Abstract
Sensory prediction (SP) is at the core of early cognitive development. Impaired SP may be a key to understanding the emergence of neurodevelopmental disorders, however there is little data on how and when this skill emerges. We set out to provide evidence of SP in the brain of premature neonates in the fundamental sensory modality: touch. Using Diffuse Correlation Spectroscopy, we measured blood flow changes in the somatosensory cortex of premature neonates presented with a vibrotactile stimulation-omission sequence. When ISI was fixed, participants presented a decrease in blood flow during stimulus omissions, starting when a stimulus should begin: the expectation of a certain stimulus onset induced deactivation of the somatosensory cortex. When ISI was jittered, we observed an increase in blood flow during omissions: the expectation of a likely but not certain stimulus onset induced activation of the somatosensory cortex. Our results reveal SP in the brain as early as four weeks before term, based on the temporal structure of a unimodal somatosensory stimulation, and show that SP produces opposite regulation of activity in the somatosensory cortex depending on how liable is stimulus onset. Future studies will investigate the predictive value of somatosensory prediction on neurodevelopment in this vulnerable population.
Abbreviations: BFi, blood flow index; cGA, corrected gestational age; DCS, diffuse correlation spectroscopy; fNIRS, functional near-infrared spectroscopy; GA, gestational age; ISI, interstimulus interval; ND, neurodevelopmental disorders; NICU, neonatal intensive care unit; RS, repetition suppression; SP, sensory prediction
Keywords: Preterm neonates, Sensory prediction, Repetition suppression, Early cognitive development, Somatosensory cortex, Tactile processing, Diffuse correlation spectroscopy, Cerebral blood flow
1. Introduction
To interact with a constantly changing environment, our brain needs to process the multiple regularities distributed in space and time which provide the ability to anticipate a stimulus based on previous experience (Bubic et al., 2010). Regularities would be used by the brain to form internal models of the outside world based on previous sensory inputs (Knill and Pouget, 2004). An internal model of the repeated stimulus would be built by the neuronal network processing a sequence, that encodes all the stimulation parameters and particularly the temporal structure of the sequence, forming a representation of the most likely input to come (Sokolov, 1963). On this basis, a currently prominent theoretical view considering the brain as intrinsically predictive, known as the Predictive Coding Theory (Friston, 2005), proposes that sensory cortices compare inputs to predictions and feed prediction errors to higher-order cortices, allowing them to improve subsequent predictions by updating internal models. Therefore, when sensory input is expected, a neuronal network is activated that is very similar to the network activated by the real stimulus, like for imagination or recall (Albright, 2012, Schubotz, 2015). This view is supported by reports of cortical responses associated with stimulus omissions in adults using auditory evoked potentials (SanMiguel et al., 2013) and somatosensory BOLD functional MRI (Chen et al., 2010) for example.
Prediction-based processes are considered a core feature of cognitive development (Baek et al., 2020). The ability to process time intervals and expect events on time is a prerequisite of attention development, and deficits in time processing are associated with attention deficits (Colombo and Richman, 2002). Predictive skills also have an adaptive role because they allow us to optimize our cognitive and behavioral resources (Schwartze and Kotz, 2013), which is critical for newborns for whom such resources are scant. Most studies of sensory prediction (SP) in young children were conducted in visual and auditory modalities. Colombo and Richman (2002) showed that 4-month-old infants learn the regular interstimulus interval (ISI) of a visual sequence and present a heart rate acceleration when a stimulus should recur but is omitted. Otte et al. (2013) showed that 2-months old infants learn the regular ISI of an auditory sequence, and ISI violation induces mismatch negativity on EEG. Similarly, newborns detect ISI lengthening, which they process as an omission (Háden et al., 2012), and downbeat omission in a metric structure (Winkler et al., 2009).
Studies also showed intersensory prediction in infants, still relying on vision and audition. Using a cross-modal cueing paradigm and evoked potentials, Kouider et al. (2015) showed that auditory afferents can act as predictive signals of the onset of a visual event in 12-month-old infants. Such intermodal SP abilities are available from 6 months of age: using functional near-infrared spectroscopy (fNIRS), Emberson et al. (2015) showed that after exposure to paired auditory-visual stimuli, when images were unexpectedly omitted after the sound infants showed a hemodynamic activation in the occipital cortex as if an image were presented.
We are only beginning to unravel the many parameters influencing SP during development. Using a visual paradigm in 12-month-old infants, Téglás and Bonatti (2016) investigated the relationship between expectation and surprise, depending on the probabilistic versus deterministic nature of the sequence. They found that probabilistic stimuli induce a pro-active response linked to anticipation, whereas deterministic stimuli induce post hoc processing linked to a surprise effect. The higher sensitivity of infants to moderately predictable visual sequences was also described in 7- and 8-month-old infants (Kidd et al., 2012) and in term neonates (Bulf et al., 2011). Authors argued that this tuning towards moderate rather than high predictability is better suited to the demands of real-life environments.
Stimulus processing can be improved or on the contrary attenuated by top-down mechanisms according to their adaptive relevance, which will constrain how much attention is dedicated to the task (Summerfield et al., 2008). Through learning, stimulus processing becomes more efficient with less attentional resources, which may then be reallocated, allowing the child to tackle increasingly complex tasks. This manifests in repetition suppression (RS), the decrease in neuronal response to a repeated innocuous stimulus (Grill-Spector et al., 2006). For example, such top-down regulation leading to RS when a stimulus was expected was shown in 6-month-old infants using fNIRS with auditory stimuli (Emberson et al., 2019).
Given its importance for cognitive development, SP may be a key to understanding early atypical trajectories of neurodevelopment. Authors proposed for example that impaired prediction abilities may be at the core of autism spectrum disorder (ASD), explaining repetitive behaviors, atypical sensory profiles, and social impairment (Sinha et al., 2014; Van de Cruys et al., 2014). Using their bimodal auditory-visual paradigm and fNIRS, Emberson et al. (2017) showed that 6-months old infants who were born preterm showed a reduced occipital response to predicted visual stimuli, suggesting that prediction impairment may be an early marker of high risk of neurodevelopmental disorders (ND). Indeed, premature neonates have a 3- to 4-fold increased risk for neurodevelopmental and psychiatric disorders in childhood (Johnson and Marlow, 2011). However, there is currently no data on SP in premature neonates. To use SP as a neonatal marker of neurodevelopment, we propose a new paradigm based on the predominant sensory modality at this developmental period: somesthesis.
Touch is the first sensory modality to develop (Bremner and Spence, 2017) and is considered the foundation upon which other perceptive, cognitive, and affective functions develop (Ardiel and Rankin, 2010). It is believed to play a critical role in attachment (Weiss et al., 2000), early sensory-motor development (Fearon et al., 2002) and to be the precursor of verbal communication (Hertenstein et al., 2006). Studies show that ND such as attention deficit disorder with or without hyperactivity (ADHD), ASD, and learning disorders, are frequently associated with atypical sensory processing, particularly tactile (Bröring et al., 2017, Cascio, 2010). In very preterm neonates, lower tolerance of handling at birth was associated with tactile sensitivity and poorer executive functioning at 4 years (Meether et al., 2021).
