Highlights
-
•
Increased expression of OV6 was positively correlated with more CD68+ TAMs infiltration in HCC.
-
•
Integration of OV6 and CD68 with tumor size and MVI status exhibited superiority in evaluating the postoperative prognosis, and may serve as a more effective prognostic model for HCC patients.
Keywords: Hepatocellular carcinoma, Tumor-associated macrophage, Cancer stem-like cells, OV6, CD68, Prognosis
Abstract
Background: Reliable prognostic indicators for accurately predicting postoperative outcomes in Hepatocellular carcinoma (HCC) patients are lacking. Although cancer stem-like cells (CSCs) and tumor-associated macrophages (TAMs) in tumor microenvironment are implicated in the occurrence and development of HCC, whether the combination of CSC biomarkers and TAM populations could achieve better performance in predicting the prognosis of patients with HCC has been rarely reported.
Methods: A total of 306 HCC patients were randomly divided into the training and validation cohorts at a 1:1 ratio, and the expression of OV6 and CD68 was assessed using immunohistochemistry in HCC samples. The prognostic value of these biomarkers for post-surgical survival and recurrence were evaluated by the curve of receiver operating characteristic and multivariate Cox regression analyses.
Results: The density of OV6+ CSCs was positively correlated with the infiltration of CD68+ TAMs in HCC. Both high OV6 expression and CD68+ TAM infiltration was closely associated with poor overall survival (OS) and progression-free survival (PFS) of HCC patients. Moreover, overexpression of OV6 and infiltration of CD68+ TAMs were identified as independent prognostic factors for OS and PFS after liver resection. The integration of OV6 and CD68 with tumor size and microvascular invasion exhibited highest C-index value for survival predictivity in HCC patients than any other biomarkers or clinical indicators alone.
Conclusion: Incorporating intratumoral OV6 expression and CD68+ TAMs infiltration with established clinical indicators may serve as a promising prognostic signature for HCC, and could more accurately predict the clinical outcomes for HCC patients after liver resection.
Abbreviations: HCC, hepatocellular carcinoma; CSC, cancer stem-like cell; OV6, oval cell 6; EpCAM, epithelial cell adhesion molecule; TME, tumor microenvironment; TAM, tumor-associated macrophage; MVI, microvascular invasion; TNM, tumor node metastasis; HBsAg, hepatitis B surface antigen; AFP, α-fetoprotein; CT, computed tomography; MRI, magnetic resonance imaging; OS, overall survival; PFS, progression-free survival; IHC, immunohistochemistry; ROC, receiver operating characteristic; AUC, area under the curve of ROC; C-index, concordance index; HR, Hazard ratio; MV, multivariable; CI, Confidence interval.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, ranking as the fifth most prevalent neoplasm and the second most common cause of cancer-related death globally [1]. Hepatic resection has been established as the mainstay of curative modality used to treat HCC and can achieve eradication of early-stage HCC [2]. However, the prognosis for HCC patients remains poor due to the high incidence of postoperative recurrence, with a 5-year recurrence rate up to 70% even after initial curative resection [1,3]. Meanwhile, although various systemic treatments including transarterial chemoembolization, targeted therapy, immunotherapy, and chemotherapy have been widely used to treat advanced HCCs, the therapeutic efficacy of these approaches remain very limited due to the great heterogeneity and complicated tumor microenvironments of HCC [4,5]. Thus, there is an urgent need to identify novel and reliable indicators for prognostic prediction and postoperative recurrence surveillance of HCC patients, which is essential in making treatment decisions and improving clinical outcomes after treatments.
To date, various molecular biomarkers that derive either from tumor tissues or body fluids have been widely used to evaluate therapeutic effects and predict clinical outcomes of patients with HCC [6]. These molecular indicators generally include oncofetal antigens, oncogenes, tumor suppressors, enzymes, micro RNAs, non-coding RNAs, etc., and investigating their cellular functions, regulatory mechanisms and clinical significance could inform early diagnosis and precision treatment for HCC. In addition, with the growing understanding of cancer stem cell (CSC) theory and its promoting role in oncogenesis [7,8], multiple CSC markers such as CD133 and epithelial cell adhesion molecule (EpCAM) have also been reported as useful predictors of HCC tumorigenesis and prognosis [9], [10], [11], [12]. A subset of less differentiated small and oval-shaped cells in primary HCC tissues, also termed as liver progenitor cells, were considered as the possible origin of liver CSCs and could be identified by utilizing specific surface marker Oval Cell 6 (OV6) [13,14]. Our previous work demonstrated that OV6-positive HCC cells possessed much stronger capacities of self-renewal, differentiation, and tumorigenicity than OV6-negative cells, implying the important role of OV6-enriched cancer cells in promoting HCC initiation and progression [15,16]. Other groups also proposed that high OV6 expression correlated with aggressive clinicopathological features and unfavorable survival outcomes [17,18]. Although these data highlight the critical role of OV6 as a potential CSC biomarker in HCC tumorigenesis, the clinical significance and prognostic value of OV6 single or combined with other clinical indicators in HCC remain largely uninvestigated.
