Abstract
Resistance of Candida albicans against the widely used antifungal agent fluconazole is often due to active drug efflux from the cells. In many fluconazole-resistant C. albicans isolates the reduced intracellular drug accumulation correlates with constitutive strong expression of the MDR1 gene, encoding a membrane transport protein of the major facilitator superfamily that is not detectably expressed in vitro in fluconazole-susceptible isolates. To elucidate the molecular changes responsible for MDR1 activation, two pairs of matched fluconazole-susceptible and resistant isolates in which drug resistance coincided with stable MDR1 activation were analyzed. Sequence analysis of the MDR1 regulatory region did not reveal any promoter mutations in the resistant isolates that might account for the altered expression of the gene. To test for a possible involvement of trans-regulatory factors, a GFP reporter gene was placed under the control of the MDR1 promoter from the fluconazole-susceptible C. albicans strain CAI4, which does not express the MDR1 gene in vitro. This MDR1P-GFP fusion was integrated into the genome of the clinical C. albicans isolates with the help of the dominant selection marker MPAR developed for the transformation of C. albicans wild-type strains. Integration was targeted to an ectopic locus such that no recombination between the heterologous and resident MDR1 promoters occurred. The transformants of the two resistant isolates exhibited a fluorescent phenotype, whereas transformants of the corresponding susceptible isolates did not express the GFP gene. These results demonstrate that the MDR1 promoter was activated by a trans-regulatory factor that was mutated in fluconazole-resistant isolates, resulting in deregulated, constitutive MDR1 expression.
Candida albicans is an important opportunistic fungal pathogen of humans and is the major cause of oropharyngeal candidiasis (OPC) in patients with AIDS (21). The azole antifungal agent fluconazole is a widely used compound to treat OPC. In recent years, however, the incidence of treatment failures has been rising. Especially in patients with AIDS who have recurrent OPC and who are receiving prolonged fluconazole therapy, treatment failures are due to the emergence of fluconazole-resistant strains (10, 22). Resistant C. albicans isolates frequently exhibit reduced intracellular drug accumulation that correlates with enhanced expression of certain multiple drug resistance genes, the ATP-binding cassette (ABC) transporters CDR1 and CDR2, and the major facilitator MDR1 (8, 14, 24, 25, 29). Fluconazole resistance is usually a stable phenotype that is maintained in the absence of selection pressure by the drug. This implies that genetic alterations have occurred in the resistant isolates that result in a constitutive overexpression of the drug efflux pumps. The MDR1 gene is not detectably expressed in vitro in fluconazole-susceptible C. albicans isolates but is strongly activated in many strains after the development of fluconazole resistance. The molecular changes responsible for the constitutive activation of the MDR1 gene in fluconazole-resistant, clinical C. albicans isolates have not been identified. Possible mechanisms include mutations in the MDR1 promoter region that might result in deregulated MDR1 expression or mutations in a regulatory factor controlling expression of the MDR1 gene.
In a previous report (8) we have described two series of C. albicans isolates from patients with AIDS who had recurrent episodes of OPC and developed fluconazole resistance during therapy. It was shown by DNA fingerprinting that in both cases fluconazole resistance had developed in a previously susceptible strain and that multiple mechanisms had contributed to a stepwise development of drug resistance. In both series of isolates the observed reduced intracellular drug accumulation correlated with high MDR1 mRNA levels. These two series of matched isolates gave us an opportunity to investigate which molecular changes were responsible for activation of the MDR1 gene in fluconazole-resistant, clinical C. albicans strains.