It is still unclear whether ND are mediated by early atypical sensory processing, or if atypical sensory profiles and ND are independent outcomes of premature birth, but because atypical tactile processing and ND frequently co-occur even in term-born children, they may be aspects of the same pathological process (Bröring et al., 2017). In a previous study, we showed that premature neonates who were born at an earlier gestational age (GA), with a smaller birth weight, and experienced more painful care procedures, required more repetitions of a tactile stimulus to habituate when tested at 35 weeks of corrected gestational age (cGA), that is two weeks before term-equivalent (Dumont et al., 2017).
In the present study, we aimed to provide evidence of somatosensory prediction in premature neonates at 33 weeks cGA, that is four weeks before term. As we did not know if participants so young would be more sensitive to deterministic or probabilistic stimuli, we designed two stimulation sequences, one with fixed ISI, the other with jittered ISI, and participants were randomly assigned to one of the two conditions. We used omissions to assess the activity of the somatosensory cortex when a stimulus was expected but not presented. We measured brain activity using Diffuse Correlation Spectroscopy (DCS). We hypothesized that premature neonates would display a change in blood flow during omissions, indicating they could anticipate somatosensory inputs in a sequence. Because ISI lengthening caused by the omission could act as a dishabituator, we also hypothesized that the response to stimuli following an omission would be larger compared with the average response to all stimuli.
2. Material and methods
2.1. Participants
We included preterm neonates born between 31 weeks + 0 day and 32 weeks + 6 days GA. This population is the youngest we could include that would meet inclusion criteria warranting a healthy brain state and function, necessary to observe sensory prediction, and a stable cardiorespiratory state compatible with safe handling and measurement. In particular, we excluded neonates with invasive respiratory assistance (i.e., ventilation on an intubation probe), neurological disease (intraventricular hemorrhage grade 3 or 4, periventricular leukomalacia, or any other brain structure alteration assessed by transfontanellar ultrasound), viral infection (respiratory syncytial virus, rotavirus), patent bacterial infection (C-reactive protein concentration >20 mg/L), or ongoing sedation (Fentanyl, Midazolam, Ketamine) during 48 h preceding the day of the measurement. All participants had an APGAR score between 7 and 10 at 5 min.
Parental informed consent was obtained in accordance with the Declaration of Helsinki. The study was approved by the ethics committee CPP Nord-Ouest III, France. Promotion and quality control were carried out by the University Hospital of Caen, France. The protocol was pre-registered before patient inclusions with the Agence nationale de sécurité du médicament et des produits de santé (ANSM, France) (ID-RCB: 2014-A01762–45), and at the US National Institute of Health (NIH) registry of clinical trials (NCT02880696).
The initial sample was composed of 40 participants randomly assigned to two groups: Fixed ISI or Jittered ISI. Sex, GA and weight at birth, and presence of intrauterine growth retardation (IUGR) were retrieved from medical records. Chronological age, cGA, and weight at measurement were recorded at measurement.
2.2. Experimental procedure
Measurements were performed at the infants’ bedside in their own room, during the first sleep period following a feeding. In premature neonates, the first sleep cycle allows sensory and cognitive processing (Fifer et al., 2010).
2.2.1. Stimuli
A coin vibrator (VPM2 at 200 Hz, dimensions: ø12 ×3.4 mm, Solarbotics Ltd., Calgary, Alberta, Canada) in a 3-D printed capsule was placed on the palm of the hand, and secured using a tubular elastic net bandage. It was connected by USB to a laptop computer outside of the incubator or crib and controlled using an in-house Java® program. At 33 weeks cGA, a drip is often present on one of the upper limbs. Therefore, the vibrator was always placed on the palm of the hand that was not mobilized by a drip, or not sensitized by a bruise caused by its withdrawal.
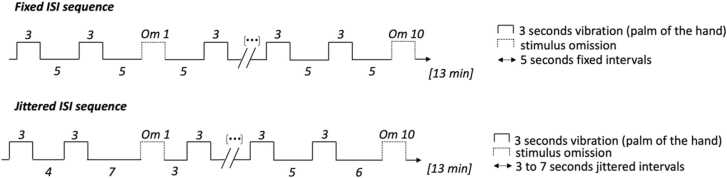
We used a unimodal stimulus omission paradigm to assess the ability of premature newborns to form a sensory prediction based on a tactile sequence. For participants in group Fixed, a fixed-ISI vibrotactile stimulation sequence was presented, consisting of 3 s long vibrations (Stimuli trials, N = 84), interspersed with 5 s long intervals. This made the stimulation onset deterministic i.e. after familiarization the subject should know when the next stimulus will start. Every 7–12 vibrations (pseudo-randomized but identical for all participants), one stimulus was omitted (Omission trials) to observe brain activity when a stimulus is expected. A total of 10 omissions were presented among the 84 stimuli. The whole sequence was 13 min long. For participants in group Jittered, ISI varied randomly between 3 and 7 s (integers only) during the sequence (identical for all participants). This made the stimulation onset probabilistic i.e. after familiarization the subject should expect the next stimulus but cannot be sure when it will recur (Fig. 1). The place of omissions in the sequence was identical to group Fixed, as well as the duration and number of stimuli. Intervals in the Jittered sequence and the place of omissions were determined by a random number generator except for the first omission that was manually set after the 12th stimulus to allow for initial familiarization.
Fig. 1.
Schema of the unimodal stimulus omission paradigm by condition. The stimulation sequence was composed of 84 vibrotactile stimuli (3 s long) interspersed with 10 pseudo-random omissions. In the Fixed group, the interstimulus interval (ISI) was 5 s, in the Jittered group it varied pseudo-randomly between 3 and 7 s (integers only).