Besides these well-recognized tumor-related indicators and CSC biomarkers, concrete evidence also emphasizes the important role of tumor microenvironment (TME) in cancer progression and metastasis [19]. Tumor-associated macrophages (TAMs), one of the major components of tumor-infiltrating immune cells in the TME, have been reported to affect various aspects of tumor progression, including cell proliferation, invasion, immunosuppression and drug resistance [20,21]. Additionally, a high density of TAMs in tumor tissues was demonstrated to correlate with adverse clinical characteristics and poor prognosis, and specific biomarkers of TAMs were also identified as predictors for prognostic evaluation and risk classification of cancer patients [22], [23], [24]. Moreover, accumulating evidence including ours have revealed that the interaction between CSCs and the TAMs may contribute to tumor initiation and progression by shaping the TME into a pro-tumorigenic but immunosuppressive landscape [25], [26], [27]. These results may facilitate the confirmation of an optimal combination of different biomarkers with improved predictive accuracy compared to using single biomarkers alone. However, whether the combination of intratumoral CSC-related biomarkers and TAMs could achieve better prognostic performance for predicting postoperative prognosis of HCC patients have been seldom reported.
In this study, we aimed to investigate the correlation between OV6+ CSCs and CD68+ TAMs in HCC samples and determine the prognostic value of integrating OV6 and CD68 with existing clinical indicators in predicting postoperative prognosis for patients with HCC. Our findings may shed new light on the clinical significance of CSC-TAM-based biomarker signature in HCC and also provide a novel prognostic model to evaluate the clinical outcomes for HCC patients.
Methods and materials
Patients and specimens
A total of 306 HCC patients who underwent curative-intent liver resection at Eastern Hepatobiliary Surgery Hospital between June 2013 and October 2017 were retrospectively recruited. All surgical specimens were histologically reviewed by experienced pathologists to confirm the diagnosis of HCC. Curative liver resection was defined as complete removal of all visible tumor lesions with microscopically negative margins (R0 resection). Paired HCC tumors and their adjacent normal tissues from 306 patients were used to detect the expression of OV6 and CD68 by immunohistochemical (IHC) staining. This study was conducted in line with the recommendations for prognostic studies of tumor biomarkers (REMARK) [28], and was approved by the Ethics Committee and Institutional Review Board of the hospital. Written informed consent for research use of clinical data was obtained from all HCC patients. The clinicopathological characteristics of patients consisted of age, gender, serum α-fetoprotein (AFP) level, hepatitis B surface antigen (HBsAg), tumor size, microvascular invasion (MVI), Child-Pugh grade, and Tumor Node Metastasis (TNM) stage.
Study endpoints and follow-up
The primary endpoints of this study were overall survival (OS) and progression-free survival (PFS). OS refers to the interval between the date of surgery and the patient's death or last follow-up, and PFS is defined as the interval from surgery to the date of the disease progression or last follow-up. After surgery, all patients were regularly followed up every 3 months with abdominal ultrasound, serum AFP measurement, or even contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) scans if new suspended lesions were clinically indicated. Tumor recurrence was diagnosed based on typical imaging features on CT or MRI scans with or without serum AFP elevation, or confirmed by histopathological examinations after re-hepatectomy for recurrent HCC. The dates of death and recurrence during follow-up as well as the last clinical visit were recorded.
IHC staining
The IHC assay was performed according to the standard protocol procedures. Briefly, the methanol-fixed, paraffin-embedded HCC specimens were cut into 5-μm thick sections. According to the routine protocol, the tissue microarray slides were deparaffinizated, rehydrated, and repaired with citrate buffer (1:100, pH 6.0) before incubating with the reagents of the hypersensitive IHC kit (KIT-9710, Maixin Biotechnologies, Fuzhou, China). After blocking the endogenous peroxidases and nonspecific binding sites, the tissue slides were incubated with the following primary antibodies at 4℃ overnight: mouse-anti-OV6 antibody (1:1000; R&D Systems) and mouse-anti-CD68 antibody (1:200; ab53444, Abcam). All slides were photographed under microscope and the images were quantified by two independent pathologists in a double-blinded way. Then, three randomly representative fields per case were taken and the percentage of positive cells of each observed field, which ranged from 0 to 100, was also determined. Meanwhile, the staining intensity was scored according to a semi-quantitative grading method as follows: no detectable staining (intensity 0); weak reactivity mainly detectable at high magnification (20–40×) (intensity 1+); moderate (intensity 2+) and strong (intensity 3+) reactivity that was easily detected at low magnification (4×) [29]. The expression of OV6 was assessed using the H-score method, which was based on the percentage of cells stained with intensities of 0, 1+, 2+, 3+, and calculated using the following equation: H-score = ∑ [intensity (0, 1, 2, 3) × extent of each staining intensity (0–100%)] [29]. Similarly, as for the quantification of immune cells, the staining intensity of CD68+ TAMs was also calculated by the number of stained nucleated cells per field and data were expressed as cell/mm2, as described in our previous study [30].
Statistical analysis
Categorical variables were presented as frequencies (n). Numerical data were expressed as the mean ± standard deviation (SD). The χ2 test or Fisher's exact test was conducted for comparison of categorical variable, while a two-tailed Student's t-test or Wilcoxon test was performed for numerical data. Cumulative curve for OS and PFS were plotted using the Kaplan-Meier method and were compared by the log-rank analysis. Multivariate analyses were conducted using the Cox proportional hazards model with a forward stepwise variable selection procedure to include the variables with P < 0.1 in univariate analyses. The predictive performance of OV6 (H-score) or CD68 (cells/mm2) for 5-year OS was evaluated using time-dependent receiver operating characteristic (ROC) curves with the “survival ROC” package [31], and the optimal cut-off value of OV6 or CD68 was determined according to the Youden Index. Specifically, the ROC curve was generated by plotting the true positive fraction (sensitivity) on the Y axis versus the false positive fraction (1-specificity) on the X axis for each OV6 or CD68 value tested. Then the most applicable cut-off value was defined as the point in the ROC space with the maximum Youden Index, which was calculated as “sensitivity+specificity-1”. Meanwhile, the area under the ROC curve (AUC) was computed with the “time ROC” package, R software 3.4.4. Harrell's concordance index (C-index) was used to compare the discrimination ability of our prognostic model versus other clinicopathological indicators. All experiments were repeated independently at least three times. All statistical analyses were performed using SPSS 21.0 (IBM Corporation) software and R-project software (version 3.5.3), and difference was considered statistically significant at P value < 0.05.
