Abstract
Background
In the musculoskeletal system, bone, tendon, and muscle form highly integrated multi-tissue units such as the rotator cuff complex, which facilitates functional and dynamic movement of the shoulder joint. Understanding the intricate interplay among these tissues within clinical, biological, and engineering contexts is vital for addressing challenging issues in treatment of musculoskeletal disorders and injuries.
Methods
A wide-ranging literature search was performed, and findings related to the socioeconomic impact of rotator cuff tears, the structure-function relationship of rotator cuff bone-tendon-muscle units, pathophysiology of injury, current clinical treatments, recent state-of-the-art advances (stem cells, growth factors, and exosomes) as well as their regulatory approval, and future strategies aimed at engineering bone-tendon-muscle musculoskeletal units are outlined.
Results
Rotator cuff injuries are a significant socioeconomic burden on numerous healthcare systems that may be addressed by treating the rotator cuff as a single complex, given its highly integrated structure-function relationship as well as degenerative pathophysiology and limited healing in bone-tendon-muscle musculoskeletal tissues. Current clinical practices for treating rotator cuff injuries, including the use of commercially available devices and evolving trends in surgical management have benefited patients while advances in application of stem/progenitor cells, growth factors, and exosomes hold clinical potential. However, such efforts do not emphasize targeted regeneration of bone-tendon-muscle units. Strategies aimed at regenerating bone-tendon-muscle units are thus expected to address challenging issues in rotator cuff repair.
Conclusions
The rotator cuff is a highly integrated complex of bone-tendon-muscle units that when injured, has severe consequences for patients and healthcare systems. State-of-the-art clinical treatment as well as recent advances have resulted in improved patient outcome and may be further enhanced by engineering bone-tendon-muscle multi-tissue grafts as a potential strategy for rotator cuff injuries.
Translational Potential of this Article
This review aims to bridge clinical, tissue engineering, and biological aspects of rotator cuff repair and propose a novel therapeutic strategy by targeted regeneration of multi-tissue units. The presentation of these wide-ranging and multi-disciplinary concepts are broadly applicable to regenerative medicine applications for musculoskeletal and non-musculoskeletal tissues.
Keywords: Musculoskeletal biomaterials, Multi-tissue regeneration, Stem cells, Growth factors, Exosomes, Rotator cuff repair
Abbreviations: H&E, Hematoxylin and eosin
Abbreviations
- ADSCs
Adipose-derived mesenchymal stromal cells
- ASES
American Shoulder and Elbow Society
- ASCR
Arthroscopic superior capsule reconstruction
- BMSCs
Bone marrow-derived mesenchymal stromal cells
- ECM
Extracellular matrix
- FGF-2
Fibroblast growth factor-2
- HCT/P
Human cells, tissues, and cellular and tissue-based product
- IGF-1
Insulin-like growth factor-1
- MSCs
Mesenchymal stromal cells
- PRP
Platelet-rich plasma
- PCL
Poly-ε-caprolactone
- PDO
Polydioxanone
- PEEK
Poly ether–ether ketone
- PLGA
Poly (lactic-co-glycolic acid)
- PLLA
Poly-L-lactic acid
- RTSA
Reverse total shoulder arthroplasty
- TGF-ß
Transforming growth factor-ß
- UCB-MSCs
Umbilical cord blood-derived mesenchymal stromal cells
- USCs
Urine-derived stem/progenitor cells
- VEGF
Vascular endothelial growth factor
1. Introduction
In the musculoskeletal system, bone, tendon, skeletal muscle and their respective interfaces form a multi-tissue unit, which is responsible for generation and translation of muscle contractile force into body movement. The importance of musculoskeletal multi-tissue units on human health and quality of life cannot be underestimated. In the last decade, musculoskeletal disorders and injuries have increased by approximately 20%, becoming the second-highest contributor to global disability (behind mental disorders) [1].
To address challenges in the repair of injured bone-tendon-muscle units such as rotator cuff tears, in vitro studies as well as in vivo preclinical and clinical trials must incorporate a wide spectrum of knowledge and perspectives from multidisciplinary fields, including developmental and musculoskeletal biology, wound healing and inflammation, biomaterials, and tissue engineering. Previous examinations on the topic of musculoskeletal multi-tissue grafts has included engineering- and biological-centric perspectives with regard to technological advances in biomaterials and tissue engineering [2], as well as recent concepts in developmental biology and wound healing [3], respectively. In this review, we focus on advancing the notion of using multi-tissue regenerative approaches, such as engineered bone-tendon-muscle grafts, as a reparative or restorative intervention in current clinical practice. First, the significance and socioeconomic impact of rotator cuff injuries is described (Section 2). This is followed by a review of the shoulder joint structure–function biomechanical relationship to emphasize the highly integrated nature of bone-tendon-muscle units within the rotator cuff complex (Section 3). Next, we discuss the pathophysiology of rotator cuff injuries and subsequent limited tissue healing, identifying the bone-tendon-muscle unit as a potential clinical therapeutic target (Section 4). Current clinical practice for rotator cuff injuries (Section 5.1) and the commercially available materials used in treatment (Section 5.2) are described to highlight the evolving trends in surgical management, including clinical applications of grafts/scaffolds, stem/progenitor cells, growth factors, and exosomes, as well as their associated healthcare regulations (Section 5.3). Lastly, we present preclinical and clinical evidence in support of using musculoskeletal multi-tissue units for the treatment of rotator cuff tears (Section 5.4). The clinically relevant knowledge presented here serve as the basis for rational design of future biomaterials and cell-based constructs to provide the next generation of clinically practical and cost-effective strategies and medical devices for the treatment of challenging musculoskeletal injuries and diseases.
2. Rotator cuff injuries and socioeconomic impact
The rotator cuff is comprised of the supraspinatus, infraspinatus, subscapularis, and teres minor skeletal muscles as well as their respective tendons, which are attached to the humeral bone. Together, these multi-tissue units stabilize and control functional movement of the shoulder. As such, injury to one or more of these four muscle-tendon tissues can result in pain and high disability for patients, representing a great socioeconomic burden for healthcare systems.
On an individual level, rotator cuff injuries affect both young and old. Typically, tear sizes can range from asymptomatic small partial-thickness tears to massive full-thickness tears that cause severe shoulder dysfunction and pain [4]. In younger patients, rotator cuff tears are often the result of occupational injuries, such as carrying heavy objects or repetitive overhead arm movements during athletic activities. In such cases, patients may experience a considerable loss of workdays, returning to work only after about 5 months post-surgery [5]. Also, such patients may only return to sports after about 7.78 ± 3.20 months depending on injury severity and repair technique [6]. While patients of an advanced age may also sustain occupational or exercise-related rotator cuff tears, there is also an increased incidence of rotator cuff injury with increased age due to tissue aging and degeneration [7]. As a result, afflicted individuals experience loss in shoulder range of motion, which dramatically reduces quality-of-life.
In addition to substantial impact on individuals, rotator cuff tears entail a huge socioeconomic burden in numerous regions including Asia, Europe, and the United States. In Japan, it is estimated that rotator cuff tears are present in 20.7% of the population, with increased prevalence as people age [8]. This does not bode well for regions such as Hong Kong, where the proportion of residents projected to be 65 years or older is 19.8% by 2029, which will be expected to result in increased rotator cuff injury incidence [9]. In Italy, for example, the expected hospital cost sustained by the national health care system for rotator cuff procedures alone is projected to cost over 1 billion Euros by 2025 [10]. Furthermore, approximately 65% of such repairs are performed in patients aged below 65 years old, who represent the working population [10]. Socioeconomic issues pertaining to lost work time and disability costs are additionally compounded by the rising incidence of rotator cuff repairs, which has increased from 44 per 100,000 person-years in 1998 to 131 per 100,000 person-years in 2011 in Finland [11]. At present, surgical repair offers a way to alleviate the burden of rotator cuff injury and disease. For example, in the United States, around 250,000 rotator cuff repairs are performed each year, and this has been estimated to save the national healthcare system USD 3.4 billion annually [12]. Thus, rotator cuff injuries are prevalent and pose a significant socioeconomic burden on the healthcare systems of various nations.
Altogether, rotator cuff tears are detrimental to the mobility as well as quality-of-life of patients and pose a heavy socioeconomic burden on national healthcare systems. This highlights knowledge gaps in our understanding of rotator cuff injuries and our abilities to treat them with current clinical techniques. As such, development of innovative tissue engineering and regenerative medicine approaches is needed to improve patient outcomes and reduce the significant burden that rotator cuff injuries pose to society.
3. Rotator cuff musculoskeletal units and their structure–function relationship
Although current clinical management such as arthroscopic surgery can improve patient outcome by reducing pain and improving shoulder function, recurrent defect (re-tear) rates post-surgery remain undesirable (21% at 1 – 2 years follow-up) [13] with extremely high rates (91 – 94%) [14,15] for large-to-massive injuries. In order to achieve optimal patient outcomes, an in-depth, comprehensive understanding of the rotator cuff musculoskeletal unit and their structure–function relationship is crucial for the development and design of new therapeutic approaches.
3.1. Rotator cuff bone-tendon-muscle multi-tissue unit
A brief overview of shoulder anatomy, along with the muscular motions facilitated by the rotator cuff, is presented here to depict the structure–function relationship within the rotator cuff. The principal components include musculoskeletal multi-tissue units, such as bone, tendon, ligaments, and skeletal muscle that work in concert to produce a robust range of motion and facilitate joint function (Fig. 1). The skeletal elements of the shoulder consist of the humerus, scapula and clavicle. There are two tuberosities on the humeral head, which are the sites for insertion of shoulder muscles and tendons [[16], [17], [18]]. Apart from the skeletal elements, the shoulder joint is surrounded and covered by a complex network of muscular tissues and their respective tendons including the deltoid, teres major, pectoralis major, latissimus dorsi, biceps brachii, rotator cuff and other supporting muscles [16]. Together, these musculoskeletal elements comprise numerous multi-tissue units that are present in the shoulder.
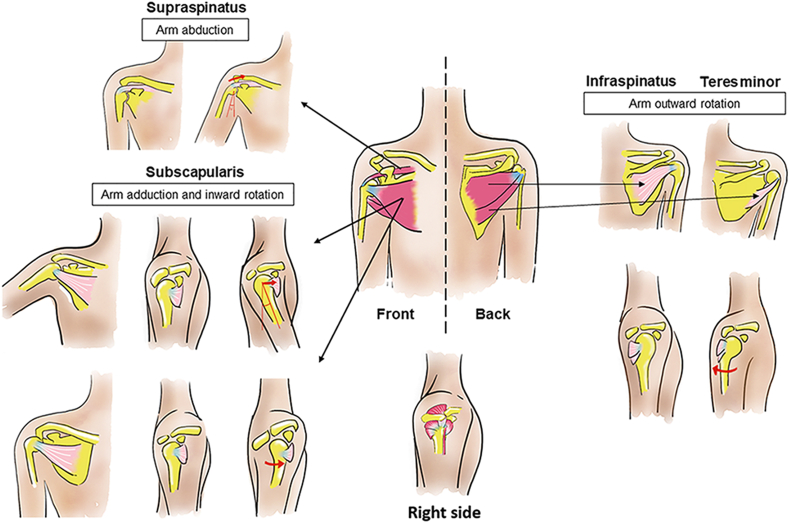
Fig. 1.
Structure–function relationship of the rotator cuff complex. The supraspinatus muscle-tendon is primarily involved in arm abduction, whereas the subscapularis muscle-tendon predominantly is involved in arm adduction and inward rotation. The infraspinatus and teres minor muscle-tendon are primarily involved in arm outward rotation. Bones, tendons, and muscles are colored yellow, blue, and red, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A bone-tendon-muscle unit is formed by the attachment of different musculoskeletal tissues. For the rotator cuff, the supraspinatus, infraspinatus, subscapularis, and teres minor tendons insert into the humeral head, acting as intermediary elements that connect their respective skeletal muscle to bone. Traditionally, biologists and surgeons have used a reductionist approach to describe the precise location for each of the four rotator cuff tendon footprints. These footprints delineate the site where each tendon inserts into the lesser and greater tuberosity of the humerus and serve as a means to measure tendon loss or act as a guide during surgical repair [19]. However, instead of finding individual muscle-tendon insertion sites, cadaveric studies showed that the rotator cuff tendons actually splay out and interdigitate to form a common and continuous insertion as they approach the humeral head [20]. In addition to interdigitating with one another, rotator cuff tendons also fuse with the articular capsule along its deepest layer before the common insertion into the bone, providing further stability and anchorage [21,22]. Thus, the four bone-tendon-muscle units of the rotator cuff constitute a highly integrated musculoskeletal complex.
3.2. Rotator cuff structure–function relationship
Four rotator cuff skeletal muscles and tendons, together with humeral bone are the major force transduction units that drive functional movement of the shoulder. These musculotendinous elements stabilize joints, control the movements and positions of joints, as well as store and release elastic (strain) energy [23]. This enables the shoulder to be one of the most mobile joints in the body and capable of limb flexion (150–180°), extension (40–60°), abduction (150–180°), internal (50–70°) and external rotation (60–90°) [24,25].
These movements are mainly controlled by the rotator cuff, which directly overlays the glenohumeral joint (Fig. 1). The rotator cuff muscles co-contract with the deltoid, compressing the humeral head into the glenoid. Asymmetric contraction of rotator cuff acts to cause humeral head rotation during shoulder motion [16,18,26]. The supraspinatus muscle-tendon unit, which is the most commonly injured rotator cuff tissue, primarily functions to raise the arm above the head, especially within the first 15° of motion [16]. The supraspinatus muscle originates from the supraspinatus fossa and inserts at the superior aspect of the greater tuberosity of the humeral head, with its tendon blending into the articular capsule and infraspinatus tendon below. The subscapularis muscle-tendon unit originates from the subscapular fossa and extends laterally to its insertion on the lesser tuberosity of the humerus. When the arm is not raised, the subscapularis rotator cuff unit acts as an internal rotator whereas when the arm is raised, muscle contraction pulls the arm in a forward and downward motion. Both the infraspinatus and teres minor muscle-tendon units have a similar role. Primarily, the function of these two muscles is to provide the external rotation force that pull the arm away from the body midline, moving the arm backwards [16,27]. Although each rotator cuff muscle-tendon is responsible for particular set(s) of musculoskeletal motion, they do not act in isolation but instead can be influenced by their neighboring muscles due to the highly integrated nature of the rotator cuff muscle-tendon tissues as mentioned in Section 3.1. Rotator cuff muscles and tendons are also additionally assisted by non-rotator cuff musculature and tendons such as the deltoid, teres major, pectoralis major, latissimus dorsi, and the long head of the biceps. Dynamic muscle forces transmitted via tendinous attachments to bone within this complex environment of skeletal elements and ligamentous restraints produce intricate varieties of force couples that account for robust shoulder movements. Injury to one or more of these components through long-term overuse or acute trauma disrupts this complex interrelationship and places the shoulder at increased risk of injury [17,18].
3.3. Benefit of understanding structure–function relationship of rotator cuff bone-tendon-muscle units
As such, rotator cuff injuries present numerous pathophysiological changes in multiple locations including bone-tendon interface, tendon, muscle, and bone, which challenge rotator cuff repair. This highlights a structure–function relationship which exists among the four rotator cuff muscle-tendon attachments that translate musculoskeletal force generation into robust shoulder movement. Additional studies that enhance our understanding of the bone-tendon-muscle structure–function relationship may assist the clinical repair of rotator cuff tear to improve the patient outcomes. For example, Liu et al. systematically discussed the suitability of different approaches for modelling aspects of rotator cuff tears and identified suitable assessment methods such as gait analysis for evaluating recovery of shoulder function [28]. Furthermore, a thorough understanding of the disease itself and the healing process is fundamental for clinical treatment, which will be further described in the following section.