2.2.2. Neurovascular activity measurement
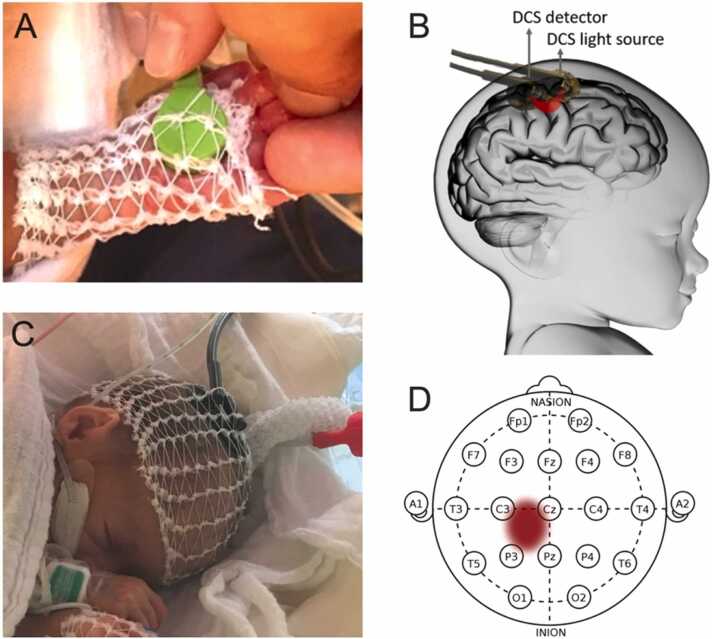
Neurovascular activity in the contralateral somatosensory cortex during the tactile sequence was measured using Diffuse Correlation Spectroscopy (DCS), a non-invasive optical imaging technique, silent and portable. DCS quantifies blood flow changes by measuring temporal fluctuations of light emerging from the tissues: the near-infrared light emitted through the scalp and skull propagates into the brain where it is scattered by moving red blood cells in tissue vasculature. This scattering from moving cells causes the detected intensity to temporally fluctuate, and the time scale of these fluctuations is quantified by the intensity temporal autocorrelation function of the collected light. The correlation diffusion equation is employed to fit the measured autocorrelation function to physical models and extract a cerebral blood flow index (BFi), in arbitrary units (for a review of DCS see Durduran & Yodh, 2013, for an example of functional DCS in preterm neonates see Roche-Labarbe et al., 2014). DCS offers a better temporal resolution than traditional fNIRS because blood flow increases immediately with neuronal activity (Hoge et al., 1999, Hoge et al., 2005). This was confirmed in preterm neonates (Roche-Labarbe et al., 2014). Blood flow is a more direct and less ambiguous proxy of neuronal activity than hemoglobin concentration. We used a commercial device, αιμα-FloMo (Hemophotonics S.L., Barcelona, Spain, http://hemophotonics.com) instrument with one light source at 785 nm (intensity < 30 mW) and four single-photon counting avalanche diodes for detection, an internal hardware correlator, and input ports for a posteriori synchronization with markers from the stimulation computer. Stimulation markers were placed in DCS data files by the stimulation device at each stimulus onset. DCS intensity auto-correlation curves were acquired at 1 Hz. The light was emitted and detected on the scalp by optical fibers bent 90° at the tips, embedded in a soft sensor made of black PVC (all four detectors on the same spot, emitter-detector distance = 1.5 cm). This probe was placed over the somatosensory cortex contralateral to the stimulated hand and secured using a tubular elastic net bandage (Fig. 2). All lights were off and shades were closed during the measurement, alarms of the syringe pump were silenced, and movements in the room were reduced to a minimum. Parents, when present, were asked not to talk or touch their child during acquisition.
Fig. 2.
Stimulation and measurement material. A. Placement of the stimulation vibrator in the palm of a participant. B. Placement of the DCS probe over the primary somatosensory cortex. C. Placement of the DCS probe on a participant. D. Region of interest in the 10–20 system.
2.3. Data processing
The auto-correlation curves that were saved from each detector were first evaluated for data quality based on the initial intercept (~0.48), tail (~1), and count-rate (>10 kHz) discarding those that did not fit these criteria (Cortese et al., 2021; Durduran and Yodh, 2013). The remaining curves were fitted with the appropriate solution of the correlation diffusion equation and averaged over the four detector channels placed at the same spot on the skin. This provided us with a time trace of BFi. Using an in-house Java software (TiSerVA is open-source and available for download at https://gitlab.ecole.ensicaen.fr/rclouard/TiSerVa), we applied a zero-phase-lag Butterworth bandpass filter (order 10, 0.03–0.3 Hz) on this time series and segmented the data around markers from − 2 s to + 7 s. We then discarded segments comprising values exceeding three standard deviations (movement artifacts). Out of 40 measurements, we discarded three datasets for insufficient quality (less than 60 Stimuli segments out of 84 or six Omissions segments out of 10 passed artifact rejection). In MATLAB® (The Mathworks, Inc.), segments were baseline corrected by subtracting the value at t0 and standardized by dividing them by their standard deviation, before averaging. We thus report relative values.
2.4. Analysis
2.4.1. Brain response to stimuli
We averaged Stimuli segments for each subject to obtain the individual response to all Stimuli trials. We also averaged the Stimuli trials immediately preceding an Omission (Preomission trials) to compare with an equal number of Omission trials. Response amplitudes were considered as the value at t3 (baseline correction implies that at t0, the value is 0).
2.4.2. Brain changes during omissions
For this analysis, we kept only participants with a stable individual average baseline and positive individual average response to both Stimuli and Preomission trials because there is uncertainty in the literature on the meaning of negative hemodynamic responses. Although negative changes can reflect true deactivation in the ipsilateral somatosensory cortex of adults (Franceschini et al., 2003, Kastrup et al., 2008), in infants it may also appear when the task-evoked increase in oxygen consumption outweighs the increase in oxygenated hemoglobin concentration resulting from the increase in blood flow (Harris et al., 2011). However, a recent review identified that the majority of research articles reported a positive hemodynamic response in newborns and infants across various functional tasks (de Roever et al., 2018). Therefore, negative changes to contralateral tactile stimuli are more likely due to the probe being slightly off relative to the region of interest (stimuli can deactivate neighboring areas), or because of a hemodynamic steal effect, i.e., a redistribution of blood flow from peripheral areas towards the center of the region of interest (Shmuel et al., 2002).
Four participants were discarded for noisy baseline (drift across the whole segment or larger changes in the 2 s preceding t0 than following t0) and 13 for the lack of a typical hemodynamic response (negative change to Stimuli or Preomission trials average). Twenty participants remained for analysis, 11 in group Fixed and 9 in group Jittered. Table 1 presents participant details at measurement (final sample).
Table 1.
Participant details (final sample) at measurement. Mean [standard deviation] Min-max.
| All subjects N = 20 | Group Fixed N = 11 | Group Jittered N = 9 | |
|---|---|---|---|
| Gestational age at birth (weeks) | 32.1 [0.5]31.3–32.7 | 32.1 [0.6]31.1–32.7 | 32.1 [0.4]31.1–32.7 |
| Birth weight (g) | 1608 [273]1010–2100 | 1657 [329]1010–2100 | 1547 [185]1300–1800 |
| Number of intrauterine growth restriction | 7 | 5 | 2 |
| Number of males/females | 9/11 | 4/7 | 5/4 |
| Chronological age at measurement (days) | 8.2 [3.1] 3–15 | 8.5 [3.5]5–15 | 7.9 [2.9]3–13 |
| Corrected gestational age at measurement (weeks) | 33.3 [0.2] 33.0–33.4 | 33.3 [0.2]33.0–33.4 | 33.2 [0.2]33.0–33.4 |
| Number of painful events recorded at the first measurement | 25.1 [8.6]10–42 | 24.5 [8.5]10–37 | 25.9 [9.1]12–42 |
We averaged Omission segments for each subject to obtain the individual changes in BFi when a stimulus was expected but not presented. The baseline t0 in group Fixed was set at t8 relative to Preomission onset (3 s stimulation + 5 s ISI). In group Jittered, because ISI varied between 3 and 7 s, stimulation was expectable from t6 relative to Preomission onset, therefore that is when we set the Omission baseline. Response amplitudes were considered as the value at t3 relative to the Omission baseline.