Results
Intratumoral OV6 expression is positively associated with CD68+ TAM infiltration in HCC specimens
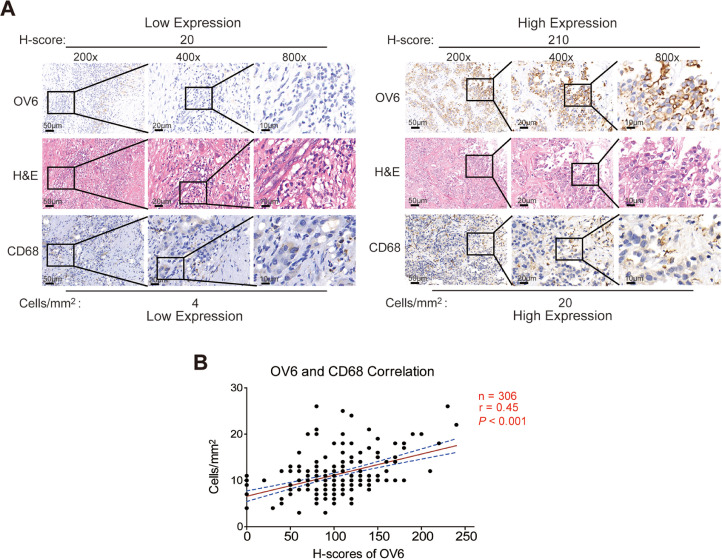
Given previous studies including ours revealing that the reciprocal network between CSCs and TAMs facilitates tumor progression and their combination could better predict prognosis of tumor patients [24], [25], [26], we expected to appraise the superiority of combing CSC-related marker OV6 and CD68+ TAM in predicting HCC patients’ prognosis. To verify our speculation, a total of 306 HCC patients were divided into the training cohort (n = 153) and validation cohort (n = 153) at a 1:1 ratio, and the clinicopathological characteristics are summarized in Supplementary Table 1. Then, IHC assays were performed in tissue assays to examine the expression levels of OV6 and CD68 in both training and validation cohorts (Fig. 1a). Although great variation in OV6 and CD68 expression levels existed among different patients, a positive correlation between OV6 expression and CD68+ TAM in HCC was observed (Pearson's r = 0.45; P < 0.001) (Fig. 1b). These data suggests that intratumoral OV6 expression is closely correlated with the infiltration of CD68+ TAMs in primary HCCs.
Fig. 1.
Intratumoral OV6 expression is positively correlated with CD68+ TAM infiltration in HCC specimens.
(A) Representative images of H&E and IHC staining of OV6 and CD68 in HCC specimens from the training cohort are shown (n = 153; scale bars: 10, 20 and 50 μm);
(B) Results of the correlation analysis between the H-score of OV6 and cell density of CD68+ TAMs in HCC specimens are presented (n = 306; Pearson's r = 0.45; P < 0.001).
Concomitant high expression of OV6 and CD68 indicates more aggressive clinical features and worse survival of HCC patients
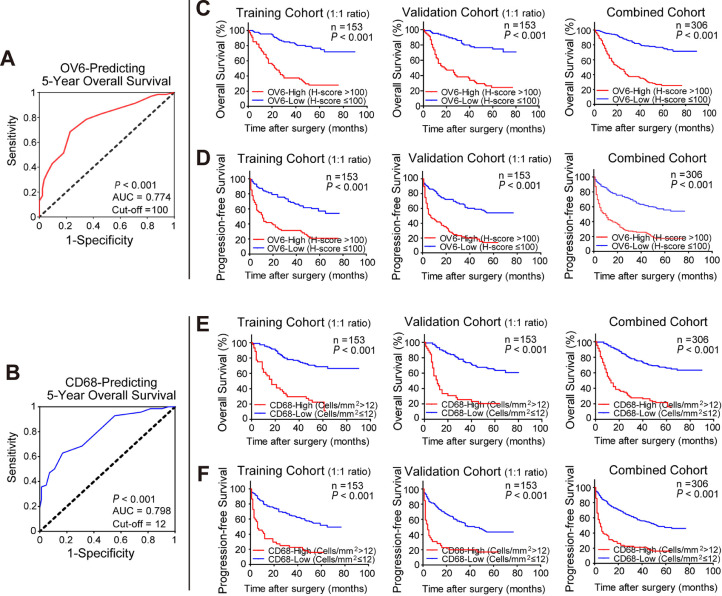
Next, we determined the predictive value of OV6 alone or in combination with CD68 in HCC patients. First, ROC analysis was carried out to determine the optimal cut-off values of OV6 and CD68 using 5-year OS as the end point. As depicted in Fig. 2a and 2b, the best cut-off value for OV6 was 100 (H score) with an AUC of 0.774 (P < 0.001) and 12 (cell/mm2) for CD68 with an AUC of 0.798 (P < 0.001) in predicting 5-year OS of HCC patients in the training cohort. As shown in Fig. 2c-f, either OV6high or CD68high group presented worse OS (P < 0.001) and PFS (P < 0.001) than their counterparts in the training cohort. In addition, similar results were also observed in the validation and combined cohorts by using the cut-off values derived from the training cohort (Fig. 2c-f). Therefore, either OV6 or CD68 expression may serve as a reliable indicator for evaluating HCC patients’ prognosis.