4. Pathophysiology of rotator cuff injury and healing process
Given that rotator cuff injury is caused by acute trauma, chronic overuse (repetitive stress), or gradual aging (degeneration) of one or more bone-tendon-muscle tissues, understanding rotator cuff pathophysiology is crucial for identifying novel clinical targets for therapeutic development. Since the rotator cuff exists as an integrated multi-tissue complex, injuries are not only confined to the tear site but also to multiple components of the musculoskeletal tissue unit including tendon, skeletal muscle and bone.
4.1. Bone–tendon interface or enthesis pathophysiology
Typically, tears primarily occur at the bone-tendon interface or enthesis. This is because there is a severe mechanical mismatch between hard and soft tissues such as bone and tendon/ligament [29]. Although the enthesis is characterized by a graded transition in mineral content, collagen fiber orientation, and biochemical composition that reduces stress concentrations resulting from this mechanical mismatch [30], it is still susceptible to injury due to acute trauma, chronic overuse, or aging. Robust healing response is observed after the injury, but enthesis tissue structure is altered and not restored to its native state [31]. Instead, fibrovascular scar tissue typically forms, instead of regenerated native, graded bone-tendon transition. The absence of fibrocartilaginous insertion at the bone-tendon interface results in stress concentrations; in the presence of mechanically weaker scar tissue, the likelihood for recurrent defects increases [32]. A comprehensive overview of the structural and functional changes that occur at this interface following injury is summarized in the work of Lu et al. [33], Longo et al. [31], Rodeo et al. [34], Rothrauff et al. [30], and Thomopoulos et al. [35].
4.2. Tendon degeneration
Although distant from the typical location for rotator cuff tears, tendons are susceptible to degeneration. In full-thickness tears, the torn tendon retracts and its compositional and biomechanical properties typically are not restored to pre-injury levels [32,36,37]. The collagen type ratio within tendon is altered, such that collagen type III is increased and becomes predominant instead of collagen type I. Thinning and disorientation of collagen fibers, myxoid and hyaline degeneration, chondroid metaplasia, calcification, fatty degeneration and vascular proliferation have been reported in degenerative tendons [38]. The presence of vascular proliferation together with fatty degeneration disrupt collagen fiber continuity and can reduce tensile properties [38]. Indeed, the tensile strength in the healed tendons has been reported to be approximately 30% relative to intact tendons [39]. In addition to incomplete bone-tendon healing as well as degenerative changes to tendon, another important obstacle that hinders rotator cuff treatment is progressive muscle atrophy or degeneration, particularly in chronic or “irreparable” rotator cuff tears [40].
4.3. Muscle degeneration
Indeed, skeletal muscle pathophysiological changes pose a significant obstacle for recovery. Such atrophic and degenerative changes in skeletal muscle include decreased tissue volume/mass and sarcomere number, increased fibrosis and fatty degeneration, reduced capillary density, and alterations in muscle pennation angle and fiber-type switching [[41], [42], [43], [44], [45], [46], [47], [48]]. These changes are typically correlated with inferior rotator cuff healing as well as poor surgical and non-surgical outcomes [49]. In a clinical study with 38 rotator cuff tear patients, the degree of muscle atrophy and fatty degeneration of the infraspinatus muscle showed a strong negative correlation with functional scores, such as the American Shoulder and Elbow Society (ASES) and Constant scores, in most cases [49]. The primary cause for this may stem from mechanical unloading due to the detachment of the torn tendon although muscle denervation caused by suprascapular nerve injury may also play a contributing role [50].
In addition to muscle fatty degeneration and architectural changes, muscle retraction affects the biomechanical properties of the muscle-tendon unit. The retracted muscle becomes stiffer with decreased elasticity and viability. As such, the impaired mechanical properties of the muscle may explain the loss of shoulder strength following a large-to-massive rotator cuff tear. Indeed, a positive correlation was found between tear size and bundle elastic modulus in torn supraspinatus muscle samples [51]. The degeneration and retraction of both muscle and tendon tissues can alter biomechanical (tension) forces generated within the rotator cuff, resulting in impaired musculoskeletal movements.
Central to this idea is the role of muscle active and passive tension. When tendon and muscle tissues are retracted and/or degenerated, the ability of muscle to generate active tension is impaired. Specifically, loss of muscle mass and decreased length of its contractile elements (sarcomeres) reduces active tension [43]. Indeed, massive rotator cuff tears have been shown to correlate with reduced muscle mass, muscle fiber length, and sarcomere length [43]. In cadaveric-simulated tendon retraction studies, partial detachment (up to two-thirds tendon width) or retraction of the supraspinatus tendon (up to two-thirds tendon width and half its length) only resulted in minor reduction (≤5%) of force transmission from the rotator cuff [52]. However, complete detachment (whole tendon width) or retraction of the supraspinatus tendon (whole tendon width and half its length) resulted in decreased force transmission by 11% and 17%, respectively [52]. In cadaveric, simulated muscle and tendon retraction studies, removal of one-third, two-thirds, and the entire supraspinatus tendon resulted in decreased force transmission by 19%, 36%, and 58%, respectively [52]. Complete repair of these simulated defects restored force transmission to levels similar to that of intact rotator cuff [52]. While it is generally recognized that some tension within the rotator cuff is necessary for optimal function, excessive passive tension following repair is correlated with inferior patient outcomes, including decreased strength and increased pain [53]. While the reasons remain to be determined and are likely multi-factorial, it has been suggested that repairing retracted muscle and tendon tissues back to the native rotator cuff footprint may damage muscle by overstretching its sarcomeric contractile elements [43], thus further decreasing muscle efficiency and active tension [54].
The loss of these tissues not only affects active tension but can also affect passive tension. Since passive muscle tension (transmitted via the rotator cuff and biceps tendon) compresses the humeral head into the glenoid cavity [17], loss of superior rotator cuff tendons such as the supraspinatus can result in the humeral head migrating out of the “socket” and upwards [[55], [56], [57]]. The displacement of humeral head narrows the subacromial space and hinders tendon gliding, resulting in inflammation and subacromial or shoulder impingement syndrome [17,58]. Together, these studies highlight the importance of restoring rotator cuff tissue tension to ensure proper musculoskeletal anatomy for efficient force generation and transmission.
4.4. Bone loss
Besides changes in tendon and muscle, bone loss has also been observed in chronic rotator cuff injury. In rat models with supraspinatus tendon transection, bone density in the greater tuberosity was markedly decreased in the delayed repair group compared to those with immediate repair [59]. Consistent with animal studies, significantly greater osteopenic changes in the greater tuberosity were observed in patients with chronic retracted rotator cuff tears, compared to those with acute minimally retracted tears [60]. This decreased bone quality may complicate surgical repair of the rotator cuff and exacerbate already inferior tendon-to-bone healing [59].
4.5. Benefit of understanding multi-tissue degeneration for clinical treatment
In summary, rotator cuff tears often occur at the bone-tendon interface. However, degenerative changes to associated bone, tendon, and skeletal muscle tissues also occur. Thus, additional attention on the bone-tendon-muscle unit, as opposed to only restoring the torn tendon back to the humeral bone, should benefit clinical treatment of rotator cuff tears by giving broader consideration to the pathophysiological degenerative changes that occur as a result of disease and injury.
5. The need for new clinical targets in the treatment of rotator cuff injuries
5.1. Current clinical practice for rotator cuff injuries
Current clinical practice for rotator cuff injury treatment target issues caused by pathophysiological degeneration. Such symptoms of rotator cuff tears include pain, loss of strength, limited shoulder motion and instability of the shoulder joint. Treatments are broadly divided into non-surgical managements and surgical repairs, which are indicated according to clinical factors, such as age, tendon location, mode of injury, and size of defect [58,61,62].
5.1.1. Non-surgical management
Non-surgical management includes rest, physical therapy, and medications (anti-inflammatory drugs or corticosteroid injection) coupled with non-invasive monitoring methods (ultrasound and shoulder examinations). Patients with tendonitis, partial thickness tears, small (<1–1.5 cm) full-thickness tears should be considered for initial conservative treatment due to the reduced risk for irreversible, chronic rotator cuff changes. Non-operative treatment at the initial stage may also be beneficial for aged patients (> 65 years) who have low physical demands and are afflicted with chronic full-thickness tears or large irreparable tears with chronic, irreversible muscle damage [61,63,64]. However, non-operative treatments are still associated with a number of risks that include lack of spontaneous healing, tear progression, muscle fatty degeneration and retraction, tendon retraction, increased difficulty of tendon mobilization, and development of arthritis in the long-term [61,[63], [64], [65]]. Therefore, early surgical repair is strongly recommended for individuals with acute tears and in younger patients (< 65 years old) with chronic, reparable tears or substantial tears (> 1 cm) without significant chronic muscle changes [61,[63], [64], [65]].
5.1.2. Surgical management
Surgical management for rotator cuff tears can range from simple debridement to complex procedures such as reverse total shoulder arthroplasty (RTSA), tendon transfer, and implantation of tissue engineered scaffolds or grafts (Fig. 2).
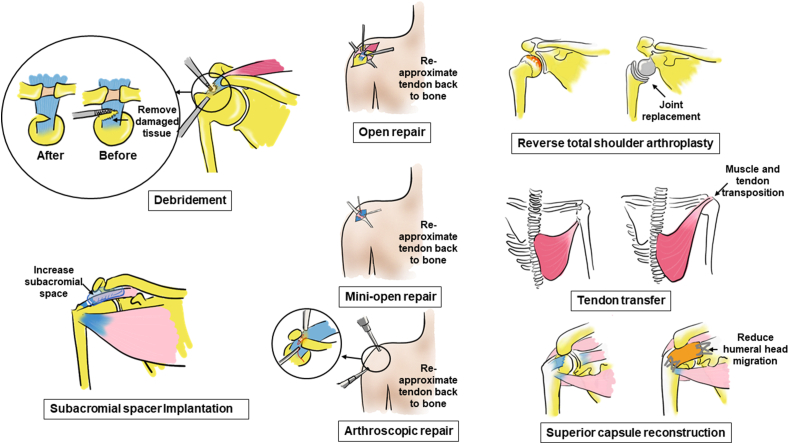
Fig. 2.
Different surgical options for rotator cuff injuries. These operative procedures include debridement, open repair, mini-open repair, arthroscopic repair, reverse total shoulder arthroplasty, tendon transfer, superior capsule reconstruction, and subacromial spacer implantation. Bones, tendons, and muscles are colored yellow, blue, and red, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Tissue debridement involves removal of loose fragments of tendon, thickened bursa, and other damaged tissue to relieve pain, whereas subacromial spacer implantation involves positioning a biodegradable, fluid-containing balloon between the acromion and humeral head to permit smooth gliding and restoration of shoulder function.
Open repair, mini-open repair, and arthroscopic repair are repair methods that seek to anatomically affix tendon back to bone via combinatorial application of suture anchor(s) and sutures. While patient outcome for these methods have largely been satisfactory, a systematic review of studies from 2003 to 2014 reported that the pooled rate for recurrent defects was as high as 79% for arthroscopic repair of chronic massive rotator cuff tears [66]. As such, other surgical methods that do not involve anatomic repair have been developed.
In contrast to anatomic repair of tendon back to bone, several reconstructive methods have been developed which restore shoulder function in a non-anatomic manner. These reconstructive procedures include: RTSA, tendon transfer, and arthroscopic superior capsule reconstruction (ASCR).
RTSA reconstructs the shoulder joint of elderly patients using a ball and socket implant affixed to the humerus and scapula, respectively, which is in reverse relative to native shoulder anatomy. RTSA relies on the deltoid muscle, instead of the rotator cuff, to power and position the arm. However, RTSA is associated with a high complication rate. The reported overall complication rate of primary RTSA is approximately 15% due to instability, infection, hematoma, scapular notching, glenoid loosening, neurological injury, heterotopic ossification, acromial and scapular spine fractures, intraoperative fractures, and component disengagement [[67], [68], [69]].
Tendon transfer is another reconstructive option for treating irreparable massive rotator cuff tears. This procedure helps to restore shoulder mechanics and allow patients to reduce pain and regain partial shoulder function [70]. This is performed by utilizing an existing, healthy muscle-tendon unit to substitute for the function of the injured rotator cuff muscle-tendon unit. In this procedure, the transferred tendon's insertion site is changed to redistribute skeletal muscle contractile force to power the injured rotator cuff but the transferred tendon's origin remains unaltered, resulting in a non-anatomic procedure. The benefit of tendon transfers is that they are not limited by the quality of the remaining rotator cuff tendon and muscles, but instead provide a functionally contracting muscle-tendon unit in comparison to other passive techniques such as subacromial spacer implantation. A variety of tendon transfer options (pectoralis major, latissimus dorsi, and trapezius tendon) are available for the treatment of patients with a massive irreparable rotator cuff tear. Good-to-excellent results have been reported in short to intermediate-term and long-term clinical studies for latissimus dorsi transfer to treat irreparable posterosuperior rotator cuff tears [[71], [72], [73], [74]]. However, several complications exist. These include the postoperative formation of hematoma, infection, late/secondary tendon rupture, anchor pull-out, and nerve palsies [75]. Recent surgical advancements such as transfer of trapezius tendon have been described, with advantages derived from the procedure's technical ease and ability to produce a muscle-tendon unit whose line of pull, tension, and excursion forces are similar to the infraspinatus rotator cuff unit [76]. However, more extensive clinical studies examining patient outcome at mid-to-long term follow up are required. Thus, tendon transfers remain a viable strategy for restoring shoulder function.
ASCR is a relatively new surgical method developed by Mihata et al. which affixes a fascia lata autograft from the superior glenoid onto the greater tuberosity of the humeral head to restore shoulder joint stability [77]. ASCR is gaining popularity and is considered a reliable and useful alternative for irreparable rotator cuff tears [78]. The reported short-term outcomes (minimum follow-up of two years) are promising with one study reporting that in 23 patients, the ASES score increased from 23.5 to 92.9 and the acromiohumeral distance also significantly increased by 4.1 mm after surgery [78]. Recently, a 5-year follow-up study of ASCR showed an increase of 63.3 points for the ASES score, 39.9 points for the JOA score, 66° for active elevation, and 4.7 mm for acromiohumeral distance compared with the preoperative values, with only 3 out of 30 patients having a graft tear [79]. However, a high rate of graft tear (9 out of 31 patients) as assessed by MRI was reported after an average of 12.8 months following ASCR [80]. Thus, studies with longer-term follow-up and larger cohort of patients are needed.
5.1.3. Evolving trends in surgical management: use of biomaterials to supplement rotator cuff repair or reconstruction
In spite of the unparalleled success of surgical repair and reconstructive techniques for improving patient outcome, it can still be difficult to regain full range of shoulder motion. As such, commercially available biomaterials such as rotator cuff patches and synthetic scaffolds have been introduced with these techniques.