MATERIALS AND METHODS
C. albicans strains.
The clinical C. albicans isolates used in this study have been described previously (8). The two isolate pairs F2 and F5 and G2 and G5 represent fluconazole-susceptible and resistant isolates of the same C. albicans strains. The isolates were kept as frozen stocks at −80°C and were subcultured on YPD agar plates (10 g of yeast extract, 20 g of peptone, 20 g of glucose, 15 g of agar per liter) at 30°C. Strains F2G54, F5G54, G2G54, and G5G54 are derivates of these clinical isolates that contain a transcriptional fusion of the MDR1 promoter (MDR1P) with the GFP gene, integrated at the CDR4 locus (see below). The fluconazole-susceptible C. albicans strain CAI4 (7) was used as a source of the MDR1 promoter for construction of the MDR1P-GFP fusion.
DNA sequencing.
The MDR1 promoter regions from the clinical C. albicans isolates were amplified with the primers MDR1p1, 5′-CGATAAATGATAAGTCACTCTACC-3′ (positions 57 to 34 within the coding region), and MDR1p2, 5′-CAACTCTACTGGTAACTATTGGCG-3′ (positions −561 to −538 with respect to the start codon), deduced from the published sequence of the MDR1 gene (6). The PCR products were phosphorylated and cloned into the SmaI site of the vector pUC18. Using the universal and reverse primers, the sequences of both strands of the cloned PCR products were determined from several independent clones of each isolate until the sequences of both MDR1 alleles had been obtained. Direct sequencing of the PCR products from each isolate was also performed with 200 ng of the phenol-extracted, ethanol-precipitated amplicons as a template and the primers Mdr1p1 and Mdr1pseq1, 5′-CTGAAAAGGATATCCCATCCC-3′. Sequencing was performed with the Thermo Sequenase fluorescence-labeled-primer cycle sequencing kit with deaza dGTP (Amersham, Braunschweig, Germany) and IRD 800 dye-labeled primers (MWG Biotech, Ebersberg, Germany). Sequences were analyzed on a LI-COR model 4000 automated sequencer (MWG Biotech). The sequences obtained by direct sequencing of PCR products were analyzed visually to detect positions of heterozygosity.
Plasmid construction.
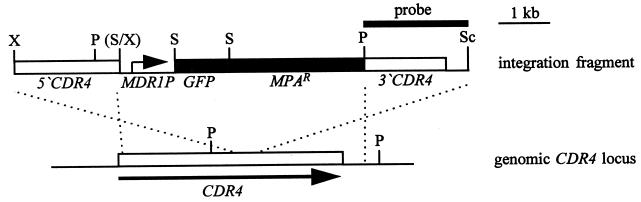
Plasmid pGFP41 has been described previously (18). It contains a GFP gene, genetically modified for expression in C. albicans under control of the SAP2 promoter, and the URA3 selection marker in the vector pBluescript. After removal of the PstI site in the polylinker by digestion with ClaI and XbaI, filling in the ends, and religation, the SalI-PstI fragment with the URA3 gene was replaced by a SalI-PstI fragment containing the MPAR marker from plasmid pAFI3 (26), resulting in plasmid pGFP49. The MDR1 promoter (MDR1P) from strain CAI4 was obtained by PCR amplification with the primers MDR1p5, 5′-GCATTGTCGACGTTCTATGTAAGTAGATGTATTGC-3′ (positions +4 to −30 of the MDR1 gene), and MDR1p7, 5′-CGTAAATCTCGAGAAACGGACTCCG-3′ (positions −1109 to −1085), thereby introducing an upstream XhoI site and a SalI site in front of the start codon (underlined). The MDR1P fragment was substituted for the XhoI-SalI fragment containing the SAP2 promoter in pGFP49, resulting in pGFP50. Subsequently, the 3′ SAP2 fragment was replaced by a PstI-SacI fragment comprising the 3′ region of the CDR4 gene (positions 2818 to 4901 with respect to the start codon [;[9;]]) to yield pGFP51. Finally, an XhoI-SalI fragment containing the 5′ CDR4 region (positions 103 to 2217) was inserted into the XhoI site of pGFP51, resulting in pGFP54. The insert of pGFP54 was excised by digestion with XhoI and SacI and used for integration of the MDR1P-GFP fusion at the CDR4 locus of the clinical C. albicans strains (Fig. 1).