We also averaged the Stimuli trials immediately following an omission (Postomission trials) to see how the brain reacted to a stimulus being presented again after being unexpectedly omitted (akin to ISI lengthening).
2.5. Statistics
We performed a repeated-measures ANOVA with one factor (Fixed/Jittered) using BFi as the dependent variable. The homogeneity of variance assessed with Levene’s test was confirmed for Preomission and Omission values, but not for Postomission (variance being much higher in the Fixed group). Therefore, we tested two repeated measures: Preomission and Omission values. Additionally, we performed Student’s t test on values at t3 for the three measures, after testing for normality, to report significance of changes relative to baseline.
2.6. Physiology
DCS measures blood flow changes in the tissues sampled by infrared light, reflecting brain activity underneath the probe. However, as light travels through the skin and skull, it is possible that part of the variance in the signal results from systemic changes occurring in superficial layers, that are not from a neuronal origin but the result of brain activity on the body’s activity. Although such changes would still indicate stimulus processing and allow similar conclusions to be drawn regarding SP, we aimed to interpret our data as being of cortical origin. Optical imaging studies in infants show that contamination from superficial layers is negligible compared with adults because skin and skull are thinner (Emberson et al., 2016). Since they are even much thinner in premature neonates, we did not expect contamination. However, because most participants were monitored for clinical purposes, we recorded data from the baby’s clinical monitoring device (heart rate, respiratory rate, and systemic blood saturation) during acquisitions. Monitoring was available for 10 out of 11 subjects in group Fixed and 7 out of 9 subjects in group Jittered. We synchronized these time series with BFi (interpolating for sampling rate offset) using our TiSerVA software and performed the same analysis on these time series as described above for BFi data.
3. Results
3.1. Brain response to tactile stimuli
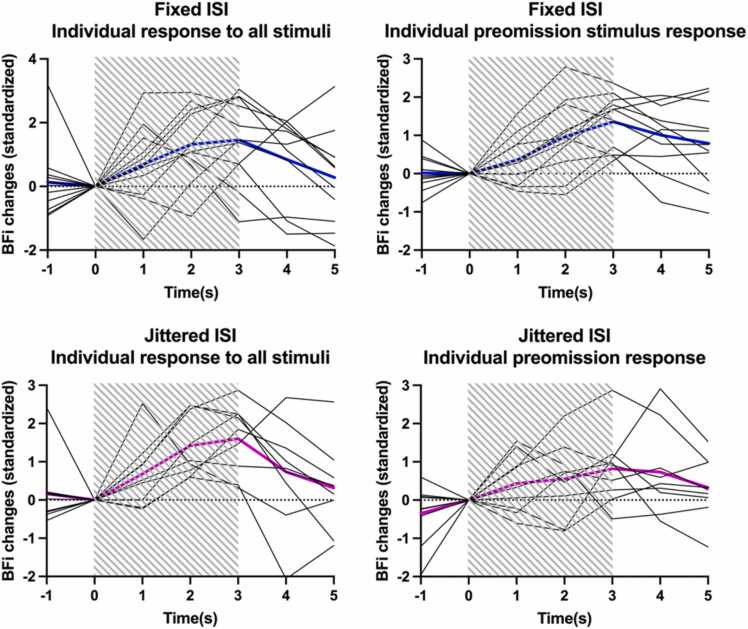
Fig. 3 presents individual averages of BFi for Stimuli trials and Preomission trials in both groups for the 20 subjects, showing a similar shape as previously described (Roche-Labarbe et al., 2014).
Fig. 3.
Individual averages of BFi for all Stimuli trials (left panels) and Preomission trials (right panels) in group Fixed (top, N = 11) and Jittered (bottom, N = 9). The striped rectangle represents the duration of the stimuli. Time is relative to stimulus onset. The blue and pink lines represent the grand averages of the hemodynamic response to Fixed and Jittered stimuli, respectively.
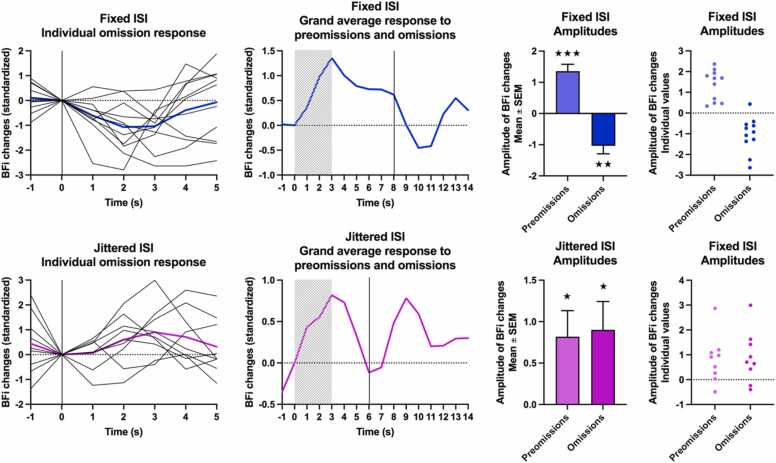
3.2. Brain response to omissions
Fig. 4 presents individual averages in BFi for Omissions, the grand averages for Preomissions followed by corresponding Omissions, and amplitudes at t3 for Preomissions and Omissions. The ANOVA revealed a main effect of the group (F(1,18)= 8.36, p = 0.010, η2 = 0.078): Jittered ISI elicited greater overall brain activity than Fixed ISI. This is due to the significant decrease in BFi during Omissions in the Fixed group (t(10) = −4.023, p = 0.002) with a very large effect size (Cohen’s d=−1.213). On the contrary, BFi increased significantly in the Jittered group (t(8) = 2.622, p = 0.031) with a large effect size (Cohen’s d=0.874). As a result of these opposite changes during Omissions, the ANOVA revealed a significant repetition effect (F(1,18)= 13.3, p = 0.002, η2 = 0.215) and the interaction between group and repetition was highly significant (F(1,18)= 15.2, p = 0.001, η2 = 0.247). There is a decrease in BFi when a tactile stimulus is expected in a deterministic ISI sequence and an increase in BFi when a tactile stimulus is expected in a probabilistic ISI sequence.
Fig. 4.
Individual averages of BFi for Omission trials, grand averages for Preomissions followed by Omissions, amplitudes for Preomissions and Omissions (Mean ± standard error and individual values) in group Fixed (top) and Jittered (bottom). The striped rectangle represents the duration of the Preomission. The vertical bar represents the moment when a stimulus should recur but was omitted (Omission “onset”). Time is relative to Preomission onset.
