Abstract
Background
Cardiac surgery studies have established the clinical relevance of personalised arterial blood pressure management based on cerebral autoregulation. However, variabilities exist in autoregulation evaluation. We compared the association of several cerebral autoregulation metrics, calculated using different methods, with outcomes after cardiac surgery.
Methods
Autoregulation was measured during cardiac surgery in 240 patients. Mean flow index and cerebral oximetry index were calculated as Pearson's correlations between mean arterial pressure (MAP) and transcranial Doppler blood flow velocity or near-infrared spectroscopy signals. The lower limit of autoregulation and optimal mean arterial pressure were identified using mean flow index and cerebral oximetry index. Regression models were used to examine associations of area under curve and duration of mean arterial pressure below thresholds with stroke, acute kidney injury (AKI), and major morbidity and mortality.
Results
Both mean flow index and cerebral oximetry index identified the cerebral lower limit of autoregulation below which MAP was associated with a higher incidence of AKI and major morbidity and mortality. Based on magnitude and significance of the estimates in adjusted models, the area under curve of MAP < lower limit of autoregulation had the strongest association with AKI and major morbidity and mortality. The odds ratio for area under the curve of MAP < lower limit of autoregulation was 1.05 (95% confidence interval, 1.01–1.09), meaning every 1 mm Hg h increase of area under the curve was associated with an average increase in the odds of AKI by 5%.
Conclusions
For cardiac surgery patients, area under curve of MAP < lower limit of autoregulation using mean flow index or cerebral oximetry index had the strongest association with AKI and major morbidity and mortality. Trials are necessary to evaluate this target for MAP management.
Keywords: acute kidney injury; cardio pulmonary bypass; cerebral autoregulation; data visualisation; individualised blood pressure management; major morbidity, mortality; organ injury; postoperative outcome
Editor's key points.
-
•
This study addresses a fundamental precision medicine opportunity for perioperative management: for every patient there is an optimal mean arterial pressure autoregulatory range for perfusion of vital organs that can differ substantially among people.
-
•
The investigators sought to establish, in patients undergoing cardiac surgery, whether the extent and duration below the optimal mean arterial pressure range, derived from measures of cerebral oxygenation or blood flow, were associated with major adverse postoperative outcomes.
-
•
Patients who had longer and more extensive intraoperative dips below the lower limit of autoregulation of their optimal mean arterial pressure range were more likely to suffer major postoperative organ injury.
-
•
The ability to impact outcomes through implementation of these physiological insights will require a thoughtful trial, for example comparing precision mean arterial pressure thresholds with a generic mean arterial threshold.
Cerebral autoregulation is a protective mechanism that exists in the brain's vasculature, to maintain stable cerebral blood flow to support oxidative metabolism. A variety of indices have been developed for autoregulation assessment.1, 2, 3, 4, 5, 6, 7 Prior studies in cardiac surgery have established the clinical relevance of personalised arterial blood pressure management based on metrics of autoregulation.8, 9, 10, 11 However, the methods used to assess autoregulation and potential hypoperfusion are not uniform, with important sources of variability including:
-
(1)
Different indices of cerebral autoregulation (e.g. transcranial Doppler-based mean flow index vs near-infrared spectroscopy-based cerebral oximetry index).
-
(2)
Different autoregulation metrics used to identify mean arterial blood pressure (MAP) thresholds, for example optimal MAP12,13 vs lower limit of autoregulation (LLA).3 Optimal MAP is defined as the MAP at which optimal autoregulation occurs14; and the LLA is defined as the critical MAP value below which cerebral blood flow decreases monotonically with decreases in MAP.3
-
(3)
Different parameters to quantify potential hypoperfusion: the duration of low MAP vs the area under curve (AUC) of low MAP calculated as the product of magnitude and duration of MAP below a certain target.10 As an extension to this latter point, although most prior studies report the AUC of MAP below a certain target,8, 9, 10, 11 it is unclear whether duration and magnitude of MAP below a threshold should be considered equivalent.
These questions are highly clinically relevant. First, both transcranial Doppler-based mean flow index and near-infrared spectroscopy-based cerebral oximetry index can be used to estimate cerebral autoregulation. However, transcranial Doppler is difficult to use in clinical practice owing to the difficulty in finding and maintaining quality monitoring from temporal windows.15, 16 Near-infrared spectroscopy-based indices of autoregulation may be preferable for simplicity, convenience, and stability,17 but assumptions about using near-infrared spectroscopy may limit accuracy in clinical use. Second, both optimal MAP and the LLA may be estimated from cerebral autoregulation monitoring. Most studies during cardiopulmonary bypass have used a threshold of MAP at the LLA, as this is thought to be the lowest MAP at which adequate cerebral perfusion is maintained. However, a number of studies in patients with head injury or preterm infants have suggested a close association of a higher target (i.e. optimal MAP) with outcomes.5,12,13,18,19 The strength of evidence for optimal MAP has provided the basis for ongoing multi-national clinical trials using optimal perfusion pressure in critically ill patients with neurological injury,20, 21, 22 such as the CPPopt Guided Therapy: Assessment of Target Effectiveness (COGiTATE) trial (https://cppopt.org/).20 However, during cardiac surgery, it is unknown which target (optimal MAP or LLA) has the strongest association with neurological and other end-organ outcomes, such as acute kidney injury (AKI).23,24 Finally, it is critical to understand the relationship between duration and magnitude of MAP below a target threshold and patient outcome. A more precise understanding would guide clinical practice on the relative importance of duration vs magnitude of hypotension.
To address these questions, the objective of this study was to compare the association of cerebral autoregulation metrics (estimated by eight different methods derived from factorial combination of the above three parameters) with three clinical outcomes in patients undergoing cardiac surgery. Identifying the method with the strongest association would provide insight into the most relevant metrics of cerebral hypoperfusion and guide the design of future interventional studies. Furthermore, inspired by the work of Güiza and colleagues25 and Donnelly and colleagues,26 we applied a three-dimensional (3-D) visualisation method to display the impact of duration and magnitude of low MAP on patient outcomes. We aim to introduce this straightforward visualisation tool to provide insight into the duration of time that patients can tolerate different intensities of hypotension.
Methods
This study was approved by the Johns Hopkins Institutional Review Board (IRB00086547, jhmeirb@jhmi.edu; Baltimore, MD, USA). Written informed consent was obtained from each patient. A list of abbreviations is included as Supplementary Table S1.