Fig. 2.
Either high OV6 or CD68 expression indicates unfavorable clinical outcomes of HCC patients after surgery.
(A) The optimal H-score cut-off value of OV6 to predict 5-years OS in the training cohort was calculated by a time-dependent ROC analysis (n = 153; High H-score of OV6: 100, AUC = 0.774, P < 0.001).
(B) The optimal cell density cut-off value of CD68 to predict 5-years OS in the training cohort was calculated by a time-dependent ROC analysis (n = 153; High cell density of CD68+ TAMs: 12, AUC = 0.798, P < 0.001).
(C-D) Kaplan-Meier analyses for the OS and PFS of HCC patients were compared based on OV6 expression levels (high v.s. low) in the training cohort (n = 153), validation cohort (n = 153) and the combined cohort (n = 306).
(E-F) Kaplan-Meier analyses for the OS and PFS of HCC patients were compared based on CD68 expression levels (high v.s. low) in the training cohort (n = 153), validation cohort (n = 153) and the combined cohort (n = 306).
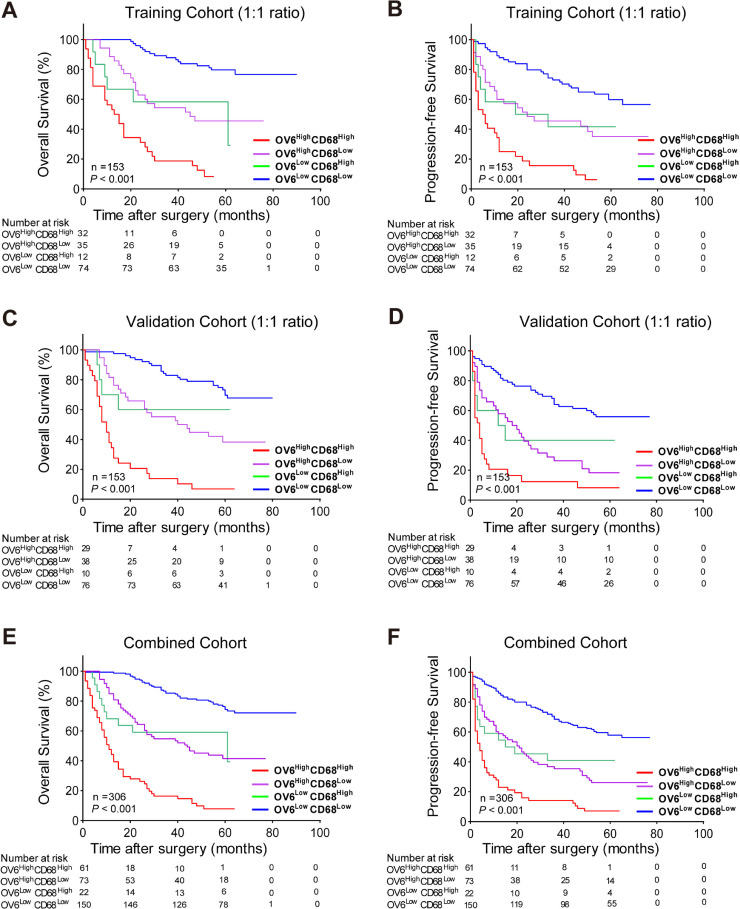
Furthermore, based on the optimal cut-off values of OV6 and CD68, HCC patients in the training cohort were divided into four groups: OV6highCD68high (n = 32), OV6highCD68low (n = 35), OV6lowCD68high (n = 12), and OV6lowCD68low (n = 74). As shown in Table 1, patients with both high OV6 and CD68 expression exhibited a higher percentage of MVI (P = 0.012) and larger tumor size (P = 0.012). In addition, Kaplan-Meier analysis was performed to compare OS and PFS among four groups and revealed that the OV6highCD68high subgroup presented worse OS and PFS than other low-expression subgroups (Fig. 3a, b). Moreover, we validated the above results in the validation and combined cohorts, which showed that patients with both high OV6 and CD68 expressions also exhibited the most unfavorable clinicopathological characteristics and had the shortest survival time versus the other three groups (Supplementary Table 2–3; Fig. 3c-f). Besides, subgroup analyses of AFP-negative patients were performed and significant differences in OS and PFS were observed among patients with high versus low OV6 or CD68 expression level. Meanwhile, the OV6highCD68high subgroup exhibited markedly worst survival rates compared with other subgroups (Supplementary Fig. 1A-F), suggesting that both high expression of OV6 and CD68 could also effectively stratify the prognosis of patients with AFP-negative HCC. Taken together, these data indicated that combing high OV6 expression and more infiltrating CD68+ TAMs may have great potential to predict unfavorable outcomes of HCC patients after liver resection.
Table 1.
