M. smegmatis and E. coli.
M. smegmatis 1-2c was transformed via electroporation by the method of Snapper et al. (40). Electroporation was performed with a Gene Pulser (Bio-Rad). Electrocompetent M. smegmatis (0.4 ml) was pipetted into a 0.4-cm-gap-size cuvette with 1- to 10-μg aliquots of plasmid DNA and electroporated at settings of 2.5 kV, 25 μF, and 1,000 Ω. To analyze the recombinant clones, plasmid DNA was electroporated directly from M. smegmatis transformants to E. coli cells. Electrocompetent E. coli DH5α cells were prepared as previously described (4). Sixty microliters of E. coli cells was pipetted into a 0.1-cm-gap-size cuvette containing 40 μl of transformed M. smegmatis cells. Electroporation into E. coli was performed at settings of 2.5 kV, 25 μF, and 200 Ω.
Intracellular survival assay.
The intracellular survival assay was devised by modifying the procedure of Ramakrishnan and Falkow (35) developed for Mycobacterium marinum and the mouse macrophage-like cell line J-774. Suspension cultures of U-937 cells were grown in RPMI 1640–10% FCS medium in 75-cm2 tissue culture flasks for 3 days before overnight treatment with 0.4 μg of phorbol 12-myristate 13-acetate (PMA) (Sigma)/ml to transform U-937 into an adherent state. PMA-containing supernatants were removed the next day, and the adherent cells were washed with Hanks' Ca2+- and Mg2+-free balanced salt solution (HBSS). Adherent U-937 cells were released by gentle rocking with 3-mm-diameter glass beads. Released cells were recovered by centrifugation, washed in HBSS, quantitated with a hemocytometer, and diluted in RPMI 1640–10% FCS medium to a density of 2 × 105 cells per ml. U-937 cells (1 ml/well) were plated into each well of 24-well tissue culture plates (Costar) and incubated overnight under 5% CO2 at 37°C.
Cultures of M. smegmatis 1-2c containing the M. tuberculosis plasmid library were grown on 7H10 agar plates with 50 μg of hygromycin B/ml for 3 to 4 days. Inocula were prepared by swabbing the plate growth into HBSS, sedimenting the mycobacteria at 1,300 × g for 10 min, resuspending in HBSS, vortexing for 30 s, and centrifuging again at 250 × g for 5 min. This yielded a supernatant consisting almost entirely of single mycobacterial cells, as observed by phase-contrast microscopy. On the assumption that an optical density at 650 nm (OD650) of 0.1 equals 108 CFU per ml, the inoculum was diluted to a density of 2 × 106 CFU per ml in RPMI 1640–10% FCS containing 2.5% fresh human serum (Omega Scientific). U-937 cells resemble human mononuclear phagocytes (38) in that phagocytosis of mycobacteria is greatly augmented in the presence of fresh human serum (complement component C3). Aliquots of the inocula were routinely plated on 7H10 plates containing hygromycin B for CFU determinations (see below) to confirm that equivalent bacterial inocula were used in all experiments. After 20 min of opsonization at room temperature, 1 ml of inoculum was added to each well containing 2 × 105 U-937 cells to give a multiplicity of infection (MOI) of 10. At this MOI, no cytotoxicity was observed. The 24-well plates were incubated for 2 h at 37°C in a 5% CO2 incubator. Acid-fast staining showed that after 2 h of incubation approximately half of the U-937 cells contained at least one M. smegmatis organism. The infected monolayers were then washed once with warm HBSS and treated with RPMI 1640–10% FCS containing 200 μg of amikacin per ml for 1 h at 37°C to kill extracellular organisms. The monolayers were washed again with HBSS, and those monolayers in which survival for 24 and 48 h was to be measured were incubated in medium containing 20 μg of amikacin/ml to prevent extracellular growth of any bacteria that might be released by premature lysis of infected U-937 cells. Cells in duplicate wells were lysed at 3, 24, and 48 h postinfection by adding 1 ml of water, waiting for 30 min, and vigorously pipetting five times to ensure cell lysis and the release of surviving intracellular bacteria. The lysates were serially diluted in 7H9 broth and plated onto 7H10 agar plates containing hygromycin B. CFU were counted after incubation at 37°C for 3 days. M. smegmatis(pOLYG) was included in every assay as a negative control. Clones that were recovered on plates at 24 and 48 h in each passage were pooled and passed through the U-937 survival assay for the next passage. A total of 6 passages were carried out to enrich for recombinant M. smegmatis with increased capacity for intracellular survival. After each step in the passage, bacterial pools were frozen at −70°C for further analysis.
Transmission electron microscopy.
U-937 cell monolayers were prepared in 24-well tissue culture plates as described for the intracellular survival assay and infected with M. smegmatis(p69) at an MOI of 50. This higher MOI was used to assist in the observation of M. smegmatis(p69) in the transmission electron microscopy preparations. At 3 and 24 h after infection, monolayers were washed and fixed for 1 h at room temperature in 0.1 M phosphate buffer (pH 7.2) containing 2% glutaraldehyde. Cells from fixed monolayers were then released into suspension by use of a rubber policeman. Specimens were postfixed in 2% osmium tetroxide, dehydrated via graded alcohol steps, and embedded in Spurr low-viscosity resin. Sections were cut, stained with uranyl acetate and lead citrate, and viewed on a Philips CM12 transmission electron microscope.