In arthroscopic repair, it can be challenging to re-attach the torn tendon back to bone for large-to-massive rotator cuff tears [14]. As such, surgeons have devised new methods that utilize native tissues, biological patches or synthetic scaffolds as either augmentation or interpositional grafts for rotator cuff repair. In a recent clinical trial, GraftJacket™ (acellular human dermal matrix) of proper size and tissue-matched thickness was used as an interpositional allograft to repair the tendon back to the footprint. A statistically significant improvement in Oxford shoulder scores (from a mean of 22–45.5) over a follow up of 18 months was demonstrated and host tissue integration was observed in one patient with arthroscopy [81]. However, the long-term outcomes and safety for these kinds of rotator cuff repair are unclear, and largely depends on the patient etiology and graft characteristics.
For ASCR, donor tissue such as fascia lata is used to treat irreparable, large-to-massive rotator cuff tears. To avoid donor-site morbidity stemming from fascia lata harvest, human acellular dermal patch allograft (ArthroFlex®), which has shown promising results in patch-augmented rotator cuff repair, has also been used for superior capsule reconstruction [82,83]. Recently, in a one-year follow up study involving 59 patients undergoing ASCR with dermal allograft, around 70% of cases showed promising outcome with the ASES scores improved from 43.6 to 77.5, and the subjective shoulder value score improved from 35.0 to 76.3 [84]. Such studies, while promising, will require longer-term follow up of patients.
5.2. Commercially available biomaterials used in treatment of rotator cuff tears
In the last few decades, several new tissue-engineered materials, including naturally derived biomaterials and synthetic polymers, have been used in the development of new tendon substitutes and other medical devices (suture anchor) for rotator cuff injury [85].
5.2.1. Naturally derived tendon scaffolds
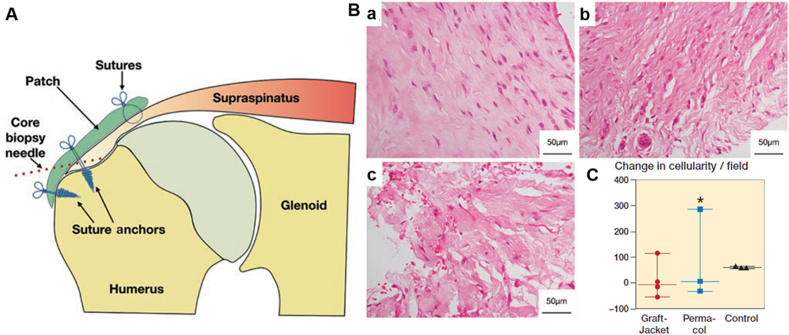
Naturally derived tendon scaffolds for rotator cuff repair are derived from the natural extracellular matrix (ECM) of various human (autograft or allograft) and animal tissues, (xenograft) including dermis, small intestine submucosa, fascia lata, and pericardium. The ECM materials are processed via proprietary methods prior to being marketed as patches for rotator cuff repair. Typically, such processing involves decellularization, chemical cross-linking, lamination of multiple layers, and/or lyophilization. Popular, commercially available, naturally derived scaffolds include GraftJacket™, AlloPatch®, ArthroFLEX®, TissueMend®, Bio-Blanket®, Zimmer®/Permacol™, Conexa™, Biotape®, Restore™, CuffPatch®, Tutopatch® and OrthADAPT® (see Table 1) [86]. Due to their natural origin, such scaffolds often exhibit well-defined three-dimensional microstructure and natural porosity, which not only provide temporary mechanical support but also facilitate host cell attachment, proliferation, and migration. The incorporation and subsequent remodelling of the scaffold by host cells may enhance and accelerate the native tissue repair. However, a major concern for most commercially available grafts is their low mechanical strength. Typically, their linear modulus and stiffness are an order of magnitude lower compared to native rotator cuff tissues [86,87]. Also, although naturally derived scaffolds are often touted for their biological activity and biocompatibility, recent histological and immunohistochemical studies have indicated a lack of patient benefit with regard to increased cellularity or vascularity after rotator cuff patch augmentation relative to standard repair control (Fig. 3) [88]. Other issues arising from use of naturally derived tendon scaffolds include ill-defined degradation rate due to the variable nature of the source material(s) [86,89]. Such issues may be addressed by slight modifications to the surgical technique [81,90,91]. For example, doubling the GraftJacket™ sheet to match patient tissue thickness (as an interpositional graft) resulted in improved Oxford shoulder scores for 18 patients with massive rotator cuff tears over an 18-month duration [81].
Table 1.
Commercially available scaffolds for rotator cuff repair.
| Brand name | Composition | Company | Study type/Level of evidence | Sample size | Repair technique | Follow-up period | Clinical outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Naturally derived | ||||||||
| GraftJacket™ | Human dermis | Wright Medical, Arlington, TN, USA | Cohort/3 | 47 | Interposition | 9.1 years | Improved clinical outcomes (Oxford shoulder score) and high patient satisfaction that was maintained at 9.1 years. | Modi et al., 2022 [189] |
| AlloPatch® | Human dermis | MTF Sports Medicine, Edison, NJ, USA | Retrospective case series/4 | 14 | Augmentation | 1 year | Majority of rotator cuffs (85.7% were intact) with improved clinical outcomes (Constant score, Flexilevel Scale of Shoulder Function, pain score, scapular plane abduction, and strength). | Agrawal, 2012 [190] |
| ArthroFLEX® | Human dermis | Arthrex, LifeNet Health, Virginia Beach, VA, USA | Controlled laboratory study (Cadaver)/3 | 25 | Interposition or Augmentation | — | Both augmentation and interposition repair increased ultimate load in cadaver shoulders. | Beitzel et al., 2022 [191] |
| TissueMend® | Fetal bovine dermis | Stryker Orthopedics, Mahwah, NJ, USA | Technical note/6 | NA | Augmentation | NA | NA | Seldes et al., 2006 [192] |
| Bio-Blanket® | Bovine dermis | Kensey Nash Corporation, Exton, PA, USA | Review/7 | NA | Augmentation | NA | NA | Coons and Barber, 2006 [193] |
| Zimmer or Permacol™ | Porcine dermis | Medtronics, Mansfield, MA, USA | Case series/4 | 10 | Augmentation | 4 weeks | Disruption of extracellular matrix underlying both patches was observed. 1 patient had adverse tissue immune response. | Rashid et al., 2020 [88] |
| Conexa™ | Porcine dermis | Tornier, Edina, MN, USA | Case series/4 | 4 | Interposition | 10.5 months | Favourable remodeling of graft with vessel infiltration without evidence of inflammation, foreign body reaction, or tissue rejection. | Christian et al., 2021 [194] |
| Biotape® | Porcine dermis | Wright Medical, Arlington, TN, USA | Review/7 | NA | Augmentation | NA | NA | Karuppaiah et al., 2019 [195] |
| 24 | NA | About 2 weeks | Comparable proliferation and tendon gene expression as other commercial grafts. | Smith et al., 2016 [196] | ||||
| Restore™ | Porcine small intestine submucosa | DePuy Orthopedics, Warsaw, IN, USA | Controlled trial/3 | 31 | Augmentation | 2 years | Poor clinical outcomes including lower strength and more impingement in external rotation with slower resolution of pain and more difficulty in daily living activities. | Walton et al., 2007 [197] |
| CuffPatch® | Porcine small intestine submucosa | Arthrotek, Warsaw, IN, USA | Controlled laboratory study (Rat)/3 | 126 | Interposition | 112 days | Presence of foreign-body giant cells, chronic inflammation and accumulation of dense, poorly organize fibrous tissue. | Valentin et al., 2006 [198] |
| Tutopatch® | Bovine pericardium | Tuto-gen Medical GmbH, Neunkirchen am Brand, Germany | Retrospective case series/4 | 152 | Augmentation | 3 years | Similar clinical outcome (UCLA score), retear rate, pain, strength, and elevation as control (open repair) group. | Ciampi et al., 2014 [93] |
| OrthADAPT® | Equine pericardium | Pegasus Biologic Inc., Irvine, CA, USA | Controlled laboratory study (Rat)/3 | 41 | NA | 3 months | Increased maximum load compared to suture only repair at 3 months. | Tornero-Esteban et al., 2015 [199] |
| Synthetic (Non-degradable) | ||||||||
| Leeds-Keio® | Polyester ethylene terephthalate | Xiros plc, Neoligaments, Leeds, UK | Randomized controlled trial/1 | 39 | Augmentation | 2 years | Improved clinical outcomes (Hospital for Special Surgery score), decreased pain, increased range of motion, and increased strength. | Tanaka et al., 2006 [200] |
| Poly-tape® | Polyester ethylene terephthalate | Yufu Itonaga Co., Ltd, Tokyo, Japan | Controlled laboratory study (in vitro)/3 | 24 | NA | About 2 weeks | Comparable proliferation and tendon gene expression as other commercial grafts. | Smith et al., 2016 [196] |
| Mersilene® mesh | Polyester ethylene terephthalate | Ethicon, Inc., Somerville, NJ | Case series/4 | 41 | Interposition | 43 months | Improved clinical outcomes (Constant score), reduced pain and improved daily living activities. | Audenaert et al., 2006 [201] |
| Lars® ligament | Terephthalic polyethylene polyester | Ligament Augmentation and Reconstruction System, Dijon, France | Controlled laboratory study (in vitro)/3 | 24 | NA | About 2 weeks | Comparable proliferation and tendon gene expression as other commercial grafts. | Smith et al., 2016 [196] |
| Gore-Tex® patch | Polytetrafluoroethylene | Gore and Associates, Flagstaff, AZ, USA | Case series/4 | 28 | NA | 44 months | Improved clinical outcome (JOA score), reduced pain, and increased abduction strength. | Hirooka et al., 2002 [202] |
| Marlex® | High-density polyethylene | C.R.Bard, Mullayhill, NJ, USA | Case series/4 | 9 | Capsular reconstruction | 3–48 months | Improved clinical outcome with respect to joint instability although there were two surgical complications. | Gortzak et al., 2010 [203] |
| Repol Angimesh® | Polypropylene | ANGIOLOGICA BM Srl, Pavia, Italy | Retrospective case series/4 | 152 | Augmentation | 3 years | Improved clinical outcome (UCLA score) as well as, lower retear rate, lower pain, increased strength, and increased elevation as control (open repair) group. | Ciampi et al., 2014 [93] |
| Synthetic (Degradable) | ||||||||
| X-Repair | Poly-L-lactic-acid | Synthasome Inc., San Diego, CA, USA | Case series/4 | 18 | Augmentation | 42 months | Improved clinical outcomes (ASES score) with 78% intact repair at 42 months. | Proctor, 2014 [204] |
| Artelon® or SportMesh™ | Polyurethane urea polymer | Artimplant AB, Sweden | Case study/6 | 3 | Augmentation | 6 months-2.5 years | Improved clinical outcomes (WORC and Oxford score) with integrity of rotator cuff retained at 15-months postoperatively for one patient. Remaining two patients showed improved Constant score (17% and 79%) at six months. | Zhaeentan et al., 2011 [205] |
| Integraft™ | Carbon fibre | Hexcel Medical, Dublin, CA | Review/7 | NA | Augmentation | NA | NA | Karuppaiah et al., 2019 [195] |
| BioFiber™ | Poly(4-hydroxybutyrate) | Tornier, Edina, MN, USA | Controlled laboratory study (in vitro)/3 | 24 | NA | About 2 weeks | Comparable proliferation and tendon gene expression as other commercial grafts. | Smith et al., 2016 [196] |
∗NA, not available
Fig. 3.
Changes in cellularity following standard rotator cuff repair or patch-augmented rotator cuff repair. (A) Schematic showing the biopsy sample site (Core biopsy needle, dotted line) at 4-week post-surgical repair. (B) Representative hematoxylin and eosin (H&E) histological staining showed cellularity changes following patch augmentation or standard repair (no patch; control) group. H&E staining was performed on (a) control, (b) GraftJacket™, and (c) Permacol™ groups, with patch augmentation showing increased disruption of the tendon extracellular matrix. (C) Histological quantification showed lower cellularity per field of view for patch-augmented repair (GraftJacket™ and Permacol™) relative to standard repair control. ∗ denotes a patient who received Permacol™ and exhibited a grossly different tissue response. Copyright obtained from Taylor & Francis and adapted from Mustafa S Rashid et al., 2020.
5.2.2. Synthetic tendon scaffolds
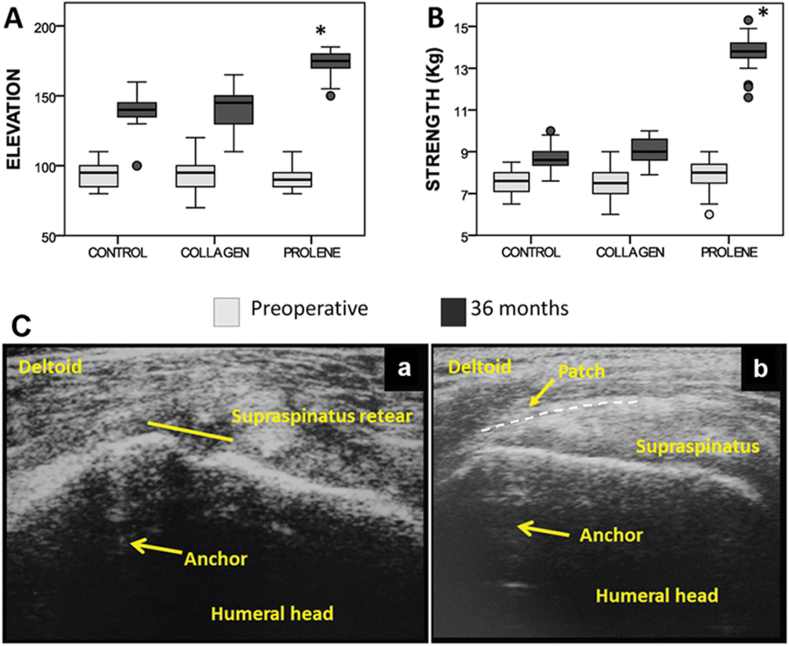
Synthetic tendon scaffolds can be further divided into non-degradable and degradable scaffolds. Non-degradable synthetic scaffolds are based on polymers such as polyester, polyurethane, polypropylene, and expanded polytetrafluoroethylene (Teflon), and can be found in commercially available products such as Leeds-Keio®, Poly-tape®, Mersilene® mesh, Lars® ligament, Gore-Tex®/Teflon™ patch, Marlex® and Repol Angimesh® (see Table 1) [86]. Recent concerns associated with these non-degradable implants include persistent infections, loss of integrity, and long-term safety [92]. As such, there has been a shift towards synthetic degradable scaffolds in recent years, which aims to provide non-permanent support to avoid undesired foreign body reactions as well as accommodate tissue healing and remodelling. Degradable scaffolds can be made of a wide variety of polymers including poly-L-lactic acid (PLLA), poly (lactic-co-glycolic acid) (PLGA), poly-ε-caprolactone (PCL), polydioxanone (PDO), and polyurethane urea, and can be found in commercially available products such as X-repair, Artelon®/SportMesh™, Intergraft™ and BioFiber™ (Table 1) [86,87]. Compared to naturally derived scaffolds, synthetic scaffolds exhibit higher consistency and superior mechanical strength. For example, use of a polypropylene scaffold resulted in increased patient arm elevation (174.71° ± 8.18°), strength (13.79 ± 0.64 kg), and reduced the rate of recurrent defects (17%) relative to control (elevation: 140.68° ± 9.84°; strength: 8.73 ± 0.54 kg; and recurrent defect rate: 41%) and collagen (elevation: 140.61° ± 12.48°; strength: 9.03 ± 0.60 kg; and recurrent defect rate: 51%) groups (Fig. 4) [93]. However, synthetic scaffolds have been traditionally associated with little-to-no bioactivity and poor biocompatibility [89].