FIG. 1.
Integration of the MDR1P-GFP fusion into the CDR4 locus of C. albicans. The genetic structure of the linear DNA fragment from pGFP54 used for transformation and the genomic structure at the CDR4 locus of the parent strains are delineated. The CDR4 coding region is represented by an open bar. The straight arrow indicates the direction of transcription. The MDR1 promoter is represented by the angled arrow. The probe used to verify the correct integration is indicated by a thick line. Only relevant restriction sites are shown. P, PstI, S, SalI, Sc, SacI X, XhoI. The SalI and XhoI sites shown in parentheses were destroyed by the cloning procedure.
C. albicans transformation.
C. albicans strains were transformed with the gel-purified linear DNA fragment from pGFP54 described above by electroporation (13). Mycophenolic acid (MPA)-resistant transformants were selected on minimal agar plates (6.7 g of yeast nitrogen base without amino acids [YNB; BIO 101, Vista, Calif.], 2 g of glucose, and 0.77 g of complete supplement medium [CSM-URA; BIO 101] per liter) containing 10 μg of MPA ml−1. Single colonies were picked after 5 to 7 days of growth and restreaked on plates containing 10 μg of MPA ml−1. Clones containing the correct insertion of the MDR1P-GFP fusion at the CDR4 locus were then further propagated on YPD agar plates.
Isolation of chromosomal DNA and Southern hybridization.
Chromosomal DNA from C. albicans strains was isolated as described by Millon et al. (17). DNA (10 μg) was digested with PstI, separated on a 1% (wt/vol) agarose gel and, after ethidium bromide staining, transferred by vacuum blotting onto a nylon membrane and fixed by UV cross-linking. Probe labeling, hybridization, washing, and signal detection were performed with the ECL labeling and detection kit provided by Amersham according to the instructions of the manufacturer.
Fluorescence microscopy.
The strains were grown overnight in YPD liquid medium, and aliquots were spotted on microscope slides. Fluorescence was detected with a Zeiss Axiolab microscope equipped for epifluorescence microscopy with a 50-W mercury high-pressure bulb and the Zeiss fluorescein-specific filter set 09.
RESULTS
Sequence analysis of the MDR1 promoter region of fluconazole-susceptible and -resistant C. albicans isolates.
From each of the two series of clinical C. albicans isolates described in a previous report (8), one fluconazole-susceptible and one resistant isolate were selected for the present study. Isolates F2 (MIC of fluconazole, 6.25 μg ml−1) and G2 (MIC, 0.39 μg ml−1) were the last isolates in each series that did not detectably express the MDR1 gene. Isolates F5 and G5, both with an MIC of ≥50 μg ml−1, were the most resistant isolates in each series and exhibited high MDR1 mRNA levels. To investigate if the activation of the MDR1 gene in the fluconazole-resistant isolates was caused by mutations in the MDR1 regulatory region, the sequence of more than 500 bp upstream of the start codon was determined. This region was chosen because there was a good chance of possible promoter mutations occurring within this distance from the MDR1 coding region and because the sequences of both DNA strands of the cloned PCR products could conveniently be determined with single sequencing reactions. For each isolate, several independent plasmid clones containing the PCR-amplified MDR1 upstream region were analyzed to obtain the sequences of both MDR1 alleles and to exclude PCR artifacts (point mutations and hybrids between the two alleles that were also obtained). Nucleotide polymorphisms between the two MDR1 alleles were detected in all four isolates, but the same two alleles found in the susceptible isolates F2 and G2 were also present in the corresponding resistant isolates F5 and G5 without any sequence alterations (Table 1). Direct sequencing of the PCR products confirmed the observed allelic differences occurring within each isolate and verified that no other nucleotide differences within the sequenced region were present in any of the four isolates. Several additional sequence differences with respect to the published MDR1 sequence (6) were also found, but all eight MDR1 alleles from the four isolates were identical at these positions (data not shown). These results demonstrate that, within the sequenced MDR1 upstream region, no promoter mutations had occurred in the fluconazole-resistant isolates that could account for the constitutive activation of the MDR1 gene.