Patients
Patients undergoing cardiopulmonary bypass surgery at Johns Hopkins Hospital (Baltimore, MD, USA) were enrolled between August 4th, 2016 and August 23, 2019. Patients were included if they were >18 yr old and undergoing isolated or combined cardiac artery bypass graft, valve, aortic, or myectomy surgery. Exclusion criteria were lung or heart transplant, insertion of a ventricular assist device, or pre-existing kidney disease (dialysis). Patients without windows for transcranial Doppler analysis were excluded. Patients with baseline dialysis were excluded. Data on a subset of these patients have been published.27,28
Signal acquisition
Data recording started after induction of general anaesthesia and tracheal intubation and stopped upon the surgical closing. Arterial blood pressure was monitored invasively through the radial or femoral artery using a standard pressure monitoring (Baxter Healthcare, CardioVascular Group, Irvine, CA, USA). Continuous cerebral blood flow velocity was monitored through bilateral transcranial Doppler (Doppler Box; DWL, Singen, Germany) of the middle cerebral arteries through the temporal bone window at the depth around 40–65 mm.29,30 Two near-infrared spectroscopy probes (Covidien, Boulder, CO, USA) were placed on the patient's forehead to monitor total haemoglobin and regional cortical oxygen saturation. The data were recorded at a sampling frequency of 128 Hz and recorded synchronously using ICM+ software (University of Cambridge, Cambridge Enterprise, Cambridge, UK; https://icmplus.neurosurg.cam.ac.uk) through an A/D converter (DT9801; Data Translation, Marlboro, MA, USA) or digitally, directly from GE Solar monitors. Autoregulation data were not provided to the anaesthesiologists, who were masked. Artifacts introduced by tracheal suctioning, arterial line flushing, or transducer malfunction were removed manually. Artifact removal and cerebral autoregulation parameters were calculated using ICM+ software.
Perioperative care
All perioperative clinical management (including MAP management) was based on usual care, and autoregulation monitoring was not used for clinical decision-making. General anaesthesia was induced and maintained with fentanyl (5–20 μg kg−1), propofol (0.5–2.0 mg kg−1), neuromuscular blocking agents, and isoflurane. Dexmedetomidine infusion, ketamine infusion, or both were used at the discretion of the attending anaesthesiologist. Cardiopulmonary bypass surgery was carried out with a non-occlusive roller pump, a membrane oxygenator, and an arterial line filter of 40 μm or less. Non-pulsatile flow was maintained between 2.0 and 2.4 L min−1 m−2, with α-stat pH management. Partial pressure of carbon dioxide was maintained between approximately 4.7 and 6 kPa. Rewarming was based on institutional standards, with a goal pharyngeal temperature <37°C. During cardiopulmonary bypass, MAP targets were established based on discussions among the surgeons, anaesthesiologists, and perfusionists and were generally MAP >50–60 mm Hg. MAP targets in the ICU were 65–90 mm Hg, and inotropes were weaned based on estimates of adequate perfusion. Sedation after surgery was maintained with dexmedetomidine or propofol until patients were ready for extubation.
Defining the optimal MAP and lower limit of autoregulation
Transcranial Doppler-based mean flow index was calculated as a moving Pearson's correlation coefficient between 10-s averages of MAP and transcranial Doppler blood flow velocity, using a 300-s data window.1 Similarly, near-infrared spectroscopy based cerebral oximetry index was calculated as a moving Pearson's correlation coefficient between 10-s averages of MAP and near-infrared spectroscopy regional cortical oxygen saturation.2 Functional autoregulation is indicated by negative or near-zero mean flow index or cerebral oximetry index values because changes in cerebral blood flow and MAP are negatively or not correlated. Impaired autoregulation is indicated by high mean flow index or cerebral oximetry index values because changes in cerebral blood flow and MAP are correlated.2
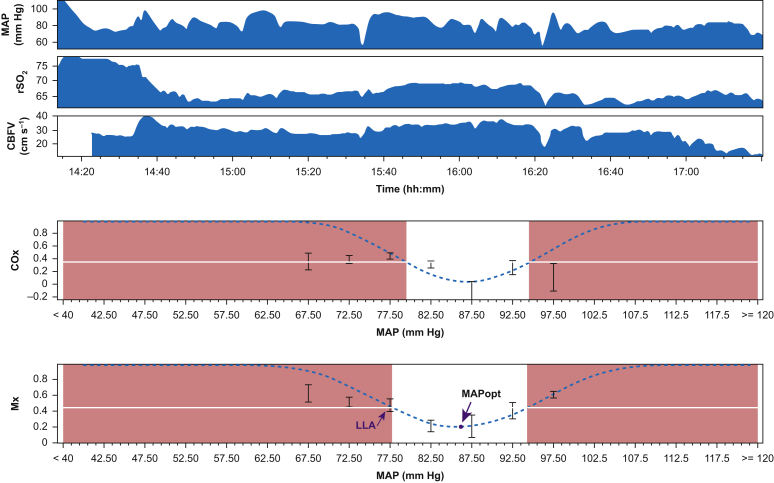
To define optimal MAP and lower limit of autoregulation (LLA), mean flow index and cerebral oximetry index values were averaged and placed into 5 mm Hg MAP bins (Fig. 1). Thus a ‘U-Shape’ curve can be depicted, and a curve fitting algorithm was applied.3,14,18 The MAP level at the lowest turning point of the ‘U-Shape’ curve was defined as the optimal MAP where cerebral autoregulatory function is most robust. The LLA was defined by drawing a straight horizontal line at cut-off values identified in our previous study27 (mean flow index, 0.45; cerebral oximetry index, 0.35) using ICM+ software3 as shown in Fig. 1. The x-coordinate of the point at which the straight line meets the U-shaped curve on the left side was defined as the LLA10,31 or treated as missing in the absence of an intersection. For patients who had available data from two sides (i.e. right and left) of the transcranial Doppler or near-infrared spectroscopy, we calculated LLA and optimal MAP of both sides and used the average. For patients with available data from only one side of the transcranial Doppler or near-infrared spectroscopy, we used data from that side.
Fig 1.
Identification of optimal arterial blood pressure (MAPopt) and lower limit of autoregulation (LLA), as indicated by the arrows. Mx, mean flow index; COx, cerebral oximetry index; CBFV, cerebral blood flow velocity; rSO2, regional cortical oxygen saturation.
