Introduction
Melanoma corresponds to the majority of deaths attributable to skin malignancies worldwide. Updated estimates suggest approximately 60,000 new cases in men and 40,000 new cases in women will be diagnosed in 2020, a surge in incidence over the past 20 years. (1,2)
Although still a challenging disease, treatment of advanced melanoma has made remarkable progress in the past decade with significant improvement in its historically dismal long-term survival outcomes. The characterization of molecules that contribute to immune evasion by downregulating T-cell mediated anti-tumor responses led to the development of immune checkpoint blockade (ICB), exemplified by strategies that inhibit cytotoxic T lymphocyte associated antigen 4 (CTLA4) and programmed cell death protein 1 (PD1) and its ligand (PD-L1).(3) Monoclonal antibodies against CTLA-4 and PD-1, capable of restoring antitumor immunity and reversing T-cell exhaustion, demonstrated robust antitumor activity and sustained responses in patients with advanced melanoma.(4) In addition to ICB, a distinct form of immunotherapy, talimogene laherparepvec (T-VEC), an oncolytic attenuated herpes simplex virus-1, induces antitumor responses by selectively replicating and lysing tumor cells while overexpressing granulocyte macrophage colony-stimulating factor (GM-CSF) to boost an immune response.(5) Concurrent with these advances in immunotherapy, the prominent role activating mutations involving the V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) gene play in approximately 50% of patients paved the way for the development of targeted therapies that block components of the mitogen-activated protein kinase pathway MAPK signaling pathway. As a result, the ICB blockers ipilimumab, nivolumab, and pembrolizumab, the oncolytic virus T-VEC, and molecules targeting the MAPK pathway have been approved by Food and Drug Administration (FDA) and incorporated into clinical practice. (4)
The efficacy of these therapies, however, can be hampered by primary and secondary resistance mechanisms, resulting in poor or transient responses in a significant proportion of patients with advanced melanoma. In this scenario of unmet clinical need, combined approaches have emerged as an alternative to enhance the benefit of systemic treatments. Regimens consisting of combined ICB blockers and separately, BRAF and MEK inhibitors are now part of the available armamentarium. However, improvements in antitumor activity through combinations may come at a cost of incremental toxicities or financial burden, and weighing the magnitude of benefit in the face of multiple treatment options is crucial for balanced treatment decisions.
In this article, we first review highlights of data for single agents to provide a context to understand the efficacy of combinatorial approaches in melanoma, including the currently approved combinations of immune-checkpoint blockers and combined BRAF and MEK inhibitors. We then discuss emerging strategies using multi-modality regimens for the treatment of advanced melanoma. Given the high number of combinations under investigation, we focus exclusively on combinations that are approved by regulatory agencies in clinical use or combinations in large phase 3 randomized studies for the treatment of patients with unresectable stage III or IV melanoma.
First-line monotherapy regimens in melanoma as a background to understanding efficacy of combinations
The incorporation of monoclonal antibodies capable of inhibiting negative co-receptors in the immune synapse and reversing immune-tolerance and T-cell exhaustion mechanisms, initially through the anti-CTLA-4 agent ipilimumab, and subsequently though PD-1 blockers, represented a major breakthrough in the management of advanced melanoma. Following initial survival gains resulting from ipilimumab, a series of studies demonstrated even more robust antitumor activity with both nivolumab and pembrolizumab as single-agents. Despite the need for more efficacious approaches, single-agent ICB with either nivolumab or pembrolizumab remains standard first-line treatment for patients with advanced melanoma.
In recent updates with long-term follow up of phase I clinical trials, both nivolumab and pembrolizumab demonstrated objective response rates (ORR) of approximately 30%, with almost 34% of the patients alive at five years.(6,7) When used in immunotherapy-naïve patients, which corresponds to a current indication for anti-PD-1 agents, both pembrolizumab and nivolumab showed superiority in terms of ORR, progression-free survival (PFS), overall survival (OS), and tolerability when compared to ipilimumab. The activity of first-line, single-agent nivolumab was demonstrated in two randomized trials, CheckMate-066 (limited to patients with BRAF wild-type tumors, in comparison to chemotherapy) and CheckMate-067 (in comparison to ipilimumab).(8,9) In the first study, nivolumab resulted in an ORR of 42.8% and a median OS of 37.5 months, with 15% of the patients developing grade 3 or 4 AEs. In CheckMate-067, median OS was 36.9 months, with a 5-year OS rate of 44%. In the phase III KEYNOTE-006 trial, patients were randomized to two dosing regimens of pembrolizumab administered every three weeks, or ipilimumab 3 mg/kg every three weeks for four doses. After a median follow-up of 57.7 months, pembrolizumab resulted in a significant improvement in OS (median OS: 32.7m vs 15.9m; HR 0.73; p=0.00049) and a favorable toxicity profile, with an incidence of treatment-related grade 3–4 AEs of 17%. In the combined treatment-naïve cohorts treated with pembrolizumab, median OS was 38.7 months, with 43.2% of the patients alive at 5 years. (10)
In addition to ICB, MAPK pathway blockade has proven to be an important approach for the treatment of advanced melanoma in patients with BRAF mutations. The BRAF inhibitors vemurafenib and dabrafenib demonstrated striking activity in randomized trials, with ORR approaching 50%. Nevertheless, the onset of secondary resistance resulted in short-lived disease control, resulting in median PFS intervals approaching 7 months and a median OS of approximately 14 months. Long-term benefit was limited to a small proportion of patients.(11,12) With the development of superior approaches that combined BRAF and MEK inhibitors, the use of single-agent BRAF inhibitors is no longer acceptable, unless patients have specific contraindications to MEK inhibitors such as a low cardiac ejection fraction or poor eyesight.