Recombinant DNA techniques.
Standard techniques were used for plasmid isolation, endonuclease restrictions, DNA modifications, ligations, and plasmid transformations (37). Restriction endonucleases and other enzymes were used as recommended by the suppliers (Life Technologies and New England Biolabs).
Construction of eis deletion derivatives.
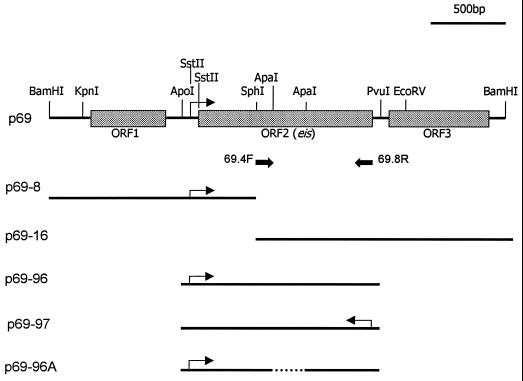
The DNA insert of p69 was released from pOLYG by digestion with BamHI and cut in the middle of open reading frame 2 (ORF2) by digestion with SphI. The resulting 1.3- and 1.7-kb fragments were ligated into BamHI-SphI-digested pUC18 to yield the subclones p62-8 and p62-16, respectively. The 1.3-kb BamHI-HindIII fragment from p62-8 was cloned into BamHI-HindIII-digested pOLYG to obtain p69-8. The 1.7-kb BamHI-HindIII fragment from p62-16 was then cloned into BamHI-HindIII-digested pOLYG to obtain p69-16. p62-97 was created by digestion of p69 with ApoI and PvuI. The PvuI site was filled in with DNA polymerase. This 1.6-kb ApoI-PvuI fragment carrying only ORF2 and its putative promoter region was isolated and subcloned into EcoRI-SmaI-digested pSP72 (Promega). p69-97 was constructed by inserting the 1.6-kb EcoRV-HindIII fragment from p62-97 into EcoRV-HindIII-digested pOLYG. p69-96 was made by inserting the 1.6-kb ClaI-HindIII fragment from p62-97 into ClaI-HindIII-digested pOLYG. The transcriptional orientation of p69-96 is opposite that of p69-97. ORF2 was disrupted by removing an internal 367-bp ApaI fragment from p69-96, creating p69-96A, which is an in-frame deletion. All p69 derivatives and p69 itself were then retransformed into M. smegmatis cells for use in the intracellular survival assay.
Nucleotide sequence data analysis.
The nucleotide sequence of the DNA insert of p69 was determined with a DNA sequencer (Applied Biosystems model 373A) at the Laboratory of Molecular Systematics and Evolution, University of Arizona. Computation was performed by using the Genetics Computer Group sequence analysis software package (version 10, University of Wisconsin, Biotechnology Center) and the National Center for Biotechnology Information BLAST (1) network service.
PCR analysis for enrichment of p69 after serial passages through U-937 cells.
Fifty isolates each from the third, fourth, fifth, and sixth rounds of M. smegmatis transformant passage in U-937 cells were analyzed for the presence of eis by using PCR amplification. After 5 days of growth on 7H10 agar containing 50 μg of hygromycin B/ml, individual colonies were picked and boiled in 50-μl aliquots of H2O for 15 min, and then 5-μl aliquots of the lysed cells were added to separate 50-μl PCR mixtures containing 1× PCR buffer (Gibco BRL), 5 mM MgCl2, 0.6 mM deoxynucleoside triphosphates, 4 μM each oligonucleotide primer (69.4F, 5′-GGATCCGTCAGACCCACCGAGCAT-3′, and 69.8R, 5′-CGGATCCCCATCCATGGCGTGT-3′; Gibco BRL), and 2.5 U of Taq DNA polymerase (Gibco BRL). Thermocycling reactions were performed in a Perkin-Elmer GeneAmp PCR System 2400 with the following parameters: an initial denaturing at 94°C for 2 min, a final additional extension at 72°C for 2 min, and 30 cycles of 94°C for 1.5 min, 56°C for 1.5 min, and 72°C for 1.5 min. For each set of reactions, pOLYG and p69 were included as negative and positive controls, respectively. PCR products were analyzed by electrophoresis on a 1% agarose gel with an eis-specific product expected to be 824 bp in length.
Southern hybridization.
For isolation of genomic DNA of M. smegmatis, the procedure of Jacobs et al. (21) was followed. Genomic DNA of M. tuberculosis Erdman, M. tuberculosis H37Rv, M. tuberculosis H37Ra, and M. bovis BCG was kindly supplied by John T. Belisle of Colorado State University. These materials were provided through the National Institutes of Health (NIH, Rockville, Md.) contract entitled “Tuberculosis research materials and vaccine testing.” Genomic DNA preparations isolated from M. abscessus, M. aurum, M. avium, M. peregrinum, M. phlei, M. triviale, M. vaccae, M. chelonae, M. fortuitum, and M. gordonae were received as a kind gift from Benjamin Schroeder and Clifton Barry III (NIH).