Fig. 4.
Shoulder function and tissue healing following open repair (control), collagen-mediated rotator cuff repair, and polypropylene (prolene) (Repol Angimesh®, Angiological BM Srl, Pavia, Italy) -mediated rotator cuff repair. (A) Boxplots summarizing elevation on the scapular plane; (B) abduction strength assessed pre- and 36 months post-operatively. ∗, Statistically significant difference at 36 months between the polypropylene group with other groups. Whiskers indicate minimum and maximum values. Round dots indicate outliers, defined as values higher than 1.5-fold the interquartile range. (C) Ultrasound images taken 12 months post-surgery showed (a) recurrent supraspinatus defect and collagen patch absorption, and (b) maintenance of rotator cuff integrity and the presence of a polypropylene patch. Copyright obtained from SAGE journals and adapted from Pietro Ciampi et al., 2014.
Despite the success of both commercially available naturally derived and synthetic scaffolds in rotator cuff patient care, a present limitation of existing tendon substitutes is that they lack features to mimic the bone-tendon interface, which is the most frequent injury site [86]. Thus, the ideal scaffolds for rotator cuff repair should possess superior bioactivity, biocompatibility, and mechanical attributes with the capability to address regeneration of the bone-tendon interface. Within a clinical setting, the current state-of-the-art is to utilize suture anchors for affixing single tissue-mimicking, tendon substitutes to bone.
5.2.3. Suture anchors
As critical elements used during rotator cuff repair, suture anchors have gone through several modifications as clinical practice transitioned from open to arthroscopic repair. Initially, metallic suture anchors with non-absorbable sutures were used, but complications of loosening, migration, articular surface damage, and artifact production in postoperative MRI were reported in numerous studies [[94], [95], [96], [97]]. In response to this, bioabsorbable anchors were developed [96]. However, other complications stemming from rapid degradation can result in loose foreign bodies (suture or non-resorbed parts of the suture anchor) within the glenohumeral space [98], necessitating revision surgery. Recent developments such as new suture anchor designs such as knotless anchor systems [99], use of osteoconductive, biocomposite suture anchors with a slower degradation profile [100], non-degradable, non-metallic suture anchors such as poly ether–ether ketone (PEEK) [101], and suture anchors comprised entirely of suture material [102] have been implemented to address these concerns with good short-term results but long-term clinical outcomes remain to be determined.
In summary, various commercially available biomaterials are used as tendon substitutes and in suture anchors for rotator cuff repair. Further development of such materials is expected to improve the clinical efficacy of rotator cuff repair and aid in the evolution and subsequent refinement of new clinical techniques.
5.3. Clinical application of stem/progenitor cells, growth factors, and exosomes
Although there have been numerous advances and developments to existing clinical techniques and commercially available biomaterials, long-term functional recovery for severely injured rotator cuff tissues remains challenging [30,103]. Also, there has been increased recognition that a highly regenerative environment is necessary to ensure complete rotator cuff tissue healing and restoration of shoulder function. As such, there have been preclinical studies and clinical trials involving application of stem/progenitor cells and growth factors for rotator cuff repair and regeneration. Clinical studies examining the benefit of scaffolds and augmentation at the healing tendon-bone interface have been well summarized in Table 1 of Rothrauff et al. [30]. The applications of stem/progenitor cells, growth factors, and exosomes along with their associated regulatory environment are further elaborated in Table 2 and the following sections.
Table 2.
Clinical studies using stem/progenitor cells, growth factors or their combination for rotator cuff repair.
| Cell type/Growth factor | Study type/Level of evidence | Tear size | Sample size | Method of delivery | Follow-up period | Clinical outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Stem/Progenitor Cells | |||||||
| BMSCs | Cohort/3 | Full-thickness | 124 (57/67) | Drilling into the bone marrow was performed in the greater tuberosity. | At a minimum of 2 years | No significant difference in pain, range of motion, strength, overall satisfaction and functional scores. The retear rate was significantly lower. | Jo et al., 2013 [120] |
| BMSCs | Case-controlled/4 | Tear size from 1.5 to 3.5 cm | 90 (45/45) | BMSCs were injected into the tendon-bone interface. | At a minimum of 10 years | Enhanced healing rate, improved quality of the repaired surface, reduced number of recurrent defects. | Hernigou et al., 2014 [121] |
| BMSCs | Retrospective cohort/3 | Full-thickness | 111 (67/44) | Drilling into the bone marrow was performed in the greater tuberosity. | 2–24 months | Improved cuff repair integrity and lower retear rate in large-massive tears. | Taniguchi et al., 2015 [206] |
| ADSCs | Cohort/3 | Full-thickness | 70 (35/35) | Injection of adipose-derived MSCs loaded in fibrin glue during rotator cuff repair. | 28.3 ± 3.8 months | No clinical differences in the 28-month period of follow-up compared to the conventional group. | Kim et al., 2017 [122] |
| Growth Factors | |||||||
| PRP | Randomized controlled trial/1 | Subacromial impingement syndrome/partial-thickness | 60 (30/30) | Injection of autologous PRP into the subacromial bursa. | At a minimum of 2 years | No improvement for clinical outcomes. May have potential deleterious effects on healing tendons. | Carr et al., 2015 [153] |
| PRP | Randomized controlled trial/1 | Medium-sized to large cuff tear | 102 (52/50) | Delivery of autologous PRP over the cuff surface through the arthroscopic portal. | At a minimum of 2 years | Visual analog scale scores were lower at 1, 3 and 6 months; Constant-Murley scores improves at 12 and 24 months; UCLA acores were higher at 6 and 12 months; Retear rate decreased at 24 months for large tears. | Pandey et al., 2016 [152] |
| PRP | Randomized controlled trial/1 | Complete rotator cuff tear | 120 (60/60) | Intraoperative pure PRP injection. | 6 and 24 months | No significantly improved function at 3, 6, and 24 months. | Flury et al., 2016 [207] |
| PRP | Randomized controlled trial/1 | Full-thickness | 60 (30/30) | Injection of autologous PRP. | 12 months | Lower recurrence rates. | Zhang et al., 2016 [151] |
| PRP | Randomized controlled trial/2 | Complete supraspinatus tear | 51 (26/25) | Liquid PRP prepared by apheresis with autologous thrombin was applied in the tendon-to-bone interface. | 60 months | No improvement for clinical or structural results at 60-month follow-up. | Malavolta et al., 2018 [148] |
| LR-PRP | Prospective randomized therapeutic trial/2 | Rotator cuff tear | 87 | Ultrasound guided injection of leukocyte-rich PRP. | 12 months | No improvement by patient-reported outcome measures and Constant score at 1 year postoperatively. | Snow et al., 2020 [147] |
| rhBMP-12 | Randomized controlled trial/2 | Full-thickness (2–4 cm wide) | 20 (16/4) | rhBMP-12/absorbable collagen sponge (ACS) was applied to the footprint. | 12 months | Functional recovery in theVAS score for pain,ROM, and isometric strength was similar compared to the control group | Ide et al., 2017 [138] |
| Combination of Stem/Progenitor Cells and Growth Factors | |||||||
| Subacromial bursa, cBMA, PRP | Therapeutic case series/4 | Tears with at least 2 tendons | 16 | Arthroscopic rotator cuff repair augmented using subacromial bursa, cBMA, and platelet-rich plasma delivered to the injury site. | 12.6 ± 1.8 months (range 12–19 months) | Improvement in ASES, Constant-Murley, SANE and pain scores. | Muench et al., 2020 [208] |
5.3.1. Application of stem/progenitor cells
Stem/progenitor cells are characterized by their self-renewal capacity and potential to differentiate into one or more cell type(s). The main aim of using stem/progenitor cell therapy in surgical rotator cuff repair is to either replace injured cells via differentiation into musculoskeletal cells, encourage tissue regeneration via their paracrine actions, or a combination of both. The most widely studied cells within the context of rotator cuff injury treatment are the multipotent adult mesenchymal stromal cells (MSCs), which can be obtained from bone marrow [104], adipose tissues [105], muscle [106], tendon [107,108], synovium [109], periosteum [110], umbilical cord [111,112], peripheral blood [113] and urine [114]. Traditionally, bone marrow-derived MSCs (BMSCs) have been most commonly reported although recent promising studies have focused on other MSCs derived from adipose, tendon, synovium and umbilical cord blood.
For BMSC-mediated regeneration, there have been a large number of preclinical studies and clinical trials. In preclinical studies, efforts have been focused primarily on delivery of BMSCs alone, BMSCs in combination with stimulating factors such as platelet-rich plasma [115], BMSCs in combination with tissue engineered scaffolds/grafts such as demineralized bone matrix [116], BMSCs genetically modified with membrane type 1 matrix metalloproteinase [117], or using BMSC-derived exosomes as a cell-free product [118]. The details of these preclinical studies are well summarized by Xu et al. [119]. In clinical studies, early efforts have focused on endogenous recruitment of BMSCs with promising results. The first clinical study to investigate the impact of BMSCs was reported by Jo et al. [120]. BMSCs were recruited from patients endogenously using a “multiple channeling” procedure, which as the name suggests, involved the creation of multiple channels in the greater tuberosity during the surgical repair of full-thickness rotator cuff tear. Patients were followed for 2 years and although clinical outcomes including pain, range of motion, strength, overall satisfaction and functional scores were not statistically different, the recurrent defect rate of the multiple channeling group (22%) was significantly lower than that of the conventional group (45.2%) [120]. This improved outcome was attributed to increased recruitment of endogenous MSCs from the proximal humerus to the wound site [120] but actual quantification of the BMSC numbers was not performed. To address this, Hernigou et al. performed a study that evaluated the efficiency of biologic augmentation of rotator cuff repair via exogeneous injection of iliac crest-derived BMSCs (51,000 ± 25,000) to the injury site. Similarly, the authors reported enhanced healing and improved the quality of the repaired surface as determined by ultrasound and MRI in the BMSCs injection group [121]. Taken together, these findings suggest that BMSC-mediated regeneration is promising for rotator cuff repair.
In addition to the BMSCs, other promising candidate cell types for rotator cuff regeneration include adult-derived adipose, tendon, synovium, peripheral blood and urine-derived MSCs. Adipose tissue is a highly practical source of MSCs as large quantities of MSCs can be easily obtained without the need for culture and expansion. Adipose-derived, mesenchymal stromal cells (ADSCs) have shown good potential in augmenting rotator cuff repair in animal models and are a promising avenue for future research. Kim et al. reported a clinical study that assessed the effect of exogenously injected ADSCs together with fibrin carrier during arthroscopic rotator cuff repair. Similar to studies that used BMSCs, there were no clinical differences after a follow-up period of 28 months, but a lower recurrent defect rate (14.3%) was observed in the ADSC injection group compared to the control group (28.5%) [122]. In addition to ADSCs, synovial MSCs derived from the subacromial bursa [123] have received recent interest due to their high concentrations in synovial tissue and increased chondrogenic potential than BMSCs [124,125]. Another novel source of stem/progenitor cells for clinically augmentation of rotator cuff healing are urine-derived stem/progenitor cells (USCs). USCs originate from the kidney and can be easily isolated from voided urine or the upper urinary tract with robust proliferation and multilineage differentiation ability [126,127]. The main benefit of USCs is that the harvesting method typically avoids invasive and painful surgical procedures [126,127]. In a canine model of infraspinatus tendon insertion detachment and repair, autogenous USC sheet implantation significantly increased the bone volume/total volume and trabecular thickness, and the USC sheet group exhibited increased fibrocartilage coverage, additional proteoglycan deposition, and higher collagen birefringence together with higher failure load and stiffness compared to the control group [114]. Together, these studies show that use of adult-derived stem/progenitor cells is promising for enhancing rotator cuff healing and regeneration.
In addition to the adult stem cells, umbilical cord blood-derived MSCs (UCB-MSCs) are promising alternatives for clinical-scale allogeneic transplantation as they have high differential potential and can be easily collected and expanded post-natally [128,129]. Relative to BMSCs and ADSCs, they could be cultured the longest and showed the highest proliferation capacity [130]. Pre-clinical studies showed therapeutic effects of UCB-MSCs on tendon repair after rotator cuff injury. For example, ultrasound-guided injection of UCB-MSCs with polydeoxyribonucleotide in a chronic rabbit model of full-thickness rotator cuff tendon tear showed histologic improvements in terms of newly regenerated collagen type 1 fibers, cell proliferation activity and angiogenesis as well as gait analysis in terms of walking distance, fast walking time, and mean walking speed compared to the other groups [112]. However, another clinical trial of UCB-MSCs on ACL reconstruction in a 2-years follow-up showed no treatment-related adverse events but also no improvement in enhancing tendon graft-mediated healing compared to the control groups [131]. Thus, future clinical studies are required to verify the efficacy of UCB-MSCs.
While there have been promising indications for using MSC-based therapy to enhance rotator cuff healing, these clinical studies are limited in number, likely due to the high risks associated with the stem/progenitor cell-based therapies, including invasive harvesting method, possible tumorigenic growth, administration site reactions, and other adverse effects. Also, the complexity of such procedures coupled with increased treatment cost may pose a barrier to patient recruitment and participation. Thus, further preclinical studies are needed to demonstrate the cost-effectiveness of stem/progenitor cell-mediated repair before widespread clinical application can become a reality. In addition, application of stem/progenitor cells could be combined with bio-mimic or bio-inductive scaffolds to achieve greater therapeutic effect on multi-tissue regeneration. A recent study demonstrated that a bioactive collagen scaffold supported tendon-derived cell growth and when used in a prospective clinical trial, resulted in improved patient outcome in terms of shoulder function, quality of life, and pain reduction, highlighting the feasibility and safety for use in rotator cuff augmentation [108]. Combining such scaffolds with stem/progenitor cells may enhance their efficacy further.