Combinatorial approaches in advanced melanoma
Combinations of immune-checkpoint blockers
The combination of ipilimumab with nivolumab was initially interrogated in a phase 1 trial that included 86 patients with advanced melanoma and was based upon the rationale that CTLA-4 and PD-1 modulate distinct, non-redundant immunologic mechanisms of escape from immune surveillance. Supporting preclinical evidence additionally suggested enhanced, and potentially synergistic activity with dual ICB blockade.(13, 14) The phase 1 study consisted of 53 patients receiving escalating doses of concurrent ipilimumab combined with nivolumab administered every three weeks for four doses, followed by single-agent nivolumab every three weeks for four doses, and subsequently the combined treatment every twelve weeks for up to eight doses. The ORR in these initial concurrent cohorts according to modified World Health Organization (WHO) criteria was 40%; treatment-related grade 3 or 4 AEs were noted in 53%. In dose escalation, both nivolumab 1mg/kg + ipilimumab 3mg/kg and nivolumab 3mg/kg + ipilimumab 1mg/kg had similar rates of efficacy and toxicity. It was therefore difficult to determine the best dose to advance for clinical development. Given dose dependent efficacy for ipilimumab as monotherapy and no clear dose dependency of single agent anti-PD1 agents along with knowledge that only ipilimumab 3mg/kg had an established OS benefit, the nivolumab 1mg/kg + ipilimumab 3mg/kg regimen was chosen for phase 2 and 3 study.(15–17)
Two subsequent trials led to the approval of the combination of ipilimumab and nivolumab by the FDA for patients with advanced melanoma in 2015. CheckMate-069 was a double-blind, randomized phase II study that included 142 treatment-naïve patients who received ipilimumab 3mg/kg combined nivolumab 1mg/kg or placebo every three weeks for four doses, followed by nivolumab 3mg/kg or placebo every two weeks until disease progression or unacceptable toxicity.(18) The primary endpoint was the rate of objective responses among individuals with BRAF wild-type (wt) tumors, which corresponded to approximately 75% of the study population. In this subgroup, the ORR was 61% for the combination (similar to those with BRAF-mutant tumors) versus 11% for those receiving ipilimumab alone (p<0.001). Treatment-related AEs of grades 3 or 4 occurred in 54% of the patients treated with the combination. In a subsequent publication of longer-term results, with a median follow-up 24.5 months, the 2-year OS rate in the combination arm was 63.8%. (19)
The pivotal CheckMate-067 study that confirmed the efficacy of ipilimumab + nivolumab was a three-arm, double-blind, randomized trial in which 945 patients with unresectable stage III or stage IV melanoma were randomized in a 1:1:1 ratio to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every three weeks for four doses followed by nivolumab every two weeks, nivolumab 3 mg/kg plus placebo every two weeks, or ipilimumab 3mg/kg plus placebo.(20) The study was designed to compare the nivolumab-containing arms with single-agent ipilimumab, with PFS and OS as co-primary endpoints; the direct comparison of the combination versus single-agent nivolumab was exploratory. Patients were stratified according to tumor PD-L1 expression, BRAF V600 mutation status and American Joint Committee on Cancer stage. Following initial demonstration of improvement in PFS for the nivolumab containing arms vs. ipilimumab, the 5-year outcomes in this trial have been recently updated.(21) The median OS has not been reached for the ipilimumab and nivolumab group (more than 60 months), and was 36.9 months and 19.9 months for single-agent nivolumab or ipilimumab, respectively, with an apparent plateau on survival curves at three years after treatment initiation. The 5-year OS rate was 52% among patients treated with combination, 44% in the nivolumab group and 26% in the ipilimumab group, with 5-year PFS rates of 36%, 29% and 8%, respectively. Fifty-eight percent of those receiving ipilimumab plus nivolumab achieved an objective response versus 45% in the nivolumab group and 19% in the ipilimumab group. Grade 3 and 4 adverse events for the combination, nivolumab alone, and ipilimumab alone were 59%, 23%, and 28%, respectively, and 42% of the patients discontinued treatment due to toxicities in the combination arm, versus 13% with single agent nivolumab. (21)
The high incidence of immune-related AEs has been a major concern with the use of combined ICB blockade, and efforts have focused on alternative strategies to mitigate these toxicities. In the phase 1b KEYNOTE-029 trial, 153 patients were treated with a regimen consisting of “low dose” ipilimumab (1mg/kg) combined with standard dose of pembrolizumab 2mg/kg given every 3 weeks for four doses, followed by single-agent pembrolizumab for up to two years. The ORR in this study was 57%, with 89% of the patients alive at 12 months. Grade 3–4 AEs occurred in 45% of the cases, with toxicities leading to treatment discontinuation in only 14% of the patients.(22) While non-randomized, these data were the first to suggest lower dose ipilimumab + anti-PD1 may have similar efficacy with lower toxicity as the higher ipilimumab dose regimens in combination with anti-P1.