AUC of MAP below optimal MAP or lower limit of autoregulation
To quantify the relationship between extension of MAP below a certain threshold and patient outcome, we calculated the AUC of MAP < optimal MAP (or LLA) through the product of magnitude (mm Hg) and duration (h) using the formula below AUC= (mm Hg min), where ΔTime is the time and Magnitudei is the individual sample values for the magnitude of MAP deviation below the optimal MAP (or LLA).11,32 We refer to this product of magnitude–time dose of MAP < optimal MAP as the AUC of MAP below optimal MAP. The same concept was also applied to the AUC of MAP below LLA.
Patient outcomes
The primary endpoints of the present study were AKI and major morbidity and mortality, based on prior literature suggesting associations of MAP below the LLA with these outcomes.9,11 We also examined stroke as an outcome, as impaired autoregulation has been associated with stroke.31 AKI was defined by comparing the maximal change in serum creatinine (SCr) in the first 2 postoperative days with baseline values measured before surgery using the Acute Kidney Injury Network (AKIN) criteria (increase in the ratio of SCr > 1.5 or acute increase in SCr >0.3 mg dl−1 within 48 h).33 Major morbidity and mortality includes stroke, AKI, mechanical lung ventilation >48 h, low cardiac output syndrome (inotrope use >24 h or new requirement for intra-aortic balloon pump insertion; inotropes include epinephrine, norepinephrine, vasopressin, dopamine, and milrinone),34,35 or operative death (i.e. all deaths that occurred during the hospitalisation in which the operation was performed, even if after 30 days, and deaths that occurred after discharge from the hospital but within 30 days of the procedure, unless the cause of death was clearly unrelated to the operation).11 The logistic European System for Cardiac Operative Risk Evaluation (logEuroSCORE) was additionally recorded for each patient because this index is widely used to identify postoperative risk for adverse effects after adult cardiac surgery.36
Visualisation of patient outcome at different pressure–time burdens of low MAP
We used a similar approach described by Güiza and colleagues25 to visualise the occurrence of each outcome at different pressure–time burdens of low MAP. Minute-by-minute resolution data (time-averaged) were first created, with each hypoperfusion episode defined as MAP below LLA at a given magnitude threshold I (mm Hg) for at least a given duration D (min). For each pair of magnitude and duration thresholds [I,D], we found all the patients (patient number: N) who experienced this pressure–time burden, and the number of patients with AKI (or major morbidity and mortality) among these patients was counted as Naki. The occurrence of AKI (or major morbidity and mortality) of this episode [I,D] was calculated as , the ratio between the number of patient with AKI (or major morbidity and mortality) divided by the total number of patients who experienced this pressure–time burden. For example, for the combination (5 mm Hg, 10 min), 132 patients experienced low MAP below LLA more than 5 mm Hg for longer than 10 min, and among these 132 patients, 33 patients developed AKI. Therefore, the occurrence of episode (5 mm Hg, 10 min) is 33/132=25%. After calculating the occurrence of AKI (or major morbidity and mortality) for each episode [I,D], a colour-coded figure was created with the magnitude of MAP below LLA defined as x-axis, duration of the low MAP defined as y-axis and the colour of each episode referring to the occurrence of AKI (or major morbidity and mortality). Red colour indicates high occurrence, whereas blue colour refers to low occurrence.
Moreover, in order to visualise the influence of potentially modifying factors, such as duration of cardiopulmonary bypass and cerebral oximetry on the development of AKI, demarcation curves (using a cut-off of risk of developing AKI=40%) were compared between patients with bypass duration >2 h and patients with bypass duration <2 h, and between episodes with regional cortical oxygen saturation >50 and episodes with regional cortical oxygen saturation <50.
Statistical analysis
Statistical analyses were calculated with Matlab software (version R2019B; MathWorks, Inc., Natick, MA, USA) and SPSS (version 25.0; IBM, Armonk, NY, USA).
The mean AUC and time duration of MAP < LLA or MAP < optimal MAP were calculated for each patient who had an LLA or optimal MAP; otherwise, the AUC or time duration were treated as missing. The mean AUC and time duration of MAP < optimal MAP (or MAP < LLA) were compared between patients with and without each outcome via logistic regression models adjusted by age, duration of bypass, and logEuroScore (decided a priori to be potentially confounding variables9). For all analyses, a P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
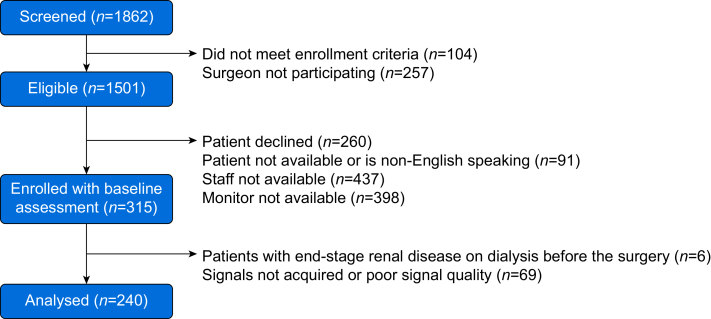
Baseline and perioperative characteristics from 240 patients are listed in Table 1. A patient flow diagram is shown in Fig. 2. A total of 205 patients had transcranial Doppler recordings through the temporal bone window. Of patients with available monitoring data, an optimal MAP could be identified in 91.3% (219/240) of patients using cerebral oximetry index and in 94.2% (193/205) of patients using mean flow index. An LLA could be identified in 72.9% (175/240) of patients using COx and in 51.2% (105/205) of patients using mean flow index. Characteristics of patients with and without an LLA are shown in Supplementary Table S2. Six patients had postoperative ischaemic stroke identified by CT, MRI, or both; 56 (23.3%) patients had AKI; and 80 (33.3%) patients had major morbidity and mortality. Perioperative characteristics by each outcome are described in Supplementary Tables S3 and S4. There were no significant difference between MAP at the LLA identified using cerebral oximetry index and MAP at the LLA identified using mean flow index (65.6 [8.3] mm Hg vs 68.2 [8.4] mm Hg, P=0.15). There were no significant differences between either the AUC < LLA calculated using cerebral oximetry index and the AUC < LLA calculated using the mean flow index (P=0.62), or the time duration of MAP < LLA calculated using the cerebral oximetry index and the time duration of MAP < LLA calculated using the mean flow index (P=0.38).