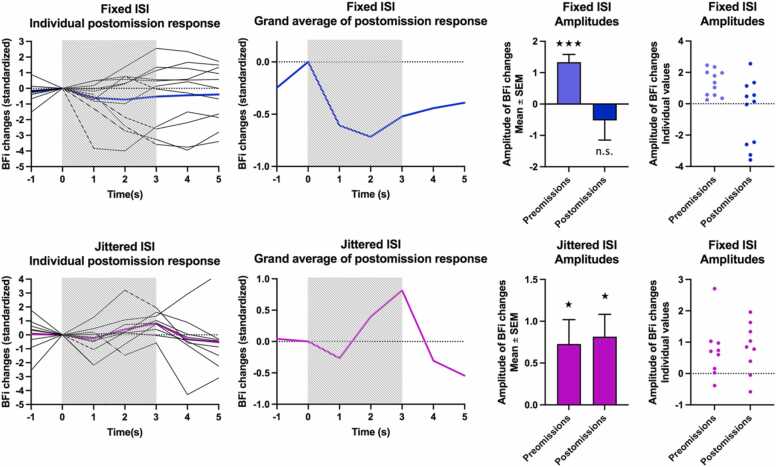
Fig. 5 presents individual averages and the grand average of BFi for Postomissions, and amplitudes at t3 for Preomissions and Postomissions for comparison. Because the variance was not homogeneous between groups for this measure, it was not included in the ANOVA. BFi changes during Postomissions were variable in the Fixed group with 4 subjects out of 10 showing a negative response, and they were not significant (t(10) = −0.829, p = 0.426). On the contrary in group Jittered, the significant increase in BFi in response to Postomissions was similar to Preomissions (t(8) = 3.044, p = 0.016), with a large effect (Cohen’s d=1.015).
Fig. 5.
Individual averages and grand averages of BFi for Postomission trials, amplitudes for Preomissions and Omissions (Mean ± standard error and individual values) in group Fixed (top) and Jittered (bottom). The striped rectangle represents the duration of the stimuli. Time is relative to stimulus onset.
3.3. Physiology
Physiological variations during the stimulation sequence did not show any pattern consistent across participants, related to either stimuli or omissions, and the ANOVA did not show any significant effect of group or repetition. We calculated Pearson’s correlation coefficient on several subjects at the individual level between physiological variables and BFi, and found no significant correlation. BFi changes reported above are thus of brain origin, and not (or negligibly) contaminated by superficial layers. Studies in infants do not show important contamination because in infants extra cerebral layers (scalp, skull, meninges) are thin (Emberson et al., 2016). They are even thinner, and largely so, in premature neonates.
4. Discussion
4.1. Somatosensory prediction in the premature neonate brain
In this study, we presented premature neonates with two different tactile stimulation sequences, one with deterministic ISI (Fixed group), and the other with probabilistic ISI (Jittered group). Stimuli were interspersed with omission trials to observe the brain’s activity when a stimulus was expected but not presented, depending on how liable was stimulus onset.
During omissions, the changes in blood flow differed according to the group. When ISI was fixed and therefore stimulus onset was precisely predictable, we found a decrease in blood flow in the somatosensory cortex, starting when a stimulus should appear: the expectation of a certain stimulus onset induced deactivation of the somatosensory cortex. This phenomenon corresponds to RS, the brain analog of behavioral habituation (Nordt et al., 2016). On the contrary, when ISI was jittered and therefore stimulus onset was not precisely predictable, we found an increase in blood flow during omissions, of similar amplitude to the increase induced by presented stimuli. The expectation of a likely but not certain stimulus onset induced activation of the somatosensory cortex. Our results reveal SP in the brain as early as four weeks before term, based on the temporal structure of a unimodal somatosensory stimulation, and show that SP produces opposite regulation of activity in the somatosensory cortex depending on how liable is stimulus onset.
Several models attempted to explain the neural bases of RS, such as neural fatigue (neurons would respond less), neural sharpening (fewer neurons would respond), and facilitation (neurons would respond faster) (Grill-Spector et al., 2006). These models focus on the bottom-up processing of perceptual information through sensory cortices. More recent works suggest instead that RS can ensue from to-down regulation of sensory cortices by higher-order cortices, following a learning phase (Summerfield et al., 2008). Therefore, RS would be observed only when a stable internal representation of the stimulus is formed (Nordt et al., 2016). Predictive models of cognition support this view, a stable representation allowing higher-order cortices to feed predictions back to sensory cortices. In adults, when a task requires attention to the repeated stimulus, it stays salient and the response is enhanced, whereas when attention is not required, the response is dampened (Kok et al., 2012). In 6-month-olds, Emberson et al. (2019) showed that expected changes in visual input are associated with RS, not unexpected changes, supporting the top-down origin of RS in infants as well. Our results also support this top-down model in premature neonates: RS was observed only when stimuli were certain. Uncertain stimulus onset may increase the number of presentations required to form a stable prediction of the upcoming stimulus, delaying RS. Jittered onsets may even increase stimulus salience and induce attentional enhancement toward the probabilistic stimuli. This remains to be studied because we do not have information on attentional processes at such a young age.
In our unimodal stimulation design, we varied the predictability of onset. This is an important difference from other works in infants, mostly bimodal paradigms when a visual stimulus is cued by an auditory one in a deterministic way (during familiarization the target occurs consistently following the cue), and omission trials consist of the omission of a stimulus that was previously certain. Kouider et al. (2015) using electroencephalography (EEG) in 12-month-old infants showed enhancement of the early neural activity evoked by the predicted visual event, vs. amplification of late components evoked when the event was replaced by a different, unexpected one. Using fNIRS in 6-month-olds, Emberson et al. (2015) reported a hemodynamic activation in the occipital cortex, as if the visual event was presented, when images were unexpectedly omitted after the tone. In our paradigm, the stimulus was not cued. Instead, the newborn had to rely on time processing, intervals being highly vs. moderately reliable, inducing more or less certainty of when the stimulus would recur. This design is closer to what Winkler et al. (2009) presented to term neonates in the auditory modality using EEG and a metric structure (i.e., music), but on a slower scale for touch. It is also very similar to the visual protocol used by Colombo and Richman (2002) in 4-month-old infants, albeit they used heart rate as the measure of sensory processing. They described precise prediction of a 2 s visual event during omission trials in a unimodal visual sequence with either 3 or 5 s ISI.