5.3.2. Application of growth factors
As an alternative to stem/progenitor cells, there has been growing scientific interest in using exogenously supplied growth factors or in situ delivery of stimulated endogenous growth factors to improve rotator cuff regeneration [[132], [133], [134]]. Single growth factor administration, including vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), transforming growth factor-ß (TGF-ß), and platelet-derived growth factor (PDGF), have been shown to promote tendon healing through different signaling pathways for in vitro and in vivo experiments. These studies are comprehensively described and summarized in other excelle nt reviews [135,136]. Presently, there have been several clinical studies assessing the effect of single growth factors for rotator cuff repair such as GDF-7/BMP-12 [137,138]. However, such studies are few, likely due to high cost, short growth factor half-life, and potential off-target side effects [134]. As such, application of low-cost growth factor mixtures such as platelet-rich plasma (PRP) has gained interest. PRP is the platelet-rich fraction of blood, and upon platelet activation, releases copious amounts of growth factors, including IGF-1, PDGF, VEGF, and TGF-ß. Such growth factors have been implicated in tendon healing [132] and thus PRP is a promising candidate for biological augmentation of rotator cuff injury. In addition, PRP is easily prepared and is lower in cost relative to recombinant growth factors. The preparation of PRP involves drawing blood, enrichment of the platelet-containing fraction, and injection into the injury site [104]. Furthermore, since PRP can be obtained autologously, there is little-to-no allergic reactions or major side effects expected [104]. Indeed, in vitro and in vivo studies utilizing PRP have demonstrated promising results for biological augmentation of rotator cuff repair [[139], [140], [141]]. In contrast, numerous clinical trials involving PRP show conflicting results. For example, a large number of studies failed to show significant effects on healing, recurrent defect rate, pain, or functional outcomes [[142], [143], [144], [145], [146], [147], [148]], whereas other studies showed significantly improved structural outcomes such as decreased recurrent defect rate compared with repairs without PRP augmentation [[149], [150], [151], [152], [153]]. Several factors likely contribute to these conflicting results, such as the method of PRP preparation, timing of treatment and activation (pre- or post-injection), variation in leukocyte content of the PRP, PRP dose and retention at the injury site. As such, there have been additional efforts aimed at optimal preparation of PRP such as separation into leukocyte-rich and leukocyte-poor fractions [154]. In such studies, it has been postulated that leukocyte-rich PRP is highly pro-inflammatory due to the presence of catabolic cytokines, which may antagonize the effects of anabolic growth factors [154], whereas other studies suggest that leukocyte-rich PRP is more beneficial in tendinopathies [155]. In addition, there have been recent attempts to modulate healing efficacy by combining PRP with ADSCs [156]. Besides exogenously supplied growth factors, another technically-easy and cost-effective method for delivering growth factors is the microfracture technique, which results in a “super clot” containing a combination of growth factors, platelets, MSCs, and other vascular elements [134]. When the microfracture method was used in combination with hyaluronic acid injection for surgical repair of rotator cuff tear in a rabbit model, improved bone-tendon healing was observed with increased chondrocytes and ECM production as well as increased biomechanical strength at the repair site 12 weeks post-surgery [133]. Thus, application of single growth factors or crude mixtures (via PRP preparation or microfracture surgical technique) represent potent means for improving rotator cuff healing and strength.
5.3.3. Application of exosomes
In addition to stem/progenitor cells and growth factors, another promising alternative is exosome-based therapy, which has been demonstrated to exert potent regenerative effects for rotator cuff healing. Exosomes are nano-sized (30–150 nm in diameter) extracellular vesicles naturally secreted by various cell types including cancer cells, endothelial cells, immune cells, and also MSCs from different sources such as bone marrow, adipose and human umbilical cord, etc. [157]. The most attractive feature of exosomes is that they are cell-free and regarded as less complex from a regulatory standpoint, with clinical trials underway following emergency use authorization from the United States Food and Drug Administration. The lipid bilayer structure of exosomes enable the transfer of messages encoded in protein, lipids, mRNA, miRNA, and metabolites, which facilitate intracellular communication [158,159]. Compared with MSCs, MSC-derived exosomes show similar biological activity such as anti-inflammatory, pro-regenerative and anti-apoptotic effects, however, they are more stable, easier to produce and have limited immune rejection [160,161]. As such, the therapeutic effect and mechanism of exosomes during tissue healing have attracted increased interest for enhancing rotator cuff repair.
In this respect, there are several in vitro and in vivo studies that highlight the potential of exosome-based therapies. Preclinical in vitro studies have shown that BMSC-derived exosomes containing TGF-β1 promoted proliferation, migration, and fibrotic activity in rotator cuff tenocytes, which may enhance rotator cuff tendon healing [162]. Similarly, circulating exosomes were also reported to improve tenocyte proliferation and migration by upregulating tenogenic markers [163]. In vivo studies also showed that in a mouse bone-tendon reconstruction model, local administration of hydrogel-delivered, BMSC-derived exosomes increased the number of M2 macrophages as well as anti-inflammatory and chondrogenic-related factors, while decreasing the M1 macrophages and related proinflammatory factors [118]. This immunomodulatory response led to increased cell proliferation and decreased cell apoptosis at early timepoints with the hydrogel-delivered, BMSC-derived exosome group exhibiting increased fibrocartilage formation and improved biomechanical properties including maximum force, strength, and elastic modulus at 1 month post-surgery [118]. Together, these findings provide a basis for the potential clinical use of exosomes in rotator cuff tendon repair.
In addition to the regenerative effects on tendon, exosomes have been demonstrated to prevent atrophy, fatty degeneration, inflammation, and vascularization of the muscles in massive or chronic rotator cuff tears. Wang et al. demonstrated that in a rat model of massive rotator cuff tear (detachment of both supraspinatus and infraspinatus tendons), injection of ADSCs-derived exosomes (into the proximal and distal side of the supraspinatus muscle) led to decreased loss of supraspinatus muscle weight, reduced adipose content as well as lower macrophage density and vascularization than the saline group at 8 and 16 weeks [164]. In contrast, myofiber regeneration and biomechanical properties were significantly elevated compared with those in the saline-treated group [164]. In addition, the effect of ADSCs-derived exosomes on fatty degeneration and on tendon-bone healing was further examined in a chronic rabbit model of rotator cuff tear [165]. Local injection of ADSC-derived exosomes into torn supraspinatus muscle during repair reduced the percentage of fatty degeneration compared to the saline group, promoted bone-tendon tissue healing as evidenced by increased fibrocartilage regeneration, abundant collagen II and tenascin-C expression, and a more homogenous healing response that included less fibrotic tissue, as well as improved biomechanical properties [165]. Together, these results suggest that ADSC-derived exosomes exhibit great regenerative potential as a cell-free adjunctive therapy to inhibit muscle fatty degeneration and improve rotator cuff healing [165]. In the future, additional clinical trials are required to elucidate the effects of exosomes and their therapeutic effects on rotator cuff healing.
5.3.4. Regulatory environment for stem/progenitor cell, growth factor, and exosome applications
To ensure successful clinical applications of stem/progenitor cells, growth factors, and exosomes, a conducive regulatory environment is needed. Specifically, regulatory requirements must maintain a judicious balance between ensuring adequate innovation to make safe and effective therapies available as well as adequate compliance to avoid unscientific interventions with broad and ambiguous claims [166]. In this regard, established regulatory frameworks and guidance documents from the United States (Human cells, tissues, and cellular and tissue-based products HCT/Ps, 21 CFR part 1271) [167] and European Union (Commission Directive 2004/23/EC and 2006/17/EC) [168] regulatory frameworks have been used to inform the development of acceptable criteria for MSC-based products in Asian markets such as India, Japan, Korean, and Singapore [169]. While regulations differ among different nations, the central principle is the same – the demonstration of safety and efficacy via standardized testing and procedures [170,171]. This includes steps to ensure that: (i) the donor poses minimal risk of transmitting infectious or genetic disease; (ii) the manufacturing and processing steps pose low risk of contamination or damage; (iii) the cell type(s) as well as their purity and potency are known; and (iv) the product will be safe and effective after transplantation to a recipient [170,171]. For example, in the United States, a HCT/P is exempted from specific governmental regulations only if the cell is both harvested and used in the same surgical procedure. Other exemptions may also consider whether a HCT/P is minimally manipulated (i.e., processing does not alter the original relevant characteristics of a structural tissue or relevant biological characteristics of cells or tissues) and whether it is for homologous use (i.e., the HCT/P performs the same basic function(s) in the recipient as in the donor) [172]. In particular, the regulatory environment for combination products such as those involving biomaterials, stem cells, and cell-derived products such as growth factor or exosomes is highly relevant and must be well understood by regenerative medicine practitioners and clinicians. This topic is well reviewed by Tian et al. with respect to bioactive, combinatorial products [173] as well as Woods and MacLoughlin with respect to exosome-based products [174]. As such, the regulatory environment is a crucial aspect that will guide the development of future stem/progenitor cell, growth factor, and exosome applications.
5.3.5. Future trends for stem/progenitor cell, growth factor, and exosome applications
In summary, there have been significant research advances for stem/progenitor cell-, growth factor, and exosome-mediated rotator cuff regeneration. These include novel applications of MSCs such as BMSCs, ADSCs, USCs, and UCB-MSCs, recombinant growth factors such as GDF-7/BMP-12, and biologics that either contain growth factor mixtures such as PRP or cell-free exosomes. While varying degrees of success have been reported in such research, it is noteworthy that few (if any) of these therapies are explicitly targeted for bone-tendon-muscle multi-tissue regeneration. Future efforts such as improved formulations and optimal dosages of PRP, and novel combinations of stem/progenitor cells, growth factors, and exosomes are expected to enhance rotator cuff multi-tissue regeneration. However, these advances will need to comply with existing healthcare regulatory rules to ensure safety and effectiveness.
5.4. Perspective: targeting of multi-tissue, musculoskeletal units
Given the multi-faceted challenges of rotator cuff repair, including incomplete healing, scar tissue formation, musculoskeletal tissue retraction and degeneration, and the high interdependency of musculoskeletal tissues in terms of maintaining a structure–function relationship for unhindered movement, a single-pronged approach such as targeting the site of rotator cuff tears alone (i.e., at the bone-tendon interface) may not suffice for restoring movement, particularly for large-to-massive injuries. While there have been unprecedented advances in surgical techniques, development of medical devices, and novel applications of bioregenerative cues such as stem/progenitor cells, exosomes, and growth factors, there have been few efforts directed at addressing multi-tissue degeneration within the bone-tendon-muscle unit comprehensively. As such, we propose that functional tissue engineering and regenerative medicine efforts employ a strategy to target and engineer multiple tissues which comprise a single musculoskeletal unit.
In support of this, there have been a large number of reports on the development and application of multi-tissue grafts. Such preclinical and clinical studies include the use of bone-tendon allografts [175] and semitendinosus and gracilis free muscle-tendon autografts [176] for treating musculoskeletal injuries, including chronic rotator cuff tears. Indeed, it has been noted that treating rotator cuff tears is not merely about addressing tendon injuries [177]. This is because bone loss typically accompanies rotator cuff injury [59], and treating such pathology via subcutaneous injections of sclerostin antibodies resulted in improved bone mineral density, enhanced tissue biomechanical properties, and excellent bone-tendon integration in an acute rat rotator cuff resection model [178]. In addition, bioprinting of growth factors makes it possible to spatially control osteoblast, tenocyte and myocyte differentiation simultaneously with a single stem cell population to generate a primitive bone-tendon-muscle unit [179]. Such growth factor biopatterning technology can be combined with various biomaterials such as aligned sub-micron fibrous scaffolds, which are morphologically similar to musculoskeletal ECM, and result in simultaneous spatial control of muscle progenitor cell differentiation and alignment [180]. In addition, novel polymer-based biomaterials that possesses phototunable bone- and tendon-like biomechanical properties such as QHM polyurethane has been developed for reducing stress concentrations and material failure at the interface of tissues with dissimilar mechanical attributes for rotator cuff repair [181]. Furthermore, Wang et al. demonstrated that combination of this mechanically-graded, bone- and tendon-like polymer with selectively biopatterned growth factors cues (osteogenic bone morphogenetic protein-2; BMP-2 and tenogenic fibroblast growth factor-2; FGF-2) could influence musculoskeletal differentiation with substrates of increased stiffness favouring osteoblast differentiation and substrates of decreased stiffness favouring tenocyte differentiation in vitro [182]. Subsequent in vivo studies also showed spatially control of ectopic bone-and tendon-like tissue formation in mouse subcutaneous studies [182]. In addition to developing scaffolds that enable smooth interfacing of tissues with dissimilar properties, scaffolds that automatically change their structure or mechanical properties to adapt to the requirement of the surrounding environment will provide new directions for tissue engineering. You et al. synthesized pH-sensitive scaffolds that swelled in response to a decrease in pH and demonstrated that the responsive scaffold could encourage wound healing by inducing a pro-healing gene expression profile indicative of enhanced angiogenesis, granulation tissue formation, and tissue remodeling [183]. Future combination of stem/progenitor cells or growth factors with the responsive scaffold may provide new ideas for advanced therapeutic strategies. Similarly, muscle-tendon autografts [176] and clinical techniques that surgically advance muscle to reduce rotator cuff tension [184] have been used to address issues of fatty muscle degeneration and retraction [58,[185], [186], [187]] to repair massive rotator cuff injuries in the clinic. Special effort has also been made towards deconstructing the cell–matrix interaction in the muscle-tendon junction. Gaffney et al. used tissue-specific extracellular matrix derived from muscle and tendon to determine how cells of each tissue interact with the microenvironment of the opposing tissue, aiming to inform future methods to engineer a more relevant muscle-tendon unit [188]. Together, these studies support the idea that engineering a bone-tendon-muscle unit will be clinically impactful for the treatment of rotator cuff tears, which raises the question of where such musculoskeletal units may be obtained from.
To practically attain musculoskeletal multi-tissue units for implementation in a clinical setting, numerous engineering and biological advances are needed. For example, technical innovations in biomaterial fabrication and maturation are required to develop grafts or tissue engineered constructs capable of biomimicking bone, tendon, and muscle tissues as well as their respective interfaces [2]. These include approximation of mechanical and biological attributes of the bone-tendon-muscle unit such as tensile properties and stress-reducing features, as well as native tissue structure and composition, respectively, while simultaneously addressing clinical concerns such as avoiding suture tearing through the tendon or premature loss of surgical repair construct. This can be accomplished through careful balancing of biomaterial attributes including scaffold architecture for eventual host cell colonization, choice of material and fabrication approach to attain desired mechanical properties, and expected degradation rate to sustain repair integrity during wound healing, which are reviewed in additional detail by Wang et al. [2] Also, improved understanding of the biology of musculoskeletal tissue development and maturation as well as the wound healing response is vital to ensure that such bone-tendon-muscle grafts or tissue engineered constructs can be integrated well with host tissue under relevant rotator cuff injury pathophysiological conditions [3]. These include employing appropriate biological cues that direct musculoskeletal tissue formation, maturation, and multi-tissue integration as well as those that govern wound healing [3]. In particular, the use of appropriate growth factors, ECM, and mechanical forces on orchestrating musculoskeletal differentiation as well as positive modulation of inflammation for musculoskeletal tissue healing, integration of clinical grafts and devices, and vascularization can be gleaned from developmental and wound healing studies, which are reviewed in additional detail by Xu et al. [3] Thus, engineering innovations and biological advances are both needed to play crucial roles in the development and application of bone-tendon-muscle multi-tissue units for rotator cuff repair.
In summary, rotator cuff tears are not simply confined to the location of the injury site. Degenerative changes to associated tissues such as bone, tendon, and muscle disrupt the structure and function of the shoulder, exacerbating the clinical condition. Although various non-surgical management methods, surgical techniques, and commercially available biomaterials exist, tissue engineering and regenerative medicine approaches that focus on engineering a bone-tendon-muscle unit to replenish injured tissue, facilitate secure tissue attachment, and restore normal shoulder kinematics (e.g., biomechanical strength, tension, degree of shoulder rotation) may offer a way to address unmet clinical challenges in rotator cuff tears. Fabricating such constructs will require drawing inspiration from domain knowledge in musculoskeletal development and integration, maturation, and wound healing to specify multiple cell fates within a single multi-tissue unit.
