Abstract
Background
This is an update of a Cochrane review first published in 2012, Issue 4. Excessive weight gain during pregnancy is associated with poor maternal and neonatal outcomes including gestational diabetes, hypertension, caesarean section, macrosomia, and stillbirth. Diet or exercise interventions, or both, may reduce excessive gestational weight gain (GWG) and associated poor outcomes; however, evidence from the original review was inconclusive.
Objectives
To evaluate the effectiveness of diet or exercise, or both, interventions for preventing excessive weight gain during pregnancy and associated pregnancy complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (5 November 2014), contacted investigators of the previously identified ongoing studies and scanned reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) of diet or exercise, or both, interventions for preventing excessive weight gain in pregnancy.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We organised RCTs according to the type of interventions and pooled data using the random‐effects model in the Review Manager software. We also performed subgroup analyses according to the initial risk of adverse effects related to poor weight control. We performed sensitivity analysis to assess the robustness of the findings.
Main results
We included 65 RCTs, out of which 49 RCTs involving 11,444 women contributed data to quantitative meta‐analysis. Twenty studies were at moderate‐to‐high risk of bias. Study interventions involved mainly diet only, exercise only, and combined diet and exercise interventions, usually compared with standard care. Study methods varied widely; therefore, we estimated the average effect across studies and performed sensitivity analysis, where appropriate, by excluding outliers and studies at high risk of bias.
Diet or exercise, or both, interventions reduced the risk of excessive GWG on average by 20% overall (average risk ratio (RR) 0.80, 95% confidence interval (CI) 0.73 to 0.87; participants = 7096; studies = 24; I² = 52%). This estimate was robust to sensitivity analysis, which reduced heterogeneity, therefore we graded this evidence as high‐quality. Interventions involving low glycaemic load diets, supervised or unsupervised exercise only, or diet and exercise combined all led to similar reductions in the number of women gaining excessive weight in pregnancy.
Women receiving diet or exercise, or both interventions were more likely to experience low GWG than those in control groups (average RR 1.14, 95% CI 1.02 to 1.27; participants = 4422; studies = 11; I² = 3%; moderate‐quality evidence). We found no difference between intervention and control groups with regard to pre‐eclampsia (RR 0.95, 95% CI 0.77 to 1.16; participants = 5330; studies = 15; I² = 0%; high‐quality evidence); however, maternal hypertension (not a pre‐specified outcome) was reduced in the intervention group compared with the control group overall (average RR 0.70, 95% CI 0.51 to 0.96; participants = 5162; studies = 11; I² = 43%; low‐quality evidence).
There was no clear difference between groups with regard to caesarean delivery overall (RR 0.95, 95% CI 0.88 to 1.03; participants = 7534; studies = 28; I² = 9%; high‐quality evidence); although the effect estimate suggested a small difference (5%) in favour of the interventions. In addition, for combined diet and exercise counselling interventions there was a 13% (‐1% to 25%) reduction in this outcome (borderline statistical significance).
We found no difference between groups with regard to preterm birth overall (average RR 0.91, 95% CI 0.68 to 1.22; participants = 5923; studies = 16; I² = 16%; moderate‐quality evidence); however limited evidence suggested that these effect estimates may differ according to the types of interventions, with a trend towards an increased risk for exercise‐only interventions.
We found no clear difference between intervention and control groups with regard to infant macrosomia (average RR 0.93, 95% CI 0.86 to 1.02; participants = 8598; studies = 27; I² = 0%; high‐quality evidence), although the effect estimate suggested a small difference (7% reduction) in favour of the intervention group. The largest effect size occurred in the supervised exercise‐only intervention group (RR 0.81, 95% CI 0.64 to 1.02; participants = 2445; studies = 7; I² = 0%), which approached statistical significance (P = 0.07). Furthermore, in subgroup analysis by risk, high‐risk women (overweight or obese women, or women with or at risk of gestational diabetes) receiving combined diet and exercise counselling interventions experienced a 15% reduced risk of infant macrosomia (average RR 0.85, 95% CI 0.73 to 1.00; participants = 3252; studies = nine; I² = 0; P = 0.05; moderate‐quality evidence)
There were no differences in the risk of poor neonatal outcomes including shoulder dystocia, neonatal hypoglycaemia, hyperbilirubinaemia, or birth trauma (all moderate‐quality evidence) between intervention and control groups; however, infants of high‐risk women had a reduced risk of respiratory distress syndrome if their mothers were in the intervention group (RR 0.47, 95% CI 0.26 to 0.85; participants = 2256; studies = two; I² = 0%; moderate‐quality evidence).
Authors' conclusions
High‐quality evidence indicates that diet or exercise, or both, during pregnancy can reduce the risk of excessive GWG. Other benefits may include a lower risk of caesarean delivery, macrosomia, and neonatal respiratory morbidity, particularly for high‐risk women receiving combined diet and exercise interventions. Maternal hypertension may also be reduced. Exercise appears to be an important part of controlling weight gain in pregnancy and more research is needed to establish safe guidelines. Most included studies were carried out in developed countries and it is not clear whether these results are widely applicable to lower income settings.
Plain language summary
Diet and exercise interventions for preventing excessive weight gain during pregnancy
The issue
A large proportion of women gain more weight than is recommended during pregnancy. Excessive weight gain in pregnancy is associated with complications such as diabetes, high blood pressure, caesarean section, and large babies. This review aimed to determine whether diet or exercise measures,or both, could prevent excessive gestational weight gain (GWG), and if they were safe.
How we conducted the review
This is an update of a review first published in 2012 and is current to November 2014 and included randomised controlled trials (RCTs) only in the updated review. We grouped studies according to the types of interventions, and according to the types of participants, i.e. normal weight women (the low‐risk group), all pregnant women (the mixed‐risk group), and overweight or obese women, or women with or at risk of gestational diabetes (the high‐risk group).
Findings
We included 65 randomised controlled trials, of which 49 trials involving 11,444 women contributed data. Twenty studies were at a moderate‐to high‐risk of bias. The diets tested were low sugar (low glycaemic load), diabetic, low‐calorie or low‐fat diets, with or without food diaries and regular weighing. The exercise interventions were most often of moderate intensity and involving regular walking, dance or aerobic classes. The comparison or control group generally received standard care. Overall, weight management interventions led to a reduction in the number of women gaining excess weight by a fifth (20%; range 13% to 27%) over the pregnancy. We considered this evidence to be high‐quality.
Overall, we found no clear benefits of all diet or exercise interventions, or both, on other outcomes including pre‐eclampsia, caesarean section, preterm birth, and having a baby weighing more than 4 kg (macrosomia), although we could not rule out a small effect on caesarean section (5% reduction) and macrosomia (7% reduction), particularly for women receiving combined diet and exercise counselling interventions. There was a tendency for supervised exercise‐only interventions to reduce macrosomia too. Maternal hypertension (high blood pressure) was also reduced with the interventions. We found no clear differences between study groups with regard to most infant complications, except that for high‐risk women the babies born to the women in the intervention group were less likely to experience breathing difficulties (respiratory distress syndrome) than babies in the control group. This evidence was mostly of a moderate quality.
The studies had differences in the types of interventions, types of participants (for example in terms of body mass index (BMI), number of previous pregnancies and age), delivery of the intervention (whether the intervention was incorporated into antenatal visits or delivered separately by a dietician), timing of the measurements, timing of commencement of the intervention (first, second or third trimester), the intensity of the intervention, and how it was monitored or supervised. Most included studies were carried out in developed countries and it is not clear whether these results are widely applicable to lower income settings.
Conclusions
We found high‐quality evidence that diet or exercise interventions, or both, help to reduce excessive weight gain in pregnancy. They may also reduce caesarean deliveries, especially with combined diet and exercise interventions, and maternal hypertension. In addition, the chances of having a baby over 4 kg and the chances of the newborn having breathing difficulties after birth may be reduced, especially in overweight and obese women. Moderate‐intensity exercise appears to be an important part of weight‐control strategies in pregnancy; however, more research is needed on side‐effects to inform safe guidelines.
Summary of findings
Summary of findings for the main comparison. All diet and/or exercise interventions compared to standard/other care for preventing excessive weight gain in pregnancy.
| All diet and/or exercise interventions compared to standard/other care for preventing excessive weight gain in pregnancy | ||||||
| Patient or population: pregnant women Settings: antenatal care settings Intervention: all diet and/or exercise interventions Comparison: routine care or minimal interventions (e.g. brochures) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard/other care | All diet and/or exercise interventions | |||||
| Excessive weight gain | Study population | RR 0.80 (0.73 to 0.87) | 7096 (24 RCTs) | ⊕⊕⊕⊕ HIGH 1 | ||
| 453 per 1000 | 362 per 1000 (330 to 394) | |||||
| Mean GWG (kg) | Mean difference not estimated | Due to substantial heterogeneity among studies, we did not consider the pooled estimate to be meaningful. Limited subgroup analyses suggested that effect estimates might differ according to risk group. | ||||
| Low weight gain | Study population | RR 1.14 (1.02 to 1.27) | 4422 (11 RCTs) | ⊕⊕⊕⊝ MODERATE2 |
||
| 227 per 1000 | 259 per 1000 (232 to 288) | |||||
| Preterm birth | Study population | RR 0.91 (0.68 to 1.22) | 5923 (16 RCTs) | ⊕⊕⊕⊝ MODERATE3 | ||
| 57 per 1000 | 52 per 1000 (39 to 70) | |||||
| Pre‐eclampsia | Study population | RR 0.95 (0.77 to 1.16) | 5330 (15 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 66 per 1000 | 62 per 1000 (50 to 76) | |||||
| Caesarean delivery | Study population | RR 0.95 (0.88 to 1.03) | 7534 (28 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 288 per 1000 | 274 per 1000 (254 to 297) | |||||
| Macrosomia Infant birthweight > 4000 g | Study population | RR 0.93 (0.86 to 1.02) | 8598 (27 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 178 per 1000 | 166 per 1000 (153 to 182) | |||||
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Although heterogeneity was moderate‐to‐high (I² = 52%), the RR was robust to sensitivity analysis, which was associated with less heterogeneity (I² ‐ 40%), therefore we did not downgrade this evidence
2Downgraded due to imprecision of results according to types of intervention (‐1)
3Downgraded due to risk of bias concerns and concerns that data could not be included in the analysis due to studies excluding women with or at risk of preterm birth post‐randomisation from analysis (Cordero 2014; Di Carlo 2014; Murtezani 2014; Rauh 2013). This omission might have led to publication bias in the review's 'preterm birth' outcome (‐1)
Summary of findings 2. Comparative table of findings by intervention type.
| Intervention type | Risk group | EGWG | Mean GWG | Low GWG | Preterm birth | Caesarean | Pre‐eclampsia | Macrosomia |
|
All interventions (max 36 studies) |
Overall | 20% reduction (13 to 27%) | Not estimated | 14% increase (2% to 27%) |
NS | NS | NS | BS (7% reduction, ‐2% to 14%) |
|
Low GL diet (max 5 studies) |
Overall | 23% reduction (9% to 33%) | Not estimated | NS | BS in favour of the intervention | NS | NA | NS |
| Low | 40% reduction (6% to 52%) | NS | NS | NS | NS | NA | NA | |
| Mixed | 21% reduction (1 study) | NS | NA | NS | NS | NA | NS | |
| High | NS | NS | NS | NS | NS | NA | NS | |
|
Diet and exercise counselling (max 13 studies) |
Overall | 14% reduction (2% to 25%) | Not estimated | NS | NS | BS (13% reduction; ‐1% to 25%) | NS | NS |
| Low | 28% reduction (5% to 45%) | NS | NS | NS | NS | NS | NS | |
| Mixed | NS | 1.80 kg reduction (0.24 to 3.36 kg) | NA | NS | BS (34% reduction; ‐5% to 59%) | NA | NS | |
| High | NS | 0.71 kg reduction (0.08 to 1.34) |
NS | NS | BS (11% reduction; ‐4 to 24%) | NS | 15% reduction (0% to 27%) |
|
|
Exercise (supervised or unsupervised) only (max 10 studies) |
Overall | 21% reduction (11% to 30%) | Not estimated | BS (19% increase; 0% to 41%) | NS | NS | NS | NS |
| Low | BS (31%; ‐2 to 53%) | 1.50 kg reduction (0,92 to 2.08 kg; one study only) | 29% increase; 6% to 42%) | BS (one study) | NS | NA | ||
| Mixed | 23% reduction (12% to 34%) | 1.35 kg reduction (0.89 to 1.80) | NS | NS | NS | NS | BS* (19% reduction; ‐2% to 36%) | |
| High | 16% reduction (5% to 27%) | NS | NS | NS | NS | NS | NS | |
|
Unsupervised exercise only (max 3 studies) |
Overall | 17% reduction (3% to 29%) | Not estimated | NS | NS | NS | NS | NS |
| Supervised exercise only (max 7 studies) | Overall | 25% reduction (11% to 37%) | Not estimated | BS (21% increase; ‐1% to 52%) | NS (trend in favour of control) | NS | NS | BS (19% reduction;‐2% to 36%) |
| Diet and supervised exercise (max 5 studies) | Overall | 29% reduction (15% to 41%) | NS | NS | NA | NS | NS | NS |
| Low | NS | 3.33 kg reduction (1,21 to 5.45; one study only) | NA | NA | Not estimable | NA | NA | |
| Mixed | 36% reduction (16% to 52%) | 1.69 kg reduction (0.11 to 3.48) | NS | NA | NS | NA | NS | |
| High | NS | NS | NA | NA | NS | NA | NS | |
|
Diet counselling/other (max 7 studies) |
Overall | NS | Not estimated | NS | NS | NS | NS | NS |
| Mixed | NS | NA | NA | NS | NS | NS | NA | |
| High | NS | NA | NA | NS | NS | NS | NS |
Finding are presented with reference to the intervention group
* Sensitivity analysis suggested that there may be a statistically significant difference in favour of the intervention for the mixed‐risk subgroup.
Abbreviations: NA = not available; NS = not statistically significant (P ≥ 0.05); BS = borderline significance
Background
Description of the condition
Pregnancy weight gain guidelines
In 2009, the Institute of Medicines (IOM) in the United States updated earlier guidelines on weight gain during pregnancy (Medicine 1990; Medicine 2009). The report set out specific ranges of weight gain for women with different prepregnancy weights: suggesting that underweight women (body mass index (BMI) less than 18.5 kg/m²) gain 28 lbs to 40 lbs (12.5 kg to 18 kg); normal weight women (BMI 18.5 kg/m² to 24.9 kg/m²) gain 25 lbs to 35 lbs (11.5 kg to 16 kg); whereas overweight women (BMI 25 kg/m² to 29.9 kg/m²) were advised to gain between 15 lbs and 25 lbs (7 kg to 11.5 kg) and obese women (BMI at least 30 kg/m²) to gain between 11 lbs and 20 lbs (5 kg to 9 kg) (Medicine 2009).
Previous guidelines from the IOM (Medicine 1990) had been widely adopted but not universally accepted. However, a review of relevant information confirmed that pregnancy weight gain within the IOM's recommended ranges was associated with the best outcomes for both mothers and infants, and that weight gain within the IOM's recommended ranges is not harmful for the mothers or for their infants (Abrams 2000).
No official recommendations or clinical guidelines for weight gain during pregnancy exist in the United Kingdom (UK) (Ford 2001). However, a report from the UK Centre for Maternal and Child Enquiries (CMACE 2010) suggested a more comprehensive guidance for the care of overweight and obese women, and recommended weighing women in the third trimester and again when women are admitted in labour. Guidelines in other countries have also recommended monitoring weight gain in pregnancy. In Sweden, it has been recommended that the optimal gestational weight gain for Swedish women is 4 kg to 10 kg for BMI less than 20, 2 kg to 10 kg for BMI 20 to 24.9; less than 9 kg for women with a BMI of 25 to 29.9, and less than 6 kg for women with a BMI of 30 or more (Cedergren 2007). Maternal weight gain recommendations based on data from high‐income countries may not be applicable to Asian women, who appear to have lower weight gains compared with women in Europe and North America (Abrams 1995; Siega‐Riz 1993). Weight gain limits for Chinese women, taking ethnic‐specific differences into account, have been recommended as 13 kg to 16.7 kg, 11 kg to 16.4 kg, and 7.1 kg to 14.4 kg respectively for women of low (BMI less than 19), moderate (BMI 19 to 23.5), and high (BMI greater than 23.5) BMI (Wong 2000).
Trends in pregnancy weight gain
Although the 1990 IOM guidelines have now been promoted for two decades it has been estimated that over this time only 30% to 40% of pregnant women in the United States gain gestational weight within the IOM recommended ranges (Abrams 2000; Cogswell 1999; Medicine 1990; Olson 2003). Furthermore, gestational weight gain above the guidelines is more common than gestational weight gain below (Stotland 2006). Several studies on gestational weight gain in the USA and Europe indicate that about 20% to 40% of women are gaining weight above the recommendations (Cedergren 2006; Medicine 2009; Olson 2003) and the prevalence of excessive gestational weight gain is increasing (Abrams 2000; Rhodes 2003; Schieve 1998). A retrospective cohort study undertaken to examine the trend in weight gain during pregnancy of 1,463,936 women over 16 years in North Carolina found that the proportion of women gaining excessive gestational weight (more than 18 kg) increased from 15.5% in 1988 to 19.5% in 2003; an additional 40 women per 1000 gained excessive weight by 2003 (Helms 2006). The recent IOM report summarised the situation in a number of countries; compared with two decades earlier "Women today are also heavier; a greater percentage of them are entering pregnancy overweight or obese, and many are gaining too much weight during pregnancy" (Medicine 2009).
Weight gain during pregnancy is generally inversely proportional to prepregnancy weight category. Although underweight women are least likely to exceed weight gain recommendations, obese women tend to gain less weight than normal and overweight women (Abrams 1989; Bianco 1998; Edwards 1996; Walling 2006). Two large population‐based studies, in Sweden and the United States, found that approximately 30% of average and overweight women had high‐gestational weight gain, compared with 20% of obese women (Cedergren 2006; Cogswell 1995).
Pregnancy weight gain and outcomes for mothers and infants
It is well known from large studies in a number of countries that excessive weight gain during pregnancy is associated with multiple maternal and neonatal complications. Retrospective cohort studies have examined the relationship between gestational weight gain and adverse neonatal outcomes among infants born at term. Gestational weight gain above the upper limit of the IOM guideline has been associated with a low five‐minute Apgar score, seizure, hypoglycaemia, polycythaemia, meconium aspiration syndrome and large‐for‐gestational age compared with women within weight gain guidelines (Hedderson 2006; Stotland 2006). For obese women, low‐gestational weight gain has been shown to decrease the risk of several undesirable outcomes including pre‐eclampsia, caesarean section, instrumental delivery, and large‐for‐gestational‐age births; whereas, excessive weight gain increased the risk for caesarean delivery in all maternal BMI classes (Cedergren 2006).
Findings from a national study in the UK revealed that compared with pregnant women in general, obese pregnant women were at increased risk of having a co‐morbidity diagnosed before or during pregnancy (in particular pregnancy‐induced hypertension and gestational diabetes), were at increased risk of having induction of labour and a caesarean birth, were more likely to have postpartum haemorrhage, and their babies were at increased risk of stillbirth, neonatal death, of being large‐for‐gestational age and more likely to be admitted for special care (CMACE 2010).
A number of studies have concluded that excessive gestational weight gain increases postpartum weight retention (Gunderson 2000; Keppel 1993; Polley 2002; Rooney 2002; Rossner 1997; Scholl 1995) and is related to a two‐ to three‐fold increase in the risk of becoming overweight after delivery (Gunderson 2000). Moreover, mothers who gained more weight during pregnancy have been shown to have children at higher risk of being overweight in early childhood (Oken 2007).
Description of the intervention
Pregnancy may be an optimal time to inform and challenge women to change their eating habits and physical activities, and thereby prevent excessive weight gain. Dietary control, exercise and eating behaviour modification are the main elements for controlling weight. Dietary interventions include low glycaemic, energy‐restricted, diabetic, healthy eating, low carbohydrate and other diets. Regular exercise is an important part of a healthy lifestyle and most guidelines support moderate‐intensity physical activity during pregnancy (Evenson 2014).
How the intervention might work
Diet and exercise interventions are recommended components of weight control programs in the general population. Diet interventions work mainly by limiting energy intake, whereas exercise interventions work by using energy. If one utilises more energy than one takes in, one creates an energy deficit, which facilitates the use of stored energy.
Why it is important to do this review
Pregnancy results in dramatic physiological changes, with weight gain occurring as part of the normal pregnancy process. This normal occurrence and expectation of weight gain in pregnancy can make it difficult for a woman of any prepregnancy weight to maintain her weight within recommended limits. Thus, pregnancy is a time when women especially need clear guidance on how best to maintain a healthy weight, in a way that will be safe for both mother and baby. Given the increasing prevalence and negative consequences of excessive gestational weight gain, preventing excessive weight gain during pregnancy is becoming increasingly important. The previous version of this review found weak evidence to support diet and exercise interventions to reduce gestational weight gain; however, findings were inconsistent and interventions heterogeneous. Despite this and another systematic review that included randomised and non‐randomised studies (Thangaratinam 2012), it remains unclear which types of interventions will yield the best outcomes for mothers and their infants, and whether interventions work equally for all risk groups. Pregnancy offers an ideal opportunity to support women towards a healthier lifestyle; however, strategies for reducing weight gain in the non‐pregnant population may not be suitable for use in pregnancy. The aim of this review was to determine whether diet or exercise interventions, or both, for preventing excessive weight gain are effective and safe in pregnancy and to stimulate further research in this field.
Objectives
To evaluate the effectiveness and safety of diet or exercise, or both, interventions for preventing excessive weight gain during pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cluster‐RCTs. Quasi‐RCTs were not eligible.
Types of participants
Pregnant women of any BMI. We considered studies recruiting women with normal BMIs to have a 'low risk' of weight‐related complications at baseline, those recruiting women from the general population including women of any BMI to have a 'mixed‐risk' status, and studies of overweight and/or obese women, or high‐risk women, as defined by the investigators, to have a 'high‐risk' status.
Types of interventions
Any diet or exercise, or both, intervention (e.g. healthy eating plan, low glycaemic diet, exercise intervention, health education, lifestyle counselling) compared with standard or routine care for preventing excessive weight gain in pregnancy. We organised our main comparison into different intervention types, as follows:
diet counselling only versus routine care;
diet and exercise counselling versus routine care;
diet interventions (e.g. low glycaemic diet) versus routine care;
exercise (supervised or unsupervised) interventions only versus routine care;
diet and supervised exercise interventions versus routine care.
We defined diet as a special selection of food, or energy intake, to which a participant was restricted. Exercise interventions included any activity requiring physical effort, carried out to sustain or improve health and fitness.
Types of outcome measures
Primary outcomes
Excessive weight gain as defined by investigators.
Secondary outcomes
For the mothers
Weight gain.
Low weight gain as defined by investigators.
Preterm birth.
Preterm prelabour rupture of membranes.
Pre‐eclampsia/eclampsia.
Hypertension (not prespecified).
Induction of labour.
Caesarean delivery.
Postpartum complication including postpartum haemorrhage, wound infection, endometritis, need for antibiotics, perineal trauma, thromboembolic disease, maternal death.
Behaviour modification outcomes: diet, physical activity.
For the newborns
Birthweight (not prespecified).
Birthweight greater than 4000 g or greater than the 90th centile for gestational age and infant sex (macrosomia).
Birthweight less than 2500 g or less than the 10th centile for gestational age and infant sex.
Complication related to macrosomia including hypoglycaemia, hyperbilirubinaemia, infant birth trauma (palsy, fracture, shoulder dystocia), respiratory distress syndrome.
Long‐term health outcomes
Maternal weight retention postpartum.
Childhood weight.
Gestational diabetes, an important outcome of many interventions aimed at preventing excessive weight gain in pregnancy, is the primary outcome of separate Cochrane reviews (Crane 2013; Han 2012; Tieu 2008) and is therefore not included in this review.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (5 November 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
For this update, we also contacted investigators of the previously identified ongoing studies by email to enquire about any new or imminent publications.
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeMuktabhant 2012.
For this update, we used the following methods based on a standard template used by the Cochrane Pregnancy and Childbirth Group for assessing the reports that were identified as a result of the updated search.
Selection of studies
Two review authors (Benja Muktabhant (BM); Theresa Lawrie (TL)) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author (Pisake Lumbiganon (PL)).
Data extraction and management
Using Microsoft Excel®, we designed a spreadsheet to collect study data and piloted it with two studies. Thereafter, two review authors (BM, TL) extracted data from included studies using the piloted form. We resolved discrepancies through discussion or, if required, we consulted a third review author (PL). Data were entered into Review Manager software (RevMan 2014) by one review author (TL) and checked for accuracy by another (BM).
For studies that reported results for obese and overweight women separately, we combined these data for the 'high‐risk women' subgroup.
Assessment of risk of bias in included studies
Two review authors (BM, TL) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor (PL or Malinee Laopaiboon (ML)).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we attempted to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually‐randomised trials provided that cluster‐RCT data were adjusted for clustering and any baseline imbalances. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely. We assessed risk of bias of these trials with particular attention to imbalances in baseline characteristics between the comparison arms, loss of clusters and appropriate analyses, and we acknowledged heterogeneity in the randomisation unit, performing sensitivity analyses to investigate the effects of including these studies on review findings.
Other issues
For studies that included three arms, we divided the control group into two equal groups and considered each comparison separately. If the number of events in the control group was an odd number, to reduce the risk of overestimating effects in favour of the intervention group, we halved it and rounded it down; for odd denominators (total number of participants in the control group), we rounded these numbers upwards for the same reason.
Dealing with missing data
For included studies, we noted levels of attrition. We imputed data for studies where results were incompletely reported, e.g. if percentages and denominators were known, but the number of events was missing. For all outcomes, we carried out, as far as possible, analyses on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified heterogeneity above 30%, we explored it by sensitivity and subgroup analyses.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots, which we assessed visually for asymmetry. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials.was considered clinically meaningful The random‐effects summary was treated as the average of the range of possible treatment effects and we considered the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. The results of these random‐effects analyses are presented as the average treatment effect with 95% confidence intervals and estimates of I².
Subgroup analysis and investigation of heterogeneity
Subgroup analyses according to risk were not specified in the original review protocol. We conducted subgroup analyses according to the risk of adverse effects related to poor weight control with the high‐risk group comprising only overweight and obese women, or women with or at risk of diabetes mellitus; a mixed‐risk group comprising women in the general population, including women of any body mass indices (BMIs), and a low‐risk group comprising normal weight women or women with BMIs of less than 25 kg/m2. Where possible, we performed subgroup analysis for the following outcomes.
Excessive gestational weight gain (GWG)
Mean GWG
Low GWG
Preterm birth
Caesarean section
Pre‐eclampsia
Macrosomia
For these analyses, we assessed subgroup differences by interaction tests available within RevMan (RevMan 2014) and reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
We explored heterogeneity by organising studies within comparisons according to the types of interventions.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality by excluding studies with risk of bias concerns from the analyses in order to assess whether this made any difference to the overall result.
Quality of evidence
Following meta‐analysis, the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) for the following key outcomes.
Excessive GWG
Mean GWG
Low GWG
Preterm birth
Caesarean section
Pre‐eclampsia
Macrosomia
'Summary of findings' tables were created using this feature in RevMan 2014 with a summary of the intervention effect and a measure of quality produced for each of the above outcomes using the GRADE approach (GRADE 2014). The GRADE approach uses five considerations to assess the quality of the body of evidence for each outcome. We downgraded the evidence from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
SeeCharacteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The original search identified 63 potential studies, of which we included 28 and excluded 12 studies in the original review (Muktabhant 2012). Two studies remained unclassified and 21 studies were ongoing.
Searches updated to November 2014 identified 169 eligible records. Of these 169 records, we included 102 records (pertaining to 41 new RCTs), and excluded 19 records (pertaining to 10 new studies, and one previously excluded study). Twelve of the 169 records were new reports of five previously included RCTs (Barakat 2011; Callaway 2010; Laitinen 2009; Luoto 2011; Phelan 2011), 36 records were of ongoing RCTs, and one record remained unclassified for this update (requiring translation from Farsi).
For this update, we excluded two previously included quasi‐RCTs (Bechtel‐Blackwell 2002; Moses 2006) and two RCTs that involved anti‐suppressant drugs (Boileau 1968; Silverman 1971), which had previously been included under a broader title (see Differences between protocol and review).
Therefore, in summary, we included a total of 65 RCTs in this update (41 new and 24 previously included). Forty‐nine studies (29 newly included) contributed data to quantitative synthesis. Twelve RCTs that had been identified as ongoing in the previous review have now been published, leaving 40 ongoing RCTs altogether (including new and previously included ongoing trials not yet reported) for this update (Characteristics of ongoing studies).
Included studies
Out of 65 included studies, two studies were cluster‐RCTs (Luoto 2011; Rauh 2013); all other studies were RCTs. We were able to adjust data for one cluster‐RCT (Luoto 2011; Appendix 1) and used adjusted data in meta‐analyses, however we were unable to adjust data from Rauh 2013, which therefore did not contribute to meta‐analyses. Seven RCT reports were conference abstracts (Angel 2011; Bisson 2014; Bogaerts 2012; Leiferman 2011; Marcinkevage 2012; Mujsindi 2014; Szmeja 2011). One of these studies (Leiferman 2011), also generated a substudy in the form of a PhD thesis (Nodine 2011), which we have linked to this study in the references section. When reported in full, these seven RCTs may yet contribute data to future versions of this review; however, in general, we gleaned very little methodological information and no usable data from the abstracts. We have included information about these trials in the Characteristics of included studies tables, but they are not otherwise discussed in the sections below.
Participants
Two studies (Bogaerts 2012; Leiferman 2011) did not report the number of participants. The remaining 63 included studies involved at least 13,523 pregnant participants, and the number of participants in each study ranged from 12 (Magee 1990) to more than 2000 (Dodd 2014). Fifty‐five out of 65 studies reported age and body mass index (BMI) at baseline and these were similar between study and control groups, with a few exceptions (see Risk of bias in included studies). Four studies recruited only nulliparous women (Althuizen 2013; Haakstad 2011; Murtezani 2014; Pinzon 2012). Most studies recruited women less than 20 weeks' gestation (48/65; 74%), with 27/65 (42%) studies recruiting women less than or equal to 14 weeks' gestation. Thirteen studies recruited participants after 20 weeks' gestation, namely Rhodes 2010 (13 to 28 weeks); Ferrara 2011 (approximately 31 weeks on average); Louie 2011 (20 to 32 weeks); Angel 2011 (more than 20 weeks); Hui 2012, Hui 2006 and Jackson 2011 (up to 26 weeks); Magee 1990 (13 to 38 weeks); Moses 2009 (approximately 30 weeks on average); Pollak 2014 (up to 21 weeks); Stafne 2012 (18 to 22 weeks); Thornton 2009 (12 to 28 weeks); Vitolo 2011 (10 to 29 weeks). Gestation at recruitment was not clear for the remainder (Bogaerts 2012; Leiferman 2011; Mujsindi 2014; Quinlivan 2011).
Studies included participants of various weight categories with 'normal weight' generally defined as a BMI greater than 18 and less than 25 kg/m², 'overweight' was considered to be greater than or equal to 25 kg/m² and less than 30 kg/m², and 'obese' was considered to be a BMI of greater than or equal to 30 kg/m². Thirty‐four studies recruited women from the 'general population' (i.e. of various BMIs) and the proportion of women with normal BMIs varied widely across study samples reporting this baseline characteristic, from 15% to 79% of participants. Most of these studies did not report results for high‐ (overweight/obese women) and low‐risk (normal BMI) women separately; however, for eight of these studies (Althuizen 2013; Hui 2014; Jeffries 2009; Phelan 2011; Polley 2002; Ronnberg 2014; Ruiz 2013; Vitolo 2011), main outcomes were reported separately for women with low/normal versus overweight/obese prepregnancy weights; therefore, where possible, we used relevant data from these studies for meta‐analyses pertaining to low, mixed‐risk and high‐risk groups. One study (ROLO 2012) recruited only secundigravid women who had previously given birth to a baby with macrosomia and reported certain outcomes separately for women in all weight categories.
Among 31 studies recruiting women in high‐risk groups, 24 studies recruited overweight and obese women, or obese women only (Angel 2011; Bisson 2014; Bogaerts 2012; Callaway 2010; Dodd 2014; Guelinckx 2010; Kong 2014; Magee 1990; Marcinkevage 2012; Mujsindi 2014; Nascimento 2012; Oostdam 2012; Petrella 2013; Poston 2013; Pollak 2014; Quinlivan 2011; Renault 2014; Rhodes 2010; Santos 2005; Szmeja 2011; Thornton 2009; Vesco 2013; Vinter 2012; Wolff 2008); and seven recruited women with, or defined as at high risk of, gestational diabetes (Ferrara 2011; Harrison 2013; Korpi‐Hyovalti 2011; Louie 2011; Luoto 2011; Moses 2009; Rae 2000).
Settings
Most studies were conducted in high‐income countries, including Australia (Callaway 2010; Dodd 2014; Harrison 2013; Jeffries 2009; Louie 2011; Moses 2009; Moses 2014; Quinlivan 2011; Szmeja 2011; Wilkinson 2012), Belgium (Bogaerts 2012; Guelinckx 2010), Canada (Hui 2006; Hui 2012; Hui 2014; Hui 2014; Ruchat 2012), Denmark (Renault 2014; Vinter 2012; Wolff 2008), Finland (Korpi‐Hyovalti 2011; Laitinen 2009; Luoto 2011), Germany (Rauh 2013), Ireland (ROLO 2012), Italy (Di Carlo 2014; Petrella 2013), Kosovo (Murtezani 2014), Norway (Haakstad 2011), Sweden (Petrov Fieril 2014; Ronnberg 2014), The Netherlands (Althuizen 2013; Oostdam 2012), Spain (Barakat 2011; Cordero 2014; Ruiz 2013), the United Kingdom (Poston 2013) and the United States of America (USA) (Angel 2011; Asbee 2009; Bisson 2014; Clapp 2002a; Clapp 2002b; Ferrara 2011; Hawkins 2014; Kieffer 2014; Kong 2014; Leiferman 2011; Magee 1990; Marcinkevage 2012; Mujsindi 2014; Phelan 2011; Pollak 2014; Polley 2002; Price 2012; Rhodes 2010; Thornton 2009; Vesco 2013). Two of these studies (Hui 2006; Polley 2002) recruited women with low‐, or low‐middle incomes in Canada and the USA, respectively. Of the six studies conducted in low‐income countries, four were conducted in Brazil (De Oliveria Melo 2012; Nascimento 2012; Santos 2005; Vitolo 2011), one was conducted in Columbia (Pinzon 2012) and one was conducted in Taiwan (Huang 2011).
Interventions
All the interventions considered in this review included modifying or restricting diet or increasing exercise, or both; however there was considerable variation in the interventions used, which included:
diet only (eight studies): low glycaemic load (GL) diet versus conventional healthy eating or routine or other care: Angel 2011; Clapp 2002a; Louie 2011; Moses 2009; Moses 2014; Rhodes 2010; ROLO 2012; one study evaluated a supervised low calorie diet (Magee 1990);
diet and exercise counselling (25 studies): Asbee 2009; Althuizen 2013; Bogaerts 2012; Dodd 2014; Ferrara 2011; Guelinckx 2010; Harrison 2013; Hawkins 2014; Huang 2011; Jackson 2011; Kieffer 2014; Korpi‐Hyovalti 2011; Luoto 2011; Marcinkevage 2012; Mujsindi 2014; Petrella 2013; Phelan 2011; Pollak 2014; Polley 2002; Quinlivan 2011; Rauh 2013; Renault 2014; Szmeja 2011; Vesco 2013; Wilkinson 2012;
exercise interventions (e.g. supervised exercise, individualised exercise programs, dance classes, provision of pedometers or treadmills) (20 studies): Barakat 2011; Bisson 2014; Callaway 2010; Clapp 2002b; Cordero 2014; De Oliveria Melo 2012; Haakstad 2011; Kong 2014; Leiferman 2011; Murtezani 2014; Nascimento 2012; Oostdam 2012; Petrov Fieril 2014; Pinzon 2012; Poston 2013; Price 2012; Renault 2014; Ruiz 2013; Ronnberg 2014; Santos 2005;
diet and supervised exercise interventions (five studies): Hui 2006; Hui 2012; Hui 2014; Ruchat 2012; Vinter 2012;
diet counselling/other (seven studies): Di Carlo 2014; Jeffries 2009; Laitinen 2009; Rae 2000; Thornton 2009; Vitolo 2011; Wolff 2008.
Interventions varied in intensity. Control groups mostly comprised routine or standard care (which also varied considerably in different settings and was not always well‐described). Hui 2006 compared a supervised group exercise and diet intervention with an exercise and diet information pack. Clapp 2002b compared different exercise intensities at different stages of pregnancy. Some studies included more than two arms (De Oliveria Melo 2012; Laitinen 2009; Guelinckx 2010; Renault 2014).
Outcomes
Gestational weight gain (GWG) or excessive GWG, or both, were reported as primary or secondary outcomes in 75% of included studies. Excessive GWG was usually defined according to prevailing IOM guidelines. Generally, baseline weight was measured at recruitment; however, several studies used self‐reported prepregnancy weight as the baseline measurement (e.g. Di Carlo 2014; Haakstad 2011; Hui 2012; Louie 2011; Moses 2014; Oostdam 2012). The final weight measurement was either collected by researchers at the last clinic or hospital visit (usually greater than or equal to 36 weeks) or from medical records. Several studies collected these follow‐up weight data earlier than 36 weeks, including Vesco 2013 (34 weeks), Oostdam 2012 (32 weeks), Harrison 2013 (28 weeks), and Petrov Fieril 2014 (25 weeks). The latter study included mean weight as an outcome, but not mean weight gain.
Other reported outcomes included postpartum weight retention, macrosomia, infant birthweight, gestational diabetes, pre‐eclampsia/hypertension, diet and physical activity (PA) behaviour, breastfeeding, biochemical parameters, e.g. serum insulin levels, and various other maternal and neonatal outcomes.
Excluded studies
Initially, we excluded 26 studies (12 previously excluded, 10 new excluded and four previously included). One previously excluded study (Moses 2007), was a follow‐up of a previously included study (Moses 2006) and these two reports are now listed together, reducing the number of excluded studies to 25 studies. The main reasons for exclusion were as follows.
Non‐randomised study or quasi‐RCT: Bechtel‐Blackwell 2002; Breslow 1963; Daley 2014; Davenport 2011; Graham 2014; Gray‐Donald 2000; Kinnunen 2007; Maitland 2014; Mohebi 2009; Moses 2006; Mottola 2010; Olson 2004; Stutzman 2010; Walker 1966.
Participants included non‐pregnant or postpartum women: Campbell 2004; Faucher 2008; Hausenblas 2008; Te Morenga 2011; Wisner 2006.
Not a diet or exercise intervention: Asemi 2011; Boileau 1968; Hauner 2012; Ismail 1990; Lindsay 2014; Silverman 1971.
Risk of bias in included studies
Details of the methodological quality of each study are given in Characteristics of included studies, Figure 1, and Figure 2. Studies were considered to be potentially at a moderate‐to‐high risk of bias if they were assessed to be at 'high risk' for at least one of the risk of bias items below, excluding blinding, as most studies were open‐label. We considered 35/65 studies (54%) to be at a low risk of bias overall, and 20/65 (29%) to be at a moderate‐to‐high risk of bias overall (Asbee 2009; Barakat 2011; Callaway 2010; Cordero 2014; Di Carlo 2014; Ferrara 2011; Luoto 2011; Murtezani 2014; Nascimento 2012; Oostdam 2012; Petrov Fieril 2014; Pinzon 2012; Price 2012; Rauh 2013; Ruchat 2012; Santos 2005; Stafne 2012; Vitolo 2011; Wilkinson 2012; Wolff 2008). Five of the latter studies (Callaway 2010; Pinzon 2012; Rauh 2013; Vitolo 2011; Wilkinson 2012) did not contribute to quantitative analysis in this update. The remaining 10 studies were at an unclear risk of bias (Angel 2011; Bisson 2014; Bogaerts 2012; Korpi‐Hyovalti 2011; Leiferman 2011; Magee 1990; Marcinkevage 2012; Mujsindi 2014; Polley 2002; Szmeja 2011).
1.
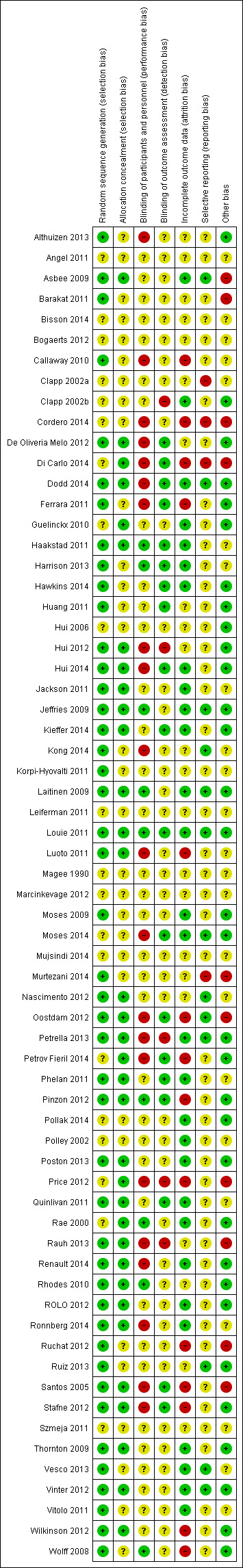
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
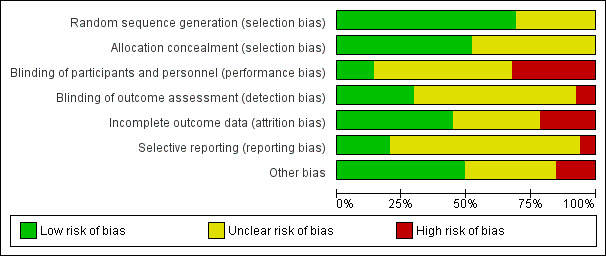
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Out of 65 studies, 45 (69%) were assessed as being at low risk of bias for generation of the randomisation sequence and 34 (52%) used methods that we judged to be at a low risk of bias for allocation concealment. The remaining studies (25%) were at an unclear risk of selection bias.
Blinding
Twenty‐four out of 65 studies (37%) had taken some steps to implement performance or detection blinding, or both. Achieving participant and personnel blinding of treatment allocation for diet and exercise interventions was indicated to be not feasible in several studies and was not described in many others. Where studies were described as open‐label or unblinded, we classified these as at a high risk of bias for this item; however, it was difficult to ascertain whether the lack of blinding, or unsuccessful blinding, impacted on outcomes or resulted in any systematic bias. In addition, the studies that did not describe blinding were probably unblinded. For these reasons we did not use blinding as a criterion in the overall assessment of individual study bias but rather took into account other types of bias.
Incomplete outcome data
We assessed 29/65 studies (45%) to be at a low risk of attrition bias. Fourteen studies (22%) (Callaway 2010; Cordero 2014; Di Carlo 2014; Ferrara 2011; Luoto 2011; Oostdam 2012; Petrov Fieril 2014; Pinzon 2012; Price 2012; Ruchat 2012; Santos 2005; Stafne 2012; Wilkinson 2012; Wolff 2008) had high attrition (greater than 20%) overall, or for the intervention or control group only, or for certain outcomes, and we considered these to be at a high risk of bias accordingly. In the remaining studies, loss of outcome data was either not stated or was less than 20% but there were other concerns (e.g. imbalance in attrition between arms) and for these we considered the risk of bias to be unclear.
Selective reporting
It was difficult to assess bias associated with the outcome reporting bias as we did not have access to the study protocols of most studies and we did not know whether results for all outcomes where data had been collected had been reported; we therefore assessed many of these studies as being unclear for the outcome reporting bias. However, we considered Cordero 2014 and Di Carlo 2014 to be at a potentially high risk of reporting bias as only per protocol findings were reported. During the course of these studies, women with preterm labour were excluded, therefore, this potential side‐effect could not be evaluated. In addition, Cordero 2014 additionally excluded data from women with poor adherence to the intervention; therefore, the reported results may be biased in the direction of the intervention.
Other potential sources of bias
Six studies had important baseline imbalances in the characteristics of the women in the intervention and control groups (Barakat 2011; Cordero 2014; Di Carlo 2014; Price 2012; Rauh 2013; Santos 2005) that might have impacted the results in favour of the intervention. Barakat 2011, Cordero 2014,Di Carlo 2014 and Price 2012 did not adjust results for these imbalances and we therefore considered them to be at a potentially high risk of bias. Cordero 2014 additionally had an unexplained difference in intervention and control group sizes. Rauh 2013 is discussed below under 'Assessment of cluster‐RCTs'.
In Price 2012, control participants were told not to exercise because it would blur the distinction between the groups. This contributed to high drop‐out rates in the control group and bias in favour of the intervention, whilst making results less applicable by enforcing no exercise. Oostdam 2012 had issues with adherence to the intervention, which may have biased results in favour of the control group.
Most studies involving an exercise component excluded women at risk of miscarriage or preterm birth at screening. However, four studies excluded women with or at risk of preterm birth post‐randomisation from analysis (Cordero 2014; Di Carlo 2014; Murtezani 2014; Rauh 2013). This omission might have led to publication bias in the review's 'preterm birth' outcome. Any other potential sources of bias were noted in the Characteristics of included studies.
Assessment of cluster‐RCTs
Two trials were cluster‐RCTs (Luoto 2011; Rauh 2013).
Recruitment bias: In Rauh 2013, intervention and control groups differed substantially in size as "During recruitment it turned out that it was easier to recruit women for the intervention group than for the control group, yielding a 2:1 ratio", instead of 1:1. In Luoto 2011, more than 20% and 30% in the intervention and control arms, respectively, were excluded from analysis based on oral glucose tolerance tests conducted between eight and 12 weeks' gestation. Only 40% and 47% of women in intervention and control groups, respectively, who were assessed as preliminary eligible were analysed.
Baseline imbalances: Baseline imbalances were limited to differences in educational levels between arms in Luoto 2011. However, in Rauh 2013, baseline characteristics differed significantly with regard to pregravid BMI (P = 0.003) and BMI at booking (P = 0.008), with a higher proportion of women in the control group considered obese or overweight (16.2% versus 31.4%; P = 0.009). In addition, mean age was younger and gestational age at booking was significantly earlier in the intervention clusters.
Loss of clusters: There was no loss of clusters in either study.
Analysis methods: Both Luoto 2011 and Rauh 2013 reported adjusting the summary effect size for clusters and baseline imbalances. However, outcome data for both studies needed to be estimated for use in meta‐analysis, and this was not possible for Rauh 2013, due to insufficient data and the lack of an intracluster correlation coefficient. The results reported in Rauh 2013 favoured the intervention arm for weight gain outcomes (Appendix 2), and although these were adjusted for BMI, age and clustering, there may also have been other (unknown) differences between the women in these groups. We therefore considered this study to be at potentially high risk of bias; however, Rauh 2013 data did not contribute to meta‐analyses.
Comparability with individually‐randomised trials: These trials were comparable to individually‐randomised trials except that large differences in study group sizes occurred in Rauh 2013. Study investigators reported that the clusters (gynaecology practices) differed significantly in size; the result was that 227 versus 129 women were eligible for the intervention and control clusters, respectively.
Overall assessment: We assessed these cluster‐RCTs as moderate‐to‐high risk of bias. Adjusted Luoto 2011 data were used in meta‐analyses; however, Rauh 2013 data could not be adjusted and were therefore not used.
Effects of interventions
We compared diet or exercise, or both, interventions together (comparisons 1) organised by types of interventions (see Table 3 for the rationale for study categories). We also analysed each intervention category separately stratified for risk (comparisons 2, 3, 4, and 5 ) for all women (i.e. a mixed‐risk population), low risk, and high‐risk women.