Early on in this line of research, Nelson et al. (1990) using EEG in 6-month-olds found that when the visual stimulus was not presented following the auditory cue, the brain responded differently to stimuli presented after an omission but not to the omission itself. In our study, we observed clear changes in brain activity during omissions, but there were also interesting effects on Postomission trials (the equivalent of Nelson’s “deliver-after-delete”). In the Fixed group, we had hypothesized that the lengthening of a previously fixed ISI would result in dishabituation (the following stimulus being treated as new) yielding strong activation. Instead, the response to Postomission stimuli was variable, with no significant difference from baseline and four participants presenting a negative response. This suggests that the suppression of neural activity induced by the repetition of highly predictable stimuli interferes with activation triggered by the stimulus being actually presented, inconsistently. This may be due to the young age of our participants, or their untimely birth, altering the fine sensory regulation. There are currently no comparable data on the preterm population that would give indications. As repetitions continue in between omissions, the response becomes consistent again as shown by the consistently positive Preomission response. There is a complex interplay between RS and stimulus-induced activation across the sequence that we would like to investigate further. Because of our short familiarization phase and the presence of omission trials among the sequence, we could not evaluate RS across familiarization in this study, but this is something we plan for future studies, as it will help disentangle the effects of stimulus-evoked activation vs. RS across fixed-ISI trials in neonates. Also, we would like to perform simultaneous EEG acquisitions to assess the contributions of early and late components of the neural response to the hemodynamic response. In the Jittered group, the response to Postomissions was similar to Preomissions and Omissions. ISI lengthening caused by the omission did not seem to induce a particular effect of stimulus processing, at least none visible with optical imaging. The activation of the somatosensory cortex during Omissions is evidence that a stimulus was expected in the range of ISI frequently encountered in the sequence, but ISI being occasionally longer did not dishabituate the participants and the following stimulus was not treated a new.
The difference in brain response between conditions highlights the top-down regulation of the primary somatosensory cortex depending on the temporal structure of environmental stimuli. The preterm brain actively processes this structure. Recent works propose that sensory prediction is a form of active engagement of infants with their environment, considering that active engagement does not necessarily require a motor component (Baek et al., 2020). In this framework, moderate predictability of stimuli enhances attention allocated to their processing, whereas high or low predictability decreases attention allocation. Learning would be facilitated by moderate predictability. For example, Téglás and Bonatti (2016) explored conditions allowing 12-month-old infants to form expectations about future visual events. They found proactive expectation (as opposed to realizing post hoc that outcomes do not match with their previous experience, i.e. a surprise effect) only when events were likely but not certain. Infants’ sensitivity to moderately predictable stimuli was also reported in term neonates (Bulf et al., 2011), and in 7- and 8-month-old infants (Kidd et al., 2012). Authors proposed that infants implicitly seek to maintain intermediate complexity of inputs, to avoid wasting cognitive resources on stimuli too complex or too simple. Our results suggest this optimization is present before the age of term in the tactile modality.
4.2. Limitations
We show that preterm neonates exhibit somatosensory prediction, but the persistence of this ability is uncertain. Emberson et al. (2017) showed SP impairment using the audiovisual omission paradigm in 6-month-old infants who were born preterm. Deficits were specific to prediction and did not affect the processing of presented stimuli. We do not know if tactile prediction could be altered after a few months in our subjects. If it were maintained, the difference with respect to audiovisual prediction results may be due to the developmental sensory heterochrony. Touch is the earliest functional sensory modality (Bremner and Spence, 2017, Dumont et al., 2018), whereas bimodal auditory-visual networks emerge in the last trimester of pregnancy and may be more vulnerable to disruption caused by preterm birth. However, tactile processing is frequently atypical in children born preterm (Crozier et al., 2016, Vanhatalo and Lauronen, 2006, Wickremasinghe et al., 2013), and is commonly reported in children with ND (Cascio, 2010, Marco et al., 2011, Puts et al., 2014). Therefore, a more plausible explanation would be that subjects in Emberson and colleagues’ study were more severely affected because they were born between 23 and 32 weeks GA, whereas ours were born at 32 weeks. If our subjects had been born earlier than 32 weeks and had spent more weeks in the NICU as a result, SP may have been impaired because of earlier brain alterations, deleterious NICU environment, or both. In this study, we repeated the same measurement at 35 weeks cGA, just before participants were discharged from the hospital. We had planned to assess the effect of cGA and NICU stay on SP. However, there was too much attrition at 35 weeks cGA for two reasons. First, participants moved more and had a thicker skull, leading to poorer data quality. The signal-to-noise ratio of DCS is lower than that of NIRS, which makes DCS even more sensitive to these issues. Despite using the channel averaging method described in Section 2.3., we could not keep enough data. Second, some participants were transferred to a different hospital closer to their parent’s home between the two measurements. We chose not to report data on the remaining small sample as conclusions would be too uncertain, and because we are currently conducting a study that should address this better using a protocol including neonates at all GA at birth and following them up into toddlerhood.
Finally, we would like to investigate further the links between systemic physiology and sensory processing in premature neonates. Our analysis of monitoring variables (heart rate, respiration rate, and blood oxygen saturation) did not reveal any effect of our stimulation protocol on these values, but we did not measure them directly, therefore we only had access to the monitor’s calculations at 1 Hz, which may not be sufficient resolution. It has been known for a long time that newborns’ heart rate increases from arousal and decreases with attentional processing (orienting response), these systemic effects interacting dynamically across sensory processing experiments (Clifton, 1974). Using dedicated physiology data acquisition, authors have used heart rate changes as an index of orienting attention in infants exposed to visual stimuli (Colombo and Richman, 2002, Richards, 2010) or in fetuses exposed to auditory changes (Weikum et al., 2012) for example. Our subjects being preterm, physiological variables are unstable, and induced changes may not be visible on this noisy baseline. Alternately, our stimuli may not have a sufficient impact on arousal or attention to yield consistent systemic changes. Recent works show that in adults, the respiratory cycle adjusts to a tactile stimulation paradigm to enhance detection and decision-making (Grund et al., 2022). Therefore, it would be informative to measure directly physiological variables during tactile processing protocols in newborns and infants to determine if such interactions are already present.
4.3. Conclusion
Our study reveals somatosensory prediction in the brain of premature neonates as early as four weeks before term, based on the temporal structure of the stimulation sequence, and shows opposite regulation of activity in the somatosensory cortex depending on the liability of stimulus onset. Future work will aim to assess if SP varies across GA at birth, length of NICU stay, and other risk factors, to determine if it could be a reliable early marker of neurodevelopment, and could be used as a ND screening tool. Disentangling how top-down mechanisms are affected across sensory modalities in at-risk populations like preterm neonates, but also siblings of diagnosed children, will be necessary. Finally, emphasis on longitudinal follow-up will allow us to test the relationship between early top-down regulation and cognitive development, as well as global adjustment in the first years of life, to understand typical and atypical neurodevelopmental trajectories.
Funding
This research was supported by the PREMATEMP grant #FDF-00041933 from the Fondation de France (http://www.fondationdefrance.org) to NRL, the NEOPRENE grant #ANR-19-CE37-0015 from the Agence Nationale de la Recherche (www.anr.fr) to NRL, and a Ph.D. grant from the Region Normandie (http://www.normandie.fr) to VD.
CRediT authorship contribution statement
V.D. designed the protocol, acquired the data, analyzed the data, and wrote the manuscript. M.G. and T.D. designed and applied DCS data processing. D.Z. designed, built, and maintained the tactile stimulation device. R.C. designed data synchronization and processing software. B.G. was responsible for patient clinical evaluation and inclusion. N.R.-L. designed the protocol, obtained funding, acquired the data, analyzed the data, and wrote the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Dr. Udo Weigel at HemoPhotonics for support with αιμα-FloMo set-up and upgrades, and Dr. Marc Aguert for helpful conversations on Statistics. We are very grateful to all the babies and their parents for their participation, and the nurses, physicians, and staff in the Neonatology Unit at the Caen University Hospital for their unwavering help and support.