TABLE 1.
Allelic differences in the MDR1 promoter regions of the C. albicans isolates F2, F5, G2, and G5
| Strain | Allele | Nucleotide at positiona:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −473 | −448 | −407 | −387 | −343 | −341 | −301 | −226 | −199 | ||
| F2 | 1 | −b | A | T | G | T | G | T | C | T |
| 2 | T | T | C | C | A | C | C | T | C | |
| F5 | 1 | − | A | T | G | T | G | T | C | T |
| 2 | T | T | C | C | A | C | C | T | C | |
| G2 | 1 | − | A | C | G | T | G | T | C | T |
| 2 | T | T | C | C | A | C | C | T | C | |
| G5 | 1 | − | A | C | G | T | G | T | C | T |
| 2 | T | T | C | C | A | C | C | T | C | |
Nucleotide positions are with respect to the MDR1 start codon (+1).
The thymidines occurring at this position in the published sequence (6) and in the second allele were deleted.
Expression of an MDR1-GFP fusion in fluconazole-susceptible and -resistant C. albicans isolates.
The sequence analysis of the MDR1 upstream region suggested that mutations in a regulatory factor might be responsible for the activation of the MDR1 gene in the two fluconazole-resistant C. albicans isolates. However, we could not exclude the possibility that cis-acting mutations might have occurred still further upstream at sites located considerably distant from the MDR1 coding region, even if we had sequenced a larger region (see, for example, reference 23). To obtain direct evidence that the molecular changes involved a regulatory factor, we tested whether the MDR1 promoter from a fluconazole-susceptible C. albicans strain would be activated in the fluconazole-resistant isolates. For this purpose, the MDR1 promoter from strain CAI4, which does not detectably express the MDR1 gene in vitro (8), was fused to the GFP gene and the reporter gene fusion was integrated into the genome of the two fluconazole-resistant isolates, F5 and G5. To avoid recombination between the MDR1 promoter from strain CAI4 and MDR1 upstream sequences in the host strains, integration was targeted to an ectopic site in the genome. The CDR4 locus was chosen for integration, as this region had been characterized previously by our group and it had been shown that inactivation of one of the CDR4 alleles did not result in a detectable phenotype (18). In addition, CDR4 expression levels did not differ between the fluconazole-susceptible and resistant isolates (9).
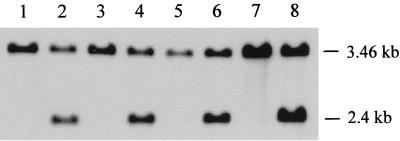
For integration of the reporter fusion into the C. albicans wild-type strains, a cassette was constructed that contained the MDR1P-GFP fusion and the dominant selection marker MPAR, a mutated allele of the C. albicans IMH3 gene conferring resistance to mycophenolic acid (13; Theiss et al., unpublished), flanked by 5′ and 3′ CDR4 sequences (Fig. 1). The linear cassette was used for transformation of the two resistant C. albicans isolates and, for control purposes, also of the corresponding fluconazole-susceptible isolates, and the genomic structures of the transformants were analyzed by Southern hybridization. The majority of MPA-resistant transformants did not exhibit detectable genomic changes at the CDR4 locus, probably because of integration of the MPAR marker into one of the IMH3 alleles, and these transformants were not further analyzed. For each parent strain, one transformant in which the MDR1P-GFP fusion had been correctly integrated at the CDR4 locus is shown in Fig. 2.
FIG. 2.