1. Types of Interventions assessed in studies contributing data.
| Number | Study ID | Experimental intervention (unless otherwise stated, the control intervention was routine care) | Participants analysed | Contributed data | ||
| Low risk | Mixed risk | High risk | ||||
| Diet counselling/other | ||||||
| 1 | Di Carlo 2014 | The intervention involved a supervised personalised diet plan meeting both personal preferences and specific gestational needs with the average caloric intake being 1916 kcal. The only fat allowed was olive oil. Participants in the intervention group underwent monthly follow‐up appointments with a dietician who monitored their weight gain and discussed issues. Control group received standard brochure on healthy eating. | 120 | / | ||
| 2. | Jeffries 2009 | Women were given a personalised weight measurement card, advised of their optimal gestational weight gain based on their BMI at the time of recruitment and the United States IOM guidelines, and instructed to record their weight at 16, 20, 24, 28, 30, 32 and 34 weeks' gestation. | 236 | / | / | |
| 3. | Laitinen 2009 | Dietary counselling was given by a dietitian at each study visit and aimed to modify dietary intake to conform with that currently recommended, particular attention being paid to the quality of dietary fat. Study visits took place 3 times during pregnancy and at 1, 6 and 12 months postpartum. | 171 | / | ||
| 4. | Quinlivan 2011 | This was a multi‐faceted intervention that included weighing on arrival and a brief dietary intervention by a food technologist at every antenatal visit. Other aspects of the intervention involved continuity of care provider and psychological assessment. | 124 | / | ||
| 5. | Rae 2000 | The intervention comprised instruction in a moderately energy restricted diabetic diet providing between 1590‐1776 kcal (70% RDA). | 117 | / | ||
| 6. | Thornton 2009 | Participants were counselled in nutrition and monitored by a registered dietitian and given a detailed nutrition program similar to a diabetic diet. | 232 | / | ||
| 7. | Wolff 2008 | Intervention comprise a healthy diet according to the official Danish dietary recommendations (% fat, protein, CHO, 30%, 15%‐20%, 50%‐55%). The energy intake was restricted based on individually estimated energy requirements and estimated energetic cost of fetal growth. | 50 | / | ||
| Low GL diet | ||||||
| 8. | Clapp 2002b | Participants were randomised to either a low‐glycaemic diet (aboriginal diet) or high‐glycaemic diet (cafeteria diet). | 20 | / | ||
| 9. | Louie 2011 | Intervention was a healthy low‐GL diet of protein (15%‐25%), fat (25%‐30%) and carbohydrate (40%‐45%) (versus healthy high‐fibre diet with moderate GL, similar to population average). Participants attended at least 3 face‐to‐face visits with the study dietician for monitoring adherence and encouragement. Intervention began after 29th week. | 92 | / | ||
| 10. | Moses 2009 | Low‐GL diet versus high‐GL diet. The dietary advice by dietitian was individualised with specific mention of the energy and nutrient balance to achieve normal weight gain during the 3rd trimester. | 63 | / | ||
| 11. | Moses 2014 | The intervention involved a low glycaemic diet from 12 to 16 weeks' gestation for the remainder of pregnancy (compared with conventional healthy eating). Women received a detailed dietary education tailored for the group assignment at baseline ‐ there were no difference in the macronutrient distribution in the diets, only the substitution of carbohydrate‐rich foods with low GL alternatives in the experimental group. Information booklets were provided. 4 contact points with a research dietician were planned (first visit, phone call, midway and final visits) to collect data and ensure adherence. | 576 | / | ||
| 12. | Rhodes 2010 | Nutrition education, dietary counselling, and a low‐GL diet (vs a low‐fat diet). | 50 | / | ||
| 13. | ROLO 2012 | Low‐GL dietary intervention given by a dietitian involving 1 dietary education session lasting 2 hours in groups of 2 ‐6 women at baseline. Follow‐up reinforcement sessions were held at 28 and 34 weeks' gestation. Women also received written resources about low‐GL foods. | 759 | / | / | / |
| Diet and exercise counselling | ||||||
| 14. | Althuizen 2013 | The intervention involved counselling by members of the research team consisting of 5 x 15 minute sessions on weight, physical activity and diet. Interventions were face‐to‐face at 18, 22, 30, and 36 weeks' gestation, with a telephone session at 8 weeks postpartum. Counsellors discussed how to control weight gain during and after pregnancy, and how to maintain a healthy lifestyle. | 219 | / | / | |
| 15 | Asbee 2009 | At the initial visit, participants met with a registered dietician to receive a standardised counselling session, including information on pregnancy‐specific dietary and lifestyle choices. Participants were instructed to engage in moderate‐intensity exercise at least 3 times per week and preferably 5 times per week. Weight gain was reviewed at each routine antenatal visit. | 100 | / | ||
| 16. | Asbee 2009 | Comprehensive dietary and lifestyle intervention (counselling) (n = 1108) Intervention involved meetings and home visits with advice on dietary, exercise, and behavioural strategies delivered by a dietician and trained research assistants. Exercise advice primarily encouraged women to increase their amount of walking and incidental activity. | 2212 | / | ||
| 17. | Ferrara 2011 | Lifestyle intervention involved 3 in‐person sessions and up to 15 telephone calls with counselling re diet, physical activity and breast‐feeding up to 12 months postpartum. The intervention was delivered by 2 dietitians. Participants were encouraged to engage in moderate‐intensity physical activity for 150 minutes per week and received written materials about food size, foods with low GL or low fat, and how to read food labels were discussed, | 197 | / | ||
| 18. | Guelinckx 2010 | 2 intervention arms: 1 involved a brochure only, the other involved a brochure and counselling by a trained nutritionist in 3 group sessions. A maximum of 5 women were brought together in these 1‐hour sessions, which were scheduled at 15, 20, and 32 weeks of pregnancy. The sessions provided participants with recommendations on a balanced, healthy diet, and physical activity specifically designed for the study to limit weight gain. | 124 | / | ||
| 19. | Harrison 2013 | Intervention provided dietary advice, simple healthy eating, and "physical activity messages" and weight gain self‐monitoring. Also included "regular self‐weighing as a key behavioural strategy". | 203 | / | ||
| 20. | Hawkins 2014 | A lifestyle intervention consisting of a culturally and linguistically modified, motivationally targeted, individually tailored 6‐month prenatal programme. Educators encouraged women to achieve guidelines for physical activity, decrease saturated fat and increase dietary fibre. The intervention consisted of 6 monthly in‐person behavioural counselling sessions and 5 telephone booster sessions with follow‐up to 6 weeks postpartum. Women were encourage to achieve ≥ 30 minutes of moderate‐intensity activity on most days of the week through walking and developing a more active lifestyle. | 68 | / | ||
| 21. | Huang 2011 | The intervention was delivered at regularly scheduled clinic visits by nurses with training in nutrition and physical fitness. The nurse discussed with each participant how to design an individualised diet and physical activity plan. The intervention consisted of 6 1‐to‐1 counselling sessions: 1 primary session (about 30–40 minutes) at the 16‐week gestation visit, and 5 1‐to‐1 booster sessions (at 28 gestational weeks, 36–38 gestational weeks, before hospital discharge after a 3 to 7‐day stay, 6 weeks' postpartum and 3 months postpartum). After each clinic visit, women in the experimental groups were sent a personalised graph of their weight changes. At the 1st session, the experimental groups also received a researcher‐prepared brochure that provided detailed information on weight management goals during pregnancy and postpartum. | 160 | / | ||
| 22. | Korpi‐Hyovalti 2011 | Individual dietary advice tailored to each participant at 6 visits to include a low fat diet rich in vegetables, fruit and berries. Moderate‐intensity physical exercise was encouraged at 6 exercise counselling sessions. | 60 | / | ||
| 23. | Luoto 2011 | Individual counselling on physical activity and diet and weight gain. At the first visit the recommendations for gestational weight gain were discussed and an appropriate weight gain graph was selected to guide the participant in monitoring her weight gain. Physical activity counselling was implemented at 8–12 weeks' gestation and the dietary counselling session occurred at 16–18 weeks' gestation. Physical activity counselling was enhanced at 4, and diet counselling at 3 subsequent visits. | 399 | / | ||
| 24. | Petrella 2013 | Lifestyle intervention involving a caloric restricted diet (1500 kcal/day) and mild exercise 30 minutes/day, 3 times per week monitored by a pedometer. | 63 | / | ||
| 25. | Phelan 2011 | The Fit for Delivery intervention included a face‐to‐face visit with an interventionist at the onset of treatment who discussed appropriate weight gains during pregnancy, physical activity (30 minutes of walking most days of the week), and calorie goals (20 kcal/kg). Emphasis was placed on decreasing high fat foods, increasing physical activity, and daily self‐monitoring. Women also received personalised weight graphs after each clinic visit, automated supportive postcards and 3 supportive phone calls. | 401 | / | / | |
| 26. | Polley 2002 | Intervention conducted at routine clinic visits by staff with training in nutrition or psychology involved education about weight gain, healthy eating, and exercise, and individual graphs of their weight gain. After each clinic visit, women were sent a personalised graph of their weight gain. | 110 | / | / | |
| 27. | Poston 2013 | A lifestyle intervention (diet plus exercise) involving 1 1‐to‐1 counselling session with a health trainer and then weekly group sessions for 8 consecutive weeks from 19 weeks' gestation. Sessions delivered by health trainers involved diet and exercise advice informed by psychological models of health behaviour. Dietary advice focused on increased consumption of foods with a low‐dietary GL, and reduction of saturated fats. Physical activity advice encouraged women to increase daily walking activity at moderate‐intensity level, setting goals monitored by a pedometer. Women also received a DVD of a pregnancy specific exercise regimen. | 154 | / | ||
| 28. | Renault 2014 | A 3‐arm study with 2 intervention groups. 1 intervention involved unsupervised exercise only (women were given a pedometer), the other involved diet and exercise counselling only. The diet and exercise intervention included follow‐up on a hypocaloric Mediterranean‐style diet. Instruction was given by a dietician every 2 weeks with alternating outpatient visits and phone calls, including weight measurement, encouragement and correcting advice on exercise and diet. | 389 | / | ||
| 29. | Vesco 2013 | Intervention involved a 45‐minute diet consultation with an individualised caloric goal, a second individualised session, weekly group meetings with weigh ins, food/activity logs. Women are encouraged to accumulate at least 30 minutes of moderate‐intensity activity per day. Pedometers recorded steps with a target of 10,000 steps daily and were only provided to the intervention group. | 114 | / | ||
| Unsupervised exercise intervention | ||||||
| 30. | Kong 2014 | Unsupervised exercise intervention involved a walking program on treadmill or other setting for a minimum of 150 min/week. Women were loaned treadmills for the study and steps monitored. | 42 | / | ||
| 31. | Renault 2014 | A 3‐arm study with 2 intervention groups. 1 intervention involved unsupervised exercise only (women were given a pedometer), the other involved diet and exercise counselling. The physical activity intervention included encouragement or increase physical activity, aiming at a daily step count of 11,000, monitored by pedometer assessment on 7 consecutive days, every 4 weeks. | 389 | / | ||
| 32. | Ronnberg 2014 | Intervention involved prescribed exercise to be at a "moderate level of exertion for approximately 30 min/day". | 374 | / | / | / |
| Supervised exercise intervention | ||||||
| 33. | Barakat 2011 | Intervention involved 35‐ to 45‐minute exercise sessions 3 times per week from the start of the pregnancy (weeks 6‐9) to the end of the 3rd trimester (weeks 38‐39) ‐ an average of 85 training sessions. Exercise intensity was light‐to‐moderate and was supervised by a fitness specialist in groups of 10‐12 women. | 80 | / | ||
| 34. | Cordero 2014 | A supervised exercise program consisting of aerobic and toning exercises for 3 sessions per week. 2 weekly sessions were performed on land (60 minutes) and 1 session was aquatic based (50 minutes). Program commenced from 10‐14 weeks to the end of the third trimester. Sessions were supervised by a qualified fitness specialist and an obstetrician. | 257 | / | ||
| 35. | De Oliveria Melo 2012 | Supervised moderate‐intensity exercise (initiated at 13 weeks or 20 weeks) vs control . Sessions consisted of warming up and stretching exercises, followed by supervised walking 3 times a week in the open air. Supervised by physical education professionals and medical, physiotherapy and nursing students. | 187 | / | ||
| 36. | Haakstad 2011 | Exercise (60 minutes supervised aerobic dance at least twice a week for a minimum of 12 weeks) (n = 52). Women in the exercise group were advised to have moderate, self‐imposed physical activity on the remaining weekdays. | 105 | / | ||
| 37. | Murtezani 2014 | The exercise training program started in the second trimester and was continued until the end of pregnancy. Each session consisted of 40‐45 minutes of aerobic and strength exercise. Individuals were supervised by certified aerobic‐instructors, and each session included a maximum of 10 participants. Intensity was moderate‐to‐vigorous; supine postures and Valsalva manoeuvres were avoided. | 63 | / | ||
| 38. | Nascimento 2012 | Intervention consisted of a supervised exercise program guided by a trained physical therapist in weekly classes with light‐to‐moderate‐intensity exercise for 40 minutes. It also included home exercise counselling which was to be performed 5 times per week (consisting of a sequence of 22 exercises or walking). | 82 | / | ||
| 39. | Oostdam 2012 | A supervised exercise intervention comprising 2 sessions of aerobic and strengthening exercises per week; each exercise session lasted for 60 minutes from 20 weeks' gestation. | 101 | / | ||
| 40. | Petrov Fieril 2014 | Intervention group received supervised resistance exercise twice a week, with light barbells and weight plates in a group setting, performed at an activity level equivalent to within moderate–to‐vigorous between weeks 14 to 25 gestation, and was self‐adjusted. In addition, walking, cycling, water‐gymnastics, Pilates, yoga and home exercises that included pelvic floor training were recommended. | 72 | / | ||
| 41. | Price 2012 | Intervention involved a program of supervised aerobic training of 45‐60 minutes, 4 days per week. | 62 | / | ||
| 42. | Ruiz 2013 | Intervention involved light‐to moderate‐intensity supervised aerobic and resistance exercises (including pelvic floor exercises) performed 3 days a week (50‐55 minutes per session) from 9 weeks to weeks 38‐39. Exercise sessions involved 8‐10 participants. | 962 | / | / | / |
| 43 | Santos 2005 | The intervention consisted of a program of supervised physical exercise of 60 minutes duration, performed 3 times per week for 12 weeks. | 72 | / | ||
| 44. | Stafne 2012 | intervention comprised a 12‐week regular standardised exercise program including aerobic activity, strength training, and balance exercises. The exercise program followed standard recommendations and included moderate‐intensity to high‐intensity activity 3 or more days per week. Physiotherapist‐supervised training sessions of 60 minutes in groups of 8‐15 women were offered once per week. | 702 | / | ||
| Supervised exercise and diet intervention | ||||||
| 45. | Hui 2006 | Exercise intervention involved a weekly supervised group session including floor aerobics, stretching and strength exercises, and similar home‐based exercise 3‐5 times/week for 30‐45 minutes per session. A video was provided to participants to assist with home‐based exercise. Diet intervention involved a computer‐assisted food choice map interview and a personalised plan by a dietician. | 46 | / | ||
| 46. | Hui 2012 | Exercise intervention involved an exercise regimen comprising 3 to 5 times per week, including a weekly supervised community‐based session and multiple home sessions, of mild‐to‐moderate exercise for 30 to 45 minutes. Program started between 20‐26 weeks. Group exercise sessions including aerobics were held in community centres and instructors were licensed fitness trainers. 2 dietary interviews with counselling were provided. | 190 | / | ||
| 47. | Hui 2014 | Lifestyle intervention (diet counselling and a supervised exercise program) vs control. Intervention included "a community‐based exercise program specifically designed for pregnant women was provided". An exercise regimen, 3 to 5 times per week including a weekly exercise session and home sessions with DVD instruction of mild‐to‐moderate aerobic exercise for 30 to 45 minutes was recommended. Program started between 20‐26 weeks and continued to 36 weeks. Group exercise sessions including aerobics were held in community centres and instructors were licensed fitness trainers. 2 dietary interviews with dietician counselling using a Food Choice Map were provided (baseline and 2 months later). Control group received standard care. | 113 | / | / | |
| 48. | Ruchat 2012 | Moderate‐intensity exercise 3‐4 times per week, including 1 supervised session (versus low‐intensity exercise in the form of a walking program 3‐4 times per week). All participants received a diet plan based on a modified diabetic diet. | 49 | / | ||
| 49. | Vinter 2012 | A lifestyle intervention consisting of dietary counselling and exercise. The intervention involved dietary advise on 4 occasions (15, 20, 28 and 35 weeks) by a dietician. Energy requirements were personalised for each participant. Exercise intervention included a pedometer and free gym membership for 6 months. Participants were encouraged to do 30‐60 minutes moderate‐physical activity daily. In addition, at the gym they had 1 supervised aerobic class with a physiotherapist for 1 hour each week. | 304 | / | ||
BMI: body mass index CHO: carbohydrate GL: glycaemic load IOM: Institute of Medicine RDA: recommended dietary allowance
1. Diet and/or exercise interventions vs routine care in all pregnant women
Forty‐nine studies involving 11,444 participants contributed data to these analyses. A maximum of 36 studies contributed data to Comparison 1 analyses. Funnel plots were asymmetrical for most analyses.
1.1 Excessive gestational weight gain (GWG)
Diet or exercise, or both, interventions resulted in an average risk reduction of excessive GWG of 20% in favour of the intervention group (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.73 to 0.87; participants = 7096; studies = 24; I² = 52%; Analysis 1.1). Reductions in favour of the intervention arms were consistent across the different types of intervention groups, except for the heterogenous group comprising 'diet counselling or other interventions', with the largest reduction occurring with 'supervised exercise and diet' interventions.
1.1. Analysis.
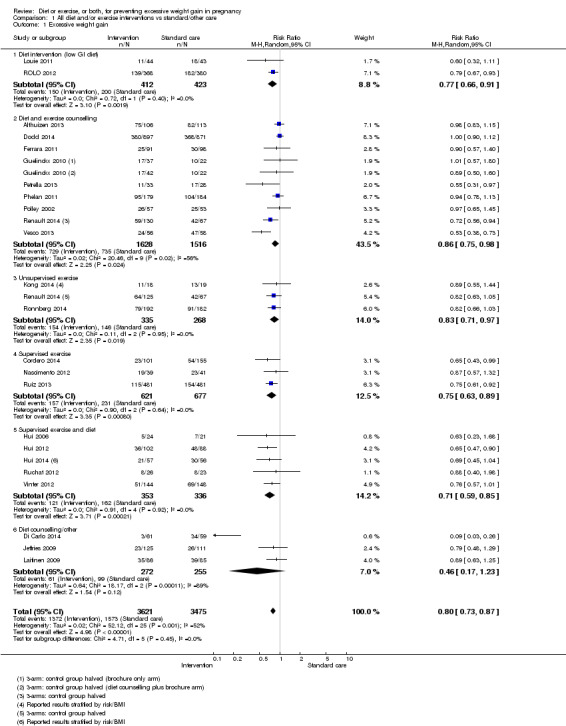
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 1 Excessive weight gain.
Sensitivity analysis
When we removed five studies with risk of bias concerns from the meta‐analysis (Cordero 2014; Di Carlo 2014; Ferrara 2011; Hui 2006; Ruchat 2012), heterogeneity was reduced and the average overall effect in favour of the intervention group was robust to the original result (RR 0.82, 95% CI 0.76 to 0.89; participants = 6437; studies = 19; I² = 40%). In addition, when we pooled data from exercise only interventions (supervised and unsupervised), findings were similar (RR 0.79, 95% CI 0.70 to 0.89; participants = 1901; studies = six; I² = 0%).
Quality of the evidence
We graded this evidence as high quality.
1.2 Mean gestational weight gain (GWG)
Thirty‐six studies reported this outcome; however, due to substantial heterogeneity we did not pool these data (Analysis 1.2). Thirteen studies reported significant differences in mean GWG between intervention and control groups in favour of the interventions, with five studies reporting mean differences in GWG in excess of 5 kg (Clapp 2002a; Di Carlo 2014; Thornton 2009; Quinlivan 2011; Wolff 2008). The remainder of studies found no significant difference in mean GWG between groups.
1.2. Analysis.
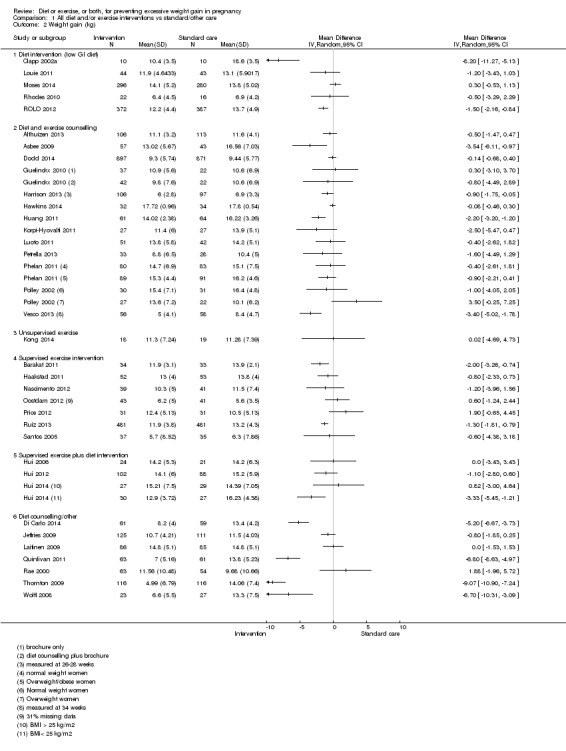
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 2 Weight gain (kg).
1.3 Low weight gain
Women in the intervention group were significantly more likely to experience low GWG compared with the control group (average RR 1.14, 95% CI 1.02 to 1.27; participants = 4422; studies = 11; I² = 3%; Analysis 1.3). Results according to types of interventions were not statistically significant, with wide confidence intervals and a consistent trend in favour of the control group. However, when data from the supervised and unsupervised exercise groups were combined the effect was of borderline statistical significance (RR 1.19, 95% CI 1.00 to 1.41; participants = 1565; studies = four; I² = 0%).
1.3. Analysis.
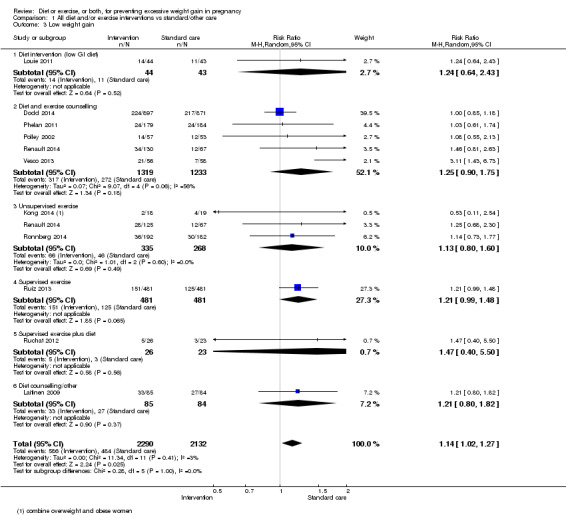
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 3 Low weight gain.
Sensitivity analysis
There were no serious risk of bias concerns for this analysis.
Quality of the evidence
We graded this evidence as moderate quality.
1.4 Preterm birth
There was no statistically significant difference between intervention and control groups for preterm birth outcomes (average RR 0.91, 95% CI 0.68 to 1.22; participants = 5923; studies = 16; I² = 16%; Analysis 1.4). Point estimates for exercise only interventions favoured the control groups; however, when data from the supervised and unsupervised groups were combined the trend was not statistically significant (RR 1.59, 95% CI 0.76 to 3.33; participants = 1358; studies = five; I² = 0%).
1.4. Analysis.
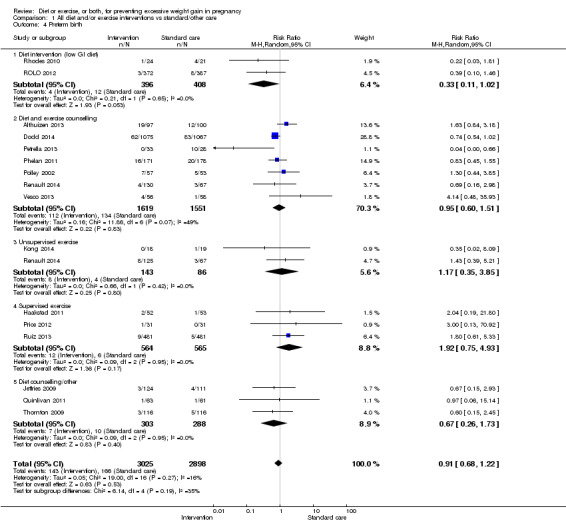
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 4 Preterm birth.
Sensitivity analysis
When two studies with risk of bias concerns were excluded from the analysis (Price 2012; Petrella 2013), results were similar to the original analysis (average RR 0.88, 95% CI 0.70 to 1.10; participants = 5800; studies = 14; I² = 0%).
Quality of the evidence
We downgraded this evidence to moderate quality due to risk of bias concerns arising from potential under‐reporting (in at least four studies, women at risk of preterm birth were withdrawn; see Risk of bias in included studies).
1.5 Pre‐eclampsia
There was no statistically significant difference between the intervention and control groups with regard to pre‐eclampsia (average RR 0.95, 95% CI 0.77 to 1.16; participants = 5330; studies = 15; I² = 0%; Analysis 1.5).
1.5. Analysis.
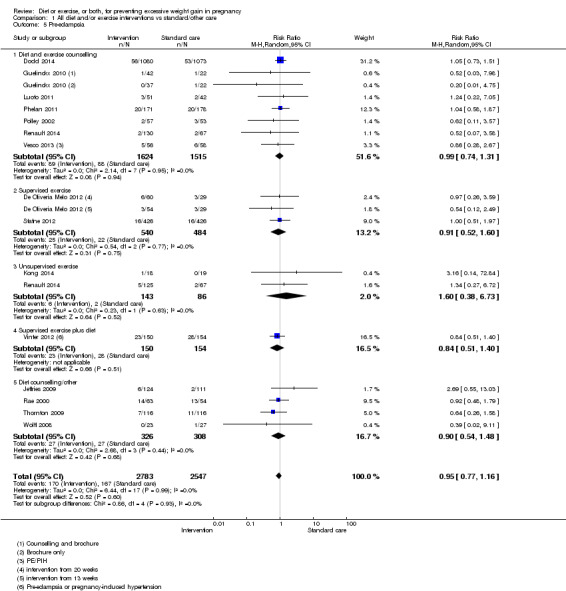
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 5 Pre‐eclampsia.
Quality of the evidence
We graded the quality of this evidence as high.
1.6 Hypertension (not prespecified)
Maternal hypertension occurred significantly more frequently in the control group compared with the intervention group (average RR 0.70, 95% CI 0.51 to 0.96; participants = 5162; studies = 11; I² = 43%; Analysis 1.6).
1.6. Analysis.
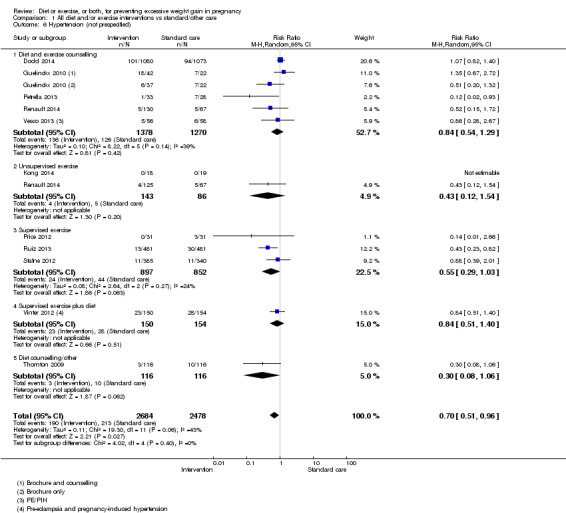
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 6 Hypertension (not prespecified).
Sensitivity analysis and investigation of heterogeneity
When we excluded three studies (Petrella 2013; Price 2012; Stafne 2012) with risk of bias concerns, the result remained in favour of the interventions; however it was no longer statistically significant (RR 0.74, 95% CI 0.53 to 1.02; participants = 4314; studies = eight; I² = 44%). Heterogeneity could not be attributed to differences between the different types of interventions. We investigated heterogeneity further below, by subgrouping studies by participant risk group.
Quality of the evidence
We downgraded the evidence to low quality due to inconsistency and risk of bias concerns.
1.7 Induction of labour
There was no statistically significant difference between intervention and control groups for this outcome (average RR 1.06, 95% CI 0.94 to 1.19; participants = 3832; studies = eight; I² = 9%; Analysis 1.7).
1.7. Analysis.
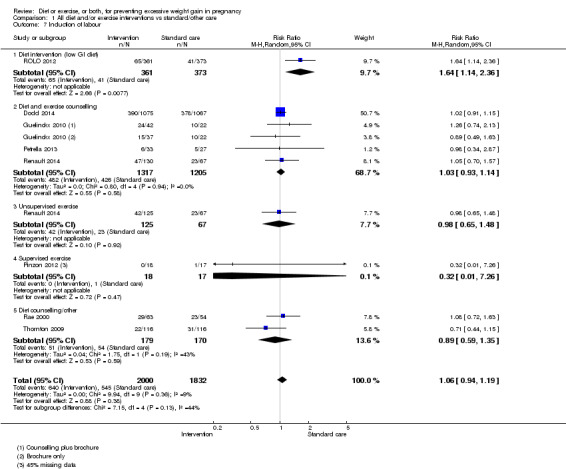
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 7 Induction of labour.
Quality of the evidence
We graded the quality of this evidence as high.
1.8 Caesarean delivery
There was no statistically significant difference in the risk of caesarean section between intervention and control groups (average RR 0.95, 95% CI 0.88 to 1.03; participants = 7534; studies = 28; I² = 9%; Analysis 1.8); although the point estimate favoured a small reduction in favour of the intervention group. Effect estimates for supervised and unsupervised exercise interventions only, alone or combined, were robust to the overall findings. However, for the diet and exercise counselling interventions, the effect estimate approached statistical significance with a 13% reduction in caesarean delivery in the intervention group (RR 0.87, 95% CI 0.75 to 1.01; participants = 3406; studies = 9; I² = 15%; P = 0.06).
1.8. Analysis.
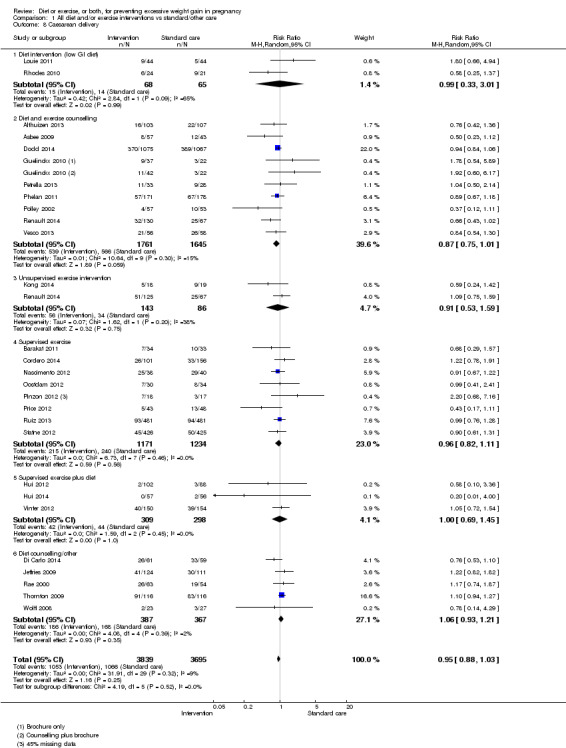
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 8 Caesarean delivery.
Sensitivity analysis
When we excluded two studies with risk of bias concerns (Di Carlo 2014; Price 2012), the results moved further toward the null (average RR 0.97, 95% CI 0.91 to 1.04; participants = 7323; studies = 26; I² = 2%).
Quality of the evidence
We graded the quality of this evidence as high, although effects may differ according to types of interventions.
1.9 and 1.10 Maternal postpartum weight retention (mean [kg] and rate)
Diet or exercise, or both, interventions on average were not associated with less postpartum weight retention in kilograms compared with the controls (average mean difference (MD) ‐1.12, 95% CI ‐2.49 to 0.25; participants = 818; studies = 7; I² = 55%; Analysis 1.9); however, pooled data from five studies indicated significantly lower rates of postpartum weight retention in the intervention group (average RR 0.78, 95% CI 0.63 to 0.97; participants = 902; studies = five; I² = 63%, Analysis 1.10).
1.9. Analysis.
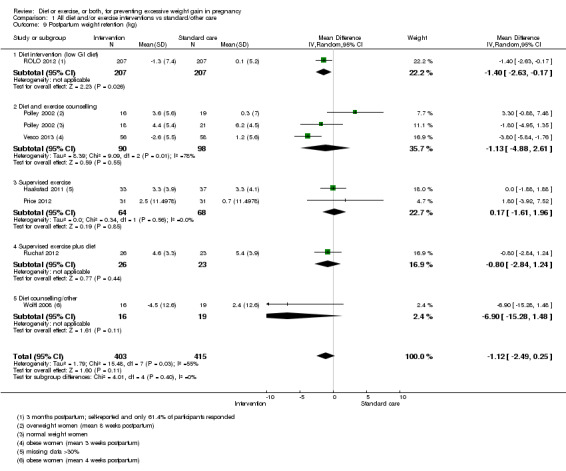
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 9 Postpartum weight retention (kg).
1.10. Analysis.
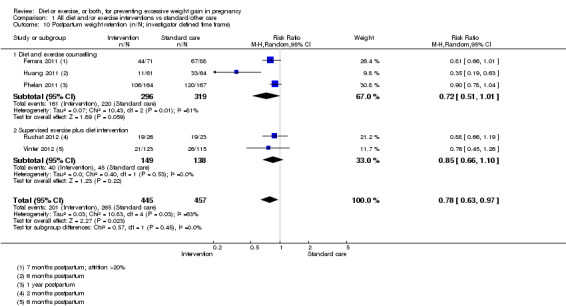
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 10 Postpartum weight retention (n/N; investigator defined time frame).
Sensitivity analysis and investigation of heterogeneity
Time frames for these assessments ranged from six weeks to six months postpartum which may have accounted for the heterogeneity. Several studies had risk of bias concerns (mainly attrition bias) for these outcomes; therefore, we did not perform sensitivity analysis.
Quality of the evidence
We graded this evidence as low quality due to inconsistency and risk of bias concerns.
1.11 to 1.13 Behaviour modification outcomes (diet and physical activity)
Energy and fibre intake
Data on mean energy intake were available for 12 studies and revealed a statistically significant difference in energy intake (in kilojoules) between intervention and control groups (average MD ‐570.77, 95% CI ‐894.28 to ‐247.26; participants = 4065; studies = 12; I² = 73%;Analysis 1.11). Similarly, fibre intake in grams was significantly higher in the intervention group (MD 1.53, 95% CI 0.94 to 2.12; participants = 3466; studies = 8; I² = 0%; Analysis 1.12).
1.11. Analysis.
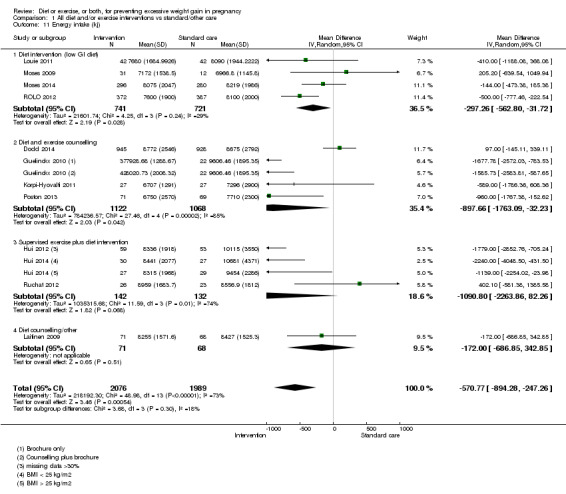
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 11 Energy intake (kj).
1.12. Analysis.
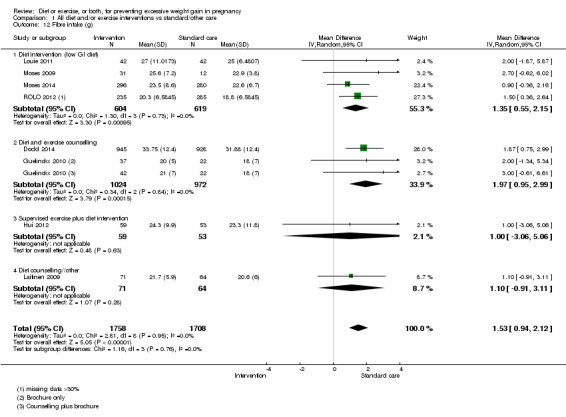
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 12 Fibre intake (g).
Physical activity score at 26 to 29 weeks
Women in the intervention group revealed higher physical activity scores on average compared with controls (average standardised mean difference (SMD) 0.40, 95% CI 0.18 to 0.61; participants = 2851; studies = 9; I² = 75%; Analysis 1.13).
1.13. Analysis.
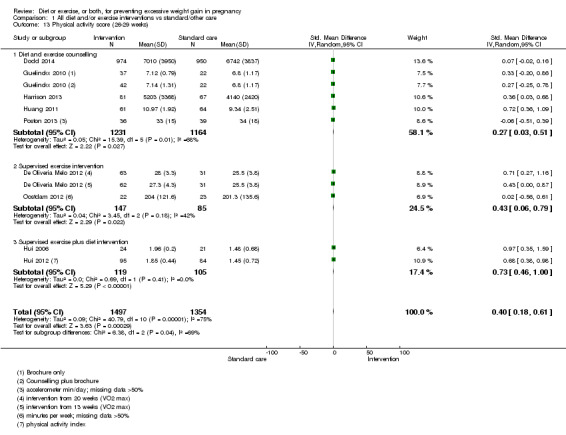
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 13 Physical activity score (26‐29 weeks).
Quality of evidence
We graded evidence relating to behaviour modification outcomes as low quality due to heterogeneity and risk of bias concerns, particularly detection (mainly self‐reported outcomes) and attrition bias.
1.14 Infant birthweight greater than 4000 g
There was no statistically significant difference in the risk of macrosomia (birthweight greater than 4000 g) between intervention and control groups overall (average RR 0.93, 95% CI 0.86 to 1.02; participants = 8598; studies = 27; I² = 0%, Analysis 1.14), although the trend favoured the intervention groups. For the 'supervised exercise' intervention group, the effect in favour of the intervention bordered on statistical significance (average RR 0.81, 95% CI 0.64 to 1.02; participants = 2445; studies = seven; I² = 0%; P = 0.07), but not when supervised and unsupervised exercise interventions were combined (RR 0.87, 95% CI 0.71 to 1.07; participants = 2674; studies = nine; I² = 0%).
1.14. Analysis.
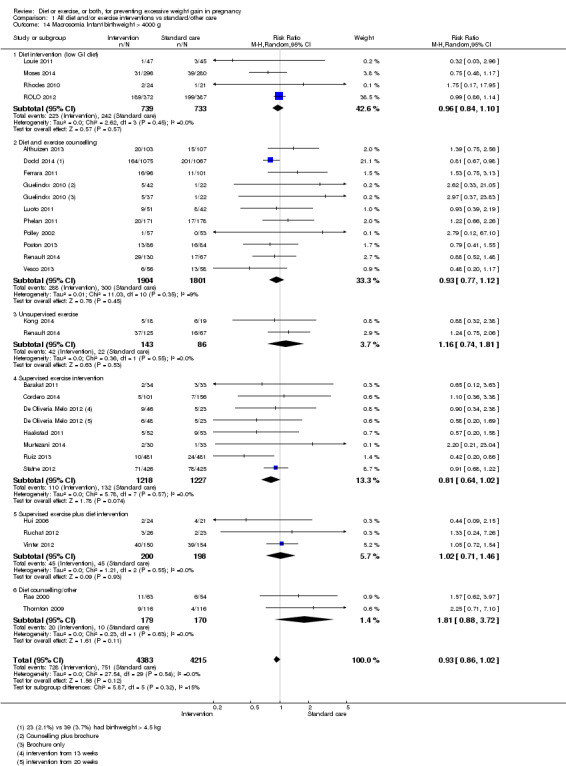
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 14 Macrosomia Infant birthweight > 4000 g.
Sensitivity analysis
Results were similar when we performed sensitivity analysis by excluding seven studies (Cordero 2014;Ferrara 2011; Luoto 2011; Murtezani 2014; Stafne 2012; Ruchat 2012) with risk of bias concerns from the analysis (average RR 0.92, 95% CI 0.82 to 1.04; participants = 7021; studies = 20; I² = 11%).
Quality of the evidence
We graded the evidence as high, although further research may reveal important differences between intervention types.
1.15 Infant birthweight greater than 90th centile
There was no statistically significant difference in the risk of large‐for‐gestational‐age infants between intervention and control groups (average RR 0.92, 95% CI 0.80 to 1.05; participants = 4525; studies = 18; I² = 0%; Analysis 1.15); however, the effect in the diet and exercise counselling group non‐significantly favoured the intervention (RR 0.87, 95% CI 0.74 to 1.02; participants = 2777; studies = 6; I2 = 0%).
1.15. Analysis.
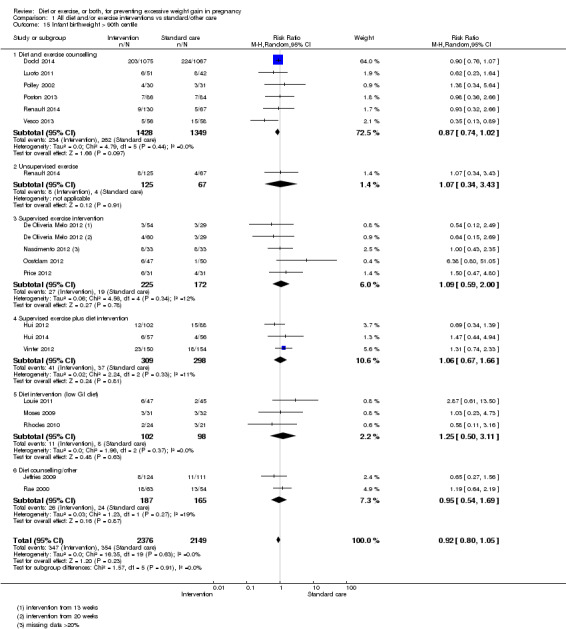
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 15 Infant birthweight > 90th centile.
Quality of the evidence
We graded the quality of this evidence as high.
1.16 Mean birthweight (g)
There was no statistically significant difference in mean birthweight between intervention and control groups (average MD 12.20, 95% CI ‐15.26 to 39.65; participants = 8350; studies = 29; I² = 44%; Analysis 1.16).
1.16. Analysis.
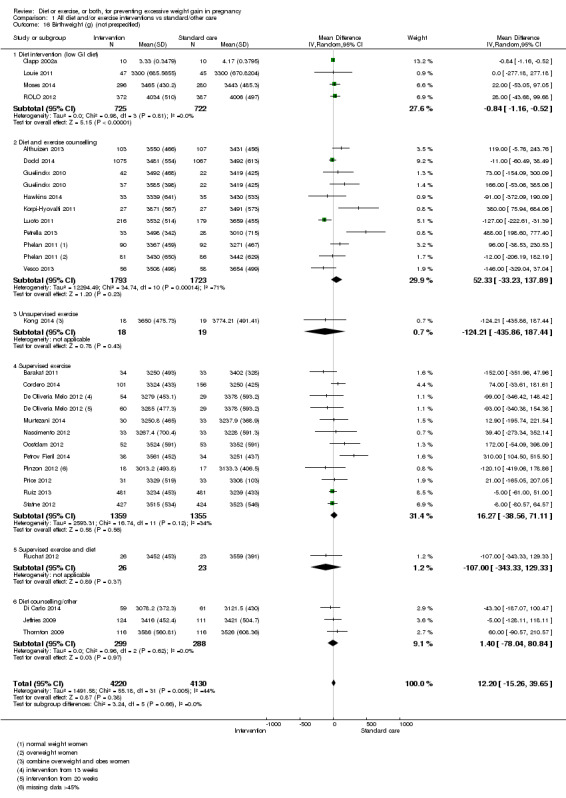
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 16 Birthweight (g) (not prespecified).
Sensitivity analysis and investigation of heterogeneity
There were many risk of bias concerns for this outcome (e.g. due to post‐randomisation exclusions of women with preterm birth in several studies) but the overall result remained fairly robust to exclusion of high‐risk studies.
Quality of the evidence
We graded the quality of the evidence as moderate due to inconsistency.
1.17 and 1.18 Infant birthweight less 2500 g or less than 10th centile
There was no statistically significant difference between groups with respect to birthweight less than 2500 g (average RR 0.88, 95% CI 0.67 to 1.14; participants = 4834; studies = 12; I² = 0%; Analysis 1.17) or small‐for‐gestational‐age infants (average (RR 1.09, 95% CI 0.61 to 1.94; participants = 662; studies = seven; I² = 0%, Analysis 1.18).
1.17. Analysis.
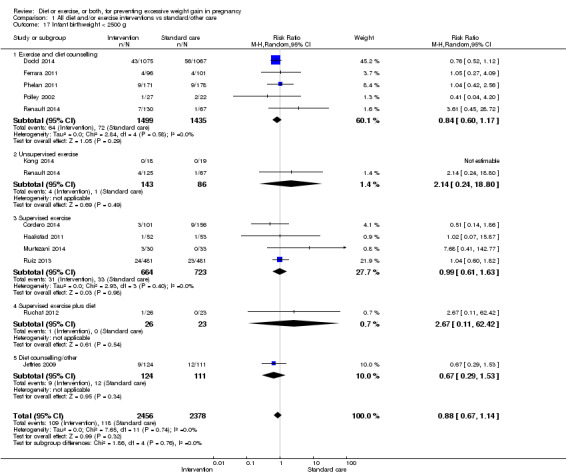
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 17 Infant birthweight < 2500 g.
1.18. Analysis.
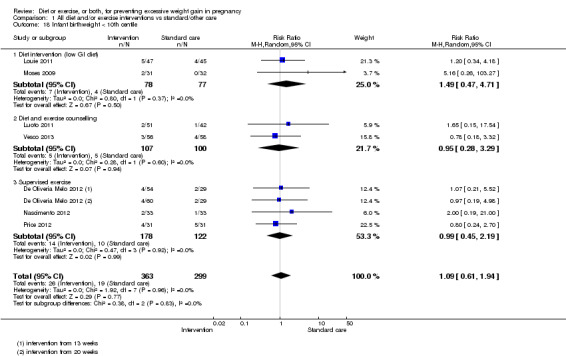
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 18 Infant birthweight < 10th centile.
Quality of the evidence
We downgraded the quality of this evidence to moderate due to potential risk of bias concerns relating mainly to the limited reporting of this outcome.
1.19 Shoulder dystocia
There was no statistically significant difference in the risk of shoulder dystocia between intervention and control groups (average RR 1.02, 95% CI 0.57 to 1.83; participants = 3253; studies = four; I² = 8%, Analysis 1.19).
1.19. Analysis.
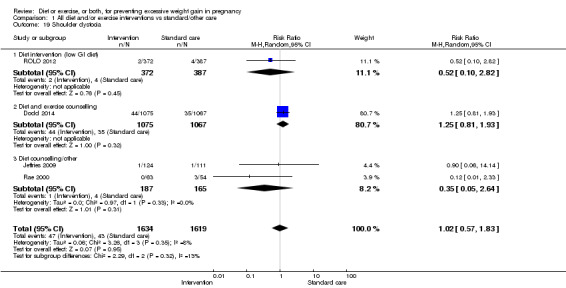
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 19 Shoulder dystocia.
Quality of the evidence
We graded this evidence as moderate quality due to imprecision.
1.20 Neonatal hypoglycaemia
There was no statistically significant difference in the risk of neonatal hypoglycaemia between intervention and control groups (average RR 0.95, 95% CI 0.76 to 1.18; participants = 2601; studies = four; I² = 0%); Analysis 1.20).
1.20. Analysis.
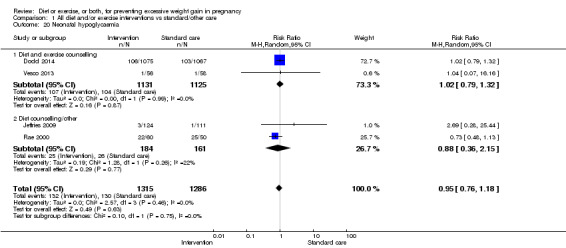
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 20 Neonatal hypoglycaemia.
Quality of the evidence
We graded this evidence as moderate quality due to indirectness (may not apply to all types of interventions).
1.21 Neonatal birth trauma
There was no statistically significant difference between intervention and control groups (average RR 0.89, 95% CI 0.35 to 2.30; participants = 2256; studies = two; I² = 0%; Analysis 1.21). Both included studies (Dodd 2014; Vesco 2013) were diet and exercise counselling interventions and were conducted in high‐risk populations.
1.21. Analysis.
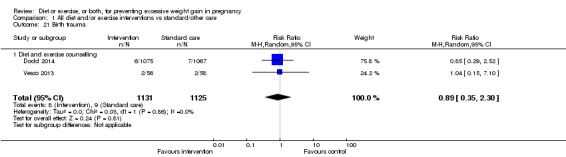
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 21 Birth trauma.
Quality of the evidence
We graded this evidence as moderate quality due to imprecision.
1,22 Neonatal hyperbilirubinaemia
There was no statistically significant difference between intervention and control groups (average RR 0.83, 95% CI 0.62 to 1.10; participants = 2256; studies = two; I² = 0%, Analysis 1.22). Both included studies (Dodd 2014; Vesco 2013) were diet and exercise counselling interventions and were conducted in high‐risk populations.
1.22. Analysis.
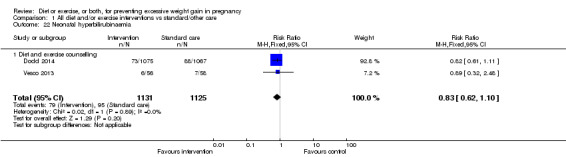
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 22 Neonatal hyperbilirubinaemia.
Quality of the evidence
We graded this evidence as moderate quality due to indirectness (may not apply to all types of interventions).
1.23 Neonatal respiratory distress syndrome
Infants in the intervention group had a significantly reduced risk of experiencing respiratory distress than infants in the control group (average RR 0.47, 95% CI 0.26 to 0.85; participants = 2256; studies = two; I² = 0%; Analysis 1.23). Both included studies (Dodd 2014; Vesco 2013) were diet and exercise counselling interventions and were conducted in high‐risk populations.
1.23. Analysis.
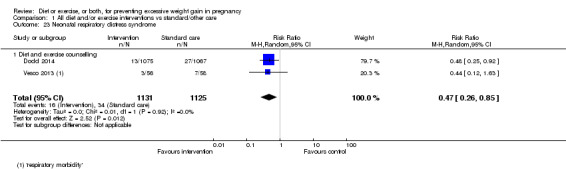
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 23 Neonatal respiratory distress syndrome.
Quality of the evidence
We graded this evidence as moderate quality due to indirectness (may not apply to all types of interventions).
1.24 Postpartum haemorrhage
There was no difference in this outcome between intervention and control groups (average RR 0.94, 95% CI 0.78 to 1.14; participants = 2901; studies = two; I² = 0%; Analysis 1.24).
1.24. Analysis.
Comparison 1 All diet and/or exercise interventions vs standard/other care, Outcome 24 Postpartum hemorrhage.
Quality of the evidence
We graded this evidence as high quality.
2. Diet interventions (low glycaemic load (GL) diet) versus routine care
A maximum of five studies contributed data to this comparison (Clapp 2002a; Louie 2011; Moses 2014; Rhodes 2010; ROLO 2012).
Excessive GWG: Low GL dietary interventions significantly reduced the risk of excessive GWG in the intervention group compared with the control group (RR 0.74, 95% CI 0.55 to 0.99; participants = 833; studies = two; I² = 46%; Analysis 2.1). The test for subgroup differences was not significant (Chi² = 1.30, df = 1 (P = 0.25), I² = 22.9%).
Mean GWG: Due to substantial heterogeneity, we did not pool these data.
Low GWG: Only one study contributed data (Louie 2011) and found no statistically significant difference between intervention and control arms (Analysis 2.3).
Preterm birth: One study contributed data to two subgroups in this analysis. There was no statistically significant difference between intervention and control groups (average RR 0.33, 95% CI 0.11 to 1.02; participants = 804; studies = two; I² = 0%; Analysis 2.4; Test for subgroup differences: Chi² = 0.21, df = 1 (P = 0.65), I² = 0%).
Caesarean delivery: Two studies contributed data to the high‐risk subgroup. There was no statistically significant difference between the intervention and control groups (average RR 0.99, 95% CI 0.33 to 3.01; participants = 133; studies = two; I² = 65%; Analysis 2.5).
Infant birthweight greater than 4000 g: There was no statistically significant difference between the intervention and control groups (average RR 0.96, 95% CI 0.77 to 1.20; participants = 1472; studies = four; I² = 14%; Analysis 2.6; however, the test for subgroup differences suggested that subgroup results may differ in respect to this outcome, with more macrosomia babies born to high‐risk women in the intervention group (Chi² = 2.12, df = 1 (P = 0.15), I² = 52.9%).