The correlation between OV6/CD68 expression and clinicopathological characteristics of patients with hepatocellular carcinoma in the training cohort (n = 153) (1:1 ratio).
| OV6/CD68 expression | ||||||
|---|---|---|---|---|---|---|
| Characteristic | OV6highCD68high(n = 32) | OV6highCD68low(n = 35) | OV6lowCD68high(n = 12) | OV6lowCD68low(n = 74) | Total(153) | P*value |
| Age | 0.113 | |||||
| <50y | 19 | 14 | 3 | 28 | 64 | |
| ≥50y | 13 | 21 | 9 | 46 | 89 | |
| Gender | 0.161 | |||||
| Male | 25 | 32 | 9 | 67 | 133 | |
| Female | 7 | 3 | 3 | 7 | 20 | |
| HbsAg | 0.013 | |||||
| + | 30 | 33 | 11 | 55 | 129 | |
| – | 2 | 2 | 1 | 19 | 24 | |
| AFP | 0.143 | |||||
| >400 ng/mL | 18 | 20 | 11 | 43 | 92 | |
| ≤400 ng/mL | 14 | 15 | 1 | 31 | 61 | |
| Tumor Size | 0.012 | |||||
| <5cm | 8 | 10 | 3 | 39 | 60 | |
| ≥5cm | 24 | 25 | 9 | 35 | 93 | |
| MVI | 0.012 | |||||
| + | 20 | 20 | 12 | 37 | 89 | |
| – | 12 | 15 | 0 | 37 | 64 | |
* Statistical significance was calculated by chi-square or fisher's exact test for categorical/binary measures.
AFP, alpha-fetoprotein; MVI, microvascular invasion.
Fig. 3.
Combination of OV6 expression and CD68+ TAMs predict poor postoperative prognosis of HCC patients.
(A-F) Kaplan-Meier analyses for the OS and PFS of HCC patients were compared according to the expression levels of OV6 and CD68 in the training cohort (n = 153), validation cohort (n = 153) and the combined cohort (n = 306).
Integrating OV6, CD68+ TAMs and existing clinical indicators more accurately predicts the postoperative prognosis of HCC patients
To further appraise the prognostic value of OV6 and CD68 in assessing the survival of HCC patients after surgery, univariate and multivariate Cox-regression analyses were performed to determine whether high expression of OV6 and CD68 was independent risk factors associating with worse outcomes of HCC patients. As shown in Table 2 and Supplementary Tables 4, 5, OV6, CD68, combined with the established clinicopathological features including MVI and tumor size, were identified as independent predictors for the OS and PFS of HCC patients in the training, validation and combined cohorts.
Table 2.
Univariate and multivariate Cox regression analysis of OV6, CD68 and clinicopathological characteristics associated with overall survival and progression-free survival in the training cohort (n = 153) (1:1 ratio).
| Characteristics | Overall survival | Progression-free survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value* | HR (95% CI) | P Value | HR (95% CI) | P Value* | |
| Age (<50y vs ≥50y) | 0.740 (0.462–1.184) |
0.210 | 1.217 (0.800–1.851) |
0.358 | ||||
|
Gender (Male vs Female) |
1.362 (0.697–2.662) |
0.366 | 1.150 (0.612–2.163) |
0.664 | ||||
| AFP (+ vs -) | 1.128 (0.697–1.826) |
0.624 | 1.155 (0.755–1.768) |
0.507 | ||||
| Child-Pugh grade | 0.546 | 0.077 | 0.788 | 0.478 | ||||
| (class A vs B) | (0.279–1.069) | (0.408–1.523) | ||||||
| HbsAg (+ vs -) | 2.358 (1.020–5.451) |
0.045 | 1.175 (0.469–2.943) |
0.731 | 1.862 (0.963–3.600) |
0.064 | ||
|
Tumor Size (<5 cm vs ≥5 cm) |
2.646 (1.530–4.577) |
0.001 | 1.913 (1.078–3.3294) |
0.027 | 1.943 (1.239–3.047) |
0.004 | 1.493 (0.935–2.385) |
0.093 |
| MVI (+ vs -) | 2.039 (1.222–3.404) |
0.006 | 1.772 (1.041–3.015) |
0.035 | 2.120 (1.347–3.336) |
0.001 | 1.853 (1.156–2.971) |
0.010 |
| OV6 expression (Low vs High) | 4.767 (2.854–7.963) |
<0.001 | 3.411 (1.914–6.078) |
<0.001 | 3.070 (1.999–4.715) |
<0.001 | 2.478 (1.571–3.910) |
<0.001 |
| CD68 expression (Low vs High) | 4.811 (2.980–7.766) |
<0.001 | 3.011 (1.824–4.971) |
<0.001 | 3.348 (2.174–5.157) |
<0.001 | 2.269 (1.438–3.580) |
<0.001 |
* Variables with P < 0.10 in univariate analysis were subjected to multivariate Cox-regression model using forward stepwise variable selection.
AFP, alpha-fetoprotein; MVI, microvascular invasion; CI, confidence interval; HR, hazard ratio.
Next, the prognostic accuracy of these abovementioned risk factors alone or in combination were determined using C-index analysis. As demonstrated in Table 3 and Supplementary Tables 6–7, the combination of OV6 and CD68 revealed superiority in predicting post-surgical survival of HCC patients, with higher C-index value than that of OV6, CD68, or any other clinical indicator alone. Furthermore, the incorporation of OV6 expression and CD68+ TAMs into the currently used clinical parameters, MVI and tumor size, presented the best accuracy in predicting OS and PFS with the highest C-index value than any other groups. Consistent with C-index values, ROC curves also confirmed the improved prognostic efficiency of our model when combined with widely used clinical parameters in three mentioned cohorts (Supplementary Fig. 2A-F). Collectively, these data indicated the best prognostic value of the combination of OV6-CD68-based classifier and clinical indicators in predicting patients’ postoperative survival.
Table 3.