Table 1.
Patient details. CBFV, cerebral blood flow velocity; COx, cerebral oximetry index; CPB, cardiopulmonary bypass; MAP, mean arterial blood pressure; MAPopt, optimal mean arterial blood pressure; Mx, mean flow index; rSO2, regional cortical oxygen saturation; LLA, lower limit of autoregulation; IQR, inter-quartile range.
| Characteristic | |
|---|---|
| Age, yr, median (range) | 65 (25–87) |
| Male sex, n (%) | 191 (79.6) |
| Height, cm, mean (sd) | 173.4 (10.4) |
| Weight, kg, mean (sd) | 87.1 (22.3) |
| Baseline haemoglobin, g dl−1, median [IQR] | 13.5 [11.9–14.6] |
| Duration of bypass, min, mean (sd) | 110.9 (44.7) |
| Surgery duration, min, mean (sd) | 296.4 (71.6) |
| Mean LogEuroScore, %, median [IQR] | 2.93 [1.77–5.54] |
| Race, n (%) | |
| Caucasian | 196 (81.7) |
| African American | 30 (12.5) |
| Asian | 4 (1.7) |
| Other | 10 (4.2) |
| Prior congestive heart failure, n (%) | 81 (31.1) |
| Prior hypertension, n (%) | 200 (76.9) |
| Prior myocardial infarction, n (%) | 79 (20.4) |
| Current smoker, n (%) | 37 (14.2) |
| Anaesthetics during surgery | |
| Fentanyl administration, n (%) | 227 (94.6) |
| Dose of fentanyl (among recipients of fentanyl), μg, median [IQR] | 500 [250–950] |
| Ketamine administration, n (%) | 65 (27.1) |
| Dose of ketamine (among recipients of ketamine), mg, median [IQR] | 50.0 [30.0–91.2] |
| Midazolam administration, n (%) | 215 (89.6) |
| Dose of Midazolam (among recipients of midazolam), mg, median [IQR] | 2.0 [2.0–2.0] |
| Inotropes or vasopressors during surgery | |
| Epinephrine administration, n (%) | 206 (85.8) |
| Norepinephrine administration, n (%) | 99 (41.3) |
| Vasopressin administration, n (%) | 13 (5.4) |
| Patient outcome | |
| Stroke, n (%) | 6 (2.5) |
| Acute kidney injury, n (%) | 56 (23.3) |
| Mechanical lung ventilation >48 h, n (%) | 14 (5.8) |
| Low cardiac output syndrome, n (%) | 25 (10.4) |
| Operative death, n (%) | 0 (0) |
| MAP, mm Hg, mean (sd) | 76.1 (6.3) |
| Mean CBFV, cm s−1, mean (sd) | 46.0 (16.1) |
| Mean rSO2, mean (sd) | 61.1 (12.4) |
| Mx, mean (sd) | 0.55 (0.13) |
| COx, mean (sd) | 0.29 (0.16) |
| Mean MAPopt_Cox, mm Hg, mean (sd) | 76.8 (8.7) |
| Mean MAPopt_Mx, mm Hg, mean (sd) | 75.5 (8.6) |
| Mean LLA_COx, mm Hg, mean (sd) | 65.6 (8.3) |
| Mean LLA_Mx, mm Hg, mean (sd) | 68.2 (8.4) |
| Mean AUC of MAP < MAPopt_COx, mm Hg h, median [IQR] | 23.3 [13.4–38.8] |
| Mean AUC of MAP < MAPopt_Mx, mm Hg h, median [IQR] | 20.5 [11.5–35.6] |
| Mean Duration of MAP < LLA_COx, min, median [IQR] | 45 [25–89] |
| Mean Duration of MAP < LLA_Mx, min, median [IQR] | 62 [31–111] |
Fig 2.
Patient flowchart.
Comparison of different methods of assessing cerebral autoregulation in relation to three outcomes
Table 2 shows the results for three clinically important outcomes (stroke, AKI, major morbidity and mortality) in association with eight different methods to calculate cerebral autoregulation metrics derived by varying three parameters.
Table 2.
The association of cerebral autoregulation metrics (as calculated using different parameters) with stroke, acute kidney injury, and major morbidity and mortality. ∗Adjusted for age, duration of bypass, and logistic EuroSCORE. †Data format: median (inter-quartile range). ‡Data format: mean (sd). The odds ratio is used to determine whether a particular exposure (e.g. low MAP) is a risk factor for a particular outcome (e.g. AKI), and to compare the magnitude of various risk factors for that outcome. For example, in this table, the odds ratio for AUC of MAP < LLA_COx of AKI was 1.05 [1.01–1.09], meaning every 1 mm Hg h increase of AUC was associated with an increase in the odds of AKI by 5% on average. MMOM, major morbidity and mortality; AKI, acute kidney injury; AUC, area under the curve, calculated as the produce of magnitude and duration of MAP below a certain threshold; LLA, lower limit of autoregulation; COx, cerebral oximetry index; Mx, mean flow index; MAP, mean arterial blood pressure; MAPopt, optimal arterial blood pressure; OR, odds ratio; SD, standard deviation; P < 0.05 is considered to be significant difference, marked in bold.