The DNA probe for Southern hybridization was prepared by using a PCR DIG Probe Synthesis Kit (Boehringer Mannheim). PCRs to generate a digoxigenin (DIG)-labeled probe included plasmid p69 as template DNA and primers 69.4F and 69.8R (described above). The reaction profile was identical to that used in PCR analysis of M. smegmatis transformant pools for the presence of eis. Samples of genomic DNA (5 μg) were digested with PstI, and the resulting fragments were separated on a 1% agarose gel and transferred to a GeneScreen Plus membrane (NEN Research Products) by standard methods (37). Southern blots were prepared as previously described (6). DIG-labeled fragments were detected according to the manufacturer's directions by using the DIG DNA Labeling and Detection Kit (Boehringer Mannheim).
SDS-PAGE analysis of M. smegmatis transformants containing derivatives of eis.
M. smegmatis transformants containing pOLYG, p69, p69-16, p69-8, p69-97, p69-96, and p69-96A were grown on Middlebrook 7H10 agar with 0.2% glucose and 50 μg of hygromycin B/ml for 3 to 4 days at 37°C before harvesting. Cells were swabbed into HBSS and pelleted by centrifugation at 12,000 × g for 10 min. Pelleted cells were resuspended in an equal volume of HBSS. One-milliliter aliquots of these cell suspensions were transferred to microcentrifuge tubes containing 0.25 g of 0.1- to 0.15-mm-diameter glass beads (Biospec Products, Bartlesville, Okla.). Cells were vortexed with glass beads for 30 min at room temperature by using a Vortex-Genie Turbomix device (Fisher Scientific). This procedure resulted in a 96% loss of cell viability, as determined by viable plate counts. After vortexing, the glass beads were allowed to settle before the supernatant was transferred. Protein concentrations of various lysates were determined by using the BCA Protein Assay Reagent (Pierce). Sodium dodecyl sulfate (SDS)-loading dye was mixed with 140-μg protein samples and boiled for 10 min before samples were loaded onto an SDS-polyacrylamide gel electrophoresis (PAGE) gel (12% polyacrylamide) with a 4% stacking gel (24). Samples were electrophoresed for 4 to 5 h at 200 V and 100 mA. Gels were stained with Coomassie brilliant blue and photographed by using an Alpha Imager 2000 Documentation and Analysis System (Alpha Innotech Corporation).
Statistical analysis of data.
Results of assays for intracellular survival are expressed as means ± standard deviations from three independent experiments performed on different days. Differences between various groups were assessed by use of Student's t test. The level of significance was set at 0.001.
Nucleotide sequence accession number.
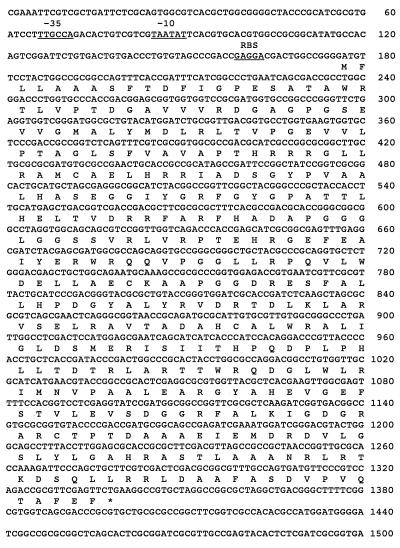
The nucleotide sequence of the eis gene shown in Fig. 4 has been deposited in the GenBank database under accession no. AF144099.
FIG. 4.
Nucleotide sequence of the 1.5-kb DNA fragment containing the eis gene and deduced amino acid sequence of Eis. Asterisk, stop codon. The putative promoter region and the ribosome binding site (RBS) are underlined.
RESULTS
Selection of M. smegmatis transformed with M. tuberculosis H37Rv DNA for enhanced survival in U-937 cells.
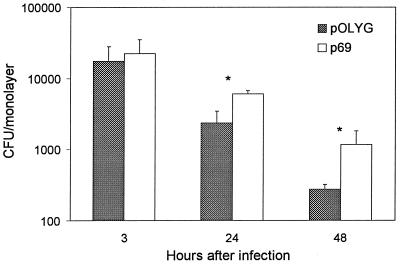
To enrich for recombinants with increased ability for intracellular survival, M. smegmatis transformants containing the M. tuberculosis H37Rv DNA plasmid library were used to infect monolayers of U-937 as described in Materials and Methods. One hundred twenty independent clones randomly selected from the 3rd passage were screened individually in the intracellular survival assay to look for individual M. smegmatis recombinants with enhanced survival compared to M. smegmatis containing only the vector pOLYG. Twenty-one of the recombinants showed ≥2-fold-increased survival 48 h after infection (data not shown). One clone (p69) demonstrated significantly enhanced survival, ranging from 2.4- to 5.3-fold at 24 and 48 h after infection, respectively (Fig. 1), and was further characterized.
FIG. 1.
Survival of M. smegmatis containing pOLYG or p69 in U-937 cells. As described in Materials and Methods, surviving intracellular bacteria were counted at 3, 24, and 48 h after infection. Data are means ± standard deviations from three independent experiments performed on different days. ∗, P < 0.001 compared to pOLYG at each time point.