C-index analysis of the prognostic accuracy of OV6, CD68 and other variables for overall survival and progression-free survival in the training cohorts (n = 153) (1:1 ratio).
| C-index (95% CI) | Overall survival | Progression-free survival |
|---|---|---|
| Training Cohort (n = 153) | ||
| Tumor Size | 0.638 (0.550–0.726) | 0.597 (0.504–0.689) |
| MVI | 0.609 (0.519–0.699) | 0.634 (0.543–0.725) |
| OV6 | 0.728 (0.646–0.811) | 0.673 (0.586–0.760) |
| CD68 | 0.696 (0.610–0.782) | 0.652 (0.566–0.739) |
| OV6+CD68 | 0.782 (0.707–0.857) | 0.714 (0.633–0.795) |
| OV6+Tumor Size+MVI | 0.799 (0.729–0.869) | 0.754 (0.676–0.832) |
| CD68+Tumor Size+MVI | 0.772 (0.698–0.847) | 0.745 (0.666–0.824) |
| OV6+CD68+Tumor Size+MVI | 0.829 (0.764–0.894) | 0.772 (0.698–0.846) |
MVI, microvascular invasion; CI, confidence interval.
Comparisons with other staging systems to predict long-term survival of HCC patients following resection
To further ascertain the model's prognostic value in HCC patients treated by surgery, we also compared the predictive ability of this OV6-CD68-based model to the routinely used clinical staging systems, including Child-Pugh grade and TNM staging classification. Supplementary Fig. 3A-D depicts the Kaplan-Meier curves for OS and PFS according to the Child-Pugh and TNM staging systems in the entire cohort. Then, by using the ROC method, our prognostic model demonstrated much better prediction trend and accuracy when compared to conventional Child-Pugh and TNM staging systems (Supplementary Fig. 3E-G). these results indicated that our proposed model may be more discriminative than other well-established staging systems like Child-Pugh grade and TNM stage in survival predictivity of surgical-treated HCC patients. In addition, considering that AFP is one of the most commonly used biomarkers for detection and prognostic prediction of HCC, we also assessed the predictive efficacy of serum AFP value for long-term prognosis, and found that AUC values of the AFP on OS and PFS were inferior to that of our prognostic model (0.541 vs. 0.829 for OS; 0.510 vs. 0.772 for PFS) (Supplementary Fig. 4A). These data were also confirmed in the validation and combined cohort (Supplementary Fig. 4B, C), suggesting that our prognostic model was more accurate and performed better than preoperative AFP value in predicting survival after resection for HCC.
In addition, according to the subgroup analyses, this OV6-CD68-based prognostic model also exhibited higher C-index value than any other indicators or combinations (Supplementary Table 8), suggesting a relatively good predictive ability for long-term outcomes in subsets of patients with AFP-negative HCC. Taken together, these results suggested that the combination of tumor-specific biomarkers (OV6 and CD68) and clinical indicators (MVI and tumor size) could serve as a highly reliable model and is superior to either biomarker or clinical predictor alone for predicting long-term prognosis of HCC patients.
Discussion
Identification of novel and reliable molecular biomarkers is of critical importance for the development of effective therapeutic strategies as well as prognosis assessment for patients with HCC. Over the past decades, an increasing number of emerging prognostic biomarkers, including CSC markers, have been explored to forecast the prognosis of postoperative HCC patients [32,33], but their prognostic values remain less than satisfactory largely due to the great heterogeneity of HCC, which can not be comprehensively reflected by only a single biomarker. On the other hand, the complex interaction between CSCs-driven tumorigenesis and tumor microenvironment in HCC is frequently neglected. Therefore, it is urgently needed to find more powerful and effective combinations based on biomarkers relating to different aspects for accurate prognostic evaluation in HCC.
TAMs refer to the most abundant immune cell subsets in tumor microenvironment and play a pivotal role in the initiation and progression of solid tumors, including HCC [20]. The usage of specific TAM subtypes as prognostic factor in caners has been widely reported in literature, and a variety of biomarkers have been demonstrated to classify and quantify TAM populations [22,34,35]. Among them, CD68, a 110 kd transmembrane glycoprotein, is a classical macrophage marker and has been frequently used as an important indicator for TAMs [36]. Concrete evidence including ours demonstrated that high infiltration of CD68+ TAMs was closely associated with tumor progression was also recognized as an independent prognostic factor of poor survival in various malignancies, especially in HCC [30,[37], [38], [39], [40]]. Consistent with the above results, our data also identified CD68+ TAM infiltration to be an independent predictor for OS and PFS in HCC patients and confirmed its prognostic value in survival predictivity, thus making it reasonable to use CD68 as TAM-specific biomarker for the assessment of patients’ outcome in subsequent analyses.
Additionally, the cross-talk between CSCs and TAMs has gradually emerged as a hotspot of cancer research in recent years, and various TAM populations have been reported to be involved in the regulation of CSC-like properties in HCC [27,41]. As evidenced, CD68+ TAMs could induce the CSC-like properties of EpCAM+ cells through TGF-beta-induced epithelial-mesenchymal transition [25], while CD14+ TAMs could promote the self-renewal and expansion of CD44+ CSCs by producing IL-6 [42]. As for the selection of CSC-specific biomarkers, our team has long been committed to exploring the functional roles of CSC-like cells in the initiation and progression of HCC, and has identified OV6 as a potential biomarker for liver CSCs [14,15,43]. Meanwhile, high expression of OV6 was shown to significantly correlate with aggressive clinicopathological features and unfavorable prognosis of HCC patients, suggesting the potential role of OV6 in survival estimation of HCC patients. However, to our knowledge, no data on the correlation between OV6 expression and CD68+ TAM infiltration as well as their prognostic value in HCC has been reported so far. It is therefore worthwhile to determine whether the combined detection of OV6 and CD68 could help improve the prognostic performance for surgical-treated HCC patients. Thus, this study sought to determine the clinical relevance of CSC-like subsets and TAMs infiltration in HCC by jointly evaluating the expression patterns of OV6 and CD68 in primary HCC tissues. Our findings for the first time revealed a potential relationship between OV6+ CSCs and CD68+ TAMs in HCC, and confirmed that combined detection of these two indicators could more accurately predict post-surgical outcomes than each biomarker did alone.
