Abstract
OBJECTIVE:
To test whether two sequential BCG-induction courses improves the response of high-risk non-muscle invasive bladder cancer. Achieving a complete response (CR) to BCG is critical to disease-free survival. Patients with preexisting BCG-specific immunity owing to prior exposure to BCG have longer disease-free survival than BCG-naïve patients likely due to heterologous immunity from the initial priming of the immune system. We evaluated this hypothesis in a phase II prospective clinical trial.
METHODS:
From 2015 to 2018, we recruited patients with primary or recurrent NMIBC (high-grade Ta, T1 tumors, with or without CIS) to receive 2-induction courses (12 intra-vesical instillations) of BCG. The primary aim of the study was CR rate 6 months after start of the first BCG induction. CR was defined as no tumor at cystoscopy or TURB biopsy. No maintenance BCG was given. We targeted at least 75 evaluable patients, and a CR of 80% or better was deemed significant.
RESULTS:
Eighty-one patients agreed to participate. Five withdrew before starting BCG, leaving 76 evaluable patients. Sixty-three patients (83%) completed the 12 instillations on schedule. Of these, 62 patients (91%) had a CR at 6 months. None of the patients had tumor progression. Serious adverse event was seen in one patient (1%). Recurrence-free survival at 2 years after complete response was 85% (95% CI 77%, 95%).
CONCLUSION:
The high response rate in patients with high-risk non-muscle-invasive bladder cancer justifies two BCG induction cycles in current practice.
INTRODUCTION
Intravesical bacillus Calmette-Guerin (BCG) is standard of care therapy after transurethral resection (TURB) of high-risk non-muscle-invasive bladder cancer (NMIBC). Patients receive an initial induction BCG course of 6-weekly instillations, often followed three months later with 3-weeks maintenance BCG treatments (1). Evaluation of response is best determined 3 and 6 months after start of induction BCG (2). A complete 6-month response to TURB and BCG portends a favorable outcome (3). Failure to respond to induction BCG identifies patients at high risk of tumor recurrence and stage progression. Since achieving an initial complete response to BCG is critical to disease-free survival (4,5), strategies to improve the 6-month response rate to BCG are worth investigating.
In an audit of 1021 patients, we found that 34% had no response to BCG after 3 and 6 months, with or without maintenance treatments (2–4). Earlier studies show up to 40% of patients who do not respond by 3 months to the first 6-week BCG induction respond to a second cycle of 6-weekly instillations (6,7), however more recent studies suggest that repeated BCG induction courses do not improve clinical outcomes (8).
Patients with preexisting BCG-specific immunity, as measured by positive PPD skin test (9), or BCG-specific T-cells (10), have longer disease-free survival, suggesting that priming local immunity initially may improve response rates to BCG therapy. A robust local and systemic immune response to BCG can be achieved by intradermal inoculation (11) or intravesical therapy alone (12). We showed that one 6-week induction course of BCG converted tuberculin skin tests from negative to positive in 60% of patients (13), and that PPD positive patients after BCG had longer tumor-free survival than PPD negative patients (10,13). These studies suggest that first exposure to BCG primes the immune system to enhance the antitumor effect of a second exposure to BCG therapy. To test this hypothesis, we conducted a prospective phase II study in high-risk bladder cancer patients to investigate the response rate to two sequential induction courses of intravesical BCG therapy.
METHODS
From November 2015 to June 2018, we recruited patients with multiple (>1), high-grade, primary or recurrent Ta or T1 tumors with carcinoma in situ (CIS) to receive 2-induction courses (12 intra-vesical instillations) of BCG. All patients underwent a visibly complete re-staging TURB prior to starting BCG therapy, and all patients had pure urothelial carcinoma (UC), except one who had UC plus nested variant. The institutional review board approved the protocol and all patients gave written informed consent (NCT02281383).