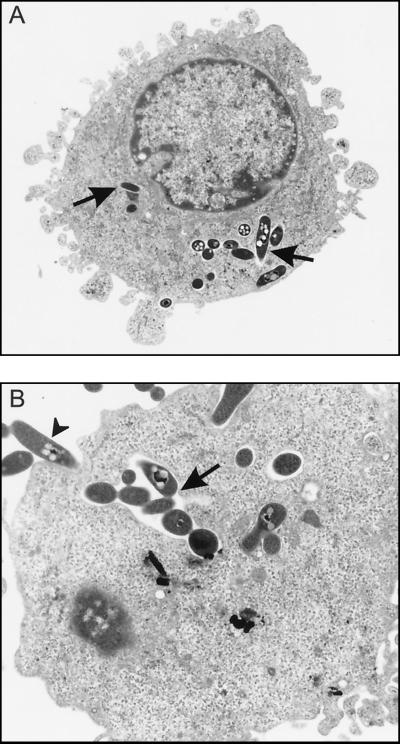
Electron microscopy was performed to confirm that M. smegmatis(p69) had indeed been internalized by U-937 cells and was present intracellularly. Figure 2 shows typical observations of intracellular M. smegmatis(p69) in the cytoplasm of U-937 cells. At 3 h postinfection, numerous M. smegmatis bacilli were present in the cytoplasm of U-937 cells either in tightly fitting phagosomes (Fig. 2A) or in more spacious phagosomes (Fig. 2B). The mycobacteria were also seen within phagosomes and not free in the cytoplasm. Similar electron micrographs were obtained at 24 h after infection (data not shown). These electron micrographs of M. smegmatis in U-937 cells are comparable to those of M. smegmatis in human mononuclear phagocytes (5).
FIG. 2.
Electron micrographs demonstrating intracellular M. smegmatis(p69) within U-937 cells at 3 h after infection. (A) Several M. smegmatis(p69) bacilli (arrows) are present in tight-fitting phagosomes in the cytoplasm of the macrophage-like cell. Magnification, ×8,500. (B) Higher magnification of an area of another U-937 cell which contains M. smegmatis(p69) in a more spacious phagosome (arrow). The arrow-head denotes bacilli probably in the early stages of internalization. Magnification, ×13,600.
The shuttle vector pOLYG is a multicopy, hygromycin B-resistant plasmid (17). Since hygromycin B was not present during serial passages and assays for survival in U-937 cells, it was necessary to verify that antibiotic selection pressure is not required to maintain the plasmid library in M. smegmatis. When infected monolayer lysates from different passages were plated on 7H10 agar plates with or without hygromycin B, equivalent numbers of CFU were recovered (data not shown). This demonstrates that hygromycin B is not required for maintenance of the genomic plasmid library in M. smegmatis during passage in the U-937 cells.
In the survival assay, amikacin (200 μg/ml) is used initially to kill uningested extracellular M. smegmatis. Following this 1-h treatment, 20 μg of amikacin/ml is present to prevent bacterial replication in the tissue culture medium. Theoretically, it might be possible to select for amikacin-resistant mutants in the survival assay. To clarify this, the MICs of amikacin were determined for M. smegmatis(pOLYG) and M. smegmatis(p69) recovered after passage in the survival assay and for unpassed M. smegmatis transformed with each vector. In both passaged and freshly transformed M. smegmatis(pOLYG) and M. smegmatis(p69), the MIC was 0.3 μg/ml. Thus, M. smegmatis(p69) has no increased resistance to amikacin.
We also sought evidence that amikacin kills extracellular microbes only and does not inhibit the intracellular survival of M. smegmatis. If the levels of amikacin used in the survival assay do indeed kill ingested M. smegmatis, then doubling the exposure of U-937 cells to the antibiotic should reduce intracellular survival. U-937 cells were pretreated with amikacin at 20 μg/ml for 48 h and then at 200 μg/ml for 1 h prior to infection with M. smegmatis(pOLYG) and M. smegmatis(p69) in the survival assay. A comparable population of U-937 cells not pretreated with the antibiotic was also infected with the same inocula, and the survival of the four sets of M. smegmatis-infected U-937 cells was then compared in the customary assay. Equivalent numbers of M. smegmatis transformants were recovered in both the amikacin-pretreated (two rounds of amikacin exposure) and non-pretreated (one round of amikacin exposure) groups at 3, 24, and 48 h after infection. Therefore, amikacin is not significantly internalized into U-937 cells to levels that interfere with the intracellular survival of M. smegmatis.
Restriction digestion and sequence analysis of p69.
