Highlights
-
•
GlycA more strongly predicted incident MI.
-
•
hsCRP more strongly predicted incident ischemic stroke.
-
•
GlycA may detect other inflammatory risk not captured by hsCRP
Keywords: Inflammation, Myocardial infarction, Ischemic stroke, Risk
Abstract
Objective
Inflammatory markers are associated with cardiovascular disease (CVD); however, the ability to specifically predict myocardial infarction (MI) as well as ischemic stroke remains unknown. There has not been a direct comparison of the associations between GlycA and hsCRP and MI and ischemic stroke in a multi-ethnic pooled cohort.
Methods
Multi-center, multi-ethnic, population-based community prospective pooled cohort of the Dallas Heart Study (DHS) and Multi-Ethnic Study of Atherosclerosis (MESA). 9,785 participants without baseline CVD enrolled with median follow-up of 13.4 years. Fatal/nonfatal MI and fatal/nonfatal ischemic stroke were assessed separately and then combined.
Results
GlycA was moderately associated with hsCRP (R=0.58 in DHS and R=0.55 in MESA). In adjusted Cox proportional hazards models with competing risk adjusted for both inflammatory markers, GlycA was directly associated with MI (HR Q4 vs. Q1 1.90, 95% CI 1.39 to 2.58), whereas hsCRP was not (HR Q4 vs. Q1 0.92, 95% CI 0.70 to 1.21). Conversely, hsCRP was directly associated with ischemic stroke (HR Q4 vs. Q1 1.73, 95% CI 1.15 to 2.59), but GlycA was not (HR Q4 vs. Q1 1.21, 95% CI 0.77 to 1.90). GlycA improved net reclassification for MI and hsCRP did so for ischemic stroke.
Conclusions
Although both GlycA and hsCRP were associated with incident CVD, GlycA more strongly predicted incident MI, and hsCRP more strongly predicted ischemic stroke.
Graphical abstract
Abbreviations List
- MI
myocardial infarction
- hsCRP
high sensitivity C-reactive protein
- CVD
cardiovascular disease
- DHS
Dallas Heart Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- BMI
body mass index
- SBP
systolic blood pressure
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
1. Introduction
High-sensitivity C-reactive protein (hsCRP) is a marker of inflammation and is associated with atherosclerotic cardiovascular disease (ASCVD) events [1,2]. The 2018 American College of Cardiology cholesterol guidelines identify hsCRP as a risk enhancer that, if measured, can help risk stratify borderline-risk individuals [3].
GlycA is a novel integrated marker of systemic inflammation assessed clinically by nuclear magnetic resonance (NMR) measurement of the N-acetyl methyl group signals of several abundant acute phase proteins in serum. The main inflammatory contributions to the GlycA signal are alpha1-acid glycoprotein, haptoglobin, alpha1-antitrypsin, alpha1-antichymotrypsin and transferrin. Strengths of GlycA include its composite measurement of changes in both the complexity and number of N-glycan side chains and low intraindividual variation compared to hsCRP [4], [5], [6]. Further, while GlycA correlated with body mass index (BMI), homeostatic model assessment for insulin resistance (HOMA-IR), hsCRP, leptin and leptin/adiponectin ratio and inversely with adiponectin, GlycA is not significantly affected by glucose tolerance due to the fact that GlycA reflects specifically enzymatic glycosylation and not nonenzymatic glycation giving rise to, for example, glycated hemoglobin (hemoglobin A1c) [7].
GlycA is moderately associated with hsCRP and is also associated with incident ASCVD events [2,[8], [9], [10], [11]]. In individuals with type 2 diabetes and peripheral artery disease, GlycA levels were higher. Further, GlycA predicted all-cause mortality after multivariable adjustment for traditional cardiovascular risk factors when measured by Nightingale [12].
However, much is still to be learned about this relatively novel inflammatory marker. Although GlycA levels are not reduced by statins as are levels of hsCRP, GlycA is reduced by anti-inflammatory therapies as well as by exercise, lifestyle intervention and bariatric surgery [1,8,[13], [14], [15], [16], [17]]. In particular for bariatric surgery patients, a recent study showed that after bariatric surgery GlycA was significantly reduced to levels similar to normal body weight controls despite the intervention group maintaining BMI over 30 kg/m2. The reduction in GlycA was related to increased high density lipoprotein particle size, which also mediated weight reduction [17]. Although some prior studies have assessed GlycA and hsCRP concurrently, they have not yet been directly compared for cardiovascular risk prediction in a multi-ethnic pooled cohort primary prevention population. Furthermore, associations between hsCRP and stroke have been inconsistent and limited by few stroke events [18], [19], [20]. Whether GlycA and hsCRP consistently are associated with both myocardial infarction (MI) and ischemic stroke is unknown [21,22]. We sought to determine associations between GlycA and hsCRP and incident MI and ischemic stroke separately as well as directly compare these inflammatory markers as risk predictors for ASCVD.