6. Conclusion
Rotator cuff tears are highly prevalent and can be detrimental to laborers and athletes, posing great socioeconomic burden to numerous countries. Detailed anatomical examination shows a highly integrated structure–function relationship in which the rotator cuff exists as a unified complex of four bone-tendon-muscle units. Indeed, rotator cuff injury can cause bone loss, tendon retraction and fibrosis, as well as muscle fatty degeneration through loss of musculoskeletal tension. Current clinical state-of-the-art includes non-surgical management such as physical rehabilitation and surgical management such as anatomical (Open repair, mini-open repair, and arthroscopic repair) and non-anatomical (RTSA, tendon transfer, and ASCR), which involve use of natural or synthetic tendon grafts and bone fixation devices. Experimental trials involving use of stem cells and/or growth factors have been conducted but various regulatory hurdles must be addressed along with multi-disciplinary integration of biological, engineering, and clinical knowledge to develop advanced biological and tissue engineering strategies to treat bone, tendon, and muscle pathologies associated with rotator cuff tears. Continued communication and collaboration among life scientists, engineers, and clinicians will facilitate the rational formulation and implementation of functional tissue engineering approaches for multi-tissue repair and regeneration.
Authorship
Category 1
Conception and design of study: X. Zhang, D. Wang, D.F.E. Ker.
Category 2
Drafting the manuscript: X. Zhang, D. Wang.
revising the manuscript critically for important intellectual content: Z. Wang, S.K.K. Ling, P.S.H. Yung, R.S. Tuan, D.F.E. Ker.
Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed): X. Zhang, D. Wang Z. Wang, S.K.K. Ling, P.S.H. Yung, R.S. Tuan, D.F.E. Ker.
X. Zhang: Writing - Original Draft; D. Wang: Writing - Original Draft; Z. Wang: Writing - Review & Editing; S.K.K. Ling: Writing - Review & Editing; P.S.H. Yung: Writing - Review & Editing; R.S. Tuan: Writing - Review & Editing; D.F.E. Ker: Supervision, Writing - Review & Editing.
Declaration of competing interest
The authors have no conflict of interest relevant to this review.
Acknowledgements
We would like to thank Shuting Huang for preparing Fig. 1, Fig. 2 of this manuscript. Dai Fei Elmer Ker's research is supported by funding from the Food and Health Bureau, Hong Kong SAR (Health Medical and Research Fund: 08190466), Research Grants Council of Hong Kong, Hong Kong SAR (Early Career Scheme Award: 24201720), The Chinese University of Hong Kong (Faculty Innovation Award: FIA2018/A/01), and the Innovation and Technology Commission, Hong Kong SAR (Tier 3 Award: ITS/090/18; Health@InnoHK program). Dan Wang's research is supported by funding from the Food and Health Bureau, Hong Kong SAR (Health Medical and Research Fund, 07180686), Research Grants Council of Hong Kong, Hong Kong SAR (General Research Fund: 14118620 and 14121121), and the Innovation and Technology Commission, Hong Kong SAR (Health@InnoHK program). Patrick Shu-hang Yung is supported by the Innovation and Technology Commission, Hong Kong SAR (Health@InnoHK program). Rocky S. Tuan is supported by the Lee Quo Wei and Lee Yick Hoi Lun Professorship in Tissue Engineering and Regenerative Medicine and the Innovation and Technology Commission, Hong Kong SAR (Health@InnoHK program).
References
- 1.Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Zhang X., Huang S., Liu Y., Fu B.S., Mak K.K., et al. Engineering multi-tissue units for regenerative medicine: bone-tendon-muscle units of the rotator cuff. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120789. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Wang D., Mak K.-L.K., Tuan R.S., Ker D.F.E. Engineering musculoskeletal grafts for multi-tissue unit repair: lessons from developmental biology and wound healing. Front Physiol. 2021;12(1287) doi: 10.3389/fphys.2021.691954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkousy H., Edwards T.B., Gartsman G.M. 3rd ed. Elsevier; Philadelphia, PA: 2019. Gartsman’s shoulder arthroscopy; pp. 1–368. [Google Scholar]
- 5.Aagaard K.E., Randeblad P., Abu-Zidan F.M., Lunsjo K. Return to work after early repair of acute traumatic rotator cuff tears. Eur J Trauma Emerg Surg. 2020;46(4):817–823. doi: 10.1007/s00068-019-01074-9. [DOI] [PubMed] [Google Scholar]
- 6.Bravi M., Fossati C., Giombini A., Macaluso A., Lazzoli J.K., Santacaterina F., et al. Criteria for return-to-play (RTP) after rotator cuff surgery: a systematic review of literature. J Clin Med. 2022;11(8):2244. doi: 10.3390/jcm11082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi K., Ditsios K., Middleton W.D., Hildebolt C.F., Galatz L.M., Teefey S.A. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88(8):1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H., et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19(1):116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Kelvin W.M.Y. Office of the Government Economist – Economic Letter; 2019/02. Population ageing trend of Hong Kong. [Google Scholar]
- 10.Longo U.G., Salvatore G., Rizzello G., Berton A., Ciuffreda M., Candela V., et al. The burden of rotator cuff surgery in Italy: a nationwide registry study. Arch Orthop Trauma Surg. 2017;137(2):217–224. doi: 10.1007/s00402-016-2610-x. [DOI] [PubMed] [Google Scholar]
- 11.Paloneva J., Lepola V., Aarimaa V., Joukainen A., Ylinen J., Mattila V.M. Increasing incidence of rotator cuff repairs--A nationwide registry study in Finland. BMC Muscoskel Disord. 2015;16:189. doi: 10.1186/s12891-015-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mather I.I.I.R.C., Koenig L., Acevedo D., Dall T.M., Gallo P., Romeo A., et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993–2000. doi: 10.2106/JBJS.L.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longo U.G., Carnevale A., Piergentili I., Berton A., Candela V., Schena E., et al. Retear rates after rotator cuff surgery: a systematic review and meta-analysis. BMC Muscoskel Disord. 2021;22(1):749. doi: 10.1186/s12891-021-04634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galatz L.M., Ball C.M., Teefey S.A., Middleton W.D., Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.J., Kim S.H., Lee S.K., Seo J.W., Chun Y.M. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2013;95(16):1482–1488. doi: 10.2106/JBJS.L.01193. [DOI] [PubMed] [Google Scholar]
- 16.Bakhsh W., Nicandri G. Anatomy and physical examination of the shoulder. Sports Med Arthrosc. 2018;26(3):E10–E22. doi: 10.1097/JSA.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 17.Culham E., Peat M. Functional anatomy of the shoulder complex. J Orthop Sports Phys Ther. 1993;18(1):342–350. doi: 10.2519/jospt.1993.18.1.342. [DOI] [PubMed] [Google Scholar]
- 18.Terry G.C., Chopp T.M. Functional anatomy of the shoulder. J Athl Train. 2000;35(3):248–255. [PMC free article] [PubMed] [Google Scholar]
- 19.Ruotolo C., Fow J.E., Nottage W.M. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246–249. doi: 10.1016/j.arthro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Clark J.M., Harryman D.T. 2nd. Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J Bone Joint Surg Am. 1992;74(5):713–725. [PubMed] [Google Scholar]
- 21.Lee C.J., Yeh M.L., Chang C.H., Chiang F.L., Hong C.K., Su W.R. The relationship of the anterior articular capsule to the adjacent subscapularis: an anatomic and histological study. Orthop Traumatol Surg Res. 2017;103(8):1265–1269. doi: 10.1016/j.otsr.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Nimura A., Kato A., Yamaguchi K., Mochizuki T., Okawa A., Sugaya H., et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867–872. doi: 10.1016/j.jse.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Trotter J.A. Structure-function considerations of muscle-tendon junctions. Comp Biochem Physiol Mol Integr Physiol. 2002;133(4):1127–1133. doi: 10.1016/s1095-6433(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 24.Dutton M. 4th ed. McGraw-Hill Education; 2016. Orthopaedic examination, evaluation, and intervention; pp. 1–1680. [Google Scholar]
- 25.Norkin C., White J. 5th ed. F. A. Davis Company; 2017. Measurements of joint motion: a guide to goniometry; pp. 1–592. [Google Scholar]
- 26.McCluskey G.M., Getz B.A. Pathophysiology of anterior shoulder instability. J Athl Train. 2000;35(3):268–272. [PMC free article] [PubMed] [Google Scholar]
- 27.Williams M.D., Edwards T.B., Walch G. Understanding the importance of the teres minor for shoulder function:Functional anatomy and pathology. J Am Acad Orthop Surg. 2018;26(5):150–161. doi: 10.5435/JAAOS-D-15-00258. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Fu S.C., Leong H.T., Ling S.K., Oh J.H., Yung P.S. Evaluation of animal models and methods for assessing shoulder function after rotator cuff tear: a systematic review. J Orthop Translat. 2021;26:31–38. doi: 10.1016/j.jot.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffat K.L., Sun W.H., Pena P.E., Chahine N.O., Doty S.B., Ateshian G.A., et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105(23):7947–7952. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothrauff B.B., Pauyo T., Debski R.E., Rodosky M.W., Tuan R.S., Musahl V. The rotator cuff organ: integrating developmental biology, tissue engineering, and surgical considerations to treat chronic massive rotator cuff tears. Tissue Eng B Rev. 2017;23(4):318–335. doi: 10.1089/ten.teb.2016.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longo U.G., Franceschi F., Ruzzini L., Rabitti C., Morini S., Maffulli N., et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36(3):533–538. doi: 10.1177/0363546507308549. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum A.J., Wicker J.F., Dines J.S., Bonasser L., Razzano P., Dines D.M., et al. Histologic stages of healing correlate with restoration of tensile strength in a model of experimental tendon repair. HSS J. 2010;6(2):164–170. doi: 10.1007/s11420-009-9152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H.H., Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodeo S.A., Arnoczky S.P., Torzilli P.A., Hidaka C., Warren R.F. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Thomopoulos S., Williams G.R., Soslowsky L.J. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125(1):106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 36.Kurdziel M.D., Davidson A., Ross D., Seta J., Doshi S., Baker K.C., et al. Biomechanical properties of the repaired and non-repaired rat supraspinatus tendon in the acute postoperative period. Connect Tissue Res. 2019;60(3):254–264. doi: 10.1080/03008207.2018.1488970. [DOI] [PubMed] [Google Scholar]
- 37.Thomopoulos S., Williams G.R., Gimbel J.A., Favata M., Soslowsky L.J. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21(3):413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto T., Nobuhara K., Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;415:111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 39.Sakabe T., Sakai T. Musculoskeletal diseases-tendon. Br Med Bull. 2011;99(1):211–225. doi: 10.1093/bmb/ldr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer G.A., Ward S.R. Developmental biology and regenerative medicine: addressing the vexing problem of persistent muscle atrophy in the chronically torn human rotator cuff. Phys Ther. 2016;96(5):722–733. doi: 10.2522/ptj.20150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton E.R., Gimbel J.A., Williams G.R., Soslowsky L.J. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23(2):259–265. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Gerber C., Meyer D.C., Schneeberger A.G., Hoppeler H., von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A(9):1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons M.C., Sato E.J., Bachasson D., Cheng T., Azimi H., Schenk S., et al. Muscle architectural changes after massive human rotator cuff tear. J Orthop Res. 2016;34(12):2089–2095. doi: 10.1002/jor.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi I., Enokida M., Nagira K., Yamasita T., Tsukutani Y., Murakami T., et al. Change in the pennation angle of the supraspinatus muscle after rotator cuff tear repair. J Shoulder Elbow Surg. 2019;28(5):888–892. doi: 10.1016/j.jse.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Meyer D.C., Pirkl C., Pfirrmann C.W., Zanetti M., Gerber C. Asymmetric atrophy of the supraspinatus muscle following tendon tear. J Orthop Res. 2005;23(2):254–258. doi: 10.1016/j.orthres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Safran O., Derwin K.A., Powell K., Iannotti J.P. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005;87a(12):2662–2670. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 47.Ward S.R., Sarver J.J., Eng C.M., Kwan A., Wurgler-Hauri C.C., Perry S.M., et al. Plasticity of muscle architecture after supraspinatus tears. J Orthop Sport Phys. 2010;40(11):729–735. doi: 10.2519/jospt.2010.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo J., Sano H., Itoi E. Changes in pennation angle in rotator cuff muscles with torn tendons. J Orthop Sci. 2012;17(1):58–63. doi: 10.1007/s00776-011-0176-6. [DOI] [PubMed] [Google Scholar]
- 49.Gladstone J.N., Bishop J.Y., Lo I.K., Flatow E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 50.Kuzel B.R., Grindel S., Papandrea R., Ziegler D. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg. 2013;21(10):613–623. doi: 10.5435/JAAOS-21-10-613. [DOI] [PubMed] [Google Scholar]
- 51.Silldorff M.D., Choo A.D., Choi A.J., Lin E., Carr J.A., Lieber R.L., et al. Effect of supraspinatus tendon injury on supraspinatus and infraspinatus muscle passive tension and associated biochemistry. J Bone Joint Surg Am. 2014;96(20):e175. doi: 10.2106/JBJS.M.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halder A.M., O'Driscoll S.W., Heers G., Mura N., Zobitz M.E., An K.N., et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84a(5):780–785. doi: 10.2106/00004623-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Davidson P.A., Rivenburgh D.W. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502–506. doi: 10.1067/mse.2000.109385. [DOI] [PubMed] [Google Scholar]
- 54.Hersche O., Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393–396. doi: 10.1016/s1058-2746(98)90030-1. [DOI] [PubMed] [Google Scholar]
- 55.Golding F.C. The shoulder—the forgotten joint. The Makenzie Davidson memorial lecture. Br J Radiol. 1962;35(411):149–158. doi: 10.1259/0007-1285-35-411-149. [DOI] [PubMed] [Google Scholar]
- 56.Terrier A., Reist A., Vogel A., Farron A. Effect of supraspinatus deficiency on humerus translation and glenohumeral contact force during abduction. Clin Biomech. 2007;22(6):645–651. doi: 10.1016/j.clinbiomech.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 57.Weiner D.S., Macnab I. Superior migration of the humeral head. A radiological aid in the diagnosis of tears of the rotator cuff. J Bone Joint Surg Br. 1970;52(3):524–527. [PubMed] [Google Scholar]
- 58.Gartsman G.M. 2nd edition. W.B. Saunders; Philadelphia: 2009. Shoulder arthroscopy; pp. 1–392. [Google Scholar]
- 59.Galatz L.M., Rothermich S.Y., Zaegel M., Silva M.J., Havlioglu N., Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23(6):1441–1447. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- 60.Cadet E.R., Hsu J.W., Levine W.N., Lu Bigliani, Ahmad C.S. The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. J Shoulder Elbow Surg. 2008;17(1):73–77. doi: 10.1016/j.jse.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Prabhakar A., Kanthalu Subramanian J.N., Swathikaa P., Kumareswaran S.I., Subramanian K.N. Current concepts on management of cuff tear. J Clin Orthop Trauma. 2022;28 doi: 10.1016/j.jcot.2022.101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narvani A.A., Imam M.A., Godeneche A., Calvo E., Corbett S., Wallace A.L., et al. Degenerative rotator cuff tear, repair or not repair? A review of current evidence. Ann R Coll Surg Engl. 2020;102(4):248–255. doi: 10.1308/rcsann.2019.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bush C., Gagnier J.J., Carpenter J., Bedi A., Miller B. Predictors of clinical outcomes after non-operative management of symptomatic full-thickness rotator cuff tears. World J Orthoped. 2021;12(4):223–233. doi: 10.5312/wjo.v12.i4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeanfavre M., Husted S., Leff G. Exercise therapy in the non-operative treatment of full-thickness rotator cuff tears: a systematic review. Int J Sports Phys Ther. 2018;13(3):335–378. [PMC free article] [PubMed] [Google Scholar]