Multiple clinicopathological variables such as tumor size, serum AFP level, and MVI can be used to forecast the prognosis of patients with HCC [1,44]. Considering that the combination of clinical features and intratumoral biomarkers could add more value to the overall prognostic accuracy in cancer patients [24], we also incorporated the MVI status and large tumor size, which were identified as independent predictors of worse OS by multivariate Cox regression analysis, into this newly established model, in an attempt to achieve better predictive power and higher clinical usefulness. As expected, the highest C-index value of integrating both OV6 and CD68 with clinical features was observed for OS and PFS of HCC patients, compared with that of any other indicators alone. In our opinion, the significance of this study lies in the establishment of a novel OV6-CD68-involved classifier in predicting outcomes based on the possible interaction between CSC and TAM subsets in HCC, and it is our hope to provide an efficient predictive tool that integrates reliable biomarkers and routine clinical variables to help clinicians better assess the prognosis and make individualized treatment decisions, thus making up for the lacks of valid biomarkers for predicting HCC outcome currently.
We also compared the prognostic value of our model with other well-accepted conventional clinical staging systems in patients with HCC [45,46]. By using ROC method, the predictive power of either Child-Pugh grade or TNM stage for 5-year OS and PFS was shown to be inferior to that of our prognostic model, implying the limited prognostic value in predicting patients’ postoperative survival (Supplementary Fig. 3). In addition, although a large number of pathological or oncogenic biomarkers have been reported to stratify HCC patients who are likely to benefit from specific therapies or to predict their clinical outcomes, most of them lack accuracy and have not been widely applied in clinical practice so far. In the present study, we also evaluated the predictive accuracy of several pathological factors, including Golgi protein 73, Glypican-3, and glutamine synthetase for the prognosis of HCC patients, but the limited prognostic performance prevented their clinical use as effective indicators for survival estimation, at least in this HCC cohort (data not shown). Even so, we still believed that more powerful prognostic predictors would be identified and prospective studies should be conducted to further assess their predictive values in stratifying the prognosis of HCC in the future.
The current study had several limitations. First, since this study only enrolled patients who underwent curative resection for HCC, this model may not be applicable to accurately predict outcomes for patients with advanced HCC who received anti-angiogenic agents or immunotherapies. Second, considering the great diversity of TAM subsets and the presence of a variety of biomarkers for TAM classification, we were unable to fully evaluate more immune-related biomarkers nor subsequently include them into our model. As such, whether other indicators or their combinations could achieve better prognostic performance than CD68 still deserves further exploration in future large-scale studies. Third, due to the inherent property of retrospective study design, data on whether patients had received appropriate therapies for recurrent HCC was not available, which may influence the OS of patients who developed HCC recurrence after surgery. Also, data regarding serum levels of des-gamma-carboxy prothrombin or other serological indices were missing or incomplete, since these biomarkers were not routinely detected in enrolled patients during the study period. Forth, although concrete positive correlation between OV6 and CD68 expression in HCC specimens was observed, the molecular mechanisms by which CD68+ TAMs interact with OV6+ CSCs in HCC progression, as well as the therapeutic potential by targeting the cross-talk between CSC and TAMs in HCC, are not involved, which are highly desirable for further investigation and still need to be addressed in our follow-up projects in the future.
In conclusion, increased expression of OV6 was positively correlated with more CD68+ TAMs infiltration in HCC. Furthermore, the integration of OV6 and CD68 with tumor size and MVI status exhibited superiority in evaluating the postoperative prognosis, and may serve as a more effective prognostic model for HCC patients after liver resection. Whether targeting the interplay between OV6+ CSCs and CD68+ TAMs can represent a promising therapeutic strategy for HCC needs to be further investigated.
Authors’ contributions
M.D.W. and H.X. contributed equally to this work. Conception and design: M.D.W. and C.W.; Administrative support: L.Z.; Provision of study materials: H.X.; Collection and assembly of data: H.X.; Data analysis and interpretation: M.D.W. and H.X.; Manuscript writing: M.D.W.; Critical revision: L.Z. and C.W.; All authors reviewed the manuscript.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Eastern Hepatobiliary Surgery Hospital.
Declaration of Competing Interest
All authors have no conflicts of interest to declare.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82173357, 81773154), Natural Science Foundation of Shanghai (No. 20ZR1449600, 22ZR1477900), Shanghai Science and Technology Committee Rising-Star Program (No. 22QA1411600) and Pudong New Area Science and technology development fund special fund for people's livelihood Research (medical and health) (PKJ2019-Y19).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101509.
Contributor Information
Ling Zhang, Email: zling1206@shsmu.edu.cn.
Chao Wang, Email: superwang2012@aliyun.com.