Pre‐eclampsia: no data available.
2.1. Analysis.
Comparison 2 Diet intervention (low GI diet) vs standard/other care, Outcome 1 Excessive weight gain.
2.3. Analysis.
Comparison 2 Diet intervention (low GI diet) vs standard/other care, Outcome 3 Low weight gain.
2.4. Analysis.
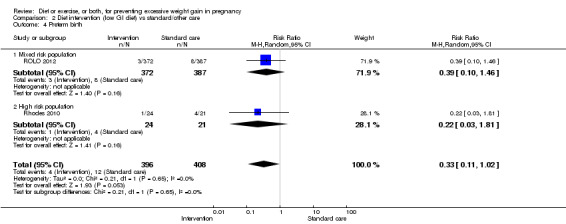
Comparison 2 Diet intervention (low GI diet) vs standard/other care, Outcome 4 Preterm birth.
2.5. Analysis.
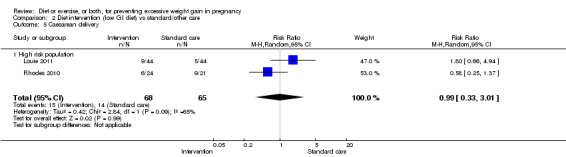
Comparison 2 Diet intervention (low GI diet) vs standard/other care, Outcome 5 Caesarean delivery.
2.6. Analysis.
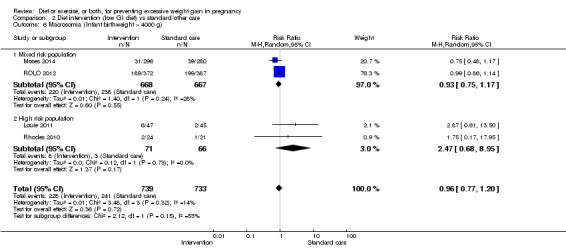
Comparison 2 Diet intervention (low GI diet) vs standard/other care, Outcome 6 Macrosomia (Infant birthweight > 4000 g).
We graded the evidence relating to excessive GWG as moderate quality, and for the other outcomes analysed as low quality due to heterogeneity or imprecision and sparse data, therefore it is likely that further research may change these effect estimates.
3. Diet and exercise counselling versus routine care
A maximum of 13 studies contributed data to these meta‐analyses. To avoid duplication of data from Althuizen 2013 we did not combine subgroup data.
Excessive GWG: Interventions reduced the incidence of excessive GWG in low‐risk participants (average RR 0.72, 95% CI 0.55 to 0.95; participants = 247; studies = 2; I² = 0%) and there was a trend towards a reduction in excessive GWG in the high‐risk subgroup (RR 0.85, 95% CI 0.71 to 1.02; participants = 2725; studies = nine; I² = 69%; Analysis 3.1; Test for subgroup differences: Chi² = 3.51, df = 2 (P = 0.17), I² = 43.0%). We downgraded this evidence to moderate due to heterogeneity.
Mean GWG: Mean GWG in kg was reduced with the intervention for the mixed‐risk subgroup (MD ‐1.80, 95% CI ‐3.36 to ‐0.24; participants = 444; studies = three; I² = 76%) and the high‐risk subgroup (MD ‐0.71, 95% CI ‐1.34 to ‐0.08; participants = 2741; studies = 11; I² = 57%) but heterogeneity was high (Analysis 3.2). In the latter, this could be explained by the timing of the measurements, and when two studies that measured weight gain at less than 34 weeks were excluded, data were homogeneous and favoured no clear difference between the intervention and control groups (MD ‐0.15, 95% CI ‐0.44 to 0.15; participants = 2424; studies = nine; I² = 0%; Test for subgroup differences suggested a difference in effect between subgroups: Chi² = 5.44, df = 2 (P = 0.07), I² = 63.2%).
Low GWG: There was no significant difference in low GWG on average overall (RR 1.23, 95% CI 0.89 to 1.72; participants = 2552; studies = five; I² = 49%; Analysis 3.3; Test for subgroup differences: Chi² = 0.07, df = 1 (P = 0.79), I² = 0%) or for subgroups; however the overall trend favoured the control group.
Preterm birth: There was no significant difference in preterm birth on average overall (RR 0.94, 95% CI 0.57 to 1.55; participants = 3170; studies = seven; I² = 52%; Analysis 3.4) or for subgroups; Test for subgroup differences: Chi² = 0.32, df = 1 (P = 0.57), I² = 0%). We downgraded this evidence to low due to imprecision and heterogeneity.
Pre‐eclampsia: There was no significant difference in pre‐eclampsia on average between intervention and control groups for the high‐risk population (average RR 1.06, 95% CI 0.79 to 1.43; participants = 2896; studies = seven; I² = 0%; Analysis 3.5). Only one study contributed events to the low‐risk subgroup.
Caesarean delivery: Interventions reduced the incidence of caesarean section on average (RR 0.89, 95% CI 0.80 to 1.00; participants = 3406; studies = nine; I² = 3%; Analysis 3.6; P = 0.05; borderline statistical significance). This trend was consistent across risk subgroups; Test for subgroup differences: Chi² = 1.53, df = 2 (P = 0.46), I² = 0%.
Infant birthweight greater than 4000 g: There was no statistically significant difference in macrosomia on average between intervention and control groups overall (RR 0.92, 95% CI 0.77 to 1.11; participants = 3705; studies = 10; I² = 7%; Analysis 3.7). or for the low‐risk or mixed‐risk subgroups. However, in the high‐risk subgroup an effect in favour of a reduction in macrosomia was of borderline statistical significance (average RR 0.85, 95% CI 0.73 to 1.00; participants = 3252; studies = nine; I² = 0%; P = 0.05) and the test for subgroup differences was statistically significant (Chi² = 3.86, df = 1 (P = 0.05), I² = 74.1%).
3.1. Analysis.
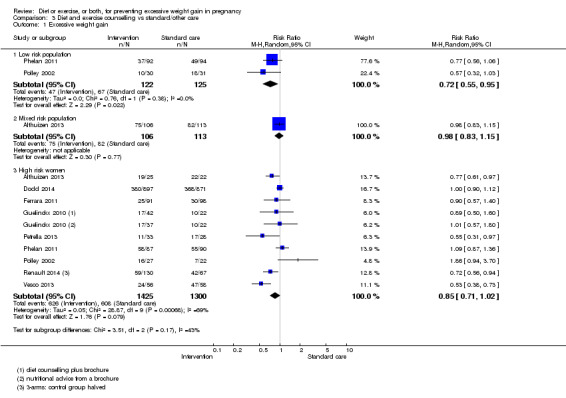
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 1 Excessive weight gain.
3.2. Analysis.
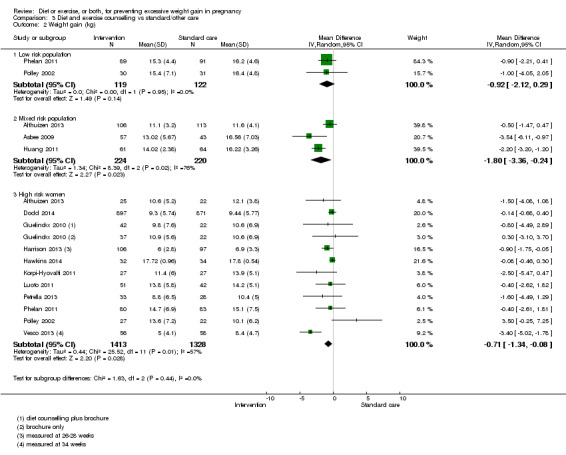
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 2 Weight gain (kg).
3.3. Analysis.
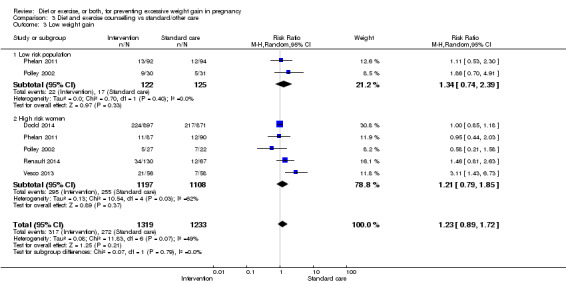
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 3 Low weight gain.
3.4. Analysis.
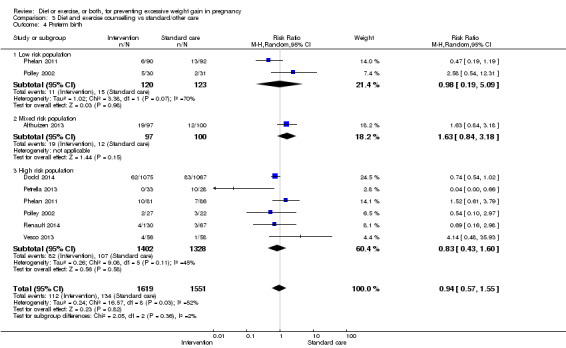
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 4 Preterm birth.
3.5. Analysis.
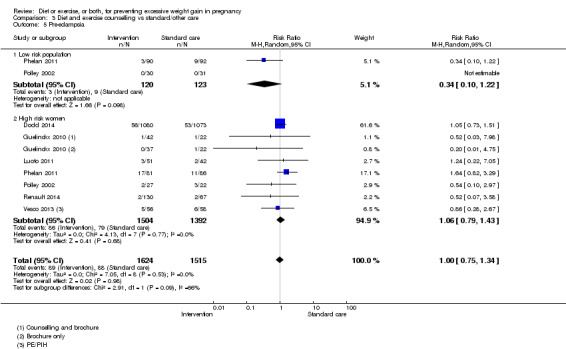
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 5 Pre‐eclampsia.
3.6. Analysis.
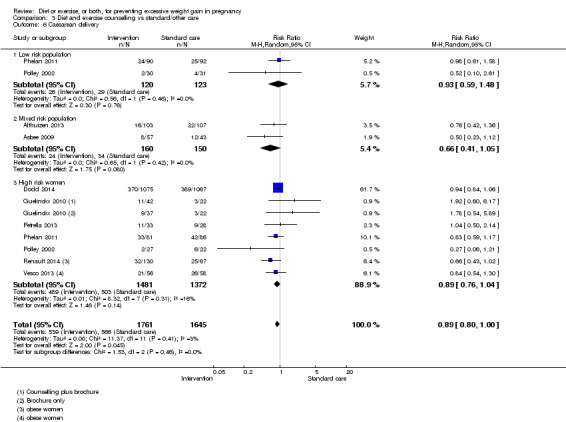
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 6 Caesarean delivery.
3.7. Analysis.
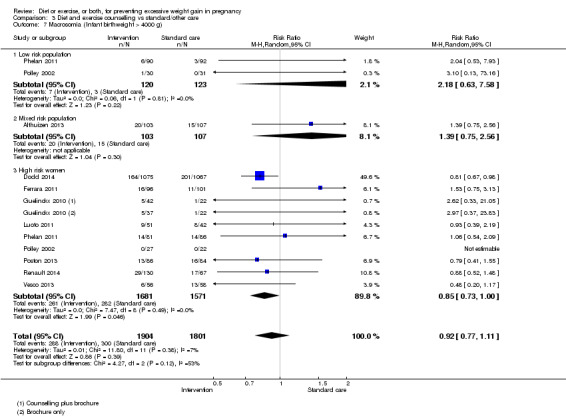
Comparison 3 Diet and exercise counselling vs standard/other care, Outcome 7 Macrosomia (Infant birthweight > 4000 g).
We graded the evidence for diet and exercise counselling interventions as moderate quality, except for the outcome preterm birth which we graded as low quality.
4. Exercise interventions versus routine care
A maximum of 13 studies contributed data to these analyses. Three studies (Kong 2014; Renault 2014; Ronnberg 2014) involved unsupervised (as opposed to supervised) exercise interventions and we performed sensitivity analyses to determine whether including unsupervised exercise intervention studies had an impact on the results. To avoid duplication of data, where individual studies contributed data to both risk subgroups (Ronnberg 2014; Ruiz 2013), we did not combine subgroup data.
Excessive GWG: Exercise interventions significantly reduced this outcome consistently across risk subgroups with point estimates for low, mixed and high risk subgroups of 0.69, 0.77, and 0,84 respectively(Analysis 4.1). Test for subgroup differences were not significant: Chi² = 1.36, df = 2 (P = 0.51), I² = 0%.
Mean GWG: When one study at a high risk of bias (Price 2012) was excluded from the mixed‐risk subgroup, the intervention was associated with a statistically significant reduction in mean weight gain in kg compared with the control in the mixed‐risk subgroup (MD ‐1.35, 95% CI ‐1.80 to ‐0.89; participants = 1134; studies = three; I² = 0%), and low risk subgroup (one study only), but not the high‐risk subgroup (MD ‐0.32, 95% CI ‐1.15 to 0.50; participants = 476; studies = four; I² = 0%); Analysis 4.2. Test for subgroup differences suggested a difference in effect between subgroups: Chi² = 5.78, df = 2 (P = 0.06), I² = 65.4%.
Low GWG: There was an increase in low GWG of borderline statistical significance between intervention and control groups for the mixed risk subgroup (average RR 1.20, 95% CI 1.00 to 1.43; participants = 1336; studies = two; I² = 0%; P = 0.05) and low risk subgroup (one study only) but not the high‐risk subgroup (average RR 1.03, 95% CI 0.66 to 1.60; participants = 504; studies = three; I² = 0%; Analysis 4.3). Test for subgroup differences were not significant: Chi² = 0.98, df = 2 (P = 0.61), I² = 0%.
Preterm birth: There was no statistically significant difference in preterm birth on average between intervention and control groups for the low risk (one study only), mixed risk (average RR 1.92, 95% CI 0.75 to 4.93; participants = 1129; studies = three; I² = 0%) or the high‐risk subgroup (average RR 1.34, 95% CI 0.51 to 3.55; participants = 504; studies = three; I² = 0%; Analysis 4.4); however the trend consistently favoured the control group. The test for subgroup differences was not significant: Chi² = 0.27, df = 1 (P = 0.60), I² = 0%.
Pre‐eclampsia: There was no statistically significant difference in pre‐eclampsia overall between intervention and control groups (average RR 0.99, 95% CI 0.58 to 1.66; participants = 1253; studies = four; I² = 0%; Analysis 4.5) and subgroup findings were similar: Test for subgroup differences: Chi² = 0.51, df = 1 (P = 0.48), I² = 0%.
Caesarean delivery: There was no statistically significant difference in caesarean delivery between intervention and control groups for low risk (one study only), mixed risk (average RR 0.96, 95% CI 0.76 to 1.22; participants = 2263; studies = six; I² = 24%) or the high‐risk subgroup (average RR 0.98, 95% CI 0.81 to 1.20; participants = 645; studies = five; I² = 0%; Analysis 4.6); . Test for subgroup differences: Chi² = 0.37, df = 2 (P = 0.83), I² = 0%.
Infant birthweight greater than 4000 g: There was no statistically significant difference in macrosomia between intervention and control groups for the low‐risk (one study only), mixed‐risk (average RR 0.81, 95% CI 0.64 to 1.02; participants = 2445; studies = seven; I² = 0%) or the high‐risk subgroups (RR 0.65, 95% CI 0.22 to 1.91; participants = 504; studies = three; I² = 74%; Analysis 4.7 Test for subgroup differences: Chi² = 0.14, df = 1 (P = 0.71), I² = 0%. However, the trend across subgroups consistently favoured the intervention, and the effect for the mixed‐risk subgroup was of borderline statistical significance.(P = 0.07) Furthermore, when three studies with risk of bias concerns were excluded (Cordero 2014; Murtezani 2014; Stafne 2012), the RR for the mixed‐risk population clearly favoured the intervention group (average RR 0.56, 95% CI 0.36 to 0.88; participants = 1274; studies = eight; I² = 0%).
4.1. Analysis.
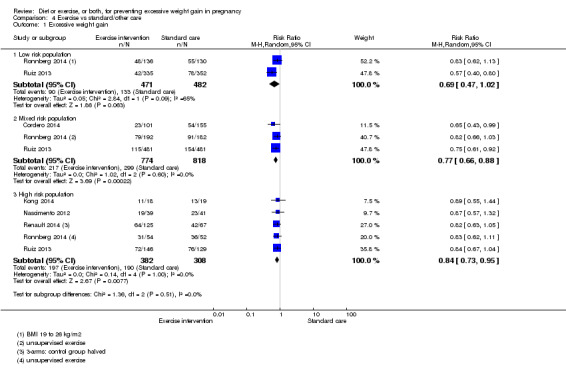
Comparison 4 Exercise vs standard/other care, Outcome 1 Excessive weight gain.
4.2. Analysis.
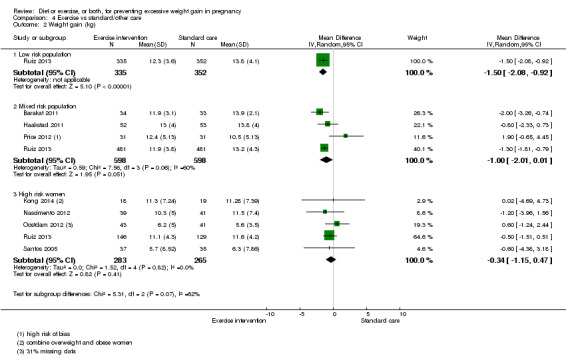
Comparison 4 Exercise vs standard/other care, Outcome 2 Weight gain (kg).
4.3. Analysis.
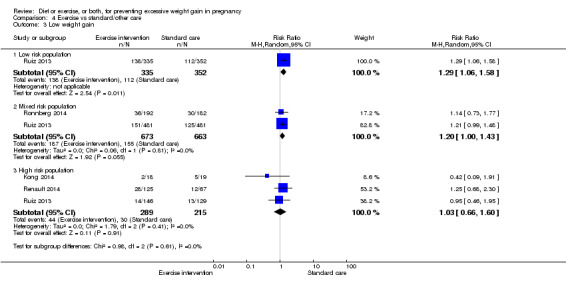
Comparison 4 Exercise vs standard/other care, Outcome 3 Low weight gain.
4.4. Analysis.
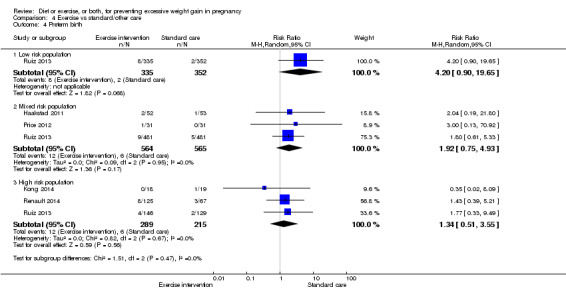
Comparison 4 Exercise vs standard/other care, Outcome 4 Preterm birth.
4.5. Analysis.
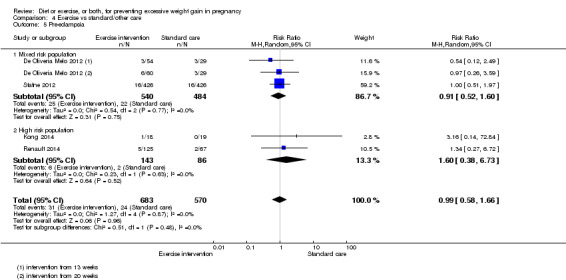
Comparison 4 Exercise vs standard/other care, Outcome 5 Pre‐eclampsia.
4.6. Analysis.
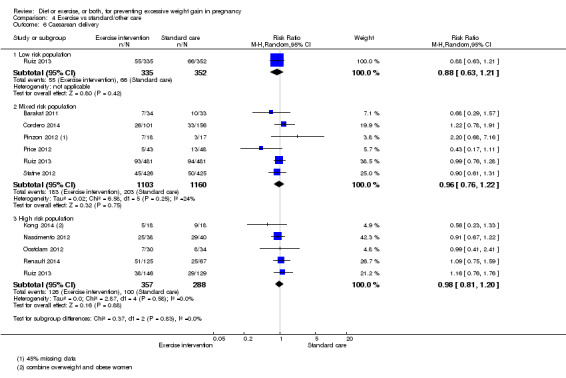
Comparison 4 Exercise vs standard/other care, Outcome 6 Caesarean delivery.
4.7. Analysis.
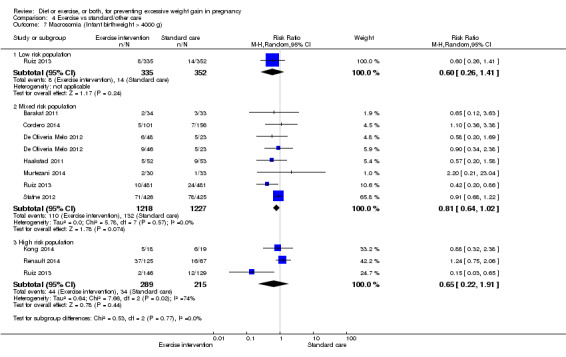
Comparison 4 Exercise vs standard/other care, Outcome 7 Macrosomia (Infant birthweight > 4000 g).
As the number of studies included in most analyses were few, we did not assess funnel plots. Including studies of unsupervised exercise did not have a significant impact on the results. We graded this evidence as moderate quality overall, except for the evidence on preterm birth (low quality) which was very imprecise and potentially subject to a serious risk of attrition and/or reporting bias.
5. Diet and supervised exercise interventions versus routine care
A maximum of five studies contributed data to these analyses; two studies contributed data to each of the mixed‐ and high‐risk subgroups, one study (Hui 2014) reported results separately for both subgroups, therefore we were able to pool these data.
Excessive GWG: Combined exercise and diet interventions significantly reduced excessive weight gain (average RR 0.75, 95% CI 0.61 to 0.92; participants = 689; studies = five; I² = 18%, Analysis 5.1; When one study at high risk of bias was excluded (Ruchat 2012), the test for subgroup differences suggested that there might be a difference in effect according to risk, with a smaller effect in the high‐risk subgroup: Test for subgroup differences: Chi² = 4.54, df = 2 (P = 0.10), I² = 55.9%.
Mean GWG: The interventions reduced mean GWG compared with controls (average MD ‐1.31, 95% CI ‐3.00 to 0.37; participants = 348; studies = three; I² = 43%; borderline significance, Analysis 5.2). However, the test for subgroup differences suggested that there might be a difference in effect according to risk,with a smaller effect in the high‐risk subgroup :Test for subgroup differences: Chi² = 4.90, df = 2 (P = 0.09), I² = 59.2%.
Low GWG: Only one study showing no difference contributed data to this outcome (Analysis 5.3).
Preterm birth: No data available
Pre‐eclampsia: Only one study showing no difference contributed data to this outcome (Analysis 5.4).
Caesarean delivery: There was no statistically significant difference in the risk of caesarean delivery between intervention and control groups (average RR 1.00, 95% CI 0.69 to 1.45; participants = 607; studies = three; I² = 0%; Analysis 5.5; Test for subgroup differences: Chi² = 0.29, df = 1 (P = 0.59), I² = 0%.
Infant birthweight greater than 4000 g: There was no statistically significant difference between groups for this outcome (RR 1.02, 95% CI 0.71 to 1.46; participants = 398; studies = three; I² = 0%; Analysis 5.6; Test for subgroup differences: Chi² = 0.33, df = 1 (P = 0.56), I² = 0%.
5.1. Analysis.
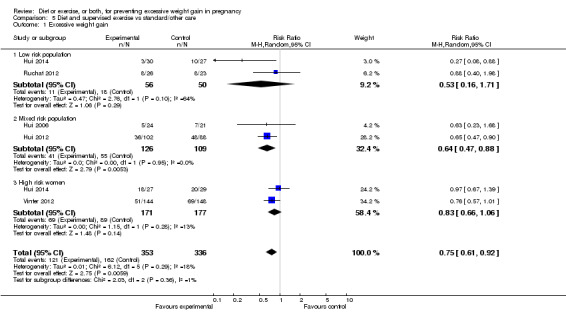
Comparison 5 Diet and supervised exercise vs standard/other care, Outcome 1 Excessive weight gain.
5.2. Analysis.
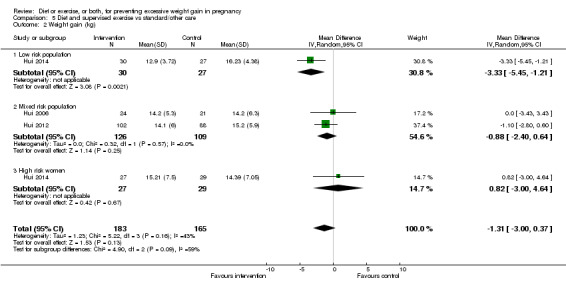
Comparison 5 Diet and supervised exercise vs standard/other care, Outcome 2 Weight gain (kg).
5.3. Analysis.
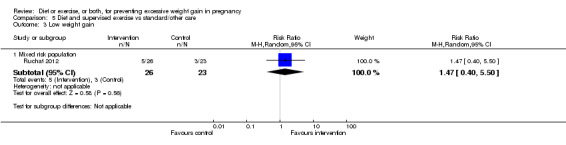
Comparison 5 Diet and supervised exercise vs standard/other care, Outcome 3 Low weight gain.
5.4. Analysis.
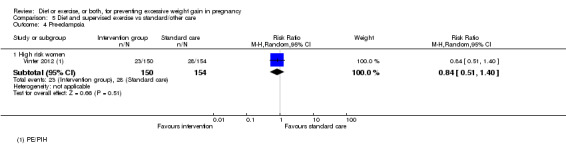
Comparison 5 Diet and supervised exercise vs standard/other care, Outcome 4 Pre‐eclampsia.
5.5. Analysis.
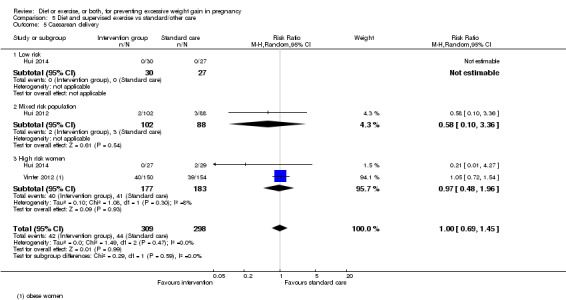
Comparison 5 Diet and supervised exercise vs standard/other care, Outcome 5 Caesarean delivery.
5.6. Analysis.
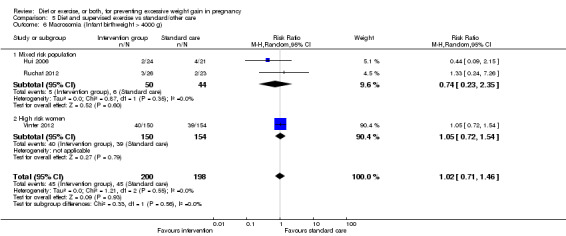
Comparison 5 Diet and supervised exercise vs standard/other care, Outcome 6 Macrosomia (Infant birthweight > 4000 g).
We graded this evidence from analyses as low‐to‐moderate quality due to imprecision (sparse data) and some inconsistencies.
6. Diet counselling only versus routine care
A maximum of seven heterogeneous studies contributed data to these analyses (Di Carlo 2014; Jeffries 2009; Laitinen 2009; Quinlivan 2011; Rae 2000; Thornton 2009; Wolff 2008).
Excessive GWG: We did not combine subgroup data for this analysis as one study contributed data to both subgroups. There was no statistically significant difference between intervention and control arms (Analysis 6.1). The test for subgroup differences showed that subgroup results were similar: Chi² = 0.49, df = 1 (P = 0.48), I² = 0%.
Mean GWG: Data for this outcome were very heterogeneous (I² greater than 90%) with four out of seven studies finding a difference in mean GWG of greater than 4 kg, with the other three studies finding little difference; therefore, we did not combine these data (Analysis 6.2).
Low GWG: Only one study contributed data (Laitinen 2009), which found that significantly more women in the intervention arm had low GWG compared with the control arm (Analysis 6.3).
Preterm birth: There was no statistically significant difference in the risk of preterm birth between intervention and control groups (average RR 0.67, 95% CI 0.26 to 1.73; participants = 591; studies = three; I² = 0%,Analysis 6.4); Test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.99), I² = 0%.
Pre‐eclampsia: There was no statistically significant difference in the risk of pre‐eclampsia between intervention and control groups (RR 0.90, 95% CI 0.54 to 1.48; participants = 634; studies = four; I² = 0%; Analysis 6.5; Test for subgroup differences: Chi² = 2.05, df = 1 (P = 0.15), I² = 51.2%.
Caesarean section: There was no statistically significant difference in the risk of caesarean delivery between intervention and control groups (average RR 1.06, 95% CI 0.93 to 1.21; participants = 754; studies = five I² = 2%; Analysis 6.6; Test for subgroup differences: Chi² = 0.30, df = 1 (P = 0.59), I² = 0%).
Infant birthweight greater than 4000 g: There was no statistically significant difference in the risk of macrosomia between intervention and control groups when data from two studies conducted in high‐risk women were pooled (RR 1.81, 95% CI 0.88 to 3.72; participants = 349; studies = two; I² = 0%, Analysis 6.7).
6.1. Analysis.
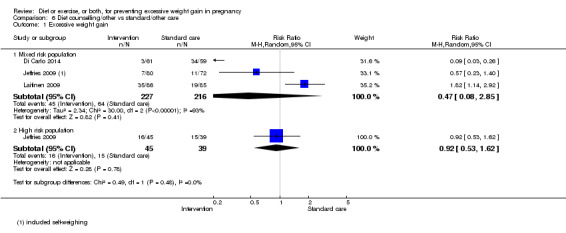
Comparison 6 Diet counselling/other vs standard/other care, Outcome 1 Excessive weight gain.
6.2. Analysis.
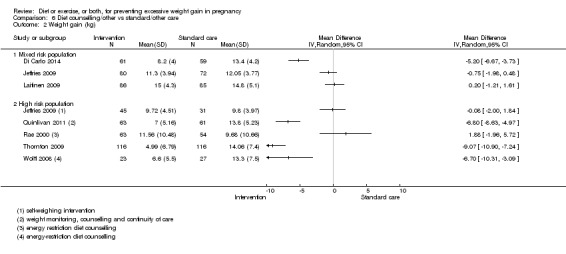
Comparison 6 Diet counselling/other vs standard/other care, Outcome 2 Weight gain (kg).
6.3. Analysis.
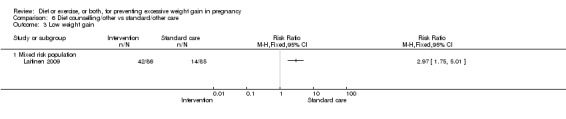
Comparison 6 Diet counselling/other vs standard/other care, Outcome 3 Low weight gain.
6.4. Analysis.
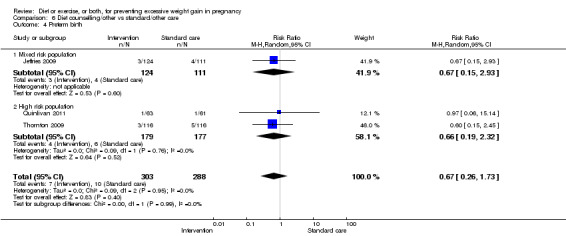
Comparison 6 Diet counselling/other vs standard/other care, Outcome 4 Preterm birth.
6.5. Analysis.
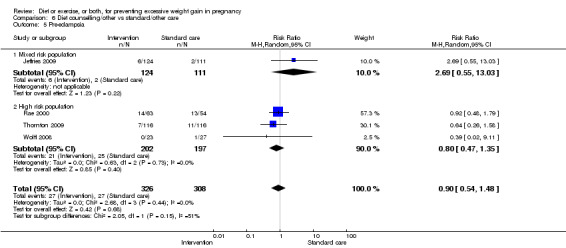
Comparison 6 Diet counselling/other vs standard/other care, Outcome 5 Pre‐eclampsia.
6.6. Analysis.
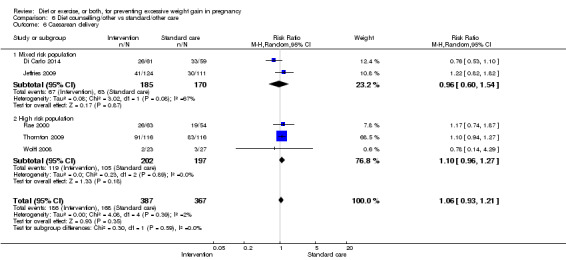
Comparison 6 Diet counselling/other vs standard/other care, Outcome 6 Caesarean delivery.
6.7. Analysis.
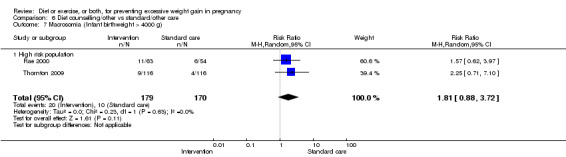
Comparison 6 Diet counselling/other vs standard/other care, Outcome 7 Macrosomia (Infant birthweight > 4000 g).
We graded the evidence relating to these interventions as low quality overall due to heterogeneity and risk of bias concerns. As the number of studies included in most analyses were few, we did not assess funnel plots.
Discussion
Summary of main results
This updated review included 65 randomised controlled trials (RCTs) in total with 49 RCTs involving at least 11,444 participants contributing data to quantitative synthesis. Twenty RCTs were considered to be at moderate‐to‐high risk of bias. Where these studies contributed data (14 studies), we performed sensitivity analysis to determine how including these data impacted on the results. Diet interventions most commonly involved a low GL, diabetic, calorie‐controlled or low fat diet; exercise interventions were most commonly of moderate intensity involving walking, dance, or aerobic classes. Most included studies were conducted in developed countries. Overall findings are summarised in Table 1. Diet or exercise,or both, interventions reduced the risk of excessive gestational weight gain (GWG) by an average of 20% (95% CI, 13 to 27%) overall. Data were moderately heterogeneous; however this overall effect was robust to sensitivity analysis and consistent across the different types of interventions, therefore we graded this evidence as high quality. The greatest effect on excessive GWG was noted for combined diet plus supervised exercise interventions.
Data for mean GWG were too heterogenous to pool and were inconsistent between, and frequently within, the different types of intervention groups, therefore, we did not pool these data. However, for subgroup analyses according to the risk of weight‐related complications, we pooled data if heterogeneity was mild or moderate, and downgraded the evidence accordingly. Limited evidence from diet and exercise counselling interventions, exercise only interventions, and diet and supervised exercise interventions suggested that there might be a difference in effect on mean weight gain according to risk, with a smaller effect in the high‐risk subgroups (low quality evidence).
Women receiving diet or exercise, or both interventions were more likely to experience low GWG than controls (average risk ratio (RR) 1.14, 95% confidence interval (CI) 1.02 to 1.27; participants = 4422; studies = 11; I² = 3; moderate‐quality evidence). We found no difference between intervention and control groups with regard to pre‐eclampsia ((RR 0.95, 95% CI 0.77 to 1.16; participants = 5330; studies = 15; I² = 0%; high‐quality evidence); however, maternal hypertension (not a pre‐specified outcome) was reduced in the intervention group compared with the control group overall (average RR 0.70, 95% CI 0.51 to 0.96; participants = 5162; studies = 11; I² = 43%; low‐quality evidence).
There was no clear difference between groups with regard to caesarean delivery overall (RR 0.95, 95% CI 0.88 to 1.03; participants = 7534; studies = 28; I² = 9%; high‐quality evidence); although the effect estimate suggested a small difference (5%) in favour of the interventions. In addition, for combined diet and exercise counselling interventions there was a 13% (‐1% to 25%) reduction in this outcome (borderline statistical significance).
We found no difference between groups with regard to preterm birth overall (average RR 0.91, 95% CI 0.68 to 1.22; participants = 5923; studies = 16; I² = 16%; moderate‐quality evidence); however limited evidence suggested that these effect estimates may differ according to the types of interventions, with a trend towards an increased risk for exercise‐only interventions.
We found no clear difference between intervention and control groups with regard to infant macrosomia overall (average RR 0.93, 95% CI 0.86 to 1.02; participants = 8598; studies = 27; I² = 0%; high‐quality evidence), although the effect estimate suggested a small difference (7% reduction) in favour of the intervention group. The largest effect size occurred in the supervised exercise‐only intervention group (RR 0.81, 95% CI 0.64 to 1.02; participants = 2445; studies = seven; I² = 0%), which approached statistical significance (P = 0.07). Furthermore, in subgroup analysis by risk, high‐risk women receiving combined diet and exercise counselling interventions experienced a 15% reduced risk of infant macrosomia (average RR 0.85, 95% CI 0.73 to 1.00; participants = 3252; studies = nine; I² = 0; P = 0.05; moderate‐quality evidence).
There were no differences in the risk of poor neonatal outcomes including shoulder dystocia, neonatal hypoglycaemia, hyperbilirubinaemia, or birth trauma (all moderate‐quality evidence) between intervention and control groups; however, infants of high‐risk women had a reduced risk of respiratory distress syndrome if their mothers were in the intervention group (RR 0.47, 95% CI 0.26 to 0.85; participants = 2256; studies = two; I² = 0%; moderate‐quality evidence).
The effect on behaviour modification outcomes, i.e. dietary and physical activity outcomes, in general, favoured the intervention groups; however, these outcomes were at high risk of bias and mainly reflected whether the implementation of the interventions was successful. Low‐quality evidence suggested that the beneficial effects of interventions on weight control during pregnancy may be sustained postpartum. For certain outcomes, e.g. childhood weight, we found no data for meta‐analysis.
For a brief summary of the effects of the different types of interventions on the main outcomes, see Table 2.
Overall completeness and applicability of evidence
There is a growing body of evidence to support the use of diet or exercise, or both, interventions to reduce excessive GWG in pregnancy. We found high‐quality evidence of other related health benefits for women and newborns, applicable to most pregnant women who are otherwise healthy, irrespective of their prepregnancy weight. Exercise appears to be an important component of weight reduction interventions; however the evidence with regard to the effect of exercise on the risk of preterm birth is of a moderate quality and this outcome should be rigorously evaluated in future studies to enable the establishment of appropriate guidelines.The evidence is not applicable to women with specific contraindications to exercise in pregnancy or pre‐existing medical conditions. Although we included four studies conducted in women with gestational diabetes, data were sparse and it is not clear whether the review findings apply to women with this condition. More research (qualitative and quantitative) to improve outcomes in this high risk group is needed.
Most included studies were carried out in developed countries and it is not clear whether these results are widely applicable to lower income settings with fewer human and financial resources. More research is needed in these less developed settings, where obesity is increasingly a major health issue. Innovative interventions utilising mobile‐phone technology (e.g. Pollak 2014) are of interest and further developments in this area are anticipated.
Quality of the evidence
Using the GRADE approach, we considered the quality of the evidence relating to excessive GWG as high quality. Although moderate heterogeneity was present, we upgraded our assessment of evidence quality from moderate‐to‐high quality as findings were precise and robust to sensitivity analysis. We graded the evidence with regard to the risk of low GWG as high quality as these estimates were consistent and precise across included studies. We downgraded the evidence with regard to preterm birth (no statistically significant difference) to moderate due to risk of bias concerns from attrition and under‐reporting. We graded the quality of the evidence for pre‐eclampsia, caesarean delivery and macrosomia as high quality and most other outcomes, as moderate quality.
We downgraded some evidence due to heterogeneity. Many factors might have contributed to this heterogeneity including obvious and subtle differences in the types of interventions, types of participants (e.g. BMIs, parity, age), delivery of the intervention (e.g. whether the intervention was incorporated into antenatal visits or delivered separately by a dietician), timing of the measurements (e.g. weight gain assessed at 34 versus 38 weeks), timing of commencement of the intervention (e.g. first, second or third trimester), sample sizes, etc. An in‐depth evaluation of individual interventions was beyond the scope of this review; however, our impression was that the more intensely monitored/supervised the intervention, the better the study results.
Potential biases in the review process
We took a number of steps to minimise bias in the review process by including all relevant RCTs, with two review authors independently classifying them, extracting data, and resolving disagreements by discussion with the other authors. Where expected outcome data were missing we made an effort to contact the study investigators, and we included adjusted data from cluster‐RCTs where possible. In the previous version of this review we included two quasi‐RCTs (Bechtel‐Blackwell 2002; Moses 2006), which we excluded for this update after deciding to limit the review to RCTs. Due to issues of scope and relevance, we also excluded two early studies of appetite suppressant drugs that had been included in the original review (Boileau 1968; Silverman 1971).
We considered it clinically meaningful to produce overall estimates of average effects across all studies where possible, organised by the type of intervention, using random effects methods. Interventions were often multifaceted and were quite heterogeneous in approach, for example, in the timing, duration, intensity, content and delivery. Some studies evaluated more than one type of intervention. Dodd 2014, for example, was mainly a diet and exercise counselling intervention study and we included it as such; however, 26% of participants in the intervention arm underwent a supervised walking exercise as part of a nested RCT. Where possible, when heterogeneity existed between studies evaluating apparently similar interventions, which could not be explained by our 'Risk of bias' assessment, we attempted to identify the possible reasons for it. In addition, although we made distinctions between types of diet and exercise interventions according to whether the exercise component was supervised or not, the results were robust on exploratory analyses when all combined diet and exercise intervention data for the main outcomes were pooled.
Two studies compared higher impact interventions with lower impact interventions (Ruchat 2012; Thornton 2009), and several studies of low GL diets compared the intervention with an alternative (low‐fat, moderate/high GL or conventional healthy eating) diet (Rhodes 2010; Louie 2011; Moses 2009; Moses 2014). For these studies, we pooled the data of the alternative intervention with the control group which, in most other studies, comprised routine care. This may have contributed to heterogeneity in the meta‐analyses and might have led to an underestimation of the summary effect of the intervention. Clapp 2002b compared different exercise intensities at different stages of pregnancy (before versus after 20 weeks' gestation) and found that low‐intensity exercise in early pregnancy moving on to higher‐intensity exercise after 20 weeks was associated with a lower pregnancy weight gain than either high‐ followed by low‐intensity exercise or moderate‐intensity exercise throughout. We did not explore the impact of higher‐ versus lower‐intensity interventions, which may have provided important information for exercise interventions and certain outcomes, such as preterm birth. This was largely due to a sparseness of data and time constraints.
Several studies reported maternal 'hypertension', and not pre‐eclampsia, and we extracted and analysed these data. This was not prespecified in the protocol (see Differences between protocol and review); however, we made every effort to minimise bias in this process, and consider the pooled findings to be of value, albeit low‐quality evidence. The effect estimates for hypertension differed from those for pre‐eclampsia with a consistent trend towards a reduction in hypertension, but not pre‐eclampsia, in intervention arms.
We did not perform meta‐analyses according to BMI classification of degree of obesity, although several studies stratified results by BMI (overweight or obese or morbidly obese). In one study that reported these BMI categories separately (Kong 2014), we combined these data for use in our meta‐analysis. For studies of high‐risk women that reported combined and separate results for overweight and obese categories (Harrison 2013; Petrella 2013; Nascimento 2012), we extracted all data but used the combined data only in our meta‐analyses. It is possible that certain types of interventions may be more effective for different high‐risk BMI categories and we may try to address this in future versions of this review.
Behaviour modification outcomes may have been subject to potential reviewer bias. Multiple and varied behaviour modification outcomes were reported by the various investigators and we pre‐specified only three outcomes (energy intake, fibre intake and a physical activity measure) for inclusion in the review. In addition, numerous different scales and time points were reported by study investigators, a narrative discussion of which is beyond the scope of this review. However, in many respects, behaviour outcome measures are measures of the intervention rather than the effect of the intervention and we consider this outcome to be a poor (indirect) measure of effectiveness of an intervention. In addition, these outcomes were subject to other serious risk of bias concerns, including detection (often self‐reported) and attrition bias.
Agreements and disagreements with other studies or reviews
A 2012 UK Health Technology Assessment review (Thangaratinam 2012) included 30 RCTs in a meta‐analysis of GWG and reported an overall reduction (± 1 kg) in mean GWG in the intervention group compared with controls.Two other systematic reviews (Ronnberg 2010; Skouteris 2010) that included RCTs and non‐randomised studies also reported GWG outcomes in favour of the interventions. Thangaratinam 2012 reported that the largest weight reduction occurred with dietary interventions (a mean reduction of 3.36 kg), which were also effective at reducing gestational hypertension, pre‐eclampsia, preterm birth and shoulder dystocia. Due to substantial heterogeneity, we did not pool mean weight gain data and our review does not agree with these findings. Thangaratinam 2012 utilised data from non‐randomised studies to provide evidence on adverse effects; however, most of these data were derived from studies on extreme diet and famine and therefore have limited applicability. Randomised controlled trial data on adverse effects remain sparse and we were unable to provide additional evidence in this regard.
We were unable to show a reduced risk of macrosomia overall, as was shown in Thangaratinam 2012, although our findings did not exclude a small reduction in risk. In addition, our findings suggested greater reductions may be achieved for high‐risk women who received combined diet and exercise interventions, and women receiving supervised exercise‐only interventions. Dodd 2014, the largest study included in this review additionally reported the rate of very high birthweight babies (greater than 4500 g), finding a significant reduction in this outcome in the intervention arm; however, we did not include this outcome.
We found limited evidence that certain interventions were somewhat less effective in reducing GWG in high‐risk women compared with the lower‐risk group. To our knowledge this has not been previously shown and requires further investigation. Several of our included studies reported gestational diabetes as an outcome and exploratory analysis suggested that the effect of diet and exercise interventions on this outcome might be significant. We did not modify our protocol to include this important outcome as a separate Cochrane review on the topic is in progress (Crane 2013).
Authors' conclusions
Implications for practice.
High‐quality evidence indicates that diet or exercise, or both, during pregnancy can reduce the risk of excessive gestational weight gain (GWG). Other benefits may include a lower risk of caesarean delivery, macrosomia, and neonatal respiratory morbidity, particularly for high‐risk women receiving combined diet and exercise interventions. Moderate‐intensity exercise appears to be an important part of controlling weight gain in pregnancy, however the evidence on the risk of preterm birth is limited and more research is needed to establish safe guidelines. Most included studies were carried out in developed countries and it is not clear whether these results are widely applicable to lower income settings.
Implications for research.
The effectiveness of these interventions in low‐income countries and in women with non‐Western lifestyles needs further evaluation. In addition, further research is needed, particularly in high‐risk women to determine whether other types of diet or adjuvant interventions (e.g. probiotics, metformin), are of value in reducing excessive GWG and improving maternal and infant outcomes. The evidence with regard to the effect of antenatal exercise on the risk of preterm birth is incomplete and this outcome should be carefully monitored and reported by researchers to enable the establishment of appropriate guidelines. Studies of interventions utilising mobile‐phone technology are of interest and further developments in this area are anticipated. Research to investigate strategies to implement diet and exercise programs into routine antenatal care in different settings is needed.
What's new
| Date | Event | Description |
|---|---|---|
| 12 June 2015 | Amended | Minor correction to acknowledgements. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 23 April 2015 | New citation required and conclusions have changed | High‐quality evidence suggests that diet or exercise, or both, interventions are effective in reducing excessive gestational weight gain. Hypertension, caesarean delivery, macrosomia and neonatal respiratory distress may also be reduced, particularly with combined diet and exercise interventions. Exercise appears to be an important part of controlling weight gain in pregnancy and more research is needed to establish safe guidelines. |
| 5 November 2014 | New search has been performed | Search updated and 169 new records identified. There are now 65 (previously 28) included studies, 49 (previously 27) of which contribute data. The title has been changed from 'Interventions for preventing excessive weight gain during pregnancy' to 'Diet or exercise, or both, for preventing excessive weight gain during pregnancy'. This was done to limit the otherwise enormous scope of the review. As a result of the title modification, two previously included 1970s studies of (obsolete) appetite suppressant drug interventions were excluded in this update. Drug interventions (e.g. metformin, probiotics), will now need to be addressed under a separate title. Methods updated and GRADE 'Summary of findings' tables have been incorporated. |
| 10 November 2008 | Amended | The Types of interventions section was amended by the inclusion of the phrase 'or other interventions for preventing excessive weight gain in pregnancy'. This amendment was made to ensure that the selection criteria are consistent with the objectives of the review which are to evaluate the effectiveness of all interventions (or combinations of interventions) to prevent excessive weight gain. |
Acknowledgements
We would like to acknowledge the staff of Cochrane Pregnancy and Childbirth Group for assistance with searching references and providing technical guidance. We also acknowledge Therese Dowswell for the significant contribution she made to the original review.