To more formally explore this essential dosing question, the randomized CheckMate-511 study tested whether ipilimumab 1mg/kg in combination with nivolumab 3mg/kg reduced incidence of severe AEs in comparison to the standard dosing of the combination regimen, ipilimumab 3mg/kg + nivolumab 1mg/kg.(23) The primary end point was the rate of treatment-related grade 3–5 AEs; ORR, PFS, OS and health-related quality of life were secondary outcomes. The alternative dosing regimen of ipilimumab 1mg/kg and nivolumab 3mg/kg resulted in a reduced incidence of grade 3–5 AEs (34% vs 48%; p=0.006), largely influenced by lower rates of hepatic, gastrointestinal and endocrine toxicities. Efficacy, while a descriptive comparison only since the study was not designed as a non-inferiority trial, did not appear different between the ipilimumab 1mg/kg and 3mg/kg arms respectively (ORR 45.6% vs 50.6%, median PFS 9.9m vs 8.9m, median OS not reached in both arms).(23) Longer follow-up of this study will be needed to truly understand whether lower doses of ipilimumab are as apparently effective as the standard dose of this combination.
Combinations of BRAF and MEK inhibitors in BRAF-mutant melanoma
As previously highlighted, despite initial response, secondary resistance, largely attributable to the reactivation of the MAPK pathway in 70% of cases, often limits the benefit of single-agent BRAF inhibitors.(24) Hence, a multi-targeted approach, with combined blockade of downstream signaling components through the addition of a MEK inhibitor emerged as an option with the capability to delay treatment resistance and enhance the antitumor effect of targeted-therapy. In addition, this strategy was developed to reduce toxicities related to the use of single-agent BRAF inhibitors that resulted from paradoxical activation of the MAPK pathway, particularly hyperkeratosis and second-primary cutaneous malignancies (keratoacanthomas and squamous-cell carcinomas). Following early evidence that simultaneous, rather than sequential administration of BRAF and MEK inhibitors, could optimize the antitumor effect, four randomized, phase 3 studies confirmed the superiority in terms of ORR, PFS and OS of BRAF inhibitors (vemurafenib, dabrafenib or encorafenib) administered concurrently with MEK inhibitors (cobimetinib, trametinib or binimetinib) in comparison to single-agent BRAF inhibitors, resulting in the approval by the FDA of three doublets.
The combination dabrafenib (150mg twice daily continuously) plus trametinib (2mg once daily continuously) was compared to single-agent dabrafenib or vemurafenib in the COMBI-d and COMBI-v trials, respectively, with ORRs approaching 70% and a complete response rate of 19% for the dabrafenib + trametinib combination. In a pooled-analysis that included 563 patients treated with the combination, 34% were alive and 19% were progression-free at 5 years, with median PFS and OS of 11.1 months and 25.9 months, respectively. Of note, patients with normal lactate dehydrogenase (LDH) levels and less than three sites of metastatic disease were more likely to achieve responses of prolonged duration and to be alive at 5 years. (25, 26)
In the coBRIM study, 495 patients with previously untreated, BRAF mutant, advanced melanoma were randomly assigned to vemurafenib (960 mg twice daily continuously, on days 1 to 28) plus cobimetinib (60 mg daily on days 1 to 21, followed by a 7-day interval off cobimetinib) in 28-day cycles, or to vemurafenib plus placebo. In an analysis from the four-year extended follow-up, the gains in OS with combined blockade were confirmed (median OS: 22.5 vs 17.4 months; p=0.005), with 34.7% alive at 5 years. The incidence of grade 3–5 AEs was 77%. (27) The 5-year data of BRAF + MEK inhibitors provide reassurance that there is a “tail-of-the-curve” with targeted therapy as well as immunotherapy. 5-year PFS and OS rates of BRAF + MEK and anti-PD-1 agents appear similar.
The third doublet of BRAF and MEK inhibitors, encorafenib and binimetinib, was investigated in the randomized, phase 3 COLUMBUS trial. Five hundred and seventy-seven patients, were randomly assigned to the combination (n=192), single-agent encorafenib (n=194) or single-agent vemurafenib (n=191). The ORR, median PFS and median OS among patients treated with the combination were 64% (following central review), 14.9 months and 33.6 months. Interestingly, encorafenib and binimetinib resulted in a more favorable toxicity profile, with an incidence of grade 3–4 AEs of 64%, versus 67% with single-agent encorafenib, and 66% with single-agent vemurafenib.(28) This is especially notable since the encorafenib dose of 450mg was higher in combination with binimetinib than as monotherapy (encorafenib 300mg daily).