Appendix. Supplementary materials
References
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann. Surg. 2006;243(2):229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akateh C, Black SM, Conteh L, et al. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J. Gastroenterol. 2019;25(28):3704–3721. doi: 10.3748/wjg.v25.i28.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santhakumar C, Gane EJ, Liu K, McCaughan GW. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol. Int. 2020;14(6):947–957. doi: 10.1007/s12072-020-10104-3. [DOI] [PubMed] [Google Scholar]
- 6.Umeda S, Kanda M, Kodera Y. Recent advances in molecular biomarkers for patients with hepatocellular carcinoma. Expert Rev. Mol. Diagn. 2019;19(8):725–738. doi: 10.1080/14737159.2019.1638254. [DOI] [PubMed] [Google Scholar]
- 7.Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24(1):41–53. doi: 10.1016/j.stem.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9(1):50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem. Biophys. Res. Commun. 2006;351(4):820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49(1):318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W, Yan HX, Chen L, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68(11):4287–4295. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Wang C, Lin Y, et al. OV6⁺ tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J. Hepatol. 2012;57(3):613–620. doi: 10.1016/j.jhep.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Yang W, Yan HX, et al. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated HBx transgenic mice. Hepatology. 2012;55(1):108–120. doi: 10.1002/hep.24675. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Yu H, Chen S, et al. Prognostic significance of combining high mobility group Box-1 and OV-6 expression in hepatocellular carcinoma. Sci. China Life Sci. 2018;61(8):912–923. doi: 10.1007/s11427-017-9188-x. [DOI] [PubMed] [Google Scholar]
- 18.Ye F, Jing YY, Guo SW, et al. Proliferative ductular reactions correlate with hepatic progenitor cell and predict recurrence in HCC patients after curative resection. Cell Biosci. 2014;4(1):50. doi: 10.1186/2045-3701-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353 doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 22.Chittezhath M, Dhillon MK, Lim JY, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41(5):815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Fan L, Yu H, et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology. 2019;70(1):241–258. doi: 10.1002/hep.30607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhir M, Melin AA, Douaiher J, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann. Surg. 2016;263(6):1112–1125. doi: 10.1097/SLA.0000000000001556. [DOI] [PubMed] [Google Scholar]
- 25.Fan QM, Jing YY, Yu GF, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352(2):160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Wang C, Liu F, et al. Reciprocal network between cancer stem-like cells and macrophages facilitates the progression and androgen deprivation therapy resistance of prostate cancer. Clin. Cancer Res. 2018;24(18):4612–4626. doi: 10.1158/1078-0432.CCR-18-0461. [DOI] [PubMed] [Google Scholar]
- 27.Müller L, Tunger A, Plesca I, et al. Bidirectional crosstalk between cancer stem cells and immune cell subsets. Front. Immunol. 2020;11:140. doi: 10.3389/fimmu.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J. Clin. Oncol. 2005;23(36):9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida A, Tsuta K, Wakai S, et al. Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung cancers. Mod. Pathol. 2014;27(5):711–720. doi: 10.1038/modpathol.2013.192. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Hong T, Wang Y, et al. Integration of intratumoral RASSF10 expression and tumor-associated macrophages into the established clinical indicators better predicts the prognosis of clear cell renal cell carcinoma patients. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1736793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 32.Ayoub WS, Steggerda J, Yang JD, Kuo A, Sundaram V, Lu SC. Current status of hepatocellular carcinoma detection: screening strategies and novel biomarkers. Ther. Adv. Med. Oncol. 2019;11 doi: 10.1177/1758835919869120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walcher L, Kistenmacher AK, Suo H, et al. Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front. Immunol. 2020;11:1280. doi: 10.3389/fimmu.2020.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Hong T, Wang Y, et al. Combining UBR5 and CD163+ tumor-associated macrophages better predicts prognosis of clear cell renal cell carcinoma patients. Cancer Immunol. Immunother. 2021;70(10):2925–2935. doi: 10.1007/s00262-021-02885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu H, Zhu Y, Wang Y, et al. Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin. Cancer Res. 2018;24(13):3069–3078. doi: 10.1158/1078-0432.CCR-17-2687. [DOI] [PubMed] [Google Scholar]
- 36.Senovilla L, Vacchelli E, Galon J, et al. Trial watch: prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1(8):1323–1343. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum. Pathol. 2009;40(3):381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Wang X, Li X, et al. CD68(+)HLA-DR(+) M1-like macrophages promote motility of HCC cells via NF-κB/FAK pathway. Cancer Lett. 2014;345(1):91–99. doi: 10.1016/j.canlet.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y, He MY, Zhu LF, et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016;35:12. doi: 10.1186/s13046-015-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjödahl G, Lövgren K, Lauss M, et al. Infiltration of CD3⁺ and CD68⁺ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. 2014;32(6):791–797. doi: 10.1016/j.urolonc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Oshimori N, Guo Y, Taniguchi S. An emerging role for cellular crosstalk in the cancer stem cell niche. J. Pathol. 2021;254(4):384–394. doi: 10.1002/path.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Wang MD, Cheng P, et al. Hepatitis B virus X protein promotes the stem-like properties of OV6+ cancer cells in hepatocellular carcinoma. Cell Death Dis. 2017;8(1):e2560. doi: 10.1038/cddis.2016.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang MD, Li C, Liang L, et al. Early and late recurrence of hepatitis B virus-associated hepatocellular carcinoma. Oncologist. 2020;25(10):e1541–e1551. doi: 10.1634/theoncologist.2019-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 46.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Eastern Hepatobiliary Surgery Hospital.