Theresa A Lawrie's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The authors alone are responsible for the views expressed in this review and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
Appendices
Appendix 1. Adjusted data for Luoto 2011
| Continuous outcome |
Intervention (Original data) (Total number = 216) |
Control (Original data) (Total number = 179) |
M1 | ICC2 | Design effect3 |
Adjusted sample4 sizes |
|||
| Cluster number |
x̄ ± SD | Cluster number |
x̄ ± SD | Intervention | Control | ||||
| Maternal weight gain |
7 | 13.8±5.8 | 7 | 14.2±5.1 | 28.21 | 0.12 | 4.27 | 50.64 | 41.96 |
1 M = average cluster size = ((total number of intervention + total number of control)/(cluster number of intervention + cluster number of control)
2 ICC = intraclass correlation; obtained from the reliable external source (Luoto 2010).
3 Design effect = 1 + (M‐1)ICC
4 Adjusted sample sizes = n / design effect
| Dichotomous outcomes | Intervention (Original data) (Total number = 216, cluster number = 7) |
Control (Original data) (Total number = 179, cluster number = 7) |
M1 | ICC2 | Design effect3 |
Intervention (Adjusted data)4 |
Control (Adjusted data)4 |
||
| n | n | Total N | n | Total N | n | ||||
| Pre‐eclampsia | 14 | 10 | 28.21 | 0.12 | 4.27 | 50.64 | 3.28 | 41.96 | 2.34 |
| Birthweight > 4000 g | 37 | 36 | 28.21 | 0.12 | 4.27 | 50.64 | 8.67 | 41.96 | 8.44 |
| Infant birthweight > 90th centile |
26 | 34 | 28.21 | 0.12 | 4.27 | 50.64 | 6.10 | 41.96 | 7.97 |
| Infant birthweight < the 10th centile |
10 | 5 | 28.21 | 0.12 | 4.27 | 50.64 | 2.34 | 41.96 | 1.17 |
Appendix 2. Data for Rauh 2013
| Continuous outcomes | Intervention | Control | Adjusted P value | ||
| x̄ ± SD | Total number | x̄ ± SD | Total number | ||
| Weight gain | 14.1 ± 4.1 | 152 | 15.6 ± 5.8 | 74 | 0.035 |
| Weight retention (at 4 months postpartum) |
2.1 ± 4.3 | 152 | 3.3 ± 5.1 | 72 | 0.070 |
| Infant birthweight | 3406 ± 402 | 156 | 3414 ± 445 | 79 | ‐ |
| Dichotomous outcomes | Intervention | Control | Adjusted P value | ||
| Number | Total number | Number | Total number | ||
| Excessive weight gain | 58 | 152 | 44 | 74 | 0.032 |
| Low weight gain | 32 | 152 | 14 | 74 | 0.972 |
| Weight retention ( > 5 kg) | 26 | 152 | 22 | 72 | 0.034 |
| Preterm | 4 | 156 | 5 | 79 | 0.088 |
| Caesarean delivery | 47 | 156 | 33 | 79 | 0.145 |
| Gestational diabetes | 8 | 156 | 9 | 79 | 0.183 |
| Infant birthweight > 90th centile | 10 | 156 | 7 | 79 | 0.702 |
| Infant birthweight < 10th centile | 6 | 156 | 3 | 79 | 0.990 |
| Induction of labour | 40 | 156 | 29 | 79 | 0.191 |
Data and analyses
Comparison 1. All diet and/or exercise interventions vs standard/other care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 24 | 7096 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.73, 0.87] |
| 1.1 Diet intervention (low GI diet) | 2 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.91] |
| 1.2 Diet and exercise counselling | 9 | 3144 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.98] |
| 1.3 Unsupervised exercise | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.71, 0.97] |
| 1.4 Supervised exercise | 3 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.63, 0.89] |
| 1.5 Supervised exercise and diet | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 1.6 Diet counselling/other | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.17, 1.23] |
| 2 Weight gain (kg) | 36 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Diet intervention (low GI diet) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Diet and exercise counselling | 13 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Unsupervised exercise | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Supervised exercise intervention | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Supervised exercise plus diet intervention | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Diet counselling/other | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Low weight gain | 11 | 4422 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.27] |
| 3.1 Diet intervention (low GI diet) | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.64, 2.43] |
| 3.2 Diet and exercise counselling | 5 | 2552 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.90, 1.75] |
| 3.3 Unsupervised exercise | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.60] |
| 3.4 Supervised exercise | 1 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.99, 1.48] |
| 3.5 Supervised exercise plus diet | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.40, 5.50] |
| 3.6 Diet counselling/other | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.80, 1.82] |
| 4 Preterm birth | 16 | 5923 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.22] |
| 4.1 Diet intervention (low GI diet) | 2 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 1.02] |
| 4.2 Diet and exercise counselling | 7 | 3170 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.60, 1.51] |
| 4.3 Unsupervised exercise | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.35, 3.85] |
| 4.4 Supervised exercise | 3 | 1129 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.75, 4.93] |
| 4.5 Diet counselling/other | 3 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.26, 1.73] |
| 5 Pre‐eclampsia | 15 | 5330 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.16] |
| 5.1 Diet and exercise counselling | 7 | 3139 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.31] |
| 5.2 Supervised exercise | 2 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.52, 1.60] |
| 5.3 Unsupervised exercise | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.38, 6.73] |
| 5.4 Supervised exercise plus diet | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.40] |
| 5.5 Diet counselling/other | 4 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.54, 1.48] |
| 6 Hypertension (not prespecified) | 11 | 5162 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.51, 0.96] |
| 6.1 Diet and exercise counselling | 5 | 2648 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.29] |
| 6.2 Unsupervised exercise | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.54] |
| 6.3 Supervised exercise | 3 | 1749 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.29, 1.03] |
| 6.4 Supervised exercise plus diet | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.40] |
| 6.5 Diet counselling/other | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.08, 1.06] |
| 7 Induction of labour | 8 | 3832 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.19] |
| 7.1 Diet intervention (low GI diet) | 1 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.14, 2.36] |
| 7.2 Diet and exercise counselling | 4 | 2522 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.93, 1.14] |
| 7.3 Unsupervised exercise | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.48] |
| 7.4 Supervised exercise | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.26] |
| 7.5 Diet counselling/other | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.35] |
| 8 Caesarean delivery | 28 | 7534 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 8.1 Diet intervention (low GI diet) | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.33, 3.01] |
| 8.2 Diet and exercise counselling | 9 | 3406 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.01] |
| 8.3 Unsupervised exercise intervention | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.59] |
| 8.4 Supervised exercise | 8 | 2405 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.11] |
| 8.5 Supervised exercise plus diet | 3 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.45] |
| 8.6 Diet counselling/other | 5 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 9 Postpartum weight retention (kg) | 7 | 818 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.49, 0.25] |
| 9.1 Diet intervention (low GI diet) | 1 | 414 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.63, ‐0.17] |
| 9.2 Diet and exercise counselling | 2 | 188 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐4.88, 2.61] |
| 9.3 Supervised exercise | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐1.61, 1.96] |
| 9.4 Supervised exercise plus diet | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.84, 1.24] |
| 9.5 Diet counselling/other | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐6.9 [‐15.28, 1.48] |
| 10 Postpartum weight retention (n/N; investigator defined time frame) | 5 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 10.1 Diet and exercise counselling | 3 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.01] |
| 10.2 Supervised exercise plus diet intervention | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.66, 1.10] |
| 11 Energy intake (kj) | 12 | 4065 | Mean Difference (IV, Random, 95% CI) | ‐570.77 [‐894.28, ‐247.26] |
| 11.1 Diet intervention (low GI diet) | 4 | 1462 | Mean Difference (IV, Random, 95% CI) | ‐297.26 [‐562.80, ‐31.72] |
| 11.2 Diet and exercise counselling | 4 | 2190 | Mean Difference (IV, Random, 95% CI) | ‐897.66 [‐1763.09, ‐32.23] |
| 11.3 Supervised exercise plus diet intervention | 3 | 274 | Mean Difference (IV, Random, 95% CI) | ‐1090.80 [‐2263.86, 82.26] |
| 11.4 Diet counselling/other | 1 | 139 | Mean Difference (IV, Random, 95% CI) | ‐172.0 [‐686.85, 342.85] |
| 12 Fibre intake (g) | 8 | 3466 | Mean Difference (IV, Random, 95% CI) | 1.53 [0.94, 2.12] |
| 12.1 Diet intervention (low GI diet) | 4 | 1223 | Mean Difference (IV, Random, 95% CI) | 1.35 [0.55, 2.15] |
| 12.2 Diet and exercise counselling | 2 | 1996 | Mean Difference (IV, Random, 95% CI) | 1.97 [0.95, 2.99] |
| 12.3 Supervised exercise plus diet intervention | 1 | 112 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐3.06, 5.06] |
| 12.4 Diet counselling//other | 1 | 135 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.91, 3.11] |
| 13 Physical activity score (26‐29 weeks) | 9 | 2851 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.18, 0.61] |
| 13.1 Diet and exercise counselling | 5 | 2395 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.03, 0.51] |
| 13.2 Supervised exercise intervention | 2 | 232 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.06, 0.79] |
| 13.3 Supervised exercise plus diet intervention | 2 | 224 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [0.46, 1.00] |
| 14 Macrosomia Infant birthweight > 4000 g | 27 | 8598 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.02] |
| 14.1 Diet intervention (low GI diet) | 4 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.10] |
| 14.2 Diet and exercise counselling | 10 | 3705 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.12] |
| 14.3 Unsupervised exercise | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.74, 1.81] |
| 14.4 Supervised exercise intervention | 7 | 2445 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.02] |
| 14.5 Supervised exercise plus diet intervention | 3 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.46] |
| 14.6 Diet counselling/other | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.88, 3.72] |
| 15 Infant birthweight > 90th centile | 18 | 4525 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.05] |
| 15.1 Diet and exercise counselling | 6 | 2777 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.74, 1.02] |
| 15.2 Unsupervised exercise | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.34, 3.43] |
| 15.3 Supervised exercise intervention | 4 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.59, 2.00] |
| 15.4 Supervised exercise plus diet intervention | 3 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.66] |
| 15.5 Diet intervention (low GI diet) | 3 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.50, 3.11] |
| 15.6 Diet counselling/other | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.69] |
| 16 Birthweight (g) (not prespecified) | 29 | 8350 | Mean Difference (IV, Random, 95% CI) | 12.20 [‐15.26, 39.65] |
| 16.1 Diet intervention (low GI diet) | 4 | 1447 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.16, ‐0.52] |
| 16.2 Diet and exercise counselling | 9 | 3516 | Mean Difference (IV, Random, 95% CI) | 52.33 [‐33.23, 137.89] |
| 16.3 Unsupervised exercise | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐124.21 [‐435.86, 187.44] |
| 16.4 Supervised exercise | 11 | 2714 | Mean Difference (IV, Random, 95% CI) | 16.27 [‐38.56, 71.11] |
| 16.5 Supervised exercise and diet | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐107.0 [‐343.33, 129.33] |
| 16.6 Diet counselling/other | 3 | 587 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐78.04, 80.84] |
| 17 Infant birthweight < 2500 g | 12 | 4834 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.14] |
| 17.1 Exercise and diet counselling | 5 | 2934 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.60, 1.17] |
| 17.2 Unsupervised exercise | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.24, 18.80] |
| 17.3 Supervised exercise | 4 | 1387 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.61, 1.63] |
| 17.4 Supervised exercise plus diet | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 62.42] |
| 17.5 Diet counselling/other | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.29, 1.53] |
| 18 Infant birthweight < 10th centile | 7 | 662 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.61, 1.94] |
| 18.1 Diet intervention (low GI diet) | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.47, 4.71] |
| 18.2 Diet and exercise counselling | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.28, 3.29] |
| 18.3 Supervised exercise | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.45, 2.19] |
| 19 Shoulder dystocia | 4 | 3253 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.83] |
| 19.1 Diet intervention (low GI diet) | 1 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.82] |
| 19.2 Diet and exercise counselling | 1 | 2142 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.81, 1.93] |
| 19.3 Diet counselling/other | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.64] |
| 20 Neonatal hypoglycaemia | 4 | 2601 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.18] |
| 20.1 Diet and exercise counselling | 2 | 2256 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 20.2 Diet counselling/other | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.36, 2.15] |
| 21 Birth trauma | 2 | 2256 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.35, 2.30] |
| 21.1 Diet and exercise counselling | 2 | 2256 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.35, 2.30] |
| 22 Neonatal hyperbilirubinaemia | 2 | 2256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.10] |
| 22.1 Diet and exercise counselling | 2 | 2256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.10] |
| 23 Neonatal respiratory distress syndrome | 2 | 2256 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.85] |
| 23.1 Diet and exercise counselling | 2 | 2256 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.85] |
| 24 Postpartum hemorrhage | 2 | 2901 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.14] |
| 24.1 Diet intervention (low GL diet) | 1 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.23, 3.08] |
| 24.2 Diet and exercise counselling | 1 | 2142 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.14] |
Comparison 2. Diet intervention (low GI diet) vs standard/other care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 2 | 833 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 1.1 Low risk population | 1 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.38, 0.94] |
| 1.2 High risk population | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.08] |
| 2 Weight gain (kg) | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Mixed risk population | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 High risk population | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Low weight gain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 High risk population | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Preterm birth | 2 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 1.02] |
| 4.1 Mixed risk population | 1 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.46] |
| 4.2 High risk population | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.81] |
| 5 Caesarean delivery | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.33, 3.01] |
| 5.1 High risk population | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.33, 3.01] |
| 6 Macrosomia (Infant birthweight > 4000 g) | 4 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.20] |
| 6.1 Mixed risk population | 2 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.17] |
| 6.2 High risk population | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [0.68, 8.95] |
2.2. Analysis.
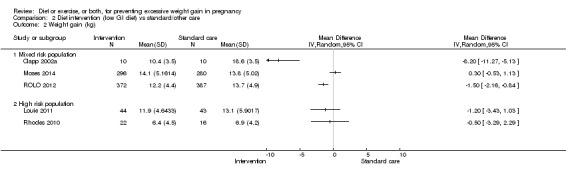
Comparison 2 Diet intervention (low GI diet) vs standard/other care, Outcome 2 Weight gain (kg).
Comparison 3. Diet and exercise counselling vs standard/other care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk population | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
| 1.2 Mixed risk population | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.15] |
| 1.3 High risk women | 9 | 2725 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.02] |
| 2 Weight gain (kg) | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk population | 2 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐2.12, 0.29] |
| 2.2 Mixed risk population | 3 | 444 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.36, ‐0.24] |
| 2.3 High risk women | 11 | 2741 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.34, ‐0.08] |
| 3 Low weight gain | 5 | 2552 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.72] |
| 3.1 Low risk population | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.74, 2.39] |
| 3.2 High risk women | 5 | 2305 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.79, 1.85] |
| 4 Preterm birth | 7 | 3170 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.57, 1.55] |
| 4.1 Low risk population | 2 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.19, 5.09] |
| 4.2 Mixed risk population | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.84, 3.18] |
| 4.3 High risk population | 6 | 2730 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.43, 1.60] |
| 5 Pre‐eclampsia | 7 | 3139 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.34] |
| 5.1 Low risk population | 2 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.10, 1.22] |
| 5.2 High risk women | 7 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.79, 1.43] |
| 6 Caesarean delivery | 9 | 3406 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 6.1 Low risk population | 2 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.59, 1.48] |
| 6.2 Mixed risk population | 2 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.41, 1.05] |
| 6.3 High risk women | 7 | 2853 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.04] |
| 7 Macrosomia (Infant birthweight > 4000 g) | 10 | 3705 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.11] |
| 7.1 Low risk population | 2 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.63, 7.58] |
| 7.2 Mixed risk population | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.75, 2.56] |
| 7.3 High risk women | 9 | 3252 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 1.00] |
Comparison 4. Exercise vs standard/other care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk population | 2 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.02] |
| 1.2 Mixed risk population | 3 | 1592 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.88] |
| 1.3 High risk population | 5 | 690 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.95] |
| 2 Weight gain (kg) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk population | 1 | 687 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.08, ‐0.92] |
| 2.2 Mixed risk population | 4 | 1196 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.01, 0.01] |
| 2.3 High risk women | 5 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.15, 0.47] |
| 3 Low weight gain | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Low risk population | 1 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.06, 1.58] |
| 3.2 Mixed risk population | 2 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.00, 1.43] |
| 3.3 High risk population | 3 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.66, 1.60] |
| 4 Preterm birth | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Low risk population | 1 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 4.20 [0.90, 19.65] |
| 4.2 Mixed risk population | 3 | 1129 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.75, 4.93] |
| 4.3 High risk population | 3 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.51, 3.55] |
| 5 Pre‐eclampsia | 4 | 1253 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.58, 1.66] |
| 5.1 Mixed risk population | 2 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.52, 1.60] |
| 5.2 High risk population | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.38, 6.73] |
| 6 Caesarean delivery | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Low risk population | 1 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.21] |
| 6.2 Mixed risk population | 6 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.22] |
| 6.3 High risk population | 5 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.20] |
| 7 Macrosomia (Infant birthweight > 4000 g) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Low risk population | 1 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.26, 1.41] |
| 7.2 Mixed risk population | 7 | 2445 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.02] |
| 7.3 High risk population | 3 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.22, 1.91] |
Comparison 5. Diet and supervised exercise vs standard/other care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.92] |
| 1.1 Low risk population | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.16, 1.71] |
| 1.2 Mixed risk population | 2 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.88] |
| 1.3 High risk women | 2 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.06] |
| 2 Weight gain (kg) | 3 | 348 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.00, 0.37] |
| 2.1 Low risk population | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐5.45, ‐1.21] |
| 2.2 Mixed risk population | 2 | 235 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐2.40, 0.64] |
| 2.3 High risk women | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.00, 4.64] |
| 3 Low weight gain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Mixed risk population | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.40, 5.50] |
| 4 Pre‐eclampsia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 High risk women | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.40] |
| 5 Caesarean delivery | 3 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.45] |
| 5.1 Low risk | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mixed risk population | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.36] |
| 5.3 High risk women | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.48, 1.96] |
| 6 Macrosomia (Infant birthweight > 4000 g) | 3 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.46] |
| 6.1 Mixed risk population | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.23, 2.35] |
| 6.2 High risk women | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.72, 1.54] |
Comparison 6. Diet counselling/other vs standard/other care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mixed risk population | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.08, 2.85] |
| 1.2 High risk population | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.53, 1.62] |
| 2 Weight gain (kg) | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Mixed risk population | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 High risk population | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Low weight gain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mixed risk population | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Preterm birth | 3 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.26, 1.73] |
| 4.1 Mixed risk population | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.15, 2.93] |
| 4.2 High risk population | 2 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.19, 2.32] |
| 5 Pre‐eclampsia | 4 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.54, 1.48] |
| 5.1 Mixed risk population | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [0.55, 13.03] |
| 5.2 High risk population | 3 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.47, 1.35] |
| 6 Caesarean delivery | 5 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 6.1 Mixed risk population | 2 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.60, 1.54] |
| 6.2 High risk population | 3 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| 7 Macrosomia (Infant birthweight > 4000 g) | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.88, 3.72] |
| 7.1 High risk population | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.88, 3.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Althuizen 2013.
| Methods | RCT, 8 midwifery practices in The Netherlands from February 2005 to May 2006. | |
| Participants | 246 randomised, 219 analysed. Inclusion criteria: women expecting their first child, able to read and speak Dutch, and in the first 14 weeks of gestation. Exclusion criteria: miscarriage, gestation ≥ 15 weeks. |
|
| Interventions | Intervention group: counselling (5 x approximately 15 minute sessions on weight, physical acitvity and diet) (n = 106). Interventions were face‐to‐face at 18, 22, 30, and 36 weeks' gestation, with a telephone session at 8 weeks postpartum. Control group: routine care (no counselling) (n = 113). |
|
| Outcomes | Primary outcomes were excessive weight gain, BMI, postpartum weight retention, birthweight, macrosomia, preterm birth, gestational diabetes. | |
| Notes | Age (intervention, control): 29.2 ± 3.3/30.4 ± 4. Enrolment gestational age < 14 weeks. Prepregnancy BMI: 24 ± 4.2/23.5 ± 3.8. Authors concluded that "The lifestyle counselling intervention evaluated in this study did not have an effect on excessive weight gain or postpartum weight retention. Our findings for overweight and obese women need to be confirmed in a larger, randomised trial". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerised random number generator drew up an allocation schedule pre‐stratified for midwifery practices." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11% of participants (17 in intervention and 10 in control group) dropped out during the course of the study. |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Angel 2011.
| Methods | RCT conducted at San Francisco General Hospital, USA between 2006 and 2009 (abstract only). | |
| Participants | 64 randomised. Inclusion criteria: obese/overweight, ages 18‐42 years, from 20 weeks. Exclusion criteria: NR. |
|
| Interventions | Intervention group: low glycaemic load diet. Control group: low fat diet. |
|
| Outcomes | Excessive weight gain, excessive fat gain. | |
| Notes | Age (intervention, control): NR. Enrolment gestational age approx. 20 weeks. Prepregnancy BMI: NR. Abstract only with no usable data. "This evidence suggests that dietary patterns are important for achieving weight gain recommendations among overweight and obese pregnant women." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14% drop out. Not yet reported in full. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only with little data. |
| Other bias | Unclear risk | Abstract only with little data. |
Asbee 2009.
| Methods | RCT, set in resident obstetric clinic in Charlotte, North Carolina, USA. | |
| Participants | Inclusion criteria: prenatal care established at 6‐16 weeks of gestation, age 18‐49 years, all prenatal care received at the Resident Obstetrics Clinic, English‐speaking, Spanish‐speaking, or both, and singleton pregnancy. Exclusion criteria: BMI higher than 40, pre‐existing diabetes, untreated thyroid disease, or hypertension requiring medication or other medical conditions that might affect body weight, delivery at institution other than Carolinas Medical Center Main, pregnancy ending in premature delivery (less than 37 weeks), and limited prenatal care (fewer than 4 visits). |
|
| Interventions | Intervention group (n = 57) received consistent program of dietary and lifestyle counselling. At the initial visit, participants met with a registered dietician to receive a standardised counselling session, including information on pregnancy‐specific dietary and lifestyle choices. The counselling consisted of recommendations for a patient‐focused caloric value divided in a 40% CHO, 30% protein, and 30% fat fashion. Participants were instructed to engage in moderate‐intensity exercise at least 3 times per week and preferably 5 times per week. They also received information on the appropriate weight gain during pregnancy using the IOM guidelines. Each participant met with the dietician only at the time of enrolment. At each routine obstetrical appointment, the healthcare provider informed the participant whether her weight gain was at the appropriate level. If her weight gain was not within the IOM guidelines, the participant’s diet and exercise regimen were reviewed and she was advised on increasing or decreasing her intake and increasing or decreasing exercise. Control group (n = 43) received routine prenatal care, including an initial physical examination and history, routine laboratory tests, and routine visits per ACOG standards. The only counselling on diet and exercise during pregnancy was that included in a standard prenatal booklet. The healthcare provider did not counsel the participant regarding any changes in diet or lifestyle. |
|
| Outcomes | Weight gain, caesarean delivery, pre‐eclampsia, shoulder dystocia. Total weight gain was defined as weight just before delivery minus prepregnancy weight. |
|
| Notes | Age (intervention, control): 26.7 ± 6.0, 26.4 ± 5.0. Enrolment gestational age (intervention, control): 13.7 ± 3.6, 13.6 ±.2 weeks. Prepregnancy BMI: 25.5 ± 6.0, 25.6 ± 5.1 kg/m². BMI category, n (intervention, control):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated random allocation. |
| Allocation concealment (selection bias) | Low risk | Study allocation was concealed in numbered and sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. Trial authors stated that they had carried out an ITT analysis: data were analysed for participants according to their randomly‐allocated group; all participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported as in the published protocol. |
| Other bias | High risk | Demographic data were similar. Age, prepregnancy weight, height and BMI were not different at baseline. Women with delivery before 37 weeks were excluded. |
Barakat 2011.
| Methods | RCT, set in Hospital de Fuen‐labrada, Madrid, Spain. | |
| Participants | 80 women randomised. Inclusion criteria: healthy pregnant women (age, 23‐38 years), had uncomplicated, singleton pregnancies. Exclusion criteria: any type of absolute obstetric contraindication to aerobic exercise during pregnancy, which included other contraindications that the authors considered to have a relevant influence on maternal perception of health: significant heart disease, restrictive lung disease, incompetent cervix/cerclage, multiple gestation, risk of premature labour, pre‐eclampsia/pregnancy‐induced hypertension, thrombophlebitis, recent pulmonary embolism (last 5 years), acquired infectious disease, retarded intrauterine development, serious blood disease, and/or absence of prenatal care. |
|
| Interventions | Intervention group: (40 randomised) moderate physical activity, included a total of 35‐ to 45‐min weekly sessions 3 days each week from the start of the pregnancy (weeks 6‐9) to the end of the 3rd trimester (weeks 38‐39), an average of 85 training sessions, exercise intensity was light‐to‐moderate. Exercise was supervised by a fitness specialist and was in groups of 10‐12 women. Control group: (40 randomised) routine care. |
|
| Outcomes | Weight gain, caesarean, birthweight < 4000 g, birthweight > 4000 g. | |
| Notes | Authors concluded that a moderate physical activity program that is performed over the first, second, and third trimester of pregnancy improves the maternal perception of health status. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by use of a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. It is not clear how lack of blinding would impact on the outcomes measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 women were randomised and 67 were analysed; 34 in the exercise group, 33 in the control group. Reason of discontinued were similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | No between‐group differences regarding most potential confounding variables (such as occupational activities, standing, smoking habits, alcohol intake) but parity was not balanced between groups; the exercise group had a higher percentage of nulliparous women (76.5%) than the control group (36.4%). |
Bisson 2014.
| Methods | RCT conducted at Centre Mere Enfant CHU de Quebec, Laval University, Quebec City, Canada. | |
| Participants | 37 randomised, 4 withdrawals. Numbers in each group not stated. Inclusion criteria: obese pregnant women < 15 weeks. Exclusion criteria: NR. |
|
| Interventions | Intervention group:12 week supervised moderate exercise program consisting of 3 weekly 1‐hr sessions in a hospital‐based setting from 15th to 28th week. Control group: routine care. |
|
| Outcomes | "perinatal and maternal outcomes." | |
| Notes | Abstract only with no usable data. Exercising women maintained their fitness compared with control women (P = 0.047) and "had reduced weekly weight gain (0.3±0.1 kg/wk vs 0.5±0.2 kg/wk for exercise and control, respectively, p=0.047." "3 exercising versus 6 control women developed either gestational diabetes or hypertension." "Based on preliminary data, birth weight and gestational age at delivery were comparable between groups (3587±459 g and 39 3 /7 ± 1 1 /7 weeks, vs 3387±409 g and 39 3 /7 ± 1 1 /7 weeks in the exercising and control groups respectively, NS." Authors concluded that "An individualized exercise training effective in maintaining maternal fitness and limiting gestational weight gain in obese, pregnant women does not increase prematurity. However larger gestational weight gain in obese, pregnant women does not increase prematurity. However larger interventional study in this population will determine the potential benefits of maternal exercise during pregnancy on child growth, metabolism and development." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear due to limited reporting (abstract only) but 4 dropped out. Numbers in each arm was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only with sparse data. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only with sparse data. |
| Other bias | Unclear risk | Abstract only with sparse data. |
Bogaerts 2012.
| Methods | 3‐arm RCT conducted in University hospital, Leuven and East Limburg Hospital Belgium. | |
| Participants | Inclusion criteria: obese pregnant women. Exclusion criteria: NR. |
|
| Interventions | Counselling involving psycho‐education (4 sessions) vs a brochure vs control. Control group: routine care. |
|
| Outcomes | GWG and psychological vulnerability (depression and anxiety). | |
| Notes | Data collected during the first, second and third trimester. They found a significant reduction in GWG in the brochure and prenatal groups compared with controls. No differences in delivery method, birthweight, anxiety or depressed mood noted. Authors concluded that: findings justify "the clinical implementation of a psycho‐educational program in order to reduce GWG and psychological vulnerability in obese pregnant women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "women were randomised." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not provided. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only with little data. |
| Other bias | Unclear risk | Abstract only with little data. |
Callaway 2010.
| Methods | RCT, set in a hospital in Brisbane, Australia. | |
| Participants | 50 women randomised. Inclusion criteria: obese pregnant women were recruited at 12 weeks’ gestation, aged 18‐45, BMI ≥ 30 kg/m2, pregnancy care at study hospital, willing and able to be randomised to an exercise intervention. Exclusion criteria: non‐English speaking, contraindication or inability to exercise, medical or obstetric contraindication to exercise including haemodynamically significant heart disease, restrictive lung disease, incompetent cervix (cerclage), multiple gestation, severe anaemia, chronic bronchitis, type 1 diabetes, orthopaedic limitations, poorly controlled seizure disorder, poorly controlled hyperthyroidism, or a heavy smoker. |
|
| Interventions | Intervention group: the intervention group received an individualised exercise program with an energy expenditure (EE) goal of 900 kcal/ week. Advice from physiotherapist and diaries for self‐monitoring. Control group: routine obstetric care. |
|
| Outcomes | Self‐report of exercise (behaviour change). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by a random number allocation technique conducted by a 3rd party. |
| Allocation concealment (selection bias) | Unclear risk | Not clear but external randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. The impact of the lack of blinding was not clear. The use of self‐monitoring diaries by the intervention group may have introduced recall bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomised 50 women, at 36 weeks 36 were followed up (30% attrition). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report and online supplement. |
| Other bias | Unclear risk | "There were no statistically significant differences between the intervention and control groups in any baseline variable." Different monitoring techniques in the 2 groups (diaries in the intervention group) may have led to recall bias. |
Clapp 2002a.
| Methods | A prospective randomised design. | |
| Participants | 20 healthy women with uncomplicated pregnancy. | |
| Interventions | The participants were enrolled prior to pregnancy and placed on a regular regimen of supervised exercise and began a weight maintaining diet (low glycaemic sources of CHO). At 8 weeks' gestation, they were randomised to either diet containing low glycaemic CHO sources (n = 10) (aboriginal CHO diet) or high glycaemic CHO sources (n = 10) (cafeteria CHO diet). All continued the same exercise regimen throughout pregnancy. | |
| Outcomes | Weight gain. Total weight gain was defined as weight at delivery minus prepregnancy weight. |
|
| Notes | During pregnancy, all women were allowed to increase caloric intake according to appetite with advancing gestation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unspecified loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Outcomes not prespecified and appear to be selected. |
| Other bias | Unclear risk | Poorly reported. |
Clapp 2002b.
| Methods | RCT, set in Case Western Reserve University at metro health medical centre, USA. | |
| Participants | Inclusion criteria: 80 healthy, regularly exercising (≥ 3 times/week), non‐substance‐abusing women were enrolled before pregnancy. After conception (which occurred within 4 months in all cases) and ultrasonic confirmation of a viable singleton pregnancy, these women were assigned in week 8 of gestation to the exercise regimens. Exclusion criteria: not stated. Number of participants: 75 women enrolled and complete the protocol; 26 in Lo‐Hi group, 24 in Mo‐Mo group, 25 in Hi‐Lo group. |
|
| Interventions | There were 3 study groups: group 1: low‐high exercise (n = 26): exercise 20 mins 5 days a week through to week 20, gradually increasing to 60 mins 5 days a week by week 24 and maintaining that regimen until delivery (Lo‐Hi); group 2: moderate‐moderate exercise (n = 24): exercise 40 mins 5 days a week from week 8 until delivery (Mod‐Mod); group 3: high‐low exercise (n = 25): exercise 60 mins 5 days a week through to week 20, gradually decreasing to 20 mins 5 days a week by week 24 and maintaining that regimen until delivery (Hi‐Lo). |
|
| Outcomes | Weight gain. | |
| Notes | Age 31 ± 1, 30 ± 1, 32 ± 1 in Lo‐Hi, Mo‐Mo, Hi‐Lo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Women were randomly assigned by envelope but it was not stated whether envelopes were sequentially numbered, opaque and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | A member of the study team carried out morphometric assessment of placenta and infant at the time of birth. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 6.25%. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline data were similar. |
Cordero 2014.
| Methods | Open‐label RCT conducted at Hospital Puerta de Hierro, Madrid, Spain. | |
| Participants | 342 randomised, 257 women analysed. Included pregnant women in Spain at 10‐12 weeks according to ultrasound, with no medical or obstetric contraindications to exercise. Excluded women with medical or obstetric contraindications. |
|
| Interventions | Intervention group (101 women): a supervised exercise program consisting of aerobic and toning exercises for 3 sessions per week. 2 weekly sessions were performed on land (60 min) and 1 session was aquatic based (50 min). Program commenced from 10‐14 weeks to the end of the third trimester. Sessions were supervised by a qualified fitness specialist and an obstetrician. Control group (156 women): routine care. |
|
| Outcomes | GDM (primary outcome, diagnosed by fasting GTT before 30 weeks' gestation, according to medical records). Excessive GWG, gestational age at delivery, mode of delivery, birthweight and length, SGA, macrosomia. | |
| Notes | Enrolment age (intervention, control): 33.6 ± 4.1, 32.9 ± 4.5. Enrolment gestational age (intervention, control): NR. Prepregnancy BMI (intervention, control):22.5 ± 3.2, 23.6 ± 4. NCT01790412. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation and ratio was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail in report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition of 21 women (16.4%) in the intervention group and 64 in the control group (29.1%). |
| Selective reporting (reporting bias) | High risk | Per protocol analysis performed based on adherence to exercise "A minimum of 80% adherence to exercise classes was required for women assigned to the intervention group". Excluded women with poor adherence, and those who developed hypertension or were at risk of preterm labour. Possible side effects of exercise, e.g. preterm labour, were NR. |
| Other bias | High risk | Sample sizes differed substantially (101 and 156 women in intervention and control groups, respectively). For intervention and control groups, respectively, 2.7% vs 4.8% had previous GDM. 17.8% vs 27.6% had sedentary lifestyles before pregnancy. These might have biased GDM and GWG results in favour of the intervention group. |
De Oliveria Melo 2012.
| Methods | Open‐label 3‐arm RCT conducted at Department of Obstetrics, University of Campinas in Brazil with recruitment from May 2008 to Sept 2010. | |
| Participants | General population, 187 randomised. Inclusion criteria: healthy pregnant women who were sedentary at admission to the study, gestational age 13 weeks or less confirmed by ultrasonography and with single live fetus. Exclusion criteria: excluded if smoked, chronic diseases, a history of premature delivery, fetal abnormalities, placenta previa, a history of vaginal bleeding, placental detachment, and cervical length less than 2.5 cm. |
|
| Interventions | Intervention group: exercise (initiated at 13 weeks) (n = 62) vs exercise (initiated at 20 weeks) (n = 63) vs control (n = 62). Exercise consisted of walking 3 times a week. Control group: routine care. |
|
| Outcomes | Uteroplacental blood flow, birthweight, pre‐eclampsia, fetal growth restriction, macrosomia (assessed by ultrasound at 38 weeks), LGA and SGA at birth. | |
| Notes | Fitness evaluated at 13, 20 and 28 weeks. Age (intervention 1, intervention 2, control): 24 [5.8]/26 [5.3]/24 [5.4]. Enrolment gestational age: ≤ 13 weeks. Prepregnancy BMI (intervention 1, intervention 2, control): 24.7 [4.3]/23.4 [3.8]/23.5 [3.5]. Authors concluded that "Moderate intensity walking improved the physical fitness level of healthy pregnant previously sedentary women without affecting feto‐placental blood flow or fetal growth". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation sequence generated in blocks of ten" using Random Allocation software program 1.0." |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. "Groups were assigned only after the woman agreed to participate." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators involved in monitoring and ...analysis were unaware of the group to which the patient had been assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 women declined to participate after randomisation (8.5%). 31 women (19%) were missing follow‐up data for the 38 week visit; these missing data were balanced across study arms. Delivery/birth data were available for 161/187 women (86%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen. |
| Other bias | Low risk | None noted. Baseline characteristics similar. |
Di Carlo 2014.
| Methods | RCT at antenatal clinic in Italy from January 2010 to January 2011. | |
| Participants | General population, 154 randomised, 120 analysed. Inclusion criteria: pregnant at 6‐13 weeks' gestation (median 8 weeks). Women were excluded if they had any significant maternal condition. Exclusion criteria: any significant maternal condition including essential hypertension, thyroid diseases, gestational diabetes, miscarriages, preterm births, multiple pregnancies with more than 2 fetuses, maternal BMI ≤ 20 and ≥ 40 kg/m². |
|
| Interventions | Intervention group: dietary intervention (personalised diet plan with monthly dietician supervision) (n = 77; 59 in final analysis). Control group: brochure on healthy eating (n = 77; 61 in final analysis). |
|
| Outcomes | Weight gain, birthweight. | |
| Notes | Age (intervention, control): 31.3 [4.7]/28.2 [5.3] (P = 0.002). Enrolment gestational age: 8‐13 weeks (median 8 weeks). Prepregnancy BMI: 26.5 [6.3]/25 [4.2] (P = 0.3). Authors concluded that "This study suggests that a personalised nutritional intervention, in which the dietician plays an active role within the obstetric team, may represent a successful approach to limiting weight gain in pregnant women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" in 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | " ...attached a sequentially numbered, opaque sealed and stapled envelope containing the allocation treatment to the patient clinical record." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The allocation sequence was concealed from the researchers." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% of participants were excluded due to miscarriages (9 vs 8 in diet and control groups respectively), loss to follow‐up (6 vs 7) and preterm births (3 vs 1). "Analysis was performed per protocol" but protocol deviations and individual denominators were NR. |
| Selective reporting (reporting bias) | High risk | Per protocol data reported with individual denominators missing from most results. Results not adjusted for baseline difference in maternal age (older in the intervention group). |
| Other bias | High risk | Baseline difference in age of women (average of 3 years older in intervention group). |
Dodd 2014.
| Methods | RCT at 3 public maternity hospitals, Adelaide in Australia from June 2008 to December 2011. | |
| Participants | High risk (overweight and obese), 2212. Inclusion criteria: women with a singleton pregnancy, between 10 + 0 and 20 + 0 weeks' gestation, and BMI ≥ 25. Exclusion criteria: women with type 1 and 2 diabetes were ineligible. |
|
| Interventions | Intervention group: comprehensive dietary and lifestyle intervention (counselling) (n = 1108) Intervention involved meetings and home visits with advice on dietary, exercise, and behavioural strategies delivered by a dietician and trained research assistants. Exercise advice primarily encouraged women to increase their amount of walking and incidental activity. Control group: routine care (n = 1104). A nested RCT was also conducted in which women randomised to the intervention group were further randomised to receive written/verbal education about physical activity (n = 295) or to participate in a supervised walking group of moderate intensity 3 times per week for 40 mins (n = 287). |
|
| Outcomes | Primary outcome measures are: incidence of infants born LGA (birthweight ≥ 90th centile for gestation and sex). Secondary outcomes included birthweight > 4000 g, hypertension, pre‐eclampsia, and gestational diabetes. | |
| Notes | Age (intervention, control): 29.3 [5.4]/29.6 [5.3]. Enrolment gestational age (intervention, control): 14 (11.9‐17)/14.1 (11.9‐17). Recruitment BMI: 31 (28.1‐35.9)/31.1 (27.7‐35.6). Authors concluded that "For women who were overweight or obese, the antenatal lifestyle advice used in the study did not reduce the risk of delivering a baby weighing above the 90th centile for gestational age and sex or improve maternal pregnancy and birth outcomes". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The computer generated randomisation schedule used balanced variable blocks in the ratio 1:1". The investigator who prepared the randomisation schedule also was not involved with the recruitment or clinical care. |
| Allocation concealment (selection bias) | Low risk | "Telephoning the central randomisation service." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up done by interviewers not involved with the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% missing data for all outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported according to protocol. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Ferrara 2011.
| Methods | RCT at Kaiser Permanente Medical Care Program of Northern California in USA from October 2005 and May 2008. | |
| Participants | High risk (GDM), 197 randomised. Inclusion criteria: women with GDM. Exclusion criteria: age < 18 years; multiple gestation; diagnosis of diabetic retinopathy; high‐risk pregnancy (i.e. drug or alcohol abuse, chronic health problems, or pregnancy complications); thyroid diseases diagnosed in the last 30 days; and non‐English speaker. |
|
| Interventions | Intervention group: lifestyle intervention called DEBI (n = 96). Lifestyle intervention involved 3 in‐person sessions and up to 15 telephone calls with counselling re diet, physical activity and breastfeeding up to 12 months postpartum. The intervention was delivered by 2 dietitians. Participants were encouraged to engage in moderate intensity physical activity for 150 mins per week and received written materials about food size, foods with low GI or low fat, and how to read food labels were discussed. Control group: routine care (n = 101). |
|
| Outcomes | Meeting a set postpartum weight goal, weight gain, birthweight and breastfeeding. | |
| Notes | Data collected at baseline (GDM diagnosis) and at 6 weeks, 7 months and 12 months. Enrolment gestational age (intervention, control): 31 [5.6]/31 [6.1]. Authors concluded that: "This study suggests that a lifestyle intervention that starts during pregnancy and continues postpartum is feasible and may prevent pregnancy weight retention and help overweight women lose weight. Strategies to help postpartum women overcome barriers to increasing physical activity are needed". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer randomisation program was used to ensure that the conditions remained balanced with regards to the following characteristics: age(< 30 and ≥ 30 years), parity (≤ 1 and < 1), pregravid BMI (< 27.0 and ≥ 27 kg/m²)." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "data were collected by research assistants who were unaware of the condition assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% attrition at 7 and 12 months follow‐up with great loss to follow‐up in the intervention groups. This could bias results in the direction of the intervention if the heavier women chose to drop out. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen. GWG was NR. |
| Other bias | Low risk | Baseline characteristics comparable. |
Guelinckx 2010.
| Methods | RCT, set in the prenatal clinic, University Hospital of Leuven, Belgium. | |
| Participants | Inclusion criteria: obese (BMI > 29.0 according to IOM criteria), white women consecutively attending the prenatal clinic before 15 week of gestation. Exclusion criteria: pre‐existing diabetes or developing GDM, multiple pregnancy, recruitment after 15 weeks of gestational age, premature labour (delivery before 37 week of gestation), primary need for nutritional advice in case of a metabolic disorder, kidney problems, Crohn's disease, allergic conditions, and inadequate knowledge of the Dutch language. |
|
| Interventions | 2 intervention groups: the passive group (n = 37): received a brochure during the 1st prenatal consultation. This brochure was specifically designed for the study and provided advice on nutrition and on physical activity and tips to limit pregnancy‐related weight gain. The active group (n = 42): received the same brochure and women were actively counselled by a trained nutritionist in 3 group sessions. A maximum of 5 women were brought together in these 1‐hour sessions, which were scheduled at 15, 20, and 32 weeks of pregnancy. The sessions provided participants with recommendations on a balanced, healthy diet, based on the Official National Dietary Recommendations (9%–11% of the energy should come from proteins, 30%–35% from fat, and 50%–55% from CHOs). Control group (n = 43): received routine prenatal care. (Energy intake was not restricted in any group.) |
|
| Outcomes | Excessive weight gain (weight gain more than the upper limit recommendation for overweight women; > 11.2 kg). Gestational weight gain. Obstetrical and neonatal outcome: pre‐eclampsia, induction of labour, caesarean section, birthweight > 4000 g. Average energy intake. Weight gain was defined as weight at birth minus prepregnancy weight. Total physical activity score at 3rd trimester. For analysis 3.10 and 4.10 a physical activity score was calculated by using a questionnaire including a total 16 questions classified into 3 domains: work, sports, and non‐sports leisure‐time activities, scored on a 5‐point scale, ranging from “never”, “seldom”, “sometimes”, “very often”, to “always”. A total score for physical activity from a minimum of 3 to a maximum of 15 was obtained. A higher score indicated more activity. |
|
| Notes | Age 29.4 ± 4.4, 28.7 ± 4.0, 28.0 ± 3.6 years for control group, passive group, active group. Enrolment gestational age: 10.2 ± 2.4, 10.2 ± 2.6, 9.3 ± 2.8 weeks for control group, passive group, active group. Prepregnancy BMI: 33.5 ± 3.9, 33.4 ± 3.07, 34.1 ±4 .5 kg/m2 for control group, passive group, active group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | Patients were randomly assigned by using block randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 9.7%. Reasons for excluding the participants from each group were similar. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline characteristics of participants were similar between intervention and control groups. |
Haakstad 2011.
| Methods | Parallel‐arm RCT at Norwegian School of Sport Sciences in Norway from September 2007‐March 2008. | |
| Participants | General population, 105. Inclusion criteria: ability to speak Norwegian; nulliparous, sedentary women whose exercise levels did not include participation in a structured exercise program including brisk walks for the past 6 months were eligible for the trial, and must be in their first 24 weeks of pregnancy. Exclusion criteria: history of more than 2 miscarriages, severe heart disease (including symptoms of angina, myocardial infarction or arrhythmias), persistent bleeding after 12 weeks of gestation, multiple pregnancy, poorly controlled thyroid disease, pregnancy‐induced hypertension or pre‐eclampsia, diabetes or gestational diabetes, and other diseases that could easily interfere with participation. |
|
| Interventions | Intervention group: exercise (60 min supervised aerobic dance at least twice a week for a minimum of 12 weeks) (n = 52). Women in the exercise group were advised to have moderate, self‐imposed physical activity on the remaining weekdays. Control group: routine care (n = 53). |
|
| Outcomes | Infant birthweight, excessive weight gain, postpartum weight retention. Maternal weight gain and the proportion of women with excessive weight gain according to IOM recommendations (used self‐reported pre‐pregnancy weight as baseline). | |
| Notes | Participants were assessed 3 times: first visit (12‐24 weeks), second visit (36‐38 weeks) and last visit (6‐12 weeks postpartum). Adherence was a problem ‐ 31 (60%) women attended less than 80% of the exercise sessions and 4 women never showed up. Investigators performed subgroup analysis of participants who attended more than 24 sessions and the difference was significant in this subgroup of 21 women vs 53 controls (GWG 11.0 vs 13.8; P = 0.01). No women in this group had excessive weight gain (P = 0.006). Age (intervention, control): 31.2 [3.7]/30.3 [4.4]. Enrolment gestational age (intervention, control): 17.3 [4.1]/18 [4.3]. Prepregnancy BMI: 23.8 [3.8]/23.9 [4.7]. Authors concluded that "Regular participation in aerobic dance exercise can contribute to significantly reduced weight gain in pregnancy." However, "not associated with a reduction in birth weight". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used "a simple (not block) computerised randomisation programme". |
| Allocation concealment (selection bias) | Low risk | "An independent person...assigned the participants to either an exercise group or a control group." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinding not possible but principal investigator was blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessor blinded". The principal investigator was "blinded to group allocation when assessing the outcome measures". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rates of 19% and 21% in exercise and control arms, respectively. Drop‐outs were due to pain, hypertension, preterm birth and other reasons and were similar between the study arms. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Unclear risk | Denominators in report tables were the total exercise and control group N, despite stating drop‐outs in each group of 10 and 11 women, respectively. |
Harrison 2013.
| Methods | RCT at 3 large tertiary hospitals in Victoria, Australia. | |
| Participants | High risk, 228 randomised; 203 analysed. Inclusion criteria: women 12‐15 weeks of gestation, overweight (BMI ≥ 25 or ≥ 23 kg/m²) if high risk ethnicity (Polyasian, Asian, African populations) or obese (BMI ≥ 30 kg/m²), and at the increased risk for developing GDM identified by validated risk prediction tool. Exclusion criteria: exclusion criteria included multiple pregnancies, diagnosed type 1 or 2 diabetes, a BMI ≥ 45 kg/m², a pre‐existing chronic medical condition, and non‐English speaking women. |
|
| Interventions | Intervention group: lifestyle counselling intervention program (individual 4 sessions based on Social Cognitive Theory provided by a health coach) (n = 121) Intervention provided dietary advice, simple healthy eating, and "physical activity messages" and weight gain self‐monitoring. Also included "regular self‐weighing as a key behavioural strategy". Control group: routine care (n = 107). |
|
| Outcomes | The primary outcome was GWG with secondary outcomes including GDM screening. | |
| Notes | Outcomes were assessed at 12‐15 and 26‐28 weeks' gestation. Postpartum outcomes NR in primary report. Age (intervention, control): 32.4 [4.6]/31.7 [4.5]. Enrolment gestational age (overall): 14 [0.8]. Recruitment BMI: 30.4 [5.6]/30.3 [5.9]. Authors concluded that "Results indicate that a low‐intensity lifestyle intervention, integrated with antenatal care, optimizes healthy GWG and attenuates physical activity decline in early pregnancy Efficacy in limiting weight gain was greatest in overweight women and in high‐risk ethnically diverse women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned …through computer‐generated randomised sequencing." |
| Allocation concealment (selection bias) | Unclear risk | "Sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Care providers….were blinded to group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Participants wore a blinded pedometer" and "investigators and outcome data analysers were blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was similar in the 2 groups and < 20% for primary outcomes. Pedometer (PA) outcomes showed high loss to follow‐up; therefore we did not use these data. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Unclear risk | Follow‐up weight gain data were collected at 28 weeks (much earlier than other included studies). |
Hawkins 2014.
| Methods | Parallel arm pilot RCT. Conducted at medical centres in Western Massachusetts, USA. Recruitment from April 2010 to Aug 2011. | |
| Participants | 68 high‐risk (overweight and obese) pregnant women randomised. Overweight and obese pregnant Hispanic women (pre‐pregnancy BMI > 25 kg/m2) aged 18–40 years, with a gestational age of < 18 weeks, and who self‐reported participating in < 30 mins of moderate‐intensity activity per week. Excluded if history of Type 2 diabetes, hypertension, heart disease or chronic renal disease; current medications that adversely influence glucose tolerance; contraindications to participating in moderate‐intensity physical activity or a low‐fat/high‐fibre diet; self‐reported participation in > 30 min of moderate‐intensity exercise on > 3 days/week or > 20 min of vigorous‐intensity exercise on > 1 day/week; or 6) multiple gestation (e.g. twins). |
|
| Interventions | A lifestyle intervention (n = 33), consisting of a culturally and linguistically modified, motivationally targeted, individually tailored 6‐month prenatal programme. Educators encouraged women to achieve guidelines for physical activity, decrease saturated fat and increase dietary fibre. The intervention consisted of 6 monthly in‐person behavioural counselling sessions and 5 telephone booster sessions with follow‐up to 6 weeks postpartum. Women were encourage to achieve ≥ 30 min of moderate‐intensity activity on most days of the week through walking and developing a more active lifestyle. Controls had routine care (n = 35). |
|
| Outcomes | GWG, infant birthweight and biomarkers of insulin resistance. | |
| Notes | Mean recruitment age and BMI not given; these baseline variables were stratified and reported as categorical variables. Mean recruitment age was 14.9 weeks overall. Group characteristics were similar. Exercise outcomes were reported for 24‐28 weeks' gestation and at 6 weeks' postpartum. "Findings suggest that a motivationally matched lifestyle intervention is feasible and may help attenuate pregnancy‐related decreases in vigorous physical activity in a population of overweight and obese Hispanic women." NCT01141582. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation was stratified by age and prepregnancy BMI with a block size of four." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clearly described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to the assigned intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. |
| Selective reporting (reporting bias) | Unclear risk | Birth outcomes other than birthweight, and maternal weight at 6 weeks postpartum, were NR. |
| Other bias | Low risk | Baseline characteristics similar. Analysis by ITT. |
Huang 2011.
| Methods | RCT; 3 groups intervention design; 2 experimental groups (from pregnancy to 6 months postpartum (EP) and from birth to 6 months postpartum (EPP). The group receiving the intervention in the postnatal period only is not included in our analysis) and 1 comparison group. | |
| Participants | From January to June 2006, pregnant women were recruited from the obstetric clinics of a hospital in Taiwan (160 women randomised). Inclusion criteria: 16 gestational weeks, age 18 years or older, no cognitive impairment or psychiatric illness, ability to speak and read Chinese, not participating in another study, and intention to give birth at the study site. |
|
| Interventions | Intervention group: (80 participants). The educational intervention began at 16 gestational weeks (baseline) and to 6 months postpartum. The intervention was delivered at regularly scheduled clinic visits by nurses with training in nutrition and physical fitness. The nurse discussed with each participant how to design an individualised diet and physical activity plan. The intervention consisted of 6 1‐to‐1 counselling sessions: 1 primary session (about 30–40 mins) at the 16‐week gestation visit, and 5, 1‐to‐1 booster sessions (at 28 gestational weeks, 36–38 gestational weeks, before hospital discharge after a 3–7‐day stay, 6 weeks' postpartum and 3 months postpartum). After each clinic visit, women in the experimental groups were sent a personalised graph of their weight changes. At the 1st session, the experimental groups also received a researcher‐prepared brochure that provided detailed information on weight management goals during pregnancy and postpartum. Control group: (80 participants) routine care, provided once each trimester, which included health education on nutrition and exercise during pregnancy. |
|
| Outcomes | GWG, weight retention at 6 months postpartum, health‐promoting behaviour; physical activity. For analysis 1.9.1 physical activity measurement was a part of the health‐promoting lifestyle profile composed of 50‐item scale uses a 4‐point response format (range = 50–200) to measure the frequency of engaging in activities related to self‐actualisation, nutrition, physical activity,interpersonal support, health responsibility and stress management. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research assistant collecting outcome data was reported to be blind to the group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 women in each group were randomised, 61 and 64 of intervention and control group were analysed (78% followed up). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | No notable baseline differences were found between groups. |
Hui 2006.