Activity of combinations in patients with CNS metastases
One of the challenges with combination therapy is appropriate patient selection, and there is a general belief that combinations may be most effective in patients with harder to treat disease. One such population involves patients with central nervous (CNS) metastases. Historically, median survival for patients with CNS metastases was approximately 4 months, and influenced by age, functional status, and activity of extracranial disease. (29, 30)
Three prospective studies have tested various approved combinations for patients with CNS metastases. The COMBI-MB study was an open-label phase 2 study of dabrafenib and trametinib at standard doses administered to 125 BRAF-mutant patients with CNS involvement, allocated in four separate cohorts. In the cohort of asymptomatic patients with BRAFV600E-mutant melanoma (n=76), 58% achieved an intracranial response. Despite this high intracranial response rate, the investigator-assessed PFS was only 5.6 months, and only 19% of the patients remained progression-free at 12 months. The toxicity profile was consistent with previous studies of dabrafenib and trametinib. (31)
In two separate studies, the ABC trial and CheckMate-204 study, the combination of ipilimumab 3mg/kg and nivolumab 1mg/kg administered every three weeks for four doses, followed by single-agent nivolumab, resulted in intracranial activity similar to those observed in patients with exclusively extracranial disease.(32,33) The intracranial ORR was approximately 50%-60% in selected, asymptomatic patients not requiring systemic steroids, and the rate of progression-free survival at 9-months was almost 60%. No new safety signals were identified in patients with intracranial disease. Of note, single-agent nivolumab resulted in objective responses in only 20% of asymptomatic patients treated in the monotherapy arm in ABC trial, suggesting the incorporation of ipilimumab substantially adds efficacy on top of anti-PD-1 alone for patients with brain metastases. (32)
Despite the activity of the combination of nivolumab + ipilimumab and dabrafenib and trametinib in selected patients with intracranial disease, these results cannot be generalized to all patients in clinical practice who have CNS metastases. Efficacy in patients who require steroids or are otherwise symptomatic is believed to be low, highlighting the unmet need for more efficacious approaches for these individuals. Follow-up time remains short in these CNS metastasis trials as well, compared to the 5-year follow-up data in prior registrational trials of these agents.
Combinations of Immunotherapy and MAPK Pathway Targeted Therapy
Supported by preclinical evidence suggesting a potential interaction between the MAPK pathway and modulation of the tumor immune microenvironment and immune responses through T-cell activation, signaling and trafficking, and increased expression of melanoma antigens in the setting of MAPK pathway inhibition,(34,35) several trials were launched to test combinations of ICB blockade with BRAF +/− MEK inhibitors. Unfortunately, combinations of MAPK pathway inhibitors with ipilimumab were found to have toxicity issues, but given their lower rates of toxicity, PD1/PDL1 agents were found to be more favorable combinatorial partners. (36, 37)
Encouraging disease-control rates approaching 100% in early phase studies with triplet combinations (BRAF and MEK inhibitors in combination with an anti-PD-1 or PD-L1 agents) in BRAF-mutant melanoma prompted a rapid development of larger, randomized studies investigating whether adding PD-1 or PD-L1 improves the efficacy of BRAF + MEK alone.(38, 39) In the phase 2, placebo-controlled, randomized part of the KEYNOTE-022 study, patients with BRAFV600E/K-mutant melanoma were randomly assigned to dabrafenib and trametinib or a triplet combination with the addition of pembrolizumab; the trial did not meet its primary endpoint and failed to demonstrate a statistically significant improvement in PFS, despite a median PFS of 16.0m in the triplet arm vs 10.3m with dabrafenib and trametinib alone.(40) Longer-term follow-up from this study will be important to know whether benefits of triplet therapy increase over time.
The largest randomized trial conducted to date exploring the triplet strategy was recently presented. The IMspire 150 (TRILOGY) was a phase 3, double-blind, placebo-controlled study, in which patients with BRAFV600E-mutation-positive advanced melanoma were randomized to the combination of vemurafenib and cobimetinib at standard doses plus placebo or, following a run-in period of 28 days, to vemurafenib 720mg twice daily continuously, cobimetinib 60mg daily for 21 days and atezolizumab 840mg administered intravenously every 14 days.(41) The trial met its primary endpoint by demonstrating a significant improvement in investigator-assessed PFS favoring the triplet combination (median PFS: 15.1m vs 10.6m; p=0.0249); OS data were immature at the time of this primary analysis. Interestingly, and somewhat different from the preliminary findings from early-phase clinical trials, ORR were similar (66.3% with vemurafenib, cobimetinib and atezolizumab versus 65% with vemurafenib, cobimetinib and placebo); there was no significant increase in the incidence of serious treatment-related adverse events. A similar trial using the triplet combination of the anti-PD-1 agent spartalizumab (PDR-001), dabrafenib and trametinib has completed accrual and results are awaited (NCT02967692). One important consideration with all of these triplet trials is that none include protocol mandated cross-over to a PD1/PDL1 agent at time of progression in the BRAF + MEK alone group as would be pursued in routine clinical practice. Therefore, even though PFS favors triplet therapy, clinicians and health care systems may still prefer sequential use of BRAF + MEK and immunotherapy (or vice versa) as it remains unclear whether overall survival will be improved with upfront triplet therapy vs. sequential administration of these agents.