- 65.Tashjian R.Z. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Henry P., Wasserstein D., Park S., Dwyer T., Chahal J., Slobogean G., et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472–2480. doi: 10.1016/j.arthro.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 67.Barco R., Savvidou O.D., Sperling J.W., Sanchez-Sotelo J., Cofield R.H. Complications in reverse shoulder arthroplasty. EFORT Open Rev. 2016;1(3):72–80. doi: 10.1302/2058-5241.1.160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farshad M., Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010;34(8):1075–1082. doi: 10.1007/s00264-010-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Familiari F., Rojas J., Nedim Doral M., Huri G., McFarland E.G. Reverse total shoulder arthroplasty. EFORT Open Rev. 2018;3(2):58–69. doi: 10.1302/2058-5241.3.170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elhassan B.T., Cox R.M., Shukla D.R., Lee J., Murthi A.M., Tashjian R.Z., et al. Management of failed rotator cuff repair in young patients. J Am Acad Orthop Surg. 2017;25(11):e261–e271. doi: 10.5435/JAAOS-D-17-00086. [DOI] [PubMed] [Google Scholar]
- 71.Gerber C., Maquieira G., Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am. 2006;88(1):113–120. doi: 10.2106/JBJS.E.00282. [DOI] [PubMed] [Google Scholar]
- 72.Aoki M., Okamura K., Fukushima S., Takahashi T., Ogino T. Transfer of latissimus dorsi for irreparable rotator-cuff tears. J Bone Joint Surg Br. 1996;78(5):761–766. [PubMed] [Google Scholar]
- 73.Miniaci A., MacLeod M. Transfer of the latissimus dorsi muscle after failed repair of a massive tear of the rotator cuff. A two to five-year review. J Bone Joint Surg Am. 1999;81(8):1120–1127. doi: 10.2106/00004623-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 74.Gerber C., Rahm S.A., Catanzaro S., Farshad M., Moor B.K. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95a(21):1920–1926. doi: 10.2106/JBJS.M.00122. [DOI] [PubMed] [Google Scholar]
- 75.Anastasopoulos P.P., Alexiadis G., Spyridonos S., Fandridis E. Latissimus dorsi transfer in posterior irreparable rotator cuff tears. Open Orthop J. 2017;11:77–94. doi: 10.2174/1874325001711010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Checchia C.S., Silva L.A.D., Sella G.D.V., Fregoneze M., Miyazaki A.N. Current options in tendon transfers for irreparable posterosuperior rotator cuff tears. Rev Bras Ortop (Sao Paulo) 2021;56(3):281–290. doi: 10.1055/s-0040-1709988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mihata T., McGarry M.H., Pirolo J.M., Kinoshita M., Lee T.Q. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 78.Mihata T., Lee T.Q., Watanabe C., Fukunishi K., Ohue M., Tsujimura T., et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 2013;29(3):459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 79.Mihata T., Lee T.Q., Hasegawa A., Fukunishi K., Kawakami T., Fujisawa Y., et al. Five-year follow-up of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. J Bone Joint Surg Am. 2019;101(21):1921–1930. doi: 10.2106/JBJS.19.00135. [DOI] [PubMed] [Google Scholar]
- 80.Lim S., AlRamadhan H., Kwak J.-M., Hong H., Jeon I.-H. Graft tears after arthroscopic superior capsule reconstruction (ASCR): pattern of failure and its correlation with clinical outcome. Arch Orthop Trauma Surg. 2019;139(2):231–239. doi: 10.1007/s00402-018-3025-7. [DOI] [PubMed] [Google Scholar]
- 81.Sharma N., El Refaiy A., Sibly T.F. Short-term results of rotator cuff repair using GraftJacket as an interpositional tissue-matched thickness graft. J Orthop. 2018;15(2):732–735. doi: 10.1016/j.jor.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denard P.J., Brady P.C., Adams C.R., Tokish J.M., Burkhart S.S. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93–99. doi: 10.1016/j.arthro.2017.08.265. [DOI] [PubMed] [Google Scholar]
- 83.Petri M., Greenspoon J.A., Millett P.J. Arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc Tec. 2015;4(6):E751–E755. doi: 10.1016/j.eats.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Denard P.J., Burkhart S.S. The evolution of suture anchors in arthroscopic rotator cuff repair. Arthroscopy. 2013;29(9):1589–1595. doi: 10.1016/j.arthro.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Aurora A., McCarron J., Iannotti J.P., Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16(5 Suppl):S171–S178. doi: 10.1016/j.jse.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Chen J., Xu J., Wang A., Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expet Rev Med Dev. 2009;6(1):61–73. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- 87.Chaudhury S., Holland C., Thompson M.S., Vollrath F., Carr A.J. Tensile and shear mechanical properties of rotator cuff repair patches. J Shoulder Elbow Surg. 2011;21(9):1168–1176. doi: 10.1016/j.jse.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 88.Rashid M.S., Smith R.D.J., Nagra N., Wheway K., Watkins B., Snelling S., et al. Rotator cuff repair with biological graft augmentation causes adverse tissue outcomes. Acta Orthop. 2020;91(6):782–788. doi: 10.1080/17453674.2020.1793613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Longo U.G., Lamberti A., Maffulli N., Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165–188. doi: 10.1093/bmb/ldp051. [DOI] [PubMed] [Google Scholar]
- 90.Bond J.L., Dopirak R.M., Higgins J., Burns J., Snyder S.J. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403–409 e1. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 91.Wong I., Burns J., Snyder S. Arthroscopic graftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 92.Hakimi O., Mouthuy P.A., Carr A. Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int J Exp Pathol. 2013;94(4):287–292. doi: 10.1111/iep.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciampi P., Scotti C., Nonis A., Vitali M., Di Serio C., Peretti G.M., et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169–1175. doi: 10.1177/0363546514525592. [DOI] [PubMed] [Google Scholar]
- 94.Silver M.D., Daigneault J.P. Symptomatic interarticular migration of glenoid suture anchors. Arthroscopy. 2000;16(1):102–105. doi: 10.1016/s0749-8063(00)90136-1. [DOI] [PubMed] [Google Scholar]
- 95.Kaar T.K., Schenck R.C., Wirth M.A., Rockwood C.A. Complications of metallic suture anchors in shoulder surgery: a report of 8 cases. Arthroscopy. 2001;17(1):31–37. doi: 10.1053/jars.2001.18246. [DOI] [PubMed] [Google Scholar]
- 96.Ozbaydar M., Elhassan B., Warner J.J.P. The use of anchors in shoulder surgery: a shift from metallic to bioabsorbable anchors. Arthroscopy. 2007;23(10):1124–1126. doi: 10.1016/j.arthro.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 97.Goeminne S., Debeer P. Delayed migration of a metal suture anchor into the glenohumeral joint. Acta Orthop Belg. 2010;76(6):834–837. [PubMed] [Google Scholar]
- 98.Barber F.A. Biodegradable shoulder anchors have unique modes of failure. Arthroscopy. 2007;23(3):316–320. doi: 10.1016/j.arthro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Hug K., Gerhardt C., Haneveld H., Scheibel M. Arthroscopic knotless-anchor rotator cuff repair: a clinical and radiological evaluation. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2628–2634. doi: 10.1007/s00167-014-3026-1. [DOI] [PubMed] [Google Scholar]
- 100.Barber F.A., Dockery W.D., Cowden C.H., 3rd The degradation outcome of biocomposite suture anchors made from poly L-lactide-co-glycolide and beta-tricalcium phosphate. Arthroscopy. 2013;29(11):1834–1839. doi: 10.1016/j.arthro.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Kim J.H., Kim Y.S., Park I., Lee H.J., Han S.Y., Jung S., et al. A comparison of open-construct PEEK suture anchor and non-vented biocomposite suture anchor in arthroscopic rotator cuff repair: a prospective randomized clinical trial. Arthroscopy. 2020;36(2):389–396. doi: 10.1016/j.arthro.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 102.Ntalos D., Sellenschloh K., Huber G., Briem D., Puschel K., Morlock M.M., et al. Conventional rotator cuff versus all-suture anchors-A biomechanical study focusing on the insertion angle in an unlimited cyclic model. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0225648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang G., Rothrauff B.B., Tuan R.S. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99(3):203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel S., Gualtieri A.P., Lu H.H., Levine W.N. Advances in biologic augmentation for rotator cuff repair. Ann N Y Acad Sci. 2016;1383(1):97–114. doi: 10.1111/nyas.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pelinkovic D., Lee J.Y., Engelhardt M., Rodosky M., Cummins J., Fu F.H., et al. Muscle cell-mediated gene delivery to the rotator cuff. Tissue Eng. 2003;9(1):143–151. doi: 10.1089/107632703762687627. [DOI] [PubMed] [Google Scholar]
- 107.Bi Y., Ehirchiou D., Kilts T.M., Inkson C.A., Embree M.C., Sonoyama W., et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 108.Chen P., Wang A., Haynes W., Landao-Bassonga E., Lee C., Ruan R., et al. A bio-inductive collagen scaffold that supports human primary tendon-derived cell growth for rotator cuff repair. J Orthop Translat. 2021;31:91–101. doi: 10.1016/j.jot.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Bari C., Dell'Accio F., Tylzanowski P., Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 110.De Bari C., Dell'Accio F., Luyten F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44(1):85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 111.Park G.Y., Kwon D.R., Lee S.C. Regeneration of full-thickness rotator cuff tendon tear after ultrasound-guided injection with umbilical cord blood-derived mesenchymal stem cells in a rabbit model. Stem Cells Transl Med. 2015;4(11):1344–1351. doi: 10.5966/sctm.2015-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwon D.R., Park G.Y., Lee S.C. Treatment of full-thickness rotator cuff tendon tear using umbilical cord blood-derived mesenchymal stem cells and polydeoxyribonucleotides in a rabbit model. Stem Cell Int. 2018;2018 doi: 10.1155/2018/7146384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martinello T., Bronzini I., Perazzi A., Testoni S., De Benedictis G.M., Negro A., et al. Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res. 2013;31(2):306–314. doi: 10.1002/jor.22205. [DOI] [PubMed] [Google Scholar]
- 114.Chen Y., Xu Y., Li M., Shi Q., Chen C. Application of autogenous urine-derived stem cell sheet enhances rotator cuff healing in a canine model. Am J Sports Med. 2020;48(14):3454–3466. doi: 10.1177/0363546520962774. [DOI] [PubMed] [Google Scholar]
- 115.Teng C., Zhou C., Xu D., Bi F. Combination of platelet-rich plasma and bone marrow mesenchymal stem cells enhances tendon-bone healing in a rabbit model of anterior cruciate ligament reconstruction. J Orthop Surg Res. 2016;11(1):96. doi: 10.1186/s13018-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thangarajah T., Sanghani-Kerai A., Henshaw F., Lambert S.M., Pendegrass C.J., Blunn G.W. Application of a demineralized cortical bone matrix and bone marrow-derived mesenchymal stem cells in a model of chronic rotator cuff degeneration. Am J Sports Med. 2018;46(1):98–108. doi: 10.1177/0363546517727512. [DOI] [PubMed] [Google Scholar]
- 117.Gulotta L.V., Kovacevic D., Montgomery S., Ehteshami J.R., Packer J.D., Rodeo S.A. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010;38(7):1429–1437. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]
- 118.Shi Y., Kang X., Wang Y., Bian X., He G., Zhou M., et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu Y., Zhang W.X., Wang L.N., Ming Y.Q., Li Y.L., Ni G.X. Stem cell therapies in tendon-bone healing. World J Stem Cell. 2021;13(7):753–775. doi: 10.4252/wjsc.v13.i7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jo C.H., Shin J.S., Park I.W., Kim H., Lee S.Y. Multiple channeling improves the structural integrity of rotator cuff repair. Am J Sports Med. 2013;41(11):2650–2657. doi: 10.1177/0363546513499138. [DOI] [PubMed] [Google Scholar]
- 121.Hernigou P., Flouzat Lachaniette C.H., Delambre J., Zilber S., Duffiet P., Chevallier N., et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 122.Kim Y.S., Sung C.H., Chung S.H., Kwak S.J., Koh Y.G. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018. doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
- 123.Morikawa D., Muench L.N., Baldino J.B., Kia C., Johnson J., Otto A., et al. Comparison of preparation techniques for isolating subacromial bursa-derived cells as a potential augment for rotator cuff repair. Arthroscopy. 2020;36(1):80–85. doi: 10.1016/j.arthro.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 124.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 125.Nimura A., Muneta T., Koga H., Mochizuki T., Suzuki K., Makino H., et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58(2):501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 126.Chen L., Li L., Xing F., Peng J., Peng K., Wang Y., et al. Human urine-derived stem cells: potential for cell-based therapy of cartilage defects. Stem Cell Int. 2018;2018 doi: 10.1155/2018/4686259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ji X., Wang M., Chen F., Zhou J. Urine-derived stem cells: the present and the future. Stem Cell Int. 2017;2017 doi: 10.1155/2017/4378947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bieback K., Kern S., Kluter H., Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cell. 2004;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 129.Wang M., Yang Y., Yang D., Luo F., Liang W., Guo S., et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126(2):220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cell. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 131.Moon S.W., Park S., Oh M., Wang J.H. Outcomes of human umbilical cord blood-derived mesenchymal stem cells in enhancing tendon-graft healing in anterior cruciate ligament reconstruction: an exploratory study. Knee Surg Relat Res. 2021;33(1):32. doi: 10.1186/s43019-021-00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bianco S.T., Moser H.L., Galatz L.M., Huang A.H. Biologics and stem cell-based therapies for rotator cuff repair. Ann N Y Acad Sci. 2019;1442(1):35–47. doi: 10.1111/nyas.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H., Chen Y., Chen S. Enhancement of rotator cuff tendon-bone healing using bone marrow-stimulating technique along with hyaluronic acid. J Orthop Translat. 2019;17:96–102. doi: 10.1016/j.jot.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCormack R.A., Shreve M., Strauss E.J. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis. 2014;72(1):89–96. [PubMed] [Google Scholar]
- 135.Prabhath A., Vernekar V.N., Sanchez E., Laurencin C.T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int J Pharm. 2018;544(2):358–371. doi: 10.1016/j.ijpharm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang C., Wu J., Li X., Wang Z., Lu W.W., Wong T.M. Current biological strategies to enhance surgical treatment for rotator cuff repair. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.657584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Greiner S., Ide J., Van Noort A., Mochizuki Y., Ochi H., Marraffino S., et al. Local rhBMP-12 on an absorbable collagen sponge as an adjuvant therapy for rotator cuff repair - a phase 1, randomized, standard of care control, multicenter study: safety and feasibility. Am J Sports Med. 2015;43(8):1994–2004. doi: 10.1177/0363546515584756. [DOI] [PubMed] [Google Scholar]