The main objective of this study was to evaluate the response rate at 6 months from the start of two induction courses (12 intravesical instillations) of BCG in patients with high-risk NMIBC. We enrolled BCG naive patients as well as patients previously treated with BCG who completed therapy 12 months prior to enrollment in this study. Patients were treated with an induction course (6 intravesical instillations) of BCG followed by a second induction course (6 intravesical instillations) with a recovery period of 2–3 weeks between the two treatment courses. Patients underwent cystoscopy or TURB and urine cytology after the first treatment course (3 months +/− 4 weeks) and TURB after second treatment course (6 months +/− 4 weeks). Adverse events using Common Terminology Criteria for Adverse Events (CTCAE v4) provided by the National Cancer Institute was used to record toxicity. Since side effects from BCG (dysuria, frequency, hematuria, occasional fever) are common, only grade 3 or greater toxicity (severe dysuria, sepsis) was recorded. PPD skin tests were not administered before or after BCG therapy.
The study used a single-stage phase II design. Patients were enrolled until a total of 75 patients had completed their second cystoscopy on study. We defined that the two-induction BCG therapy would be worth further study if the overall response rate at 6 months is an H1 of 80% versus a null H0 of 65%. The probabilities of a type I error and type II error are set at 0.05 and 0.1, respectively. The null hypothesis was chosen based on a review of patients which found that 34% of patients had no response to BCG at 6 months (14). Given that these patients were treated at our institution, and case mix and treatment protocols are similar, we did not adjust the estimate we used to choose our null hypothesis. On completion of the trial, if 56 or more patients had a response, the regimen will be declared effective and of interest. All evaluable patients who met the eligibility criteria for the study and enrolled in it were included in the analysis, regardless of whether they completed the full two BCG induction courses. We performed three sensitivity analyses to assess the effects of missing 6-month outcomes data. In the first sensitivity analysis, we considered patients who responded to treatment at 3 months but did not have a prescribed evaluation at 6 months, as responders at 6 months. The second analysis used cytology and cystoscopy results from after the specified 6-month period in patients who were followed between 7 and 12 months after initiation of BCG therapy. In the final sensitivity analysis, we classified all patients who did not have outcome data at 6 months as non-responders.
We also present the rate of recurrence among all patients who had complete response to BCG at 6-month follow-up. Patients were followed every 3 to 6 months thereafter with urine cytology and outpatient cystoscopy. Tumor recurrence was defined as the presence of high-grade papillary disease (Ta) or the presence of CIS or T1 disease on TURB. All analyses were conducted using R 4.0.2.
RESULTS
A total of 81 patients were enrolled in this study. Patients who were later determined to be ineligible, died before initiation of treatment or did not proceed with treatment were considered unevaluable and excluded from the analysis (N = 5). A total of 76 patients were included in the final analysis.
Patient characteristics are included in Table 1. The median age was 72 years (74% > 65 years), and the majority were males. Most patients had multiple T1 tumors associated with CIS. Twenty-eight patients (36%) had received prior BCG or MMC therapy for tumor recurrences. Thirteen patients (17%) underwent an interim TURB between the first and second BCG courses because of visible or suspicious tumor on cystoscopy and/or a positive urine cytology (8 had negative biopsies).
Table 1.