| Methods | RCT, set in a community nurse‐managed centre and the Manitoba Clinic, both in urban Winnipeg, Manitoba, Canada. | |
| Participants | Inclusion criteria: women < 26 weeks pregnant with no pre‐existing diabetes were recruited on a voluntary basis. Exclusion criteria: pregnant women who had medical obstetric, skeletal or muscular disorders that could contraindicate physical exercise during pregnancy. |
|
| Interventions | Intervention (n = 24): additional intervention: lifestyle intervention including exercise intervention and dietary intervention. Exercise intervention: participants were instructed in group‐session exercises and in home‐based exercise. Weekly group session included floor aerobics, stretching and strength exercises, 3‐5 times/week for 30‐45 mins per session, video provided to participants to assist with home‐based exercise. Nutrition intervention: computer‐assisted food choice map interview, dieticians provided a personalised plan for participants. Control (n = 21): standard care group received an information package on diet and physical activity for a healthy pregnancy. |
|
| Outcomes | Excessive weight gain, weight gain, caesarean section, infant birthweight > 4000 g, and physical activity at end of study. Weight gain was defined as weight at birth (from medical chart) minus prepregnancy weight. For analysis 1.9.1 physical activity was defined as recreational physical activity which was measured by using the PARmed‐X for Pregnancy form based on Health Canada recommendations. Low levels (physical activity = 0) are defined as either no physical activity or activity < 1 to 2 times per week and for < 20 min per session; moderate levels (physical activity = 1) are defined as activity 1 to 2 times per week and for > 20 min per session or > 2 times per week and for < 20 min per session; high level (physical activity = 2) are defined as activity > 2 times per week and for > 20 min per session. |
|
| Notes | Age (intervention, control): 26.2 ± 5.7, 26.2 ± 5.4. Prepregnancy BMI (intervention, control): 25 ± 6.3, 23.4 ± 3.9 kg/m². Excessive weight gain was assessed based on prepregnant BMI:
(Canadian guidelines for healthy weights) Majority of participants (89%) were from low‐income families or low‐middle income families. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were enrolled and randomised into additional intervention and standard care groups. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 52 enrolled, 45 completed. Loss = 7/52*100 = 13.5%; 7 pregnant women dropped out due to school or work commitments. The participants who dropped out were significantly younger and had lower incomes than those who completed the program (P < 0.01). |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | No significant differences were found in age, prepregnancy BMI, and family income between additional intervention and standard care groups. |
Hui 2012.
| Methods | RCT at Tertiary hospital, Winipeg, Canada, from July 2004 to February 2010. | |
| Participants | General population, 224 randomised, 190 completed the study (88 controls and 102 interventions). Inclusion criteria: non‐diabetic pregnant women living in Winnipeg, < 26 weeks' gestation, signed informed consent. Exclusion criteria: medical or obstetric contraindications to exercise during pregnancy. |
|
| Interventions | Intervention group: lifestyle intervention (diet counselling and an exercise program) (n = 112) Intervention included "a community‐based exercise program specifically designed for pregnant women was provided". An exercise regimen, 3 to 5 times per week including a weekly exercise session and multiple home sessions of mild‐to‐moderate exercise for 30 to 45 mins was recommended. Program started between 20‐26 weeks. Group exercise sessions including aerobics were held in community centres and instructors were licensed fitness trainers. 2 dietary interviews with counselling were provided. Control group: routine care (n = 112). |
|
| Outcomes | Primary outcome ‐ prevalence of excessive GWG and measures of physical activity and food intake between the 2 groups. Other measures included physical activity, gestational diabetes, weight‐related obstetric procedures, gestational weight gain, the prevalence for LGA and birthweights. | |
| Notes | Participants logbooks were collected weekly. Delivery data and maternal weight at delivery were collected from medical records. Age (intervention, control): 30.1 [5.2]/28.7 [5.9]. Enrolment gestational age given as < 26 weeks' gestation. Prepregnancy BMI: 24.9 [5.4]/25.7 [5.1]. Authors concluded that a "lifestyle intervention during pregnancy increased physical activity, improved dietary habits and reduced excessive GWG in urban‐living pregnant women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed using a computer‐generated randomisation allocation table by a staff member without involvement in the study design." |
| Allocation concealment (selection bias) | Low risk | "After randomisation, participants received a sealed envelope labelled with the assigned randomisation number." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study staff were not blinded to the types of intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The study staff were not blinded to the types of intervention." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low risk for primary outcomes ‐ 30 participants (15%) withdrew from the study (7 in intervention group and 23 in the control group). However, only half the women completed dietary logbooks, so these data could not be used. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Hui 2014.
| Methods | Parallel arm RCT conducted in Winipeg, Canada between May 2009 and December 2011. | |
| Participants | 116 normal and high BMI women were randomised (BMI < 25 and BMI > 25), 113 analysed. Included if < 20 weeks' gestation, no existing GDM. Excluded if they had medical or obstetric contraindications to exercise during pregnancy. |
|
| Interventions | Lifestyle intervention (diet counselling and a supervised exercise program) (n = 57) vs control (n = 56). Intervention included "a community‐based exercise program specifically designed for pregnant women was provided". An exercise regimen, 3 to 5 times per week including a weekly exercise session and home sessions with DVD instruction of mild to moderate aerobic exercise for 30 to 45 mins was recommended. Program started between 20‐26 weeks and continues to 36 weeks. Group exercise sessions including aerobics were held in community centres and instructors were licensed fitness trainers. 2 dietary interviews with dietician counselling using a Food Choice Map were provided (baseline and 2 months later). Control group received standard care. |
|
| Outcomes | GWG, birthweight, delivery route, physical activity and food intake, gestational diabetes, LGA. | |
| Notes | Investigators stratified results by BMI (< 25 and > 25 kgm2). Recruitment age: overweight group 31 [4]/32 [5]; normal weight group 31 [3]/29 [6]. Gestational age: < 26 weeks. Recruitment BMI: overweight group 29.5 [5.1]/ 29.7 [1.3]; normal weight group 21.6 [2.2]/22.6 [1.9]. Authors concluded that "the lifestyle intervention program decreased EGWG, GWG, and offspring birth weight in pregnant women with normal, but not above normal, pre‐pregnancy BMI, which was associated with increased physical activity and decreased carbohydrate intake". NCT00486629. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Low risk | Allocation by a staff member not involved in study design by sealed, numbered envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "the study staff were not blinded to the types of intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnancy and maternal weight outcomes were collected by student assistants without knowledge of assignment. (High risk for participant logbooks for exercise and diet). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Not able to determine. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Jackson 2011.
| Methods | RCT, set in 5 prenatal care practices in the San Francisco Bay Area, USA, including 3 public hospitals, 2 academic practices, and a community hospital. 2006‐2007. | |
| Participants | 327 women randomised. Inclusion criteria: English‐speaking women 18 years or older and less than 26 weeks of gestation. Exclusion criteria: women who report smoking, alcohol use, drug use, or partner violence. |
|
| Interventions | Intervention group: (163 randomised) The Video Doctor: an interactive computer program including in‐depth behavioural risk assessments and tailored counselling messages, and producing printed output for both the patient and clinician. An actor‐portrayed Video Doctor appears and offers education on exercise, nutrition and weight gain based on principles of Motivational Interviewing. Dietary counselling focused on increasing intake of fruits and vegetables and whole grains, increasing healthful vs unhealthful fats and decreasing sugary foods. The Video Doctor emphasised dietary and exercise behaviour changes over weight gain. The Video Doctor programme required 10–15 mins to complete. After 4 weeks, participants received a brief ‘‘booster’’ Video Doctor counselling. Control group: (164 randomised) usual care. The usual care group did not interact with the Video Doctor and the program did not produce a Cueing Sheet or Educational Worksheet. Behavioural counselling for the usual care group was determined by the clinician. |
|
| Outcomes | Self‐reported servings per day or week of healthful foods (e.g. fruits and vegetables) and unhealthful foods (e.g. sweets), and exercise duration and frequency, and weight gain above the IOM guidelines. | |
| Notes | Mean baseline age (intervention, control): 26.1 ± 5.8, 26.9 ± 6.2. Enrolment BMI (intervention, control): reported as % categories: overweight and obese women comprised 48% and 41% of intervention and control groups respectively. Gestation (intervention, control): 19.7 ± 5.5, 19.2 ± 6.0. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer. |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer (interactive computer programme intervention). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants would not have been blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated that staff or outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women were excluded after randomisation: 3 due to insufficient English, 1 because of inaccurate gestational age, and 2 withdrew during the baseline assessment leaving 158 in the Video Doctor group and 163 in the usual care group. ITT analysis was performed for primary outcome (weight gain) for other outcomes 327 were randomised and 287 (88%) completed follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not able to determine as assessed from published study report. |
| Other bias | Unclear risk | There were no significant differences between the control and Video Doctor groups for any of the demographic variables listed except education. Results for weight gain were not fully reported by randomisation group. Results for mean weight gain of each group were reported without SDs. |
Jeffries 2009.
| Methods | RCT, set in a tertiary obstetric hospital in Melbourne, Australia. | |
| Participants | Inclusion criteria: pregnant women at < 14 weeks' gestation. Exclusion criteria: age < 18 or > 45 years, type 1 or type 2 diabetes mellitus, multiple pregnancy, or non‐English speaking. |
|
| Interventions | Intervention (n = 125): women allocated to the intervention group were given a personalised weight measurement card, advised of their optimal GWG (based on their BMI at the time of recruitment and the United States IOM guidelines, and instructed to record their weight at 16, 20, 24, 28, 30, 32 and 34 weeks' gestation. Control (n = 111): not given instructions about regular weight measurement. |
|
| Outcomes | Weight gain above IOM guideline, mean weight gain from recruitment to follow‐up at 36 weeks' gestation. SGA (< 10th centile), large‐for‐gestational age (> 90th centile), preterm (< 37 weeks), instrumental delivery, caesarean delivery, pre‐eclampsias, neonatal hypoglycaemia, shoulder dystocia. Weight gain was weight difference between weight at about 36 weeks' gestation and weight at 1st antenatal appointment. |
|
| Notes | Gestation age at recruitment (intervention, control): 11.6, 11.4 weeks. BMI category, n (intervention, control):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Using opaque, sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants were blinded to the purpose of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up, 23/148 in intervention group, 27/138 in control group. Similar reason of loss to follow‐up in intervention and control groups. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported as in the published protocol. |
| Other bias | Low risk | Baseline characteristics were similar. |
Kieffer 2014.
| Methods | Parallel‐arm RCT at community health centre in Detroit, USA from 2004 to 2006. | |
| Participants | General population, 278 randomised, all included in primary analysis. Inclusion criteria: pregnant Latinas were eligible if they were age 18 or older, South Detroit residents, and at less than 20 gestation weeks at eligibility screening. Exclusion criteria: see inclusion criteria. |
|
| Interventions | Intervention group: a counselling intervention (MOMS intervention included home visits, group classes, related activities and social support from community health workers to influence eating and exercise, weight beliefs and behaviours) (n = 139) Intervention was administered over 11 weeks. Control group: routine care (n = 139). |
|
| Outcomes | Dietary behaviour outcomes including sugar, fats, and fibre. | |
| Notes | Little usable data extracted from this report. Age (intervention, control): 27.3 [5.3]/27.1 [5.1]. Enrolment gestational age < 20 weeks. Recruitment BMI: 24.2 [5.1]/24.7 [5]. Authors concluded that "We confirmed the hypothesis that a community‐planned CHW‐led healthy lifestyle intervention could improve dietary behaviors of low‐income Lantina women during pregnancy". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "statistician generated random allocation sequence in blocks of 40". |
| Allocation concealment (selection bias) | Low risk | "After baseline data collection, each women received...a sealed envelope containing her group assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collected by trained interviewers who were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% loss to follow‐up. Data were available for 139/139 and 136/139 women in the intervention and control groups, respectively, for the primary analysis. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Kong 2014.
| Methods | RCT at Low State University in USA (accrual dates not stated). | |
| Participants | Obese/overweight pregnant women, 42 randomised. Inclusion criteria: maternal age between 18 and 45 years, singleton pregnancy, nonsmoker, self‐reported overweight (BMI ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2) before pregnancy, no prior history of chronic diseases (including type 1 diabetes, cardiovascular disease, thyroid, or lung disorder), and no prior history of gestational diabetes. Only enrolled women with < 3 30‐min episodes of physical activity in previous 6 months. Exclusion criteria: see inclusion criteria. |
|
| Interventions | Intervention group: exercise intervention (unsupervised walking program on treadmill or other setting for a minimum of 150 min/week) (n = 18). Control group: routine care (n = 19). |
|
| Outcomes | Activity, weight gain, pregnancy risks and labour procedures, infant birth outcomes. | |
| Notes | Women were loaned treadmills for the study. Interventions continued at least to gestational week 35. Data reported separately for overweight (n = 18) and obese (n = 19) participants. Data were collected for 1 week periods at weeks 10‐14 (baseline), weeks 17‐19 (V2), weeks 27‐29 (V3), and weeks 34‐36 (V4). Age (obese group: intervention, control): 28.6 [5.3]/25.7 [4]. Age (overweight group: intervention, control): 26.2 [2.6]/27.3 [3.6]. Enrolment gestational age < 15 weeks. Recruitment BMI (obese group: intervention, control): 34.7 [4.6]/34.2 [3.6]. Recruitment BMI (overweight group: intervention, control): 26.5 [1.2]/27.4 [1.4]. Authors concluded that a "pilot, unsupervised walking intervention increased the moderate‐intensity physical activity (MPA) of overweight and obese women during pregnancy" with "trend to more favourable maternal and birth outcomes" in the intervention group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After the initial enrollment, participants were randomly assigned …using a computer‐based random number generator (Microsoft Excel 2010)." |
| Allocation concealment (selection bias) | Unclear risk | "All participants and research staff were blinded to the group allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants to intervention was not possible due to the nature of the intervention. Study coordinator not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not described. Pregnancy outcome measures self‐reported by participants via a postpartum questionnaire and may be subject to bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 withdrawals (19.6%, 5 in physical activity group and 4 in control group). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | None noted. Baseline characteristics comparable. |
Korpi‐Hyovalti 2011.
| Methods | RCT, set in 2 hospitals in rural municipalities (Kauha‐joki and Lapua) in Finland. | |
| Participants | 60 women randomised. Inclusion criteria: women at high risk of gestational diabetes: women had 1 or more risk factors (BMI > 25 kg/m2, previous history of GDM or birth of child > 4.5 kg, age > 40 years, family history of diabetes or the venous plasma glucose concentration after 12 hours fasting in the morning was 4.8‐5.5 mmol/L and 2‐hour OGTT plasma glucose < 7.8 mmol/L. Exclusion criteria: women who were diagnosed as having GDM in this study and women who had risk factors for GDM or whose fasting venous plasma glucose was 4.8‐5.5 mmol/L but who for personal or professional reasons did not wish to participate in the trial. |
|
| Interventions | Intervention group: (n = 30) a lifestyle intervention; included diet counselling and exercise counselling. Dietary advice tailored to each woman individually on 6 occasions. Women were encouraged to eat a diet rich in vegetables, berries and fruits, and to use low‐fat. Moderate‐intensity physical exercise during pregnancy was encouraged, 6 sessions for exercise counselling Control group: close follow‐up group (n = 30). All women were given general information on diet and physical activity to decrease the risk of GDM during pregnancy as part of routine care. |
|
| Outcomes | Weight gain, GDM, birthweight. | |
| Notes | Age: 29.2 [5.4]/29.8 [5.4]. Gestation at recruitment given as 8‐12 weeks. Baseline BMI: 27.3 [6.0]/25.5 [3.4]. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned to the lifestyle intervention group or to the close follow‐up group by the study physician in the Central Hospital with the use of a computed randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women were aware of group assignment although it was stated that the nurses scheduling study visits did not have access to the randomisation list. It is not clear what impact the lack of blinding would have on the outcomes measured. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 women were randomised, 54 were followed up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were no statistically significant differences in baseline measures between the lifestyle intervention and the close follow‐up groups although women in the intervention group had slightly higher prepregnancy weight (mean 76.6 kg compared with 69.6 kg in controls. |
Laitinen 2009.
| Methods | RCT, set in maternal welfare clinics in the city of Turku and neighbouring areas in south‐west Finland. | |
| Participants | Women were eligible for participation if they were less than 17 weeks’ gestation and had no metabolic or chronic diseases such as diabetes. Participants were Caucasian. |
|
| Interventions | At 1st trimester 256 pregnant women were allocated to 3 groups: modification of dietary intake according to current recommendations with probiotics or placebo and a control group receiving placebo only.
Dietary counselling given by a dietitian at each study visit aimed to modify dietary intake to conform with that currently recommended, particular attention being paid to the quality of dietary fat Study visits took place 3 times during pregnancy at 13·9 (SD 1·6), 23·8 (SD 1·4) and 33·9 (SD 1·4) weeks of gestation and at 1, 6 and 12 months postpartum. |
|
| Outcomes | Weight gain, energy intake, dietary fibre intake at 3rd trimester of pregnancy. Weight gain was calculated by subtracting self‐reported prepregnancy weight from that recorded at a prenatal visit or at hospital within 1 week before delivery. |
|
| Notes | Age (intervention 1, intervention 2, control): 30.1 ± 5.2, 29.7 ± 4.1, 30.2 ± 5.0. Enrolment BMI NR. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned to 3 study groups according to computer‐generated block randomisation. |
| Allocation concealment (selection bias) | Low risk | Using sealed envelopes. At the 1st study visit the envelopes were opened. The random allocation sequence was thus concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probiotics/placebo were double‐blind in the intervention groups but single blind in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up (to delivery). |
| Selective reporting (reporting bias) | Low risk | The outcomes reported as in the published protocol. |
| Other bias | Low risk | No other bias apparent. |
Leiferman 2011.
| Methods | RCT conducted by the Colorado School of Public Health, Denver, USA. | |
| Participants | Pregnant women. Inclusion/exclusion criteria not described. |
|
| Interventions | My Baby My Move (MBMM) was an 8‐week community‐based program that involved both didactic and experiential components. Peer leaders delivered the program. | |
| Outcomes | Sparse information and no data were available on this trial. | |
| Notes | Conference abstract only with no usable data. Authors concluded that "These findings suggest that implementing community‐based interventions to increase antenatal PA are well received and show promise in ultimately changing PA behaviour". Full report is pending (personal communication). Nodine 2011 is a substudy of 29 women from this study that examined the effect of exercise on sleep parameters. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on which to base judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on which to base judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information on which to base judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information on which to base judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information on which to base judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information on which to base judgement. |
| Other bias | Unclear risk | Insufficient information on which to base judgement. |
Louie 2011.
| Methods | Parallel‐arm RCT conducted in Australia. | |
| Participants | 99 randomised, 92 analysed. Inclusion criteria: women with GDM diagnosed by GTT at 20‐32 weeks' gestation and singleton pregnancy. |
|
| Interventions | Dietary intervention: low GI diet (n = 47) vs conventional high‐fibre, moderate GI diet (n = 45). Intervention included 3 face‐to‐face visits with the study dietician. | |
| Outcomes | Birthweight, LGA, SGA, caesarean section, macrosomia, GWG. | |
| Notes | Age (intervention, control): 34 [4.1]/32.4 [4.5]. Enrolment gestational age: 26.1 [4]/26 [4.3]. Prepregnancy BMI: 23.9 [4.4]24.1 [5.7]. Authors concluded that "In intensively monitored women with GDM, an LGI diet and a conventional HF diet produce similar pregnancy outcomes". They suggested that because the intervention began after the 29th week gestation this may have attributed to the lack of difference. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation by "computer‐generated numbers, stratified by BMI (BMI < 30 vs ≥ 30) and weeks of gestation (< 28 vs ≥ 28)". |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was unpredictable and concealed from the recruiter." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A biostatician blinded to the diet allocation performed the statistical analyses." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Four women delivered prematurely and three women withdrew after first dietary instruction." |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Other bias | Low risk | Baseline characteristics comparable. |
Luoto 2011.
| Methods | Cluster‐RCT, set in primary healthcare centres in 14 municipalities in Pirkanmaa region in south‐western Finland. | |
| Participants | Recruitment 2007‐8. Inclusion criteria: pregnant women at 8–12 weeks' gestation at high risk of developing gestational diabetes; BMI ≥ 25 kg/m² based on measured height and self‐reported prepregnancy weight; GDM or any signs of glucose intolerance or newborn’s macrosomia (≥ 4500 g) in any earlier pregnancy; type 1 or 2 diabetes in 1st‐ or 2nd‐degree relatives; or age ≥ 40 years. Exclusion criteria: at least 1 of the 3 baseline (8–12 weeks' gestation) OGTT measurements was abnormal (fasting blood glucose ≥ 5.3 mmol/L, 10.0 mmol/L at 1 hr, and 8.6 mmol/L at 2 hr); prepregnancy type 1 or 2 diabetes; inability to speak Finnish; age <18 yr; multiple pregnancy; physical restriction preventing physical activity; substance abuse; treatment or clinical history of psychiatric illness. |
|
| Interventions | Intervention group: (7 municipalities) Individual counselling on physical activity and diet and weight gain. At the 1st visit the recommendations for GWG were discussed and an appropriate weight gain graph was selected to guide the participant in monitoring her weight gain. The primary physical activity counselling was implemented at 8–12 weeks' gestation and the primary dietary counselling session at 16–18 weeks' gestation. Physical activity counselling was enhanced at 4, and diet counselling at 3 subsequent visits. Control group: (7 municipalities) usual care group received no counselling beyond usual care, which included some dietary counselling (partly on different topics) and follow‐up of gestational weight, but little physical activity counselling. |
|
| Outcomes | Incidence of GDM as assessed by OGTT (maternal outcome) and newborns’ birthweight adjusted for gestational age, maternal weight gain and the need for insulin treatment during pregnancy, changes in physical activity and diet (intake of total fat, saturated and polyunsaturated fatty acids, saccharose, and fibre. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The unit of randomisation was municipality. In the randomisation process, participating municipalities were 1st pair‐wise matched. 14 municipalities were then randomised by computer. |
| Allocation concealment (selection bias) | Low risk | Cluster‐randomised trial with computer randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women in the intervention group were provided with notebooks to record diet and activity, women in the control group were not; this may have affected recall and may have introduced bias. It was not clear whether staff collecting outcome data were blind to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No loss of clusters (14 clusters were randomised and all were included in the analysis). 343 women in the intervention and 297 in the usual care group agreed to participate in the trial. However, 81 (23.6%) of the participants in intervention group and 93 (31.3%) of the participants in the usual care group had an abnormal OGTT result at baseline and were thus excluded. The final number of participants in the analyses was 219 (89.0% of participants receiving allocated intervention) in the intervention group and 180 (91.8% of participants receiving allocated intervention) in the usual care group. However about 40% of eligible participants of each group were followed up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study reports. Analysis appropriate for clusters.There was adjustment of data for clustering and various cluster, clinic and individual level differences at baseline. |
| Other bias | Unclear risk | Baseline characteristics of each group were similar, except that women in the intervention group had higher education than in the usual care group. |
Magee 1990.
| Methods | RCT, set in prenatal care at the University of Washington Obstetrics Clinics. | |
| Participants | Pregnant women with obesity (prepregnancy weight > 120% of ideal body weight) and diagnosed with gestational diabetes, recruited at 28 weeks' gestation. | |
| Interventions | All women were hospitalised for 2‐week duration in the metabolic ward. Intervention: calorie‐restricted (n = 7): during the 1st week, the women consumed normal diet with 2400 kcal/day; 50% CHO, 30% fat and 20% protein with 11 g of total dietary fibre per 500 kcal During the 2nd week, the women were placed on 1200 kcal/day diet. This reduction was accomplished by decreasing portion sizes without changing other features of diet. Control (n = 5): during the 1st week, the women consumed identical diet as the intervention group; 2400 kcal/day, and continued on the same diet (2400 kcal/day) during the 2nd week. |
|
| Outcomes | Metabolic indices: fasting plasma glucose, OGTT, insulin, triglyceride, free fatty acids, glycerol, ß‐hydroxybutyrate, and urine ketones. We have not included outcome data from this hospital inpatient study in the analyses in the review. |
|
| Notes | Age (calorie‐restricted, control): 30 ± 4, 36 ± 5 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Unclear risk | No information provided. |
Marcinkevage 2012.
| Methods | RCT in a large urban hospital in the USA. | |
| Participants | High risk (obese and overweight), 55 randomised, 36 analysed so far (ongoing). Inclusion criteria: overweight and obese 'minority' women receiving prenatal care. Gestation at recruitment: 14.4 +‐2.8. Exclusion criteria: see inclusion criteria. |
|
| Interventions | Intervention group: lifestyle intervention (monthly behavioural counselling meeting focused on reducing sedentary behaviour and increasing levels of physical activity) (n = 17). Control group: routine care (n = 19). |
|
| Outcomes | Physical activity, GWG, glucose values. | |
| Notes | Abstract only with no usable data. Preliminary analysis shows decrease in median(range) total activity in both groups from first visit to mid‐pregnancy. Reduction in sedentary behaviour from first trimester to mid‐pregnancy in intervention but not regular group. Median(range) GWG at mid‐pregnancy is similar for both groups. At mid‐pregnancy lower fasting and 30 min glucose values in intervention vs regular care. Preliminary data demonstrate the feasibility of modifying lifestyle during early pregnancy in underserved black women. Authors concluded that "Preliminary data demonstrate the feasibility of modifying lifestyle during early pregnancy in underserved black women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. "randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Insufficient information. |
Moses 2009.
| Methods | Randomised controlled trial, set in the city of Wollongong, New South Wales, Australia. | |
| Participants | Inclusion criteria: pregnant women, age 18–40 years (inclusive), singleton pregnancy, no previous GDM, nonsmoker, diagnosis of GDM and seen for the 1st dietary visit between 28 and 32 weeks of gestation, and ability to follow the protocol requirements. Exclusion criteria: any condition or medication that could affect glucose levels and unwillingness to follow the prescribed diet. |
|
| Interventions | 63 women were randomly assigned to receive 1 of 2 different diets, low–GI diet (n = 31) or higher–GI diet (n = 32). Both diets were compatible with the recommended nutritional intake in pregnancy. The CHO intake was designed to achieve a minimum of 175 g/day with only the recommended choice of CHO foods varying. The dietary advice by dietitian was individualised with specific mention of the energy and nutrient balance to achieve normal weight gain during the 3rd trimester. The low–glycaemic diet: based on previously verified low–GI food, including pasta, grain breads, and unprocessed breakfast cereals with a high fibre content. Women were specifically asked to avoid consuming white bread, processed commercial breakfast cereals, potatoes, and some rice varieties. The higher–glycaemic diet: a diet with a high‐fibre and low‐sugar content, with no specific mention of the GI. Potatoes, whole wheat bread, and specific high‐fibre, moderate‐to‐high–GI breakfast cereals were recommended. |
|
| Outcomes | Induction of labour, method of delivery, LGA baby (> 90th centile), SGA baby (< 10th centile). | |
| Notes | Age (LGI, HGI): 30.8 + 0.7, 31.3 + 0.8. Gestational age at entry to study (LGI, HGI): 30.3 + 0.2 weeks, LGI 29.9 + 0.2 weeks. BMI at enrolment (LGI, HGI): 32.0, 32.8 kg/m². |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned using permuted blocks of unequal size with the list generated using STATA (version 7.0). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The physician caring for the women was blinded. Study dietitians were not blinded to dietary assignment but were aware of the need for impartiality and equivalent treatment Participants were impossible to blind to the GI concept, as it is widely known and discussed in the lay press. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout apparent. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | There were no significant differences in the baseline characteristics of the 2 groups. |
Moses 2014.
| Methods | Parallel‐arm RCT conducted in Australia from Feb 2010 to Sept 2012. | |
| Participants | 691 randomised, 576 analysed. Inclusion: healthy pregnant women at 12 to 16 weeks' gestation who agree to be randomised. Exclusion: women with pregestational diabetes; multiple birth; assisted reproduction; special diet or referred to a dietitian for other reasons. |
|
| Interventions | A low glycaemic diet (n = 354) or a conventional healthy diet (n = 337) from 12 to 16 weeks' gestation for the remainder of pregnancy. | |
| Outcomes | Primary outcomes: prevalence of LGA at birth (more than 90th centile); prevalence of childhood obesity as determined by BMI. Secondary outcomes: prevalence of gestational diabetes; ponderal index; prevalence of SGA; GWG. |
|
| Notes | Age (intervention, control): 29.9 [0.3]/29.9 [0.3]. Enrolment gestational age: 16.5 [0.1]/16.2 [0.1]. Prepregnancy BMI: 24.3 [0.3]/24.7 [0.3]. Outcomes were assessed at baseline, 28 weeks and 34 weeks. Authors concluded that "A low‐intensity dietary intervention with an LGI diet compared with an HE diet in pregnancy did not result in any significant differences in birth weight, fetal percentile, or PI. In conclusion, the outcome of this study was neutral. Infants of women instructed to consume LGI CHO foods during pregnancy were of normal size and had a similar PI to those of infants of women who received conventional HE advice". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Study personnel were not blinded to the dietary assignment but were aware of the need for impartiality and equivalent treatment. Obstetric care providers were not specifically blinded to the study allocation but were also not informed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout < 20%. In the low GI group, 58 women were later excluded (27 women had developed GD). In the HE group, 57 women were later excluded (28 women had developed GD). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Mujsindi 2014.
| Methods | RCT (setting not mentioned) in USA (accrual dates NR). | |
| Participants | High risk (obese and overweight), 79 randomised. Inclusion criteria: overweight and obese 'minority' women receiving prenatal care. Exclusion criteria: see inclusion criteria. |
|
| Interventions | Intervention group: lifestyle intervention (behaviour counselling with 5 dietary/nutrition consultations during pregnancy and at 3 months postpartum, food records, pedometers and logs, pregnancy activity questionnaire and food frequency questionnaire) (n = ?). Control group: routine care (n = ?). |
|
| Outcomes | Primary outcome was GWG and postpartum weight retention rates. Secondary outcomes included obstetric, delivery and neonatal outcomes. | |
| Notes | Abstract only with no usable data. Obese women enrolled in a structured nutrition program during pregnancy and in the postpartum period had similar GWG and postpartum weight retention to women in standard care. There was no difference in demographic data, parity, smoking status and socioeconomic status. When comparing the PEN vs STD groups, there was no difference in mean GWG at 20 weeks, 24, 30 and 36 weeks. Return to study entry weight was compared between the groups at 3 and 6 months. There was no difference between the groups at either time (P = 0.21 and 0.18 respectively). Authors concluded that "Obese women enrolled in a structured nutrition program during pregnancy and in the postpartum period had similar gestational weight gain and postpartum weight retention as women in standard care". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. "Randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Insufficient information. |
Murtezani 2014.
| Methods | A parallel arm RCT conducted in the Republic of Kosovo. Accrual dates not given. | |
| Participants | 72 pregnant women randomised, 63 analysed. | |
| Interventions | Intervention (n = 30) involved an exercise training program that started in the second trimester and was continued until the end of pregnancy. Each session consisted of 40‐45 min of aerobic and strength exercise. Individuals were supervised by certified aerobic‐instructors, and each session included a maximum of 10 participants. Intensity was moderate to vigorous; supine postures and Valsalva manoeuvres were avoided. Controls had routine care (n = 33). |
|
| Outcomes | GWG, neonatal weight, Apgars, macrosomia. | |
| Notes | The participants were examined twice during the study period. The first visit was between 14‐20 weeks of gestation (baseline visit) and the second at week 36‐38 (after the intervention). Recruitment age: 26.9 [4.7]/25.7 [5.1]. Recruitment gestation: 18.9 [1.8]/ 18.3 [1.4]. Prepregnancy BMI: 23 [2.6]/ 22.4 [2.2]. "Supervised aerobic and strength conditioning exercise performed over the second and third trimester ….does not have a negative impact on the newborn's size and health." Reported gestational weight, not weight gain. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment procedure was performed using random numbers generated by a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Report states 'single blind' but details not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Report states 'single blind' but details not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 participants (4 from the control group and 5 from the intervention group) withdrew after randomisation for medical and personal reasons: hypertension (n.= 2), vascular disease (n = 1), intrauterine growth restriction (n = 1), amniotic‐fluid leakage (n = 2), premature birth (n = 1) , injured n = 1) and unknown (n = 1). It was not stated from which group these withdrawals occurred. |
| Selective reporting (reporting bias) | High risk | Women with hypertension, preterm birth and amniotic fluid leakage were excluded after randomisation, yet these are potential side effects of vigorous exercise in pregnancy. |
| Other bias | High risk | Baseline characteristics were similar for study groups. Women with hypertension, preterm birth and amniotic fluid leakage were excluded after randomisation, yet these are potential side effects of vigorous exercise in pregnancy and needed to be reported. |
Nascimento 2012.
| Methods | RCT conducted at an antenatal outpatient clinic in Brazil from August 2008 to March 2010. | |
| Participants | High risk. Overweight or obese, 82 randomised. Inclusion criteria: pregnant women ≥ 18 yrs, pre‐gestational BMI ≥ 26 kg/m², gestational age 14‐24 weeks. Exclusion criteria: multiple pregnancy, exercising regularly, and conditions that contraindicate exercise, such as cervical incompetence, severe arterial hypertension, diabetes with vascular disease and risk of abortion. |
|
| Interventions | Intervention group: supervised exercise program (n = 40). Intervention consisted of an exercise program guided by a trained physical therapist in weekly classes with light to moderate intensity exercise for 40 mins. It also included home exercise counselling which was to be performed 5 times per week (consisting of a sequence of 22 exercises or walking). Control group: routine prenatal care program (n = 42). |
|
| Outcomes | Primary outcomes were GWG and excessive weight gain. Secondary outcomes were increased arterial blood pressure, perinatal outcomes and QoL (WHOQOL). | |
| Notes | Outcomes measured at baseline and 36 weeks. Delivery data were collected from medical records. Age (intervention, control): 29.7 [6.8]/30.9 [5.9]. Enrolment gestational age (intervention, control): 14.3 [4.5]/13.6 [3.5]. Prepregnancy BMI: 34.8 [6.6]/36.4 [6.9]. Slight baseline imbalances noted as 8/40 vs 15/42 were diabetic and 15/40 vs 22/42 were hypertensive in exercise and control groups, respectively, at baseline. Authors concluded that "The exercise programme was not associated with control of gestational weight gain in our sample as a whole, but was beneficial for lower gestational weight gain in overweight women. Exercise was not associated with adverse perinatal outcomes and did not affect variation in arterial blood pressure or the perception of QoL". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised to the groups using the SAS statistical program (SAS Institute, Cary, NC, USA), which generated a list of random numbers based on a uniform distribution." |
| Allocation concealment (selection bias) | Low risk | "The sequence was randomly distributed in opaque envelopes, which were sealed and sequentially numbered. Each participant received a sequence number corresponding to a sealed envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 woman in each group withdrew. For neonatal weight outcome, missing data were > 20% but similar numbers missing in each group. Authors indicate that this was because some women delivered at other hospitals. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Other bias | Unclear risk | Baseline imbalances noted as 8/40 vs 15/42 were diabetic and 15/40 vs 22/42 were hypertensive in exercise and control groups, respectively, at baseline. Also, the control group had a greater proportion of obese women. This could affect some outcomes (e.g. LGA, GWG) in favour of the intervention. |
Oostdam 2012.
| Methods | RCT at hospitals and midwifery practices in The Netherlands from January 2007 to January 2011. | |
| Participants | High risk. Overweight or obese, 121 randomised, 101 analysed. Inclusion criteria: pregnant women who were overweight or obese and at risk for GDM. AND had at least 1 of the 3 following characteristics: (1) history of macrosomia. (2) history of GDM; or (3) first‐grade relative with Type 2 diabetes. Exclusion criteria: recruitment after 20 weeks of gestation; age under 18 years; non‐Dutch speaker; having been diagnosed with GDM before randomisation; hypertension; alcohol abuse; drug abuse; use of any medication that affects insulin secretion or insulin sensitivity; serious pulmonary, cardiac, hepatic, or renal impairment; malignant disease; and serious mental or physical impairment. |
|
| Interventions | Intervention group: exercise intervention (2 sessions of aerobic and strengthening exercises per week; each exercise session lasted for 60 mins) (n = 62). Control group: routine care (n = 59). |
|
| Outcomes | Maternal outcome measures were fasting blood glucose (mmol/L, fasting insulin (pmol/L) and HbA1c (%), body weight (kg), BMI (kg/m2), and daily physical activity (min/week). Offspring outcome measures were birthweight and fetal growth. | |
| Notes | Outcomes assessed at baseline, 24 and 32 weeks. Age (intervention, control): 30.8 [5.2]/30.1 [4.5]. Enrolment gestational age was 15 weeks. Prepregnancy BMI: 33 [3.7]/33.9 [5.6]. Authors concluded that exercise intervention "had no effects on fasting blood glucose, insulin sensitivity, and birthweight, most probably because of low compliance". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, stratified by hospital. |
| Allocation concealment (selection bias) | Low risk | "Women were recruited by midwives and gynaecologists who were unaware of the allocation of other women, with no risk of compromising allocation concealment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "By the nature of the intervention the researcher and research assistant could not be blinded for allocation after randomisation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All outcome measures were assessed by independent examiners, unaware of group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rates, especially at 32 weeks (19/62 [31%] did not respond in intervention group and 12/59 [20%] did not respond in control group). For GWG outcome dropout rate was 31% overall. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Other bias | High risk | Poor adherence to the intervention ‐ "only a small proportion (16.3%) of the women in our intervention group attended at least half of the training sessions". Follow‐up weight gain data were collected at 32 weeks (much earlier than most other included studies). |
Petrella 2013.
| Methods | RCT in Italy (setting and accrual dates NR). | |
| Participants | High risk. Overweight or obese. 61 randomised. Inclusion criteria: women with BMI > 25 at 1st trimester, > 18 years with singleton pregnancy at 12 weeks' gestation. Exclusion criteria: women with twin pregnancy, chronic disorders, GDM in previous pregnancy, smoking, previous surgery, women who engaged in regular physical activity, or used dietary supplements or herbal products known to affect body weight were excluded. |
|
| Interventions | Intervention group: lifestyle intervention involving a caloric restricted low GI diet (1500 kcal/day) and prescribed moderate‐intensity exercise 30 min/day, 3 times per week. Pedometers were to be worn (n = 33). Control group: routine care with a nutritional brochure about health eating (n = 28). |
|
| Outcomes | GDM, GWG, hypertension, and preterm delivery. | |
| Notes | Recruitment age: 31 [4.2]/32.4 [5.9]. Gestational age: first trimester. Recruitment BMI: 32.1 [5]/ 32.9 [6.2]. Authors concluded that "A constant physical activity and a change toward healthy eating improves nutrients intake, prevents excessive weight gain and avoids the maternal unfavorable outcomes associated with overweight/obese women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in blocks of 3. |
| Allocation concealment (selection bias) | Low risk | "Study randomization is numbered and sealed in white envelopes. Randomization occurs in consecutive order at time of the first visit." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to study design, both gynecologist and dietitian know the allocation of the patient." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor knew the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 women in the control group withdrew. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Other bias | Low risk | Baseline characteristics similar. |
Petrov Fieril 2014.
| Methods | RCT conducted at 2 ANCs in Gothenburg, Sweden with recruitment from Feb 2006 to Nov 2006 and Sept 2008 to April 2009. | |
| Participants | 92 pregnant women randomised, 72 analysed. Included if singleton pregnancy < 14 weeks' gestation, no medical or obstetric complications, ability to understand Swedish. Excluded if contraindications to exercise in pregnancy. |
|
| Interventions | Intervention group received supervised resistance exercise twice a week, with light barbells and weight plates in a group setting, performed at an activity level equivalent to within moderate–to‐vigorous between weeks 14 to 25 gestation, and was self‐adjusted (n = 51). In addition, walking, cycling, water‐gymnastics, Pilates, yoga and home exercises that included pelvic floor training were recommended. Controls received a generalised exercise recommendation, a home‐based training program and a telephone (n = 41). | |
| Outcomes | Health‐related quality of life, physical strength, pain, weight, blood pressure, functional status, activity level and perinatal data. | |
| Notes | Little usable review data (birthweight only). Age (intervention, control): 30.8 [3.6]/30.6 [3.4]. Enrolment gestational age: 13 weeks. Prepregnancy BMI: 22.6 [2.5]/23 [2.6]. Authors concluded that "supervised, moderate–to vigorous resistance exercise does not jeopardize the health status of healthy pregnant women or the fetus". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned to either the intervention group or the control group (allocation ratio 1:1)", however the group sizes were significantly different (51 vs 41, respectively). |
| Allocation concealment (selection bias) | Low risk | "The research coordinator performed the randomization by using opaque sealed envelopes, which were randomly picked out before the meeting with each participant." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All data were collected at a primary health care location by an investigator who was blinded to" group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% and 18% of participants dropped out of the intervention and control groups, respectively. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen. GWG and EGWG NR. |
| Other bias | Low risk | Baseline characteristics were similar in study groups. |
Phelan 2011.
| Methods | RCT, 2 arms with individual randomisation stratified by prepregnancy weight, set in 6 obstetric offices in Providence, Rhode Island, USA from 2006 to 2008. | |
| Participants | 401 women randomised. Inclusion criteria: gestational age between 10 and 16 weeks, BMI between 19.8 and 40, nonsmoking , adults (aged > 18 yr), fluency in English, access to a telephone, and a singleton pregnancy. Exclusion criteria: major health or psychiatric diseases, weight loss during pregnancy, or a history of ≥ 3 miscarriages. |
|
| Interventions | Intervention group: standard care plus a behavioural lifestyle intervention. The Fit for Delivery intervention included a face‐to‐face visit with an interventionist at the onset of treatment who discussed appropriate weight gains during pregnancy, physical activity (30 min of walking most days of the week), and calorie goals (20 kcal/kg); emphasis was placed on decreasing high fat foods, increasing physical activity, and daily self‐monitoring of eating, exercise, and weight. Body‐weight scales, food records, and pedometers were provided to promote adherence to daily self‐monitoring. Automated postcards that prompted healthy eating and exercise habits were mailed weekly. In addition, after each clinic visit, women were sent personalised graphs of their weight gains with feedback. All women in the intervention received 3 brief (i.e. 10–15 min) supportive phone calls from the dietitian during the intervention. Women who were over‐ or under weight‐gain guidelines during any 1 month interval received additional brief, supportive phone calls (2 calls/month) that provided structured meal plans, and specific goals until weight gains returned to appropriate amounts. Control group: routine care. Women received standard nutrition counselling provided by physicians, nurses, nutritionists, and counsellors. As part of routine care women were weighed by nurses at each clinical visit; weight graphs were not provided. |
|
| Outcomes | Excessive weight gain, low weight gain, preterm birth, pre‐eclampsia or eclampsia, caesarean delivery rate, high birthweight, low birthweight, maternal weight retention. | |
| Notes | Baseline age (intervention, control): 28.8 ± 5.2, 28.6 ± 5.2. Baseline BMI (intervention, control): 26.48 ± 5.9, 26.32 ± 5.6. Enrolment gestation (intervention, control): 13.5 ± 1.8, 13.6 ± 1.8. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated in randomly varying block sizes and stratified by clinic and BMI category. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in opaque envelopes prepared by the study statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In the abstract it was stated that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 34 in the standard care group, 36 in the intervention group. Exclusions: 18 in the standard care group, 25 in the intervention group. 401 participants were randomly assigned into the intervention (n = 201) and control groups ( n = 200), included in 6 month postpartum analysis; 182 control, 176 intervention. ITT analysis was performed assuming that those lost to follow‐up were treatment failures. It was reported that this revealed almost identical results as for those completing the study (data not shown). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | The 2 study groups did not significantly differ on key baseline measures (sample stratified). |
Pinzon 2012.
| Methods | RCT conducted in Colombia with recruitment from March 2008 to November 2009. | |
| Participants | General population. 64 women randomised. Inclusion criteria: nulliparous women in gestational week 16‐20 with live fetus and normal pregnancy. Exclusion criteria: history of high BP, chronic illness, persistent bleeding after 12 weeks' gestation, poorly‐controlled thyroid disease, placenta praevia, incompetent cervix, oligohydramnios, miscarriage in previous 12 months. |
|
| Interventions | Intervention group: exercise intervention (n = 33) involved a supervised exercise program 3 times a week for 12 weeks. Exercise sessions involved walking (10 min) aerobic exercise (30 min), stretching (10 min) and relaxation exercise (10 min). Control group: usual activities (n = 31). |
|
| Outcomes | Fitness (VO2 max, BP, heart rate at rest, and other parameters) changes in blood lipids, insulin sensitivity (HOMA‐IR) and body composition (fat‐free mass, body fat, skinfold thickness and muscular area), maternal weight, birthweight, neonatal outcomes. | |
| Notes | Age (intervention, control): 19.2 [2.6]/19.5 [3.4]. Enrolment gestational age: 17.5 [3.4]/17 [4.5]. Recruitment BMI: 21.4 [2.4]/22.4 [3.8]. "Regular aerobic exercise improves endothelium‐dependent vasodilation in pregnancy." (Ramirez‐velez 2011) "At the end of the 3‐month program, there was no difference in the change in blood lipids. Triglycerides and VLDL were significantly lower in the experimental group. The experimental group showed lower values of body fat and skinfold thicknesses than did the control group, but these differentials were non‐significant." (Pinzon 2012) There was no significant difference between groups regarding maternal weight gain during pregnancy. Authors concluded that "The potential public health benefits of exercise are to great and this study supports existing guidelines indicating that latina women may begin or maintain an exercise program during pregnancy". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. "The treatment allocation system was set up so that the researcher in charge of randomly assigning participants to each group did not know in advance which treatment the participant was going to receive." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" "research assistants were blinded to the group assignment of the subjects and were in charge of the prenatal care of the women". "Due to the nature of the study, it was not possible to blind the women participating." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all study measurements were done by a person blinded to the distribution of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 women withdrew early (22%; 9 in study and 5 in control arm) therefore 50/64 women assessed for fitness outcomes. Weight and pregnancy outcome data were only available for 35/64 women (55%; 18 in study and 17 in control group). |
| Selective reporting (reporting bias) | Unclear risk | Unable to comment as study protocol not seen. |
| Other bias | Low risk | None noted. Adherence was 75%. Baseline characteristics were similar. |
Pollak 2014.
| Methods | Pilot RCT with ratio 2:1, conducted in 2 antenatal clinics in USA in 2012. | |
| Participants | 35 overweight and obese women randomised. Included if 18 years or older, English‐speaking, registered for prenatal care at participating clinics, pre‐pregnancy BMI of 25–40, 12–21 weeks' gestation, and having a cell phone with an unlimited texting plan for the next 5 months. Excluded if pre‐existing diabetes, limited mobility or inability to walk,impaired cognition or mental health with inability to provide consent. |
|
| Interventions | SMS texting intervention (n = 22) targeting weight‐related behaviours including increasing daily steps to 10000, avoiding sweetened drinks, eating 5 fruit and veg per day, and eliminating fast food intake. Tests were sent 3 times per week and women were asked to text back their self‐monitored weight measurement. Control intervention (n = 11) was a general 'text4baby' intervention with general information about pregnancy with few tests related to healthy eating or physical activity. Women were asked to wear a pedometer. |
|
| Outcomes | Weight at 40 weeks, eating habit scores, physical activity scores. Follow‐up surveys were conducted at approximately 22 and 32 weeks' gestation and women weighed. In addition, weights were obtained from clinical records from baseline and delivery. |
|
| Notes | No usable data for this review. The main results were that mean weight gain in the intervention group was estimated to be 6 pounds less than the control group at 40 weeks' gestation for women who completed the intervention (n = 23). Mean age: 29 vs 32 years. Gestation at recruitment: 16 vs 17 weeks. Pre‐pregnancy BMI: 29 vs 28 kg/m2. Investigators concluded that "SMS texting is a promising vehicle for behavior change among pregnant women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized eligible women in a 2:1 fashion." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 women withdrew from the study; however, their weight data from clinical records were included (ITT analysis). 2 others miscarried and were excluded. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen but no usable (extractable) outcome data were provided in the report. |
| Other bias | Low risk | Baseline characteristics were similar. |
Polley 2002.
| Methods | RCT, set in an obstetric clinic for low‐income women at a hospital in Pittsburgh, PA, USA. | |
| Participants | Inclusion criteria: pregnant women before 20 weeks of gestation. (Women were recruited in to 4 cells; normal and overweight, black and white.) Exclusion criteria: underweight women, younger than 18 years, 1st prenatal visit > 12 weeks' gestation, high‐risk pregnancy (i.e. drug abuse, chronic health problems, previous complications during pregnancy, current multiple gestation). |
|
| Interventions | Intervention (n = 57): the intervention was provided at regular scheduled clinic visits by staff with training in nutrition or clinical psychology. Education about weight gain, healthy eating, and exercise and individual graphs of their weight gain. Shortly after recruitment, written and oral information were given in the following area: appropriate weight gain, exercise, healthy eating. Newsletters prompting healthy eating and exercise habits were mailed bi‐weekly. After each clinic visit, women were sent a personalised graph of their weight gain. Those exceeding weight gain goals were given additional individualised nutrition and behavioural counselling using the format listed; a stepped care approach. Control (n = 53): usual care: standard nutrition counselling provided by the physicians, nurses, nutritionists and WIC counsellors. This counselling emphasised a well‐balanced dietary intake and advice to take a multivitamin/iron supplement. |
|
| Outcomes | Excessive weight gain, total weight gain, low weight gain. Low birthweight infants, macrosomia infants, preterm delivery, caesarean delivery, pre‐eclampsia, weight retention at 4 weeks' postpartum. Total weight gain was based on self‐reported prepregnancy weight and weight at last clinic visit prior to delivery. |
|
| Notes | Excessive weight gain categorised as above the IOM recommendations. Low weight gain categorised as below the IOM recommendations. IOM recommends a weight gain of 6.8‐11.3 kg for overweight women (BMI of 26‐29) and a weight gain of 6.8 kg (with no specified upper limit) for obese women (BMI > 29). Age of participants 25.5 ± 4.8. Gestational age at recruitment (intervention, control): 14.7 ± 3.1 weeks. BMI category, n (intervention, control):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly assigned to the standard care control group or to the intervention. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Using ITT approach. Loss to follow‐up < 10%. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Unclear risk | Refusal rates were higher among black women (28/74 refused) than among white (16/90 refused), and higher in overweight black women than in any of the other 3 weight‐by‐race categories. |
Poston 2013.
| Methods | A pilot RCT conducted at multiple tertiary and university hospitals in the UK with recruitment from March 2010 to May 2013. (UPBEAT study). | |
| Participants | 183 randomised, 154 analysed. Inclusion criteria: BMI ≥30 kg/m² and singleton pregnancy; gestational age > 15 weeks and < 17 weeks' gestation. Exclusion criteria: unable or unwilling to give written informed consent; gestation < 15 weeks and > 17 weeks, pre‐existing diabetes, pre‐existing hypertension (treated), pre‐existing renal disease, multiple pregnancies; systemic lupus erythematous antiphospholipid syndrome, sickle cell disease, thalassaemia, coeliac disease, currently prescribed metformin, thyroid disease or current psychosis. |
|
| Interventions | Intervention group: a lifestyle intervention (diet plus exercise) (n = 94) involving 1 1‐to‐1 session with a health trainer and then weekly group sessions for 8 consecutive weeks from 19 weeks' gestation. Sessions delivered by health trainers involved diet and exercise advice informed by psychological models of health behaviour. Dietary advice focused on increased consumption of foods with a low dietary GI, and reduction of saturated fats. Physical activity advice encouraged women to increase daily walking activity at moderate intensity level, setting goals monitored by a pedometer.