2. Methods
The study sample included participants from the Dallas Heart Study (DHS) and the Multi-Ethnic Study of Atherosclerosis (MESA). Both population-based, multi-ethnic cohort studies included participants without baseline CVD with ongoing data collection. DHS is a cohort of Dallas County residents ages 18 to 65 years with an intentional oversampling of black persons to comprise 50% of the cohort. Individuals enrolled between July 2000 to January 2002 with a total enrollment of 3,557 participants. Race/ethnicity groups included white, black, Hispanic and other [23]. MESA is a cohort of participants aged 45 to 84 years from six United States communities enrolling 6,814 individuals. Participants enrolled over a 24-month period beginning in July 2000. Race/ethnicity groups included white, black, Hispanic and Asian (of Chinese descent) [24]. Individuals with missing inflammatory biomarkers (DHS 185 excluded, MESA 65 excluded), follow-up (DHS 460 excluded, MESA 27 excluded), traditional cardiovascular risk factors (DHS 115 excluded, MESA 36 excluded), history of CVD (DHS 196 excluded, MESA none excluded due to study design excluding this risk factor), and glomerular filtration rate less than 15 (DHS 5 excluded, MESA 3 excluded) were excluded. There were no other exclusions for this study. The UT Southwestern Human Research Protection Program reviewed this project and approved it as IRB exempt.
In DHS, data were obtained through two visits. Visit one consisted of a household interview as well as blood pressure and weight measurement by trained field interviewers. Visit two consisted of an in-home phlebotomy collection in EDTA tubes, which were maintained at four degrees Celsius for less than four hours. Samples were centrifuged and plasma was stored at -70 degrees Celsius [23]. Thawed samples using the Roche/Hitachi 912 System, Tina-quant assay (Roche Diagnostics, Indianapolis, IN) measured high-sensitivity C-reactive protein as previously described by Khera, et al. [25]. The precision of these methods has been studied in the past. Over two-thirds of subjects were classified into the same quartile by Roche and Dade systems and there was no variation by more than one quartile [26].
In MESA, data were from the baseline visit. Blood samples were stored at -70 degrees Celsius and analyzed at University of Vermont laboratory (Burlington, Vermont). The Behring Nephelometer-2 measured high-sensitivity C-reactive protein (Dade Behring Inc., Deerfield, Illinois) as previously described by Whelton, et al. [27].
For both DHS and MESA, GlycA was measured on serum or EDTA plasma specimens by NMR LipoProfile® testing (LipoScience (now LabCorp), Raleigh, NC, USA) using a 400 MHz NMR Profiler or Vantera automated analyzer employing the LipoProfile-4 (LP4) deconvolution algorithm. The GlycA NMR signal arises from N-acetyl methyl groups on glycosylated serum proteins (mainly the acute phase proteins alpha1-acid glycoprotein, haptoglobin, alpha1-antitrypsin, alpha1-antichymotrypsin and transferrin). GlycA concentrations are reported in micromol/Liter units [4].
The primary endpoint is a combined outcome of fatal or nonfatal MI and fatal or nonfatal ischemic stroke. The primary endpoint was divided into 1) fatal and nonfatal MI and 2) fatal and nonfatal ischemic stroke. All definite or probable hemorrhagic or embolic stroke events were excluded. Secondary endpoint was defined as composite cardiovascular disease (CVD) defined as ASCVD plus coronary artery bypass graft surgery or percutaneous coronary intervention, peripheral artery disease revascularization, and cardiovascular death.
In DHS, end points were adjudicated by two cardiologists blinded to the exposure variables. International Classification of Diseases, 10th Revision, codes I00 to I99 defined cardiovascular causes of death in the National Death Index. The Dallas-Fort Worth Hospital Council Data Initiative database quarterly assessments provided data on nonfatal cardiovascular events. Participant vital status is ongoing, but this data analysis included status through December 31, 2013 [28]. In MESA, a physician committee adjudicated cardiovascular events. Direct participant contact every 9 months identified participant deaths and hospitalizations for a median follow-up of 15.7 years, and deaths were identified through the National Death Index in participants lost to follow-up [29]. For the pooled cohort analysis median follow-up was 13.4 years (IQR 11.8 -16.1 years).
Variables from DHS and MESA were harmonized and synthesized into one large cohort which was then analyzed by using individual patient level data. Cox proportional hazards models of baseline GlycA or baseline hsCRP associated with the primary endpoint or composite CVD (secondary endpoint) were analyzed for competing risk with the Fine-and-Grey method. GlycA and hsCRP were analyzed primarily as sex- and race-adjusted quartiles. For all Cox models, we adjusted for cohort and used robust standard errors to account for the correlation of patients belonging to a particular cohort. Proportional hazards assumptions were satisfied by checking Schoenfeld residuals. Multivariable models included cohort, age, sex, race/ethnicity, diabetes, systolic blood pressure, anti-hypertensive medication, statin medication, current smoking, body mass index (BMI), total and HDL cholesterol. Subsequently, baseline GlycA and baseline hsCRP were combined in multivariable models. Two-sided P values of 0.05 or less were considered statistically significant. Statistical analysis was performed with SAS software version 9.4 (Raleigh, NC, USA). Analyses were not corrected for multiple comparisons.