Patient characteristics in evaluable patients, N = 76
| Characteristic | N = 81 |
|---|---|
| Patients eligible | 81 |
| Patients evaluable | 76 |
| Age (median) | 72 (64, 76) |
| Age > 65 years | 56 (74%) |
| Male | 64 (84%) |
| Tumor Stage | |
| pTa | 24 (32%) |
| pT1 | 52 (68%) |
| Associated CIS | 30 (39%) |
| Tumor Type | |
| Primary | 51 (67%) |
| Recurrent | 25 (33%) |
| Prior BCG | 16 (21%) |
| Prior Mitomycin C | 12 (16%) |
| Interim TURB | 13 (17%) |
| Interim TURB pathology | |
| pT0 | 8 (53%) |
| pTa | 3 (33%) |
| CIS | 2 (13%) |
| Number of BCG doses | |
| 12 | 63 (83%) |
| 11 | 2 (2.6%) |
| 10 | 1 (1.3%) |
| 9 | 1 (1.3%) |
| 8 | 2 (2.6%) |
| 7 | 1 (1.3%) |
| 6 | 5 (6.6%) |
| 2 | 1 (1.3%) |
| Adverse events | |
| Refused 2nd BCG induction due to side effects | 3 (3.9%) |
| Hospitalized for urosepsis | 1 (1.3%) |
| None | 72 (95%) |
Of the 76 patients, 68 had both cystoscopy/TURB and urine cytology results available at 6 months. Among these patients, there were 62 patients with full response (stage T0 on cystoscopy/TURB and negative urine cytology) at 6 months (91%, lower bound of one-sided 95% CI 83%). There were no patients who progressed to muscle-invasive bladder cancer or had evidence of distant metastasis within 6 months of BCG initiation. Patients who had received prior BCG therapy had a 6-month response rate of 89% versus 98% among BCG naïve patients.
In this cohort, there were 8 patients who were missing 6-month outcomes. One patient died before 6 months of unrelated causes. Seven patients were evaluated from 7 to 12 months after the first BCG dose and all were complete responders. In the first sensitivity analysis, 7 patients were included using 3-month response in place of their missing 6-month outcome, resulting in a rate of 92% (lower bound of one-sided 95% CI 84%). The second sensitivity analysis used response in the 7 to 12-month timeframe, with a response rate of 92% (lower bound of one-sided 95% CI 85%). In the last sensitivity analysis, we included all 8 patients with missing 6-month outcomes as non-responders and found a response rate of 82% (lower bound of one-sided 95% CI 73%).
Compliance and tolerance of the two consecutive BCG courses was acceptable. Serious adverse events were rare. Thirteen patients did not receive all 12 doses. Four patients stopped BCG treatments owing to side effects, including one episode of sepsis. Nine patients who tolerated BCG well refused to return to the clinic to continue treatments, citing disinterest or difficulty with travel.