Women also received a DVD of a pregnancy specific exercise regimen. Control group: routine care (n = 89). |
|
| Outcomes | Primary outcome: GDM. Others were GWG, macrosomia, LGA, dietary and exercise parameters, anxiety and depression. | |
| Notes | Age (intervention, control): 30.4 [5.7]/30.7 [4.9]. Enrolment gestational age was 16 weeks. Recruitment BMI: 36.5 [4.7]/36.1 [4.8]. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed online" "balanced by minimisation for maternal age, centre, ethnicity, parity and BMI." |
| Allocation concealment (selection bias) | Low risk | "randomised treatment was allocated automatically" by online randomisation program. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 women (15.7%) dropped out of the control arm, and 15 lost to follow‐up in the intervention group (15.8%). |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported, except for GWG. ITT analysis. |
| Other bias | Low risk | Baseline characteristics were comparable. |
Price 2012.
| Methods | RCT at local obstetric practices in Austin, Texas, in USA from July 2006 to March 2010. | |
| Participants | General population, 91 randomised, 62 analysed. Inclusion criteria: sedentary women 12‐14 weeks' gestation; viable singleton pregnancy at 12–14 weeks by reasonable dates and/or ultrasound; body mass index (BMI) less than 39 kg/m2. Exclusion criteria: no aerobic exercise more than once per week for at least the past 6 months; chronic heart or lung disease; poorly controlled diabetes, hypertension, epilepsy, or hyperthyroidism; severe anaemia no orthopedic limitations; and history of premature delivery, infant delivered for SGA, or unexplained fetal death. |
|
| Interventions | Intervention group: exercise intervention (n = 43). Exercise intervention involved a program of supervised aerobic training (45‐60 mins, 4 days a week). Control group: sedentary women (n = 48). "Control subjects were told not to exercise." |
|
| Outcomes | Cardiorespiratory fitness. Strength, flexibility, and discomfort. Pregnancy complications, delivery data, postpartum recovery. | |
| Notes | Outcomes assessed at 5 points: 12‐14 weeks, 18‐20 weeks, 24‐26 weeks, 30‐32 weeks, and 6‐8 weeks postpartum. Age (intervention, control): 30.5 [5]/27.6 [7.3] (P = 0.08). Enrolment gestational age was at 12‐14 weeks. Prepregnancy BMI: 26.6 [3.1]/28.7 [5.4] (P = 0.042). "Previously sedentary women who began exercising at 12–14 weeks had improved fitness and delivery outcomes." Authors concluded that "Compared with women who remained sedentary, active women improved aerobic fitness and muscular strength, delivered comparable size infants with significantly fewer cesarean deliveries, and recovered faster postpartum, at least related to the lower incidence of cesarean section. Active women developed no gestational hypertension and reported no injuries related to the exercise regimen". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned." |
| Allocation concealment (selection bias) | Low risk | "Subjects were randomized using numbered, opaque envelopes containing an equal number of group assignments prepared by the study statistician." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. "Author kept a log of attendance" and seemed to perform the fitness assessments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/43 (28%) and 17/48 (35%) dropped out in the intervention and control groups, respectively. Reasons for drop outs in intervention group were logistics (9) and other reasons (3). Reasons in control group were immediate withdrawal due to wanting to exercise (5), and later withdrawal due to wanting to exercise (3) and logistics (9). |
| Selective reporting (reporting bias) | Unclear risk | Mean weight gain (12.4 kg vs 10.5 kg in intervention and control groups, respectively) was NR with standard deviations or denominators so these data were not usable in our meta‐analysis. |
| Other bias | High risk | Baseline BMI was significantly lower in the intervention group and loss to follow‐up was high. Also, "control subjects were told not to exercise because it would blur the distinction between the groups". This contributed to high drop out rates in the control group and may make results less generalisable by enforcing no exercise. This study design seems ethically flawed. |
Quinlivan 2011.
| Methods | RCT set in the maternity service of a public general hospital serving a socio‐economically disadvantaged area in Melbourne, Australia. | |
| Participants | 132 randomised. Inclusion criteria: pregnant with a fetus with no known anomalies, spoke English, did not intend to relinquish their infant, did not have a multiple gestation, were able to attend hospital for antenatal care and were overweight (BMI 25–29. 9) or obese (BMI > 29 .9). Exclusion criteria: not described. |
|
| Interventions | Intervention group: a 4‐step multidisciplinary protocol of antenatal care which had the following 4 criteria: (i) continuity of care provider; (ii) weighing on arrival; (iii) brief dietary intervention by a food technologist at every antenatal visit; and (iv) psychological assessment. Women attended special study clinics. Control group: routine care (with access to high‐risk clinics if medically indicated). |
|
| Outcomes | Weight gain, preterm delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to the intervention or control groups occurred using computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes, stratified by category (overweight or obese;16), which were only opened by the midwife after each woman’s enrolment was completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data for mother and infant were audited by a nurse independent of clinical care pathways and blinded to randomisation status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 132 randomised, 124 analysed (8 excluded from analysis (4 of each group). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were no significant differences in terms of antenatal, demographic and health behaviour variables between intervention and control groups. Women in the intervention group attended special study clinics; these clinics may have been different from standard clinics in more ways than the intended study interventions (although it was stated that care was standard apart from the 4 stage intervention). |
Rae 2000.
| Methods | RCT, set in the Diabetes Service, King Edward Memorial Hospital for Women, Perth, Western Australia | |
| Participants | Inclusion criteria: gestation < 35 weeks and 6 days, > 110% of ideal body weight for height (adjusted for expected pregnancy weight gain and using a BMI of 25 as equal to 100% ideal body weight), OGTT with fasting plasma glucose > 5.4 mmol/L and/or 2‐hour plasma glucose > 7.9 mmol/L. Exclusion criteria: not stated. |
|
| Interventions | Intervention (n = 63): the intervention comprised instruction in a moderately energy restricted diabetic diet providing between 1590‐1776 kcal (70% RDA). Control (n = 54): the control group were instructed in a diabetic diet which was not energy restricted, providing approximately 2010‐2220 kcal a day. |
|
| Outcomes | Weight gain, pre‐eclampsias, induction of labour, caesarean delivery, shoulder dystocia, birthweight > 4000 g, birthweight > 90th centile, assisted delivery. Weight gain was calculated as the difference between prepregnancy weight and delivery weight. |
|
| Notes | Age (intervention, control) 30.2, 30.6 years. Gestation at diagnosis(intervention, control) 28.1 ± 5.8, 28.3 ± 4.6 weeks. BMI at diagnosis (intervention, control) 37.9 ± 0.7, 38.0 ± 0.7 kg/m². |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Women were allocated at random using opaque numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and the Diabetes Service staff were blinded to the allocation to diet group. Medical staff were blinded to the group allocation of each participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 6.4% (8), 4 for each group. |
| Selective reporting (reporting bias) | Unclear risk | Could not be determined. |
| Other bias | Low risk | The groups were similar in level of education, employment, racial distribution, and alcohol and cigarette consumption. There were no significant differences at enrolment in weight, or energy expenditure. |
Rauh 2013.
| Methods | Cluster‐RCT, open‐label at 8 gynaecological practices in Germany from February 2010 to August 2012. | |
| Participants | General population. 250. Inclusion criteria: older than 18, 1 live fetus, < 18 weeks' gestation, BMI ≥18.5 kg/m², and able to speak sufficient German. Exclusion criteria: if they had any condition preventing physical activity, such as cervical incompetence, placenta praevia, or persistent bleeding. Prepregnancy diabetes or uncontrolled diseases that may affect weight development like thyroid dysfunction, or psychiatric diseases. |
|
| Interventions | Intervention group: lifestyle counselling (n = 167) Intervention consisted of 2 individually delivered counselling sessions focusing on diet, physical activity and weight self‐monitoring, delivered at the 20th (60 min) and 30th week (30 min) by trained researchers. Control group: routine care (n = 83). |
|
| Outcomes | The primary outcome was the proportion of pregnant women exceeding weight gain recommendations of the IOM. The secondary outcome variables were maternal weight retention at 4 months postpartum and short‐term obstetric and neonatal outcomes. | |
| Notes | Age (intervention, control): 32.2 [4.4]/30.8 [4.9]. Enrolment gestational age: 9 (8‐11)/7 (6‐8). Prepregnancy BMI: 21.7 (19.9‐23.7)/22.8 (20.6‐26.6). We were unable to adjust reported data for clustering and baseline differences, therefore we did not pool these data. The study report gave adjusted MD for GWG of ‐1.7 kg (‐3.0 to ‐0.3) in favour of the intervention, and for excessive GWG an adjusted OR of 0.5 (0.3 to 0.9). Authors concluded that "Lifestyle counselling given to pregnant women reduced the proportion of pregnancies with excessive GWG without increasing suboptimal weight gain, and may exert favourable effects on postpartum weight retention". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated allocation table." "Gynaecological practices were randomised (rather than individuals)." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was performed by a researcher not involved in the study design thereby preventing allocation bias." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to "the nature of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible for study staff due to "the nature of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss of clusters. 15/167 women in the intervention group and 9/83 control group women were lost to follow‐up. Women in the intervention group dropped out for personal reasons (5), miscarriage or late‐term abortion (3), complications of pregnancy (3) and preterm delivery (4). Control women dropped out because of personal reasons (1), moved away (1), unable to contact (2) and preterm delivery (5). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen. Appropriate analysis methods used for clusters. Results were adjusted for age and prepregnancy BMI, which was higher in the control group. ITT not stated. |
| Other bias | High risk | "During recruitment it turned out that it was easier to recruit women for the intervention group than for the control group, yielding a 2:1 ratio", instead of 1:1. Practices differed in size with 227 vs 129 women were eligible for the intervention and control groups, respectively. Baseline characteristics differed significantly with regard to pregravid BMI (P = 0.003) and BMI at booking (P = 0.008), with a higher proportion of women in the control group considered obese or overweight (16.2% vs 31.4%; P = 0.009). Mean age was younger and gestational age at booking was significantly earlier in the intervention clusters. Although results were adjusted for BMI, age and clustering, there may also have been other (unknown) differences between the women in these groups. Preterm birth and pregnancy complications NR as outcomes and these women were excluded from the analysis. |
Renault 2014.
| Methods | 3‐arm RCT at 1 hospital in Hvidovre in Denmark from March 2009 to March 2012. | |
| Participants | High risk. Obese. 425 randomised. 389 analysed. Inclusion criteria: pregnant women with a prepregnancy BMI of 30 kg/m2 or greater. Age older than 18 years, a singleton pregnancy, and a normal scan in weeks 11‐14, gestational age at inclusion of less than 16 weeks, and an ability to read and speak Danish. Exclusion criteria: multiple pregnancy, pregestational diabetes, or other serious diseases limiting their level of physical activity, previous bariatric surgery, or alcohol or drug abuse. |
|
| Interventions | Comparison of 3 groups. Intervention groups: intervention 1: physical activity and diet (n = 130); intervention 2: physical activity only (n = 125). The physical activity intervention included encouragement of increase physical activity, aiming at a daily step count of 11,000, monitored by pedometer assessment on 7 consecutive days, every 4 weeks. Dietary intervention included follow‐up on a hypocaloric Mediterranean‐style diet. Instruction was given by a dietician every 2 weeks with alternating outpatient visits and phone calls, including weight measurement, encouragement and correcting advice Control group: routine care (n = 134). |
|
| Outcomes | Primary outcome: GWG. Secondary outcomes: complications of pregnancy and delivery and neonatal outcome. |
|
| Notes | Age (intervention 1, intervention 2, control): 31.2 [4.4]/ 30.9 [4.4]/31.3 [4.2]. Enrolment gestational age was 11‐14 weeks. Prepregnancy BMI: 34.4 [4.2]/34.1 [4.4]/33.7 [3.5]. GWG was calculated from the 36‐37 week weight minus prepregnancy self‐reported weight and was reported as median (range) so could not be included in the review meta‐analysis for this outcome. GWG in the physical activity plus diet group was 8.6 kg (‐9.6 to 34.1) vs 9.4 kg in the physical activity group (‐4.4 to 28.2) vs 10.9 kg in the control group (‐4.4 to 28.7) (P = 0.024). 1 woman in the physical activity group had a placental abruption and stillbirth. Authors concluded that "No significant difference was found between the 2 intervention groups. Physical activity intervention assessed by pedometer with or without dietary follow‐up reduced GWG compared with controls in obese pregnant women. The pedometer intervention is an inexpensive methods of increasing daily physical activity and can easily be implemented into daily clinical practice". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was stratified according to parity to ensure equal distribution of primiparous in the 3 groups." |
| Allocation concealment (selection bias) | Low risk | "Web allocation by an independent organization properly concealed the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates for most outcomes. However, physical activity scores had > 20% missing data and we considered these data high risk. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline characteristics were comparable. Good compliance. |
Rhodes 2010.
| Methods | RCT (pilot study), set in Beth Israel Deaconess Medical Center, Boston, MA, and Children’s Hospital Boston, Boston, MA, USA. | |
| Participants | Inclusion criteria: pregnant women with prepregnancy or 1st trimester BMI equal to or greater than 25 kg/m² and less than 45 kg/m², singleton pregnancy, willing to consume the diets for duration of pregnancy, participant to be at week 28 or less of pregnancy at baseline visit. Exclusion criteria: smoking during pregnancy, major medical illness (e.g. diabetes mellitus, hypertension, thyroid disease), taking prescription medication known to affect body weight, alcohol consumption during pregnancy, intention to deliver infants in the environment outside of Beth Israel Deaconess MedicalCenter, Boston, high level of physical activity. |
|
| Interventions | Intervention group 1: nutrition education, dietary counselling, and a low‐GI diet. Intervention group 2: nutrition education, dietary counselling, and a low‐fat diet. |
|
| Outcomes | Maternal outcome: weight change. Infant outcome: macrosomia, large‐for‐gestational age, caesarean delivery. |
|
| Notes | Age (intervention, control): 33.7 [3.9]/33.2 [3.7]. Enrolment gestational age: 19.8 (5)/19.6 (4.3). Prepregnancy BMI (intervention, control): 32.1(4.6)/ 31.2 (3.1). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly “permuted blocks of 2 and 4 preventing anticipation of future assignments. |
| Allocation concealment (selection bias) | Low risk | Separate random assignment envelopes for each stratum. Random assignment envelopes were prepared by the hospital clinical trials unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Formal blinding of participants was not possible, although participants were not informed of their group assignments. The following staff were blinded to group assignment: obstetricians who provided clinical care to women; nurses who measured maternal body weight and blood pressure, collected and processed maternal blood samples, and analysed urinalyses; labour and delivery room nurses who obtained birthweight; laboratory staff who analysed maternal blood; and staff who performed data entry. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff who performed maternal body composition analysis, 24‐h dietary recalls, and infant anthropometric measurements “were predominantly, but not always”, blinded due to logistical considerations. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 46 women were randomised and infant outcomes were available for 45. There was some loss to follow‐up among women with outcome data at 36 weeks available for 38. Reasons for loss were explained and loss was reasonably balanced across groups. It was stated that analysis was by randomisation group irrespective of whether or not women received the intended intervention. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Baseline characteristics of participants did not differ between intervention groups. |
ROLO 2012.
| Methods | RCT conducted at a maternity hospital in Ireland with recruitment from January 2007 to January 2011. | |
| Participants | 800 randomised, 759 analysed. Inclusion criteria: secundigravid women whose first baby was macrosomic (birthweight > 4.0 kg). Exclusion criteria: underlying medical disorders, previous history of gestational diabetes, those on any drugs, and those unable to give full informed consent, age less than 18 years, gestation greater than 18 weeks, and multiple pregnancy. |
|
| Interventions | Low GI dietary intervention (n = 394 ) involving 1 dietary education session lasting 2 hours in groups of 2‐6 women with a dietitian at baseline. Follow‐up reinforcement sessions were held at 28 and 34 weeks' gestation. Women received written resources about low GI foods. Control group: routine care (n = 406). |
|
| Outcomes | Primary outcome: birthweight. Secondary outcomes: GWG, maternal GI and dietary intake. |
|
| Notes | GTT was done at 28 weeks' gestation. Women completed food diaries. Other outcomes were recorded on delivery.Age (intervention, control): 32 [4.2]/32 [4.2]. Enrolment gestational age: 13 [2.3]/12.9 [2.2]. Recruitment BMI: 26.8 [5.1]/26.8 [4.8]. Glucose challenge test at 28 weeks showed significantly less women in the intervention group vs control group with glucose intolerance defined as GCT > 7.8 mmol/L (54/350 [15%] vs 79/371 [21%]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The research midwife did the randomisation by using computer generated allocations in a ratio of one to one." |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates for most outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Excluded women who did not receive allocated intervention (i.e. per protocol analysis). |
| Other bias | Low risk | Baseline characteristics were comparable. Good compliance. |
Ronnberg 2014.
| Methods | Registered, open‐label, parallel assignment RCT conducted in Sweden. ID NCT00451425. | |
| Participants | General population: 445 women randomised, 374 analysed. Included if > 18 years old, Swedish speaking, BMI ≥ 19, gestation < 16 weeks, and healthy. Excluded if history of eating disorders or history of previous growth restricted child. |
|
| Interventions | Intervention group: a motivational exercise intervention that included a personalised weight graph, GWG monitoring at each antenatal visit, and prescribed exercise to be at a "moderate level of exertion for approximately 30 min/day". The intervention was administered at routine antenatal visits by midwives. Control group: standard care. All women received basic dietary advice according to Swedish national guidelines. | |
| Outcomes | GWG (1990 IOM guidelines), weight retention up to 1ne year, fetal and maternal complications in pregnancy and during delivery, infant birthweight and infant weight up to 1 year of age. | |
| Notes | Age: 29.9 [4.5]/ 29.9 [4.8]. Enrolment gestational age: 10.7 vs 9.7 weeks. 72% normal weight women, 28% overweight or obese. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated in blocks of variable sizes between 4 and 8." |
| Allocation concealment (selection bias) | Low risk | "Sealed, opaque, sequentially numbered envelopes were kept by administrative personnel not related to the study." A research assistant opened the next envelope and informed the midwife telephonically of each group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 8% in the intervention group and 11% in the control group. ITT analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Only GWG outcomes reported. Other maternal and fetal outcomes were stated as having "no significant difference between groups". Unpublished data requested. |
| Other bias | Unclear risk | Gestational age at recruitment was statistically significantly different (10.7 weeks vs 9.7 weeks, for intervention and control groups, respectively). |
Ruchat 2012.
| Methods | RCT of 2 interventions compared with historical control in Canada. | |
| Participants | General population, 73 recruited, 49 analysed. Inclusion criteria: normal weight women with singleton pregnancies between 16 and 20 weeks' gestation. Exclusion criteria: specific exclusion criteria included the following: maternal age < 18 years or > 40 years, smoking, multiple pregnancy, presence of chronic disease, or other contraindications to exercise. |
|
| Interventions | 2 lifestyle interventions vs an historical control. Intervention groups: interventions were moderate‐intensity exercise plus diet (n = 26), vs low‐intensity exercise plus diet (n = 23). The low‐intensity exercise intervention consisted of a walking program that corresponded with 30% HRR (oxygen consumption reserve) whereas the moderate‐intensity program corresponded with a 70% HRR. The exercise was performed 3 to 4 times per week, with 1 session per week supervised. Participants wore an HR monitor to ensure they were exercising within the predetermined target HR zone. Control group: control group were 45 normal weight women with singleton pregnancies who did not participate in a structured exercise program. All participants received a diet plan based on a modified diabetic diet. |
|
| Outcomes | GWG, maternal weight retention 2 months postpartum, infant birthweight. | |
| Notes | We only included data from the randomised participants, i.e. the low intensity intervention vs moderate intensity intervention. As control group was not randomised, we did not use these data. Age (intervention 1, intervention 2): 30.4 [4.5]/ 31 [3.8]. Enrolment gestational age: 17 [1.5]/17.5 [1.5]. Prepregnancy BMI: 21.7 [1.9]/22.1 [1.7]. Diet outcomes were collected between 34 and 36 weeks' gestation. Women were weighed weekly in the laboratory. Delivery and newborn data were obtained from medical records and complication during delivery were obtained from maternal recall within 6‐18 hours after delivery. Authors concluded that "Results suggest that a prenatal nutrition exercise program regardless of exercise intensity, reduced excessive GWG and decreased weight retention at 2 months postpartum in women of normal weight before pregnancy". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All the participants were randomised using a block procedure with four subjects per block." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described. Exercise and diet logs were recorded by the participants at home. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 49 participants (67%) out of 73 completed the study. Even if 1 excludes the 7 women who "decided to withdraw after the peak exercise test" (before randomisation), withdrawal is still > 20%. Similar numbers withdrawn from both groups. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | High risk | Control group was historical, not randomised; therefore, these data were not used in the review. See Potential biases in the review process. |
Ruiz 2013.
| Methods | RCT at 3 primary care hospitals in Madrid, Spain, from September 2007‐January 2011. | |
| Participants | General population, 962 randomised and analysed. Inclusion criteria: women who were sedentary (not exercising for > 20 mins on > 3 days a week), with singleton and uncomplicated gestations, not at high risk of preterm delivery (i.e.. previous preterm birth) and not participating in any other trial were able to participate in this study. Exclusion criteria: women with any obstetric contraindication to exercise were not eligible to participate. |
|
| Interventions | Intervention group: exercise intervention (n = 481). The intervention included light‐ to moderate‐intensity aerobic and resistance exercises performed 3 days a week (50‐55 mins per session) from 9 weeks to weeks 38‐39. Exercise sessions included 8‐10 participants. Control group: routine care (n = 481). |
|
| Outcomes | Primary outcome: GWG. Secondary outcomes: Maternal and fetal outcomes, including birthweight, gestational age, type of delivery (caesarean, etc), Apgar scores, childbirth, gestational diabetes and hypertension. | |
| Notes | Age (intervention, control): 31.6 [4]/ 31.9 [4]. Enrolment gestational age was 5‐6 weeks. Prepregnancy BMI: 23.7 [3.9]/23.5 [4.2]. GWG was calculated from measured weight at last visit minus weight at first prenatal visit. Other outcomes were obtained from hospital records after delivery. Authors concluded that "Supervised exercise of light to moderate intensity can be used to prevent excessive gestational weight gain, especially in normal weight women. The effects seemed to be more pronounced in normal weight women…". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14% attrition overall. 68 women in the control arm were lost to follow‐up because of threat of premature delivery (n = 11), persistent bleeding (n = 9), a move to another hospital (n = 20), or miscellaneous personal reasons (n = 28). 70 women in the intervention arm were lost to follow‐up due to threat of premature delivery (n = 14), persistent bleeding (n = 7), a move to another hospital (n = 25), or miscellaneous personal reasons (n = 24). No protocol deviations. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Other bias | Low risk | None noted. Baseline characteristics were comparable. |
Santos 2005.
| Methods | Randomised clinical trial, set in a referral centre prenatal clinic in Porto Alegre, Brazil, during the period 2000–2002. | |
| Participants | Inclusion criteria: healthy, nonsmoking pregnant women, aged 20 years or more, of gestational age less than 20 weeks, having a BMI between 26 and 31 kg/m² (corresponding to a prepregnancy BMI of 25‐30 kg/m²) (overweight), and without diabetes or hypertension. Exclusion criteria: not stated. |
|
| Interventions | Intervention (n = 37): the intervention consisted of a program of supervised physical exercise of 60 mins duration, performed 3 times per week for 12 weeks. Each session consisted of 5‐10 mins of warm up, 30 mins of heart rate‐monitored aerobic activity, 10‐15 mins of exercise involving upper and lower limbs, and 10 mins of stretching and relaxation. Aerobic activities were always performed between 50% and 60% of the maximum predicted heart rate, never exceeding 140 beats per min. The exercises followed the recommendations concerning physical activity practice during pregnancy according to the American College of Sports Medicine, and the ACOG. Control (n = 35): the control group participated in once‐weekly sessions that included relaxation (respiratory exercises and light stretching but no aerobic or weight‐resistance exercises) and focus group discussions concerning maternity. Control participants were neither encouraged to exercise nor discouraged from exercising. |
|
| Outcomes | Weight gain, low birthweight, prematurity. Weight gain was calculated from different between weight at baseline and weight after 12 weeks of intervention. |
|
| Notes | At baseline; age (exercise, control) 26.0 ± 3.4, 28.6 ± 5.9 years. BMI (exercise, control) 28.0 ± 2.1, 27.5 ± 2.1 kg/m². Gestational age (exercise, control) 17.5 ± 3.3, 18.4 ± 3.9 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised following a blocked sequence generated from a random number table by a statistician not participating in other aspects of the study. |
| Allocation concealment (selection bias) | Low risk | The study co‐ordinator implemented the randomisation by using numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The same cardiologist, blinded to treatment allocation, performed both tests. The anaerobic threshold was determined by review of the gas exchange curves by 2 cardiologists working independently and blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 22%, 19.6%, and 23.9% in intervention and control groups . |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | High risk | Women in the intervention group were somewhat younger, had higher physical activity, and were earlier in their pregnancy. |
Stafne 2012.
| Methods | RCT conducted at St Olavs Hospital, Trondheim University Hospital, and Stavanger University Hospital in Norway with recruitment from April 2007 to June 2009. | |
| Participants | 855 randomised, 702 analysed. Inclusion criteria: white women aged 18 years or older with a singleton live fetus. Exclusion criteria: high‐risk pregnancies or diseases that could interfere with participation (or both). For practical reasons, women who lived too far from the hospitals to attend weekly training groups (more than 30‐min drive) were excluded. |
|
| Interventions | Intervention group: 12 weeks regular standardised exercise program including aerobic activity, strength training, and balance exercises. The exercise program followed standard recommendations and included moderate‐intensity to high‐intensity activity 3 or more days per week. Physiotherapist‐supervised training sessions of 60 mins in groups of 8‐15 women were offered once per week. Control group: routine care (women were not discouraged from exercising). |
|
| Outcomes | Primary outcome: GDM, insulin resistance. Secondary outcomes: maternal weight, BMI, newborn weight, gestational age, Apgar score. |
|
| Notes | Baseline assessment at 18‐22 weeks, outcomes assessed at 32 to 36 weeks' gestation. Age (intervention, control): 30.5 [4.4]/ 30.4 [4.3]. Enrolment gestational age was 18‐22 weeks. Recruitment BMI: 24.7 [3]/25 [3.4]. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Concealed randomization in blocks of 30 was performed at the Unit for Applied Clinical Research, by a Web‐based computerized procedure." |
| Allocation concealment (selection bias) | Low risk | "concealed randomisation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the nature of the study it was not blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of primary outcome (insulin resistance) "was blinded for group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% dropout overall with more dropouts in the control group (24% vs the intervention group 13%). |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. ITT performed. |
| Other bias | Low risk | None noted. Baseline characteristics similar. |
Szmeja 2011.
| Methods | RCT conducted in Adelaide, Australia. Study period not stated. | |
| Participants | HIgh risk. 193 women included. Inclusion criteria: women with a BMI ≥ 25 kg/m2 and a live singleton pregnancy between 10 and 20 weeks' gestation. Exclusion criteria not stated. |
|
| Interventions | Intervention group (98 women): received an "informational DVD". Control group (95 women): "No DVD". |
|
| Outcomes | Self‐reported knowledge of health dietary and lifestyle choices, satisfaction with pregnancy care. | |
| Notes | No statistically significant differences reported. Authors concluded that the intervention was not effective. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Appear to be self‐reported outcomes only. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement. |
| Other bias | Unclear risk | This report has not been published in full, possibly because the intervention was not effective. |
Thornton 2009.
| Methods | RCT, set in the ambulatory obstetric clinics of 3 tertiary care medical centres ‐ Morristown Memorial Hospital, St Luke’s‐Roosevelt Hospital Center, and Jamaica Hospital Medical Center. Each study site was an urban, public clinic of a teaching hospital, New York Medical College. | |
| Participants | Inclusion criteria: pregnant with a single fetus between 12 and 28 weeks of gestation that had a BMI greater than or equal to 30 kg/m². Exclusion criteria: women with pre‐existing diabetes, hypertension, or chronic renal disease. |
|
| Interventions | Intervention (n = 116): monitored group; counselled in nutrition by a registered dietitian and given a more detailed dietary intake protocol. The nutrition program for the monitored women followed dietary guidelines similar to those used in women with the diagnosis of gestational diabetes. The women in this group were asked to record in a diary all of the foods and beverages eaten during each day. Control (n = 116): unmonitored group; counselled in nutrition by a registered dietitian regarding conventional prenatal nutrition guidelines. |
|
| Outcomes | Weight gain, weight retention (calculated from the difference between weight at 6 weeks' postpartum and weight at baseline). GDM, pre‐eclampsia, gestational hypertension, haemorrhage/infection postpartum, preterm delivery (< 37 weeks), labour induction, caesarean delivery, macrosomal infant (> 4500 g). Weight gain was weight difference between the baseline(12‐28 weeks) pregnancy weight and weight before delivery. |
|
| Notes | Age (intervention, control) 26.8, 27.3. BMI (intervention, control) 37.41 ± 7.01, 38.22 ± 7.48 kg/m². |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random‐number table was used to assign each consecutively numbered envelope to either the study or control group in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Envelopes were prepared and sequentially numbered. A card indicating the assigned group was placed in the envelope, and the envelope was sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The ITT principle was performed. Loss to follow‐up 9.7%. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Demographic data for the randomised groups were comparable. |
Vesco 2013.
| Methods | Parallel‐arm RCT conducted by Harvard Medical School, USA. | |
| Participants | 160 planned, 114 randomised so far. Inclusion criteria: women aged 18 or older, who were obese at the beginning of pregnancy (BMI ≥ 30 kg/m²); 10‐20 weeks' gestation. Exclusion criteria: gestational age > 20 weeks, multifetal pregnancy, anticipated disenrolment prior to delivery, non‐English speaking, plans to move within the year, type 1 or 2 diabetes mellitus (or gestational diabetes), and other medical conditions that require specialised nutritional care (e.g. history of bariatric surgery) or conditions that may affect weight gain (e.g. severe hyperemesis gravidarum). |
|
| Interventions | Intevention group: lifestyle intervention (diet plus exercise) (n = 56). Following randomisation, all participants receive a 45‐min dietary consultation. They were encouraged to follow the Dietary Approaches to Stop Hypertension diet (DASH) without sodium restriction and received an individualised calorie intake goal, a second individual counselling session and attend weekly group meetings (90 min) with weigh‐ins, food and activity logs until they give birth. Women are encouraged to accumulate at least 30 min of moderate intensity activity per day. Pedometers recorded steps with at target of 10,000 steps daily and were only provided to the intervention group. Control group: usual care (n = 58) with a 1‐time advice session about healthy eating. |
|
| Outcomes | Primary outcomes: GWG, excessive weight gain, postpartum weight retention at 1 year (mean difference between postpartum and baseline weight), proportion of LGA neonates. Secondary outcomes: feasibility and acceptability of the intervention, maternal dietary intake and physical activity, and infant birthweight, feeding patterns, and growth during the first year of life. |
|
| Notes | Median age (intervention, control): 29 (27‐32), 29 (26‐31). Mean BMI 36.7 (5.2) / 36.8 (4.7). 21% of sample had BMI > 40 kg/m2. Data collection at 34‐weeks' gestation and at 2‐weeks' and 1‐year postpartum. Enrolment at 10‐20 weeks' gestation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation ...using a computerised algorithm to generate the random assignments in blocks of four." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Good follow‐up achieved. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported. ITT analysis. |
| Other bias | Unclear risk | Follow‐up weight gain data were collected at 34 weeks (i.e. earlier than most other included studies). |
Vinter 2012.
| Methods | RCT conducted at 2 university hospitals in Odense, Denmark from October 2007 to October 2010. | |
| Participants | 360 randomised, 304 analysed. Included obese women aged 18‐40 years, 10‐14 weeks' gestation, BMI of 30‐45kg/m2. Excluded if chronic medical conditions, prior serious obstetric complications, alcohol/drug abuse, non‐Danish speaking, multiple pregnancy, positive GTT. |
|
| Interventions | Intervention group: a lifestyle intervention consisting of dietary counselling and exercise (n = 180). The intervention involved dietary advise on 4 occasions (15, 20, 28 and 35 weeks) by a dietician. Energy requirements were personalised for each participant. Exercise intervention included a pedometer, free gym membership for 6 months, encouraged to do 30‐60 min moderate physical activity daily. At the gym they had closed training classes with a physiotherapist for 1 hour each week. Control group: routine care (n = 180). |
|
| Outcomes | GWG, pre‐eclampsia, hypertension, GDM, caesarean section, macrosomia, LGA, admission to NICU, maternal weight retention at 6 months postpartum, infant and childhood weight. | |
| Notes | GWG was defined as weight gain at 35 weeks. Obstetric and neonatal outcomes were obtained from medical records. Childhood weight not yet reported. Age (intervention, control): 29 (27‐32)/29 (26‐31). Enrolment gestational age was 10‐14 weeks. Recruitment BMI: 33.4 (31.7‐36.5)/33.3 (31.7‐36.9). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated numbers" 1:1, stratified for smoking. |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 56/360 (15%) dropped out for primary outcomes. Similar between groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | None noted. Baseline characteristics comparable. |
Vitolo 2011.
| Methods | RCT (pilot study), set in primary care settings in Porto Alegre, Brazil. | |
| Participants | Inclusion criteria: gestational age between 10 and 29 weeks; women attending the prenatal care unit of the health unit. Exclusion criteria: positive testing for HIV, previous diagnostic of diabetes, hypertension, anaemia or another condition that needed a special diet and age over 35 years. |
|
| Interventions | Intervention group: (159 women) weight and diet were assessed at recruitment. The aim of the intervention was to improve diet and encourage weight‐appropriate weight gain in pregnancy. For low weight women, the priority was increasing the energetic density of the meals. For normal weight women, daily consumption of vegetables, greens, fruit and water were encouraged and women were advised to restrict consume of fat‐rich foods and oil in cooking. For the overweight women, the intervals between meals were prioritised and women were encouraged to restrict their consumption of snacks. Women received a further interview 1 month later to reinforce messages. Control group: (162 women) women did not receive any special intervention but were informed about their weight and nutritional status and advised to seek professional help if they were under or overweight. Their doctors were also provided with the results of the nutritional evaluation. |
|
| Outcomes | Weight gain. | |
| Notes | Reported average weekly weight gain which was significantly lower in the overweight women in the intervention group than the control group (342.2 g [143.6[ vs 420 g [185.4]; P = 0.01). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised by means of a dark pouch with 2 equal sized cubes containing the term intervention in 1 and control in the other. |
| Allocation concealment (selection bias) | Unclear risk | 2 cubes were concealed in a dark pouch and 1 was removed at the point of randomisation which indicated allocation. (It is possible that this could be changed by the person carrying out randomisation.) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 315 women accepted participation in the study.There were 307 women with anthropometric data collected in the last trimester. |
| Selective reporting (reporting bias) | Unclear risk | The results relate to the number of women with excessive or low weight gain at different gestational ages. |
| Other bias | Unclear risk | 'Risk of bias' assessment from translated notes. |
Wilkinson 2012.
| Methods | RCT conducted at a maternity hospital in Australia with recruitment from August 2010 to March 2011. | |
| Participants | Randomised 360, analysed 242. Included if attending their booking visit at the MH research site and were 18 years or older (or under 18 years, with the consent of a parent or guardian). Excluded if unable to read and speak English at a level that allowed completion of pen‐and‐paper surveys. |
|
| Interventions | Intervention group: a lifestyle intervention (diet plus exercise, smoking cessation, etc) (n = 178) consisting of a 1‐hour 'Healthy start to pregnancy' workshop with a 12 page booklet with evidence based information, with goal setting and self‐monitoring activities. Control group: routine care (n = 182). |
|
| Outcomes | Good nutrition, PA, GWG awareness, number of cigarettes smoked, BMI. | |
| Notes | Outcomes assessed at recruitment and 12 weeks later at around 26 weeks. Age (intervention, control): 29.5 [5.1]/29 [4.7]. Enrolment gestational age: 14.5 [3]/14.2 [3]. Prepregnancy BMI: 25.4 [5.2]/24.6 [5.5]. ITT analysis showed increased weekly activity scores in mins in the intervention group. Only per protocol analyses showed significant differences in diet quality and mean servings of vegetables and fruit in the intervention group at the follow‐up interview (26 weeks). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerized randomisation process." |
| Allocation concealment (selection bias) | Low risk | "allocation was concealed using sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not described. Most outcomes, including activity scores and food consumption were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only half the women attended the workshop, and overall response at the second assessment was 67%. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. Reported ITT and per protocol analyses. Few review outcomes reported. Little usable data. |
| Other bias | Low risk | Baseline characteristics overall were comparable. |
Wolff 2008.
| Methods | RCT, set in Copenhagen, Denmark. | |
| Participants | Inclusion criteria: pregnant obese women (BMI > 30 kg/m²), nondiabetic non‐smoking and Caucasian recruited at 15 ± 3 weeks of gestation. Exclusion criteria: smoking, age < 18 or > 45, multiple pregnancy, or medical complication. |
|
| Interventions | Intervention (n = 23): restriction of GWG to 6‐7 kg by 10 consultation of 1 hour each with trained dietitian. The women were instructed to eat a healthy diet according to the official Danish dietary recommendations (% fat, protein, CHO, 30, 15‐20, 50%‐55%). The energy intake was restricted based on individually estimated energy requirements and estimated energetic cost of fetal growth. Control (n = 27): the control group had no consultations with the dietitian and had no restrictions on energy intake or GWG. All participants followed the routine clinical schedule. |
|
| Outcomes | Weight gain, weight retention at 4 weeks' postpartum, pre‐eclampsias, caesarean delivery. Weight gain was calculated as difference between self‐reported prepregnancy weight and weight just before delivery. |
|
| Notes | Age (intervention, control) 28 ± 4, 30 ± 5 years. Gestational age (intervention, control) 15 ± 2, 16 ± 3 weeks. BMI at inclusion visit (intervention, control) 34.9 ± 4, 34.6 ± 3 kg/m². |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The computerised randomisation took place after the women had given written informed consent. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The physicians and midwives were blinded in regard to the treatment assignment, and women were asked not to reveal their allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 24%: 17.8% in intervention group, 28.9% in control group. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline characteristics were similar. |
ACOG: the American Congress of Obstetricians and Gynecologists ANC: antenatal clinic BMI: body mass index BP: blood pressure CHO: carbohydrate GDM: gestational diabetes mellitus GI: glycaemic index GWG: gestational weight gain HbA1C: haemoglobin A1C HGI: high glycaemic index Hi‐Lo: high‐low exercise IOM: Institute of Medicine ITT: intention‐to‐treat LGA: large‐for‐gestational age LGI: low glycaemic index Lo‐Hi: low‐high exercise min: minutes Mo‐Mo: moderate‐moderate exercise n: number NICU: neonatal intensive care unit NR: not reported OGTT: oral glucose tolerance test QoL: quality of life RCT: randomised controlled trial RDA: recommended dietary allowance SD: standard deviation SGA: small‐for‐gestational age VLDL: very low‐density lipoprotein vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asemi 2011 | RCT of effect of probiotic yogurt during pregnancy on inflammatory factors, lipid profiles, oxidative stress and insulin resistance, not on preventing excessive weight gain. |
| Bechtel‐Blackwell 2002 | Quasi‐RCT. |
| Boileau 1968 | Irrelevant intervention. |
| Breslow 1963 | Non‐RCT. |
| Campbell 2004 | Participants included pregnant and nonpregnant women. |
| Daley 2014 | Abstract of the process evaluation of an RCT. |
| Davenport 2011 | Quasi‐RCT of a target weight program intervention in pregnancy. |
| Faucher 2008 | It was not clear that women in this study were pregnant (community weight loss intervention). |
| Graham 2014 | Formative research for e‐Mons study not RCT results. |
| Gray‐Donald 2000 | Non‐RCT. |
| Hauner 2012 | RCT omega‐3 fatty acids on infant adipose tissue, not an intervention to reduce excessive weight gain in pregnancy. |
| Hausenblas 2008 | Participants included both pregnant and postpartum women. |
| Ismail 1990 | Not a relevant intervention.This study examined the use of cefoxitin (an antibiotic) for the prevention of post‐cesarean‐section infection. |
| Kinnunen 2007 | Non‐RCT. |
| Lindsay 2014 | Abstract of RCT (probiotics) without gestational weight gain outcomes. |
| Maitland 2014 | Abstract of a pilot cross‐over study of only 16 women to evaluate a dietary supplement. |
| Mohebi 2009 | Quasi‐RCT protocol. |
| Moses 2006 | Quasi‐RCT. |
| Mottola 2010 | Not a RCT. |
| Olson 2004 | Non‐RCT. |
| Silverman 1971 | Irrelevant intervention. |
| Stutzman 2010 | Quasi‐RCT on the effect of exercise on blood pressure and heart rate variability, not excessive weight gain. |
| Te Morenga 2011 | This study did not include pregnant women. |
| Walker 1966 | Non‐RCT. |
| Wisner 2006 | Participants were postpartum women. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Shakeri 2012.
| Methods | This study requires translation from Farsi before it can be classified. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Astrup 2013.
| Trial name or title | An optimised programming of healthy children (APPROACH). |
| Methods | RCT, single blind, parallel assignment. Location: Denmark. |
| Participants | 390 obese pregnant women. |
| Interventions | High‐protein/low‐GL diet vs low‐protein/high‐GL diet. |
| Outcomes | Primary: GWG. changes in bodyweight, body composition, body fat. Secondary: growth and development of fetus. |
| Starting date | 01/11/2013. |
| Contact information | Nina RW Geiker, nina.rica.wium.geiker@regionh.dk, Copenhagen University Hospital at Herlev. |
| Notes | Estimated completion date: December 2015 (final data collection date for primary outcome measure). |
Atkinson 2013.
| Trial name or title | Be healthy in pregnancy (B‐HIP): an RCT to study nutrition and exercise approaches for healthy (BHIP). |
| Methods | RCT, 2‐arm randomised 1‐site trial, parallel assignment, single blind. Location: Canada. |
| Participants | 110 women with singleton pregnancies. Excluded: currently breastfeeding previous child, pregnancy resulting from in vitro fertilisation, and others. |
| Interventions | Nutrition (energy high protein, low fat diet) and exercise (aerobic) vs standard prenatal care. |
| Outcomes | GWG within IOM guidelines, maternal and infant outcomes. |
| Starting date | 01/07/2012. |
| Contact information | Stephanie A Atkinson, PhD, satkins@mcmaster.ca, McMaster University. |
| Notes | Estimated completion date: November 2016 (final data collection date for primary outcome measure). |
Barakat 2012.
| Trial name or title | Effect of a supervised exercise program in obese and overweight pregnant women on outcomes and level of depression. |
| Methods | RCT. Location: Spain. |
| Participants | 150 obese and overweight pregnant women. Inclusion criteria · Being healthy and able to exercise following American College of Obstetricians and Gynecologists (ACOG) guidelines. · Being able to communicate in Spanish. · Giving birth at Hospital Universitario de Fuenlabrada. · Having a BMI greater than 24.9. Exclusion criteria · Multiparity. · Obstetrician complications. · Being interested in the study after 18 weeks. · Not being regular in physical exercise program (minimum adherence 80%). · Younger than 18 years old. |
| Interventions | Behavioural: exercise group. Supervised physical conditioning program of 3, 55‐60 minute sessions per week during whole pregnancy (from week 9 to 38). Each session consists of 25‐30 minutes of cardiovascular exercise, 10 minutes of specific exercises (strength and balance exercises), and 10 minutes of pelvic floor muscles training. Aerobic activity was prescribed at light‐to‐moderate intensity, aiming for 55‐60 of maximal heart rate. All women wore a heart rate (HR) monitor (Polar FT7) during the training sessions to ensure that exercise intensity was light‐to‐moderate. |
| Outcomes | Primary outcomes: change from level of depression at the end of the pregnancy [Time frame: Up to 36 weeks] The Center for Epidemiological Studies‐Depression scale (CES‐D) was administered to all obese and overweight pregnant women at the beginning and at the end of their pregnancies. Secondary outcomes · Maternal GWG [Time frame: 40‐42 weeks]. · Maternal outcomes [Time frame: after labour]. · Fetal weight [Time frame: after labour]. |
| Starting date | October 2009. |
| Contact information | Ruben Barakat,PhD, barakatruben@gmail.com, Universidad Politécnica de Madrid, Spain. |
| Notes | Final data collection date for primary outcome measure, January 2013. |
Brownfoot 2011.
| Trial name or title | Weighing in pregnancy. |
| Methods | RCT. |
| Participants | Pregnant women attending for antenatal care at < 20 weeks' gestation 18‐45 years. Excluded multiple gestation or medical or psychiatric illness. (Target sample size 650.) |
| Interventions | Weighing as part of each antenatal visit compared with routine care. |
| Outcomes | Weight gain within IOM and WHO recommendations; medical complications of pregnancy (pre‐eclampsia, hypertension, gestational diabetes); need for induction of labour; mode of delivery; postpartum complication; infant birthweight; macrosomia and complications relating to macrosomia. |
| Starting date | 1st January 2010. |
| Contact information | fiona.brownfoot@thewomens.org.au |
| Notes | Emailed 22/9/14. |
Callaway 2012.
| Trial name or title | Probiotics for the prevention of gestational diabetes in overweight and obese women. |
| Methods | RCT, placebo, parallel, blinded. Location: Australia. |
| Participants | 640 obese and overweight women. 18 to 50 years. Excluded: gestation > 16 weeks, pre‐existing diabetes, pre‐existing impaired fasting glucose or impaired glucose tolerance, GDM on early screening, and others. |
| Interventions | Diet (administration of probiotics) vs placebo. |
| Outcomes | Gestational diabetes. GWG. pre‐eclampsia, induction of labour, caesarean section. neonatal outcomes, biochemical and other outcomes. |
| Starting date | 01/06/2012. |
| Contact information | Prof Leonie Callaway, l.callaway@uq.edu.au, Royal Brisbane and Women's Hospital. |
| Notes |
Chasan‐Taber 2009.
| Trial name or title | A RCT of prenatal physical activity to prevent gestational diabetes: design and methods. |
| Methods | RCT. A blocked randomisation is used such that both treatment groups are assigned an equal number of times in each set of 4 sequentially enrolled women. |
| Participants | Location: Bay State Medical Center in western Massachusetts. Target number of participants: 364. Inclusion criteria: women are sedentary, with a diagnosis of GDM in a prior pregnancy defined according to American Diabetes Association (ADA) criteria. Exclusion criteria: age < 18 or > 40 years, history of diagnosis of diabetes outside of pregnancy, hypertension, heart disease or chronic renal disease, current medications that adversely influence glucose tolerance, > 16 weeks' gestation, contraindications to participating in moderate‐physical activity, inability to read English at a 6th grade level, self‐reported participation in > 30 minutes of moderate‐intensity or vigorous‐intensity exercise on > 3 days/week, and non singleton pregnancy. |
| Interventions | Exercise intervention: person education on exercise followed by weekly, biweekly and monthly mail and telephone follow‐up. Health and wellness intervention: person education health and wellness followed by weekly and monthly mail and telephone follow‐up. |
| Outcomes | Maternal weight gain (change in weight from pregravid to delivery), birthweight, Apgar score, caesarean delivery, macrosomia (> 4000 g) and LGA, defined as newborn weight the 90th percentile for completed gestational weeks using cutoff points defined by Oken. |
| Starting date | April 2010. |
| Contact information | Lisa Chasan‐Taber; lct@schoolph.umass.edu Megan Ward Harvey, meward@schoolph.umass.edu |
| Notes | 1 paper has been published (Hawkins 2014). It found that the exercise arm had significantly greater increases in sports or exercise activity. Findings on GWG and neonatal outcomes are pending. |
Chasan‐Taber 2013.
| Trial name or title | Lifestyle intervention in overweight and obese pregnant Hispanic women. |
| Methods | RCT, parallel, double blind. Location: USA. |
| Participants | Estimated enrolment 333. Overweight or obese Hispanic women 16‐45 years old. Excluded if type 2 diabetes, heart disease, or chronic renal disease. multi‐gestational, and other exclusions. |
| Interventions | PA and diet intervention materials and health education vs standard prenatal care. |
| Outcomes | Insulin resistance, GWG, postpartum weight loss, postpartum cardiovascular risk and others. |
| Starting date | 01/01/2014. |
| Contact information | Lisa Chasan‐Taber, ScD, lct@schoolph.umass.edu, University of Massachusetts, Amherst |
| Notes | Estimated completion date: 01/12/2018. |
Choi 2011.