Contributor Information
Victoria Dumont, Email: victoria.dumont@unicaen.fr.
Nadège Roche-Labarbe, Email: nadege.roche@unicaen.fr.
Data Availability
Anonymized data will be available upon request to Dr. Nadege Roche-Labarbe by email at nadege.roche@unicaen.fr and after a collaboration agreement has been approved by the University of Caen Normandy, France, and by the Caen University Hospital, France, co-owners of the data, with the requesting institution.
References
- Albright T.D. On the perception of probable things: neural substrates of associative memory, imagery, and perception. Neuron. 2012;74(2):227–245. doi: 10.1016/j.neuron.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel E.L., Rankin C.H. The importance of touch in development. Paediatr. Child Health. 2010;15(3):153–156. doi: 10.1093/pch/15.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek S., Jaffe-Dax S., Emberson L.L. How an infant's active response to structured experience supports perceptual-cognitive development. Prog. Brain Res. 2020;254:167–186. doi: 10.1016/bs.pbr.2020.05.015. [DOI] [PubMed] [Google Scholar]
- Bremner A.J., Spence C. Vol. 52. Elsevier; 2017. pp. 227–268. (The Development of Tactile Perception). [DOI] [PubMed] [Google Scholar]
- Bröring T., Oostrom K.J., Lafeber H.N., Jansma E.P., Oosterlaan J. Sensory modulation in preterm children: theoretical perspective and systematic review. Plos One. 2017;12(2) doi: 10.1371/journal.pone.0170828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubic A., Cramon, von D.Y., Schubotz R.I. Prediction, cognition and the brain. Front. Hum. Neurosci. 2010;4:25. doi: 10.3389/fnhum.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulf H., Johnson S.P., Valenza E. Visual statistical learning in the newborn infant. Cognition. 2011;121(1):127–132. doi: 10.1016/j.cognition.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Cascio C.J. Somatosensory processing in neurodevelopmental disorders. J. Neurodev. Disord. 2010;2(2):62–69. doi: 10.1007/s11689-010-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.L., Babiloni C., Ferretti A., Perrucci M.G., Romani G.L., Rossini P.M., et al. Effects of somatosensory stimulation and attention on human somatosensory cortex: an fMRI study. NeuroImage. 2010;53(1):181–188. doi: 10.1016/j.neuroimage.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Clifton R.K. Heart rate conditioning in the newborn infant. J. Exp. Child Psychol. 1974;18(1):9–21. doi: 10.1016/0022-0965(74)90084-8. [DOI] [PubMed] [Google Scholar]
- Colombo J., Richman W.A. Infant timekeeping: attention and temporal estimation in 4-month-olds. Psychol. Sci. 2002;13(5):475–479. doi: 10.1111/1467-9280.00484. [DOI] [PubMed] [Google Scholar]
- Cortese L., Presti Lo, G., Pagliazzi M., Contini D., Dalla Mora A., Dehghani H., et al. Recipes for diffuse correlation spectroscopy instrument design using commonly utilized hardware based on targets for signal-to-noise ratio and precision. Biomed. Opt. Express. 2021;12(6):3265–3281. doi: 10.1364/BOE.423071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier S.C., Goodson J.Z., Mackay M.L., Synnes A.R., Grunau R.E., Miller S.P., Zwicker J.G. Sensory Processing Patterns in Children Born Very Preterm. Am. J. Occup. Ther. 2016;70(1) doi: 10.5014/ajot.2016.018747. 7001220050p1–7001220050p7. [DOI] [PubMed] [Google Scholar]
- Dumont V., Bulla J., Bessot N., Gonidec J., Zabalia M., Roche-Labarbe N. The manual orienting response habituation to repeated tactile stimuli in preterm neonates: Discrimination of stimulus locations and interstimulus intervals. Dev. Psychobiol. 2017;59(5):590–602. doi: 10.1002/dev.21526. [DOI] [PubMed] [Google Scholar]
- Dumont V., Delaunay El-Allam M., Roche-Labarbe N. In: Psychobiological Foundations of Early Sensory-motor Development and Implications for Neonatal Care. Murphy P.N., editor. The Routledge International Handbook of Psychobiology (1st ed.). Milton Park, Abingdon; Oxon; New York, NY: Routledge, 2018.: Routledge: 2018. [DOI] [Google Scholar]
- Durduran T., Yodh A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. NeuroImage. 2013;85(1):51–63. doi: 10.1016/j.neuroimage.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Richards J.E., Aslin R.N. Top-down modulation in the infant brain: Learning-induced expectations rapidly affect the sensory cortex at 6 months. Proc. Natl. Acad. Sci. 2015;112(31):9585–9590. doi: 10.1073/pnas.1510343112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Crosswhite S.L., Goodwin J.R., Berger A.J., Aslin R.N. Isolating the effects of surface vasculature in infant neuroimaging using short-distance optical channels: a combination of local and global effects. Neurophotonics. 2016;3(3) doi: 10.1117/1.NPh.3.3.031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Boldin A.M., Riccio J.E., Guillet R., Aslin R.N. Deficits in top-down sensory prediction in infants at risk due to premature birth. Curr. Biol. 2017;27(3):431–436. doi: 10.1016/j.cub.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Boldin A.M., Robertson C.E., Cannon G., Aslin R.N. Expectation affects neural repetition suppression in infancy. Dev. Cogn. Neurosci. 2019;37 doi: 10.1016/j.dcn.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon I., Hains S.M.J., Muir D.M., Kisilevsky B.M. Development of tactile responses in human preterm and full-term infants from 30 to 40 weeks postconceptional age. Infancy. 2002;3(1):31–51. [Google Scholar]
- Fifer W.P., Byrd D.L., Kaku M., Eigsti I.-M., Isler J.R., Grose-Fifer J., et al. Newborn infants learn during sleep. Proc. Natl. Acad. Sci. USA. 2010;107(22):10320–10323. doi: 10.1073/pnas.1005061107/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini M.A., Fantini S., Thompson J.H., Culver J.P., Boas D.A. Hemodynamic evoked response of the sensorimotor cortex measured noninvasively with near-infrared optical imaging. Psychophysiology. 2003;40(4):548–560. doi: 10.1111/1469-8986.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005;360(1456):815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grund M., Al E., Pabst M., Dabbagh A., Stephani T., Nierhaus T., et al. al, et al. Respiration, heartbeat, and conscious tactile perception. J. Neurosci. 2022;42(4):643–656. doi: 10.1523/JNEUROSCI.0592-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Háden G.P., Honing H., Winkler I. Presented at the 12th conference on music perception. Thessaloniki; Greece: 2012. Newborn Infants Are Sensitive to Sound Timing. [Google Scholar]
- Harris J.J., Reynell C., Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev. Cogn. Neurosci. 2011;1:199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertenstein M.J., Verkamp J.M., Kerestes A.M., Holmes R.M. The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 2006;132(1):5–94. doi: 10.3200/mono.132.1.5-94. [DOI] [PubMed] [Google Scholar]