Risk prediction indices included calibration, category-less net reclassification index (NRI), integrated discrimination improvement (IDI) and C statistic. All models were well calibrated but the ones for stroke without hsCRP were borderline calibrated (p=0.07). The indices were adapted for the survival analysis setting with calibration assessed via the modified Nam D'Agostino statistic, and the C statistic assessed via Harrell's method. Models were further constructed to evaluate for interactions of several risk factors for each outcome. Interactions for sex, ethnicity, and diabetes were determined a priori.
3. Results
After exclusion criteria, the pooled cohort resulted in a total of 9,279 individuals for our analysis (2,596 individuals from DHS and 6,683 individuals from MESA, Table 1, Supplemental Table 1). The median age of study participants was 57 years with 53.9% women and 33.4% blacks.
Table 1.
Cardiovascular Risk Factors by Quartiles of GlycA and hsCRP.
| Variable |
GlycA | hsCRP | ||||||
|---|---|---|---|---|---|---|---|---|
| (μmol/L) | (mg/L) | |||||||
| Quartile | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 |
| N=2323 | N=2321 | N=2315 | N=2320 | N=2315 | N=2323 | N=2322 | N=2319 | |
| GlycA | 294 | 343 | 384 | 443 | 330 | 354 | 373 | 401 |
| (μmol/L) *,† | (274, 311) | (331, 359) | (368, 399) | (420, 472) | (296, 367) | (317, 392) | (333, 416) | (359, 446) |
| hsCRP | 1.10 | 1.70 | 2.40 | 4.25 | 0.51 | 1.50 | 3.20 | 8.62 |
| (mg/L) *,† | (0.52, 2.40) | (0.81, 3.65) | (1.08, 5.02) | (2.00, 9.43) | (0.32, 0.77) | (1.07, 2.09) | (2.17, 4.49) | (5.30, 13.40) |
| Male No. (%) | 1074 (46) | 1064 (46) | 1069 (46) | 1072 (46) | 1063 (46) | 1077 (46) | 1069 (46) | 1070 (46) |
| Black No. (%) | 776 | 775 | 775 | 773 | 777 | 773 | 774 | 775 |
| (33) | (33) | (33) | (33) | (34) | (33) | (33) | (33) | |
| White No. (%) | 864 | 857 | 860 | 858 | 857 | 862 | 861 | 859 |
| (37) | (37) | (37) | (37) | (37) | (37) | (37) | (37) | |
| Hispanic No. (%) | 471 | 477 | 463 | 479 | 471 | 473 | 474 | 472 |
| (20) | (21) | (20) | (21) | (20) | (20) | (20) | (20) | |
| Other No. (%) | 212 | 212 | 217 | 210 | 210 | 215 | 213 | 213 |
| (9) | (9) | (9) | (9) | (9) | (9) | (9) | (9) | |
| Age (yr) * | 50 | 56 | 59 | 61 | 56 | 57 | 58 | 56 |
| (40, 61) | (48, 67) | (50, 69) | (52, 69) | (47, 67) | (48, 68) | (49, 68) | (48, 66) | |
| Systolic Blood Pressure | 118 | 122 | 125 | 127 | 119 | 122 | 125 | 126 |
| (mmHg) *,† | (108, 132) | (111, 137) | (112, 141) | (115, 142) | (108, 134) | (111, 136) | (113, 140) | (115, 141) |
| Total Cholesterol | 180 | 186 | 192 | 195 | 184 | 189 | 191 | 188 |
| (mg/dL) *,† | (158, 203) | (166, 210) | (170, 216) | (170, 219) | (163, 208) | (166, 213) | (170, 215) | (164, 213) |
| HDL-C | 50 | 49 | 48 | 46 | 52 | 49 | 47 | 46 |
| (mg/dL) *,† | (41, 61) | (41, 59) | (40, 58) | (39, 55) | (43, 63) | (41, 59) | (39, 56) | (39, 55) |
| BMI | 26 | 27 | 28 | 29 | 25 | 27 | 29 | 30 |
| (kg/m2) *,† | (23, 30) | (24, 31) | (25, 32) | (26, 34) | (23, 28) | (25, 31) | (26, 33) | (26, 35) |
median (95% confidence interval).
p-value <0.0001 for variable by GlycA quartile.
p-value <0.0001 for variable by hsCRP quartile. Body mass index (BMI), high-density lipoprotein cholesterol (HDL-C).