To facilitate analysis of p69, the plasmid was directly transformed by electroporation from M. smegmatis into E. coli DH5α cells. Restriction mapping indicated that p69 contained a 2.99-kb DNA insert. The p69 insert was sequenced and analyzed. The insert DNA was GC rich (68% G+C) and was identical to a nucleotide sequence found in the M. tuberculosis genome database (13). This is a 9-kb region in which none of the potential ORFs has a known function. Nucleotide sequencing revealed the presence of three potential ORFs designated ORF1, ORF2, and ORF3s in the insert (Fig. 3). These ORFs correspond to the hypothetical genes Rv2417c, Rv2416c, and Rv2415c, respectively, in the M. tuberculosis genome (13). The deduced amino acid sequences of the three ORFs were used in searches of the GenBank/EMBL and SWISSPROT databases with the BLAST and FASTA programs. Some homology (34% identity) was found between ORF2 and a hypothetical 45-kDa protein (orf5), of unknown function, downstream from the amfC gene of Streptomyces griseus, which is involved in aerial mycelium formation in this microbe (23). Only one of the three ORFs encoded on the p69 insert, ORF2, contains both a putative promoter region and a ribosome binding site (Fig. 3 and 4). These results suggest that ORF2 of p69 is likely to be the gene which confers the enhanced intracellular survival phenotype on M. smegmatis. ORF2 can potentially express a 42.2-kDa protein (387 amino acids).
FIG. 3.
Restriction map of p69 and its deletion derivatives. Shaded boxes represent the three ORFs revealed by sequencing. An arrow indicates the putative promoter. Solid arrows, primers 69.4F and 69.8R, used in the PCR; dotted line, in-frame deletion.
Subcloning and deletion analysis of ORF2.
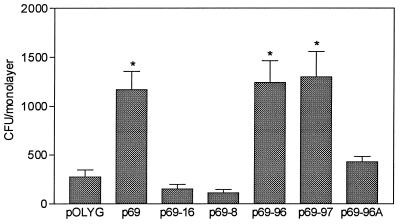
Deletion analysis of the p69 insert DNA was carried out to verify that intact ORF2 is indeed essential for the enhanced intracellular survival phenotype observed in M. smegmatis(p69) (Fig. 3). Plasmid p69-8 contains a complete ORF1 and the 5′ half of ORF2, while plasmid p69-16 contains the 3′ half of ORF2 and an intact ORF3. Both plasmids p69-96 and p69-97 contain a 1.6-kb ApoI-PvuI fragment with the intact ORF2 and its putative promoter region, but in opposite orientations. Plasmid p69-96A is an in-frame deletion of p69-96, in which ORF2 has been disrupted by removal of the 367-bp ApaI fragment. These deletion constructs were electrotransformed into M. smegmatis and compared in the intracellular survival assay with p69 and pOLYG as controls (Fig. 5). M. smegmatis transformants with p69-8, p69-16, and p69-96A all contain a disrupted ORF2 and survived no better than pOLYG transformants. M. smegmatis containing p69-96 and p69-97 had levels of intracellular survival comparable to that of p69 and significantly higher than that of pOLYG (P < 0.001). The activity of ORF2 in either orientation provides evidence that ORF2 can be expressed from its own promoter. These results confirm that the M. tuberculosis gene ORF2 is directly responsible for the enhanced intracellular phenotype associated with p69 in M. smegmatis. Therefore, ORF2 was named the enhanced intracellular survival (eis) gene.
FIG. 5.
Survival of M. smegmatis containing pOLYG, p69, and deletion derivatives of p69 in U-937 cells. Surviving intracellular bacteria were counted 3, 24, and 48 h after infection, but only the 48-h values are presented. Data are means ± standard deviations from three independent experiments performed on different days. ∗, P < 0.001 compared to pOLYG.
Efficiency of selection of eis-bearing transformants by U-937 cells.
With the identification of eis, it became possible to measure its rate of selection during serial passages in U-937 cells. Ten independent clones were selected at random from the 3rd to 6th passages of the M. smegmatis transformant pools. Plasmids from these clones were isolated and characterized by digestion with BamHI and SmaI to determine the percentage with the same restriction digest patterns as p69. In addition, 50 independent clones were randomly selected from each of these same passages and two eis-specific oligonucleotide primers (Fig. 3) were used in PCR analysis for the presence of the eis gene in each clone. Results showed that the eis-bearing transformants, undetectable in the 3rd passage, were enriched by the 6th passage to 70 or 88% of the transformant pool, as determined by restriction digestion or PCR analysis, respectively (Fig. 6). Similar results were obtained in a second independent series of passages of the recombinant library in the U-937 survival assay.
FIG. 6.
Enrichment of p69 during passage through U-937 cells. Plasmid restriction patterns were analyzed for 10 clones randomly selected from each passage. PCR analysis was performed on 50 clones randomly selected from each passage. Details of the methodology are given in Materials and Methods.
There is a possible alternative explanation for the enrichment of eis-bearing transformants during library passage in U-937 cells. It may be that eis-bearing transformants grew faster in the medium used to prepare the inocula for each round of selection in U-937 cells. However, the growth curves of M. smegmatis, M. smegmatis(pOLYG), and M. smegmatis(p69) in 7H9 medium were found to be indistinguishable (data not shown). Therefore, these results show that the intracellular survival assay efficiently enriches for the transformants with enhanced survival phenotypes and that eis confers a real survival advantage on M. smegmatis containing this gene.
Demonstration of eis only in M. tuberculosis and M. bovis BCG.