Southern hybridization of PstI-digested chromosomal DNA of C. albicans isolates F2, (lane 1), F5 (lane 3), G2 (lane 5), and G5 (lane 7) and the corresponding transformants F2G54 (lane 2), F5G54 (lane 4), G2G54 (lane 6), and G5G54 (lane 8). The sizes of the hybridizing fragments are indicated on the right-hand side of the blot. The correct integration of the MDR1P-GFP fusion at the CDR4 locus reduces the size of one 3.46-kb PstI fragment representing the intact CDR4 gene (Fig. 1) to 2.4 kb.
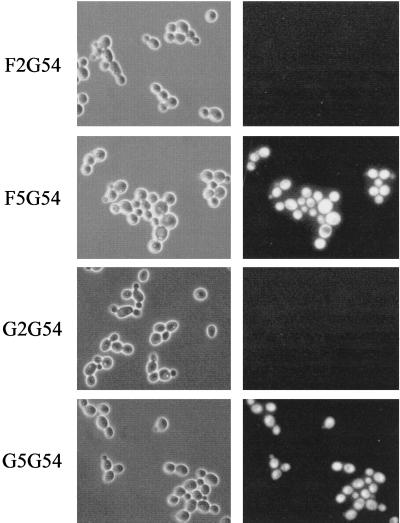
Expression of the MDR1P-GFP fusion was analyzed by epifluorescence microscopy after growth of the transformants in YPD liquid medium, conditions under which the MDR1 gene is activated in the fluconazole-resistant isolates, F5 and G5, but not detectably expressed in the corresponding susceptible isolates, F2 and G2 (8). One representative transformant of each parent strain is shown in Fig. 3. All transformants of strains F5 and G5 containing the MDR1P-GFP fusion showed a fluorescent phenotype. In contrast, strains F2G54 and G2G54 containing the identical MDR1P-GFP fusion integrated in the fluconazole-susceptible isolates F2 and G2, respectively, did not exhibit any fluorescence. None of the parent strains fluoresced under these experimental conditions (data not shown). The fact that an identical MDR1 promoter was activated in the fluconazole-resistant isolates but not in the matched susceptible isolates demonstrates that MDR1 activation in the resistant isolates was mediated by mutations in a trans-regulatory factor, resulting in MDR1 expression under conditions under which the gene is normally repressed.
FIG. 3.
Phase-contrast (left) and corresponding fluorescence (right) micrographs of transformants containing the chromosomally integrated MDR1P-GFP fusion.
DISCUSSION
For many clinical C. albicans strains that became fluconazole resistant during therapy, a correlation between drug resistance and activation of the MDR1 gene has been found by several independent research groups (8, 14, 24, 29). In most cases, fluconazole resistance and MDR1 expression are stable phenotypes that are maintained after in vitro passage of the clinical isolates in the absence of selection pressure by the drug. However, as with the stable overexpression of the ABC transporters CDR1 and CDR2, the genetic basis for the constitutive activation of the MDR1 gene in such strains has not been elucidated. In Saccharomyces cerevisiae several regulatory proteins (Pdr1p, Pdr3p, and Yap1p) controlling expression of multiple drug resistance genes of the ABC transporter and major-facilitator superfamilies are known (1, 3, 5, 11, 12, 15, 16, 20, 28, 30), and mutations in these regulators that result in upregulation of their respective target genes have been identified (4, 19). Recently, functional homologues of these regulators have also been found in C. albicans (1, 27); however, conflicting data about the roles of these transcriptional regulators have been obtained. Overexpression of the YAP1 homologue CAP1 in S. cerevisiae resulted in resistance of the transformants against fluconazole and other drugs that was mediated by the transcriptional activation of the FLR1 gene encoding a major facilitator homologous to the C. albicans Mdr1 protein (1). Overexpression of a mutated form of CAP1, but not wild-type CAP1, in C. albicans CAI4 resulted in activation of the MDR1 gene and, concomitantly, resistance against fluconazole and several other drugs (2), suggesting the possibility that similar mutations might also be responsible for MDR1 activation in fluconazole-resistant clinical C. albicans isolates. On the other hand, disruption of the CAP1 gene in the MDR1-overexpressing, fluconazole-resistant C. albicans strain FR2 did not suppress but further increased the level of MDR1 expression, and it was concluded that CAP1 was a negative regulator of MDR1 that was not responsible for MDR1 activation in this strain (2). Similarly, the C. albicans transcriptional regulator FCR1 was identified by functional complementation of an S. cerevisiae pdr1 pdr3 mutant (27). Overexpression of FCR1 in this S. cerevisiae mutant resulted in fluconazole resistance that was mediated by the transcriptional activation of the ABC transporter PDR5. In contrast, disruption of FCR1 in C. albicans resulted in hyperresistance against fluconazole, demonstrating that, similarly to CAP1, FCR1 behaved as a transcriptional activator when overexpressed in S. cerevisiae but acted as a negative regulator of drug resistance in C. albicans. The transcriptional targets of FCR1 in C. albicans have not been reported (27).