While BRAF mutant melanoma has been the focus to study MAPK pathway inhibitors and immunotherapy, the activity of combined MAPK pathway inhibition (MEK inhibition) and ICB blockade was also investigated in patients with BRAF wild-type tumors, with disappointing results. In the phase 3, randomized IMspire 170 trial, there was no significant improvement in PFS, OS or ORR with the combination of cobimetinib and atezolizumab in comparison to single-agent pembrolizumab (median PFS – primary endpoint: 5.5m vs 5.7m; p=0.295). Why this study was negative is unclear. It could have been due to the low activity of MEK inhibitors in BRAF wildtype melanoma, differences between PD-L1 and PD-1 inhibition in melanoma, or from immunologic effects of MEK inhibition which may have been detrimental to anti-tumor immunity. Nonetheless, this result was similar to another randomized study in colorectal cancer where the combination of MEK and PD-L1 inhibition was not found to be as effective as hoped.(42)
Other Immunotherapy Combinations
In addition to anti-CTLA-4 and anti-PD-1 immunotherapies, additional combinatorial agents are being evaluated to enhance antigen processing and presentation, inhibit other T cell checkpoints, and provide immunologically beneficial cytokines with the goal of increasing an anti-tumor immune response. Talimogene laherparepvec (T-VEC) was approved by the FDA based upon the results of a phase 3 trial in which patients with stage IIIB-IV melanoma were treated with intratumoral injections of T-VEC. T-VEC resulted in improved durable responses when compared to GM-CSF alone.(5) The potential for combining T-VEC and systemic ICB has been tested in clinical trials, with promising results and an encouraging safety profile. A randomized study compared the combination of T-VEC and ipilimumab to ipilimumab alone in 198 patients with unresectable stage IIIB to IV melanoma. The use of the combination resulted in a significant improvement in ORR (39% vs 19%; p=0.002), with responses in lesions that did not receive T-VEC injections.(43) Using a similar approach, T-VEC was combined with pembrolizumab in a phase 1b study including 21 patients; 57.1% achieved an objective response, including 23.8% confirmed complete responses.(44) While of interest, given challenges interpreting efficacy of combinatorial strategies in small, single arm studies, we await randomized data and answers to the question of whether T-VEC improves the efficacy of pembrolizumab in the ongoing phase 3, MASTERKEY-265 clinical trial (NCT02263508).
In the field of combined immunotherapy approaches, the activity of combinations of anti-PD-1 agents with agents that target the lymphocyte-activation gene 3 (LAG-3) checkpoint and the interleukin-2 (IL-2) pathway (bempegaldesleukin) are currently in randomized phase 3 studies. LAG-3 is a negative regulator of T-cell function. Relatlimab, an anti-LAG-3 monoclonal antibody, demonstrated activity in a phase I/IIa study in combination with nivolumab in immunotherapy refractory patients and is currently being investigated in a randomized, phase II/III study in treatment-naïve patients in combination with nivolumab vs. nivolumab alone (NCT03470922).(45) Bempegaldesleukin is a pegylated IL-2 agonist with preferential binding to the IL-2 receptor’s beta-gamma subunit as opposed to the alpha subunit. This preferential binding is hypothesized to lead to more T effector proliferation with less immunosuppressive T regulatory cell expansion. In early phase non-randomized studies, bempegaldesleukin in combination with nivolumab resulted in encouraging antitumor activity, but similar to relatlimab, randomized data are needed.(46) A phase 3 study testing bempegaldesleukin + nivolumab vs. nivolumab alone is accruing patients (NCT3635983).
Discussion and Conclusions
Robust advances confirmed across several clinical trials have led to the approval of multiple therapies for the treatment of patients with advanced melanoma, improving life expectancy in profound ways. Examples of these agents incorporated into clinical practice in recent years include ICB blockade with anti-CTLA-4 and anti-PD-1 monoclonal antibodies, targeted therapies represented by BRAF and MEK inhibitors and the oncolytic viral therapy, T-VEC. Despite the improvements produced by single-agent regimens, combinatorial approaches are currently considered among the standard treatment options and have substantially improved the outcomes for patients with advanced melanoma. For those with tumors harboring a BRAF mutation and candidates for targeted-therapy, the combined use of BRAF and MEK inhibitors has consistently replaced single-agent vemurafenib or dabrafenib. In the field of immunotherapy, single agent anti-PD-1 with nivolumab or pembrolizumab alone or the nivolumab + ipilimumab combination is a reasonable treatment approach.
This multiplication in the number of treatment alternatives favorably translates into a welcome growing complexity of treatment algorithms for patients who present with advanced melanoma (Figure 1). Unfortunately, the only established biomarker continues to be the BRAF mutation status, as the expression of PD-L1 has not been shown to be a useful biomarker in melanoma to select treatment. The best treatment to be given upfront for BRAF-mutant patients remains to be determined, and results of ongoing studies looking at the best sequence and combinations of BRAF/MEK inhibition and immune-checkpoint blockade are eagerly awaited. More recently, with the possibility of using triplet combinations composed of BRAF and MEK inhibitors in combination with PD1/PDL1 ICB blockers, a relevant clinical question that is beginning to emerge is which patients with BRAF mutant tumors will be the best candidates for immunotherapy alone versus the combination of immunotherapy with targeted therapies versus targeted therapy alone. Overall survival data from randomized phase 3 studies with high rates of PD1 ICB use after progression in the BRAF + MEK alone control group are needed to fully understand where triplet therapy will reside in the treatment algorithm.
Figure 1.
