Editor—Opioid-induced respiratory depression (OIRD) is a serious complication of opioid use. It is related to activation of μ-opioid receptors, expressed on neurones in brainstem respiratory networks.1 Reversal of OIRD by naloxone restores breathing activity but drawbacks include difficulty in reversing high-affinity or high-dose opioids, short duration of action, pain and withdrawal symptoms, and inability to reverse non-opioid-induced respiratory depression.1 Hence, there is an unmet need for respiratory stimulants that will reverse respiratory depression from any drug without these drawbacks. One possible strategy is treatment of OIRD with the hypothalamic hormone thyrotropin-releasing hormone (TRH).2,3 TRH is widely distributed throughout the neuraxis and apart from effects within the hypothalamic–hypophysial neuroendocrine system, it has functions within the limbic/cortical and brainstem/midbrain systems.4,5 TRH acts by binding to the G protein-coupled receptors, TRHR1 and TRHR2.5,6 TRHR2 modulates non-endocrine functions such as the antiepileptic and respiratory effects of TRH.4, 5, 6
TRH has a short half-life and is hydrophilic, impairing its ability to cross the blood–brain barrier. In contrast, the TRH analogue taltirelin has improved therapeutic selectivity, is more potent than TRH, and has a longer duration of action.2,3 Early animal studies show that TRH and its analogues stimulate breathing via a direct effect at the pre-Bötzinger complex, an important respiratory rhythm generator within the brainstem respiratory network.7 In rodents, TRH and taltirelin improve morphine- and sufentanil-induced respiratory depression by inducing rapid but shallow breathing.2,3 Here we present new data on the ability of TRH and taltireline to reverse OIRD in rodents and humans.
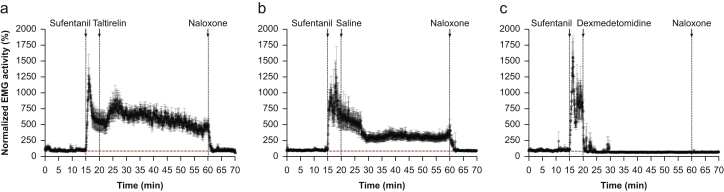
First, using the methods of Weinger and colleagues,8 and as approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee, we studied we studied the effect of taltirelin 1 mg kg−1 i.v., dexmedetomidine 30 μg kg−1 i.v., and saline 1 ml kg−1 i.m. on the m. gastrocnemius EMG in 27 awake restrained rats after sufentanil 10 μg kg−1 i.v. Filtering and sampling were at 128 Hz as described in the work of Weinger and colleagues.8 Sufentanil increased EMG activity that was subsequently reduced by the α2-adrenergic receptor agonist dexmedetomidine but not by taltirelin (Fig 1); in fact, taltirelin seemed to increase EMG activity. Enhanced EMG activity from opioids is a known effect in animals and humans (i.e. the rigid cage syndrome), and is reversed by naloxone (Fig 1). Muscle rigidity from opioids is a further cause of respiratory impairment in addition to the effect on the brainstem. Non-opioid interventions that reduce muscle tone improve ventilation and gas exchange by improving tidal volume and reducing ventilation/perfusion mismatch.8 Apart from dexmedetomidine, volatile anaesthetics and the α1-adrenergic receptor agonist prazosin reduce opioid-enhanced muscle tone and consequently improve the ability of respiratory stimulants such as taltirelin to reverse OIRD.3,8
Fig 1.
Effects of 1 mg kg−1 taltirelin i.v. (a, n=10), 1 mg kg−1 saline i.m. (b, n =7), and 30 μg kg−1 dexmedetomidine i.v. (c, n = 10) on normalised m. gastrocnemius EMG in awake restrained rats after 10 μg kg−1 sufentanil i.v. At t=60 min, 1 mg kg−1 naloxone i.v. was injected. Sufentanil increased EMG activity, which was not reduced by taltirelin. Dexmedetomidine reduced EMG activity to pre-sufentanil baseline values. Data are mean (standard deviation).
Next, we investigated the respiratory effect of a continuous TRH infusion in six healthy male and female volunteers. Experiments were performed at isohypercapnia with resting ventilation increased to 20 L min−1. After ethics approval (trial registration NL6966, www.trialregister.nl) and written informed consent was obtained, participants received a continuous remifentanil infusion (target plasma concentration 1.2 ng ml−1) such that isohypercapnic ventilation was reduced by 50%. With steady-state ventilation, dose-escalating continuous TRH infusions i.v. were given. The first subject started with a total dose of 0.8 mg given over 60 min (initial bolus dose of 0.2 mg followed by infusion of 0.2 mg over 30 min, repeated once). As no respiratory effect was observed, the dose was increased to 1.6 mg (bolus 0.4 mg followed by infusion of 0.4 mg over 30 min, repeated once), 3.2 mg (0.8 mg/0.8 mg, repeated once), 4.8 mg (1.2 mg/1.2 mg, repeated once), 8 mg (2 mg/2 mg, repeated once), and 4 mg (2 mg/2 mg, not repeated because of futility) in subsequent subjects. In addition, three subjects received normal saline instead of TRH on a different study visit. None of the subjects showed any sign of consistent reversal of OIRD from remifentanil. This stands in contrast to ketamine, which showed a dose-dependent return towards baseline ventilation in the same human model of OIRD.9 One concern is that the TRH dose was too low, but it was 20-fold higher than the dose used in earlier human studies that showed a significant, albeit modest, effect from 0.4 mg i.v. TRH on breathing when no opioids were administered.10 We contend that the infusions of TRH reached central sites as all participating subjects experienced adverse effects such as headache, nausea, warm or cold feeling, and restlessness. We refrained from using higher TRH doses to prevent worse adverse effects or development of endocrinological effects. Moreover, pricing of the TRH was such that we higher doses were overly expensive (∼£800/US$1000 for an 8 mg dose) and thus represent an uneconomical treatment of OIRD.