| Stroke |
Acute kidney injury |
Major morbidity or mortality |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without stroke | With stroke | Adjusted∗ |
Without AKI | With AKI | Adjusted∗ |
Without MMOM | With MMOM | Adjusted∗ |
||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||||||||
| COx | MAPopt | AUC of MAP < MAPopt_COx (mm Hg h)† | 22.3 (13.0, 38.8) | 36.5 (30.8, 38.4) | 1.01 [0.98–1.05] | 0.49 | 22.2 (12.4, 39.0) | 25.8 (18.8, 38.5) | 1.00 [0.99–1.02] | 0.80 | 21.6 (12.0, 40.3) | 27.3 (17.9, 38.4) | 1.00 [0.99–1.02] | 0.56 |
| Duration of MAP < MAPopt_COx (min)‡ | 158.9 (66.0) | 247.3 (66.8) | 1.01 [1.00–1.03] | 0.18 | 156.3 (68.3) | 179.9 (59.7) | 1.00 [1.00–1.01] | 0.18 | 153.1 (65.2) | 179.1 (68.7) | 1.00 [1.00–1.01] | 0.08 | ||
| LLA | AUC of MAP < LLA_COx (mm Hg h)† | 4.3 (2.3, 10.0) | 11.0 (6.5, 17.5) | 1.04 [0.95–1.14] | 0.36 | 3.9 (1.9, 8.5) | 8.7 (3.1, 15.1) | 1.05 [1.01–1.09] | 0.01 | 3.9 (2.1, 8.6) | 7.3 (3.0, 14.6) | 1.04 [1.01–1.08] | 0.01 | |
| Duration of MAP < LLA_COx (min)‡ | 60.3 (51.8) | 100.0 (65.2) | 1.01 [0.99–1.03] | 0.23 | 52.5 (45.7) | 85.1 (61.5) | 1.01 [1.01–1.02] | 0.001 | 53.4 (46.4) | 77.1 (59.4) | 1.01 [1.00–1.02] | 0.004 | ||
| Mx | MAPopt | AUC of MAP < MAPopt_Mx (mm Hg h)† | 20.6 (11.4, 35.4) | 15.9 (14.9, 53.3) | 1.01 [0.97–1.04] | 0.76 | 18.0 (11.0, 31.8) | 23.7 (14.5, 44.8) | 1.01 [1.00–1.03] | 0.05 | 18.1 (10.6, 31.3) |
23.6 (14.7, 44.2) | 1.02 [1.00–1.03] | 0.01 |
| Duration of MAP < MAPopt_Mx (min)‡ | 149.6 (72.4) | 219.0 (131.0) | 1.00 [0.99–1.01] | 0.78 | 141.7 (68.4) | 177.0 (88.0) | 1.01 [1.00–1.01] | 0.04 | 139.5 (65.4) | 176.5 (86.6)n | 1.01 [1.00–1.01] | 0.03 | ||
| LLA | AUC of MAP< LLA_Mx (mm Hg h)† | 6.3 (3.3, 14.9) | 5.3 (3.2, 27.7) | 1.00 [0.92–1.10] | 0.92 | 5.9 (2.9, 11.7) | 13.7 (4.7, 31.0) | 1.05 [1.01–1.09] | 0.01 | 5.5 (2.9, 12.0) | 9.1 (4.6, 23.0) | 1.06 [1.02–1.11] | 0.006 | |
| Duration of MAP < LLA_Mx (min)‡ | 77.4 (61.5) | 106.0 (112.4) | 1.00 [0.98–1.02] | 0.87 | 69.2 (56.0) | 113.6 (77.6) | 1.01 [1.00–1.02] | 0.01 | 68.4 (54.9) | 99.1 (72.5) | 1.01 [1.00–1.02] | 0.004 | ||
Based on comparing the magnitude and significance of the model estimates for each method, general observations for each parameter of interest can be made. For the index of autoregulation parameter, both mean flow index and cerebral oximetry index identified the cerebral LLA below which MAP was associated with a higher incidence of AKI and major morbidity and mortality. For MAP threshold parameter, MAP < LLA was more strongly associated with AKI and major morbidity and mortality than MAP < optimal MAP. For the magnitude of insult parameter, AUC and duration below a threshold showed similar associations with AKI and major morbidity and mortality.
Taken as a whole and combining parameters, there are several results to highlight in Table 2. Based on magnitude and significance of the estimates, the AUC of MAP < LLA had the strongest association with AKI and major morbidity and mortality, regardless of whether cerebral oximetry index or mean flow index was used. There was no statistical association of cerebral oximetry index-derived MAP < optimal MAP with AKI or major morbidity and mortality. The remaining four combinations of cerebral autoregulation metrics that were examined demonstrated significant associations with both AKI and major morbidity and mortality, but with less magnitude and significance of the associations, compared with AUC of MAP < LLA. Finally, there was no association of any cerebral autoregulation metric with stroke.
Visualisation of major morbidity and mortality or AKI occurrence at different pressure–time burden of low MAP
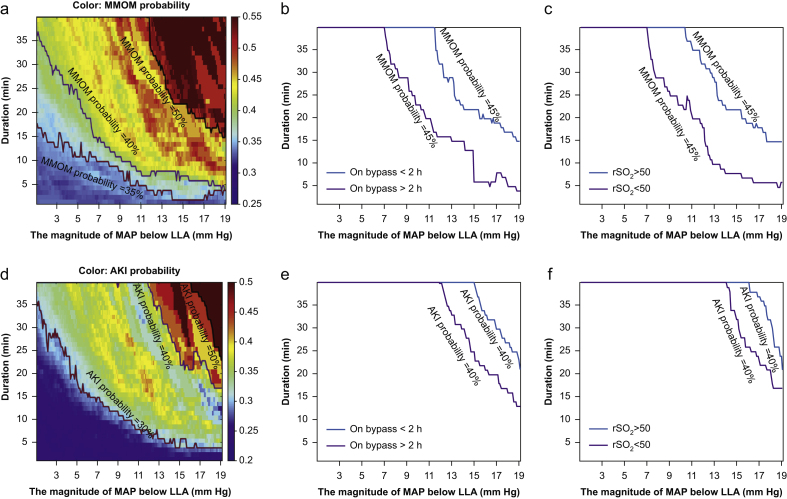
We next examined the contribution of low magnitude pressure vs duration in the calculation of the AUC of MAP < LLA as prior work has treated these parameters (pressure, duration) as equivalent. Figure 3 shows the visualisation of occurrence of major morbidity and mortality (panel a) and AKI (panel d) across different magnitudes and durations of MAP < LLA. The probability of both major morbidity and mortality and AKI increased as both the magnitude and duration of MAP < LLA increased, with highest occurrence in the top-right corner of the figure. For example, the black line in Fig. 3a shows that if the MAP was below LLA by 13 mm Hg for longer than 30 min, the probability of developing major morbidity and mortality increased to 50%. Figure 3b,c and e,f demonstrate that exact demarcation of different risk thresholds may vary according to other factors. For instance, Fig. 3b shows the contribution of duration of cardiopulmonary bypass. At the same level of low MAP, patients who experience longer duration of cardiopulmonary bypass (red line, Fig. 3b) have lower tolerance compared with patients with shorter duration of cardiopulmonary bypass (blue line). Similarly, Fig. 3c demonstrates that episodes with low regional cortical oxygen saturation (red line, Fig. 3c) are associated with more major morbidity and mortality compared with episodes with higher regional cortical oxygen saturation, even at the same level of low MAP (blue line, Fig. 3c). Similar results are demonstrated for AKI, with longer duration of cardiopulmonary bypass (red line, Fig. 3e) and lower regional cortical oxygen saturation (red line, Fig. 3f) being associated with a reduced tolerance for a given magnitude and duration of decreased MAP.