Across sex- and race-adjusted quartiles, higher quartiles of GlycA were directly associated with older age, higher blood pressure, total cholesterol, BMI, and hsCRP (p<0.001, Table 1). Higher quartiles of hsCRP are not directly associated with age; however, higher quartiles of hsCRP were directly associated with systolic blood pressure, BMI and GlycA (p<0.001). Higher quartiles of both GlycA and hsCRP were inversely associated with HDL-C (p<0.001).
Over a median of 13.4 years, there were 843 primary endpoint events (554 fatal or nonfatal MIs and 289 fatal or nonfatal ischemic strokes). There were 1212 secondary endpoint events (Supplemental Fig. 1).
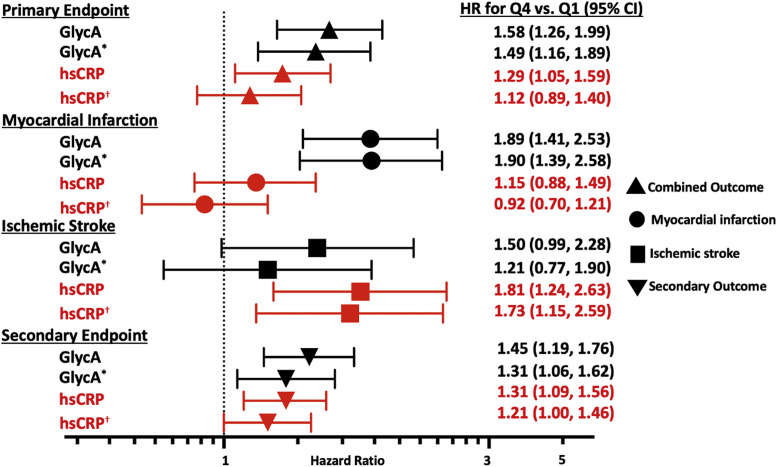
With respect to the primary endpoint of combined MI and ischemic stroke, in unadjusted analyses, GlycA and hsCRP were both directly associated with the primary endpoint (GlycA: HR Q4 vs. Q1 2.24, 95% CI 1.79 to 2.80; hsCRP: HR Q4 vs. Q1 1.58, 95% CI 1.31 to 1.91). Both GlycA and hsCRP remained independently associated with the primary endpoint in adjusted analyses (GlycA: HR Q4 vs. Q1 1.58, 95% CI 1.26 to 1.99; hsCRP: HR Q4 vs. Q1 1.29, 95% CI 1.05 to 1.59). In risk factor-adjusted models that included both markers concurrently, only GlycA remained directly associated with the primary endpoint (GlycA: HR Q4 vs. Q1 1.49, 95% CI 1.16 to 1.89; hsCRP: HR Q4 vs. Q1 1.12, 95% CI 0.89 to 1.40, Fig. 1). Since total cholesterol is the risk factor included in risk prediction assessments, as in the pooled cohort equation and SCORE, we included total cholesterol in our analysis [30,31]. We repeated analyses to include LDL cholesterol and the results were very similar.
Fig. 1.
Adjusted Hazard Ratios of GlycA and hsCRP for Cardiovascular Outcomes. Risk factor adjustments: age, sex, race/ethnicity, diabetes, systolic blood pressure, anti-hypertensive medication, statin medication, current smoking, body mass index (BMI), total and HDL cholesterol. *Additionally adjusted for hsCRP. †Additionally adjusted for GlycA.
Further dividing the primary outcome by vascular domain in multivariable analyses, higher GlycA was directly associated with MIs (HR Q4 vs. Q1 1.89, 95% CI 1.41 to 2.53), but higher hsCRP was not associated with MIs (HR Q4 vs. Q1 1.15, 95% CI 0.88 to 1.49). Additional adjustment for hsCRP did not attenuate the association between GlycA and MIs (HR Q4 vs. Q1 1.90, 95% CI 1.39 to 2.58, Fig. 1).
In contrast, higher hsCRP was directly associated with ischemic strokes in multivariable adjusted models (hsCRP: HR Q4 vs. Q1 1.81, 95% CI 1.24 to 2.63), but higher GlycA was not associated with ischemic stroke (GlycA: HR Q4 vs. Q1 1.50, 95% CI 0.99 to 2.28, Fig. 1). When including both inflammatory variables GlycA and hsCRP, higher hsCRP remained associated with ischemic stroke (HR Q4 vs. Q1 1.73, 95% CI 1.15 to 2.59) whereas GlycA did not (HR Q4 vs. Q1 1.21, 95% CI 0.77 to 1.90, Fig. 1).
Both GlycA and hsCRP were associated with the secondary composite endpoint in multivariable models, including adjustment for both inflammatory markers concurrently (GlycA: HR Q4 vs. Q1 1.31, 95% CI 1.06 to 1.62; hsCRP: HR Q4 vs. Q1 1.21, 95% CI 1.00 to 1.46, Fig. 1).