| Trial name or title | A pilot study of a mobile phone‐based physical activity program in pregnant women. |
| Methods | RCT, parallel, single blind. Location: USA. |
| Participants | 30 physically inactive women at 10 ‐ 20 weeks' gestation with pre‐pregnancy BMI >= 18.5. Excluded if known medical or obstetric complication that restricts physical activity. History of eating disorders. |
| Interventions | Study mobile phone app and activity monitor vs activity monitor. |
| Outcomes | Physical activity, measured steps. |
| Starting date | 01/10/2012. |
| Contact information | JiWon Choi, PhD, no email given, University of California, San Francisco. |
| Notes | Estimated completion date: May 2014. |
Downs 2011.
| Trial name or title | Determinants and outcomes of physical activity in pregnancy: findings from active MOMS, a randomised physical activity intervention for pregnant women. |
| Methods | RCT. |
| Participants | Pregnant women (inclusion and exclusion criteria not described). |
| Interventions | 2 physical activity interventions and a control group. 1 group received a structured intervention with face‐to‐face physical activity education, motivational support and moderate‐physical activity on 2 days per week for 70 mins with an instructor. The 2nd group (lifestyle support) received educational support via mailed materials and phone support. The control group received standard care. |
| Outcomes | Performance (physical activity). |
| Starting date | |
| Contact information | Danielle S Downs,The Pennsylvania State University, University Park, PA, USA. |
| Notes | Study reported in brief abstract with preliminary findings. |
Farajzadegan 2013.
| Trial name or title | Maternal‐centred life‐style modification program for weight gain management during pregnancy. |
| Methods | RCT. Location: Iran. |
| Participants | 160 pregnant women between 6 to 10 weeks' gestational age who have no condition that requires medical care nor drug taking. |
| Interventions | Nutrition and exercise (educational package, prenatal care log book and 10, 20‐minute counselling sessions) vs standard prenatal care. |
| Outcomes | GWG, BMI. |
| Starting date | Not stated. |
| Contact information | Dr Zahra Amini Pozveh, aminizahra2005@yahoo.com, Isfahan University of Medical Sciences. |
| Notes |
Fernandez 2011.
| Trial name or title | Electronically‐mediated weight interventions for pregnant and postpartum women. |
| Methods | RCT, 3‐armed, parallel, double blind. Location: USA. |
| Participants | Estimated enrolment 1641 pregnant women at or before 20 weeks' gestation. Excluded BMI < 18.5 and > 35, multiple gestation, any previous conditions that could interfere with weight loss. |
| Interventions | Behavioural intervention through a website during pregnancy and until 18 months postpartum or intervention through website during pregnancy only vs non weight‐related content. |
| Outcomes | GWG, postpartum weight retention, caloric intake, PA. |
| Starting date | 01/05/2011. |
| Contact information | Diana Fernandez, no email given, University of Rochester. |
| Notes | Estimated completion date December 2014. |
Ferrara 2014.
| Trial name or title | A pragmatic cluster randomised clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. |
| Methods | Cluster‐RCT, multicentre, blinded. Location: USA. |
| Participants | 2,320 women with a GDM diagnosis between March 27, 2011 and March 30, 2012. Excluded: neonatal loss, no telephone contact. |
| Interventions | Nutrition and exercise (diabetes prevention program‐derived print/telephone lifestyle intervention of 13 telephonic sessions) vs usual care. |
| Outcomes | Proportion of women who reach a postpartum weight goal and total weight change. Postpartum glycaemia, blood pressure, depression, percent of calories from fat total caloric intake and physical activity levels. |
| Starting date | March 27, 2011. |
| Contact information | Assiamira Ferrara, Assiamira.Ferrara@kp.org, Kaiser Permanente Northern California. |
| Notes |
Goldberg 2012.
| Trial name or title | Randomised control pilot of a behaviour‐based exercise and diet intervention to reduce risk factors for gestational diabetes among otherwise healthy pregnant women. |
| Methods | RCT, parallel, open label. Location: USA. |
| Participants | 30 healthy first trimester pregnant women 18 to 40 years. Excluded: hypertension, diabetes, known cardiopulmonary disease; orthopaedic problems or other conditions that would prevent regular physical activity. |
| Interventions | Nutrition and exercise (20 interactive weekly sessions to promote daily exercise, vegetable and fruit intake, maintain a diet that is relatively lower in fat and rich in whole grains) vs standard medical care. |
| Outcomes | Achieving 30 minutes of daily exercise, 4 or more times each week. Eating 5 or more servings of vegetables and/or fruits each day, pregnancy weight gain, fasting glucose, HbA1C, pregnancy weight gain, blood pressure, lipid and lipoprotein levels, pedometer records and more. |
| Starting date | 01/11/2012. |
| Contact information | Linn Goldberg MD, goldberl@ohsu.edu, Oregon Health and Science University. |
| Notes |
Graziano 2013.
| Trial name or title | WATE study ‐ gestational weight gain and the electronic medical record. |
| Methods | RCT, parallel, open label. Location: USA. |
| Participants | Estimated enrolment 300. Pregnant women with a single intrauterine gestation. Excluded multiple gestation pregnancy. |
| Interventions | Weight alerts (electronic medical record popup/highlight in their chart, displaying the weight gain, patient perception, recommended guidelines for weight gain in pregnancy) vs usual display, with no flag nor highlight. |
| Outcomes | Weight gain, primary outcome will be measured as the percent of patients who achieve their recommended weight gain goal in pregnancy. |
| Starting date | 01/10/2013. |
| Contact information | Scott Graziano, MD MS, sgrazia@lumc.edu, Loyola University. |
| Notes | Estimated primary completion date: October 2014 (final data collection date for primary outcome measure). |
Hivert 2012.
| Trial name or title | Intervention to promote changes of healthy lifestyle (physical activity and nutrition) during gestation. |
| Methods | RCT, parallel, open label. Location: Canada. |
| Participants | Estimated enrolment of 50 overweight or obese pregnant women at risk of developing diabetes mellitus. Excluded if pre‐pregnancy diabetes detected in the first trimester, multiple gestation, taking medications that can affect blood sugar or weight. |
| Interventions | Healthy lifestyle counselling and PA sessions once a week until week 36 of gestation vs information about the recommended weight gain during pregnancy and an evaluation about of their nutritional and PA habits. |
| Outcomes | Weight change during pregnancy, levels of maternal and fetal adipokines, maternal and fetal glycaemic control, healthy lifestyle and others. |
| Starting date | 01/12/2011. |
| Contact information | Marie‐France Hivert, marie‐france.hivert@usherbrooke.ca, Universitaire de Sherbrooke. |
| Notes | Estimated completion date: January 2014. Emailed 25/9/14. Unpublished pilot study data for 20 women.was received on the 17/11/14. |
John 2014.
| Trial name or title | Healthy eating and lifestyle in pregnancy (HELP): a protocol for a cluster‐randomised trial to evaluate the effectiveness of a weight management intervention in pregnancy. |
| Methods | Cluster‐RCT, blinded. Location: UK. |
| Participants | Target recruitment of 570 pregnant women aged 18 years or over, with a BMI ≥ 30 (kg/m2) and between 12 and 20 weeks' gestation. Excluded if unable to understand English, any pregnancy‐related complications, any previous medical complications, any nutritional complications, involved in other research. |
| Interventions | Nutrition and exercise (weekly 1.5‐hour weight management group) vs usual care and 2 leaflets giving advice on diet and physical activity. |
| Outcomes | BMI at 12 months postpartum, GWG, QoL, mental health, waist‐hip ratio, child weight centile, admission to neonatal unit, diet, and others. |
| Starting date | Not stated. |
| Contact information | Elinor John, simpsonsa@cf.ac.uk, Cardiff University. |
| Notes |
King 2013.
| Trial name or title | Postprandial response to almond consumption in overweight Hispanic pregnant women. |
| Methods | RCT, cross‐over, single blind (investigator). Location: USA. |
| Participants | Estimated enrolment 20 overweight or obese pregnant Hispanic women between 30 and 36 weeks' gestation. Excluded if diagnosis of GDM, pre‐existing diabetes mellitus, renal disease, thyroid disease, cardiovascular disease, history of drug abuse, nut allergies. |
| Interventions | Almond meal (bagel, almond butter and apple juice) vs bagel with cream cheese and apple juice. |
| Outcomes | Markers for glucose intolerance, serum triglyceride concentrations. Secondary: Metabolic markers, glucose, insulin, hormonal and inflammatory markers, satiety markers. |
| Starting date | September 2011. |
| Contact information | Lisa R Sawrey‐Kubicek, MS, RD, lsawreykubicek@chori.org, Children's Hospital & Research Center Oakland. |
| Notes | Estimated completion date: December 2013. Emailed 25/9/14. |
Ko 2010.
| Trial name or title | Effect of physical activity on metabolic syndrome in pregnancy and fetal outcome. |
| Methods | RCT, single blind (investigator). |
| Participants | Location: United States, Washington. Target number of participants: 100. Inclusion criteria: pregnant women 18‐45 years old receiving prenatal care at MAMC. Exclusion criteria: women do not have a gallbladder, do not speak English, over 14 weeks pregnant at study entry, do not plan to deliver at MAMC, have medical contraindications, unwilling to participate in exercise intervention program, < 18 years of age, currently engaged in a regular vigorous‐exercise program. |
| Interventions | Intervention group will exercise 3 times per week at moderate‐vigorous intensity for 45 minutes per session through their 36 week of pregnancy. Control group women will continue their usual physical activity throughout pregnancy. |
| Outcomes | Primary outcome: central adiposity. Secondary outcomes: leptin levels, glucose insulin, cholesterol, fetal adiposity, neonatal adiposity. |
| Starting date | October 2007. |
| Contact information | Cynthia W Ko, University of Washington. |
| Notes |
Laitinen 2013.
| Trial name or title | Placebo‐controlled intervention study for maternal and child health. |
| Methods | RCT, parallel, double blind placebo‐controlled. Location: Finland. |
| Participants | Estimated enrolment: 440 overweight healthy pregnant women less than 16 weeks' gestation. Excluded if diabetes, coeliac disease, hypo‐/hyperthyroidism, increased bleeding tendency. |
| Interventions | Dietary supplement (comparison of probiotics, fish oil and their combination) vs placebo. |
| Outcomes | GDM, fasting glucose levels, prevalence of allergy in child. Secondary: management of GDM, body composition, immunologic and metabolic markers, fecal microbiota. |
| Starting date | September 2013. |
| Contact information | |
| Notes | Estimated primary completion date: March 2016 (final data collection date for primary outcome measure). |
Li 2011.
| Trial name or title | Effects of dietary and lifestyle interventions in obese pregnant women from the first trimester on gestational weight gain and pregnancy outcomes. |
| Methods | RCT, parallel, single blind (participant). Location: China. |
| Participants | 373 overweight or obese pregnant women gestational age between 6 and 12 weeks of gestation, pre‐pregnancy ≥ 28 (kg/m2) aged ≥ 18 years, and a singleton pregnancy. Excluded if prediabetes and diabetes, hypertension, chronic renal disease, thyroid disorder, gestational weeks ≥ 13, multiple pregnancy, uterine malformation, physical restriction that prevents exercise. |
| Interventions | Dietary and lifestyle intervention (individualised dietary intake protocol ‐ standard care intensive dietary and lifestyle intervention from first trimester to delivery, every 2‐4 weeks) vs standard care. |
| Outcomes | GWG, from enrolment to delivery (28‐34 weeks of gestation), GDM, hypertensive disorders, large‐for‐gestational‐age infants, macrosomia, caesarean section. |
| Starting date | 01/04/2011. |
| Contact information | |
| Notes | Emailed 25/9/14. |
Liu 2011.
| Trial name or title | Magnesium supplementation in the second trimester of pregnancy to overweight and obese individuals. |
| Methods | RCT, 3‐arm, parallel, double blind. Location: USA. |
| Participants | Estimated enrolment: 60 overweight and obese pregnant women in their first trimester between 18 and 40 years of age. Excluded if on insulin therapy or other oral hypoglycaemic agents, multiple gestation, baseline HgbA1C > 6.5%, prior history of clinically diagnosed T2D, multiple dietary restrictions/food allergies, heart, renal, or liver failure, clinical history of psychiatric illness or substance abuse and other exclusions. |
| Interventions | Group A will receive oral magnesium citrate, group B will receive dietary counselling about following a magnesium rich diet) vs group C who will receive a placebo (control). |
| Outcomes | Maternal biomarkers, blood and urine markers, neonatal birthweight/height, macrosomia, preterm birth, head circumference, and Apgar score and more. |
| Starting date | 01/01/2012. |
| Contact information | Simin Liu, Dr, no email given, University of California, Los Angeles. |
| Notes |
McAuliffe 2013.
| Trial name or title | Pregnancy, exercise and nutrition research study with app support. |
| Methods | Single centre RCT. Location: Ireland. |
| Participants | 500 women with singleton pregnancies between 10‐15 weeks' gestation between the ages of 18‐45 with a smart‐phone, and a BMI of greater than 25 kg/m2. Excluded if pre‐gestational diabetes or early onset gestational diabetes or past history of gestational diabetes, and other exclusions. |
| Interventions | Women in the intervention group will have standard antenatal care but will receive a ‘healthy lifestyle package with app support’ (a combination of a healthy diet, an exercise intervention with a smart phone application as an information and motivational source) vs controls who will receive a ‘regular lifestyle package’ consisting of standard antenatal care and general advice on weight gain according to BMI. |
| Outcomes | Gestational diabetes, GWG, glycaemic index, PA levels. |
| Starting date | 01/01/2013. |
| Contact information | Professor Fionnuala McAuliffe fionnuala.mcauliffe@ucd.ie |
| Notes | Estimated completion date: 2016 (personal communication on 25/9/14). |
Moholdt 2011.
| Trial name or title | Exercise training in pregnancy for obese women (ETIP): study protocol for a RCT. |
| Methods | RCT, parallel, blinded. Location: Norway. |
| Participants | 150 previously sedentary obese pregnant women with singleton live fetus at an 11‐14 weeks ultrasound scan. Excluded if pregnancy complications, high risk for preterm labour or diseases that could interfere with participation, and habitual exercise training. |
| Interventions | Physical exercise (60 mins up to 4 times per week starting in gestation week 14) vs standard antenatal care. |
| Outcomes | GWG, exercise capacity, endothelial function, physical activity level, body composition, serum markers of cardiovascular risk, incontinence, lumbopelvic pain and cardiac function, anthropometric variables at birth, Apgar score, serum markers of inflammation and metabolism in cord blood. |
| Starting date | 01/09/2010. |
| Contact information | Trine T Moholdt, trine.moholdt@ntnu.no, Norwegian University of Science and Technology. |
| Notes | Estimated completion date: 2016 (personal communication on 25/9/14). |
Nagle 2011.
| Trial name or title | Continuity of midwifery care and gestational weight gain in obese women. |
| Methods | RCT. |
| Participants | 214 primiparous women attending 1 of the study hospitals for maternity care with a booking BMI > 30 and less than 17 weeks' gestation. Exclusion criteria include inability to speak English, multiple pregnancy, vaginal bleeding or serious medical condition. |
| Interventions | Continuity of midwifery care compared with routine management. |
| Outcomes | GWG; women's experience of care and satisfaction with care; psychological well being. |
| Starting date | Not clear. |
| Contact information | cate.nagle@deakin.edu.au |
| Notes | Emailed 25/9/14. |
Nagle 2013.
| Trial name or title | Primary prevention of gestational diabetes for women who are overweight and obese: a RCT. |
| Methods | RCT, not blinded. Location: Australia. |
| Participants | Pregnant women who are overweight or obese, with a singleton pregnancy, less than 14 weeks' gestation and recruited from the Barwon South West region of Victoria, Australia. Excluded: with pre‐existing diabetes, a past history of gestational diabetes, currently experiencing vaginal bleeding or with severe medical conditions. |
| Interventions | Behavioural counselling: (EDGE program from recruitment until birth, consisting of weekly contact with a brief (5‐minute) phone call each fortnight) vs usual care. |
| Outcomes | GDM, GWG, LGA, psychological health and more. |
| Starting date | Not stated. |
| Contact information | Cate Nagle, cate.nagle@deakin.edu.au, Deakin University. |
| Notes | Emailed 25/9/14. |
Norman 2012.
| Trial name or title | Efficacy of metformin in pregnant obese women: a RCT. |
| Methods | RCT, multicentre, placebo‐controlled. Location: UK. |
| Participants | Caucasian obese pregnant women between 12‐0 and 16+0 weeks' gestation. Excluded if non‐Caucasian, gestation greater than 16 weeks, pre‐existing diabetes, gestational diabetes in a previous pregnancy, systemic disease requiring regular medication, gestational diabetes in index pregnancy prior to randomisation, previous delivery of a baby less than 3rd centile or previous pregnancy with pre‐eclampsia prompting delivery before 32 weeks' gestation and more. |
| Interventions | Medicinal (oral metformin tablets given 3 times daily to a maximum of 500 ‐ 2500 mg daily) vs placebo. |
| Outcomes | Gestational age and sex adjusted birthweight centiles of the baby, correlation between maternal insulin resistance, adverse pregnancy outcomes. Neonatal body composition, biological mechanisms of metformin, maternal anthropometry and more. |
| Starting date | 01/02/2011. |
| Contact information | Prof Jane Norman, jane.norman@ed.ac.uk, University of Edinburgh. |
| Notes | Estimated end date: 30/11/2013. |
Parat 2010.
| Trial name or title | Impact of education during pregnancy in overweight pregnant women (ETOIG). |
| Methods | RCT. Masking: single blind (participant). |
| Participants | Location: Hospital Necker Paris, France. Target number of participants: 800. Inclusion criteria: pregnant women who agree the study, BMI > 25 kg/m² (BMI is based on retrospective self reported weight of the patient before pregnancy), no more than 21 weeks of gestation. Exclusion criteria: younger than 18 years, multiple gestation, high‐risk pregnancy, psychiatric pathology, diabetes diagnosed before the inclusion, fetal malformation, history of obesity surgery, no understanding of French language, planning to move to another area. |
| Interventions | Intervention: therapeutic education; intensive training individual and collective teaching. Control: placebo comparator; classical follow‐up with 2 individual consultations. |
| Outcomes | Primary outcomes: ‐30% reduction of rapid infancy weight gain at 2 years defined as > + 0.67 change in weight SD score. The 0.67 SD represents the difference between the displayed centile lines on standard infant growth charts. Secondary outcomes: ‐reduction of rapid infancy weight gain between 0 and 6 months; ‐reduction of the number of children with BMI over 19 at 2 years; ‐reduction of incidence of gestational diabetes, pre‐eclampsia, HTA during pregnancy, caesarean, fetal macrosomia; ‐reduction of spontaneous feeding at 4 months; ‐increase of breastfeeding (number of women and duration); ‐reduction 1 and 2 years after pregnancy of mother weight and BMI (except 2nd pregnancy); ‐reduction of abnormality of lipid and glycaemia test in women, 2 years after the pregnancy. |
| Starting date | September 2008. |
| Contact information | Sophie Parat; sophie.parat@nck.aphp.fr Raphael Serreau; raphael.serreau@cch.aphp.fr |
| Notes |
Peccei 2010.
| Trial name or title | Nutrition intervention for the promotion of healthy weight gain during pregnancy: the Revere pregnancy weight management study. |
| Methods | RCT, parallel, open label. Location: USA. |
| Participants | 300 overweight or obese pregnant women ages 18 to 49 at the first prenatal visit < 16 weeks' gestation who have and have not dieted in the past. Excluded if multiple gestation, diabetes prior to pregnancy, medical history of an eating disorder and other exclusions. |
| Interventions | Nutrition counselling (twice monthly interaction with a registered dietitian from 6‐16 weeks' gestation through 6 months postpartum) vs current standard of optimal care in addition to 1 nutrition education session with the study nutritionist at 6‐16 weeks' gestation. |
| Outcomes | GWG, intake of nutritious foods, hypertension and eclampsia, gestational diabetes, caesarian delivery, macrosomia, admission to neonatal intensive care unit and others. |
| Starting date | 01/12/2009. |
| Contact information | Alessandra Peccei, MD, Massachusetts General Hospital. |
| Notes | Estimated completion date: January 2015. |
Rauh 2014.
| Trial name or title | Rauh K, KuHealthy living in pregnancy: a cluster‐randomised controlled trial to prevent excessive gestational weight gain ‐ rationale and design of the GeliS study. |
| Methods | A prospective, cluster‐randomised, controlled, open intervention trial. |
| Participants | 2500 pregnant women. Inclusion criteria: women aged 18 to 43 years with a singleton pregnancy, a pre‐pregnancy BMI ≥ 18.5 kg/m2 and ≤ 40 kg/m2, and sufficient German language skills are eligible for the study. These pregnant women are recruited before the 12th week of gestation. Exclusion criteria: multiple pregnancy, high‐risk pregnancy prohibiting study participation (contraindications to exercise e.g. placenta praevia, persistent bleeding, cervical incompetence etc.), prepregnancy diabetes mellitus or early gestational diabetes, uncontrolled chronic diseases (e.g. thyroid dysfunction), psychiatric or psychosomatic diseases, and any other diseases which could interfere with compliance according to the study protocol. |
| Interventions | Behavioural: lifestyle intervention. The intervention program consists of 4 individual counselling modules focusing on diet, physical activity and weight monitoring (12th‐16th, 16th‐20th, 30th‐34th week of gestation and 6th‐8th week postpartum). The counselling sessions are given by carefully trained midwives or medical staff in combination with prenatal visits and follow a standardised curriculum. Women are provided with brochures including a list of adequate prenatal exercise programs and a pedometer. Furthermore, they receive a chart personalised according to their baseline BMI category to monitor weight development. |
| Outcomes | Primary outcomes ‐GWG. ‐Proportion of pregnant women showing excessive GWG according to IOM guidelines. Secondary outcomes –Incidence of gestational diabetes (24th‐28th week of gestation, via an OGTT). glycosylated haemoglobin concentration (30th‐34th week of gestation). –Other pregnancy complications such as pre‐eclampsia. –Anthropometric measures and health status of the newborns (birthweight, height, head circumference, LGA, SGA, Apgar score, pH). –Obstetric complications (mode of delivery, induction of labour, rate of caesarean sections, etc.). |
| Starting date | September 2013. |
| Contact information | Kathrin Rauh, M.Sc. kathrin.rauh@KErn.bayern.de |
| Notes | Estimated completion date: September 2018. |
Redman 2012.
| Trial name or title | Expecting success: personalised management of body weight during pregnancy. |
| Methods | RCT, parallel, open label. Location: USA. |
| Participants | 306 overweight or obese pregnant women between 18 and 40 years old before 12 weeks of your pregnancy. Excluded if pregnant with more than 1 infant, habitually smoke, abuse illegal or prescription drugs in the last 6 months, consume more than 2 alcoholic drinks per week, history of: 3 or more first trimester miscarriages, high blood pressure, type 1 diabetes, pregnancy related diabetes during screening, HIV or AIDS, psychotic disorder, major depressive episode, bipolar disorder or eating disorders and other exclusions. |
| Interventions | Behavioural counselling (weight management counselling up to 4 times per month at clinic, plus 2 telephonic sessions) vs physician directed care. |
| Outcomes | GWG. PA, diet, postpartum weight retention. |
| Starting date | 01/12/2012. |
| Contact information | Loren Johnson, BS, MS, loren.johnson@pbrc.edu, Pennington Biomedical Research Centre. |
| Notes | Estimated completion date: June 2016. |
Roberts 2012.
| Trial name or title | Interventions to reduce excess weight gain in pregnancy in overweight and obese mothers. |
| Methods | RCT, parallel, open label. Location: USA. |
| Participants | 75 over weight or obese pregnant women age 18‐45 years in first trimester of pregnancy. Excluded if carrying multiple fetuses, GDM at study entry, Type 2 diabetes mellitus or blood glucose > 125 mg/dL at screening, current substance abuse, smoker, alcohol consumption of more than 1 drink per day, pre‐existing medical conditions or use of medications. |
| Interventions | Diet counselling (Group based counselling plus Fiber Cereal or resistant starch) vs routine clinical care and no additional interventions. |
| Outcomes | Maternal and infant body weight change, maternal non‐fasting weight, infant weight. Infant outcomes: body composition, Apgar, dietary intake. Maternal and perinatal outcomes: caesarean deliveries, gestational hypertension/pre‐eclampsia, preterm birth, birth complications, fasting blood glucose and insulin concentrations throughout pregnancy. |
| Starting date | 01/07/2012. |
| Contact information | Contact: Lorien E Urban, Ph.D, PregWeight@tufts.edu, Tufts University. |
| Notes | May 2014 (final data collection date for primary outcome measure). |
Seneviratne 2014.
| Trial name or title | Antenatal exercise in overweight and obese women and its effects on offspring and maternal health: design and rationale of the IMPROVE (Improving Maternal and Progeny Obesity Via Exercise) RCT. |
| Methods | A 2‐arm parallel RCT. Location: Auckland, New Zealand. |
| Participants | 100 pregnant women aged 18 to 40 years with a BMI ≥ 25 kg/m2 who are carrying a singleton fetus less than 20 weeks of gestation, with gestation confirmed by a dating scan early in pregnancy, and who are resident in the Auckland region are eligible for the trial. Exclusion criteria include ongoing smoking during the current pregnancy or contra‐indications to aerobic exercise in pregnancy as stated by ACOG. |
| Interventions | The exercise regimen consists of home‐based stationary cycling at 20 weeks of gestation. The 16‐week exercise regimen consists of 67 exercise sessions, comprising approximately 1500 minutes of moderate‐intensity exercise. The frequency and duration of prescribed exercise varies over the intervention period. The intervention commences with 3 exercise sessions per week and increases to 5 sessions per week. All exercise sessions commence with a 5‐minute warm‐up on the stationary cycle at low intensity, maintaining heart rate below target rate, and concludes with a similar 5‐minute cool down.The duration of moderate‐intensity exercise at prescribed heart rate increases gradually from 15 minutes to 30 minutes per session and gradually decreases again from 33 weeks of gestation while maintaining the frequency at 5 per week. A qualified exercise physiologist maintains regular contact with the exercising participants via phone, email, and home visits. To increase compliance with exercise, if cycling becomes unacceptable or uncomfortable with advancing pregnancy, participants are encouraged to utilise an alternative forms of exercise such as brisk walking, while maintaining same heart rate intensity, frequency, and duration during exercise. |
| Outcomes | Primary outcome: offspring birthweight. Secondary outcomes: offspring outcomes include neonatal anthropometry and body composition, fetal growth measures, cord blood metabolic markers and newborn complications including rates of LGA (birthweight > 90th centile) and SGA (birthweight < 10th centile) babies. Maternal outcomes include weight gain, aerobic fitness, quality of life, metabolic markers, postpartum weight and body composition, as well as pregnancy and delivery outcomes including timing and mode of delivery and length of hospital stay. |
| Starting date | 01/10/2012. |
| Contact information | Sumudu N Seneviratne (s.seneviratne@auckland.ac.nz). |
| Notes |
Shen 2010.
| Trial name or title | Impact of diet and exercise activity on pregnancy outcomes (IDEA). |
| Methods | Randomised, open label. |
| Participants | Location: Canada. Inclusion criteria: age 18 years and older, pregnancy < 20 weeks, expressed interest in study and willingness to consent to participate in the study. Exclusion criteria: obstetric or medical contraindications for exercise according to 2002 SOCG guideline (ruptured membranes, preterm labour, incompetent cervix, hypertensive disorders of pregnancy, growth restricted fetus, placenta previa, persistent bleeding in 2nd or 3rd trimester, significant metabolic, cardiovascular, respiratory or systemic disorder), pre‐existing diabetes (except a history of GDM, but not in current pregnancy), multiple gestations. |
| Interventions | A community‐based exercise and dietary intervention. |
| Outcomes | Primary outcomes: excessive weight gain during pregnancy. Secondary outcomes: macrosomia, requirement of delivery procedures. |
| Starting date | July 2006. |
| Contact information | Garry Shen; gshen@ms.umanitoba.ca University of Manitoba. |
| Notes |
Skouteris 2012.
| Trial name or title | A RCT of a specialised health coaching intervention to prevent excessive gestational weight gain and postpartum weight retention in women: the HIPP study. |
| Methods | RCT. Location: Australia. |
| Participants | 220 women who have a BMI > 18.5 are 18 years of age or older, English speaking, no history of disordered eating or diabetes and are less than 18 weeks' gestation at recruitment. |
| Interventions | Behavioural counselling (1‐on‐1 sessions with a Health Coach, and 2 by 2 hour educational group sessions led by a Health Coach) vs education alone (2 by 2 hour educational group sessions with no HC components). |
| Outcomes | BMI, waist circumference, psychological factors. |
| Starting date | Not stated. |
| Contact information | Helen Skouteris, helen.skouteris@deakin.edu.au, Deakin University, Melbourne. |
| Notes | Publication pending (personal communication on 25/9/14). |
Smith 2010.
| Trial name or title | The design of a community lifestyle programme to improve the physical and psychological well‐being of pregnant women with a BMI of 30 kg/m² or more. |
| Methods | RCT. |
| Participants | Location: England. Enrolment: 400 (200 from each area). Inclusion criteria: women attending for antenatal care in a 2 UK hospitals with a BMI 30 kg/m² or greater. Exclusion criteria: aged under 18, intend to move in the next 3 months, take weight control medication or if they have any cautions for starting exercise (this will be determined using the Revised Physical Activity Readiness Questionnaire (PARQ) and the Royal College of Obstetricians and Gynaecologists (RCOG) recommendations). |
| Interventions | The lifestyle programme will run for 1.5 hours per week for 10 weeks and is supplementary to standard antenatal care. Women will be invited to the 10‐week programme at any stage before 30 weeks' gestation to ensure completion of the programme before their delivery. The control group will receive routine care. |
| Outcomes | Primary outcomes: pregnancy weight gain, birthweight, mode of birth, and method of infant feeding at hospital discharge: psychological outcomes include self‐efficacy, well‐being, and goal attainment. Secondary outcomes: women's experience of pregnancy and healthcare services, amount of physical activity, food intake, and the suitability of the intervention components. |
| Starting date | October 2011 (recruitment). |
| Contact information | Professor Tina Lavender; tina.lavender@manchester.ac.uk University of Manchester, Oxford Road, Manchester. M13 9PL United Kingdom. |
| Notes | Publication pending (personal communication on 25/9/14). |
Thangaratinam 2014.
| Trial name or title | Effect of simple, targeted diet in pregnant women with metabolic risk factors on pre‐eclampsia (ESTEEM): a randomised trial. |
| Methods | RCT, location: United Kingdom. |
| Participants | 3640 pregnant women. Inclusion criteria: pregnant women less than 18 weeks of gestation with at least 1 of the following: i. BMI ≥30 kg/m2 ii. Raised serum triglycerides ≥1.7 mmol/L iii. Raised blood pressure of systole ≥ 140 mm Hg or diastole ≥ 90 mm Hg. Exclusion criteria: i. BMI < 18.5 kg/m2 or ≥ 40 kg/m2 ii. Women on lipid altering drugs iii. History of diabetes iv. Chronic renal disease v. Auto immune disease vi. Multiple pregnancy vii. Poor understanding of written and spoken English viii. Not able to follow Mediterranean diet for religious or other reasons ix. < 16 years of age x. Not able to consume nuts or extra virgin olive oil. |
| Interventions | Behavioural: targeted ESTEEM diet. The key components of the diet are: High intake of vegetables, nuts, non‐refined grains, legumes and fruits; moderate‐to‐high consumption of fish; small‐to‐moderate intake of poultry and dairy products such as yoghourt and cheese; low consumption of red meat and processed meat and avoidance of sugary drinks, fast food and high fat food; high fibre; intake of nuts including walnuts and almonds that are rich sources of monounsaturated and polyunsaturated fatty acids (30 g/day); olive oil to cook and dress salads as the main source of fat (0.5 L/week). The intervention will include structured meal plans and grocery lists, recipes for healthy diet and appropriate choices at restaurants. |
| Outcomes | Number of participants with a diagnosis of pre‐eclampsia. Diagnosis of pre‐eclampsia defined as: new onset hypertension after 20 weeks' gestation defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mmHg, in at least 2 readings AND new onset proteinuria defined as spot urine. Protein/creatinine ratio test greater than 30 mg/mmol or > 24‐hour urine 300 mg/24 hours or 2+ or more on standard urinary dipstick tests after 20 weeks' gestation. Superimposed pre‐eclampsia in women with chronic hypertension or chronic proteinuria. Women with eclamptic seizures with no hypertension or proteinuria. |
| Starting date | July 2014. |
| Contact information | Shakila Thangaratinam, s.thangaratinam@qmul.ac.uk |
| Notes | Estimated primary completion date: February 2016. |
Thomson 2014.
| Trial name or title | Delta Healthy Sprouts: a randomised comparative effectiveness trial to promote maternal weight control and reduce childhood obesity in the Mississippi Delta. |
| Methods | Parallel arm RCT. Location: USA. |
| Participants | 150 mothers and their infants residing in the rural Mississippi Delta who are in their second trimester and are < 19 weeks pregnant with first, second or third child, > 18 years of age between 14 and 18 weeks' gestation. Excluded if multiple pregnancy. |
| Interventions | Nutrition and PA counselling (monthly PaT lessons and materials and PaTE supplemental nutrition and physical activity lessons and materials) vs monthly PaT lessons and materials only. |
| Outcomes | Primary: mothers: GWG, BMI. Infants: weight‐for‐length. Secondary; diet, diet quality, physical activity, knowledge, beliefs, and practices. |
| Starting date | January 2013. |
| Contact information | Jessica L Thomson, jessica.thomson@ars.usda.gov, United States Department of Agriculture/University of Illinois at Chicago and University of Illinois Cancer Centre. |
| Notes |
Uauy 2013.
| Trial name or title | Effectiveness of a normative intervention (diet, physical activity and breastfeeding) on maternal nutrition and offspring growth and development: nutrition in the first 1000 days key to healthy growth and long‐term health. |
| Methods | Cluster‐RCT, multicentre, single group, double blind (investigator, outcomes assessor). Location: Chile. |
| Participants | 2400 pregnant women before 15 weeks of pregnancy who are not planning to move in the next 2 years. Excluded if women were classified as high risk according to the norms of the Chilean Ministry of Health (MoH) and/or underweight (BMI < 18.5). |
| Interventions | Diet and physical activity counselling‐support and breastfeeding promotion till 12 months postpartum vs routine care. |
| Outcomes | GWG, glycaemic control, weight retention at 12 months, infant growth. |
| Starting date | 01/09/2013. |
| Contact information | Maria L Garmendia, PhD, mgarmendia@inta.uchile.cl, University of Chile. |
| Notes | Estimated completion date: March 2017. |
Vistad 2009.
| Trial name or title | Fit For Delivery: a study of the effect of exercise intervention and nutritional counselling on pregnancy outcome. |
| Methods | RCT, parallel, single blinded (investigator). Location: Norway. |
| Participants | 600 pregnant women expecting first child who reside in 1 of the following towns: Kristiansand, Søgne, Sogndalen, Vennesla, Lillesand, Mandal, in their gestational weeks 12‐20. Excluded if twin or other multiple pregnancy, pre‐existing diabetes, physical handicap which precludes participation in exercise groups, ongoing drug addiction, serious mental disorder, BMI at or below 19 before pregnancy, inability to read/write Norwegian or English. |
| Interventions | Diet counselling (2 telephone consultations on nutritional health and access to Internet topics) and twice weekly exercise groups vs routine pregnancy care. |
| Outcomes | GWG, birthweight, maternal fasting serum glucose level, incidence of operative delivery, both caesarean section and operative vaginal delivery, maternal body composition. Secondary: maternal weight retention, measurement of serum levels of hormones which regulate serum glucose levels, in both the pregnant woman and her new‐born baby, incidence of women with serum glucose levels >7.8 mmol/L after 2‐hour glucose challenge test. |
| Starting date | 01/09/2009. |
| Contact information | Ingvild Vistad, MD, PhD, no email mentioned, Sorland Hospital HF. |
| Notes | Estimated completion date: December 2014. |
Yan 2012.
| Trial name or title | Low glycaemic index diet intervention on insulin resistance of overweight pregnant women. |
| Methods | RCT, parallel, single blind (participant). Location: China. |
| Participants | 400 pregnant women with first prenatal examination BMI equal to or greater than 24 kg/m2, singleton pregnancy, uniparous, 18 years to 45 years, ≥ 12 weeks' gestation. Excluded if artificial impregnation, history of hypertension, diabetes, coronary heart disease or mental disorder, special diet habit. |
| Interventions | Diet counselling (Dietary Guidelines for Chinese residents for pregnant women and low glycaemic index diet) vs standard diet counselling (diet counselling based on Dietary guidelines for Chinese Residents). |
| Outcomes | Maternal insulin, cord blood C‐peptide, incidence of gestational diabetes, macrosomia. Secondary: incidence of gestational hypertension, proportion of women with newly incident hypertension during pregnancy, birthweight, caesarean delivery, gestational age. |
| Starting date | 01/02/2012. |
| Contact information | Weili Yan, no email given, Fudan University. |
| Notes | November 2014 (final data collection date for primary outcome measure). |
ACOG: the American Congress of Obstetricians and Gynecologists BMI: body mass index DBP: diastolic blood pressure GDM: gestational diabetes mellitus GL: glycaemic load GWG: gestational weight gain HbA1c: haemoglobin A1c HDL: high density lipoprotein HIV: human immunodeficiency virus HTA: hydrothermal endometrial ablation IOM: Institute of Medicine LGA: large‐for‐gestational age MAMC: Madigan Army Medical Center OGTT: oral glucose tolerance test PA: physical activity QoL: quality of life RCT: randomised controlled trial SBP: systolic blood pressure SD: standard deviation SOCG: the Society of Obstetricians and Gynaecologists of Canada T2D: Type 2 diabetes WHO: World Health Organization
Differences between protocol and review
This update differs from the protocol and previous versions of the review in the following ways.
The title has been changed from 'Interventions for preventing excessive weight gain during pregnancy' to 'DIet or exercise, or both, for preventing excessive weight gain during pregnancy'. This was done to limit the otherwise enormous scope of the review. As a result of the title modification, two previously included 1970s studies of (obsolete) appetite suppressant drug interventions were excluded in this update. Drug interventions (e.g. metformin, probiotics), will now need to be addressed under a separate title.
The protocol and previous versions of this review included quasi‐RCTs. For this update, we have excluded quasi‐RCTs. We believe that the large and growing number of RCTs in this field justifies our approach of only using the best quality trials (RCTs).
We amended the Subgroup analysis and investigation of heterogeneity section of the methods by including subgroup analysis according to the risk of excess weight‐related adverse outcomes (low‐risk, mixed‐risk and high‐risk populations).
For this update, we decided to include the additional outcomes 'birthweight' and 'hypertension'; these are frequently reported outcomes in the context of the review interventions. Although they were not prespecified, the decision to include these outcomes was taken before commencing data extraction. We re‐visited previously included studies to retrieve these data, where reported. By capturing these additional data we hope that this and future versions of the review will be more complete.
We amended the outcome 'complications relating to macrosomia' to include 'neonatal respiratory distress syndrome'.
Contributions of authors
B Muktabhant (BM) conceived and designed the draft protocol. P Lumbiganon (PL) and C Ngamjarus (CN) reviewed and commented on the revisions of the protocol. For the original review, BM, CN and T Dowswell (TD) selected studies, extracted data and conducted data analysis. BM and TD drafted the original review with feedback from PL and CN. For the update, BM and T Lawrie (TL) selected studies, extracted data, conducted data analysis and prepared the review. ML and PL contributed to data analysis, discussion and revision of the manuscript. All authors approved the final version of the review.
Sources of support
Internal sources
Khon Kaen University, Thailand.
University of Liverpool, UK.
External sources
Thai Cochrane Network, Thailand.
Thailand Research Fund/Distinquished Professor Award, Thailand.
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Althuizen 2013 {published data only}
- Althuizen E, Poppel MN, Seidell JC, Wijden C, Mechelen W. Design of the new life(style) study: a randomised controlled trial to optimise maternal weight development during pregnancy. BMC Public Health 2006;6:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althuizen E, Wijden C, Mechelen W, Seidell J, Poppel M. The effect of a counselling intervention on weight changes during and after pregnancy: a randomised trial. BJOG : an International Journal of Obstetrics and Gynaecology 2013;120(1):92‐9. [DOI] [PubMed] [Google Scholar]
- Broekhuizen K, Althuizen E, Poppel MNM, Donker M, Mechelen W. From theory to practice: intervention fidelity in a randomized controlled trial aiming to optimize weight development during pregnancy. Health Promotion Practice 2012;13(6):816‐25. [DOI] [PubMed] [Google Scholar]
Angel 2011 {published data only}
- Angel MD, Haene J, Perez M, Hernandez G, Castaneda D, King JC. Dietary patterns associated with gestational weight gain and fat mass gain in overweight and obese pregnant women. FASEB Journal 2011;25:783.15. [Google Scholar]
Asbee 2009 {published data only}
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Dietary counseling prevents excessive weight gain during pregnancy, a randomized controlled trial. Obstetrics & Gynecology 2008;11(4 Suppl):6S. [DOI] [PubMed] [Google Scholar]
- Asbee SM, Jenkins TR, ButlerJR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstetrics & Gynecology 2009;113(2 Pt 1):305‐12. [DOI] [PubMed] [Google Scholar]
Barakat 2011 {published data only}
- Barakat R. Effect of physical exercise program during pregnancy on excessive weight gain and its consequences. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2011.
- Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2011;204(5):402.e1‐402.e7. [DOI] [PubMed] [Google Scholar]
Bisson 2014 {published data only}
- Bisson M, Almeras N, Dufresne S, Rheaume C, Bujold E, Robitailee J, et al. Exercise improving fitness in obese women during pregnancy: a difference for the mother and child?. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3‐6; Vancouver, Canada. 2014:Abstract no: 2946.618.
Bogaerts 2012 {published data only}
- Bogaerts A. Effect of psycho‐education on gestational weight gain and anxiety/depression in obese pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2011.
- Bogaerts A, Devlieger R, Nuyts E, Witters I, Gyselaers W, Guelinckx I. Psycho‐education reduces gestational weight gain in obese pregnant women: Randomized controlled trial [abstract]. Obesity Facts 2012;5:53. [Google Scholar]
Callaway 2010 {published data only}
- Byrne NM, Groves AM, McIntyre HD, Callaway LK. Changes in resting and walking energy expenditure and walking speed during pregnancy in obese women. American Journal of Clinical Nutrition 2011;94(3):819‐30. [DOI] [PubMed] [Google Scholar]
- Callaway L. A randomized controlled trial using exercise to reduce gestational diabetes and other adverse maternal and neonatal outcomes in obese pregnant women ‐ the pilot study. Australian Clinical Trials Registry (www.actr.org.au/) [accessed 31 October 2010].
- Callaway L, McIntyre D, Colditz P, Byrne N, Foxcroft K, O'Connor B. Exercise in obese pregnant women: a randomized study to assess feasibility. Hypertension in Pregnancy 2008;27(4):549. [Google Scholar]
- Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes. Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 2010;33(7):1457‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxcroft KF, Rowlands IJ, Byrne NM, McIntyre HD, Callaway LK, for the BAMBINO group. Exercise in obese pregnant women: the role of social factors, lifestyle and pregnancy symptoms. BMC Pregnancy and Childbirth 2011;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clapp 2002a {published data only}
- Clapp III JF. Maternal carbohydrate intake and pregnancy outcome. Proceedings of the Nutrition Society 2002;61(1):45‐50. [DOI] [PubMed] [Google Scholar]
- Clapp IJF. Diet, exercise, and feto‐placental growth. Archives of Gynecology and Obstetrics 1997;260:101‐8. [Google Scholar]
Clapp 2002b {published data only}
- Clapp JF 3rd, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. American Journal of Obstetrics and Gynecology 2002;186(1):142‐7. [DOI] [PubMed] [Google Scholar]
Cordero 2014 {published data only}
De Oliveria Melo 2012 {published data only}
- Oliveria Melo AS, Silva JLP, Tavares JS, Barros VO, Leite DFB, Amorim MMR. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstetrics & Gynecology 2012;120(2 Pt 1):302‐10. [DOI] [PubMed] [Google Scholar]
- Melo A. Exercise and pregnancy: randomised clinical trial. Current Controlled Trials (www.controlled‐trials.com/) [accessed 31 October 2010].
Di Carlo 2014 {published data only}
- Carlo C, Iannotti G, Sparice S, Chiacchio MP, Greco E, Tommaselli GA, et al. The role of a personalized dietary intervention in managing gestational weight gain: a prospective, controlled study in a low‐risk antenatal population. Archives of Gynecology and Obstetrics 2014;289:765‐70. [DOI] [PubMed] [Google Scholar]
Dodd 2014 {published data only}
- Cramp CS, Moran LJ, Deussen AR, Yelland LN, Dodd JM. Evaluation of printed nutrition education material in overweight and obese women during pregnancy‐findings from the limit randomised trial. Journal of Paediatrics and Child Health 2013;49 Suppl 2:118. [Google Scholar]
- Dodd J. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: a randomised trial. Australian Clinical Trials Registry (www.actr.org.au/) (accessed 31 October 2010).
- Dodd J. Obesity in pregnancy‐the limit randomised trial. Journal of Paediatrics and Child Health 2013;49 Suppl 2:4. [Google Scholar]
- Dodd JM. Dietary and lifestyle advice for pregnant women who Are overweight or obese: the LIMIT randomized trial. Annals of Nutrition & Metabolism 2014;64(3‐4):197‐202. [DOI] [PubMed] [Google Scholar]
- Dodd JM, Cramp C, Sui Z, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Medicine 2014;12(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, McPhee AJ, Turnbull D, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on neonatal health outcomes: the LIMIT randomised trial. BMC Medicine 2014;12(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ (Clinical Research Ed.) 2014;348:g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, Turnbull DA, McPhee AJ, Wittert G, Crowther CA, Robinson JS. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: the LIMIT randomised controlled trial. BMC Pregnancy and Childbirth 2011;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames AJ, Grivell RM, Dodd JM, Deussen A. The effect of limited gestational weight gain in overweight and obese women on maternal and infant outcomes. Journal of Paediatrics and Child Health 2013;49 Suppl 2:19. [Google Scholar]
- Grivell R, Yelland L, Earl RA, Staehr CJ, Dodd J. The effect of antenatal dietary and lifestyle advice on fetal body composition in women who are overweight or obese: findings from the LIMIT randomised trial. Ultrasound in Obstetrics and Gynecology 2013;42(Suppl 1):10. [Google Scholar]
- Grivell R, Yelland L, Staehr CJ, Earl RA, Dodd J. The effect of antenatal dietary and lifestyle advice on fetal growth in women who are overweight or obese: findings from the LIMIT randomised trial. Ultrasound in Obstetrics and Gynecology 2013;42(Suppl 1):83. [Google Scholar]
- Kannieappan LM, Deussen AR, Moran LJ, Grivell RM, Yelland LN, Dodd JM. The effect of antenatal dietary advice on maternal body composition in women who are overweight or obese ‐ findings from the limit randomised trial. Journal of Paediatrics and Child Health 2013;49 Suppl 2:94. [Google Scholar]
- Newman AK, Deussen AR, Moran LJ, Grivell RM, Yelland LN, Turnbull D, et al. The effect of antenatal dietary and lifestyle advice on maternal psychological health in women who are overweight or obese‐findings from the limit randomised trial. Journal of Paediatrics and Child Health 2013;49 Suppl 2:119. [Google Scholar]
- Sui Z, Yelland LN, Turnbull D, Dodd JM. Walking to limit gestational weight gain and keep fit during pregnancy ‐ findings from the walk randomised trial. Journal of Paediatrics and Child Health 2013;49 Suppl 2:120. [Google Scholar]
Ferrara 2011 {published data only}
- Ehrlich SF, Hedderson MM, Feng J, Crites Y, Quesenberry CP, Ferrara A. Lifestyle intervention improves postpartum fasting glucose levels in women with gestational diabetes. Diabetes 2014;63(Suppl 1):A95. [Google Scholar]
- Ferrara A. Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial). ClinicalTrials.gov (www.clinicaltrials.gov) [accessed 31 October 2010].
- Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34(7):1519‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guelinckx 2010 {published data only}
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. American Journal of Clinical Nutrition 2010;91(2):373‐80. [DOI] [PubMed] [Google Scholar]
Haakstad 2011 {published data only}
- Bo K, Haakstad LAH. Is pelvic floor muscle training effective when taught in a general fitness class in pregnancy? A randomised controlled trial. Physiotherapy 2011;97(3):190‐5. [DOI] [PubMed] [Google Scholar]
- Haakstad L. Effect of regular exercise in prevention of excessive weight gain in pregnancy. ClinicalTrials.gov (www.clinicaltrials.gov) [accessed 31 October 2010].
- Haakstad L, Bo K. Effect of supervised aerobic dance exercise in prevention of excessive weight gain in pregnancy: a single blind randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S198. [Google Scholar]
- Haakstad L, Bo K. Exercise during pregnancy‐Does it impact offspring birth weight parameters?. Journal of Science and Medicine in Sport 2012;15(Suppl 1):S340. [Google Scholar]
- Haakstad LA, Bo K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC Pregnancy and Childbirth 2011;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haakstad LAH, Bo K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: A randomised controlled trial. European Journal of Contraception and Reproductive Health Care 2011;16(2):116‐25. [DOI] [PubMed] [Google Scholar]
Harrison 2013 {published data only}
- Harrison CL, Lombard CB, Gibson‐Helm M, Deeks A, Teede HJ. Limiting excess weight gain in high‐risk pregnancies: A randomized controlled trial. Endocrine Reviews 2011;32(3 Meeting Abstracts):P1‐466. [Google Scholar]
- Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity 2013;21(5):904‐9. [DOI] [PubMed] [Google Scholar]
- Harrison CL, Teede HJ, Lombard CB. How effective is self‐weighing in the setting of a lifestyle intervention to reduce gestational weight gain and postpartum weight retention?. Australian & New Zealand Journal of Obstetrics & Gynaecology 2014;54:382‐5. [DOI] [PubMed] [Google Scholar]
- Lombard C, Harrison C, Teede H. A randomized controlled trial investigating self‐weighing and the prevention of excess weight gain in early pregnancy. Endocrine Reviews 2011;32(3 Meeting Abstracts):P2‐768. [Google Scholar]
- Teede HJ, Harrison CL, Gibson‐Helm M, Lombard CB. Improving physical activity in high‐risk pregnancies: A randomized controlled trial. Endocrine Reviews 2011;32(3 Meeting Abstracts):P1‐467. [Google Scholar]
Hawkins 2014 {published data only}
- Hawkins M, Hosker M, Marcus BH, Rosal MC, Braun B, Stanek EJ, et al. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: a feasibility randomized controlled trial. Diabetic Medicine 2014;32:108‐15. [DOI] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery 2011;27(2):257‐64. [DOI] [PubMed] [Google Scholar]
Hui 2006 {published data only}
- Hui AL, Ludwig SM, Gardiner P, Sevenhuysen G, Murray R, Morris M, et al. Community‐based exercise and dietary intervention during pregnancy: a pilot study. Canadian Journal of Diabetes 2006;30(2):169‐75. [Google Scholar]
Hui 2012 {published data only}
- Hui A, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean H, et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG: an International Journal of Obstetrics & Gynaecology 2012;119(1):70‐7. [DOI] [PubMed] [Google Scholar]
Hui 2014 {published data only}
- Hui AL, Ludwig S, Gardiner P, Sevenhuysen G, Dean HJ, Sellers E, et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre‐pregnancy Body Mass Index in a randomized control trial. BMC Pregnancy and Childbirth 2014;14(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2011 {published data only}
- Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Education and Counseling 2011;83(2):203‐9. [DOI] [PubMed] [Google Scholar]
Jeffries 2009 {published data only}
- Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Medical Journal of Australia 2009;191(8):429‐33. [DOI] [PubMed] [Google Scholar]
- Shub A. Diet and exercise in pregnancy. Australian Clinical Trials Registry (www.actr.org.au/) [accessed 21 June 2007].