Fig 3.
Visualising the relationship between the magnitude of MAP below LLA (mm Hg), time duration (min) and occurrence of major morbidity and mortality or AKI. As an overview, each coordinate in the figures refers to a hypoperfusion episode of MAP below LLA at a certain magnitude (mm Hg) for at least a certain duration (min). For each pair of magnitude and duration thresholds, the probability of AKI or MMOM is calculated and then represented by colour codes (blue colour indicates low probability and red colour indicates high probability). The solid lines demarcate distinct transitions in probabilities for each outcome. For example, the colour coding and black line in panel (a) demonstrate that if the MAP was below LLA cerebral oximetry index by 13 mm Hg, and lasted for longer than 30 min, the probability of developing MMOM increased to 50%. Panels (a) and (d) demonstrate that the probability of both MMOM and AKI increase as both the magnitude and duration of MAP < LLA increase, with highest occurrence in the top-right corner of each panel. Panels (b) and (e) demonstrate the modifying effects of bypass duration on the probability of AKI (b) and MMOM (e). Panels (c) and (f) demonstrate the modifying effects of reduced cerebral oximetry values (regional cortical oxygen saturation [rSO2]) on probability of AKI (c) and MMOM (f). These latter panels demonstrate that patients with longer duration of cardiopulmonary bypass and lower cerebral oximetry values (rSO2) have an increased risk of AKI and MMOM even at similar intensities and duration of MAP below the LLA, as compared with patients who had shorter duration of bypass or higher cerebral oximetry values. MAP, mean arterial blood pressure; LLA, lower limit of autoregulation; AKI, acute kidney injury; MMOM, major morbidity and operative mortality.
Discussion
Currently, there is no agreement on the appropriate MAP targets for individual patients during cardiac surgery. Although individualised monitoring of cerebral autoregulation parameters may provide insight, a variety of different approaches to monitor autoregulation have been used, each of which may provide different estimates of MAP targets. We compared eight different approaches to calculating cerebral autoregulation metrics, using factorial combinations of three sources of variation. The main finding of this study was that the AUC of MAP < LLA had the strongest association with AKI and major morbidity and mortality, regardless of whether cerebral oximetry index or mean flow index was used. The odds ratio for AUC of MAP < LLA was 1.05 (1.01–1.09), meaning every 1 mm Hg h increase of AUC was associated with an increase in the odds of AKI by 5% on average.
In addition, our new direct visualisation tool can display the interaction of duration and magnitude of low MAP on patient outcomes in a novel way.
There are several parameters that can be varied in the calculation of cerebral autoregulation metrics. We examined three key parameters: (1) the specific index of autoregulation calculated using transcranial Doppler vs near-infrared spectroscopy, (2) different thresholds based on autoregulation curves, and (3) duration vs AUC below a threshold. Understanding the optimal parameters to use could help optimise MAP targets for future interventional trials and for clinical practice.
With respect to the first parameter (index of autoregulation), an important area of uncertainty has been the use of transcranial Doppler spectroscopy-vs near-infrared spectroscopy-based approaches to monitor cerebral autoregulation. Although transcranial Doppler-based approaches have historically been considered the gold standard, near-infrared spectroscopy-based approaches offer several advantages, including ease of use, more complete data, and less artifacts comparing with transcranial Doppler. Because cerebral near-infrared spectroscopy is routinely used during cardiopulmonary bypass, monitoring cerebral autoregulation using near-infrared spectroscopy-based approaches would be highly clinically relevant. However, questions about the accuracy of near-infrared spectroscopy-based approaches stem from several areas of uncertainty, including the contribution of extracerebral tissue and the potentially confounding effects of medications or other changes in physiology. This study compared (in the same patients) a near-infrared spectroscopy-based index of autoregulation to a transcranial Doppler-based index of autoregulation. The results showed that, regardless of the index of autoregulation, deviations of MAP < LLA were associated with AKI and major morbidity and mortality, supporting the application of near-infrared spectroscopy-based monitoring of cerebral autoregulation during cardiac surgery. Moreover, the LLA could be identified in more patients using near-infrared spectroscopy-based monitoring of cerebral autoregulation than using transcranial Doppler-based approaches, with potential reasons including difficulties in maintaining consistent transcranial Doppler monitoring and signal artifacts.
A second area of uncertainty has been the exact MAP threshold from cerebral autoregulation monitoring to use as a target. During cardiac surgeries, MAP targets are often lower than a patient's baseline MAP for several reasons, including to reduce bleeding from collateral circulation during bypass, to reduce stress on surgically repaired tissue, or to reduce the need for extensive inotropic or vasopressor support. Therefore, most studies examining cerebral autoregulation characteristics during cardiac surgery have used the MAP at the LLA.8, 9, 10, 11 However, optimal pressure, which is higher than the LLA, has been validated widely in the neurological ICU, and strong associations between poor outcome and deviations from optimal pressure have been reported.5,12,13,18 19 Thus, in patients undergoing cardiac surgery, it is unclear whether the cerebral LLA or optimal MAP is a better target for reducing the risk of AKI or major morbidity and mortality. This is clinically relevant because a higher MAP target might require more intropic or vasopressor support, may increase surgical bleeding, or both. The results of our study provide insight into the utility of cerebral LLA and optimal MAP as potential MAP targets during cardiac surgery, but trials are needed to compare these two approaches directly. On a related note, although our focus has been on potential hypoperfusion, it is also important to point out that MAP above the upper limit of autoregulation (ULA) may be important to consider for neurological outcomes, such as delirium.28 However, the relatively short period in the operating room along with a relatively low MAP means that a ULA cannot be identified as frequently as an LLA. Although the ULA may be less relevant for outcomes that are likely related to hypoperfusion, it is noteworthy that the plateau of cerebral autoregulation based on average data was less than 25 mm Hg MAP and so MAP may cross the ULA in usual practice, especially in the postoperative period.