With respect to discrimination, GlycA or hsCRP did not significantly improve the C statistic for the primary endpoint, ischemic stroke or the secondary endpoint. GlycA but not hsCRP improved the C statistic for MI (Table 2). In terms of reclassification, adding GlycA to traditional cardiovascular risk factors improved the IDI but not the NRI for the combined MI and ischemic stroke endpoint (Table 3). In contrast, the addition of hsCRP to traditional cardiovascular risk factors improved the IDI and NRI for the primary outcome of combined MI and ischemic stroke (Table 3).
Table 2.
C-Statistic of GlycA and hsCRP for Cardiovascular Outcomes.
| C-statistic (95% CI) | P-Value | ||
|---|---|---|---|
| Primary Endpoint | Traditional Risk Factors (TRF) | 0.76 (0.74 to 0.77) | |
| (MI + Ischemic Stroke) | TRF + GlycA | 0.76 (0.74 to 0.77) | 0.13 |
| TRF + hsCRP | 0.76 (0.74 to 0.77) | 0.30 | |
| Myocardial Infarction | Traditional Risk Factors (TRF) | 0.77 (0.73 to 0.81) | |
| TRF + GlycA | 0.77 (0.74 to 0.81) | 0.04 | |
| TRF + hsCRP | 0.77 (0.73 to 0.81) | 0.20 | |
| Ischemic Stroke | Traditional Risk Factors (TRF) | 0.76 (0.73 to 0.79) | |
| TRF + GlycA | 0.76 (0.73 to 0.79) | 0.90 | |
| TRF + hsCRP | 0.76 (0.73 to 0.79) | 0.92 | |
| Secondary Endpoint | Traditional Risk Factors (TRF) | 0.76 (0.73 to 0.80) | |
| TRF + GlycA | 0.76 (0.73 to 0.80) | 0.15 | |
| TRF + hsCRP | 0.76 (0.73 to 0.80) | 0.17 |
Risk factor adjustments: age, sex, race/ethnicity, diabetes, systolic blood pressure, anti-hypertensive medication, statin medication, current smoking, body mass index (BMI), total and HDL cholesterol.
High-density lipoprotein (HDL), myocardial infarction (MI), traditional risk factors (TRF).
Table 3.
IDI and NRI of GlycA and hsCRP for cardiovascular outcomes.
| IDI (P-Value) | NRI (P-Value) | ||
|---|---|---|---|
| Primary Endpoint | GlycA | 0.005 (0.0001) | 0.17 (0.1) |
| (MI + Ischemic Stroke) | hsCRP | 0.002 (0.04) | 0.13 (0.0002) |
| Myocardial Infarction | GlycA | 0.007 (0.00004) | 0.20 (0.0001) |
| hsCRP | 0.001 (0.17) | 0.10 (0.02) | |
| Ischemic Stroke | GlycA | 0.0006 (0.13) | 0.07 (0.16) |
| hsCRP | 0.001 (0.06) | 0.12 (0.06) | |
| Secondary Endpoint | GlycA | 0.005 (0.002) | 0.10 (0.01) |
| hsCRP | 0.002 (0.007) | 0.06 (0.02) |
Risk factor adjustments: age, sex, race/ethnicity, diabetes, systolic blood pressure, anti-hypertensive medication, statin medication, current smoking, body mass index (BMI), total and HDL cholesterol.
Integrated Discrimination Index (IDI), Net Reclassification Index (NRI), High-density lipoprotein (HDL), myocardial infarction (MI).
The addition of GlycA improved the IDI and NRI for MI (Table 3). Both GlycA and hsCRP improved the IDI and the NRI for the secondary outcome.
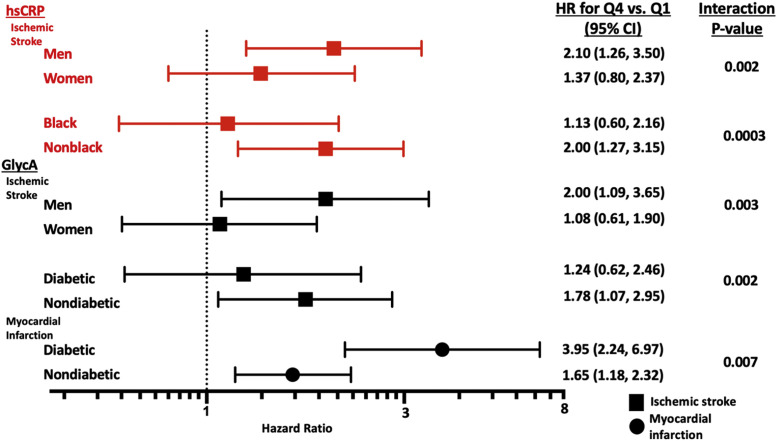
Sex modified the associations of both GlycA and hsCRP and ischemic stroke such that both markers were only associated with higher risk of ischemic stroke among men but not women (p interactions = 0.003 and 0.002, respectively, Fig. 2). In addition, hsCRP was only associated with higher risk of ischemic stroke among White but not Black participants (p interaction=0.0003). Diabetes modified GlycA associations in a contrasting pattern based on vascular endpoint. GlycA was associated more strongly with higher risk of MI among those with diabetes (p interaction=0.007) while it was associated with higher risk of ischemic stroke only among those without diabetes (p interaction=0.002, Fig. 2). Interaction testing by cohort did not modify associations for GlycA nor hsCRP with any cardiovascular endpoint.