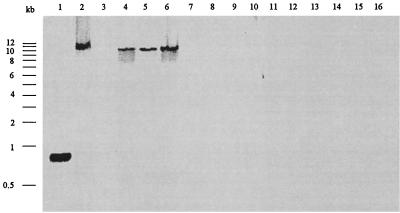
If eis contributes to the survival of M. tuberculosis in macrophages, it might be present in pathogenic species but absent in nonpathogenic species. The genomic DNAs of a number of mycobacterial species were examined by Southern analysis using a PCR-generated DIG-labeled probe to detect the presence of eis (Fig. 7). The eis gene was identified as a 12-kb band present only in M. tuberculosis H37Rv, H37Ra, and Erdman and in M. bovis BCG. None of the nonpathogenic mycobacterial species tested, including M. smegmatis, hybridized with the DIG-labeled eis probe. These results demonstrate that eis occurs only in pathogenic mycobacteria and their laboratory-produced derivatives.
FIG. 7.
Southern blot analysis for the presence of eis in Mycobacterium spp. The 824-bp DIG-labeled PCR product from eis was hybridized to PstI-digested chromosomal DNA. Lanes: 1, 824-bp PCR product of eis obtained with primers 69.4F and 69.8R; 2, M. bovis BCG; 3, M. smegmatis 1-2c; 4, M. tuberculosis H37Rv; 5, M. tuberculosis Erdman; 6, M. tuberculosis H37Ra; 7, M. abscessus; 8, M. aurum; 9, M. avium; 10, M. gordonae; 11, M. peregrinum; 12, M. phlei; 13, M. triviale; 14, M. vaccae; 15, M. chelonae; 16, M. fortuitum.
Identification of the putative gene product of eis.
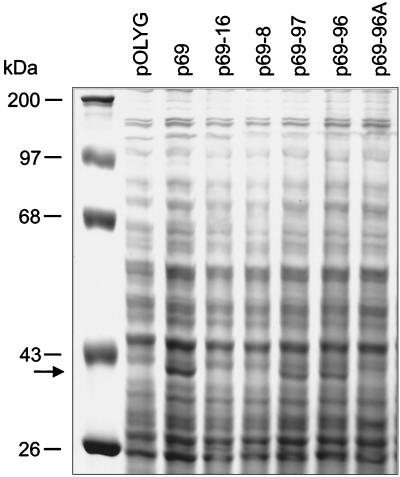
To assay for the presence of an eis gene product, M. smegmatis transformants containing vector alone, p69, or deletion derivatives of p69 were lysed by vortexing with glass beads. Proteins from the various cell lysates were separated on a 12% SDS–PAGE gel. Figure 8 shows that in the presence of an intact eis gene (p69, p69-97, and p69-96), there is a unique Coomassie blue-stained band corresponding to a protein matching the predicted size of Eis (42 kDa). This protein band is not present in M. smegmatis transformants containing eis gene deletion constructs (p69-16, p69-8, and p69-96A). Therefore, the appearance of this 42-kDa protein directly correlates with enhanced intracellular survival of the various transformants containing an intact eis gene (compare Fig. 5 and 8).
FIG. 8.
SDS-PAGE analysis of M. smegmatis transformants. M. smegmatis transformants containing vector alone (pOLYG), p69, or deletion derivatives of p69 were lysed by vortexing with glass beads. Protein samples (140 μg of total protein per lane) were subjected to electrophoresis by using a 12% SDS–PAGE gel. A unique Coomassie blue-stained band corresponding to a 42-kDa protein (arrow) is present only in lanes of lysates from M. smegmatis transformants containing an intact eis gene (p69, p69-97, and p69-96) and not from transformants containing eis gene deletions (p69-16, p69-8, and p69-96A). Protein molecular size standards (in kilodaltons) are shown on the left.
DISCUSSION
Previous workers have used similar approaches to look for genes of M. tuberculosis needed for survival in macrophages. Arruda et al. cloned DNA from avirulent M. tuberculosis H37Ra and isolated the mce gene, which augmented the ability of E. coli to enter and survive in nonphagocytic HeLa cells (3). Mundayoor and Shinnick (29) passed recombinant DNA libraries of M. leprae in E. coli through a mouse macrophage-like cell line to enrich for clones with increased survival in those host cells. Using pOLYG as a cloning vector, Wieles et al. (44) cloned the thioredoxin-thioredoxin reductase gene of M. leprae into M. smegmatis and showed that the transformant was less rapidly killed by human mononuclear phagocytes than M. smegmatis with pOLYG alone.
In the present study, the human macrophage-like cell line U-937 was used to show that a clone of avirulent M. smegmatis transformed with DNA from the virulent M. tuberculosis strain H37Rv exhibited significantly enhanced intracellular survival for at least 48 h. Evidence indicates that the prolonged survival of this clone (p69) in U-937 cells resulted from the presence of a M. tuberculosis gene we have designated eis and that disruption of this gene was followed by loss of the enhanced intracellular survival phenotype.
Identification of eis using M. smegmatis and U-937 cells suggests the potential of this system for identifying additional genes contributing to the survival of virulent mycobacteria in macrophages. Of the 21 clones isolated from the 3rd passage of M. smegmatis transformants in U-937 cells, only p69 has been characterized in detail. The restriction patterns of many of the remaining 20 clones are not the same as that of p69, which suggests that further investigation may lead to identification of additional genes contributing to the intracellular survival of mycobacteria. A limitation of this system is the size of the H37Rv DNA insert used in this library, 5.4 kb on average. This effectively limits the probability of finding more than one gene of interest in a single DNA insert. It is possible that cosmid libraries of M. tuberculosis DNA, with insert sizes of up to 40 kb, may be used to identify virulence factors that require the concerted action of several separate genes or the presence of an entire operon.