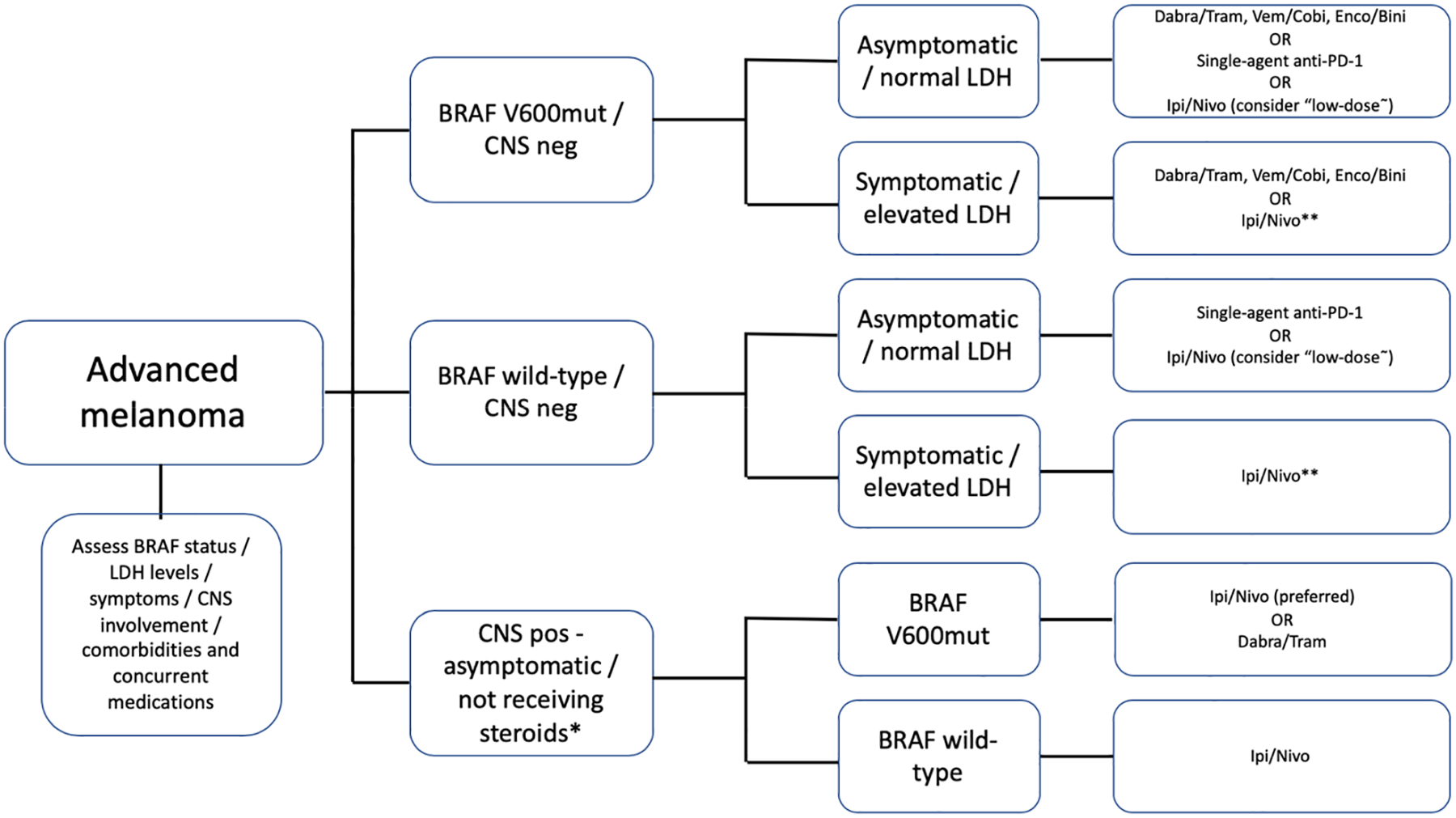
Overview of proposed treatment algorithms involving combinatorial approaches for patients with advanced melanoma.
Dabra – dabrafenib / Tram – trametinib / Vem – vemurafenib / Cobi – cobimetinib / Enco – encorafenib / Bini – binimetinib / Ipi/Nivo – ipilimumab 3mk/kg + nivolumab 1mg/kg / “low-dose”- ipilimumab 1mg/kg + nivolumab 3mg/kg / * - for patients with symptomatic CNS metastases or using steroids, the optimal approach remains unclear / ** - in these scenarios, single-agent anti-PD-1 is also an acceptable alternative, particularly in select situations (underlying autoimmune diseases, comorbidities, etc.)
Variables that directly influence the choice for first-line therapies encompass, in addition to the BRAF-mutation status, the presence or absence of brain metastases, serum LDH levels, the burden of disease, presence of symptoms, and additional factors that include barriers to accessing care, social support, comorbidities (eg: underlying auto-immune diseases, history of solid-organ transplant) and the use of concurrent medications.(47) For patients with normal LDH levels, limited disease burden, and no CNS involvement, long-term benefit can be achieved with both BRAF/MEK inhibitors and ICB blockade, as demonstrated by recent updates of KEYNOTE-006, CheckMate-067, COMBI-v and COMBI-d studies. For this subgroup, the favorable safety profile demonstrated by single-agent anti-PD-1 in comparison to ipilimumab + nivolumab must be considered, although the latter regimen can be considered due to the potential benefits in response rate and PFS, particularly adopting ipilimumab at “low doses” (1mg/kg). In parallel, this is also the subgroup that does best with combined BRAF/MEK inhibitors. In this setting of favorable prognostic factors, comorbidities, the spectrum of adverse events, preference for oral or intravenous therapy, and the potential of drug interactions may contribute to the choice of treatment, as targeted-agents may share metabolic pathways with concomitant medications through the cytochrome P450 complex. Based upon the results of contemporary, non-comparative trials with a limited number of patients, nivolumab + ipilimumab remains the treatment of choice for those presenting with asymptomatic CNS involvement, and can be considered for individuals with BRAF wild-type melanoma with elevated LDH, multiple sites of disease (particularly in the presence of bone or liver involvement) or symptomatic patients who may only have an opportunity for one line of systemic therapy.