Fig. 2.
Interactions of Subgroups Association with Ischemic Stroke or Myocardial Infarction. Risk factor adjustments: age, sex, race/ethnicity, diabetes, systolic blood pressure, anti-hypertensive medication, current smoking, body mass index (BMI), total and high-density lipoprotein cholesterol.
4. Discussion
Our study revealed differential associations between two inflammatory markers, GlycA and hsCRP, and cardiovascular events by vascular domain and directly compared GlycA and hsCRP for cardiovascular risk prediction. Accounting for each other, GlycA was associated with MI but not ischemic stroke, whereas hsCRP was associated with ischemic stroke but not MI. In line with these associations, GlycA improved indices of risk prediction for MI, and hsCRP improved indices of risk prediction for ischemic stroke. Both biomarkers were included in multivariable analysis consistent with prior studies and to observe how the biomarkers jointly affect outcomes. Interaction analyses revealed that associations with ischemic stroke were restricted to men, White participants, and those without diabetes. In addition, the link between GlycA and MI was strongest among those with diabetes.
Although chronic inflammation is a well-described contributor to atherosclerosis, recent trials demonstrating reduced CV events by directly targeting inflammation (CANTOS and COLCOT) have increased attention on the ability to detect those at highest inflammatory risk and those most likely to benefit [32,33]. While hsCRP is an established inflammatory marker, GlycA is an emerging composite inflammatory marker with consistent associations with both primary and recurrent CV events [[8], [9], [10], [11],34]. Beyond capturing CV risk, higher GlycA has been associated with mortality, chronic inflammatory-related severe hospitalization, cancer incidence, and incidence of type 2 diabetes, revealing GlycA as a more global marker of cardiometabolic risk [2,[35], [36], [37]]. GlycA is moderately correlated with hsCRP, a finding confirmed in our pooled, multi-ethnic cohort, suggesting that GlycA imparts information distinct from that provided by hsCRP. Indeed, we demonstrated that GlycA is associated with the primary endpoint of combined MI and stroke as well as a secondary composite endpoint including revascularization of both coronary and peripheral arteries. When analyzed together, GlycA but not hsCRP remained associated, suggesting that the risk information provided by GlycA is not related to the inflammatory risk carried by hsCRP. GlycA improved risk prediction indices for these combined endpoints, suggesting clinical relevance. The magnitude of this improvement was modest but similar to that of hsCRP's improvement in the same models and in the same pooled cohort.
We also sought to describe associations for endpoints specific to vascular domains, namely MI and ischemic stroke. Most prior studies combine these endpoints but there were notable differences with respect to traditional risk factor associations (hypertension), circulating biomarkers (lipoproteins), and even effects of therapies [38]. Here, we found that GlycA was predominantly associated with risk of MI whereas hsCRP was predominantly associated with risk of ischemic stroke. These novel differential associations by vascular domain may have direct clinical relevance in identifying those at highest risk and those most likely to benefit from anti-inflammation interventions. For example, in the CANTOS trial which enrolled those with a history of MI and elevated hsCRP levels, canakinumab, an IL-1 beta antagonist, reduced Il-1 beta levels and hsCRP levels but only reduced MI and not stroke (N=264 stroke events). Unfortunately, analyses within CANTOS assessing reductions in hsCRP with risk did not partition combined endpoints by vascular domain [32]. In contrast, in the COLCOT trial enrolling those with a MI within 30 days, colchicine was associated with a reduction in stroke but not MI [33]. In this study and others, the effects of colchicine on hsCRP are not consistent but support our findings linking hsCRP to ischemic stroke risk. Lastly, within the CIRT trial which tested low dose methotrexate in those with prior atherosclerotic CVD and either diabetes or metabolic syndrome, baseline IL-6 levels were associated with MI but not stroke and hsCRP was not associated with either MI or stroke when adjusted for baseline lipids [39]. Taken together, GlycA levels may represent a novel marker of MI risk and better identify those most likely to benefit from therapies targeting inflammation, particularly to lower myocardial infarction risk. Similarly, recently the Elastic Net Progression of Early Subclinical Atherosclerosis has identified young asymptomatic individuals with increased cardiovascular risk who are likely to benefit from interventions [40].