- 138.Ide J., Mochizuki Y., van Noort A., Ochi H., Sridharan S., Itoi E., et al. Local rhBMP-12 on an absorbable collagen sponge as an adjuvant therapy for rotator cuff repair-a phase 1, randomized, standard of care control, multicenter study: Part 2-a pilot study of functional recovery and structural outcomes. Orthop J Sports Med. 2017;5(9) doi: 10.1177/2325967117726740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoppe S., Alini M., Benneker L.M., Milz S., Boileau P., Zumstein M.A. Tenocytes of chronic rotator cuff tendon tears can be stimulated by platelet-released growth factors. J Shoulder Elbow Surg. 2013;22(3):340–349. doi: 10.1016/j.jse.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 140.Jo C.H., Kim J.E., Yoon K.S., Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035–1045. doi: 10.1177/0363546512437525. [DOI] [PubMed] [Google Scholar]
- 141.Dolkart O., Chechik O., Zarfati Y., Brosh T., Alhajajra F., Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271–1277. doi: 10.1007/s00402-014-2026-4. [DOI] [PubMed] [Google Scholar]
- 142.Charousset C., Zaoui A., Bellaiche L., Piterman M. Does autologous leukocyte-platelet-rich plasma improve tendon healing in arthroscopic repair of large or massive rotator cuff tears? Arthroscopy. 2014;30(4):428–435. doi: 10.1016/j.arthro.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 143.Rodeo S.A., Delos D., Williams R.J., Adler R.S., Pearle A., Warren R.F. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 144.Castricini R., Longo U.G., De Benedetto M., Panfoli N., Pirani P., Zini R., et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 145.Weber S.C., Kauffman J.I., Parise C., Weber S.J., Katz S.D. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263–270. doi: 10.1177/0363546512467621. [DOI] [PubMed] [Google Scholar]
- 146.Randelli P., Arrigoni P., Ragone V., Aliprandi A., Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518–528. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 147.Snow M., Hussain F., Pagkalos J., Kowalski T., Green M., Massoud S., et al. The effect of delayed injection of leukocyte-rich platelet-rich plasma following rotator cuff repair on patient function: a randomized double-blind controlled trial. Arthroscopy. 2020;36(3):648–657. doi: 10.1016/j.arthro.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 148.Malavolta E.A., Gracitelli M.E.C., Assuncao J.H., Ferreira Neto A.A., Bordalo-Rodrigues M., de Camargo O.P. Clinical and structural evaluations of rotator cuff repair with and without added platelet-rich plasma at 5-year follow-up: a prospective randomized study. Am J Sports Med. 2018;46(13):3134–3141. doi: 10.1177/0363546518795895. [DOI] [PubMed] [Google Scholar]
- 149.Jo C.H., Shin J.S., Lee Y.G., Shin W.H., Kim H., Lee S.Y., et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. Am J Sports Med. 2013;41(10):2240–2248. doi: 10.1177/0363546513497925. [DOI] [PubMed] [Google Scholar]
- 150.Jo C.H., Shin J.S., Shin W.H., Lee S.Y., Yoon K.S., Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102–2110. doi: 10.1177/0363546515587081. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Z., Wang Y., Sun J. The effect of platelet-rich plasma on arthroscopic double-row rotator cuff repair: a clinical study with 12-month follow-up. Acta Orthop Traumatol Turcica. 2016;50(2):191–197. doi: 10.3944/AOTT.2015.15.0113. [DOI] [PubMed] [Google Scholar]
- 152.Pandey V., Bandi A., Madi S., Agarwal L., Acharya K.K., Maddukuri S., et al. Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff tear? A randomized controlled trial. J Shoulder Elbow Surg. 2016;25(8):1312–1322. doi: 10.1016/j.jse.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 153.Carr A.J., Murphy R., Dakin S.G., Rombach I., Wheway K., Watkins B., et al. Platelet-rich plasma injection with arthroscopic acromioplasty for chronic rotator cuff tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43(12):2891–2897. doi: 10.1177/0363546515608485. [DOI] [PubMed] [Google Scholar]
- 154.Le A.D.K., Enweze L., DeBaun M.R., Dragoo J.L. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Collins T., Alexander D., Barkatali B. Platelet-rich plasma: a narrative review. EFORT Open Rev. 2021;6(4):225–235. doi: 10.1302/2058-5241.6.200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen L., Dong S.W., Liu J.P., Tao X., Tang K.L., Xu J.Z. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res. 2012;30(6):991–997. doi: 10.1002/jor.22033. [DOI] [PubMed] [Google Scholar]
- 157.Connor D.E., Paulus J.A., Dabestani P.J., Thankam F.K., Dilisio M.F., Gross R.M., et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metabol. 2019;37(5):759–767. doi: 10.1007/s00774-019-01013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Isola A.L., Chen S. Exosomes: the messengers of health and disease. Curr Neuropharmacol. 2017;15(1):157–165. doi: 10.2174/1570159X14666160825160421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 160.Basu J., Ludlow J.W. Exosomes for repair, regeneration and rejuvenation. Expet Opin Biol Ther. 2016;16(4):489–506. doi: 10.1517/14712598.2016.1131976. [DOI] [PubMed] [Google Scholar]
- 161.Burke J., Kolhe R., Hunter M., Isales C., Hamrick M., Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cell Int. 2016;2016 doi: 10.1155/2016/5802529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li J., Liu Z.P., Xu C., Guo A. TGF-beta1-containing exosomes derived from bone marrow mesenchymal stem cells promote proliferation, migration and fibrotic activity in rotator cuff tenocytes. Regen Ther. 2020;15:70–76. doi: 10.1016/j.reth.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ren Y., Zhang S., Wang Y., Jacobson D.S., Reisdorf R.L., Kuroiwa T., et al. Effects of purified exosome product on rotator cuff tendon-bone healing in vitro and in vivo. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang C., Song W., Chen B., Liu X., He Y. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am J Sports Med. 2019;47(13):3247–3255. doi: 10.1177/0363546519876323. [DOI] [PubMed] [Google Scholar]
- 165.Wang C., Hu Q., Song W., Yu W., He Y. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 2020;48(6):1456–1464. doi: 10.1177/0363546520908847. [DOI] [PubMed] [Google Scholar]
- 166.Berger I., Ahmad A., Bansal A., Kapoor T., Sipp D., Rasko J.E.J. Global distribution of businesses marketing stem cell-based interventions. Cell Stem Cell. 2016;19(2):158–162. doi: 10.1016/j.stem.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 167.Food Drug Administration H.H.S. Human cells, tissues, and cellular and tissue-based products; establishment registration and listing. Interim final rule; opportunity for public comment. Fed Regist. 2004;69(17):3823–3826. [PubMed] [Google Scholar]
- 168.European Medicines Agency . Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. OJEU; 2006. (L38/40) [Google Scholar]
- 169.Tanaka T., Lee S.M., Mikami M., Yokota K., Takakura K. Gaps between Asian regulations for eligibility of human mesenchymal stromal cells as starting materials of cell therapy products and comparability of mesenchymal stromal cell-based products subject to changes in their manufacturing process. Regen Ther. 2020;15:265–273. doi: 10.1016/j.reth.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Halme D.G., Kessler D.A. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355(16):1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 171.George B. Regulations and guidelines governing stem cell based products: clinical considerations. Perspect Clin Res. 2011;2(3):94–99. doi: 10.4103/2229-3485.83228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.U.S. Food and Drug Administration . 2020. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: Minimal manipulation and homologous use guidance for industry and food and drug administration staff. [Google Scholar]
- 173.Tian J., Song X., Wang Y., Cheng M., Lu S., Xu W., et al. Regulatory perspectives of combination products. Bioact Mater. 2022;10:492–503. doi: 10.1016/j.bioactmat.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Woods N., MacLoughlin R. Defining a regulatory strategy for ATMP/Aerosol delivery device combinations in the treatment of respiratory disease. Pharmaceutics. 2020;12(10) doi: 10.3390/pharmaceutics12100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smith M.J., Cook J.L., Kuroki K., Jayabalan P.S., Cook C.R., Pfeiffer F.M., et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169–177. doi: 10.1016/j.arthro.2011.08.296. [DOI] [PubMed] [Google Scholar]
- 176.Gigante A., Bottegoni C., Milano G., Riccio M., Dei Giudici L. Semitendinosus and gracilis free muscle-tendon graft for repair of massive rotator cuff tears: surgical technique. Joints. 2016;4(3):189–192. doi: 10.11138/jts/2016.4.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Werner B.C. Sclerostin antibody treatment enhances rotator cuff tendon-to-bone healing in an animal model. J Bone Joint Surg Am. 2017;99(10):e52. doi: 10.2106/JBJS.16.01019. Make no bones about it-rotator cuff repair healing is not just about the tendon: commentary on an article by Shivam A. Shah, PhD, et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shah S.A., Kormpakis I., Havlioglu N., Ominsky M.S., Galatz L.M., Thomopoulos S. Sclerostin antibody treatment enhances rotator cuff tendon-to-bone healing in an animal model. J Bone Joint Surg Am. 2017;99(10):855–864. doi: 10.2106/JBJS.16.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ker E.D., Chu B., Phillippi J.A., Gharaibeh B., Huard J., Weiss L.E., et al. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials. 2011;32(13):3413–3422. doi: 10.1016/j.biomaterials.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ker E.D., Nain A.S., Weiss L.E., Wang J., Suhan J., Amon C.H., et al. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32(32):8097–8107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 181.Ker D.F.E., Wang D., Behn A.W., Wang E.T.H., Zhang X., Zhou B.Y., et al. Functionally graded, bone- and tendon-like polyurethane for rotator cuff repair. Adv Funct Mater. 2018;28(20) doi: 10.1002/adfm.201707107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang D., Ker D.F.E., Ng K.W., Li K., Gharaibeh B., Safran M., et al. Combinatorial mechanical gradation and growth factor biopatterning strategy for spatially controlled bone-tendon-like cell differentiation and tissue formation. NPG Asia Mater. 2021;13(1):26. [Google Scholar]
- 183.You J.O., Rafat M., Almeda D., Maldonado N., Guo P., Nabzdyk C.S., et al. pH-responsive scaffolds generate a pro-healing response. Biomaterials. 2015;57:22–32. doi: 10.1016/j.biomaterials.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 184.Yokoya S., Nakamura Y., Harada Y., Ochi M., Adachi N. Outcomes of arthroscopic rotator cuff repair with muscle advancement for massive rotator cuff tears. J Shoulder Elbow Surg. 2019;28(3):445–452. doi: 10.1016/j.jse.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 185.Davies M.R., Lee L., Feeley B.T., Kim H.T., Liu X. Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J Orthop Res. 2017;35(7):1539–1547. doi: 10.1002/jor.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Davies M.R., Liu X., Lee L., Laron D., Ning A.Y., Kim H.T., et al. TGF-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mori D., Funakoshi N., Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911–1921. doi: 10.1016/j.arthro.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 188.Gaffney L.S., Davis Z.G., Mora-Navarro C., Fisher M.B., Freytes D.O. Extracellular matrix hydrogels promote expression of muscle-tendon junction proteins. Tissue Eng. 2022;28(5–6):270–282. doi: 10.1089/ten.TEA.2021.0070. [DOI] [PubMed] [Google Scholar]
- 189.Modi A., Haque A., Deore V., Singh H.P., Pandey R. Interposition GraftJacket allografts for irreparable rotator cuff tears. Bone Joint Lett J. 2022;104-b(1):91–96. doi: 10.1302/0301-620X.104B1.BJJ-2021-0826.R1. [DOI] [PubMed] [Google Scholar]
- 190.Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36–44. doi: 10.4103/0973-6042.96992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Beitzel K., Chowaniec D.M., McCarthy M.B., Cote M.P., Russell R.P., Obopilwe E., et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148–1154. doi: 10.1177/0363546512437835. [DOI] [PubMed] [Google Scholar]
- 192.Seldes R.M., Abramchayev I. Arthroscopic insertion of a biologic rotator cuff tissue augmentation after rotator cuff repair. Arthroscopy. 2006;22(1):113–116. doi: 10.1016/j.arthro.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 193.Coons D.A., Alan Barber F. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc Rev. 2006;14(3):185–190. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 194.Christian R.A., Stabile K.J., Gupta A.K., Leckey B.D., Jr., Cardona D.M., Nowinski R.J., et al. Histologic analysis of porcine dermal graft augmentation in treatment of rotator cuff tears. Am J Sports Med. 2021;49(13):3680–3686. doi: 10.1177/03635465211049434. [DOI] [PubMed] [Google Scholar]
- 195.Karuppaiah K., Sinha J. Scaffolds in the management of massive rotator cuff tears: current concepts and literature review. EFORT Open Rev. 2019;4(9):557–566. doi: 10.1302/2058-5241.4.180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Smith R.D., Carr A., Dakin S.G., Snelling S.J., Yapp C., Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107–118. doi: 10.22203/ecm.v031a08. [DOI] [PubMed] [Google Scholar]
- 197.Walton J.R., Bowman N.K., Khatib Y., Linklater J., Murrell G.A. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786–791. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- 198.Valentin J.E., Badylak J.S., McCabe G.P., Badylak S.F. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 199.Tornero-Esteban P., Hoyas J.A., Villafuertes E., Rodríguez-Bobada C., López-Gordillo Y., Rojo F.J., et al. Efficacy of supraspinatus tendon repair using mesenchymal stem cells along with a collagen I scaffold. J Orthop Surg Res. 2015;10:124. doi: 10.1186/s13018-015-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tanaka N., Sakahashi H., Hirose K., Ishima T., Ishii S. Augmented subscapularis muscle transposition for rotator cuff repair during shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2006;15(1):2–6. doi: 10.1016/j.jse.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 201.Audenaert E., Van Nuffel J., Schepens A., Verhelst M., Verdonk R. Reconstruction of massive rotator cuff lesions with a synthetic interposition graft: a prospective study of 41 patients. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):360–364. doi: 10.1007/s00167-005-0689-7. [DOI] [PubMed] [Google Scholar]
- 202.Hirooka A., Yoneda M., Wakaitani S., Isaka Y., Hayashida K., Fukushima S., et al. Augmentation with a Gore-Tex patch for repair of large rotator cuff tears that cannot be sutured. J Orthop Sci. 2002;7(4):451–456. doi: 10.1007/s007760200078. [DOI] [PubMed] [Google Scholar]
- 203.Gortzak Y., Mahendra A., Griffin A.M., Wunder J.S., Ferguson P.C. Synthetic mesh repair of the shoulder joint after surgical resection around the shoulder joint. Orthop Proc. 2010;92-B:64. (SUPP_I) [Google Scholar]
- 204.Proctor C.S. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508–1513. doi: 10.1016/j.jse.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 205.Zhaeentan S., Hagert E. Artelon® tissue reinforcement in the repair of a ruptured, degenerative rotator cuff in an elderly man. Shoulder Elbow. 2011;3(3):171–174. [Google Scholar]
- 206.Taniguchi N., Suenaga N., Oizumi N., Miyoshi N., Yamaguchi H., Inoue K., et al. Bone marrow stimulation at the footprint of arthroscopic surface-holding repair advances cuff repair integrity. J Shoulder Elbow Surg. 2015;24(6):860–866. doi: 10.1016/j.jse.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 207.Flury M., Rickenbacher D., Schwyzer H.K., Jung C., Schneider M.M., Stahnke K., et al. Does pure platelet-rich plasma affect postoperative clinical outcomes after arthroscopic rotator cuff repair? A randomized controlled trial. Am J Sports Med. 2016;44(8):2136–2146. doi: 10.1177/0363546516645518. [DOI] [PubMed] [Google Scholar]
- 208.Muench L.N., Kia C., Berthold D.P., Uyeki C., Otto A., Cote M.P., et al. Preliminary clinical outcomes following biologic augmentation of arthroscopic rotator cuff repair using subacromial bursa, concentrated bone marrow aspirate, and platelet-rich plasma. Arthrosc Sports Med Rehabil. 2020;2(6):e803–e813. doi: 10.1016/j.asmr.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]