Still unanswered is the question of how the eis product acts to prolong the survival of M. smegmatis, and perhaps ultimately that of M. tuberculosis, in macrophages. Numerous mycobacterial activities must be required for long-term survival of these organisms in human phagocytes. These include housekeeping activities necessary for intracellular growth and survival, activities needed to defend the mycobacteria against stressful conditions, and activities specifically evolved to promote entry, proliferation, and latency in host cells (25). The finding that eis is present only in virulent mycobacteria or their laboratory-generated derivatives and not in M. smegmatis and numerous other nonpathogenic mycobacteria argues against eis being a mere housekeeping gene.
A 42-kDa protein has been identified as the putative eis gene product, Eis. Two lines of evidence support this conclusion. First, the unique size of the protein matches the size predicted for an eis gene protein product. Second, the appearance of the 42-kDa protein in M. smegmatis transformants containing an intact eis gene directly correlates with the enhanced intracellular phenotype conferred by the gene in M. smegmatis. Exploratory studies suggest that Eis expressed in M. smegmatis is surface located. Such a location suggests participation of the Eis protein in the interactions between the surfaces of mycobacteria and the membranes of host cells, either in entry or during the subsequent sojourn of the mycobacteria in phagosomes. Studies are currently in progress to demonstrate that Eis is expressed in M. tuberculosis and to determine its cellular location in the pathogen.
The increased survival in U-937 cells conferred on M. smegmatis by the presence of the eis gene is modest but still significant, considering that it is the result of introducing a single gene from a highly pathogenic bacterium into a nonpathogenic relative. Since the survival-enhancing effect of eis may depend on its overexpression in the multicopy vector pOLYG, it will be necessary to see if eis placed in a single-copy chromosomal location still enhances M. smegmatis intracellular survival. To examine if eis also prolongs the survival of virulent M. tuberculosis H37Rv, eis deletion mutants of this organism must be studied in human macrophages and in animal models.
ACKNOWLEDGMENTS
We thank Peggy McClusky of the Arizona Research Laboratory, Division of Biotechnology Imaging Facilities, University of Arizona for expert technical assistance with the electron microscopy studies. Special thanks are extended to N. Cianciotto for supplying us with the U-937 cells and to David Carrol for technical suggestions.
This work was supported by a Senior Fogarty International Fellowship, by a grant from the Arizona Disease Control Research Commission, and by grant AI45537 from the National Institutes of Health to R.L.F. D.B.Y. and P.O. were supported by a Program Grant from the Wellcome Trust.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J A, Hart P D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual non-fusion pattern and observation on bactericidal survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda S, Bomfim G, Knights R, Huimo-Byron T, Riley L W. Cloning of a M. tuberculosis DNA fragment associated with entry and survival inside cells. Science. 1993;261:1454–1457. doi: 10.1126/science.8367727. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1989. [Google Scholar]
- 5.Baker K, Fan H, Barroll C, Kaplan G, Barker J, Hellmann W, Cohn Z A. Nonadherent cultures of human monocytes kill Mycobacterium smegmatis, but adherent cultures do not. Infect Immun. 1996;64:428–433. doi: 10.1128/iai.64.2.428-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannan J D, Moran M J, MacInnes J I, Soltes G A, Friedman R L. Cloning and characterization of btr, a Bordetella pertussis gene encoding an FNR-like transcriptional regulator. J Bacteriol. 1993;175:7228–7235. doi: 10.1128/jb.175.22.7228-7235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardarov S, Kriakov J, Carriere C, Yu S, Vaamonde C, McAdam R A, Bloom B R, Hatfull G F, Jacobs W R., Jr Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1997;94:10961–10966. doi: 10.1073/pnas.94.20.10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belisle J T, Pascopella L, Inamine J M, Brennan P J, Jacobs W R., Jr Isolation and expression of a gene cluster responsible for biosynthesis of the glycopeptidolipid antigens of Mycobacterium avium. J Bacteriol. 1991;173:6991–6997. doi: 10.1128/jb.173.21.6991-6997.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 10.Bosque F, Milon G, Valderrama L, Saravia N G. Permissiveness of human monocytes and monocyte-derived macrophages to infection by promastigotes of Leishmania (Viannia) panamensis. J Parasitol. 1998;84:1250–1256. [PubMed] [Google Scholar]