Further expanding on the novel association between GlycA and MI, interaction testing revealed effect modification by diabetes status such that the association between higher GlycA and MI risk was enhanced in those with diabetes. This may explain a possible hypothesis for the increased cardiovascular risk in those with diabetes and may also serve to identify those with diabetes who may benefit most from adjunctive therapies such as ezetimibe (IMPROVE-IT) and PCSK9 inhibitors (FOURIER and ODYSSEY), which have been shown to have consistent or enhanced benefits in those with diabetes [41], [42], [43].
Analyses of risk markers specifically for ischemic stroke have been limited mostly due to small numbers in individual cohorts or trials powered to show differences in composite endpoints. Thus, our pre-specified analysis on ischemic stroke in a pooled multi-ethnic cohort revealed several interesting and potentially clinically relevant findings. First, though hsCRP did associate with a combined MI and ischemic stroke endpoint, the association was restricted to ischemic stroke. It is possible that while hsCRP may have value as a risk enhancer in primary prevention, it may best be used to identify those at highest risk of ischemic stroke and perhaps those most likely to benefit from therapies that lower stroke risk such as pioglitazone (IRIS) and potentially colchicine (COLCOT) [33,44]. Furthermore, we found that the association between hsCRP and ischemic stroke in our pooled cohort was restricted to men and White participants. More analyses regarding hsCRP and risk of ischemic stroke within trials with sufficient numbers of strokes will help clarify these intriguing findings, including the ongoing CONVINCE trial (NCT02898610).
The limitations of our study include data from an observational, cohort study with a single measurement of inflammatory markers without repeat data measurements to re-evaluate risk. However, the relationships and understanding of the individuals’ interactions significance with a single measurement of an inflammatory marker is encouraging for risk prediction without the necessity of multiple measurements. We were also unable to adjust for use of anti-inflammatory medications if any. However, this was a diverse, primary prevention cohort with over nine-thousand participants in the combined cohort. The assay utilized in this study was the LabCorp clinical grade assay which may have different performance characteristics than other research grade assays [45].
Future directions to validate these findings among those with and without cardiovascular disease and to test integrating GlycA into CVD prediction models are warranted. Further evaluation into interventions to reduce GlycA could prove effective therapies to reduce future cardiovascular risk.
Disclosures
K. Riggs, A. Khera, P. Greenland, C. Ayers: no disclosures
P. Joshi: NASA, NovoNordisk, Amgen, grant support; Bayer, consultant; G3 Therapeutics, equity
J. Otvos: LapCorp employment
A. Rohatgi: HDL Diagnostics, consultant; CSL Limited, consultant; Consultant/Advisory Board; Merck, Research Support
Authors have not published related papers from this same study.
Sources of Funding
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Supported by the Donald W. Reynolds Foundation (Las Vegas, NV), and by United States Public Health Service General Clinical Research Center (USPHS GCRC) grant #M01-RR00633 from National Institutes of Health/National Center for Research Resources-Clinical Research.
A. Rohatgi: supported by NIH/NHLBI R01HL136724 and NIH/NHLBI K24HL146838
NMR particle data provided by LabCorp (previously LipoScience); Raleigh, NC
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Author contributions
Kayla Riggs and Anand Rohatgi – manuscript draft, data analysis and edits
Colby Ayers – statistical analysis
Parag Joshi, Amit Khera, James Otvos – manuscript review and edits
Philip Greenland – manuscript review and edits, MESA cohort expert
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2022.100373.
Appendix. Supplementary materials
References
- 1.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 2.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clin Chem. 2016;62(7):1020–1031. doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61(5):714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 5.Lawler PR, Mora S. Glycosylation signatures of inflammation identify cardiovascular risk: some glyc it hot. Circ Res. 2016;119(11):1154–1156. doi: 10.1161/CIRCRESAHA.116.310005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballout RA, Remaley AT. GlycA: a new biomarker for systemic inflammation and cardiovascular disease (CVD) risk assessment. J Lab Precis Med. 2020;5 doi: 10.21037/jlpm.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dullaart RP, Gruppen EG, Connelly MA, Otvos JD, Lefrandt JD. GlycA, a biomarker of inflammatory glycoproteins, is more closely related to the leptin/adiponectin ratio than to glucose tolerance status. Clin Biochem. 2015;48(12):811–814. doi: 10.1016/j.clinbiochem.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N-linked glycoprotein side-chain biomarker, rosuvastatin therapy, and incident cardiovascular disease: an analysis from the JUPITER trial. J Am Heart Assoc. 2016;5(7) doi: 10.1161/JAHA.116.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: relationship with C-reactive protein and renal function. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGarrah RW, Kelly JP, Craig DM, et al. A novel protein glycan-derived inflammation biomarker independently predicts cardiovascular disease and modifies the association of HDL subclasses with mortality. Clin Chem. 2017;63(1):288–296. doi: 10.1373/clinchem.2016.261636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otvos JD, Guyton JR, Connelly MA, et al. Relations of GlycA and lipoprotein particle subspecies with cardiovascular events and mortality: a post hoc analysis of the AIM-HIGH trial. J Clin Lipidol. 