Although earlier animal data showed that TRH is able to overcome OIRD by restoration of rhythmic activity within the pre-Bötzinger complex,2,3 we observed, in agreement with earlier statements,5 that TRH is ineffective in humans over the dose range tested. This may be related to species differences, such as differences in receptor activation. In rodents, TRHR2 is selectively involved in respiratory stimulation by TRH; in humans, TRHR1 – but not TRHR2 – has been identified in the central nervous system.6 An attractive alternative to TRH may be the TRH-analogue taltireline, which is more potent. However, data on taltirelin reversal of OIRD in awake and anaesthetised rodents show that taltirelin is associated with rapid shallow breathing with resultant abnormalities in blood gases and lactic acidosis.2,3 We observed that taltirelin does not ameliorate but possibly even enhances muscle rigidity from opioids in the rat. In conclusion, our results indicate that TRH is not a viable reversal agent of OIRD in humans; no conclusions can be drawn on taltirelin as human studies are unavailable. We propose future human studies using taltirelin possibly in combination with low-dose naloxone with the hypothesis that this combination will produce effective and long-term reversal of OIRD.
Declarations of interest
The Anesthesia & Pain Research Unit of the Department of Anesthesiology, Leiden University Medical Center (Leiden, The Netherlands) received/receives funding from AMO Pharma Ltd. (Leeds, UK), Bedrocan BV (Groningen, The Netherlands), Gruenenthal GmbH (Aachen, Germany), Medasense Biometrics Ltd (Ramat Gan, Israel), Medtronic (Minneapolis, MN, state, USA), MSD Nederland BV (Haarlem, The Netherlands), LTS Lohmann Therapie Systeme AG (Andernach, Germany), and Trevena Inc. (Chesterbrook, PA, USA), outside of the the topic of the current study.
References
- 1.van der Schrier R., Dahan J.D.C., Boon M., et al. Advances in reversal strategies of opioid-induced respiratory depression. Anesthesiology. 2022;136:618–632. doi: 10.1097/ALN.0000000000004096. [DOI] [PubMed] [Google Scholar]
- 2.Boghosian J.D., Luethy A., Cotten J.F. Intravenous and intratracheal thyrotropin releasing hormone and its analog taltirelin reverse opioid-induced respiratory depression in isoflurane anesthetized rats. J Pharmacol Exp Ther. 2018;366:105–112. doi: 10.1124/jpet.118.248377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandrea K.E., Cotten J.F. A comparison of breathing stimulants for reversal of synthetic opioid-induced respiratory depression in conscious rats. J Pharmacol Exp Ther. 2021;378:146–156. doi: 10.1124/jpet.121.000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gary K.A., Sevarion K.A., Yarbough G.G., Prange A.J., Winokur A. The thyrotropin-releasing hormone (TRH) hypothesis of homeostatic regulation: implications of TRH-based therapeutics. J Phramacol Ther. 2003;305:410–416. doi: 10.1124/jpet.102.044040. [DOI] [PubMed] [Google Scholar]
- 5.Khomane K.S., Meena C.L., Jain R., Bansal A.K. Novel thyrotropin-releasing hormone analogs: a patent review. Exp Opin Ther Patents. 2011;21:1673–1691. doi: 10.1517/13543776.2011.623127. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y., Lu X., Gershengorn M.C. Thyrotropin-releasing hormone receptors — similarities and differences. J Mol Endocrinol. 2003;30:87–97. doi: 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- 7.Inyushkin A.N., Merkulova N.A., Chepurnov S.A. The pre-Bötzinger complex participates in generating the respiratory effects of thyroliberin. Neurosci Behav Physiol. 1999;29:285–292. doi: 10.1007/BF02465344. [DOI] [PubMed] [Google Scholar]
- 8.Weinger M.B., Segal I.S., Maze M. dexmedetomidine, acting through central alpha-2 adrenoreceptors, prevents opiate-induced muscle rigidity in the rat. Anesthesiology. 1989;71:242–249. doi: 10.1097/00000542-198908000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Jonkman K., van Rijnsoever E., Olofsen E., et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120:1117–1127. doi: 10.1016/j.bja.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Nink M., Krause U., Lehnert H., et al. Thyrotropin-releasing hormone has stimulatory effects on ventilation in humans. Act Physiol Scand. 1991;141:309–318. doi: 10.1111/j.1748-1716.1991.tb09086.x. [DOI] [PubMed] [Google Scholar]