In summary, combinatorial approaches represent a new standard in the management of patients with advanced melanoma, and an expansion in the plethora of potential combinations is expected in the coming years. Nevertheless, translational research, effective biomarkers and future clinical trials are warranted to address the large body of questions that remain to be answered, and to enable rational, patient-centered, and cost-effective incorporation of novel approaches.
Table 1.
Summary of selected, pivotal randomized clinical trials addressing combinatorial approaches for the treatment of patients with advanced melanoma.
| Study | Author/Year | Regimen* | ORR* | PFS / OS* | AEs* (grade 3 or higher) |
|---|---|---|---|---|---|
| Combined immune-checkpoint blockade | |||||
| CM-069 | Postow et al. 2015 | IPI 3mg/kg + NIVO 1mg/kg -> NIVO 3mg/kg | 61%# | NR | 54% |
| CM-067 | Larkin et al. 2019 | IPI 3mg/kg + NIVO 1mg/kg -> NIVO 3mg/kg | 58% | Median PFS: 11.5m / 5y OS: 52% | 59% |
| CM-511 | Lebbé et al. 2019 | IPI 1mg/kg + NIVO 3mg/kg | 45.6% | Median PFS: 9.9m | 34% |
| Combined BRAF and MEK inhibitors ** | |||||
| COMBI-v ** | Long et al. 2019 | Dabrafenib 150mg bid + Trametinib 2mg qd | 68% | Median PFS: 11.1m / Median OS: 25.9m | 59% |
| COMBI-d ** | |||||
| coBRIM ** | Dréno et al. 2018 | Vemurafenib 960mg bid + Cobimetinib 60mg qd | 70% | Median OS: 22.5m | 77% |
| COLUMBUS ** | Dummer et al. 2018 | Encorafenib 450mg QD + Binimetinib 45mg BID | 63% (central review) | Median PFS: 14.9m / Median OS: 33.6m | 64% |
| Combined IT and TT | |||||
| KEYNOTE-022 ** | Ascierto et al. Nat Med 2019 | Pembrolizumab 2mg/kg q3w + Dabrafenib 150mg BID + Trametinib 2mg QD | - | Median PFS: 16m | 58.3% |
| TRILOGY ** | McArthur et al. 2020 | Vemurafenib 720mg BID + Cobimetinib 60mg QD + Atezolizumab 840mg q14d | 66.3% | Median PFS: 16.1m / median OS 28.8m | - |
- in treatment arms comprising combinations /
- trials limited to patients with tumors harboring a BRAF B600E or V600K mutation /
- among patients with BRAF wild-type tumors /
IT – immunotherapy / TT – targeted therapy / ORR – objective response rate / PFS – progression-free survival / OS – overall survival / AEs – incidence of grade 3 or higher adverse events / NR – not reached / IPI- ipilimumab / NIVO – nivolumab / BID – twice daily / QD – once daily
Clinics Care Points.
Combinatorial approaches in melanoma hold the potential to enhance antitumor activity and, in specific scenarios, mitigate toxicities;
Single-agent nivolumab or pembrolizumab result in response rates of approximately 40%, with the possibility of long-term benefits; single-agent T-VEC has limited role in the first-line setting; single-agent BRAF inhibitors are no longer are a standard treatment due to superiority of BRAF + MEK inhibitor combinations;
Combinatorial approaches currently approved for clinical use include ipilimumab and nivolumab and, for patients with melanoma harboring a BRAF mutation, doublets of BRAF and MEK inhibitors vemurafenib and cobimetinib, dabrafenib and trametinib and encorafenib and binimetinib;
Across several randomized, clinical trials, combinations of BRAF/MEK inhibitors resulted in superior outcomes in comparison to single-agent BRAF inhibitors, without an increase in the absolute incidence of adverse events;
The combination of ipilimumab and nivolumab results in an increased objective response rate at the risk of an increased frequency of immune-related adverse events and treatment discontinuations. No statistically significant improvement in overall survival over single agent anti-PD-1 alone has been demonstrated to date with this combination; Treatment decisions for patients with advanced melanoma are driven by BRAF-mutation status, presence or absence of central nervous system metastases, disease burden, symptoms, serum LDH levels, comorbidities and concurrent medications, among other factors.
Synopsis.
The treatment landscape for patients with advanced melanoma has dramatically improved over the past decade, leading to unprecedented survival. Despite the robust activity of single-agent immune-checkpoint blockade with anti-CTLA-4 or anti-PD-1 agents, and the efficacy of targeted-therapies capable of interrupting aberrant signaling resulting from BRAF mutations, the benefit from these therapies is not universal. Advanced understanding of immune and molecular processes underlying melanoma tumorigenesis has demonstrated the promise of combined, multi-drug regimens. In this review, we discuss the currently available evidence that supports the use of combinatorial approaches in the treatment of advanced melanoma and provide insights into promising new combination strategies under investigation.
Acknowledgements:
There was no specific funding received for this manuscript.
Disclosures:
RRM - Research involvement: BMS, Lilly, Merck, MSD, Novartis, Roche. Honoraria: Bayer, BMS, Merck, MSD, Novartis, Roche, Sanofi. Travel grants: BMS, Novartis, Sanofi; MAP - Consulting fees from 2015-Present: BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro. Honoraria: BMS and Merck. Institutional Support: RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, AstraZeneca
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rodrigo Ramella Munhoz, Oncology Center, Hospital Sírio Libanês, Instituto do Cancer do Estado, Rua Dona Adma Jafet, 91, São Paulo, Brazil 01308-050.