2018;12(2):348–355. doi: 10.1016/j.jacl.2018.01.002. e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zierfuss B, Hobaus C, Herz CT, et al. GlycA for long-term outcome in T2DM secondary prevention. Diabetes Res Clin Pract. 2021;171 doi: 10.1016/j.diabres.2020.108583. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PC, Kremer JM, Emery P, et al. Lipid profile and effect of statin treatment in pooled phase II and phase III baricitinib studies. Ann Rheum Dis. 2018;77(7):988–995. doi: 10.1136/annrheumdis-2017-212461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6) doi: 10.1161/CIRCIMAGING.117.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett DB, Slentz CA, Connelly MA, et al. Association of the composite inflammatory biomarker glyca, with exercise-induced changes in body habitus in men and women with prediabetes. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/5608287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson ML, Renteria-Mexia A, Connelly MA, et al. Decreased GlycA after lifestyle intervention among obese, prediabetic adolescent Latinos. J Clin Lipidol. 2019;13(1):186–193. doi: 10.1016/j.jacl.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manmadhan A, Lin BX, Zhong J, et al. Elevated GlycA in severe obesity is normalized by bariatric surgery. Diabetes Obes Metab. 2019;21(1):178–182. doi: 10.1111/dom.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie D, Deng L, Liu XD, Li JM, Zhang YB. Role of high sensitivity C-reactive protein and other risk factors in intracranial and extracranial artery occlusion in patients with ischaemic stroke. J Int Med Res. 2015;43(5):711–717. doi: 10.1177/0300060515586246. [DOI] [PubMed] [Google Scholar]
- 19.Patgiri D, Pathak MS, Sharma P, Kutum T, Mattack N. Serum hsCRP: A Novel Marker for Prediction of Cerebrovascular Accidents (Stroke) J Clin Diagn Res. 2014;8(12):CC08–CC11. doi: 10.7860/JCDR/2014/10386.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawood FZ, Judd S, Howard VJ, et al. High-Sensitivity C-reactive protein and risk of stroke in atrial fibrillation (from the reasons for geographic and racial differences in stroke study) Am J Cardiol. 2016;118(12):1826–1830. doi: 10.1016/j.amjcard.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawler PR, Bhatt DL, Godoy LC, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42(1):113–131. doi: 10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 23.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 25.Khera A, Vega GL, Das SR, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94(9):3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47(3):418–425. [PubMed] [Google Scholar]
- 27.Whelton SP, Narla V, Blaha MJ, et al. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;113(4):644–649. doi: 10.1016/j.amjcard.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroules CD, Rosero E, Ayers C, Peshock RM, Khera A. Abdominal aortic atherosclerosis at MR imaging is associated with cardiovascular events: the Dallas heart study. Radiology. 2013;269(1):84–91. doi: 10.1148/radiol.13122707. [DOI] [PubMed] [Google Scholar]
- 29.Duprez DA, Otvos J, Tracy RP, et al. High-density lipoprotein subclasses and noncardiovascular, noncancer chronic inflammatory-related events versus cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4(9) doi: 10.1161/JAHA.115.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmali KN, Goff DC, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64(10):959–968. doi: 10.1016/j.jacc.2014.06.1186. Jr. [DOI] [PubMed] [Google Scholar]
- 31.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 33.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 34.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3(5) doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connelly MA, Gruppen EG, Wolak-Dinsmore J, et al. GlycA, a marker of acute phase glycoproteins, and the risk of incident type 2 diabetes mellitus: PREVEND study. Clin Chim Acta. 2016;452:10–17. doi: 10.1016/j.cca.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Chandler PD, Akinkuolie AO, Tobias DK, et al. Association of N-linked glycoprotein acetyls and colorectal cancer incidence and mortality. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawler PR, Akinkuolie AO, Chandler PD, et al. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res. 2016;118(7):1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh K, Chandra A, Sperry T, et al. Associations between high-density lipoprotein particles and ischemic events by vascular domain, sex, and ethnicity: a pooled cohort analysis. Circulation. 2020;142(7):657–669. doi: 10.1161/CIRCULATIONAHA.120.045713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Cabo F, Rossello X, Fuster V, et al. Machine learning improves cardiovascular risk definition for young, asymptomatic individuals. J Am Coll Cardiol. 2020;76(14):1674–1685. doi: 10.1016/j.jacc.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Giugliano RP, Cannon CP, Blazing MA, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from improve-it (improved reduction of outcomes: vytorin efficacy international trial) Circulation. 2018;137(15):1571–1582. doi: 10.1161/CIRCULATIONAHA.117.030950. [DOI] [PubMed] [Google Scholar]
- 42.Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–950. doi: 10.1016/S2213-8587(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 43.Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618–628. doi: 10.1016/S2213-8587(19)30158-5. [DOI] [PubMed] [Google Scholar]
- 44.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374(14):1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med. 2017;15(1):219. doi: 10.1186/s12967-017-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.