So far, none of the transcriptional regulators of drug resistance identified in C. albicans has been shown to be involved in the development of fluconazole resistance in clinical isolates, and it was suggested that mutations in the regulatory region of the multiple drug resistance genes themselves may be responsible for their overexpression in resistant isolates (27). This lack of knowledge about the molecular changes leading to activation of multiple drug resistance genes in clinical C. albicans strains is due to the fact that wild-type C. albicans is not easily accessible to genetic manipulation. The recent development of the dominant selection marker MPAR (Theiss et al., unpublished) has eliminated this problem and allowed us to investigate the basis of MDR1 activation in two different series of fluconazole-resistant, clinical C. albicans strains. Our results clearly demonstrate that in both cases MDR1 activation was caused by mutations in a trans-regulatory factor, since the MDR1 promoter from a fluconazole-susceptible C. albicans strain that did not detectably express the MDR1 gene was activated in the two resistant isolates but not in the matched susceptible isolates. It is likely that a similar mechanism is responsible for MDR1 activation in other fluconazole-resistant, clinical C. albicans strains and is, therefore, of general relevance. The mutations might directly affect a transcriptional activator or repressor binding to the MDR1 regulatory region, but they may also involve regulatory proteins controlling the activity of transcription factors. To understand the mechanisms of drug resistance in more detail, it is necessary to elucidate the identity of the regulator(s), its mode of action, and the mutations occurring in drug-resistant, clinical C. albicans isolates that lead to constitutive expression of the MDR1 gene.
ACKNOWLEDGMENTS
This study was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (BMBF grant O1 K1 8906-0). Gerwald Köhler was supported by the BMBF Stipendienprogramm Infektionsforschung.
REFERENCES
- 1.Alarco A-M, Balan I, Talibi D, Mainville N, Raymond M. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J Biol Chem. 1997;272:19304–19313. doi: 10.1074/jbc.272.31.19304. [DOI] [PubMed] [Google Scholar]
- 2.Alarco A-M, Raymond M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J Bacteriol. 1999;181:700–708. doi: 10.1128/jb.181.3.700-708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcriptional regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- 4.Carvajal E, van den Hasel H B, Cybularz-Kalaczkowska A, Balzi E, Goffeau A. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol Gen Genet. 1997;256:406–415. doi: 10.1007/s004380050584. [DOI] [PubMed] [Google Scholar]
- 5.Coleman S T, Tseng E, Moye-Rowley W S. Saccharomyces cerevisiae basic region-leucine zipper protein regulatory networks converge at the ATR1 structural gene. J Biol Chem. 1997;272:23224–23230. doi: 10.1074/jbc.272.37.23224. [DOI] [PubMed] [Google Scholar]
- 6.Fling M E, Kopf J, Tamarkin A, Gorman J A, Smith H A, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methothrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- 7.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz R, Kelly S L, Lamb D C, Kelly D E, Ruhnke M, Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franz R, Michel S, Morschhäuser J. A fourth gene from the Candida albicans CDR family of ABC-transporters. Gene. 1998;220:91–98. doi: 10.1016/s0378-1119(98)00412-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum M A, Rex J H, Galgiani J N. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34:489–495. doi: 10.1128/jcm.34.3.489-495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzmann D J, Burnett P E, Golin J, Mahé Y, Moye-Rowley W S. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol Cell Biol. 1994;14:4653–4661. doi: 10.1128/mcb.14.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katzmann D J, Hallstrom T C, Voet M, Wysock W, Golin J, Volckaert G, Moye-Rowley W S. Expression of an ATP-binding cassette transporter-encoding gene (YOR1) is required for oligomycin resistance in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6875–6883. doi: 10.1128/mcb.15.12.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler G A, White T C, Agabian N. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J Bacteriol. 1997;179:2331–2338. doi: 10.1128/jb.179.7.2331-2338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Ribot J L, McAtee R K, Lee L N, Kirkpatrick W R, White T C, Sanglard D, Patterson T F. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahé Y, Parle-McDermott A, Nourani A, Delahodde A, Lamprecht A, Kuchler K. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: a novel target for the transcription factors Pdr1 and Pdr3. Mol Microbiol. 1996;20:109–117. doi: 10.1111/j.1365-2958.1996.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 16.Meyers S, Schauer W, Balzi E, Wagner M, Goffeau A, Golin J. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr Genet. 1992;21:431–436. doi: 10.1007/BF00351651. [DOI] [PubMed] [Google Scholar]
- 17.Millon L, Manteaux A, Reboux G, Drobacheff C, Monod M, Barale T, Michel-Briand Y. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J Clin Microbiol. 1994;32:1115–1118. doi: 10.1128/jcm.32.4.1115-1118.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morschhäuser J, Michel S, Hacker J. Expression of a chromosomally integrated, single-copy GFP gene in Candida albicans, and its use as a reporter of gene regulation. Mol Gen Genet. 1998;257:412–420. doi: 10.1007/s004380050665. [DOI] [PubMed] [Google Scholar]
- 19.Nourani A, Papajova D, Delahodde A, Jacq C, Subik J. Clustered amino acid substitutions in the yeast transcription regulator Pdr3p increase pleiotropic drug resistance and identify a new central regulatory domain. Mol Gen Genet. 1997;256:397–405. doi: 10.1007/s004380050583. [DOI] [PubMed] [Google Scholar]
- 20.Nourani A, Wesolowski-Louvel M, Delaveau T, Jacq C, Delahodde A. Multiple-drug-resistance phenomenon in the yeast Saccharomyces cerevisiae: involvement of two hexose transporters. Mol Cell Biol. 1997;17:5453–5460. doi: 10.1128/mcb.17.9.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odds F C. Candida and candidosis: a review and bibliography. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 22.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rupp S, Summers E, Lo H-J, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 26.Staib P, Kretschmar M, Nichterlein T, Köhler G, Michel S, Hof H, Hacker J, Morschhäuser J. Host-induced, stage-specific virulence gene activation in Candida albicans during infection. Mol Microbiol. 1999;32:533–546. doi: 10.1046/j.1365-2958.1999.01367.x. [DOI] [PubMed] [Google Scholar]
- 27.Talibi D, Raymond M. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J Bacteriol. 1999;181:231–240. doi: 10.1128/jb.181.1.231-240.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wemmie J A, Szczypka M S, Thiele D J, Moye-Rowley W S. Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J Biol Chem. 1994;269:32592–32597. [PubMed] [Google Scholar]
- 29.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfger H, Mahé Y, Parle-McDermott A, Delahodde A, Kuchler K. The yeast ATP binding cassette (ABC) protein genes PDR10 and PDR15 are novel targets for the Pdr1 and Pdr3 transcriptional regulators. FEBS Lett. 1997;418:269–274. doi: 10.1016/s0014-5793(97)01382-3. [DOI] [PubMed] [Google Scholar]