Finally, we also examined the contribution of AUC vs duration-only of MAP below a critical threshold in leading to postoperative complications, as prior work has treated these parameters (magnitude, duration) as equivalent.10,11 We first found in regression models that both duration and AUC of MAP below critical thresholds were similarly associated with postoperative AKI and major morbidity and mortality. To further investigate the relative importance of duration and magnitude of low MAP, we used a visualisation tool developed by Güiza and colleagues,25 which estimates the risk of an outcome based on various combinations of duration and magnitude of MAP below a critical threshold. The plots visually demonstrate that both magnitude and duration of MAP below a critical threshold together are associated with AKI and major morbidity and mortality. Furthermore, the plots provide outcome-specific risk cut-offs in the cardiac surgery patient population to guide haemodynamic management. Finally, the tool also shows that other factors, such as duration of cardiopulmonary bypass and cerebral oximetry values, may modify thresholds of risk. Longer bypass duration and separately lower rSO2 were associated with more AKI and major morbidity and mortality at similar exposures to MAP below the cerebral LLA. In the future, the magnitude–duration plot may be a tool to individualising prediction for postoperative outcomes based on interactions of magnitude and duration of MAP below a critical threshold.
Strengths and limitations
Strengths of this study include a systematic comparison of several autoregulation indexes for personalised MAP management during cardiac surgery and a novel method of visualising the influence of duration and magnitude of low MAP in patient outcome. The tool could be extended to various types of surgery and postoperative care. Instead of using only one type of patient outcome, we include several important morbidities after cardiac surgery (major morbidity and mortality, AKI, and stroke), outcomes for which there is a strong biologic rationale for the importance of hypoperfusion and for which prior studies suggested the clinical relevance of metrics of autoregulation,9,11 Moreover, the thresholds of LLA (0.35 for cerebral oximetry index and 0.45 for mean flow index) in this study were identified through a rigorous process described in our previous publication.27 The difference of the cut-offs between the two parameters might be because transcranial Doppler and near-infrared spectroscopy are detecting different modalities of cerebral blood flow, with transcranial Doppler mainly detecting the cerebral blood flow in large vessels and near-infrared spectroscopy mainly detecting cerebral oxygen saturations. This observation further underscores the importance of considering the subtleties of the different monitoring techniques for monitoring autoregulation.
However, there are several limitations to consider. First, there were a limited number of patients with postoperative stroke, thus limiting our ability to make inferences. Other brain-focused outcome metrics, such as neurological/cognitive outcomes, delirium, or brain MRI, may more directly demonstrate the importance of potential cerebral hypoperfusion. Second, an LLA could not be identified in all patients for several reasons, including insufficient MAP values to visualise a curve at the patient's actual LLA, an autoregulation curve that was always above or below the cut-off and thus did not intersect the cut-off, and imprecise curves because of artifact. An LLA was identified less often than optimal MAP, because in some of these scenarios (e.g. an autoregulation curve always above or below a cut-off), an optimal MAP but not LLA could be identified. The difficulty in consistently identifying an LLA was magnified with transcranial Doppler-based indices because motion-based artifacts can reduce the number of data points at each MAP. There are usually less data points at the LLA than at optimal MAP and so reductions in data points at the LLA can disproportionately affect the quality of the curve at the LLA. Although characteristics of patients with and without a reference LLA were similar (Supplementary Table S2), it is also not certain that the thresholds we identified can be applied to all patients. Third, autoregulation data were calculated using periods of both pulsatile and non-pulsatile flow. The assumptions that underlie the characterisation of autoregulation do not depend on changes in blood pressure at the frequency of heart rate, and these methods have been used in prior studies.2,9,10,37 Nevertheless, there may be changes in cerebral physiology during bypass that could alter cerebral reactivity and introduce noise into time-averaged measurements in these calculations. In addition, there were differences between patients in anaesthetic and inotropic medications administered during surgery that could potentially affect autoregulation, but there were no associations with the outcomes of interest (Supplementary Table S4). Fourth, unlike the ICU-based study from Güiza and colleagues,25 the data in the present study were collected intraoperatively and the monitoring duration was shorter, resulting in coarse demarcation curves owing to limited data points. Finally, this is an observational study, and it is unclear the degree to which managing mean arterial pressure using these approaches could improve patient outcomes.
Conclusions
For patients undergoing cardiac surgery, the predictive potential of mean arterial pressure staying below lower limit of autoregulation the predictive potential of mean arterial pressure staying below lower limit of autoregulation (using either transcranial Doppler spectroscopy-based or near-infrared spectroscopy-based metrics of cerebral autoregulation) had the strongest association with acute kidney injury and major morbidity and mortality. Data from randomised trials are needed to support the clinical relevance of these thresholds to modify intraoperative management. A visualisation method has the potential to demonstrate the interaction of magnitude and duration of mean arterial pressure below critical thresholds with respect to acute kidney injury and major morbidity and mortality.
Authors' contributions
Study conception/design: all authors
Data analysis and acquisition: XYL, KA, CHB
Data interpretation: all authors
Writing of original draft: XYL, CHB
Critical review/editing of paper: all authors
Final approval of the version to be submitted: all authors Agreement to be accountable for all aspects of the work: all authors
Declarations of interest
CHB reported receiving grants from the National Institutes of Health (NIH) during the conduct of the study, and consulting for and participating in a data share with Medtronic. CWH reported receiving grants and personal fees for being a consultant and providing lectures for Medtronic/Covidien, Inc., being a consultant to Merck, Inc., and receiving grants from the NIH outside of the submitted work. JKL has received support from and been a paid consultant for Medtronic, and she is a paid consultant Edwards Life Sciences. JKL arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Some methods used to measure and monitor autoregulation as described in this manuscript were patented by The Johns Hopkins University, listing KMB as a co-inventor. These patents are exclusively licensed to Medtronic Inc., and KMB received a portion of the licensing fee. PS and MC are authors of ICM+ software licensed by Cambridge Enterprise Ltd, UK, and have a financial interest in a part of licensing fee.
Handling editor: Michael Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.03.029.
Contributor Information
Xiuyun Liu, Email: liuxiuyun1@gmail.com.
Charles H. Brown, IV, Email: cbrownv@jhmi.edu.