We then assessed recurrence after treatment among those 62 patients who had complete response at 6 months. One patient with complete response at 6 months was excluded from the analysis due to having no subsequent follow-up. There were 13 patients who had a recurrence. Eleven were treated successfully with salvage intravesical therapy and two patients underwent radical cystectomy. Median follow-up time among patients who did not recur was 2.3 years (quartiles 1.7, 3.0). Recurrence-free survival at 2 years after complete response was 85% (95% CI 77%, 95%). Kaplan-Meier estimates of recurrence are presented in Figure 1.
Figure 1.
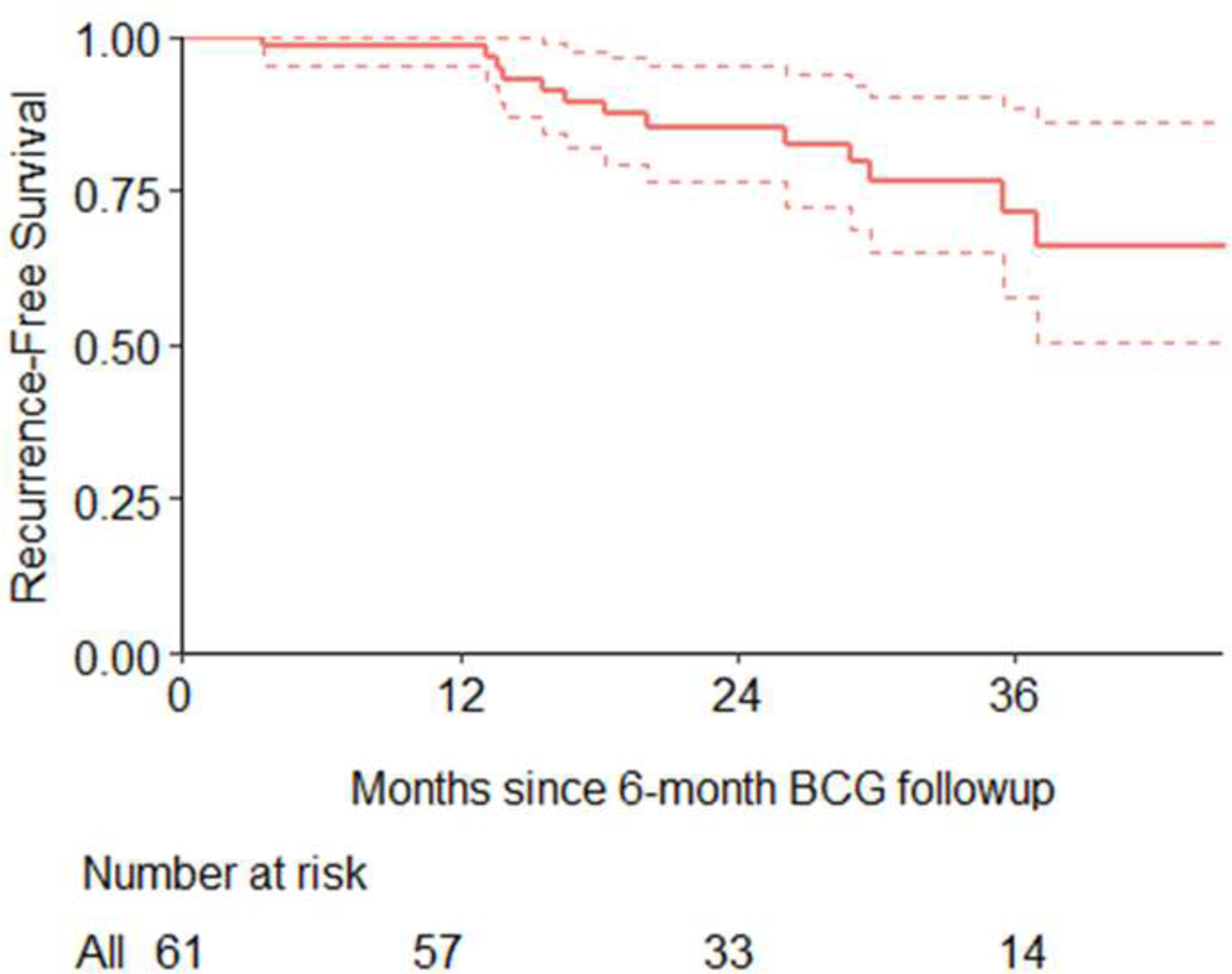
Kaplan-Meier estimates for time from 6-month follow-up to recurrence, among patients who had a complete response at 6 months. One patient was excluded who did not have any further follow-up after complete response at 6 months.
DISCUSSION
The major finding of this study is two 6-weekly courses of BCG therapy administered a few weeks apart was safe and generally well-tolerated. Majority of patients (83%) completed all 12 instillations and 91% achieved a complete response by 6 months, which exceeded the expected response rate. Results also met the criteria for success even when we did a sensitivity analysis based on the extreme case where patients who responded at 3 months were considered as “non-responders” at 6 months if they were missing 6 months outcome data. However, 7 of the 8 patients who did not undergo evaluation at 6 months per protocol were complete responders when evaluated 7 to 12 months later.
Strengths of the study are its prospective robust statistical design and inclusion of an elderly population with very high risk (predominantly pT1 tumors with associated CIS) non-muscle-invasive bladder cancers. A unique feature is all patients were scheduled to receive a planned second 6-week BCG induction cycle at 3 months, including those who had no disease after the first induction.
A recent retrospective study reported contemporary results in 116 patients who received a second BCG induction course for recurrent or persistent disease (15). The average time interval from the first to the second induction therapy was 7.85 months. Complete responses were seen in 89.7% patients after the second course, and 67.2% of these remained disease-free over 2 years with maintenance BCG therapy. In contrast, our study showed 91% patients achieved a complete response at 6 months from the first of 12 instillations, with recurrence-free survival of 85% at two years.