Kieffer 2014 {published data only}
- Kieffer EC, Welmerink DB, Sinco BR, Welch KB, Rees Clayton EM, Schumann CY, et al. Dietary outcomes in a Spanish‐language randomized controlled diabetes prevention trial with pregnant Latinas. American Journal of Public Health 2014;104(3):526‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2014 {published data only}
- Kong KL, Campbell G, Foster C, Peterson D, Lanningham‐Foster L. A pilot walking program promotes moderate‐intensity physical activity during pregnancy. Medicine & Science in Sports & Exercise 2014;46(3):462‐72. [DOI] [PubMed] [Google Scholar]
Korpi‐Hyovalti 2011 {published data only}
- Korpi‐Hyovalti E, Schwab U, Laaksonen DE, Linjama H, Heinonen S, Niskanen L. Effect of intensive counselling on the quality of dietary fats in pregnant women at high risk of gestational diabetes mellitus. British Journal of Nutrition 2012;108(5):910‐7. [DOI] [PubMed] [Google Scholar]
- Korpi‐Hyovalti EA, Laaksonen DE, Schwab US, Vanhapiha TH, Vihla KR, Heinonen ST, et al. Feasibility of a lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health 2011;11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laitinen 2009 {published data only}
- Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. European Journal of Clinical Nutrition 2011;65(1):10‐9. [DOI] [PubMed] [Google Scholar]
- Hautero U, Laakso P, Linderborg K, Niinivirta K, Poussa T, Isolauri E, et al. Proportions and concentrations of serum n‐3 fatty acids can be increased by dietary counseling during pregnancy. European Journal of Clinical Nutrition 2013;67(11):1163‐8. [DOI] [PubMed] [Google Scholar]
- Hoppu U, Isolauri E, Koskinen P, Laitinen K. Diet and blood lipids in 1‐4 year‐old children. Nutrition Metabolism & Cardiovascular Diseases 2013;23(10):980‐6. [DOI] [PubMed] [Google Scholar]
- Huurre A, Laitinen K, Rautava S, Korkeamaki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double‐blind placebo‐controlled study. Clinical and Experimental Allergy 2008;38(8):1342‐8. [DOI] [PubMed] [Google Scholar]
- Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo‐controlled trial. Clinical Nutrition 2011;30(2):156‐64. [DOI] [PubMed] [Google Scholar]
- Kaplas N, Isolauri E, Lampi AM, Ojala T, Laitinen K. Dietary counseling and probiotic supplementation during pregnancy modify placental phospholipid fatty acids. Lipids 2007;42(9):865‐70. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Ilmonen J, Isolauri E. Dietary counselling and probiotic intervention during pregnancy modify postpartum adiposity. Annals of Nutrition and Metabolism 2011;58(Suppl 3):87. [Google Scholar]
- Laitinen K, Poussa T, Isolauri E, Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. British Journal of Nutrition 2009;101(11):1679‐87. [DOI] [PubMed] [Google Scholar]
- Niinivirta K, Isolauri E, Laakso P, Linderborg K, Laitinen K. Dietary counseling to improve fat quality during pregnancy alters maternal fat intake and infant essential fatty acid status. Journal of Nutrition 2011;141(7):1281‐5. [DOI] [PubMed] [Google Scholar]
- Piirainen T, Isolauri E, Lagstrom H, Laitinen K. Impact of dietary counselling on nutrient intake during pregnancy: a prospective cohort study. British Journal of Nutrition 2006;96(6):1095‐104. [DOI] [PubMed] [Google Scholar]
- Vahamiko S, Isolauri E, Laitinen K. Weight status and dietary intake determine serum leptin concentrations in pregnant and lactating women and their infants. British Journal of Nutrition 2013;110(6):1098‐106. [DOI] [PubMed] [Google Scholar]
Leiferman 2011 {published data only}
- Leiferman J. My baby, my move: an antenatal community‐based physical activity intervention. Annals of Behavioral Medicine 2011;41 Suppl 1:S135. [Google Scholar]
- Nodine M. The Contribution of Physical Activity to Sleep Parameters in Pregnancy within the Context of Prepregnant Body Mass Index [thesis]. University of Colorado, 2011. [Google Scholar]
Louie 2011 {published data only}
- Brand‐Miller J. A randomized, two‐arm parallel dietary intervention study to compare the effects of consuming a low glycemic diet or wholegrain high fibre diet on infant birth weight and body composition, complications related to Gestational Diabetes Mellitus (GDM) and progression to GDM diagnosis in women at high‐risk of GDM. Australian New Zealand Clinical Trials Register (www.anzctr.org.au) (accessed 11 February 2011).
- Louie JC, Markovic TP, Perera N, Foote D, Petocz P, Ross GP, et al. A randomized controlled trial investigating the effects of a low‐glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011;34:2341‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luoto 2011 {published data only}
- Aittasalo M, Raitanen J, Kinnunen TI, Ojala K, Kolu P, Luoto R. Is intensive counseling in maternity care feasible and effective in promoting physical activity among women at risk for gestational diabetes? Secondary analysis of a cluster randomized NELLI study in Finland. International Journal of Behavioral Nutrition and Physical Activity 2012;9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolu P, Raitanen J, Rissanen P, Luoto R. Health care costs associated with gestational diabetes mellitus among high‐risk women‐‐results from a randomised trial. BMC Pregnancy & Childbirth 2012;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large‐for‐gestational‐age newborns by lifestyle counseling: a cluster‐randomized controlled trial. PLoS Medicine 2011;8(5):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto RM, Kinnunen TI, Aittasalo M, Ojala K, Mansikkamaki K, Toropainen E, et al. Prevention of gestational diabetes: design of a cluster‐randomized controlled trial and one‐year follow‐up. BMC Pregnancy and Childbirth 2010;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhkala J, Luoto R, Ahotupa M, Raitanen J, Vasankari T. Postpartum weight retention is associated with elevated ratio of oxidized LDL lipids to HDL‐cholesterol. Lipids 2013;48(12):1227‐35. [DOI] [PubMed] [Google Scholar]
Magee 1990 {published data only}
Marcinkevage 2012 {published data only}
- Marcinkevage J, Correa A, Ramakrishnan U, Sharma A, Venkat KM, Umpierrez G. Reducing sedentary behavior and increasing physical activity during pregnancy: A feasibility study. Diabetes 2012;61 Suppl 1:A344. [Google Scholar]
- Umpierrez G. Lifestyle intervention to limit excessive weight gain during pregnancy in minority women. ClinicalTrials.gov (accessed 21 May 2013). NCT01084941 2010.
Moses 2009 {published data only}
- Moses RG, Barker M, Winter M, Petocz P, Brand‐Miller JC. Can a low‐glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009;32(6):996‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moses 2014 {published data only}
- Brand‐Miller J. A pregnancy intervention to reduce postprandial glucose excursions in the primary prevention of paediatric obesity. Current Controlled Trials (www.controlled‐trials.com/) [accessed 31 October 2010].
- Moses RG, Casey S, Cleary J, Milosavljevic M, Quinn E, Tapsell L, et al. Effect of low glycaemic index dietary advice in normal pregnancy: The PREGGIO study. Obesity Research and Clinical Practice 2013;7:e34‐5. [Google Scholar]
- Moses RG, Casey SA, Quinn EG, Cleary JM, Tapsell LC, Milosavljevic M, et al. Pregnancy and Glycemic Index Outcomes study: effects of low glycemic index compared with conventional dietary advice on selected pregnancy outcomes. American Journal of Clinical Nutrition 2014;99(3):517‐23. [DOI] [PubMed] [Google Scholar]
Mujsindi 2014 {published data only}
- Mujsindi W, Habash D, Childs G. Impact of nutrition education on gestational weight gain in obese pregnant women. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S188. [Google Scholar]
Murtezani 2014 {published data only}
- Murtezani A, Pacarada M, Ibraimi Z, Nevzati A, Abazi N. The impact of exercise during pregnancy on neonatal outcomes: a randomised controlled trial. Journal of Sports Medicine and Physical Fitness 2014;54(6):802‐8. [PubMed] [Google Scholar]
Nascimento 2012 {published data only}
- Nascimento KLK, Surita SLN, Parpinelli FGS, Kasawara MAP. Type of delivery and neonatal outcome in overweight and obese pregnant women with excessive weight gain. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):73‐4. [Google Scholar]
- Nascimento SL, Surita FG, Parpinelli MA, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: a randomised clinical trial. BJOG: an International Journal of Obstetrics & Gynaecology 2011;118(12):1455‐63. [DOI] [PubMed] [Google Scholar]
- Surita F. Physical exercise influence among overweight and obese pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2010.
Oostdam 2012 {published data only}
- Oostdam N, Bosmans J, Wouters MGAJ, Eekhoff EMW, Mechelen W, Poppel MNM. Cost‐effectiveness of an exercise program during pregnancy to prevent gestational diabetes: Results of an economic evaluation alongside a randomised controlled trial. BMC Pregnancy and Childbirth 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostdam N, Poppel MN, Eekhoff EM, Wouters MG, Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy and Childbirth 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostdam N, Poppel MNM, Wouters MGAJ, Eekhoff EMW, Bekedam DJ, Kuchenbecker WKH, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: Results of a randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2012;119(9):1098‐107. [DOI] [PubMed] [Google Scholar]
- Poppel M, Oostdam N, Wouters M, Eekhoff M, Mechelen W. FitFor2: Effects of an exercise training program on the incidence of gestational diabetes. Journal of Science and Medicine in Sport 2012;15 Suppl 1:S342‐S343. [Google Scholar]
Petrella 2013 {published data only}
- Facchinetti F. Pregnancy complications in women with BMI > 25kg/m2 enrolled in a healthy lifestule and eating habits program. ClinicalTrials.gov (accessed 21 May 2013). NCT01783210 2013.
- Petrella E, Facchinetti F, Bertarini V, Pignatti L, Neri I, Battistini NC. Occurrence of pregnancy complications in women with BMI > 25 submitted to a healthy lifestyle and eating habits program. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S33‐4. [Google Scholar]
- Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. Journal of Maternal‐Fetal and Neonatal Medicine 2014;27(13):1348‐52. [DOI] [PubMed] [Google Scholar]
Petrov Fieril 2014 {published data only}
Phelan 2011 {published data only}
- Phelan S, Phipps MG, Abrams B, Darroch F, Grantham K, Schaffner A, et al. Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve‐month outcomes of the Fit for Delivery randomized trial. American Journal of Clinical Nutrition 2014;99(2):302‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Factors associated with success in the "fit for delivery" intervention to reduce excessive gestational weight gain. Obesity 2011;19(Suppl 1):S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. American Journal of Clinical Nutrition 2011;93(4):772‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinzon 2012 {published data only}
- Pinzon DC, Zamora K, Martinez JH, Florez‐Lopez ME, Plata AC, Mosquera M, et al. Type of delivery and gestational age is not affected by pregnant Latin‐American women engaging in vigorous exercise: a secondary analysis of data from a controlled randomized trial. Revista de Salud Publica 2012;14(5):731‐43. [PubMed] [Google Scholar]
- Ramirez‐Velez R. Aerobic exercise in pregnant Latina women: Effect on metabolic and body composition outcomes. A randomized clinical trial. FASEB Journal 2014;28(1 Suppl 1):886.6. [Google Scholar]
- Ramirez‐Velez R. Combined aerobic and resistance exercise on metabolic and body composition outcomes in primigravid Latina women. Obesity Reviews 2014;15(Suppl 2):201‐2. [Google Scholar]
- Ramirez‐Velez R, Aguilar de Plata AC, Escudero MM, Echeverry I, Ortega JG, Salazar B, et al. Influence of regular aerobic exercise on endothelium‐dependent vasodilation and cardiorespiratory fitness in pregnant women. Journal of Obstetrics and Gynaecology Research 2011;37(11):1601‐8. [DOI] [PubMed] [Google Scholar]
Pollak 2014 {published data only}
- Pollak KI, Alexander SC, Bennett G, Lyna P, Coffman CJ, Bilheimer A, et al. Weight‐related SMS texts promoting appropriate pregnancy weight gain: A pilot study. Patient Education and Counselling 2014;97:256‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polley 2002 {published data only}
- Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2002;26(11):1494‐502. [DOI] [PubMed] [Google Scholar]
Poston 2013 {published data only}
- Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy and Childbirth 2014;14(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes L, Bell R, Robson S, Poston L. Association between physical activity in obese pregnant women and pregnancy outcomes: The UPBEAT Pilot Study. Annals of Nutrition & Metabolism 2014;64(3‐4):239‐46. [DOI] [PubMed] [Google Scholar]
- Maitland RA, Barr S, Briley A, Seed P, Poston L. Incidence of gestational diabetes in an obese population using the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria in the UK Pregnancies Better Eating and Activity Trial (UPBEAT) pilot study. Diabetic Medicine 2012;29(Suppl 1):152. [Google Scholar]
- Poston L. Improving pregnancy outcome in obese women: a feasibility study. Current Controlled Trials (http://controlled‐trials.com/) (accessed 31 October 2010).
- Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy and Childbirth 2013;13(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger C, Flynn A, Barr S, Seed PT, Inskip HM, Poston L. Maternal diet patterns and glycaemic load in obese pregnant women taking part in a pilot trial of a lifestyle intervention (the upbeat trial). Diabetes 2014; Vol. 63.
Price 2012 {published data only}
- Price B, Amini B, Kappeler K. Exercise in pregnancy: Effect on fitness and obstetric outcomes ‐ a randomized trial. Medicine & Science in Sports & Exercise 2012;44(12):2263‐9. [DOI] [PubMed] [Google Scholar]
Quinlivan 2011 {published data only}
- Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four‐step multidisciplinary approach to the antenatal care of obese pregnant women. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:141‐6. [DOI] [PubMed] [Google Scholar]
Rae 2000 {published data only}
- Rae A, Bond D, Evans S, North F, Roberman B, Walters B. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2000;40(4):416‐22. [DOI] [PubMed] [Google Scholar]
Rauh 2013 {published data only}
- Rauh K, Gabriel E, Kerschbaum E, Schuster T, Kries R, Amann‐Gassner U, et al. Safety and efficacy of a lifestyle intervention for pregnant women to prevent excessive maternal weight gain: a cluster‐randomized controlled trial. BMC Pregnancy and Childbirth 2013;13(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Renault 2014 {published data only}
- Renault KM, Norgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The Treatment of Obese Pregnant Women (TOP) study: A randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. American Journal of Obstetrics and Gynecology 2014;210(2):134.e1‐9. [DOI] [PubMed] [Google Scholar]
Rhodes 2010 {published data only}
- Pawlak DB. Glycemic load and infant birth weight in pregnant overweight/obese women. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 31 October 2010).
- Rhodes ET, Pawlak DB, Takoudes TC, Ebbeling CB, Feldman HA, Lovesky MM, et al. Effects of a low‐glycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. American Journal of Clinical Nutrition 2010;92(6):1306‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
ROLO 2012 {published data only}
- Donnelly J, Horan M, Walsh J, McGowan C, Byrne J, Molloy EJ, et al. Impact of a low GI diet on neonatal body composition (ROLO Kids). Pediatric Academic Societies Annual Meeting; 2013 May 4‐7; Washington DC, USA. 2013.
- Donnelly JM, Walsh JM, Byrne J, Molloy E, McAuliffe FM. Altered neonatal anthropometric measurements following maternal low GI diet in pregnancy (ROLO study). Acta Obstetricia et Gynecologica Scandinavica 2013;92(s160):13. [Google Scholar]
- Horan M, McGowan C, Donnelly J, Gibney E, McAuliffe F. Maternal diet and weight at 3 months partum following a pregnancy intervention with a low glycaemic index diet: Results from the ROLO randomised control trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A129‐A130, Abstract no: PMM.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan MK, McGowan CA, Donnelly J, Gibney E, McAuliffe FM. The association of maternal characteristics and macronutrient intake in pregnancy with neonatal body composition. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A11. [Google Scholar]
- Horan MK, McGowan CA, Doyle O, McAuliffe FM. Well‐being in pregnancy: An examination of the effect of socioeconomic, dietary and lifestyle factors including impact of a low glycaemic index dietary intervention. European Journal of Clinical Nutrition 2014;68(1):19‐24. [DOI] [PubMed] [Google Scholar]
- Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. Maternal diet and weight at 3 months postpartum following a pregnancy intervention with a low glycaemic index diet: results from the ROLO randomised control trial. Nutrients 2014;6(7):2946‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. Maternal low glycaemic index diet, fat intake and postprandial glucose influences neonatal adiposity ‐ secondary analysis from the ROLO study. Nutrition Journal 2014;13(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony R, Byrne J, Curran S, O'Herlihy C, McAuliffe F. A pilot study of the feasibility of a randomised trial of low glycaemic diet versus normal diet from early pregnancy in euglycaemic women. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa38. [Google Scholar]
- McAuliffe F. A randomised controlled trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of foetal macrosomia. Current Controlled Trials (www.controlled‐trials.com/) (accessed 12.05.2010). [DOI] [PMC free article] [PubMed]
- McGowan CA, Walsh JM, Byrne J, Curran S, McAuliffe FM. The influence of a low glycemic index dietary intervention on maternal dietary intake, glycemic index and gestational weight gain during pregnancy: a randomized controlled trial. Nutrition Journal 2013;12(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Mahony R, Foley M, Auliffe F. A randomised control trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of macrosomia. BMC Pregnancy and Childbirth 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Mahony R, Foley M, McAuliffe F. ROLO study: a randomized control trial of low glycemic index diet to prevent macrosomia in euglycemic women. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S4. [Google Scholar]
- Walsh J, McGowan C, Byrne J, Foley M, Mahony R, McAuliffe F. The influence of a low glycaemic index dietary intervention on maternal glycaemic index, dietary intake and gestational weight gain. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S33. [Google Scholar]
- Walsh JM, Mahony RM, Canty G, Foley ME, McAuliffe FM. Identification of those most likely to benefit from a low‐glycaemic index dietary intervention in pregnancy. British Journal of Nutrition 2014;112:583‐9. [DOI] [PubMed] [Google Scholar]
- Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012;345:e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ronnberg 2014 {published data only}
- Nilsson K. Weight gain during pregnancy ‐ a randomized controlled trial of intervention to prevent excessive gestational weight gain. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 September 2014] 2009.
- Ronnberg A, Ostlund I, Fadl H, Gottvall T, Nilsson K. Intervention during pregnancy to reduce excessive gestational weight gain‐a randomised controlled trial. BJOG :an International Journal of Obstetrics and Gynaecology 2014 Nov 4 [Epub ahead of print]. [DOI] [PubMed]
Ruchat 2012 {published data only}
- Ruchat SM, Davenport MH, Giroux I, Hillier M, Batada A, Sopper MM, et al. Nutrition and exercise reduce excessive weight gain in normal‐weight pregnant women. Medicine and Science in Sports and Exercise 2012;44(8):1419‐26. [DOI] [PubMed] [Google Scholar]
Ruiz 2013 {published data only}
- Ruiz R, Perales M, Pelaez M, Lopez C, Lucia A, Barakat R. Supervised exercise‐based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clinic Proceedings 2013;88(12):1388‐97. [DOI] [PubMed] [Google Scholar]
- Santaella MP. The role of a supervised physical exercise program as an alternative on the control of maternal gestational weight gain. ClinicalTrials.gov (accessed 21 May 2013). NCT01790347 2013.
Santos 2005 {published data only}
- Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, et al. Aerobic exercise and submaximal functional capacity in overweight pregnant women: a randomized trial. Obstetrics & Gynecology 2005;106(2):243‐9. [DOI] [PubMed] [Google Scholar]
Stafne 2012 {published data only}
- Morkved S. Effects of regular exercise during pregnancy. ClinicalTrials.gov (www.clinicaltrials.gov) (accessed 31 October 2010).
- Stafne SN, Salvesen KÅ, Romundstad PR, Eggebø TM, Carlsen SM, Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstetrics & Gynecology 2012;119(1):29‐36. [DOI] [PubMed] [Google Scholar]
Szmeja 2011 {published data only}
- Szmeja MA, Grivell RM, Deussen AR, Dodd JM. Evaluation of information provision to women who are overweight or obese during pregnancy. Journal of Paediatrics and Child Health 2011;47(Suppl 1):78. [Google Scholar]
Thornton 2009 {published data only}
- Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. Journal of the National Medical Association 2009;101:569‐77. [DOI] [PubMed] [Google Scholar]
Vesco 2013 {published data only}
- Vesco K, Leo M, Gillman M, King J, McEvoy C, Karanjaa N, et al. Impact of a weight management intervention on pregnancy outcomes among obese women: The Healthy Moms Trial. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S352. [Google Scholar]
- Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, et al. Efficacy of a group‐based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity (Silver Spring, Md.) 2014;22(9):1989‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesco KK, Karanja N, King JC, Gillman MW, Perrin N, McEvoy C, et al. Healthy Moms, a randomized trial to promote and evaluate weight maintenance among obese pregnant women: study design and rationale. Contemporary Clinical Trials 2012;33(4):777‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinter 2012 {published data only}
- Tanvig M. Lifestyle in pregnancy and offspring (LiPO). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 6 February 2014] 2013.
- Tanvig M. Lifestyle in pregnancy and offspring ‐ comparison between children born to obese women and children born to normal weight women (LiPO). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 6 February 2014] 2013.
- Tanvig M. Offspring body size and metabolic profile ‐ Effects of lifestyle intervention in obese pregnant women. Danish Medical Journal 2014;61(7):B4893. [PubMed] [Google Scholar]
- Tanvig M, Vinter CA, Jorgensen JS, Wehberg S, Ovesen PG, Beck‐Nielsen H, et al. Effects of lifestyle intervention in pregnancy and anthropometrics at birth on offspring metabolic profile at 2.8 years ‐ results from the Lifestyle in Pregnancy and Offspring (LiPO) study. Journal of Clinical Endocrinology and Metabolism 2015;100(1):175‐83. [DOI] [PubMed] [Google Scholar]
- Vinter A. Lifestyle and pregnancy: the clinical effect of lifestyle intervention during pregnancy in obese women. ClinicalTrials.gov (www.clinicaltrials.gov) [accessed 31 October 2010].
- Vinter C, Jensen D, Ovesen P, Beck‐Nielsen H, Lamont R, Jorgensen J. Postpartum weight retention and breastfeeding among obese women from the LiP (Lifestyle in Pregnancy) Study. Acta Obstetricia et Gynecologica Scandinavica 2012;91(Suppl 159):141‐2. [DOI] [PubMed] [Google Scholar]
- Vinter CA, Jensen DM, Ovesen P, Beck‐Nielsen H, Jorgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011;34(12):2502‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter CA, Jensen DM, Ovesen P, Beck‐Nielsen H, Tanvig M, Lamont RF, et al. Postpartum weight retention and breastfeeding among obese women from the randomized controlled Lifestyle in Pregnancy (LiP) trial. Acta Obstetricia et Gynecologica Scandinavica 2014;93:794‐801. [DOI] [PubMed] [Google Scholar]
Vitolo 2011 {published data only}
- Vitolo MR. Impact of a nutritional intervention program for weight control during pregnancy. ClinicalTrials.gov (www.clinicaltrials.gov) [accessed 31 October 2010].
- Vitolo MR, Fraga Bueno MS, Mendes Gama C. Impact of a dietary counseling program on the gain weight speed of pregnant women attended in a primary care service. Revista Brasileira de Ginecologia e Obstetricia 2011;33(1):13‐9. [PubMed] [Google Scholar]
Wilkinson 2012 {published data only}
- Wilkinson SA, McIntyre HD. Evaluation of the 'healthy start to pregnancy' early antenatal health promotion workshop: a randomized controlled trial. BMC Pregnancy and Childbirth 2012;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 2008 {published data only}
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. International Journal of Obesity 2008;32(3):495‐501. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asemi 2011 {published data only}
- Asemi Z, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on serum levels of calcium, Iron and liver enzymes in pregnant women. International Journal of Preventive Medicine 2013;4(8):949‐55. [PMC free article] [PubMed] [Google Scholar]
- Asemi Z, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effect of daily consumption of probiotic yogurt on oxidative stress in pregnant women: A randomized controlled clinical trial. Annals of Nutrition and Metabolism 2012;60(1):62‐8. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: a randomized controlled trial. Pakistan Journal of Biological Sciences 2011;14(8):476‐82. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(9):1552‐6. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Samimi M, Tabassi Z, Naghibi M, Rahimi A, Khorammian H, et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. European Journal of Clinical Nutrition 2013;67(1):71‐4. [DOI] [PubMed] [Google Scholar]
- Taghizadeh M, Hashemi T, Shakeri H, Abedi F, Sabihi SS, Alizadeh SA, et al. Synbiotic food consumption reduces levels of triacylglycerols and VLDL, but not cholesterol, LDL, or HDL in plasma from pregnant women. Lipids 2014;49(2):155‐61. [DOI] [PubMed] [Google Scholar]
Bechtel‐Blackwell 2002 {published data only}
- Bechtel‐Blackwell DA. Computer‐assisted self‐interview and nutrition education in pregnant teens. Clinical Nursing Research 2002;11(4):450‐62. [DOI] [PubMed] [Google Scholar]
Boileau 1968 {published data only}
- Boileau PA. Control of weight‐gain during pregnancy: use of diethylpropion hydrochloride. Applied Therapeutics 1968;10:763‐5. [PubMed] [Google Scholar]
Breslow 1963 {published data only}
- Breslow S, Belafsky HA, Shangold JE, Hirsch LM, Stahl MB. Control of weight gain in pregnancy: double blind study of a dieting aid. Clinical Medicine 1963;70:931‐8. [PubMed] [Google Scholar]
Campbell 2004 {published data only}
- Campbell MK, Carbone E, Honess‐Morreale L, Heisler‐Mackinnon J, Demissie S, Farrell D. Randomized trial of a tailored nutrition education CD‐ROM program for women receiving food assistance. Journal of Nutrition Education & Behavior 2004;36(2):58‐66. [DOI] [PubMed] [Google Scholar]
Daley 2014 {published data only}
- Daley A, Jolly K, Lewis A, Clifford S, Kenyon AK, Roalfe SJ, et al. The feasibility and acceptability of regular weighing of pregnant women by community midwives to prevent excessive weight gain: RCT. Pregnancy Hypertension 2014;4(3):233. [DOI] [PubMed] [Google Scholar]
Davenport 2011 {published data only}
- Davenport H. Target weight program of pregnancy for improved prenatal care. JOGNN: Journal of Obstetric, Gynecologic & Neonatal Nursing 2011;40(Suppl 1):S101. [Google Scholar]
Faucher 2008 {published data only}
- Faucher MA. Promotoras de salud and portion control: a community intervention aimed at weight loss in low‐income Mexican‐American women. Journal of Midwifery and Women's Health 2008;53(5):482. [DOI] [PubMed] [Google Scholar]
Graham 2014 {published data only}
- Graham ML, Uesugi KH, Niederdeppe J, Gay GK, Olson CM. The theory, development, and implementation of an e‐intervention to prevent excessive gestational weight gain: e‐moms roc. Telemedicine Journal and E‐Health 2014;20(12):1135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray‐Donald 2000 {published data only}
- Gray‐Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ: Canadian Medical Association Journal 2000;163(10):1247‐51. [PMC free article] [PubMed] [Google Scholar]
Hauner 2012 {published data only}
- Hauner H, Much D, Vollhardt C, Brunner S, Schmid D, Sedlmeier E‐M, et al. Effect of reducing the n26:n23 long‐chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open‐label randomized controlled trial. American Journal of Clinical Nutrition 2012;95(2):383‐94. [DOI] [PubMed] [Google Scholar]
Hausenblas 2008 {published data only}
- Hausenblas HA, Brewer BW, Raalte JL, Cook B, Downs DS, Weis CA, et al. Development and evaluation of a multimedia CD‐ROM for exercise during pregnancy and postpartum. Patient Education and Counseling 2008;70(2):215‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 1990 {published data only}
- Ismail MA, Nelson KE, Larson P, Moses VK. Selective effect of cefoxitin prophylaxis on post‐cesarean‐section microbial flora. Journal of Reproductive Medicine 1990;35:168‐74. [PubMed] [Google Scholar]
Kinnunen 2007 {published data only}
- Aittasalo M, Pasanen M, Fogelholm M, Kinnunen TI, Ojala K, Luoto R. Physical activity counseling in maternity and child health care ‐ a controlled trial. BMC Women's Health 2008;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi‐Clarke L, Weiderpass E, et al. Preventing excessive weight gain during pregnancy ‐ a controlled trial in primary health care. European Journal of Clinical Nutrition 2007;61(7):884‐91. [DOI] [PubMed] [Google Scholar]
- Luoto R. Physical activity and dietary counseling and supervised group exercises for first‐time pregnant women ‐ a feasibility study of a controlled trial. Current Controlled Trials (www.controlled‐trials.com/) [accessed 21 June 2007].
- Luoto R, Kharazmi E, Saarinen NM, Smeds AI, Makela S, Fallah M, et al. Effect of dietary intervention on serum lignan levels in pregnant women ‐ a controlled trial. Reproductive Health. ISRCTN21512277 2010; Vol. 7, issue 1:26. [DOI] [PMC free article] [PubMed]
- Mustila T, Raitanen J, Keskinen P, Saari A, Luoto R. Lifestyle counseling during pregnancy and offspring weight development until four years of age: follow‐up study of a controlled trial. Journal of Negative Results in Biomedicine 2012;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsay 2014 {published data only}
- Lindsay K, Kennelly M, Smith T, Maguire O, Shanahan F, Brennan L, et al. Probiotics in obese pregnancy to reduce maternal fasting glucose: A randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A156, Abstract no: PPO.19. [Google Scholar]
Maitland 2014 {published data only}
- Maitland RA, Patel NR, Sherry C, Marriage B, Barr S, Lopez‐pedrosa JM, et al. A pilot study to evaluate the effects of a dietary supplement with slow digesting‐low GI carbohydrates in obese pregnant women using continuous glucose monitoring. Diabetes 2014;63(Suppl 1):A342, Abstract no: 1311‐P. [Google Scholar]
Mohebi 2009 {published data only}
- Mohebi S. Effect of nutrition education program on the recommended weight gain during pregnancy in pregnant women; application of health belief model. Iranian Registry of Clinical Trials (http://www.irct.ir/) [accessed 6 December 2010].
Moses 2006 {published data only}
- Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P, et al. Effect of a low‐glycemic‐index diet during pregnancy on obstetric outcomes. American Journal of Clinical Nutrition 2006;84(4):807‐12. [DOI] [PubMed] [Google Scholar]
- Moses RG, Luebke M, Petocz P, Brand‐Miller JC. Maternal diet and infant size 2 y after the completion of a study of a low‐glycemic‐index diet in pregnancy. American Journal of Clinical Nutrition 2007;86(6):1806. [DOI] [PubMed] [Google Scholar]
Mottola 2010 {published data only}
- Mottola MF, Giroux I, Gratton R, Hammond J, Hanley A, Harris S, et al. Nutrition and exercise prevent excess weight gain in overweight pregnant women. Medicine and Science in Sports and Exercise 2010;42(2):265‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 2004 {published data only}
- Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. American Journal of Obstetrics and Gynecology 2004;191(2):530‐6. [PUBMED: 15343232] [DOI] [PubMed] [Google Scholar]
Silverman 1971 {published data only}
- Silverman M, Okun R. The use of an appetite suppressant (diethylpropion hydrochloride) during pregnancy. Current Therapeutic Research, Clinical and Experimental 1971;13:648‐53. [PubMed] [Google Scholar]
Stutzman 2010 {published data only}
- Stutzman SS, Brown CA, Hains SM, Godwin M, Smith GN, Parlow JL, et al. The effects of exercise conditioning in normal and overweight pregnant women on blood pressure and heart rate variability. Biological Research for Nursing 2010;12(2):137‐48. [DOI] [PubMed] [Google Scholar]
Te Morenga 2011 {published data only}
- Morenga L. A randomised, controlled dietary intervention to evaluate the effect of a high protein diet compared with a high carbohydrate, high fibre diet on individual components of the metabolic syndrome and marks of cardiovascular disease risk in overweight and obese women. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) [accessed 16 February 2011].
Walker 1966 {published data only}
- Walker JL. Methylclothiazide in excessive weight gain and edema of pregnancy. Obstetrics & Gynecology 1966;27:247‐51. [PubMed] [Google Scholar]
Wisner 2006 {published data only}
- Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DK, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. Journal of Clinical Psychopharmacology 2006;26(4):353‐60. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Shakeri 2012 {published data only}
- Shakeri M, Fekri S, Shahnavaz A, Shakibazadeh E. Effectiveness of a group‐based educational program on physical activity among pregnant women. HAYAT 2012;18(3):1‐9. [Google Scholar]
References to ongoing studies
Astrup 2013 {published data only}
- Astrup AV. An optimized programming of healthy children (APPROACH). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 February 2014] 2013.
Atkinson 2013 {published data only}
- Atkinson SA. Be healthy in pregnancy (B‐HIP): a trial to study nutrition and exercise approaches for healthy (B‐HIP). ClinicalTrials.gov [accessed 21 May 2013] 2013.
Barakat 2012 {published data only}
- Barakat R. Effect of a supervised exercise program in obese and overweight pregnant women on outcomes and level of depression. A randomized controlled trial. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2012.
Brownfoot 2011 {published data only}
- Brownfoot F. In antenatal women, does weighing at each visit compared with routine antenatal care reduce the incidence of excessive weight gain during pregnancy?. Australian New Zealand Clinical Trials Register (www.anzctr.org.au) (accessed 27 July 2011).
Callaway 2012 {published data only}
- Callaway L. Randomized placebo controlled trial of probiotics in overweight and obese women to assess the prevention of gestational diabetes. Australian Clinical Trials Registry (www.actr.org.au) [accessed 6 July 2012] 2012.
Chasan‐Taber 2009 {published data only}
- Chasan‐Taber L, Marcus BH, Stanek E 3rd, Ciccolo JT, Marquez DX, Solomon CG, et al. A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: design and methods. Journal of Women's Health 2009;18(6):851‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan‐Taber L, Silveira M, Marcus BH, Braun B, Stanek E, Markenson G. Feasibility and efficacy of a physical activity intervention among pregnant women: the behaviours affecting baby and you (B.A.B.Y) study. Journal of Physical Activity & Health 2011;8 Suppl 2:S228‐S238. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Chasen‐Taber L, Marcus B, Stanek E, Braun B, Ciccolo J, et al. Impact of an exercise intervention on physical activity during pregnancy: the behaviors affecting baby and you study. American Journal of Public Health 2014;104(10):e74‐e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chasan‐Taber 2013 {published data only}
- Chasan‐Taber L. Lifestyle intervention in overweight and obese pregnant Hispanic women. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 6 February 2014] 2013.
Choi 2011 {published data only}
- Choi J. The MOTHER (Mobile technologies to help enhancing regular physical activity) trial for pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 22 May 2014] 2011.
Downs 2011 {published data only}
- Downs DS. Determinants and outcomes of physical activity in pregnancy: findings from active MOMS, a randomized physical activity intervention for pregnant women. Annals of Behavioral Medicine 2011;41 Suppl 1:S135. [Google Scholar]
Farajzadegan 2013 {published data only}
- Farajzadegan Z, Pozveh ZA. The design of maternal centered life‐style modification program for weight gain management during pregnancy ‐ a study protocol. Journal of Research in Medical Sciences 2013;18(8):683‐7. [PMC free article] [PubMed] [Google Scholar]
Fernandez 2011 {published data only}
- Fernandez D. eMoms of Rochester. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 22 May 2014] 2011.
Ferrara 2014 {published data only}
- Ferrara A, Hedderson MM, Albright CL, Brown SD, Ehrlich SF, Caan BJ, et al. A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes' Effects on Moms (GEM) study. BMC Pregnancy and Childbirth 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2012 {published data only}
- Goldberg L. The OHSU pregnancy exercise & nutrition (PEN) program. ClinicalTrials.gov (accessed 21 May 2013) 2012.
Graziano 2013 {published data only}
- Graziano S. Gestational weight gain and the electronic medical record. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 February 2014] 2013.
Hivert 2012 {published data only}
- Hivert MF. Intervention to promote changes of healthy lifestyle (physical activiity and nutrition) during gestation. ClinicalTrials.gov (accessed 21 May 2013) 2012.
John 2014 {published data only}
- Cassidy D, John E, Copeland L, Simpson SA, for the HELP study team. Weight management in pregnancy: participants' experiences of 'Healthy Eating and Lifestyle in Pregnancy (HELP)", a maternity care intervention for obese pregnant women. Pregnancy Hypertension 2014;4(3):233. [DOI] [PubMed] [Google Scholar]
- John E, Cassidy DM, Playle R, Jewell K, Cohen D, Duncan D, et al. Healthy eating and lifestyle in pregnancy (HELP): a protocol for a cluster randomised trial to evaluate the effectiveness of a weight management intervention in pregnancy. BMC Public Health 2014;14:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SA, Cassidy D, John E, for the HELP study team. 'Healthy Eating and Lifestyle in Pregnancy (HELP)' trial: process evaluation framework. Pregnancy Hypertension 2014;4(3):233. [DOI] [PubMed] [Google Scholar]
King 2013 {published data only}
- King JC. Postprandial response to almond consumption in overweight Hispanic pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 6 February 2014] 2013.
Ko 2010 {published data only}
- Ko CW. Effect of physical activity on metabolic syndrome in pregnancy and fetal outcome. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 31 October 2010).
Laitinen 2013 {published data only}
- Laitinen K. Nutrition and pregnancy intervention study. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 February 2014] 2013.
Li 2011 {published data only}
- Li G. Effects of dietary and lifestyle interventions in obese pregnant women from the first trimester on gestational weight gain and pregnancy outcomes. ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 20 March 2014] 2011.
Liu 2011 {published data only}
- Liu S. Magnesium supplementation in the second trimester of pregnancy for overweight individuals. ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 2011] 2011.
McAuliffe 2013 {published data only}
- McAuliffe F. Pregnancy, exercise and nutrition research study with app support. Current Controlled Trials [(accessed 31 May 2013] 2013.
Moholdt 2011 {published data only}
- Moholdt TT, Salvesen K, Ingul CB, Vik T, Oken E, Morkved S. Exercise Training in Pregnancy for obese women (ETIP): study protocol for a randomised controlled trial. Trials 2011;12:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagle 2011 {published data only}
- Nagle C, Skouteris H, Hotchin A, Bruce L, Patterson D, Teale G. Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial. BMC Public Health 2011;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagle 2013 {published data only}
- Nagle C, Skouteris H, Morris H, Nankervis A, Rasmussen B, Mayall P, et al. Primary prevention of gestational diabetes for women who are overweight and obese: a randomised controlled trial. BMC Pregnancy and Childbirth 2013;13(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 2012 {published data only}
- Norman J. A multicentre randomised placebo controlled clinical trial of metformin versus placebo in pregnant women to reduce the risk of obesity and metabolic syndrome in their babies. http://www.controlled‐trials.com/ISRCTN51279843/EMPOWaR (accessed 22 June 2012) 2012.
Parat 2010 {published data only}
- Parat S. Impact of education during pregnancy in overweight pregnant women (ETOIG). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 31 October 2010].
Peccei 2010 {published data only}
- Peccei A. Nutrition intervention for the promotion of healthy weight gain during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) [(accessed 21 May 2013] 2010.
Rauh 2014 {published data only}
- Rauh K, Kunath J, Rosenfeld E, Kick L, Ulm K, Hauner H. Healthy living in pregnancy: a cluster‐randomized controlled trial to prevent excessive gestational weight gain ‐ rationale and design of the GeliS study. BMC Pregnancy and Childbirth 2014;14:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redman 2012 {published data only}
- Redman LM, Martin CK. Personalized management of body weight during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 22 May 2014] 2012.
Roberts 2012 {published data only}
- Roberts S. Interventions to reduce excess weight gain in pregnancy in overweight and obese mothers. ClinicalTrials.gov (accessed 21 May 2013) 2012.
Seneviratne 2014 {published data only}
- Seneviratne S, Cutfield W, McCowan L, Ekeroma A, Parry G, Gusso S, et al. Improve trial‐improving maternal and progeny risk of obesity via exercise‐A randomised controlled trial on effects of exercise in pregnancy in overweight and obese women and their offspring. Obesity Reviews 2014;15(Suppl 2):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne SN, Parry GK, McCowan LM, Ekeroma A, Jiang Y, Gusso S, et al. Antenatal exercise in overweight and obese women and its effects on offspring and maternal health: design and rationale of the IMPROVE (Improving Maternal and Progeny Obesity Via Exercise) randomised controlled trial. BMC Pregnancy and Childbirth 2014; Vol. 14, issue 1:148. [DOI] [PMC free article] [PubMed]
Shen 2010 {published data only}
- Shen G. Impact of diet and exercise activity on pregnancy outcomes (IDEA). ClinicalTrials.gov (www.clinicaltrials.gov) [accessed 31 October 2010].
Skouteris 2012 {published data only}
- Skouteris H, McCabe M, Milgrom J, Kent B, Bruce LJ, Mihalopoulos C, et al. Protocol for a randomized controlled trial of a specialized health coaching intervention to prevent excessive gestational weight gain and postpartum weight retention in women: the HIPP study. BMC Public Health 2012;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2010 {published data only}
- Smith DM, Whitworth M, Sibley C, Taylor W, Gething J, Chmiel C, et al. The design of a community lifestyle programme to improve the physical and psychological well‐being of pregnant women with a BMI of 30 kg/m2 or more. BMC Public Health 2010;10:284. [PUBMED: 20507580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thangaratinam 2014 {published data only}
- Thangaratinam S. Effect of simple, targeted diet in pregnant women with metabolic risk factors on pre‐eclampsia (ESTEEM): a randomised trial. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 2 September 2014] 2014.
Thomson 2014 {published data only}
- Thomson JL, Tussing‐Humphreys LM, Goodman MH. Delta Healthy Sprouts: A randomized comparative effectiveness trial to promote maternal weight control and reduce childhood obesity in the Mississippi Delta. Contemporary Clinical Trials 2014;38:82‐91. [DOI] [PubMed] [Google Scholar]
Uauy 2013 {published data only}
- Uauy R. Diet, physical activity and breastfeeding intervention on maternal nutrition, offspring growth and development. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 February 2014] 2013.
Vistad 2009 {published data only}
- Vistad I. Fit for delivery: a study of the effect of exercise sessions and nutritional counselling on pregnancy outcome (FFF). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2009.
Yan 2012 {published data only}
- Yan W. Low glycemic index diet management for overweight pregnant women (LGIDMOPW). ClinicalTrials.gov (accessed 21 May 2013) 2012.
Additional references
Abrams 1989
- Abrams B, Newman V, Key T, Parker J. Maternal weight gain and preterm delivery. Obstetrics & Gynecology 1989;74(4):577‐83. [PubMed] [Google Scholar]
Abrams 1995
- Abrams B, Carmichael S, Selvin S. Factors associated with the pattern of maternal weight gain during pregnancy. Obstetrics & Gynecology 1995;86(2):170‐6. [DOI] [PubMed] [Google Scholar]
Abrams 2000
- Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. American Journal of Clinical Nutrition 2000;71(5 Suppl):1233S‐1241S. [DOI] [PubMed] [Google Scholar]
Bianco 1998
- Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstetrics & Gynecology 1998;91(1):97‐102. [DOI] [PubMed] [Google Scholar]
Cedergren 2006
- Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. International Journal of Gynecology & Obstetrics 2006;93(3):269‐74. [DOI] [PubMed] [Google Scholar]
Cedergren 2007
- Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstetrics & Gynecology 2007;110(4):759‐64. [DOI] [PubMed] [Google Scholar]
CMACE 2010
- Centre for Maternal and Child Enquiries (CMACE). Maternal Obesity in the UK: findings from a national project. http://www.oaa‐anaes.ac.uk/assets/_managed/editor/File/CMACE/CMACE_Obesity_Report_2010_Final%20for%20printing.pdf (accessed March 2012).
Cogswell 1995
- Cogswell ME, Serdula MK, Hungerford DW, Yip R. Gestational weight gain among average‐weight and overweight women‐‐what is excessive?. American Journal of Obstetrics and Gynecology 1995;172(2 Pt 1):705‐12. [DOI] [PubMed] [Google Scholar]
Cogswell 1999
- Cogswell ME, Scanlon KS, Fein SB, Schieve LA. Medically advised, mother's personal target, and actual weight gain during pregnancy. Obstetrics & Gynecology 1999;94(4):616‐22. [DOI] [PubMed] [Google Scholar]
Crane 2013
- Crane M, Bain E, Tieu J, Han S, Middleton P, Crowther CA. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010443] [DOI] [PubMed] [Google Scholar]
Edwards 1996
- Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal‐weight women: effects of gestational weight change. Obstetrics & Gynecology 1996;87(3):389‐94. [DOI] [PubMed] [Google Scholar]
Evenson 2014
- Evenson KR, Barakat R, Brown WJ, Dargent‐Molina P, Haruna M, Mikkelsen EM, et al. Guidelines for physical activity during pregnancy: comparisons from around the world. American Journal of Lifestyle Medicine 2014;8(2):102‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2001
- Fiona F, Barrowclough D. Pregnancy ‐ associated weight gain ‐ does it contribution to the rising rate of obesity in women in UK?. Nutrition and Food Science 2001;31(4):183‐8. [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Gunderson 2000
- Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiology Review 2000;22(2):261‐74. [DOI] [PubMed] [Google Scholar]
Han 2012
- Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009021.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedderson 2006
- Hedderson MM, Weiss NS, Sacks DA, Pettitt DJ, Selby JV, Quesenberry CP, et al. Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstetrics & Gynecology 2006;108(5):1153‐61. [DOI] [PubMed] [Google Scholar]
Helms 2006
- Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. American Journal of Obstetrics and Gynecology 2006;194(5):e32‐e34. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Keppel 1993
- Keppel KG, Taffel SM. Pregnancy‐related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. American Journal of Public Health 1993;83(8):1100‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medicine 1990
- Institute of Medicine. Nutrition During Pregnancy. Washington DC: National Academy Press, 1990. [Google Scholar]
Medicine 2009
- Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. http://www.iom.edu/Reports/2009/Weight‐Gain‐During‐Pregnancy‐Reexamining‐the‐Guidelines.aspx (accessed March 2012).
Moses 2007
- Moses RG, Luebke M, Petocz P, Brand‐Miller JC. Maternal diet and infant size 2 y after the completion of a study of a low‐glycemic‐index diet in pregnancy. American Journal of Clinical Nutrition 2007;86(6):1806. [DOI] [PubMed] [Google Scholar]
Nodine 2011
- Nodine M. The Contribution of Physical Activity to Sleep Parameters in Pregnancy within the Context of Prepregnant Body Mass Index [thesis]. University of Colorado, 2011. [Google Scholar]
Oken 2007
- Oken E, Taveras EM, Kleinman KP, Rich‐Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. American Journal of Obstetrics and Gynecology 2007;196(4):322 e1‐e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 2003
- Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. Journal of the American Dietetic Association 2003;103(1):48‐54. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2003
- Rhodes JC, Schoendorf KC, Parker JD. Contribution of excess weight gain during pregnancy and macrosomia to the cesarean delivery rate, 1990‐2000. Pediatrics 2003;111(5 Pt 2):1181‐5. [PubMed] [Google Scholar]
Ronnberg 2010
- Ronnberg AK, Nilsson K. Interventions during pregnancy to reduce excessive gestational weight gain: a systematic review assessing current clinical evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. BJOG: an International Journal of Obstetrics and Gynaecology 2010;117(11):1327‐34. [DOI] [PubMed] [Google Scholar]
Rooney 2002
- Rooney BL, Schauberger CW. Excess pregnancy weight gain and long‐term obesity: one decade later. Obstetrics & Gynecology 2002;100(2):245‐52. [DOI] [PubMed] [Google Scholar]
Rossner 1997
- Rossner S. Weight gain in pregnancy. Human Reproduction 1997;12 Suppl 1:110‐5. [DOI] [PubMed] [Google Scholar]
Schieve 1998
- Schieve LA, Cogswell ME, Scanlon KS. Trends in pregnancy weight gain within and outside ranges recommended by the Institute of Medicine in a WIC population. Maternal and Child Health Journal 1998;2(2):111‐6. [DOI] [PubMed] [Google Scholar]
Scholl 1995
- Scholl TO, Hediger ML, Schall JI, Ances IG, Smith WK. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstetrics & Gynecology 1995;86(3):423‐7. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Siega‐Riz 1993
- Siega‐Riz AM, Adair LS. Biological determinants of pregnancy weight gain in a Filipino population. American Journal of Clinical Nutrition 1993;57(3):365‐72. [DOI] [PubMed] [Google Scholar]
Skouteris 2010
Stotland 2006
- Stotland NE, Cheng YW, Hopkins LM, Caughey AB. Gestational weight gain and adverse neonatal outcome among term infants. Obstetrics & Gynecology 2006;108(3 Pt 1):635‐43. [DOI] [PubMed] [Google Scholar]
Thangaratinam 2012
- Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Duda W, Borowiack E, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technology Assessment (Winchester, England). 2012;16(31):iii‐iv, 1‐191. [DOI] [PMC free article] [PubMed]
Tieu 2008
- Tieu J, Crowther CA, Middleton P. Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006674.pub2] [DOI] [PubMed] [Google Scholar]
Walling 2006
- Walling AD. New insights on optimal weight gain during pregnancy. American Family Physician 2006;74(12):2103‐5. [Google Scholar]
Wong 2000
- Wong W, Tang NL, Lau TK, Wong TW. A new recommendation for maternal weight gain in Chinese women. Journal of the American Dietetic Association 2000;100(7):791‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Muktabhant 2012
- Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD007145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