Michael Andrew Postow, Melanoma Service, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, NY, USA.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. Ca Cancer J Clin 2020; 70:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Glazer AM, Winkelmann RR, Farberg AS et al. Analysis of trends in US melanoma incidence and mortality. JAMA Dermatol 2017; 153(2): 225–226. [DOI] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune setpoint. Nature 2017; 541 (7637): 321–330. [DOI] [PubMed] [Google Scholar]
- 4.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma.Nat Rev Clin Oncol 2017; 14(8): 463–482. [DOI] [PubMed] [Google Scholar]
- 5.Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J Immunother Cancer 2019; 7(1): 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol 2019; (10): 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30(4): 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously-untreated BRAF wild-type advanced melanoma treated with nivolumab therapy. Three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019; 5(2): 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R et al. Five-year survival with combined nivolumab + ipilimumab in advanced melanoma. N Engl J Med 2019; 381: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 10.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomized, controlled, phase 3 study. Lancet Oncol 2019; 20(9): 1239–1251. [DOI] [PubMed] [Google Scholar]
- 11.McCarthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A, Ascierto PA, Schadendorf D et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib monotherapy: Analysis from phase 2 and 3 clinical trials. Eur J Cancer 2020; 125: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Kluger H, Callahan MK et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010; 107(9):4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11(2): 155–164. [DOI] [PubMed] [Google Scholar]
- 16.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622 [DOI] [PubMed] [Google Scholar]
- 17.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postow MA, Chesney J, Pavlick AC et al. Nivolumab + ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodi FS, Chesney J, Pavlick AC et al. Combined nivolumab + ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016; 17 (11): 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R et al. Combined nivolumab + ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larkin J, Chiarion-Sileni V, Gonzalez R et al. Five-year survival with combined nivolumab + ipilimumab in advanced melanoma. N Engl J Med 2019; 381: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 22.Long GV, Atkinson V, Cebon JS et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol 2017; 18(9): 1202–1210. [DOI] [PubMed] [Google Scholar]
- 23.Lebbé C, Meyer N, Mortier L et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/Iv CheckMate 511 trial. J Clin Oncol 2019; 37(11): 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizos H, Menzies AM, Pupo GM,, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014; 20(7):1965–1977. [DOI] [PubMed] [Google Scholar]
- 25.Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016; 17 (12): 1743–1754. [DOI] [PubMed] [Google Scholar]
- 26.Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019; 381: 626–636. [DOI] [PubMed] [Google Scholar]
- 27.Dréno B, Ascierto PA, McCarthur GA, et al. Efficacy adn safety of cobimetinibe combined with vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: analysis from the 4-year extended follow up of the phase 3 coBRIM study. J Clin Oncol 2018; 36, no. 15_suppl 9522. [Google Scholar]
- 28.Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in COLUMBUS: A phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) vs vemurafenib (VEM) or ENCO in BRAF-mutant-melanoma. J Clin Oncol 2018; 36, no 15_suppl 9504. [Google Scholar]
- 29.Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 2017; 19:1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: An update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017; 99(4):812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinibe in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017, 18(7): 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long GV, Atkinson V, Lo S, et al. Combination nivolumab + ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018; 19(5): 672–681. [DOI] [PubMed] [Google Scholar]
- 33.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab + ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018; 379(8): 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013; 19(5): 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu-Lieskovan S, Mok S, Moreno BH, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAFV600E melanoma. Sci Transl Med 2015; 7(279): 279ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribas A, Hodi FS, Callahan MK, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368 (4): 1365–1366. [DOI] [PubMed] [Google Scholar]
- 37.Minor DR, Puzanov I, Callahan MK, et al. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res 2015; 28(5): 611–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan RJ, Hamid O, Gonzalez R, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med 2019; 25(6): 929–935 [DOI] [PubMed] [Google Scholar]
- 39.Ribas A, Lawrence D, Atkinson V, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med 2019; 25(6): 936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascierto PA, Ferrucci PF, Fisher R, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 2019; 25(6): 941–946. [DOI] [PubMed] [Google Scholar]
- 41.McCarthur GA, Stroyakovskiy D, Gogas H et al. Evaluation of atezolizumab, cobimetinib, and vemurafenib in previously untreated patients with BRAFV600 mutation-positive advanced melanoma: primary results from the phase 3 IMspire150 trial. Presented during AACR Annual meeting. April, 2020. [Google Scholar]
- 42.Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019; 20(6): 849–861. [DOI] [PubMed] [Google Scholar]
- 43.Chesney J, Puzanov I, Collichio F, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of TalimogeneLaherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol 2018; 36(17): 1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol 2016; 34_suppl 9568. [Google Scholar]
- 45.Ascierto PA, Melero I, Bhatia S, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J Clin Oncol 2017; 35, no 15_suppl 9520. [Google Scholar]
- 46.Diab A, Cho D, Papadimitrakopoulou V, et al. NKTR-214 (CD122-biased agonist) plus nivolumab in patients with advanced solid tumors: Preliminary phase 1/2 results of PIVOT. J Clin Oncol 2018; 36, no_15_suppl: 3006. [Google Scholar]
- 47.Seth R, Messersmith H, Kaur V, et al. Systemic therapy for melanoma: ASCO Guideline. J Clin Oncol 2020; published ahead of print. doi: 10.1200/JCO.20.00198. [DOI] [PubMed] [Google Scholar]