A study limitation is we cannot prove that an initial BCG induction course primes mucosal innate and adaptive immunity to enhance the therapeutic effect of a timely second series of BCG instillations. We did not use intradermal BCG or determine PPD skin test reactivity before and after treatments. However, the concept of initial exposure to BCG followed by a second full-course treatment exposure follows sound immunologic principles.
The two BCG induction cycle regimen may be an alternative to maintenance therapy, especially now given the current shortage of BCG. High risk NMIBC patients are recommended to receive a 6-week induction course, followed by 3-weekly maintenance treatments at 3, 6, 12, 18, 24, 30, and 36 months, for a total of 27 instillations. Our regimen achieves comparable results with 12 instillations, and no maintenance.
CONCLUSION
We enrolled 76 evaluable patients with high-risk non-muscle-invasive bladder cancer into a prospective phase II study to receive two 6-weekly courses of BCG therapy. Eighty-three percent of patients completed all 12 instillations and 91% achieved a complete response by 6 months, which exceeded the expected response rate. Although a randomized trial would be needed to confirm these findings, the high early complete response rate in patients with high-risk non-muscle-invasive bladder cancer justifies two BCG induction cycles in current practice.
Footnotes
References
- 1.Lamm DL, Blumenstein BA, Crissman JD, et al. : Maintenance BCG immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol 2000; 163: 1124–29. [PubMed] [Google Scholar]
- 2.Herr HW, Dalbagni G: Defining BCG refractory superficial bladder tumors. J Urol 2003; 169: 1706–8. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Badalament RA, Amato DA, et al. : Superficial bladder cancer treated with BCG: a multivariate analysis of factors affecting tumor progression. J Urol 1989; 141: 22–9. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Dalbagni G, Donat SM: Bacillus Calmette-Guerin without maintenance therapy for high-risk non-muscle-invasive bladder cancer. Eur Urol 2011; 60: 32–6. [DOI] [PubMed] [Google Scholar]
- 5.Lerner SP, Tangen CM, Sucharew H. Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol 2009; 27: 155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sylvester RJ, Van der Meijden A, Witjes JA, et al. : High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology 2005; 66: 90–107. [DOI] [PubMed] [Google Scholar]
- 7.Nadler RB, Catalona WJ, Hudson MA, et al. : Durability of the tumor-free response for intravesical BCG therapy. J Urol 1994; 152: 367–73. [DOI] [PubMed] [Google Scholar]
- 8.Gontero P, Bohle A, Malmstrom PU, et al. : The role of BCG in the treatment of non-muscle-invasive bladder cancer. Eur Urol 2010; 57: 410–429. [DOI] [PubMed] [Google Scholar]
- 9.Bilen CY, Inci K, Erkan I: The predictive value of purified protein derivative results on complications and prognosis in patients with bladder cancer treated with BCG. J Urol 2003; 169: 1702–05. [DOI] [PubMed] [Google Scholar]
- 10.Biot C, Rentsch CA, Gsponer JR, et al. : Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012; 4: 137ra72. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW, Laudone VP, Badalament RA, et al. : Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J Clin Oncol 1988; 6: 1450–55. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi K, Koga S, Nishikido M, et al. : Systemic immune response after intravesical instillation of BCG for superficial bladder cancer. Clin Exp Immunol 1999; 115: 131–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badalament RA, Herr HW, Wong GY, et al. : A prospective randomized trial of maintenance versus nonmaintenance intravesical BCG therapy of superficial bladder cancer. J Clin Oncol 1987; 5: 441–49. [DOI] [PubMed] [Google Scholar]
- 14.Sfakianos JP, Kim PH, Hakimi AA, Herr HW: The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guerin. J Urol, 2014. 191(2): p. 341–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniels MJ, Barry E, Schoenberg M, et al. : Contemporary oncologic outcomes of second induction BCG in patients with nonmuscle invasive bladder cancer. Urol Oncol 2020; 38: 5e9–5e16. [DOI] [PubMed] [Google Scholar]