- 11.Caron E, Liautard J P, Kohler S. The monocytic cell line U-937, physiologically differentiated by retinoic acid and vitamin D3, is a model for intracellular behavior of Brucella spp. Ann N Y Acad Sci. 1994;730:276–278. doi: 10.1111/j.1749-6632.1994.tb44264.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1112. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 14.Ehrt S, Shiloh M U, Ruan J, Choi M, Gunzburg S, Xie Q W, Riley L W. A novel antioxidant gene from Mycobacterium tuberculosis. J Exp Med. 1997;186:1885–1896. doi: 10.1084/jem.186.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton M J, Vermeulen M W. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbe T, Harris D, Vodemeir M, Lathigra R, Ivanyi J, Young D B. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbe T, Barathi J, Barnini S, Zhang Y, Abou-Zeid C, Tang D, Mukherjee R, Young D B. Transformation of mycobacterial species using hygromycin resistance as a selectable marker. Microbiology. 1994;140:133–138. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- 18.Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 19.Ilangumaran S, Arni S, Poincelet M, Theler J M, Brennan P J, Nasir-ud-Din, Hoessli D C. Integration of mycobacterial lipoarabinomannans into glycosyl-phosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- 20.Jacobs W R, Jr, Tuckman M, Bloom B R. Introduction of foreign DNA into mycobacteria using a shuttle plasmid. Nature. 1987;327:532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 22.King C H, Mundayoor S, Crawford J T, Shinnick T M. Expression of contact-dependent cytolytic activity of Mycobacterium tuberculosis and isolation of the genomic locus that encodes the activity. Infect Immun. 1993;61:2708–2712. doi: 10.1128/iai.61.6.2708-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo N, Kimura M, Beppu T, Horinouchi S. Cloning and characterization of a gene involved in aerial mycelium formation in Streptomyces griseus. J Bacteriol. 1995;177:6401–6410. doi: 10.1128/jb.177.22.6401-6410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lagier L, Pelicic V, Lecossier D, Prod'hom G, Rauzier J, Guilhot C, Gicquel B, Hance A J. Identification of genetic loci implicated in the survival of Mycobacterium smegmatis in human mononuclear phagoytes. Mol Microbiol. 1998;29:465–475. doi: 10.1046/j.1365-2958.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowrie D B. How macrophages kill tubercle bacilli. J Med Microbiol. 1983;16:1. doi: 10.1099/00222615-16-1-1. [DOI] [PubMed] [Google Scholar]
- 27.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonough K A, Kress Y, Broom B R. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundayoor S, Shinnick T M. Identification of genes involved in the resistance of mycobacteria to killing by macrophages. Ann N Y Acad Sci. 1995;730:31–35. doi: 10.1111/j.1749-6632.1994.tb44236.x. [DOI] [PubMed] [Google Scholar]
- 30.Numazaki K, Suzuki K, Chiba S. Replication of Chlamydia trachomatis and Chlamydia pneumoniae in human monocytic cell line U-937. J Med Microbiol. 1995;42:191–195. doi: 10.1099/00222615-42-3-191. [DOI] [PubMed] [Google Scholar]
- 31.Pascopella L, Collins F M, Martin J M, Lee M H, Hatfull G F, Stover C K, Bloom B R, Jacobs W R., Jr Use of in vivo complementation in Mycobacterium tuberculosis to identify a genomic fragment associated with virulence. Infect Immun. 1994;62:1313–1319. doi: 10.1128/iai.62.4.1313-1319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearlman E, Jiwa A H, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U-937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 33.Pelicic V, Jackson M, Reyrat J M, Jacobs W R, Jr, Gicquel B, Guilhot C. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1997;94:10955–10960. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plum G, Brenden M, Clark-Curtiss J E, Pulverer G. Cloning, sequencing, and expression of the mig gene of Mycobacterium avium, which codes for a secreted macrophage-induced protein. Infect Immun. 1997;65:4548–4557. doi: 10.1128/iai.65.11.4548-4557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramakrishnan L, Falkow S. Mycobacterium marinum persists in cultured mammalian cells in a temperature-restricted fashion. Infect Immun. 1994;62:3222–3229. doi: 10.1128/iai.62.8.3222-3229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley L W. Determinants of cell entry and intracellular survival of Mycobacterium tuberculosis. Trends Microbiol. 1995;3:27–31. doi: 10.1016/s0966-842x(00)88865-4. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 39.Shinnick T M, King H, Quinn F D. Molecular biology, virulence and pathogenicity of mycobacteria. Am J Med Sci. 1995;309:92–98. doi: 10.1097/00000441-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Snapper S B, Metton R E, Mustafu S, Kieser T, Jacobs W R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 41.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 42.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 43.Swartz R P, Naii D, Vogel C W, Yeager H., Jr Differences in uptake of mycobacteria by human monocytes: a role for complement. Infect Immun. 1988;56:2223–2227. doi: 10.1128/iai.56.9.2223-2227.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wieles B, Ottenhoff T H M, Steenwijk T M, Fraken K L M C, DeVries R R P, Langgermans J A M. Increased intracellular survival of Mycobacterium smegmatis containing the Mycobacterium leprae thioredoxin-thioredoxin reductase gene. Infect Immun. 1997;65:2537–2541. doi: 10.1128/iai.65.7.2537-2541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing E J, Koran H S, Fisher D G, Kelly V. Stimulation of a human macrophage-like cell line (U-937) to inhibit multiplication of an intracellular pathogen. J Reticuloendothel Soc. 1981;29:312–328. [PubMed] [Google Scholar]
- 46.Zhang Y, Lathigra R, Garbe T, Catty D, Young D B. Genetic analysis of superoxide dismutase, the 23-kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991;5:381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Heym B, Allen B, Young D B, Cole S T. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]