- Hoge R.D., Atkinson A.J., Gill B., Crelier G.R., Marrett S., Pike G.B. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge R.D., Franceschini M.A., Covolan R.J.M., Huppert T., Mandeville J.B., Boas D.A. Simultaneous recording of task-induced changes in blood oxygenation, volume, and flow using diffuse optical imaging and arterial spin-labeling MRI. NeuroImage. 2005;25:701–707. doi: 10.1016/j.neuroimage.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Johnson S., Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011;69(5 Part 2):11R–18R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- Kastrup A., Baudewig J., Schnaudigel S., Huonker R., Becker L., Sohns J.M., et al. Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. NeuroImage. 2008;41(4):1364–1371. doi: 10.1016/j.neuroimage.2008.03.049. [DOI] [PubMed] [Google Scholar]
- Kidd C., Piantadosi S.T., Aslin R.N. The Goldilocks effect: human infants allocate attention to visual sequences that are neither too simple nor too complex. Plos One. 2012;7(5) doi: 10.1371/journal.pone.0036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill D.C., Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27(12):712–719. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kok P., Rahnev D., Jehee J.F.M., Lau H.C., de Lange F.P. Attention reverses the effect of prediction in silencing sensory signals. Cereb. Cortex. 2012;22(9):2197–2206. doi: 10.1093/cercor/bhr310. [DOI] [PubMed] [Google Scholar]
- Kouider S., Long B., Le Stanc L., Charron S., Fievet A.-C., Barbosa L.S., Gelskov S.V. Neural dynamics of prediction and surprise in infants. Nat. Commun. 2015;6(1):79. doi: 10.1038/ncomms9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E.J., Hinkley L.B.N., Hill S.S., Nagarajan S.S. Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meether M., Bush C.N., Richter M., Pineda R. Neurobehaviour of very preterm infants at term equivalent age is related to early childhood outcomes. Acta Paediatr. 2021;110(4):1181–1188. doi: 10.1111/apa.15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Ellis A.E., Collins P.F., Lang S.F. Infants’ neuroelectric responses to missing stimuli: can missing stimuli be novel stimuli? Dev. Neuropsychol. 1990;6(4):339–349. doi: 10.1080/87565649009540471. [DOI] [Google Scholar]
- Nordt M., Hoehl S., Weigelt S. The use of repetition suppression paradigms in developmental cognitive neuroscience. Cortex. 2016;80:61–75. doi: 10.1016/j.cortex.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Otte R.A., Winkler I., Braeken M.A.K.A., Stekelenburg J.J., van der Stelt O., Van den Bergh B.R.H. Detecting violations of temporal regularities in waking and sleeping two-month-old infants. Biol. Psychol. 2013;92(2):315–322. doi: 10.1016/j.biopsycho.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Puts N.A.J., Wodka E.L., Tommerdahl M., Mostofsky S.H., Edden R.A.E. Impaired tactile processing in children with autism spectrum disorder. 2014;111(9):1803–1811. doi: 10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.E. The development of attention to simple and complex visual stimuli in infants: behavioral and psychophysiological measures. Dev. Rev.: DR. 2010;30(2):203–219. doi: 10.1016/j.dr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Labarbe N., Fenoglio A., Radhakrishnan H., Kocienski-Filip M., Carp S.A., Dubb J., et al. Somatosensory evoked changes in cerebral oxygen consumption measured non-invasively in premature neonates. NeuroImage. 2014;85:1–8. doi: 10.1016/j.neuroimage.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roever I., Bale G., Mitra S., Meek J., Robertson N.J., Tachtsidis I. Investigation of the pattern of the hemodynamic response as measured by functional near-infrared spectroscopy (fNIRS) studies in newborns, less than a month old: a systematic review. Front. Hum. Neurosci. 2018;12:371. doi: 10.3389/fnhum.2018.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel I., Saupe K., Schröger E. I know what is missing here: electrophysiological prediction error signals elicited by omissions of predicted ”what” but not ”when. Front. Hum. Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz R.I. Brain Mapping. Elsevier; 2015. Prediction and Expectation; pp. 295–302. [DOI] [Google Scholar]
- Schwartze M., Kotz S.A. A dual-pathway neural architecture for specific temporal prediction. Neurosci. Biobehav. Rev. 2013;37(10):2587–2596. doi: 10.1016/j.neubiorev.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Shmuel A., Yacoub E., Pfeuffer J., Van de Moortele P.F., Adriany G., Hu X., Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36(6):1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Sinha P., Kjelgaard M.M., Gandhi T.K., Tsourides K., Cardinaux A.L., Pantazis D., et al. Autism as a disorder of prediction. Proc. Natl. Acad. Sci. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov E.N. Higher nervous functions: the orienting reflex. Annu. Rev. Physiol. 1963;25(1):545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Summerfield C., Trittschuh E.H., Monti J.M., Mesulam M.M., Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci. 2008;11(9):1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téglás E., Bonatti L.L. Infants anticipate probabilistic but not deterministic outcomes. Cognition. 2016;157:227–236. doi: 10.1016/j.cognition.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Van de Cruys S., Evers K., Van der Hallen R., Van Eylen L., Boets B., de-Wit L., Wagemans J. Precise minds in uncertain worlds: predictive coding in autism. Psychol. Rev. 2014;121(4):649–675. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S., Lauronen L. Neonatal SEP – Back to bedside with basic science. Semin. Fetal Neonatal Med. 2006;11(6):464–470. doi: 10.1016/j.siny.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Weikum W.M., Oberlander T.F., Hensch T.K., Werker J.F. Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc. Natl. Acad. Sci. USA. 2012;109 Suppl 2(Supplement_2):17221–17227. doi: 10.1073/pnas.1121263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.J., Wilson P., Hertenstein M.J., Campos R. The tactile context of a mother’s caregiving: Implications for attachment of low birth weight infants. Infant Behav. Dev. 2000;23:91–111. [Google Scholar]
- Wickremasinghe A.C., Rogers E.E., Johnson B.C., Shen A., Barkovich A.J., Marco E.J. Children born prematurely have atypical sensory profiles. J. Perinatol. 2013;33:631–635. doi: 10.1038/jp.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I., Haden G., Ladinig O., Sziller I., Honing H. Newborn infants detect the beat in music. Proc. Natl. Acad. Sci. USA. 2009;106(7):2468–2471. doi: 10.1073/pnas.0809035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be available upon request to Dr. Nadege Roche-Labarbe by email at nadege.roche@unicaen.fr and after a collaboration agreement has been approved by the University of Caen Normandy, France, and by the Caen University Hospital, France, co-owners of the data, with the requesting institution.