Funding
US National Institutes of Health (K76 AG057020 to CHB, R01HL092259 to CWH; and NIH NINDS R01 NS107417 and R01 NS113921 to JKL).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Czosnyka M., Smielewski P., Kirkpatrick P., Menon D.K., Pickard J.D. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 2.Brady K., Joshi B., Zweifel C., et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly J., Czosnyka M., Adams H., et al. Individualising thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45:1464–1471. doi: 10.1097/CCM.0000000000002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young A.M.H., Donnelly J., Czosnyka M., et al. Continuous multimodality monitoring in children after traumatic brain injury—preliminary experience. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X., Schreiber M., Donnelly J., et al. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: a retrospective study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera-Lara L., Zorrilla-Vaca A., Healy R.J., et al. Determining the upper and lower limits of cerebral autoregulation with cerebral oximetry autoregulation curves: a case series. Crit Care Med. 2018;46:e473–e477. doi: 10.1097/CCM.0000000000003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady K.M., Lee J.K., Kibler K.K., Easley R.B., Koehler R.C., Shaffner D.H. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke. 2008;39:2531–2537. doi: 10.1161/STROKEAHA.108.514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogue C.W., Palin C.A., Arrowsmith J.E. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103:27–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 9.Ono M., Arnaoutakis G.J., Fine D.M., et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown C.H., Neufeld K.J., Tian J., et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surg. 2019;154:819–826. doi: 10.1001/jamasurg.2019.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono M., Brady K., Easley R.B., et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aries M.J.H., Czosnyka M., Budohoski K.P., et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 13.da Costa C.S., Czosnyka M., Smielewski P., Austin T. Optimal mean arterial blood pressure in extremely preterm infants within the first 24 hours of life. J Pediatr. 2018;203:242. doi: 10.1016/j.jpeds.2018.07.096. [DOI] [PubMed] [Google Scholar]
- 14.Aries M.J.H., Czosnyka M., Budohoski K.P., et al. Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care. 2012;17:67–76. doi: 10.1007/s12028-012-9687-z. [DOI] [PubMed] [Google Scholar]
- 15.Bein B., Meybohm P., Cavus E., et al. A comparison of transcranial Doppler with near infrared spectroscopy and indocyanine green during hemorrhagic shock: a prospective experimental study. Crit Care. 2006;10:R18. doi: 10.1186/cc3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smielewski P., Czosnyka M., Pickard J.D., Kirkpatrick P. Clinical evaluation of near-infrared spectroscopy for testing cerebrovascular reactivity in patients with carotid artery disease. Stroke. 1997;28:331–338. doi: 10.1161/01.str.28.2.331. [DOI] [PubMed] [Google Scholar]
- 17.Kuebler W.M., Sckell A., Habler O., et al. Noninvasive measurement of regional cerebral blood flow by near-infrared spectroscopy and indocyanine green. J Cereb Blood Flow Metab. 1998;18:445–456. doi: 10.1097/00004647-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Steiner L.A., Czosnyka M., Piechnik S.K. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Petkus V., Preiksaitis A., Chaleckas E., et al. Optimal cerebral perfusion pressure: targeted treatment for severe traumatic brain injury. J Neurotrauma. 2020;37:389–396. doi: 10.1089/neu.2019.6551. [DOI] [PubMed] [Google Scholar]
- 20.Beqiri E., Smielewski P., Robba C., et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias C., Silva M.J., Pereira E., et al. Optimal cerebral perfusion pressure management at bedside: a single-center pilot study. Neurocrit Care. 2015;23:92–102. doi: 10.1007/s12028-014-0103-8. [DOI] [PubMed] [Google Scholar]
- 22.Jaeger M., Dengl M., Meixensberger J., Schuhmann M.U. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med. 2010;38:1343–1347. doi: 10.1097/CCM.0b013e3181d45530. [DOI] [PubMed] [Google Scholar]
- 23.Hobson C.E., Yavas S., Segal M.S., et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 24.Arora P., Kolli H., Nainani N., Nader N., Lohr J. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26:687–697. doi: 10.1053/j.jvca.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Güiza F., Depreitere B., Piper I., et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015;41:1067–1076. doi: 10.1007/s00134-015-3806-1. [DOI] [PubMed] [Google Scholar]
- 26.Donnelly J., Gu F., Depreitere B., Meyfroidt G., Czosnyka M., Smielewski P. Visualising the pressure-time burden of elevated intracranial pressure after severe traumatic brain injury: a retrospective confirmatory study. Br J Anaesth. 2021;126 doi: 10.1016/j.bja.2020.09.018. e15–7. [DOI] [PubMed] [Google Scholar]
- 27.Liu X.L., Akiyoshi K., Nakano M., et al. Determining thresholds for three indices of autoregulation to identify the lower limit of autoregulation during cardiac surgery. Crit Care Med. 2021;49:650–660. doi: 10.1097/CCM.0000000000004737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano M., Nomura Y., Whitman G., et al. Cerebral autoregulation in the operating room and intensive care unit after cardiac surgery. Br J Anaesth. 2021;126:967–974. doi: 10.1016/j.bja.2020.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bathala L., Mehndiratta M., Sharma V. Transcranial Doppler: technique and common findings (Part 1) Ann Indian Acad Neurol. 2013;16:174–179. doi: 10.4103/0972-2327.112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley R.E., Chang J.Y., Scheinman N.J., Levin B.E., Duncan R.C., Lee S.C. Transcranial Doppler assessment of cerebral flow velocity during cognitive tasks. Stroke. 1992;23:9–14. doi: 10.1161/01.str.23.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Ono M., Joshi B., Brady K., et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;103:391–398. doi: 10.1093/bja/aes148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aries M.J.H., Elting J.W., De Keyser J., Kremer B.P.H., Vroomen P.C.A.J. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010;41:2697–2704. doi: 10.1161/STROKEAHA.110.594168. [DOI] [PubMed] [Google Scholar]
- 33.Mehta R.L., Kellum J.A., Shah S.V., et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maganti M.D., Rao V., Borger M.A., Ivanov J., David T.E. Predictors of low cardiac output syndrome after isolated aortic valve surgery. Circulation. 2005;112:I448–I452. doi: 10.1161/CIRCULATIONAHA.104.526087. [DOI] [PubMed] [Google Scholar]
- 35.Algarni K.D., Maganti M., Yau T.M. Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 2011;92:1678–1684. doi: 10.1016/j.athoracsur.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Roques F., Michel P., Goldstone A., Nashef S. The logistic EuroSCORE. Eur Heart J. 2003;24:882–883. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 37.Hori D., Nomura Y., Ono M., et al. Optimal blood pressure during cardiopulmonary bypass defined by cerebral autoregulation monitoring. J Thorac Cardiovasc Surg. 2017;154:1590–1598. doi: 10.1016/j.jtcvs.2017.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.